Experimental Study of Catalytically Enhanced Cyclic Steam-Air Stimulation for In Situ Hydrogen Generation and Heavy Oil Upgrading
Abstract
1. Introduction
- Oil cracking
- Oil aquathermolysis
2. Results
2.1. Experimental Workflow
2.2. Outlet Fluids
3. Discussion
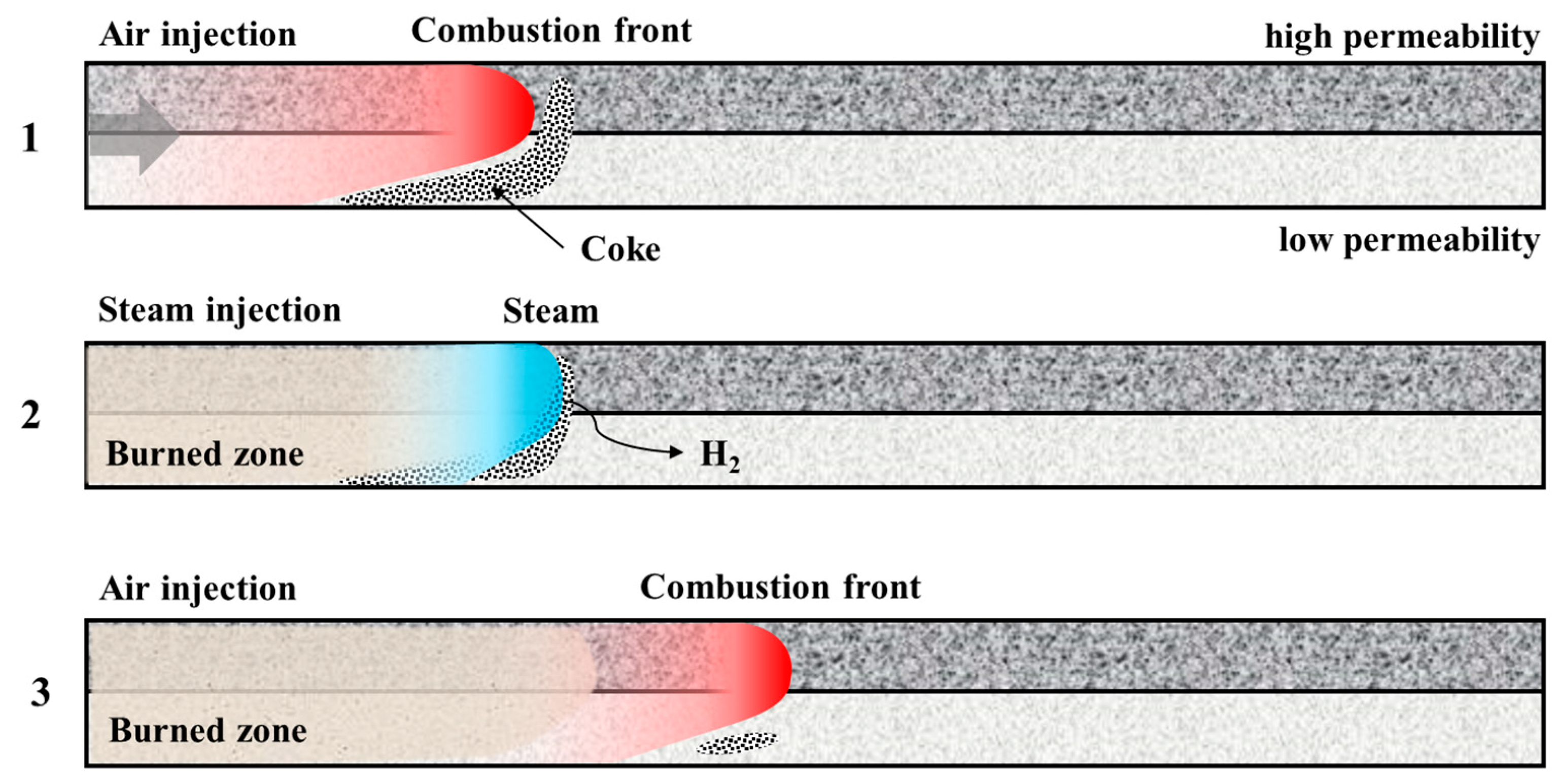
3.1. Temperature Profile Analysis
3.2. Outlet Gas Analysis
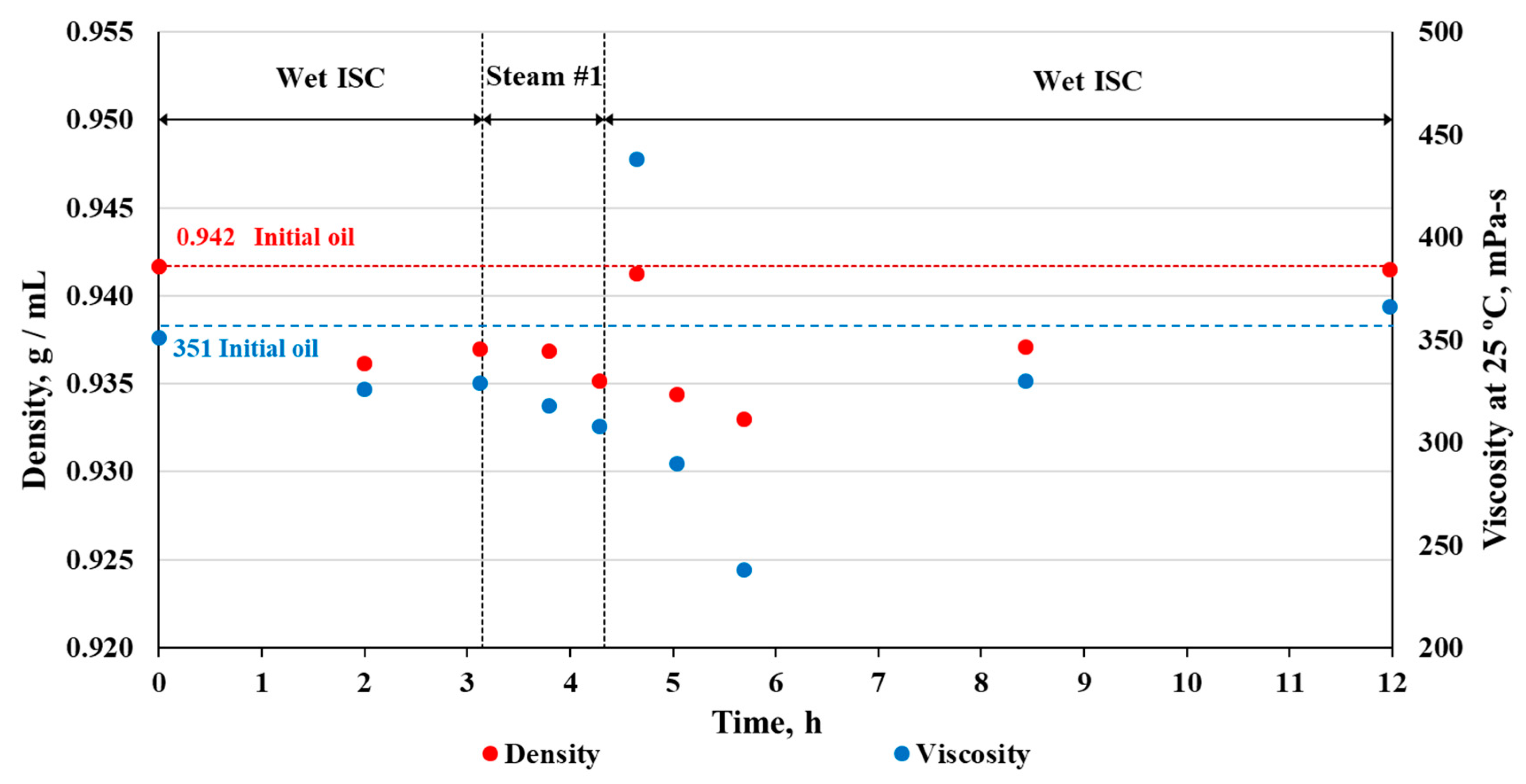
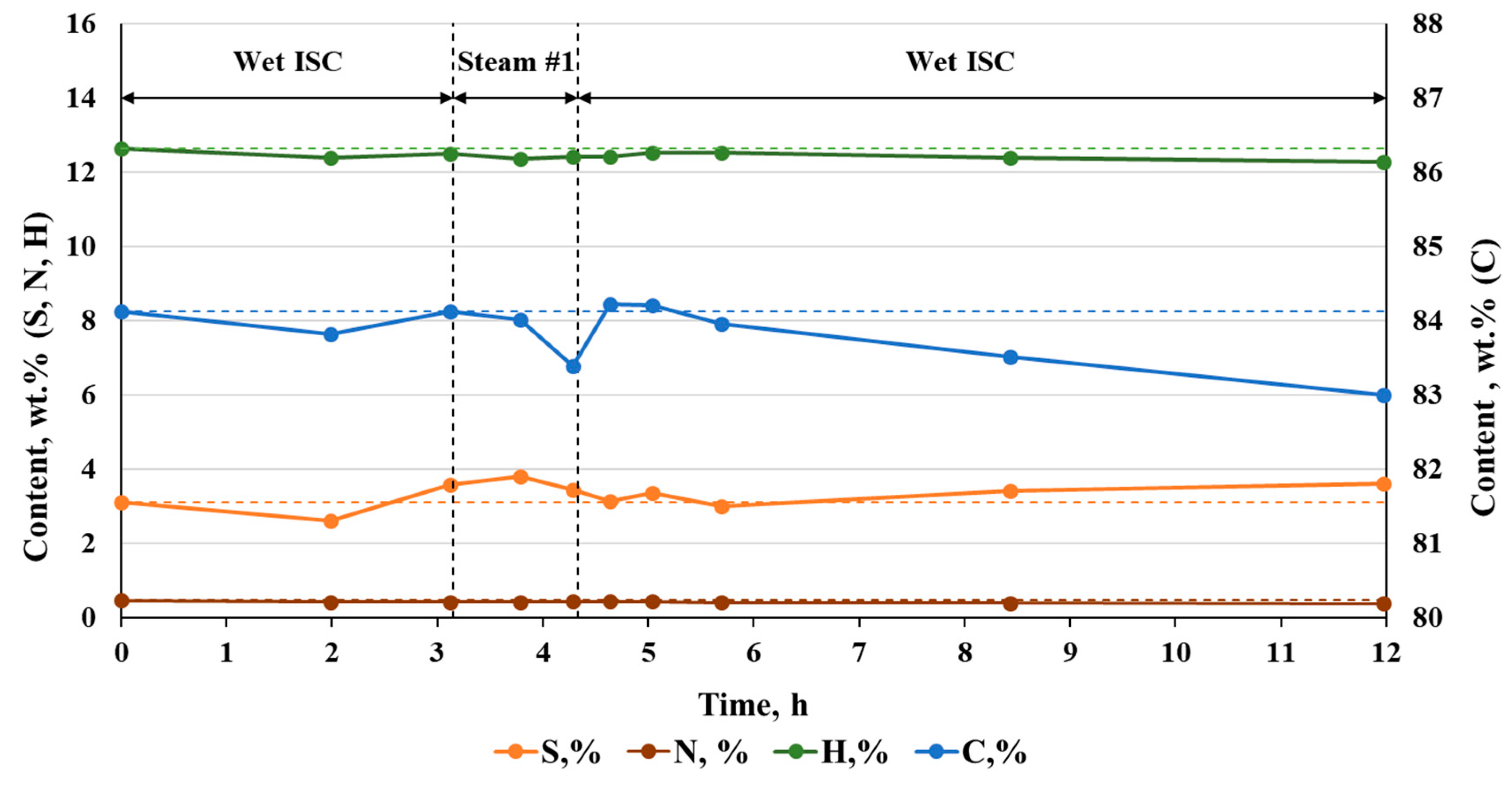
3.3. Effect on Oil Composition and Properties
3.4. Residual Coke Content
4. Materials and Methods
4.1. Design of the Experiment
4.2. Preparation of the Core Model
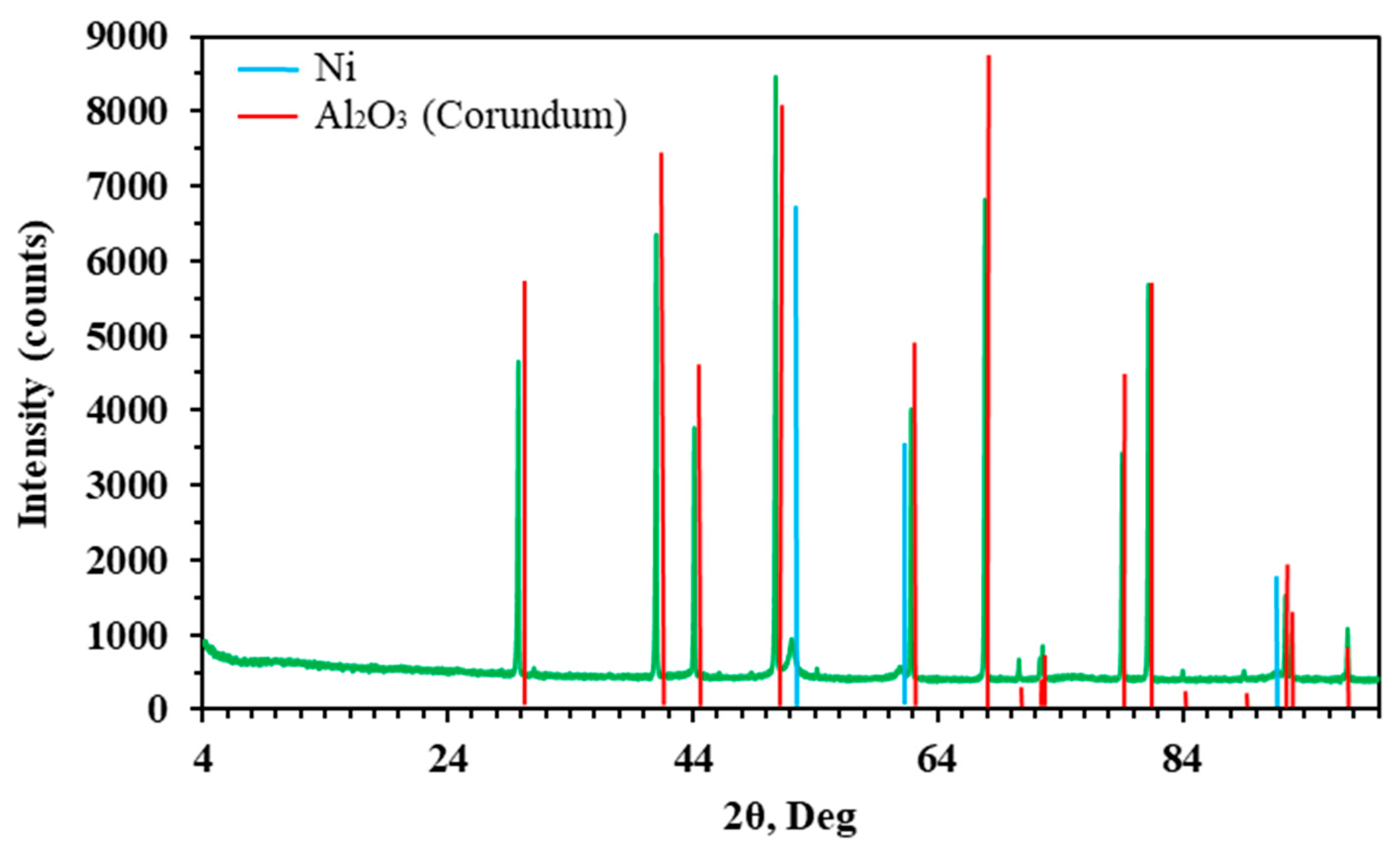
4.3. Catalyst Preparation and Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allison, E.; Mandler, B. Heavy Oil; American Geosciences Institute: Alexandria, VA, USA, 2018. [Google Scholar]
- Omoregbe, O.; Hart, A. Global Trends in Heavy Oil and Bitumen Recovery and In-Situ Upgrading: A Bibliometric Analysis during 1900–2020 and Future Outlook. J. Energy Resour. Technol. Trans. ASME 2022, 144, 123007. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, L.; Wang, Y.; Jin, H.; Chen, L.; Huang, Z. Enhanced Oil Recovery and in Situ Upgrading of Heavy Oil by Supercritical Water Injection. Energy Fuels 2020, 34, 360–367. [Google Scholar] [CrossRef]
- Sheng, J.J. Steam Flooding, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123865458. [Google Scholar]
- Wang, C.; Liu, H.; Pang, Z.; Wang, J.; Chen, C.; Wang, C.; Wu, Z. Enhance Oil Recovery for Steam Flooding: Low-Temperature Oxidative Decomposition of Heavy Oil with Air Injection. Energy Fuels 2015, 29, 6242–6249. [Google Scholar] [CrossRef]
- Sheng, J. Cyclic Steam Stimulation. In Enhanced Oil Recovery Field Case Studies; Gulf Professional Publishing: Houston, TX, USA, 2013; pp. 389–412. [Google Scholar] [CrossRef]
- Xu, A.; Mu, L.; Fan, Z.; Wu, X.; Zhao, L.; Bo, B.; Xu, T. Mechanism of heavy oil recovery by cyclic superheated steam stimulation. J. Pet. Sci. Eng. 2013, 111, 197–207. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, J.; Gates, I.D. On the physics of cyclic steam stimulation. Energy 2016, 115, 969–985. [Google Scholar] [CrossRef]
- Butler, R.M.; McNab, G.S.; Lo, H.Y. Theoretical Studies on the Gravity Drainage of Heavy Oil during In-Situ Steam Heating. Esso Resour. Can. Ltd. 1981, 5, 455–460. [Google Scholar] [CrossRef]
- Bagci, S. The effect of fractures on the steam-assisted gravity drainage process. Energy Fuels 2004, 18, 1656–1664. [Google Scholar] [CrossRef]
- Ji, D.; Zhong, H.; Dong, M.; Chen, Z.; Jia, X. A Model to Estimate Heat Efficiency in Steam-Assisted Gravity Drainage by Condensate and Initial Water Flow in Oil Sands. Ind. Eng. Chem. Res. 2016, 55, 13147–13156. [Google Scholar] [CrossRef]
- Giacchetta, G.; Leporini, M.; Marchetti, B. Economic and environmental analysis of a Steam Assisted Gravity Drainage (SAGD) facility for oil recovery from Canadian oil sands. Appl. Energy 2015, 142, 1–9. [Google Scholar] [CrossRef]
- Sarathi, P.S. In-Situ Combustion Handbook—Principles and Practices; National Petroleum Technology Office, Department of Energy: Tulsa, OK, USA, 1999; Volume 9.
- Yang, M.; Harding, T.G.; Chen, Z. An Improved Kinetics Model for in Situ Combustion of Pre-Steamed Oil Sands. Energy Fuels 2017, 31, 3546–3556. [Google Scholar] [CrossRef]
- Abuhesa, M.B.; Hughes, R. Comparison of conventional and catalytic in situ combustion processes for ol recovery. Energy Fuels 2009, 23, 186–192. [Google Scholar] [CrossRef]
- Mallory, D.G.; Moore, R.G.; Mehta, S.A. Ramped Temperature Oxidation Testing and In Situ Combustion Projects. Energy Fuels 2018, 32, 8040–8056. [Google Scholar] [CrossRef]
- Chen, B. Investigation of In-Situ Combustion Kinetics Using the Isoconversional Principle; Stanford University: Stanford, CA, USA, 2012. [Google Scholar]
- Gutiérrez, D.; Moore, R.G.; Ursenbach, M.G.; Mehta, S.A. The ABCs of in-situ-combustion simulations: From laboratory experiments to field scale. J. Can. Pet. Technol. 2012, 51, 256–267. [Google Scholar] [CrossRef]
- Barzin, Y.; Moore, R.G.; Mehta, S.A.; Ursenbach, M.G.; Tabasinejad, F. A Comprehensive Kinetics Model for Light Oil Oxidation/Combustion Reactions under High Pressure Air Injection Process (HPAI). In Proceedings of the SPE Annual Technical Conference and Exhibition? New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar]
- Bousaid, I.S.; Ramey, H.J. Oxidation of Crude Oil in Porous Media. Soc. Pet. Eng. J. 1968, 8, 137–148. [Google Scholar] [CrossRef]
- Yang, X.; Gates, I.D. Combustion kinetics of Athabasca bitumen from 1D combustion tube experiments. Nat. Resour. Res. 2009, 18, 193–211. [Google Scholar] [CrossRef]
- Zhao, R.B.; Xia, X.T.; Luo, W.W.; Shi, Y.L.; Diao, C.J. Alteration of Heavy Oil Properties under In-Situ Combustion: A Field Study. Energy Fuels 2015, 29, 6839–6848. [Google Scholar] [CrossRef]
- Speight, J.G. The Chemistry and Technology of Petroleum; Taylor & Francis: Abingdon, UK, 2014. [Google Scholar]
- Mehrabi-Kalajahi, S.; Hadavimoghaddam, F.; Varfolomeev, M.A.; Salari, R.; Zinnatullin, A.L.; Vagizov, F.G. Effect of Different Water Content and Catalyst on the Performance of Heavy Oil Oxidation in Porous Media for in Situ Upgrading. Ind. Eng. Chem. Res. 2022, 61, 9234–9248. [Google Scholar] [CrossRef]
- Ovalles, C.; Filgueiras, E.; Morales, A.; Scott, C.E.; Gonzalez-Gimenez, F.; Embaid, B.P. Use of a dispersed iron catalyst for upgrading extra-heavy crude oil using methane as source of hydrogen. ACS Div. Fuel Chem. Prepr. 1998, 43, 521–523. [Google Scholar] [CrossRef]
- Weissman, J.G.; Kessler, R.V.; Sawicki, R.A.; Belgrave, J.D.M.; Laureshen, C.J.; Mehta, S.A.; Moore, R.G.; Ursenbach, M.G. Down-hole catalytic upgrading of heavy crude oil. Energy Fuels 1996, 10, 883–889. [Google Scholar] [CrossRef]
- Hovsepian, C.N.; Carbognani Ortega, L.; Pereira-Almao, P. Laboratory Two-Dimensional Experimental Simulation of Catalytic in Situ Upgrading. Energy Fuels 2016, 30, 3652–3659. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Hu, Z.; Xu, B.; Li, Y.; Pu, W.; Zhao, J. A review of in situ upgrading technology for heavy crude oil. Petroleum 2021, 7, 117–122. [Google Scholar] [CrossRef]
- Golafshani, M.B.; Mehrabi-kalajahi, S.; Varfolomeev, M.A.; Sadikov, K.G.; Emelianov, D.A.; Rodionov, N.O.; Zinnatullin, A.L.; Vagizov, F.G. Improving the Performance of Heavy Oil Oxidation Using Oil- Soluble Copper-Based Catalysts in In Situ Combustion for the In Situ Upgrading Process. Energy Fuels 2023, 37, 6705–6714. [Google Scholar] [CrossRef]
- Schacht-Hernández, P.; Quintana-Solórzano, R.; Morelos-Santos, O.; Soto-Escalante, I.; Ancheyta, J. In Situ Upgrading of Heavy Crude Oil: Comparative Study of the Performance of Cu-, Fe-, Ni-, or Zr-Containing Water-Based Catalysts. Energy Fuels 2022, 36, 12580–12590. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Nasyrova, Z.R.; Gareev, B.I.; Vakhin, A.V. Catalytic Hydrothermal Conversion of Heavy Oil in the Porous Media. Energy Fuels 2021, 35, 1297–1307. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, Y.; Shi, Q.; Yu, Z. The Effect of Carbon-Supported Nickel Nanoparticles in the Reduction of Carboxylic Acids for in Situ Upgrading of Heavy Crude Oil. Energy Fuels 2017, 31, 6045–6055. [Google Scholar] [CrossRef]
- Al-Marshed, A.; Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Effectiveness of Different Transition Metal Dispersed Catalysts for In Situ Heavy Oil Upgrading. Ind. Eng. Chem. Res. 2015, 54, 10645–10655. [Google Scholar] [CrossRef]
- Alkhaldi, S.; Husein, M.M. Hydrocracking of heavy oil by means of in situ prepared ultradispersed nickel nanocatalyst. Energy Fuels 2014, 28, 643–649. [Google Scholar] [CrossRef]
- Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Downhole heavy crude oil upgrading using CAPRI: Effect of steam upon upgrading and coke formation. Energy Fuels 2014, 28, 1811–1819. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. In situ upgrading of athabasca bitumen using multimetallic ultradispersed nanocatalysts in an oil sands packed-bed column: Part 2. Solid analysis and gaseous product distribution. Energy Fuels 2014, 28, 1351–1361. [Google Scholar] [CrossRef]
- Al-Marshed, A.; Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of Heavy Oil Upgrading Using Dispersed Nanoparticulate Iron Oxide as a Catalyst. Energy Fuels 2015, 29, 6306–6316. [Google Scholar] [CrossRef]
- Masudi, A.; Muraza, O. Zirconia-Based Nanocatalysts in Heavy Oil Upgrading: A Mini Review. Energy Fuels 2018, 32, 2840–2854. [Google Scholar] [CrossRef]
- Alemán-Vázquez, L.O.; Torres-Mancera, P.; Ancheyta, J.; Ramírez-Salgado, J. Use of Hydrogen Donors for Partial Upgrading of Heavy Petroleum. Energy Fuels 2016, 30, 9050–9060. [Google Scholar] [CrossRef]
- Aleman-Vazquez, L.O.; Torres-Mancera, P.; Munoz, J.A.D.; Ancheyta, J. Batch reactor study for partial upgrading of a heavy oil with a novel solid hydrogen transfer agent. Energy Fuels 2020, 34, 15714–15726. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, H. The effect of hydrogen donor additive on the viscosity of heavy oil during steam stimulation. Energy Fuels 2002, 16, 842–846. [Google Scholar] [CrossRef]
- Amrollahi Biyouki, A.; Hosseinpour, N.; Bahramian, A.; Vatani, A. In-situ upgrading of reservoir oils by in-situ preparation of NiO nanoparticles in thermal enhanced oil recovery processes. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 289–300. [Google Scholar] [CrossRef]
- Hart, A.; Wood, J.; Greaves, M. In situ catalytic upgrading of heavy oil using a pelletized Ni-Mo/Al2O3 catalyst in the THAI process. J. Pet. Sci. Eng. 2017, 156, 958–965. [Google Scholar] [CrossRef]
- Hallam, R.J.; Hajdo, L.E.; Donnelly, J.K.; Resources, B.P.; Paul, R.; Co, B.P.E. Thermal Recovery of Bitumen at Wolf Lake. SPE Reserv. Eng. 1989, 4, 178–186. [Google Scholar] [CrossRef]
- Hajdo, L.E.; Hallam, R.J.; Vorndran, L.D.L. Hydrogen Generation during In-Situ Combustion. In Proceedings of the SPE 13661, California Regional Meeting, Bakersfield, CA, USA, 27–29 March 1985; pp. 675–689. [Google Scholar]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A Comprehensive Kinetic Theory to Model Thermolysis, Aquathermolysis, Gasification, Combustion, and Oxidation of Athabasca Bitumen. In Proceedings of the SPE 129660, SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; pp. 1–31. [Google Scholar]
- Kapadia, P.R.; Kallos, M.S.; Leskiw, C.; Gates, I.D.; Kapadia, P.R.; Kallos, M.S.; Leskiw, C.; Gates, I.D. Potential for Hydrogen Generation during In Situ Combustion of Bitumen. In Proceedings of the SPE 122028, SPE EUROPEC/EAGE Annual Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009; pp. 1–14. [Google Scholar]
- Kapadia, P.R.; Wang, J.; Kallos, M.S.; Gates, I.D. Practical process design for in situ gasification of bitumen. Appl. Energy 2013, 107, 281–296. [Google Scholar] [CrossRef]
- Askarova, A.; Afanasev, P.; Popov, E.; Mikitin, E.; Darishchev, V. Application of oil in situ combustion for the catalytic methane conversion in the porous medium of the gas reservoir. J. Pet. Sci. Eng. 2023, 220, 111256. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Wu, C.; Lei, G.-L.; Yao, C.-J.; Sun, K.-J.; Gan, P.-Y.; Cao, Y.-B. Mechanism for reducing the viscosity of extra-heavy oil by aquathermolysis with an amphiphilic catalyst. J. Fuel Chem. Technol. 2010, 38, 684–690. [Google Scholar] [CrossRef]
- Qu, X.; Li, Y.; Li, S.; Wang, J.; Xu, H.; Li, Z. Thermal cracking, aquathermolysis, and their upgrading effects of Mackay River oil sand. J. Pet. Sci. Eng. 2021, 201, 108473. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part III–changes in composition resins and asphaltenes. Pet. Sci. Technol. 2018, 36, 1857–1863. [Google Scholar] [CrossRef]
- Fau, G.; Gascoin, N.; Steelant, J. Hydrocarbon pyrolysis with a methane focus: A review on the catalytic effect and the coke production. J. Anal. Appl. Pyrolysis 2014, 108, 1–11. [Google Scholar] [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional roles of second metals in catalyzing methane decomposition over the Ni-based catalysts for hydrogen production: A critical review. Int. J. Hydrogen Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Al Alwan, B.A.; Shah, M.; Danish, M.; Al Mesfer, M.K.; Khan, M.I.; Natarajan, V. Enhanced methane decomposition over transition metal-based tri-metallic catalysts for the production of COx free hydrogen. J. Indian Chem. Soc. 2022, 99, 100393. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to CO x -free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-Gas Shift: 1. Intrinsic Kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A Gen. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming. Catal. Sci. Technol. 1984, 5, 1–117. [Google Scholar] [CrossRef]
- Lopeman, T.; Anbari, H.; Leeke, G.; Wood, J. Numerical Modeling of Toe-to-Heel Air Injection and Its Catalytic Variant (CAPRI) under Varying Steam Conditions. Energy Fuels 2023, 37, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Manrique, E.J.; Kovscek, A.R. Progress toward Pilot-Scale Simulation of In-Situ Combustion Incorporating Geomechanics. SPE Reserv. Eval. Eng. 2023, 26, 152–166. [Google Scholar] [CrossRef]
- Yang, S.; Huang, S.; Jiang, Q.; Yu, C.; Zhou, X. Experimental study of hydrogen generation from in-situ heavy oil gasification. Fuel 2022, 313, 122640. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, M.; Gao, S.; Sun, G.; Xu, G. Reactivity and kinetics for steam gasification of petroleum coke blended with black liquor in a micro fluidized bed. Appl. Energy 2015, 160, 820–828. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhang, X.; Sun, G. Experimental Study of Petroleum Coke Steam Gasification Catalyzed by Black Liquor in a Fluidized Bed. Energy Procedia 2014, 61, 472–475. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yin, H.; He, D.L.; Gong, H.F.; Liu, Z.Z.; Liu, Y.Q.; Zhang, X.M.; Pu, W.F. Low temperature oxidized coke of the ultra-heavy oil during in-situ combustion process: Structural characterization and evolution elucidation. Fuel 2022, 313, 122676. [Google Scholar] [CrossRef]
- Ba, Z.; Zhao, J.; Li, C.; Huang, J.; Fang, Y.; Zhang, L.; Kong, L.; Wang, Q. Developing efficient gasification technology for high-sulfur petroleum coke to hydrogen-rich syngas production. Fuel 2020, 267, 117170. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Wu, S.; Huang, S.; Gao, J. Potassium-catalyzed steam gasification of petroleum coke for H2 production: Reactivity, selectivity and gas release. Fuel Process. Technol. 2011, 92, 523–530. [Google Scholar] [CrossRef]
- Karimi, A.; Semagina, N.; Gray, M.R. Kinetics of catalytic steam gasification of bitumen coke. Fuel 2011, 90, 1285–1291. [Google Scholar] [CrossRef]
- Lan, C.C.; Zhang, S.H.; Liu, X.J.; Liu, R.; Lyu, Q. Kinetic behaviors of coke gasification with CO2 and H2O. ISIJ Int. 2021, 61, 167–173. [Google Scholar] [CrossRef]
- Wang, Z.; Ouyang, P.; Cui, L.; Zong, B.; Wu, G.; Zhang, Y. Valorizing petroleum coke into hydrogen-rich syngas through K-promoted catalytic steam gasification. J. Energy Inst. 2020, 93, 2544–2549. [Google Scholar] [CrossRef]
- Mahamulkar, S.; Yin, K.; Agrawal, P.K.; Davis, R.J.; Jones, C.W.; Malek, A.; Shibata, H. Formation and Oxidation/Gasification of Carbonaceous Deposits: A Review. Ind. Eng. Chem. Res. 2016, 55, 9760–9818. [Google Scholar] [CrossRef]
- Zamani, A.; Maini, B.; Pereira-Almao, P. Experimental study on transport of ultra-dispersed catalyst particles in porous media. Energy Fuels 2010, 24, 4980–4988. [Google Scholar] [CrossRef]
- Afanasev, P.; Popov, E.; Cheremisin, A.; Berenblyum, R.; Mikitin, E.; Sorokin, E.; Borisenko, A.; Darishchev, V.; Shchekoldin, K.; Slavkina, O. An experimental study of the possibility of in situ hydrogen generation within gas reservoirs. Energies 2021, 14, 5121. [Google Scholar] [CrossRef]
- Aounallah, K. Efficient Simulation and Analysis of the Effects of Permeability on the In-Situ Combustion of Heavy Oils. In Proceedings of the SPE Annual Technical Conference and Exhibition? Calgary, AB, Canada, 30 September–2 October 2019. [Google Scholar]
- Awoleke, O.G.; Castanier, L.M.; Kovscek, A.R. An Experimental Investigation of In-Situ Combustion in Heterogeneous Media. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Greaves, M.; Al-Honi, M. Three-Dimensional Studies of In-Situ Combustion-Horizontal Wells Process with Reservoir Heterogeneities. J. Can. Pet. Technol. 2000, 39. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. Potential for hydrogen generation from in situ combustion of Athabasca bitumen. Fuel 2011, 90, 2254–2265. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J. Review on clean hydrogen generation from petroleum reservoirs: Fundamentals, mechanisms, and field applications. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Wei, W.; Wang, J.; Afshordi, S.; Gates, I.D. Detailed analysis of Toe-to-Heel Air Injection for heavy oil production. J. Pet. Sci. Eng. 2020, 186, 106704. [Google Scholar] [CrossRef]
- Pirez, C.; Fang, W.; Capron, M.; Paul, S.; Jobic, H.; Dumeignil, F.; Jalowiecki-Duhamel, L. Steam reforming, partial oxidation and oxidative steam reforming for hydrogen production from ethanol over cerium nickel based oxyhydride catalyst. Appl. Catal. A Gen. 2016, 518, 78–86. [Google Scholar] [CrossRef]
- Kho, E.T.; Scott, J.; Amal, R. Ni/TiO2 for low temperature steam reforming of methane. Chem. Eng. Sci. 2016, 140, 161–170. [Google Scholar] [CrossRef]
- Naeem, M.A.; Al-Fatesh, A.S.; Fakeeha, A.H.; Abasaeed, A.E. Hydrogen production from methane dry reforming over nickel-based nanocatalysts using surfactant-assisted or polyol method. Int. J. Hydrogen Energy 2014, 39, 17009–17023. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Methane reforming to synthesis gas over Ni catalysts modified with noble metals. Appl. Catal. A Gen. 2011, 408, 1–24. [Google Scholar] [CrossRef]
- Rahimi, P.M.; Gentzis, T. The Chemistry of Bitumen and Heavy Oil Processing. In Practical Advances in Petroleum Processing; Springer: New York, NY, USA, 2006; ISBN 9780387257891. [Google Scholar]
- Rabee, A.I.M.; Mekhemer, G.A.H.; Osatiashtiani, A.; Isaacs, M.A.; Lee, A.F.; Wilson, K.; Zaki, M.I. Acidity-reactivity relationships in catalytic esterification over ammonium sulfate-derived sulfated zirconia. Catalysts 2017, 7, 204. [Google Scholar] [CrossRef]
- Khakimova, L.; Askarova, A.; Popov, E.; Moore, R.G.; Solovyev, A.; Simakov, Y.; Afanasiev, I.; Belgrave, J.; Cheremisin, A. High-pressure air injection laboratory-scale numerical models of oxidation experiments for Kirsanovskoye oil field. J. Pet. Sci. Eng. 2020, 188, 106796. [Google Scholar] [CrossRef]
- BSISO9277:2010; Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. BSI: London, UK, 2010.
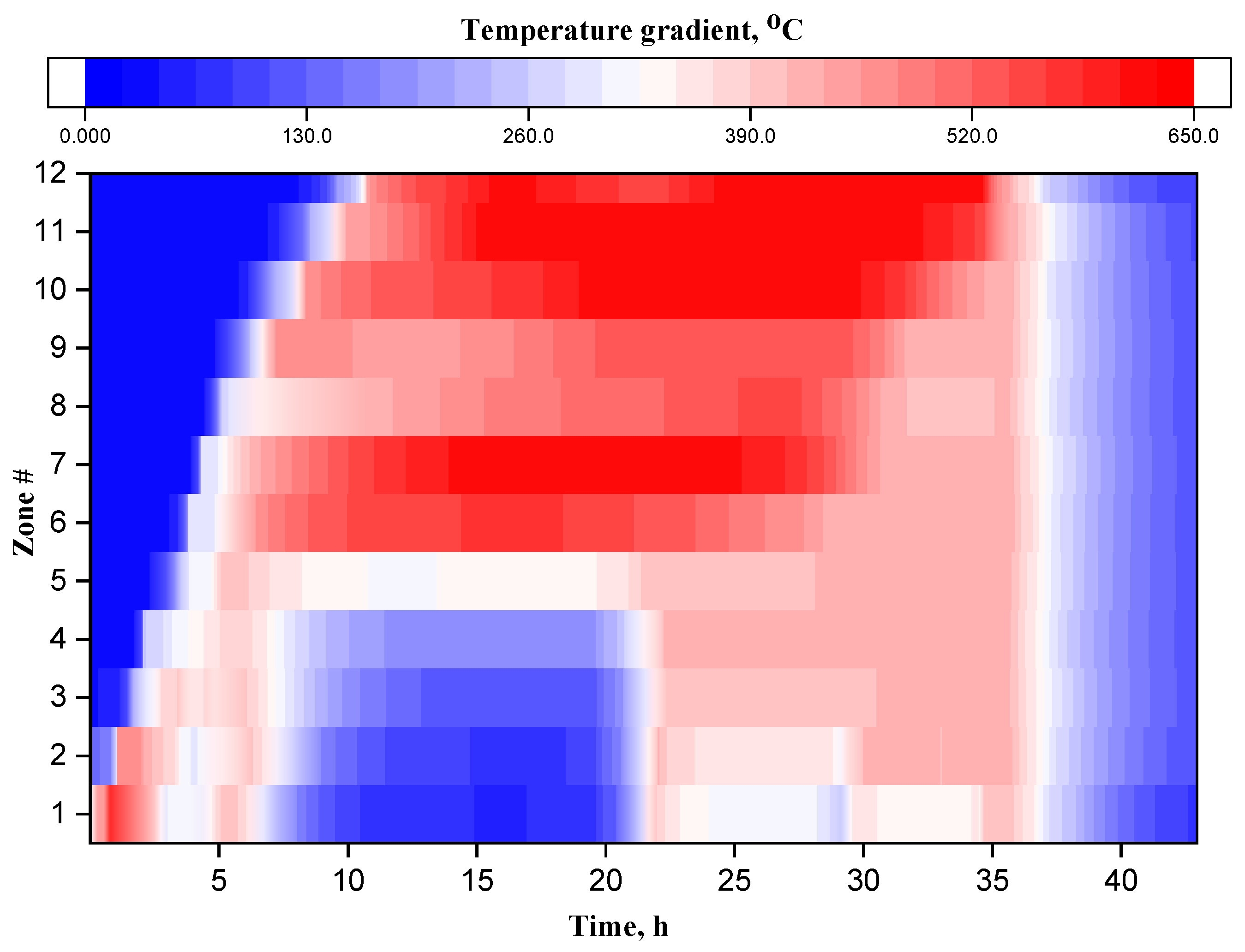
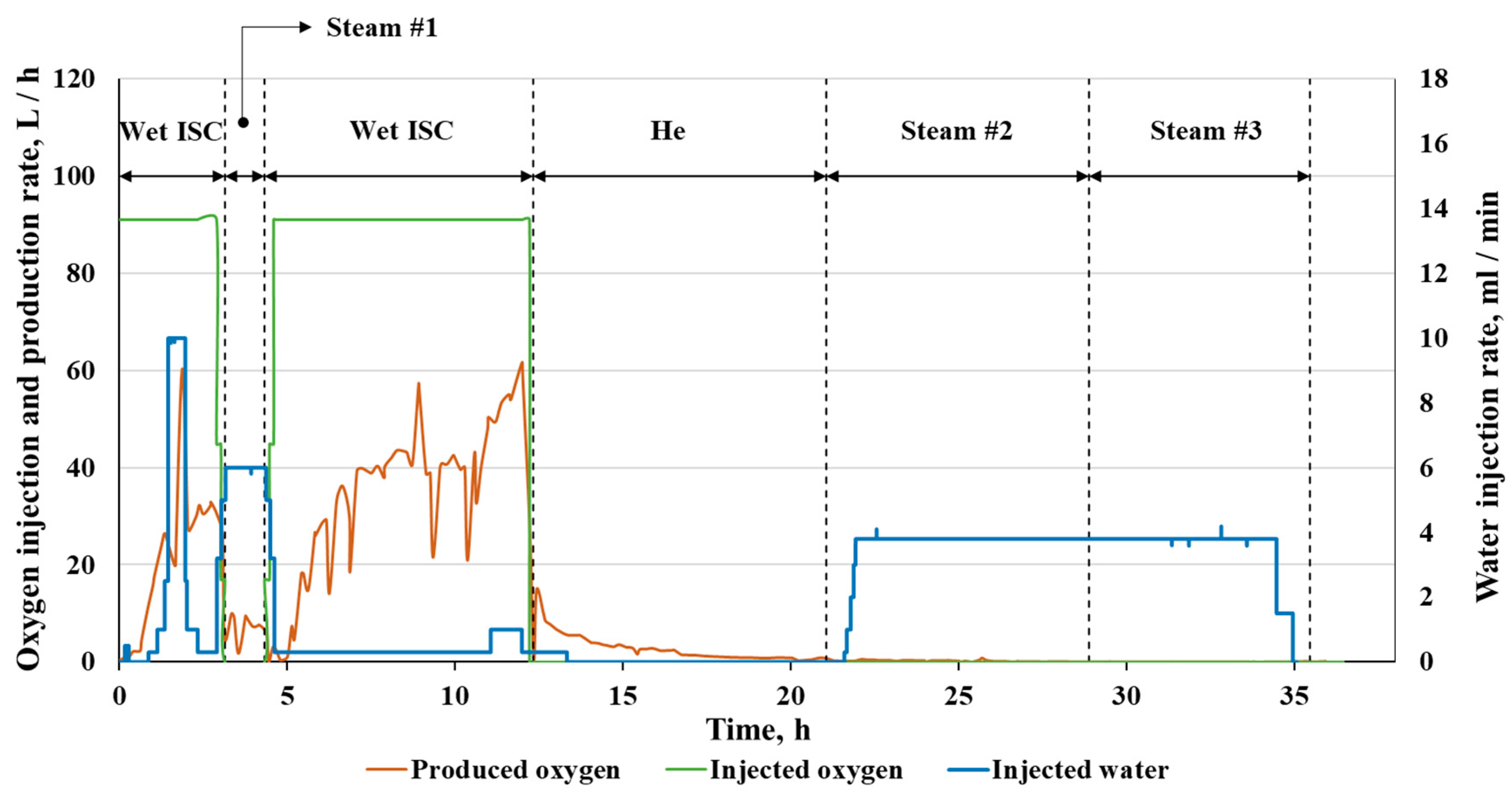

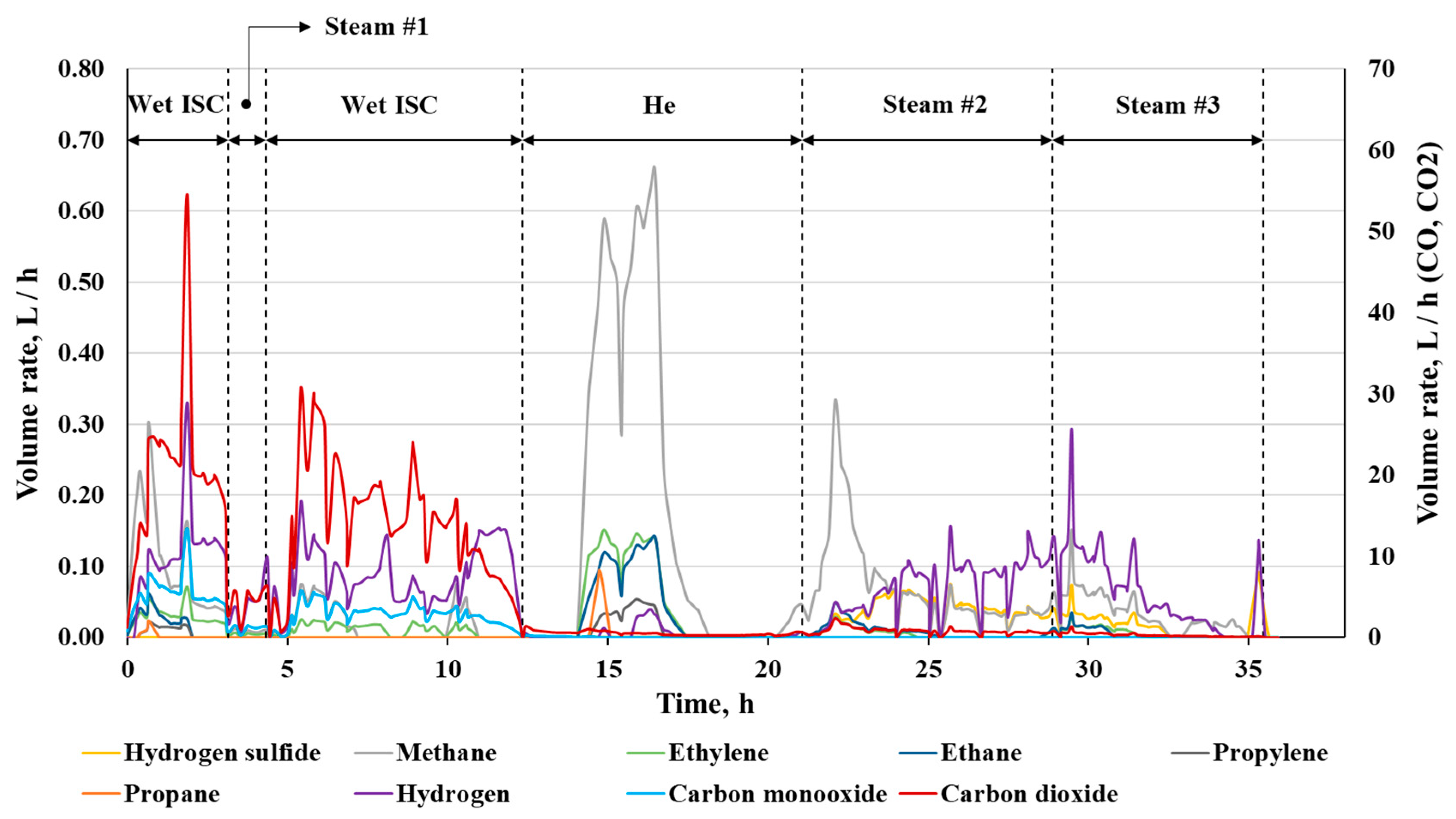
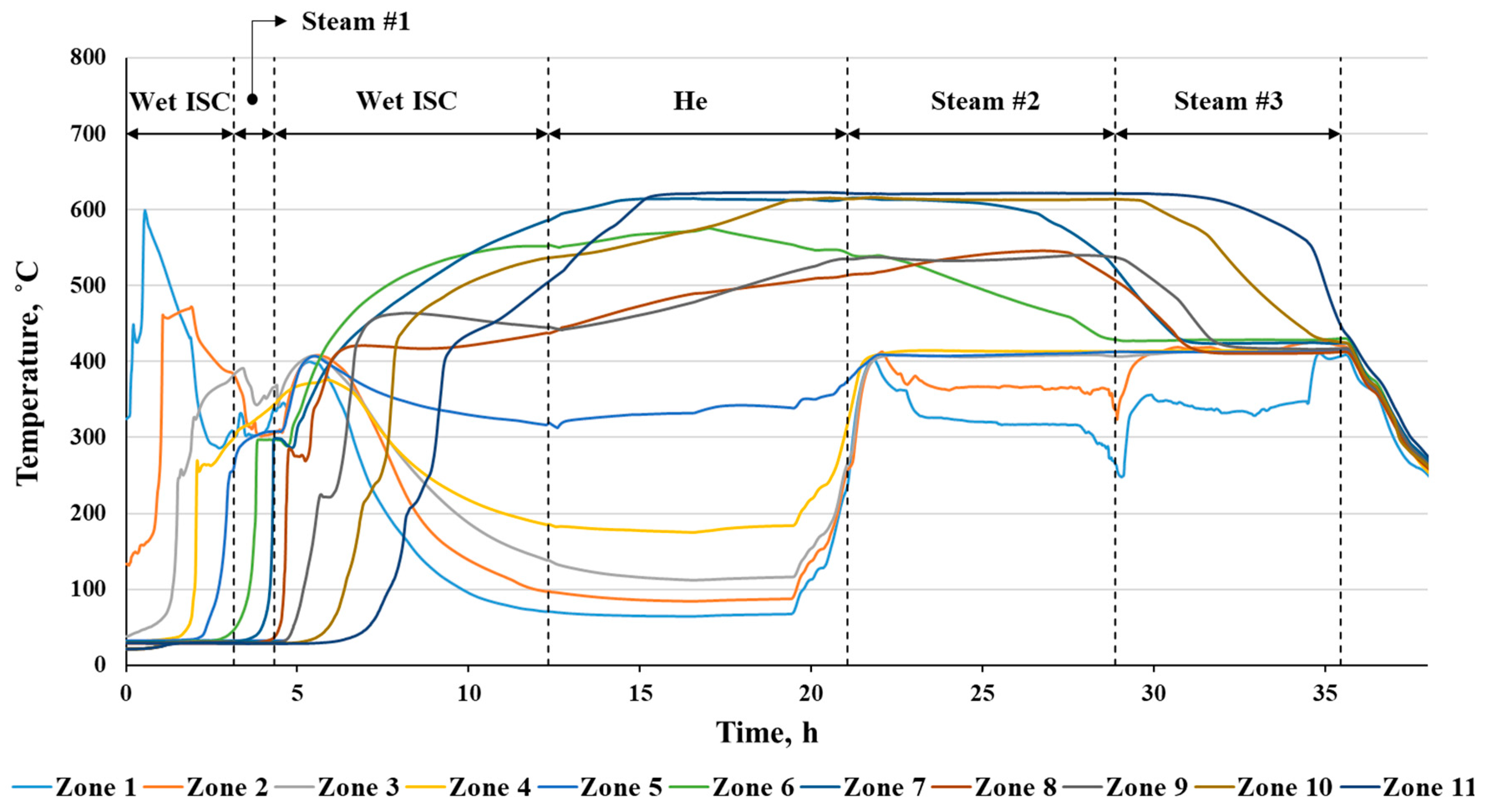









| # | Method | Reservoir Depth | Temperature, °C | Applicability | Main Effects | Ecological Aspects | Difficulties |
|---|---|---|---|---|---|---|---|
| 1 | Steam flood/CSS | Shallow to medium (up to 1500 m) | 200–350 | Heavy oil and bitumen recovery, aerial, vertical and horizontal wells | Bitumen and oil viscosity reduction, upgrade of produced oil (with catalyst) | Significant water consumption | Wide and simple implementation |
| 2 | SAGD | Shallow | 200–350 | Heavy oil and bitumen recovery horizontal well | Bitumen and oil viscosity reduction, upgrade of produced oil (with catalyst) | Significant water consumption | One or two horizontal wells needed |
| 3 | ISC | Deep reservoirs (up to 3000 m) | 400–700 | Heavy oil, bitumen, conventional oil recovery | Bitumen and oil viscosity reduction in situ oil upgrade, improvement of oil with catalyst | Small water injection, release of carbon oxides | Required high-quality engineering support, difficult to manage |
| 4 | Electrical heating | Shallow | 200–300 | Heavy oil and bitumen recovery, horizontal well | Bitumen and oil viscosity reduction, in situ oil upgrade | No water injection, low carbon oxides release, low environmental impact | Loses of energy during transfer to depth, horizontal well required for intensive recovery |
| 5 | In situ catalytic upgrading | Up to 3000 m | 200–300 | Heavy oil, conventional oil recovery | Oil viscosity reduction, in situ oil upgrade | Reduction in carbon oxides emissions (during oil processing) | Mainly in well bottom space, high permeability required |
| 6 | Combined CSS with catalyst and ISC * | Shallow to medium (up to 1500 m) | 300–700 | Heavy oil and bitumen recovery, NOVEL: hydrogen production | Bitumen and oil viscosity reduction, in situ oil upgrade, further improvement of oil with catalyst | Moderate water consumption, reduction in carbon oxides emissions | Required high-quality engineering support |
| # | Name of the Stage | Time, Hour | Note |
|---|---|---|---|
| 1 | Start of the air injection | 0 | The beginning of the active stage of the experiment |
| 2 | Wet in situ combustion #1 | 0.15–3.15 | Ignition and running of the wet combustion |
| 3 | Steam #1 | 3.15–4.33 | Quenching of the combustion front by steam, stopping air injection |
| 4 | Wet in situ combustion #2 | 4.33–12.35 | The second stage of air injection |
| 5 | He | 12.35–19.44 | Try on the experiment completion, end of air injection, combustion front passed 75% of the length of the CT |
| 6 | Steam #2 | 19.44–29.07 | A restart of steam injection since the continuous exothermic reactions in several CT zones |
| 7 | Steam #3 | 29.07–35.46 | Continuation of steam injection with heating of the first CT zones |
| 8 | End of the experiment | 35.46–38.00 | End of steam injection, depressurizing, and cooling of the CT |
| Initial oil, g | 3181.28 |
| Residual oil, g | 42.41 |
| Recovered oil, g | 3036.54 |
| -Recovered at wet ISC stages, g | 1135.17 |
| -Recovered at Steam stages, g | 1797.09 |
| -Recovered at Helium purge, g | 104.28 |
| Burnout oil, g | 141.40 |
| Residual coke, g | 26.56 |
| Gathered oil, g | 3246.91 |
| Error, % | −2.06 |
| Recovery factor | 0.95 |
| Parameter | Value |
|---|---|
| Aver. combustion front temperature, °C | 400 |
| Combustion front velocity, m/h | 0.124 |
| O2/Fuel ratio, m3(ST) × kg−1 | 7.43 |
| Air/Fuel ratio, m3(ST) × kg−1 | 35.38 |
| Injected oxygen to carbon oxides, % | 13.56 |
| Reacted oxygen to carbon oxides, % | 31.36 |
| (CO2 + CO)/CO ratio | 2.80 |
| (CO2 + CO)/N2 ratio | 0.04 |
| Apparent atomic H/C ratio | 7.19 |
| Parameter | Value |
|---|---|
| Pore pressure, MPa/psi | 8/1160 |
| Initial temperature, °C | 27–30 |
| Combustion initiation temperature, °C | 350 |
| Air injection rate TMFC, st. L/h | 314 |
| Water injection rate, mL/min | 0.3–1.0 |
| Steam injection rate, mL/min | 0.3–6.2 |
| Initial oil saturation | 50–60% |
| Phase transition temperature, °C | 295 |
| Parameter | Value |
|---|---|
| Porosity of the high-permeable zone (#1), % | 37.3 |
| Porosity of the low-permeable zone (#2), % | 35.7 |
| Permeability of high-permeable zone (#1), D | 4.95 |
| Permeability of low-permeable zone (#2), D | 4.70 |
| Average initial oil saturation, % | 0.62 |
| Component of the Water Solution | Concentration, g/L |
|---|---|
| NaCl | 175.47 |
| CaCl2 × 2H2O | 37.05 |
| MgCl2 × 6H2O | 22.42 |
| Na2SO4 × 10H2O | 3.05 |
| Elements | Relative Content (wt.%) | Relative Content (Atom.%) |
|---|---|---|
| Al | 50.05 | 41.83 |
| O | 38.02 | 53.59 |
| Ni | 11.93 | 4.58 |
| Sum | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afanasev, P.; Smirnov, A.; Ulyanova, A.; Popov, E.; Cheremisin, A. Experimental Study of Catalytically Enhanced Cyclic Steam-Air Stimulation for In Situ Hydrogen Generation and Heavy Oil Upgrading. Catalysts 2023, 13, 1172. https://doi.org/10.3390/catal13081172
Afanasev P, Smirnov A, Ulyanova A, Popov E, Cheremisin A. Experimental Study of Catalytically Enhanced Cyclic Steam-Air Stimulation for In Situ Hydrogen Generation and Heavy Oil Upgrading. Catalysts. 2023; 13(8):1172. https://doi.org/10.3390/catal13081172
Chicago/Turabian StyleAfanasev, Pavel, Alexey Smirnov, Anastasia Ulyanova, Evgeny Popov, and Alexey Cheremisin. 2023. "Experimental Study of Catalytically Enhanced Cyclic Steam-Air Stimulation for In Situ Hydrogen Generation and Heavy Oil Upgrading" Catalysts 13, no. 8: 1172. https://doi.org/10.3390/catal13081172
APA StyleAfanasev, P., Smirnov, A., Ulyanova, A., Popov, E., & Cheremisin, A. (2023). Experimental Study of Catalytically Enhanced Cyclic Steam-Air Stimulation for In Situ Hydrogen Generation and Heavy Oil Upgrading. Catalysts, 13(8), 1172. https://doi.org/10.3390/catal13081172








