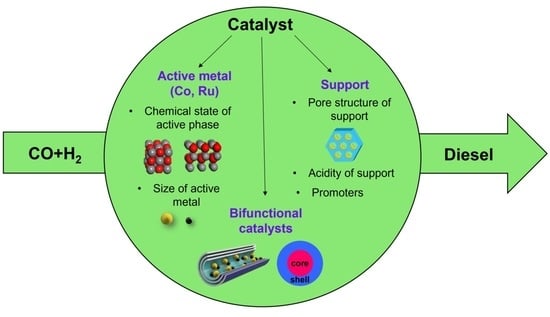
Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction
Abstract
:1. Introduction
2. The Main Factors Affecting the Selectivity of Diesel Fuels
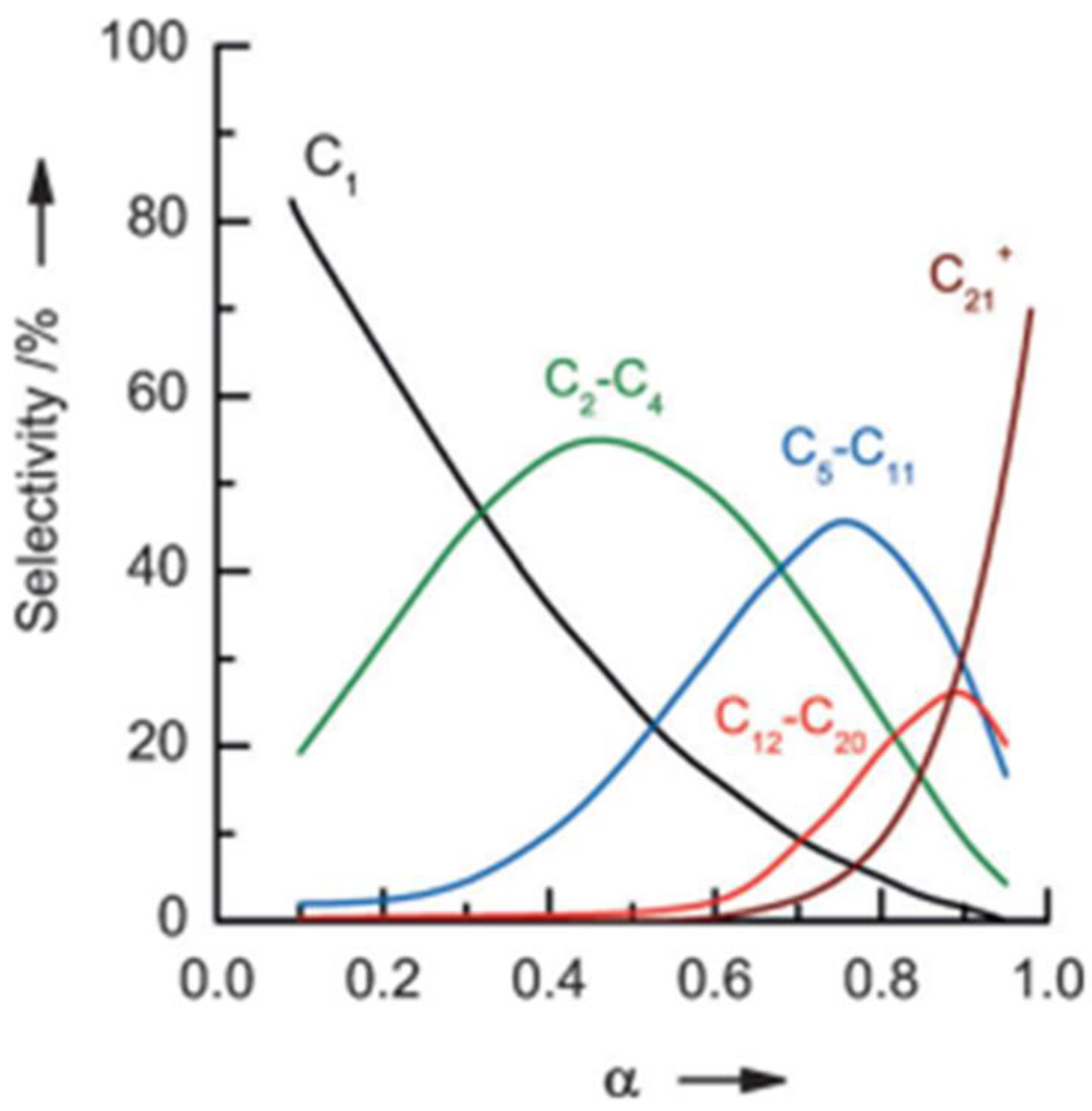
2.1. Fischer–Tropsch Process Conditions
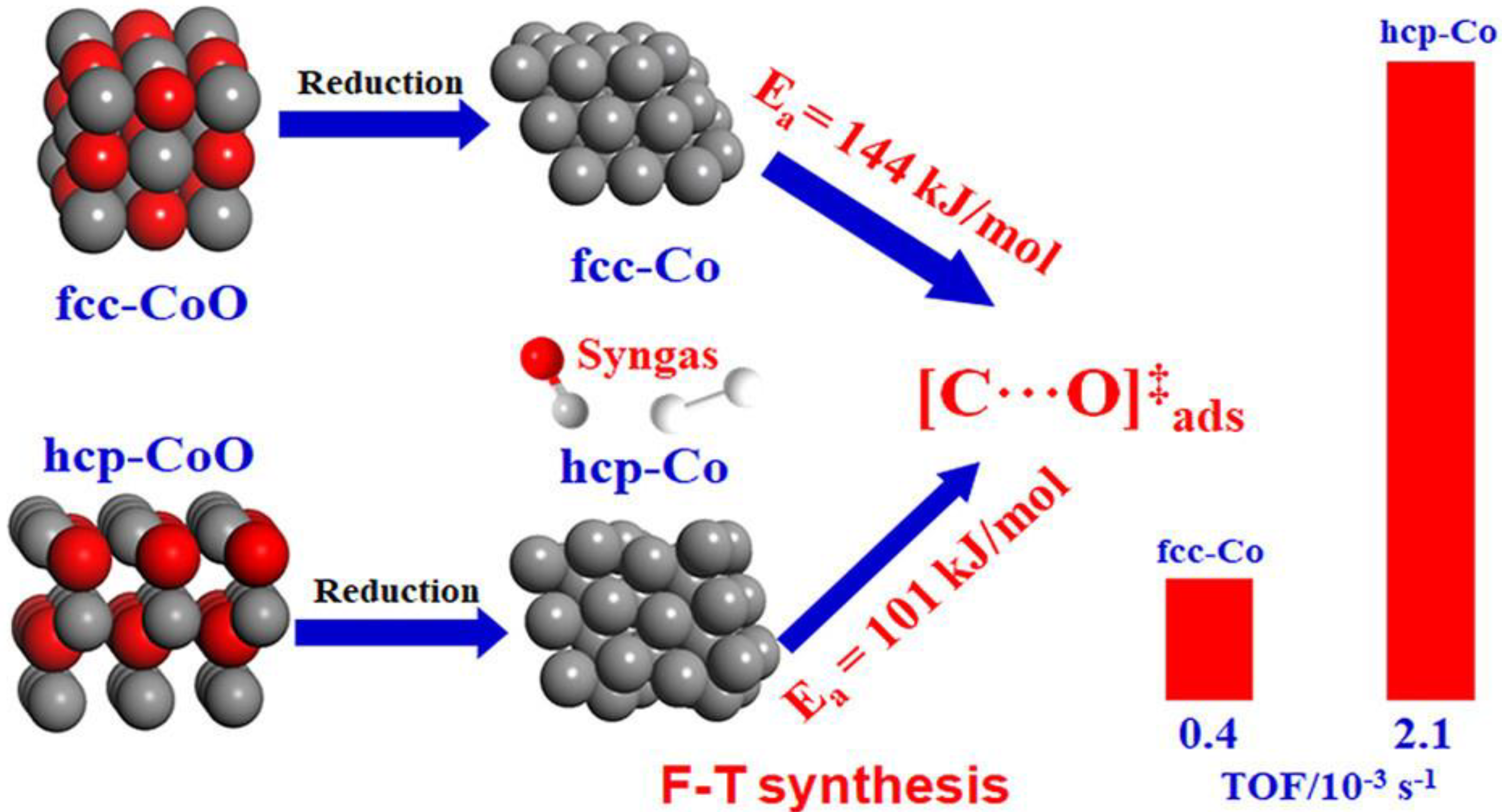
2.2. Active Phase Particle Size and Chrmical State
2.3. Carriers
2.4. Microporous and Mesoporous Materials
2.4.1. Effect of Acidity
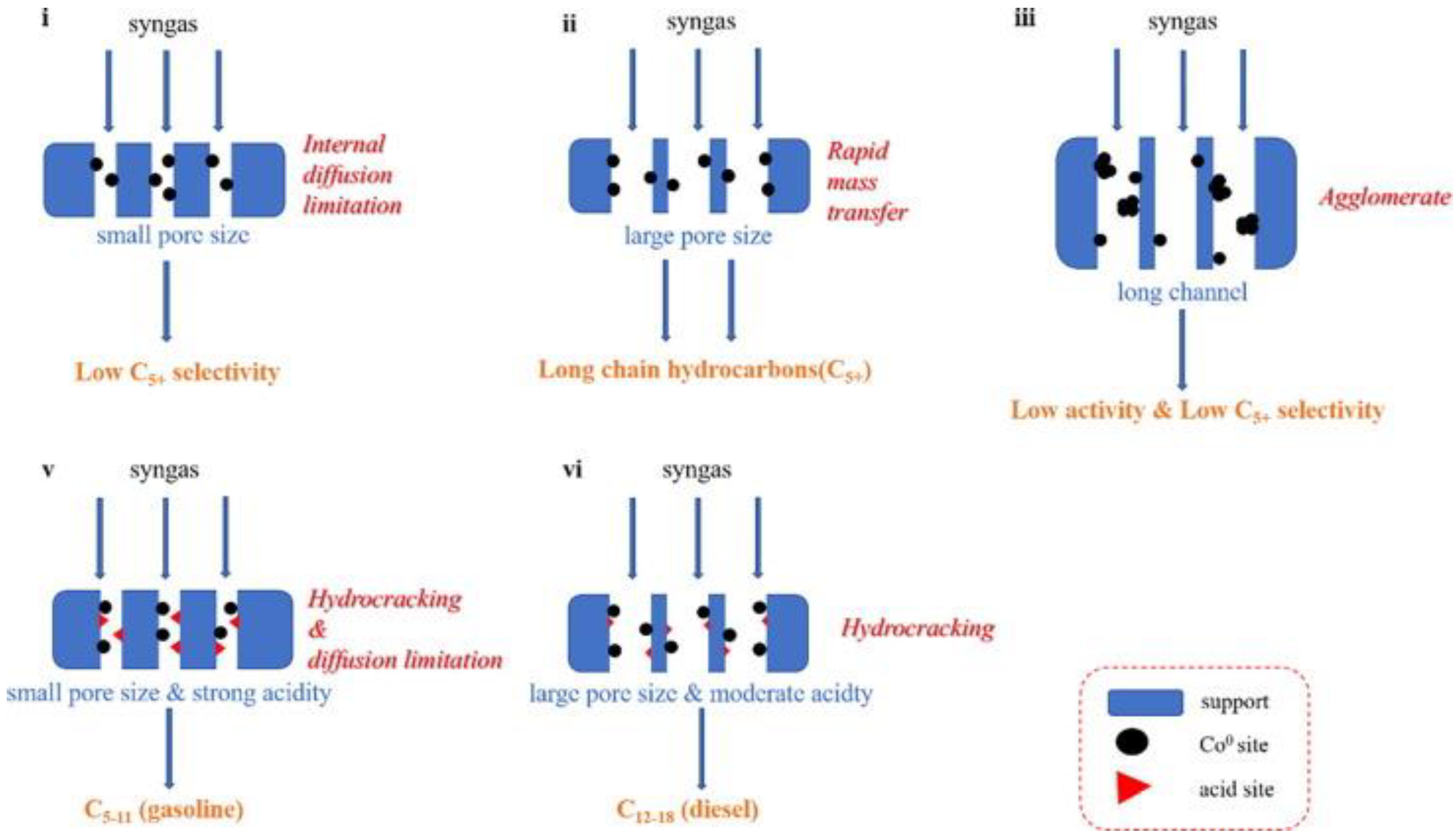
2.4.2. Effect of Pore Size
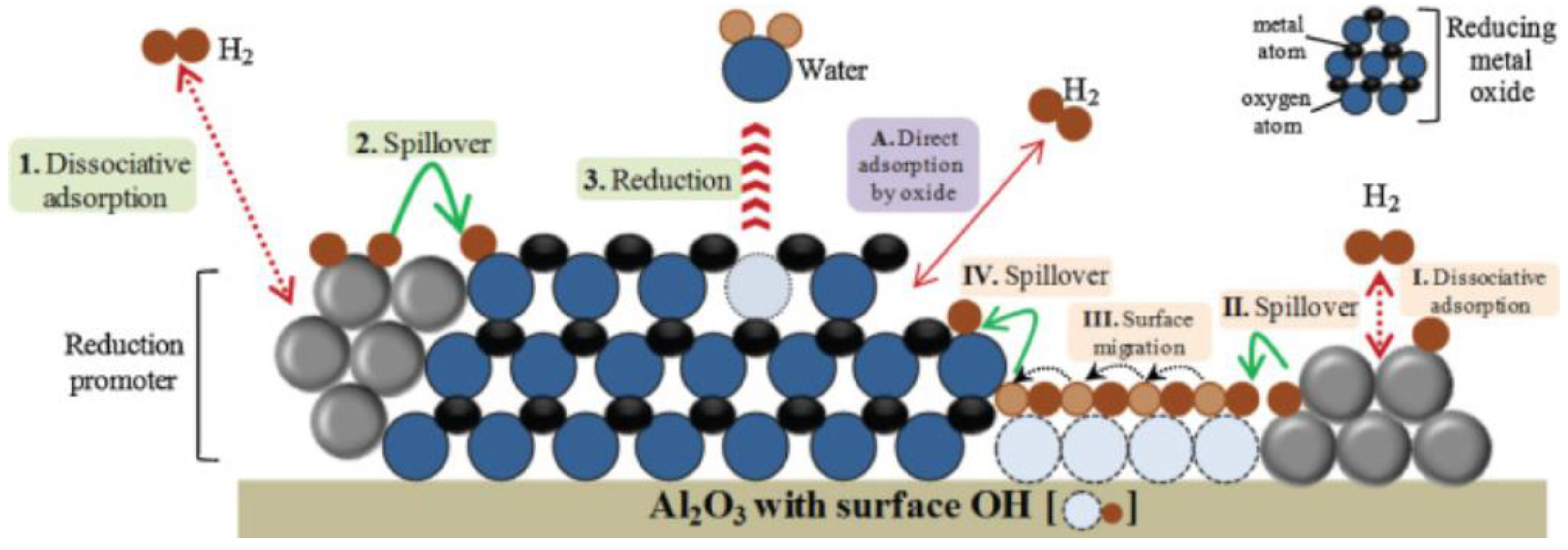
2.5. Promoters
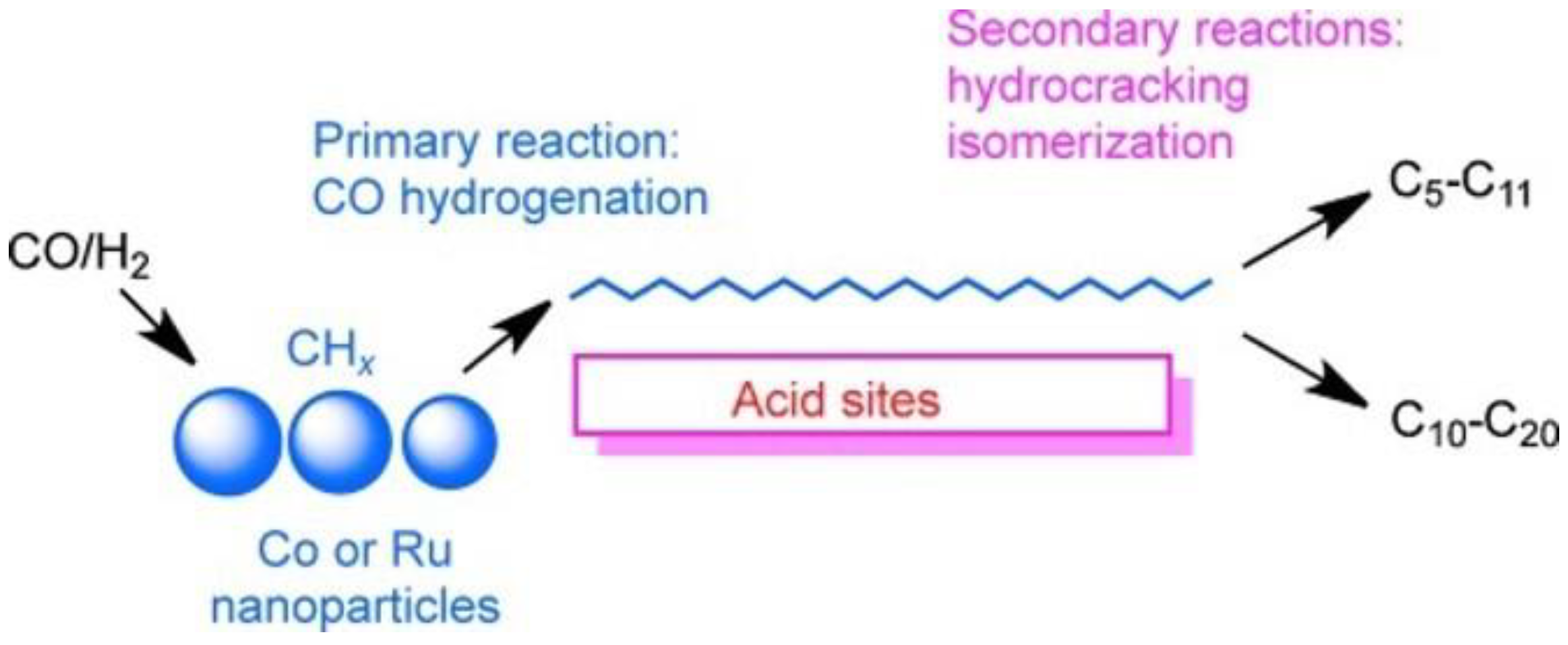
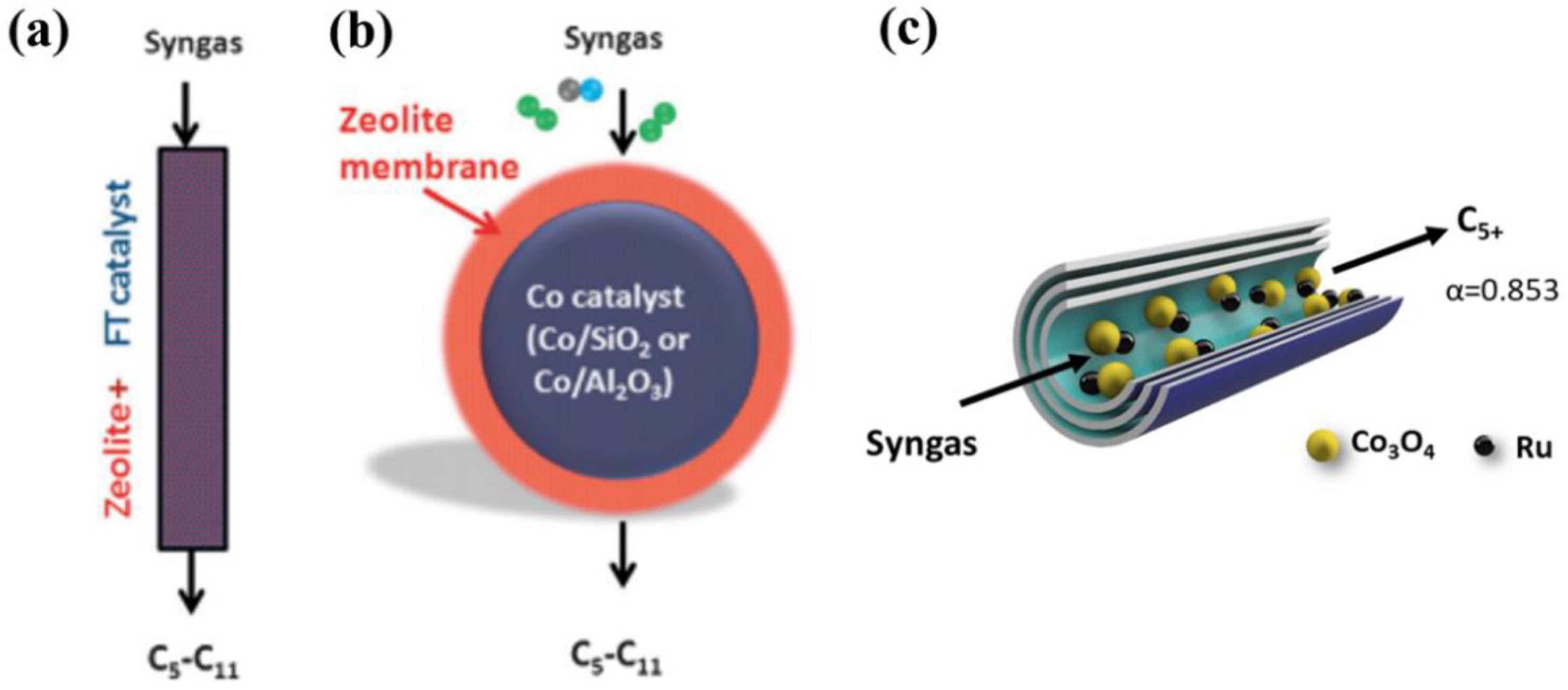
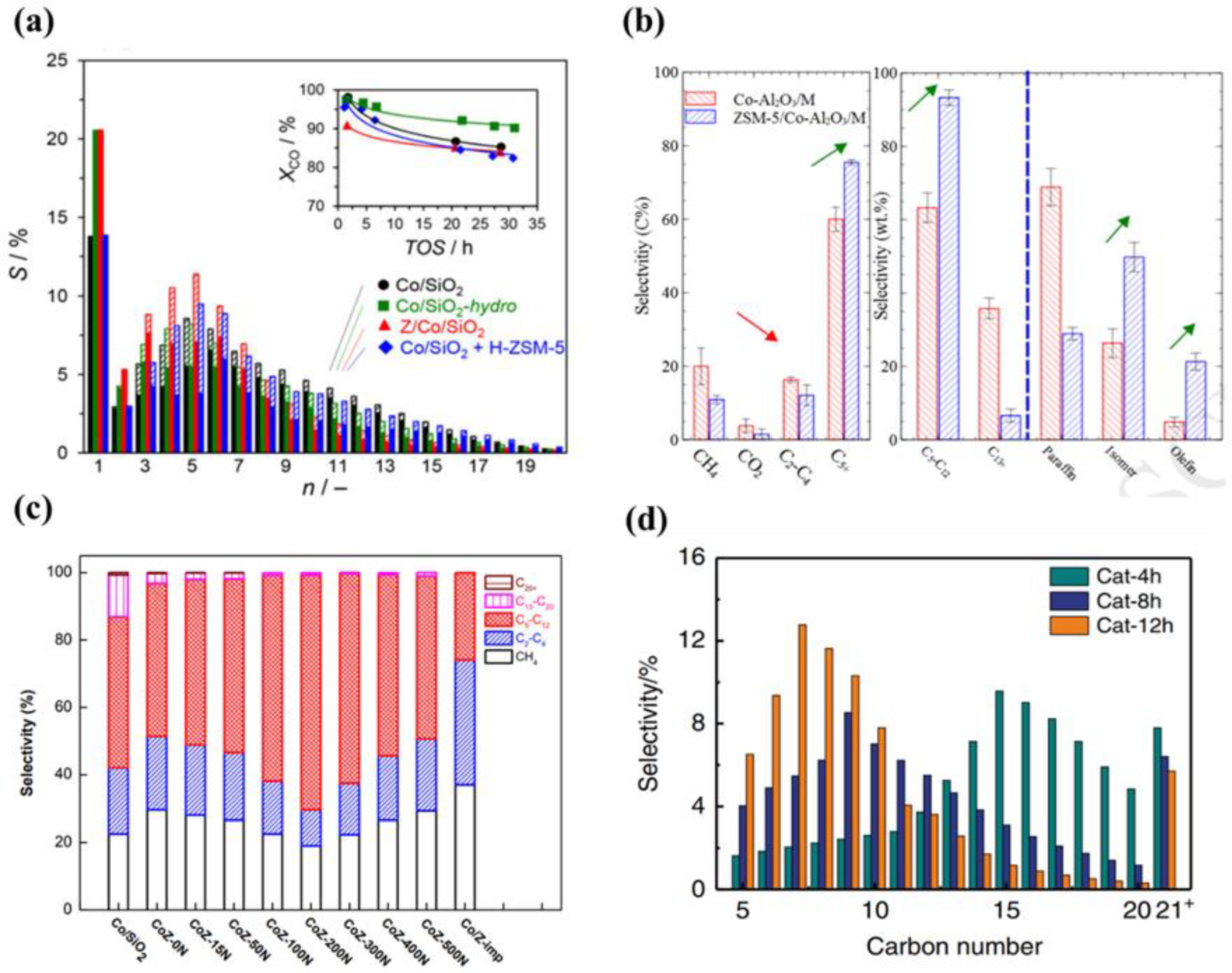
3. Bifunctional Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlNouss, A.; McKay, G.; Al-Ansari, T. A techno-economic-environmental study evaluating the potential of oxygen-steam biomass gasification for the generation of value-added products. Energy Convers. Manag. 2019, 196, 664–676. [Google Scholar] [CrossRef]
- Shafer, W.D.; Gnanamani, M.K.; Graham, U.M.; Yang, J.; Masuku, C.M.; Jacobs, G.; Davis, B.H. Fischer–Tropsch: Product selectivity–the fingerprint of synthetic fuels. Catalysts 2019, 9, 259. [Google Scholar] [CrossRef]
- Wang, C.; Fang, W.; Liu, Z.; Wang, L.; Liao, Z.; Yang, Y.; Li, H.; Liu, L.; Zhou, H.; Qin, X.; et al. Fischer–Tropsch synthesis to olefins boosted by MFI zeolite nanosheets. Nat. Nanotechnol. 2022, 17, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, K.Y.; Rhim, G.B.; Youn, M.H.; Lee, Y.L.; Chun, D.H.; Roh, H.S. Nano-catalysts for gas to liquids: A concise review. Chem. Eng. J. 2023, 468, 143632. [Google Scholar] [CrossRef]
- Gill, S.S.; Tsolakis, A.; Dearn, K.D.; Rodríguez-Fernández, J. Combustion characteristics and emissions of Fischer–Tropsch diesel fuels in IC engines. Prog. Energy Combust. Sci. 2011, 37, 503–523. [Google Scholar] [CrossRef]
- Aut Zhang, S.; Yang, X.; Zhang, H.; Chu, C.; Zheng, K.; Ju, M.; Liu, L. Liquefaction of biomass and upgrading of bio-oil: A review. Molecules 2019, 24, 2250. [Google Scholar] [CrossRef]
- Eliseev, O.L.; Savost’yanov, A.P.; Sulima, S.I.; Lapidus, A.L. Recent development in heavy paraffins synthesis from CO and H2. Mendeleev Commun. 2018, 28, 345–351. [Google Scholar] [CrossRef]
- Kibby, C.; Jothimurugesan, K.; Das, T.; Lacheen, H.S.; Rea, T.; Saxton, R.J. Chevron’s gas conversion catalysis-hybrid catalysts for wax-free Fischer–Tropsch synthesis. Catal. Today 2013, 215, 131–141. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, W.; Du, Y.; Li, J. Species and impacts of metal sites over bifunctional catalyst on long chain n-alkane hydroisomerization: A review. Appl. Catal. A Gen. 2021, 611, 117916. [Google Scholar] [CrossRef]
- Todic, B.; Nowicki, L.; Nikacevic, N.; Bukur, D.B. Fischer–Tropsch synthesis product selectivity over an industrial iron-based catalyst: Effect of process conditions. Catal. Today 2016, 261, 28–39. [Google Scholar] [CrossRef]
- Suo, Y.; Yao, Y.; Zhang, Y.; Xing, S.; Yuan, Z.Y. Recent advances in cobalt-based Fischer–Tropsch synthesis catalysts. J. Ind. Eng. Chem. 2022, 115, 92–119. [Google Scholar] [CrossRef]
- Hodala, J.L.; Moon, D.J.; Reddy, K.R.; Reddy, C.V.; Kumar, T.N.; Ahamed, M.I.; Raghu, A.V. Catalyst design for maximizing C5+ yields during Fischer–Tropsch synthesis. Int. J. Hydrogen Energy 2021, 46, 3289–3301. [Google Scholar] [CrossRef]
- van Santen, R.A.; Ghouri, M.M.; Shetty, S.; Hensen, E.M. Structure sensitivity of the Fischer–Tropsch reaction; molecular kinetics simulations. Catal. Sci. Technol. 2011, 1, 891–911. [Google Scholar] [CrossRef]
- Amoo, C.C.; Xing, C.; Tsubaki, N.; Sun, J. Tandem Reactions over Zeolite-Based Catalysts in Syngas Conversion. ACS Cent. Sci. 2022, 8, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, K.; Kang, J.; Deng, W.; Wang, Y. Fischer–Tropsch catalysts for the production of hydrocarbon fuels with high selectivity. ChemSusChem 2013, 7, 1251–1264. [Google Scholar] [CrossRef]
- Pan, X.; Jiao, F.; Miao, D.; Bao, X. Oxide–zeolite-based composite catalyst concept that enables syngas chemistry beyond Fischer–Tropsch synthesis. Chem. Rev. 2021, 121, 6588–6609. [Google Scholar] [CrossRef] [PubMed]
- Eran, T.N.; Galli, F.; Mazzoni, F.; Longhi, M.; Grainca, A.; Patience, G.; Pirola, C. Metallosilicates as an iron support to catalyze Fischer–Tropsch synthesis. Catal. Today 2022, 404, 132–141. [Google Scholar] [CrossRef]
- Yu, F.; Xiao, F.-S. Mesostructured materials. In Comprehensive Inorganic Chemistry III, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 41–66. [Google Scholar] [CrossRef]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant advances in C1 catalysis: Highly efficient catalysts and catalytic reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Niu, C.; Xia, M.; Chen, C.; Ma, Z.; Jia, L.; Hou, B.; Li, D. Effect of process conditions on the product distribution of Fischer–Tropsch synthesis over an industrial cobalt-based catalyst using a fixed-bed reactor. Appl. Catal. A Gen. 2020, 601, 117630. [Google Scholar] [CrossRef]
- Botes, F.G. Influences of water and syngas partial pressure on the kinetics of a commercial alumina-supported cobalt Fischer−Tropsch catalyst. Ind. Eng. Chem. Res. 2009, 48, 1859–1865. [Google Scholar] [CrossRef]
- Dinse, A.; Aigner, M.; Ulbrich, M.; Johnson, G.R.; Bell, A.T. Effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer–Tropsch Synthesis. J. Catal. 2012, 288, 104–114. [Google Scholar] [CrossRef]
- Yang, J.; Ma, W.; Chen, D.; Holmen, A.; Davis, B.H. Fischer–Tropsch synthesis: A review of the effect of CO conversion on methane selectivity. Appl. Catal. A Gen. 2014, 470, 250–260. [Google Scholar] [CrossRef]
- Iglesia, E.; Reyes, S.C.; Madon, R.J. Transport-enhanced α-olefin readsorption pathways in Ru-catalyzed hydrocarbon synthesis. J. Catal. 1991, 129, 238–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Li, X.; Wang, C.; Long, Q.; Ma, L. Mesoporous Fe-based spindles designed as catalysts for the Fischer–Tropsch synthesis of C5+ hydrocarbons. New J. Chem. 2018, 42, 15968–15973. [Google Scholar] [CrossRef]
- De la Osa, A.R.; De Lucas, A.; Romero, A.; Valverde, J.L.; Sánchez, P. Fischer–Tropsch diesel production over calcium-promoted Co/alumina catalyst: Effect of reaction conditions. Fuel 2011, 11, 1935–1945. [Google Scholar] [CrossRef]
- Van Der Laan, G.P.; Beenackers, A. Kinetics and selectivity of the Fischer–Tropsch synthesis: A Literature Review. Catal. Rev. Sci. Eng. 1999, 41, 255–318. [Google Scholar] [CrossRef]
- Schulz, H.; Claeys, M. Kinetic modelling of Fischer–Tropsch product distributions. Appl. Catal. A Gen. 1999, 186, 91–107. [Google Scholar] [CrossRef]
- Mendes, F.M.; Noronha, F.B.; Souza, C.D.; da Silva, M.A.; Gaspar, A.B.; Schmal, M. The effect of pressure on promoted Ru and Re-Co/Niobia catalysts in the Fischer–Tropsch synthesis. Stud. Surf. Sci. Catal. 2004, 147, 361–366. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Martini, F.; Moggi, P. Co/SiO2 Sol–Gel Catalysts for Fischer–Tropsch Synthesis. Catal. Lett. 2001, 76, 65–69. [Google Scholar] [CrossRef]
- Liuzzi, D.; Pérez-Alonso, F.J.; Rojas, S. Ru-M (M = Fe or Co) Catalysts with high Ru surface concentration for Fischer–Tropsch synthesis. Fuel 2021, 293, 120435. [Google Scholar] [CrossRef]
- Al-Dossary, M.; Fierro, J.L.G. Effect of high-temperature pre-reduction in Fischer–Tropsch synthesis on Fe/ZrO2 catalysts. Appl. Catal. A Gen. 2015, 499, 109–117. [Google Scholar] [CrossRef]
- Eslava, J.L.; Sun, X.; Gascon, J.; Kapteijn, F.; Rodríguez-Ramos, I. Ruthenium particle size and cesium promotion effects in Fischer–Tropsch synthesis over high-surface-area graphite supported catalysts. Catal. Sci. Technol. 2017, 7, 1235–1244. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, W.; Wang, Y. Recent advances in understanding the key catalyst factors for Fischer-Tropsch synthesis. J. Energy Chem. 2013, 22, 27–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Wang, Y. Development of novel catalysts for Fischer–Tropsch synthesis: Tuning the product selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer–Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, Z.; Bukur, D.B.; Goodman, D.W. Fischer–Tropsch synthesis on a model Co/SiO2 catalyst. J. Catal. 2009, 268, 196–200. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, L.; Zhang, J.; Liu, C.; Sun, J.; Peng, B.; Wang, Y.; Rappé, K.G.; Zhang, Y.; Li, J.; et al. Role of active phase in Fischer–Tropsch synthesis: Experimental evidence of CO activation over single-phase cobalt catalysts. ACS Catal. 2018, 8, 7787–7798. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Davis, B.H. Fischer–Tropsch synthesis: Activity of metallic phases of cobalt supported on silica. Catal. Today 2013, 215, 13–17. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer–Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Su, H.; Sommerdijk, N.A.J.M.; De Jong, K.P. Assembly and activation of supported cobalt nanocrystal catalysts for the Fischer–Tropsch synthesis. Chem. Commun. 2018, 54, 2530–2533. [Google Scholar] [CrossRef]
- Den Breejen, J.P.; Radstake, P.B.; Bezemer, G.L.; Bitter, J.H.; Frøseth, V.; Holmen, A.; de Jong, K.D. On the origin of the cobalt particle size effects in Fischer–Tropsch Catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar] [CrossRef]
- Nakhaei Pour, A.; Housaindokht, M. Fischer–Tropsch synthesis over CNT supported cobalt catalysts: Role of metal nanoparticle size on catalyst activity and products selectivity. Catal. Lett. 2013, 143, 1328–1338. [Google Scholar] [CrossRef]
- Xiong, H.; Motchelaho, M.A.; Moyo, M.; Jewell, L.L.; Coville, N.J. Correlating the preparation and performance of cobalt catalysts supported on carbon nanotubes and carbon spheres in the Fischer–Tropsch synthesis. J. Catal. 2011, 278, 26–40. [Google Scholar] [CrossRef]
- Wang, Z.J.; Yan, Z.; Liu, C.J.; Goodman, D.W. Surface science studies on cobalt Fischer–Tropsch catalysts. ChemCatChem 2011, 3, 551–559. [Google Scholar] [CrossRef]
- Carballo, J.M.G.; Yang, J.; Holmen, A.; García-Rodríguez, S.; Rojas, S.; Ojeda, M.; Fierro, J.L.G. Catalytic effects of ruthenium particle size on the Fischer–Tropsch Synthesis. J. Catal. 2011, 284, 102–108. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Iron particle size effects for direct production of lower olefins from synthesis gas. J. Am. Chem. Soc. 2012, 134, 16207–16215. [Google Scholar] [CrossRef]
- Kang, J.; Deng, W.; Zhang, Q.; Wang, Y. Ru particle size effect in Ru/CNT-catalyzed Fischer–Tropsch synthesis. J. Energy Chem. 2013, 22, 321–328. [Google Scholar] [CrossRef]
- Prieto, G.; Martínez, A.; Concepción, P.; Moreno-Tost, R. Cobalt particle size effects in Fischer–Tropsch synthesis: Structural and in situ spectroscopic characterisation on reverse micelle-synthesised Co/ITQ-2 model catalysts. J. Catal. 2009, 266, 129–144. [Google Scholar] [CrossRef]
- Herranz, T.; Deng, X.; Cabot, A.; Guo, J.; Salmeron, M. Influence of the cobalt particle size in the CO hydrogenation reaction studied by in situ X-Ray absorption spectroscopy. J. Phys. Chem. B 2009, 113, 10721–10727. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.J.; Karandikar, P.R.; Jun, K.W.; Ha, K.S.; Park, H.G. Fischer–Tropsch catalysts deposited with size-controlled Co3O4 nanocrystals: Effect of Co particle size on catalytic activity and stability. Appl. Catal. A Gen. 2012, 411–412, 15–23. [Google Scholar] [CrossRef]
- Wang, Z.J.; Skiles, S.; Yang, F.; Yan, Z.; Goodman, D.W. Particle size effects in Fischer–Tropsch synthesis by cobalt. Catal. Today 2012, 181, 75–81. [Google Scholar] [CrossRef]
- Li, X.; Almkhelfe, H.; Bedford, N.M.; Back, T.C.; Hohn, K.L.; Amama, P.B. Characterization and catalytic behavior of Fischer–Tropsch catalysts derived from different cobalt precursors. Catal. Today 2019, 338, 40–51. [Google Scholar] [CrossRef]
- Phaahlamohlaka, T.N.; Dlamini, M.W.; Mogodi, M.W.; Kumi, D.O.; Jewell, L.L.; Billing, D.G.; Coville, N.J. A sinter resistant Co Fischer–Tropsch catalyst promoted with Ru and supported on titania encapsulated by mesoporous silica. Appl. Catal. A Gen. 2018, 552, 129–137. [Google Scholar] [CrossRef]
- Akbarzadeh, O.; Mohd Zabidi, N.A.; Abdul Wahab, Y.; Hamizi, N.A.; Chowdhury, Z.Z.; Merican Aljunid Merican, Z.; Ab Rahman, M.; Akhter, S.; Rasouli, E.; Johan, M. Effect of Cobalt Catalyst Confinement in Carbon Nanotubes Support on Fischer–Tropsch Synthesis Performance. Symmetry 2018, 10, 572. [Google Scholar] [CrossRef]
- Cheng, Q.; Tian, Y.; Lyu, S.; Zhao, N.; Ma, K.; Ding, T.; Jiang, Z.; Wang, L.; Zhang, J.; Zheng, L.; et al. Confined small-sized cobalt catalysts stimulate carbon-chain growth reversely by modifying ASF law of Fischer–Tropsch synthesis. Nat. Commun. 2018, 9, 3250. [Google Scholar] [CrossRef] [PubMed]
- Pour, A.N.; Hosaini, E.; Tavasoli, A.; Behroozsarand, A.; Dolati, F. Intrinsic kinetics of Fischer–Tropsch synthesis over Co/CNTs catalyst: Effects of metallic cobalt particle size. J. Nat. Gas Sci. Eng. 2014, 21, 772–778. [Google Scholar] [CrossRef]
- Schmidt, W. Solid Catalysts on the Nanoscale: Design of Complex Morphologies and Pore Structures. ChemCatChem 2009, 1, 53–67. [Google Scholar] [CrossRef]
- Martínez, A.; Prieto, G. The Application of Zeolites and Periodic Mesoporous Silicas in the Catalytic Conversion of Synthesis Gas. Top. Catal. 2009, 52, 75–90. [Google Scholar] [CrossRef]
- Asoro, M.A.; Kovar, D.; Shao-Horn, Y.; Allard, L.F.; Ferreira, P.J. Coalescence and sintering of Pt nanoparticles: In situ observation by aberration-corrected HAADF STEM. Nanotechnology 2010, 21, 025701. [Google Scholar] [CrossRef]
- Wang, Y.; Noguchi, M.; Takahashi, Y.; Ohtsuka, Y. Synthesis of SBA-15 with different pore sizes and the utilization as supports of high loading of cobalt catalysts. Catal. Today 2001, 68, 3–9. [Google Scholar] [CrossRef]
- Arsalanfar, M.; Mirzaei, A.A.; Bozorgzadeh, H.R.; Samimi, A.; Ghobadi, R. Effect of support and promoter on the catalytic performance and structural properties of the Fe–Co–Mn catalysts for Fischer–Tropsch synthesis. J. Ind. Eng. Chem. 2014, 20, 1313–1323. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval-Constant, A.; Bechara, R.; Zholobenko, V.L. Pore Size Effects in Fischer Tropsch Synthesis over Cobalt-Supported Mesoporous Silicas. J. Catal. 2002, 206, 230–241. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Takahashi, Y.; Noguchi, M.; Arai, T.; Takasaki, S.; Tsubouchi, N.; Wang, Y. Novel utilization of mesoporous molecular sieves as supports of cobalt catalysts in Fischer–Tropsch synthesis. Catal. Today 2004, 89, 419–429. [Google Scholar] [CrossRef]
- Martínez, A.; López, C.; Márquez, F.; Díaz, I. Fischer–Tropsch synthesis of hydrocarbons over mesoporous Co/SBA-15 catalysts: The influence of metal loading, cobalt precursor, and promoters. J. Catal. 2003, 220, 486–499. [Google Scholar] [CrossRef]
- Hong, J.; Chernavskii, P.A.; Khodakov, A.Y.; Chu, W. Effect of promotion with ruthenium on the structure and catalytic performance of mesoporous silica (smaller and larger pore) supported cobalt Fischer–Tropsch catalysts. Catal. Today 2009, 140, 135–141. [Google Scholar] [CrossRef]
- Okabe, K.; Wei, M.; Arakawa, H. Fischer–Tropsch Synthesis over Cobalt Catalysts Supported on Mesoporous Metallo-silicates. Energy Fuels 2003, 17, 822–828. [Google Scholar] [CrossRef]
- Marchetti, S.G.; Cagnoli, M.V.; Alvarez, A.M.; Bengoa, J.F.; Gallegos, N.G.; Yeramian, A.A.; Mercader, R.C. Iron Uniform-Size Nanoparticles Dispersed on MCM-41 Used as Hydrocarbon Synthesis Catalyst. Hyperfine Interact. 2002, 139, 33–40. [Google Scholar] [CrossRef]
- Wang, P.; Kang, J.; Zhang, Q.; Wang, Y. Lithium ion-exchanged zeolite faujasite as support of iron catalyst for Fischer-Tropsch synthesis. Catal. Lett. 2007, 114, 178–184. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Gnanamani, M.K.; Pendyala, V.R.R.; Yen, C.H.; Klettlinger, J.L.S.; Tomsik, T.M.; Davis, B.H. Fischer–Tropsch synthesis: Support and cobalt cluster size effects on kinetics over Co/Al2O3 and Co/SiO2 catalysts. Fuel 2011, 90, 756–765. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer−Tropsch synthesis: Comparison of performances of iron and cobalt catalysts. Ind. Eng. Chem. Res. 2007, 46, 8938–8945. [Google Scholar] [CrossRef]
- Storsæter, S.; Tøtdal, B.; Walmsley, J.C.; Tanem, B.S.; Holmen, A. Characterization of alumina-, silica-, and titania-supported cobalt Fischer–Tropsch catalysts. J. Catal. 2005, 236, 139–152. [Google Scholar] [CrossRef]
- Oh, J.H.; Bae, J.W.; Park, S.J.; Khanna, P.K.; Jun, K.W. Slurry-phase Fischer–Tropsch synthesis using Co/γ-Al2O3, Co/SiO2 and Co/TiO2: Effect of support on catalyst aggregation. Catal. Lett. 2009, 130, 403–409. [Google Scholar] [CrossRef]
- Khangale, P.R.; Meijboom, R.; Jalama, K. Fischer-Tropsch synthesis over unpromoted Co/ɣ-Al2O3 catalyst: Effect of activation with CO compared to H2 on catalyst performance. Bull. Chem. React. Eng. Catal. 2009, 14, 35–41. [Google Scholar] [CrossRef]
- Liu, C.; Hong, J.; Zhang, Y.; Zhao, Y.; Wang, L.; Wei, L.; Chen, S.; Wang, G.; Li, J. Synthesis of γ-Al2O3 nanofibers stabilized Co3O4 nanoparticles as highly active and stable Fischer–Tropsch synthesis catalysts. Fuel 2016, 180, 777–784. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Zhang, Y.; Li, J.; Racoillet, G.; Davis, B.H. Fischer–Tropsch synthesis: Support, loading, and promoter effects on the reducibility of cobalt catalysts. Appl. Catal. A Gen. 2002, 233, 263–281. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z.; Niu, N.; Li, J. Preparation and Characterization of SiO2/Co and C/Co Nanocomposites as Fisher-Tropsch Catalysts for CO2 Hydrogenation. Chem. Res. Chin. Univ. 2018, 34, 635–642. [Google Scholar] [CrossRef]
- Gholami, Z.; Asmawati Mohd Zabidi, N.; Gholami, F.; Ayodele, O.B.; Vakili, M. The influence of catalyst factors for sustainable production of hydrocarbons via Fischer-Tropsch synthesis. Rev. Chem. Eng. 2017, 33, 337–358. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef]
- Pereira, A.L.C.; González-Carballo, J.M.; Pérez-Alonso, F.J.; Rojas, S.; Fierro, J.L.G.; Rangel, M.D.C. Effect of the mesostructuration of the beta zeolite support on the properties of cobalt catalysts for Fischer–Tropsch synthesis. Top. Catal. 2011, 54, 179–189. [Google Scholar] [CrossRef]
- Claure Zeballos, M.; Pardo Tarifa, F.L.; Lopez N, L.G.; Cabrera M, S. Mesoporous Silicoaluminate materials (MCM-41, SBA-15 and MCF) by atrane route for Cobalt Catalyst. Rev. Bol. Quim. 2022, 39, 13–32. [Google Scholar] [CrossRef]
- Travaglini, L.; Picchetti, P.; Del Giudice, A.; Galantini, L.; De Cola, L. Tuning and controlling the shape of mesoporous silica particles with CTAB/sodium deoxycholate catanionic mixtures. Microporous Mesoporous Mater. 2019, 279, 423–431. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis methods of mesoporous silica materials. Mater. Today 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-based catalysts for Fischer–Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef] [PubMed]
- Ngamcharussrivichai, C.; Imyim, A.; Li, X.; Fujimoto, K. Active and selective bifunctional catalyst for gasoline production through a slurry-phase Fischer–Tropsch synthesis. Ind. Eng. Chem. Res. 2007, 46, 6883–6890. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Bechara, R.; Griboval-Constant, A. Fischer–Tropsch synthesis over silica supported cobalt catalysts: Mesoporous structure versus cobalt surface density. Appl. Catal. A Gen. 2003, 254, 273–288. [Google Scholar] [CrossRef]
- González, O.; Pérez, H.; Navarro, P.; Almeida, L.C.; Pacheco, J.G.; Montes, M. Use of different mesostructured materials based on silica as cobalt supports for the Fischer–Tropsch synthesis. Catal. Today 2009, 148, 140–147. [Google Scholar] [CrossRef]
- Kim, N.Y.; Jung, J.S.; Lee, J.S.; Yang, E.H.; Hong, G.H.; Lim, S.S.; Noh, Y.S.; Hodala, J.L.; Lee, K.Y.; Moon, D.J. Synthesis and characterization of Al-modified SBA-15 for Fischer–Tropsch synthesis (FTS) reaction. Res. Chem. Intermed. 2016, 42, 319–334. [Google Scholar] [CrossRef]
- Rai, A.; Sibi, M.G.; Farooqui, S.A.; Anand, M.; Bhaumik, A.; Sinha, A.K. Mesoporous γ-Alumina with Isolated Silica Sites for Direct Liquid Hydrocarbon Production during Fischer–Tropsch Reactions in Microchannel Reactor. ACS Sustain. Chem. Eng. 2017, 5, 7576–7586. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Mazurova, K.; Kotelev, M.; Eliseev, O.; Gushchin, P.; Glotov, A.; Kazantsev, R.; Vinokurov, V.; Lvov, Y. Ruthenium-Loaded Halloysite Nanotubes as Mesocatalysts for Fischer–Tropsch Synthesis. Molecules 2020, 25, 1764. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Effects of catalyst preparation on hydrocarbon product distribution in hydrocracking of the Fischer-Tropsch product with low Pt-loaded catalysts. Catalysts 2015, 5, 1983–2000. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nat. Catal. 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kang, S.H.; Kim, J.H.; Lee, Y.J.; Jun, K.W. Fischer-Tropsch synthesis on Co-Al2O3-(promoter)/ZSM5 hybrid catalysts for the production of gasoline range hydrocarbons. Korean J. Chem. Eng. 2015, 32, 1993–1998. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, J.H.; Kim, J.H.; Sai Prasad, P.S.; Bae, J.W.; Cheon, J.Y.; Jun, K.W. ZSM-5 supported cobalt catalyst for the direct production of gasoline range hydrocarbons by Fischer–Tropsch synthesis. Catal. Lett. 2011, 141, 1464–1471. [Google Scholar] [CrossRef]
- Saib, A.M.; Claeys, M.; Van Steen, E. Silica supported cobalt Fischer–Tropsch catalysts: Effect of pore diameter of support. Catal. Today 2002, 71, 395–402. [Google Scholar] [CrossRef]
- Borg, Ø.; Eri, S.; Blekkan, E.A.; Storsæter, S.; Wigum, H.; Rytter, E.; Holmen, A. Fischer–Tropsch synthesis over γ-alumina-supported cobalt catalysts: Effect of support variables. J. Catal. 2007, 248, 89–100. [Google Scholar] [CrossRef]
- Bartolini, M.; Molina, J.; Alvarez, J.; Goldwasser, M.; Almao, P.P.; Zurita, M.J. Effect of the porous structure of the support on hydrocarbon distribution in the Fischer–Tropsch reaction. J. Power Sources 2015, 285, 1–11. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, K.; Kang, J.; Gu, B.; Yu, X.; Zhang, Q.; Wang, Y. Impact of Hydrogenolysis on the Selectivity of the Fischer–Tropsch Synthesis: Diesel Fuel Production over Mesoporous Zeolite-Y-Supported Cobalt Nanoparticles. Angew. Chem. Int. Ed. Engl. 2015, 54, 4553–4556. [Google Scholar] [CrossRef]
- Shiba, N.C.; Liu, X.; Mao, H.; Qian, X.; Hildebrandt, D.; Yao, Y. Effect of Ru-promotion on the catalytic performance of a cobalt-based Fischer–Tropsch catalyst activated in syngas or H2. Fuel 2022, 320, 123939. [Google Scholar] [CrossRef]
- Bertella, F.; Concepción, P.; Martínez, A. TiO2 polymorph dependent SMSI effect in Co-Ru/TiO2 catalysts and its relevance to Fischer-Tropsch synthesis. Catal. Today 2017, 289, 181–191. [Google Scholar] [CrossRef]
- Parnian, M.J.; Najafabadi, A.T.; Mortazavi, Y.; Khodadadi, A.A.; Nazzari, I. Ru promoted cobalt catalyst on γ-Al2O3: Influence of different catalyst preparation method and Ru loadings on Fischer–Tropsch reaction and kinetics. Appl. Surf. Sci. 2014, 313, 183–195. [Google Scholar] [CrossRef]
- Parnian, M.J.; Khodadadi, A.A.; Najafabadi, A.T.; Mortazavi, Y. Preferential chemical vapor deposition of ruthenium on cobalt with highly enhanced activity and selectivity for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2014, 470, 221–231. [Google Scholar] [CrossRef]
- Nabaho, D.; Niemantsverdriet, J.H.; Claeys, M.; van Steen, E. Hydrogen spillover in the Fischer–Tropsch synthesis: An analysis of platinum as a promoter for cobalt–alumina catalysts. Catal. Today 2016, 261, 17–27. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Taeb, A.; Feyzi, F.; Yaripour, F. Fischer–Tropsch synthesis over Ru promoted Co/γ-Al2O3 catalysts in a CSTR. Catal. Commun. 2004, 5, 137–143. [Google Scholar] [CrossRef]
- Kogelbauer, A.; Goodwin, J.G.; Oukaci, R. Ruthenium promotion of Co/Al2O3 Fischer–Tropsch catalysts. J. Catal. 1996, 160, 125–133. [Google Scholar] [CrossRef]
- Diehl, F.; Khodakov, A.Y. Promotion of cobalt Fischer-Tropsch catalysts with noble metals: A review. Oil Gas Sci. Technol. Rev. IFP 2009, 64, 11–24. [Google Scholar] [CrossRef]
- Tsubaki, N.; Sun, S.; Fujimoto, K. Different functions of the noble metals added to cobalt catalysts for Fischer–Tropsch synthesis. J. Catal. 2001, 199, 236–246. [Google Scholar] [CrossRef]
- Hilmen, A.M.; Schanke, D.; Holmen, A. TPR study of the mechanism of rhenium promotion of alumina-supported cobalt Fischer–Tropsch catalysts. Catal. Lett. 1996, 38, 143–147. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Y.; Liew, K.; Li, J. Ruthenium promotion of Co/SBA-15 catalysts with high cobalt loading for Fischer–Tropsch synthesis. Fuel Process. Technol. 2009, 90, 237–246. [Google Scholar] [CrossRef]
- Schulz, H.; Claeys, M.; Harms, S. Effect of water partial pressure on steady state Fischer-Tropsch activity and selectivity of a promoted cobalt catalyst. Stud. Surf. Sci. 1997, 107, 193–200. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, H.; Whidden, T.; Zheng, Y.; Zhao, J. Highly Selective Fischer–Tropsch Synthesis for C10–C20 Diesel Fuel under Low Pressure. Can. J. Chem. Eng. 2017, 95, 1537–1543. [Google Scholar] [CrossRef]
- Kang, J.; Wang, X.; Peng, X.; Yang, Y.; Cheng, K.; Zhang, Q.; Wang, Y. Mesoporous Zeolite Y-Supported Co Nanoparticles as Efficient Fischer–Tropsch Catalysts for Selective Synthesis of Diesel Fuel. Ind. Eng. Chem. Res. 2016, 55, 13008–13019. [Google Scholar] [CrossRef]
- Sun, B.; Qiao, M.; Fan, K.; Ulrich, J.; Tao, F. Fischer–Tropsch synthesis over molecular sieve supported catalysts. ChemCatChem 2011, 3, 542–550. [Google Scholar] [CrossRef]
- Abelló, S.; Montané, D. Exploring iron-based multifunctional catalysts for Fischer–Tropsch synthesis: A review. ChemSusChem 2011, 4, 1538–1556. [Google Scholar] [CrossRef]
- Tomasek, S.; Lonyi, F.; Valyon, J.; Wollmann, A.; Hancsók, J. Hydrocracking of Fischer–Tropsch paraffin mixtures over strong acid bifunctional catalysts to engine fuels. ACS Omega 2020, 5, 26413–26420. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J. Catalytic Hydrocracking—Mechanisms and Versatility of the Process. ChemCatChem 2012, 4, 292–306. [Google Scholar] [CrossRef]
- Bouchy, C.; Hastoy, G.; Guillon, E.; Martens, J.A. Fischer-Tropsch Waxes Upgrading via Hydrocracking and Selective Hydroisomerization. Oil Gas Sci. Technol. 2009, 64, 91–112. [Google Scholar] [CrossRef]
- Botes, F.G.; Böhringer, W. The addition of HZSM-5 to the Fischer–Tropsch process for improved gasoline production. Appl. Catal. A Gen. 2004, 267, 217–225. [Google Scholar] [CrossRef]
- Yang, G.; Xing, C.; Hirohama, W.; Jin, Y.; Zeng, C.; Suehiro, Y.; Wang, T.; Yoneyama, Y.; Tsubaki, N. Tandem catalytic synthesis of light isoparaffin from syngas via Fischer–Tropsch synthesis by newly developed core–shell-like zeolite capsule catalysts. Catal. Today 2013, 215, 29–35. [Google Scholar] [CrossRef]
- Sartipi, S.; van Dijk, J.E.; Gascon, J.; Kapteijn, F. Toward bifunctional catalysts for the direct conversion of syngas to gasoline range hydrocarbons: H-ZSM-5 coated Co versus H-ZSM-5 supported Co. Appl. Catal. A Gen. 2013, 456, 11–12. [Google Scholar] [CrossRef]
- Zhu, C.; Bollas, G.M. Gasoline selective Fischer-Tropsch synthesis in structured bifunctional catalysts. Appl. Catal. B Environ. 2018, 235, 92–102. [Google Scholar] [CrossRef]
- Mazurova, K.; Glotov, A.; Kotelev, M.; Eliseev, O.; Gushchin, P.; Rubtsova, M.; Kazantsev, R.; Vinokurov, V.; Stavitskaya, A. Natural aluminosilicate nanotubes loaded with RuCo as nanoreactors for Fischer-Tropsch synthesis. Sci. Technol. Adv. Mater. 2021, 23, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Cheng, S.; Zhang, G.; Dai, P.; Cao, Y.; Lu, C.; Yang, R.; Xing, C.; Shan, S. Complete encapsulation of zeolite supported Co based core with silicalite-1 shell to achieve high gasoline selectivity in Fischer–Tropsch synthesis. Fuel 2018, 215, 226–231. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Chen, J.F.; Zhang, Y. Cobalt nanoparticles imbedded into zeolite crystals: A tailor-made catalyst for one-step synthesis of gasoline from syngas. Int. J. Hydrogen Energy 2016, 41, 21965–21978. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Agliullin, M.R.; Zubkov, I.N.; Papeta, O.P.; Khliyan, G.T.; Savostyanov, A.P. Bifunctional cobalt-containing catalytic systems based on SAPO-11 molecular sieves in Fischer–Tropsch synthesis of fuels. Pet. Chem. 2021, 61, 378–387. [Google Scholar] [CrossRef]
- Abello, S.; Bonilla, A.; Perez-Ramirez, J. Mesoporous ZSM-5 zeolite catalysts prepared by desilication with organic hydroxides and comparison with NaOH leaching. Appl. Catal. A Gen. 2009, 364, 191–198. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Qiao, J.; Sun, Y.; Jin, W.; Zhang, H.; Ma, J. Effect of mesoporous ZSM-5 morphology on the catalytic performance of cobalt catalyst for Fischer–Tropsch synthesis. J. Energy Inst. 2020, 93, 1187–1194. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Jiang, X.; Wei, L.; Li, Z.; Liu, C. Nano-ZSM-5 decorated cobalt based catalysts for Fischer–Tropsch synthesis to enhance the gasoline range products selectivity. J. Taiwan Inst. Chem. Eng. 2020, 116, 153–159. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Han, D.; Wu, J.; Zhang, D. A preliminary evaluation of ZSM-5/SBA-15 composite supported Co catalysts for Fischer–Tropsch synthesis. Fuel Process. Technol. 2015, 134, 449–455. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Huang, J.; Liang, J.; Wang, H.; Li, Z.; Wu, J.; Li, M.; Zhao, Y.; Niu, J. Effect of hierarchical crystal structures on the properties of cobalt catalysts for Fischer–Tropsch synthesis. Fuel 2016, 174, 17–24. [Google Scholar] [CrossRef]
- Douvartzides, S.L.; Charisiou, N.D.; Papageridis, K.N.; Goula, M.A. Green Diesel: Biomass Feedstocks, Production Technologies, Catalytic Research, Fuel Properties and Performance in Compression Ignition Internal Combustion Engines. Energies 2019, 12, 809. [Google Scholar] [CrossRef]


| Catalyst | Loading, wt.% | Reaction Conditions | Catalysis Performance | |||||
|---|---|---|---|---|---|---|---|---|
| T, °C | P, MPa | GHSV, h−1 | H2/CO Ratio | Conversion CO, % | C5+ Selectivity, % | C10–C20 Selectivity, % | ||
| Co/Al2O3 | 20Co | 220 | 2 | 6000 | 2 | 25.6 | 95.50 | 70.27 |
| 235 | 32.1 | 91.64 | 65.55 | |||||
| 300 | 86.2 | 87.44 | 65.38 | |||||
| Ca-Co/Al2O3 | 0.6Ca 17Co | 220 | 2 | 6000 | 2 | 33.0 | 96.67 | 68.07 |
| 242 | 100 | 39.37 | 64.80 | |||||
| Co/Al2O3 | 20Co | 220 | 2 | 4000 | 2 | 34.7 | 95.76 | 39.01 |
| 6000 | 25.6 | 95.50 | 52.78 | |||||
| Ca-Co/Al2O3 | 0.6Ca 17Co | 220 | 2 | 4000 | 55.7 | 97.91 | 55.51 | |
| 6000 | 2 | 33.0 | 96.67 | 67.83 | ||||
| 12,000 | 6.3 | 88.46 | 64.77 | |||||
| Co/Al2O3 | 20Co | 220 | 2 | 6000 | 0.5 | 6.6 | 96.32 | 33.98 |
| 1.0 | 16.6 | 97.90 | 62.68 | |||||
| 1.5 | 11.0 | 90.30 | 56.57 | |||||
| 2.0 | 25.6 | 95.50 | 70.66 | |||||
| Ca-Co/Al2O3 | 0.6Ca 17Co | 220 | 2 | 6000 | 0.5 | 7.1 | 94.63 | 58.57 |
| 1.0 | 12.2 | 96.20 | 50.52 | |||||
| 1.5 | 12.5 | 91.82 | 58.91 | |||||
| 2.0 | 33.0 | 96.67 | 67.19 | |||||
| Catalyst | Loading, wt.% | Particle Size a, nm | Particle Size b, nm | Catalysis Performance | Ref. | ||
|---|---|---|---|---|---|---|---|
| Conversion CO, % | C5+ Selectivity, % | C10–C20 Selectivity, % | |||||
| IC c | 15Co | 12.4 | 12.1 | 75 | 24.9 | [43] | |
| MC1 c | 7.9 | 7.6 | 89 | 26.8 | |||
| MC2 c | 8.6 | 8.3 | 85 | 26.9 | |||
| MC3 c | 9.8 | 9.4 | 81 | 26.4 | |||
| Ru/CNT-C573-R673 d | 7.4 | 30 | 65 | [48] | |||
| 30%Co/SiO2 e | 30Co | 183 | 10 | 78.9 | [49] | ||
| 10%Co/ITQ(1) e | 10Co | 12.8 | 10 | 61.6 | |||
| 10%Co/ITQ(2) e | 10Co | 8.2 | 10 | 63.0 | |||
| Co/CNTs.A f | 10Co | 5.1 | 5 0.2 | 16.4 | 18.4 | [55] | |
| Co/CNTs.A.600 f | 4.2 | 4 0.2 | 28.3 | 33.2 | |||
| Co/CNTs.A.700 f | 5.3 | 5 0.2 | 37.5 | 38.8 | |||
| Co/CNTs.A.800 f | 6.1 | 6 0.2 | 50.9 | 54.6 | |||
| Co/CNTs.A.900 f | 7.2 | 7 0.2 | 58.7 | 59.1 | |||
| Cat-12h g | 13.9Co | 11.4 | 80.6 | 80.0 | 23.7 | [56] | |
| Cat-8h g | 14.5Co | 9.1 | 78.2 | 74.9 | 39.3 | ||
| Cat-4h g | 15.3Co | 7.2 | 77.0 | 84.2 | 66.2 | ||
| Cat-1M g | 16.3Co | 14.3 | 84.0 | 67.3 | 29.7 | ||
| Catalyst. | Loading, wt.% | Acidity | NH3 Adsorption Quantity (mmol g−1) | Reaction Conditions | Catalysis Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| T, °C | P, MPa | H2/CO Ratio | Conversion CO, % | C5+ Selectivity, % | C10–C20 Selectivity, % | |||||
| Co/Al-SBA-15(10) | Strong | 6.81 | 230 | 2 | 2 | 33.45 | 69.98 | 10 | [88] | |
| Co/Al-SBA-15(7) | Medium | 7.55 | 32.06 | 76.18 | 13 | |||||
| Co/Al-SBA-15(5) | Weak | 5.44 | 49.59 | 82.44 | 20 | |||||
| HTN@Ru-1 | 2Ru | Strong | 0.315 | 260 | 1 | 2 | 15.6 | 26.7 | 48.9 | [90] |
| HTN@Ru-2 | Weak | 0.129 | 17.8 | 78.0 | 52.7 | |||||
| HTN@Ru-3 | Medium | 0.250 | 18.8 | 67.7 | 67.1 | |||||
| Co/Ymeso-H | 15Co | Strong | - | 250 | 2 | 1 | - | 66 | - | [92] |
| Co/Ymicro-Na | Weak | - | - | 74 | - | |||||
| Co/Ymeso-K | Weak | - | - | 76 | 58.0 | |||||
| Co/Ymeso-Ce | Medium | - | - | 86 | 27.6 | |||||
| Co/Ymeso-La | Medium | - | - | 88 | 52.6 | |||||
| Co-Al2O3-Ru/ZSM-5 | 13Co 0.3Ru | Strong | 0.055 | 240 | 2 | 2 | 31 | 76 | 55 | [93] |
| Co-Al2O3-Pt/ZSM-5 | 13Co 0.3Pt | Medium | 0.056 | 41 | 80 | 62 | ||||
| Co-Al2O3-La/ZSM-5 | 13Co 0.3La | Weak | 0.055 | 21 | 60 | 46 | ||||
| Co/ZSM-5 (Si/Al = 15) | 20Co | Strong | 0.549 | 240 | 2 | 2 | 56.2 | 43.2 | 18.1 | [94] |
| Co/ZSM-5 (Si/Al = 25) | 20Co | Medium | 0.512 | 50.6 | 44.9 | 25.7 | ||||
| Co/ZSM-5 (Si/Al = 140) | 20Co | Weak | 0.219 | 47.3 | 52.4 | 38.8 | ||||
| Co/ZSM-5 (Si/Al = 250) | 20Co | Weak | 0.117 | 43.1 | 60.4 | 47.6 | ||||
| Catalyst | Loading, wt.% | Carrier Pore Diameter, nm | Reaction Conditions | Catalysis Performance | α | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| T, °C | P, MPa | H2/CO Ratio | Conversion CO, % | C5+ Selectivity, % | C10–C20 Selectivity, % | |||||
| Co(N)/MCM-41(IMP) | 10Co | 2.2 | 250 | 2 | 2 | 20 | 53 | 21 | 0.70 | [64] |
| Co(N)/MCM-41 (TIE) | 7.7 | 58 | 18 | 0.73 | ||||||
| Co(A)/MCM-41 (TIE) | 10 | 64 | 10 | 0.70 | ||||||
| Co(A)/SBA-15 | 20Co | 3.5 | 250 | 2 | 2 | 14 | 13 | 0.69 | [64] | |
| Co(A)/SBA-15 | 250 | 72 | 8 | 0.72 | ||||||
| Co(10A + 10N)/SBA-15 | 230 | 84 | 32 | 0.93 | ||||||
| Co(N)/SBA-15 | 230 | 89 | 30 | 0.92 | ||||||
| 5CoS1 | 5Co | 9.1 | 190 | 0.1 | 2 | 4 | 68.4 | 0.77 | [86] | |
| 10CoS1 | 10Co | 67.1 | 0.77 | |||||||
| 50CoS1 | 50Co | 43.1 | 0.68 | |||||||
| 5CoS2 | 5Co | 33 | 190 | 0.1 | 2 | 4 | 60.7 | 0.70 | [86] | |
| 10CoS2 | 10Co | 63.0 | 0.74 | |||||||
| 50CoS2 | 50Co | 63.3 | 0.75 | |||||||
| Co/SBA-15 | 20Co | 4.9 | 250 | 1 | 2 | 63.2 | 71.1 | 0.70 | [87] | |
| Co/Al-MCM-41 | 3.2 | 38.5 | 74.4 | 0.72 | ||||||
| Co/INT-MM1 | 2.6 | 40.3 | 71.8 | 0.70 | ||||||
| Co/SiO2 | 20Co | 4 | 220 | 1.5 | 2 | 44 | 71 | 0.80 | [95] | |
| Co/SiO2 | 10 | 60 | 74 | 0.85 | ||||||
| Co/SiO2 | 15 | 30 | 72.1 | 0.84 | ||||||
| C-2 | 20Co 0.5Re | 6.5 | 210 | 2 | 2 | 50 | 79.8 | [96] | ||
| C-5 | 9.0 | 81.3 | ||||||||
| C-8 | 10.5 | 80.9 | ||||||||
| C-12 | 20.8 | 83.8 | ||||||||
| Co-SBA-15-1 | 30Co | 5 | 230 | 2 | 2 | 28 | 58 | 36 | 0.72 | [97] |
| Co-SBA-15-8 | 10.7 | 29 | 54 | 41 | 0.78 | |||||
| Co-SBA-15-11 | 14.6 | 28 | 49 | 41 | 0.83 | |||||
| Catalyst | Promoter | Loading, wt.% | Reaction Conditions | Catalysis Performance | α | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| T, °C | P, MPa | H2/CO Ratio | Conversion CO, % | C5+ Selectivity, % | C10–C20 Selectivity, % | |||||
| Co-Ru/Ti-A a | Ru | 10Co 0.5Ru | 220 | 2 | 2 | 10 | 62.0 | [100] | ||
| Co-Ru/Ti-R a | Ru | 10Co 0.5Ru | 10 | 79.1 | ||||||
| Co/Ru/Al2O3 | Ru | 15Co 0.15Ru | 220 | 2 | 2 | 15.3 | 40.7 | 0.65 | [101] | |
| Ru/Co/Al2O3 | Ru | 0.15Ru 15Co | 12.9 | 43.1 | 0.56 | |||||
| CoRu/Al2O3 | Ru | 15Co 0.15Ru | 19 | 46.5 | 0.72 | |||||
| CoRu/Al2O3 | Ru | 15Co 0.3Ru | 21.1 | 44.2 | 0.70 | |||||
| CoRu/Al2O3 | Ru | 15Co 0.6Ru | 10.2 | 45.7 | 0.69 | |||||
| CoRu1 b | Ru | 15Co 0.05Ru | 220 | 0.1 | 2 | 24.7 | 42.1 | 0.70 | [102] | |
| CoRu2 | Ru | 15Co 0.1Ru | 26.8 | 43.9 | 0.73 | |||||
| CoRu3 | Ru | 15Co 0.15Ru | 31.1 | 46.4 | 0.73 | |||||
| CoRu4 | Ru | 15Co 0.2Ru | 40.1 | 44.3 | 0.73 | |||||
| CoRu5-180 | Ru | 15Co 0.3Ru | 42.9 | 44.9 | 0.74 | |||||
| Co/Ru/SBA-15 | Ru | 30Co 0.05Ru | 210 | 2 | 2 | 33.4 | 81.7 | [110] | ||
| Co/Ru/SBA-15 | Ru | 30Co 0.1Ru | 35.4 | 84.2 | ||||||
| Co-Re/Al2O3 | Re | 20Co 0.5Re | 230 | 0.85 | 2.3 | 94 | 89 | 62 | [112] | |
| Co-Ru/Al2O3 | Ru | 15Co 1Ru | 240 | 240 | 2 | 2 | 50 | 69 | ||
| Co-Rh/Nb2O5 | Rh | 1.9Co 2.3Rh | 150 | 0.1 | 2 | 26 | 68 | n.a. | ||
| Co-Ni-Cs/La2O3 | Ni, Cs | 50Co 20Ni1Cs | 260 | 0.2 | 2 | 20 | 21 | n.a. | ||
| Co-Ru/Na-meso-Y-IMF | Ru | 15Co 0.3Ru | 230 | 2 | 1 | 45 | 85 | 52 | [113] | |
| Co-Re/Na-meso-Y-IMF | Re | 15Co 0.3Re | 39 | 88 | 54 | |||||
| Co-Ce/Na-meso-Y-IMF | Ce | 15Co 3Ce | 21 | 75 | 38 | |||||
| Co-Zr/Na-meso-Y-IMF | Zr | 15Co 3Zr | 32 | 86 | 49 | |||||
| Co-Mn/Na-meso-Y-IMF | Mn | 15Co 3Mn | 37 | 92 | 65 | |||||
| Catalyst | Loading, wt.% | Reaction Conditions | Catalysis Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| T, °C | P, MPa | H2/CO Ratio | Conversion CO, % | C5+ Selectivity, % | C10–C20 Selectivity, % | |||
| Co-Pt/ZSM-5 | 13Co 0.3Pt | 240 | 2 | 2 | 42 | 82 | 68 | [93] |
| Co-Al2O3/ZSM-5 | 13Co | 33 | 52 | 35 | ||||
| Co-Al2O3-Ru/ZSM-5 | 13Co 0.3Ru | 31 | 76 | 55 | ||||
| Co-Al2O3-Pt/ZSM-5 | 13Co 0.3Pt | 41 | 80 | 62 | ||||
| Co-Al2O3-La/ZSM-5 | 13Co 0.3La | 21 | 60 | 46 | ||||
| Co/SiO2 | 15Co | 230 | 2 | 1 | 65 | 88 | 44 | [98] |
| Co/Al2O3 | 58 | 89 | 41 | |||||
| Co/HY | 98 | 75.1 | 38 | |||||
| Co/Na-Y | 80 | 78.2 | 46 | |||||
| Co/H-meso-Y | 99 | 81.2 | 47 | |||||
| Co/Na-meso-Y | 94 | 89 | 60 | |||||
| Co/MZN | 15Co | 220 | 2 | 2 | 80.3 | 68.5 | 28 | [128] |
| Co/MZT | 75.4 | 68.2 | 35.4 | |||||
| Co/MZNS | 70.2 | 41.5 | 10 | |||||
| Co/MZTE | 86.2 | 70 | 33.1 | |||||
| Co/M | 15Co | 250 | 2 | 2 | 58.2 | 84.5 | 35.3 | [129] |
| Co/M-Z | 25.7 | 68.3 | 13.4 | |||||
| Co/M-4Z | 7.5 | 50.4 | 5.9 | |||||
| Co/Z | 33.4 | 47.0 | 2.5 | |||||
| Co/ZS-0 | 15Co | 240 | 2 | 2 | 90.0 | 65.1 | 26.6 | [130] |
| Co/ZS-10 | 90.3 | 68.8 | 29.3 | |||||
| Co/ZS-20 | 90.6 | 79.7 | 38.0 | |||||
| Co/ZS-30 | 87.9 | 77.3 | 35.2 | |||||
| Co/ZS-50 | 84.9 | 71.9 | 30.7 | |||||
| Co/ZS-100 | 70.5 | 69.6 | 34.1 | |||||
| Co/ZSM-5 | 15Co | 220 | 2 | 2 | 28.2 | 45.5 | 10.6 | [131] |
| Co/SBA-15 | 65.5 | 84 | 42.4 | |||||
| Co/MZ-1 | 30.2 | 74.2 | 31.2 | |||||
| Co/MZ-2 | 34.4 | 74.7 | 29.5 | |||||
| Co/MZ-3 | 38.2 | 74.1 | 29.1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurova, K.; Miyassarova, A.; Eliseev, O.; Stytsenko, V.; Glotov, A.; Stavitskaya, A. Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction. Catalysts 2023, 13, 1215. https://doi.org/10.3390/catal13081215
Mazurova K, Miyassarova A, Eliseev O, Stytsenko V, Glotov A, Stavitskaya A. Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction. Catalysts. 2023; 13(8):1215. https://doi.org/10.3390/catal13081215
Chicago/Turabian StyleMazurova, Kristina, Albina Miyassarova, Oleg Eliseev, Valentine Stytsenko, Aleksandr Glotov, and Anna Stavitskaya. 2023. "Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction" Catalysts 13, no. 8: 1215. https://doi.org/10.3390/catal13081215
APA StyleMazurova, K., Miyassarova, A., Eliseev, O., Stytsenko, V., Glotov, A., & Stavitskaya, A. (2023). Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction. Catalysts, 13(8), 1215. https://doi.org/10.3390/catal13081215