Abstract
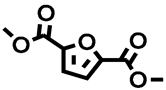
2,5-furandicarboxylic acid (FDCA) is one of the most studied bio-based monomers, being considered the best substitute for fossil-derived terephthalic acid in plastic production. FDCA is employed in the preparation of polyethylene furanoate (PEF), demonstrating superior mechanical and thermal proprieties compared to the widely used polyethylene terephthalate (PET). Nevertheless, FDCA synthesis mostly relies on the oxidation of the bio-based platform chemical hydroxymethyl furfural (HMF), whose notoriously instable nature renders FDCA yield and industrial scale-up production complicated. On the contrary, FDCA esters are less studied, even though they have greater solubility in organic media, which would favor their isolation and potential application as monomers for PEF. On these premises, we report herein an alternative green synthetic approach to FDCA methyl ester (FDME) using galactaric acid as the substrate, dimethyl carbonate (DMC) as the green media, and Fe2(SO4)3 as the heterogeneous Lewis acid. Optimization of the reaction conditions allowed the selective production of FDME in a 70% isolated yield; product purification was achieved via flash column chromatography over silica. Furthermore, it was possible to employ up to 5.0 g of galactaric acid in a single reaction, leading to a good isolated yield of FDME.
1. Introduction
Molecules produced from fossil resources possess limited chemical diversity and usually require numerous synthetic steps to introduce heteroatoms into their structure. On the contrary, biomass-derived compounds often incorporate heteroatoms and stereocenters, which can be utilized to produce advanced building blocks for synthesis [1,2]. For this reason, there is a substantial need to employ these feedstocks to design a greener future for chemistry. From this perspective, bio-based platform chemicals represent an important opportunity to favor the shift from petroleum-derived compounds to a more sustainable chemical production, as they can be employed as substrates for many high added value products [3]. Among these compounds, furan-based molecules [4,5,6] have gained attention due to the possibility of employing them as building blocks in biorefinery processes [7,8], as well as for the production of bio-based materials and fuels [9]. In particular, 5-hydroxymethyl furfural (HMF) [10,11] and its derivatives, i.e., 2,5-bis(hydroxymethyl)furan (BHMF) [4], 5,5′-[oxybis(methylene)]bis-2-furfural (OBMF) [5], and 2,5-furandicarboxylic acid (FDCA) and its esters [6], are well-known representatives of this family of compounds.
As an example, 2,5-furandicarboxylic acid (FDCA) [9] has been extensively investigated as a potential substitute for terephthalic acid in polyesters, polyamides, polyurethanes, and plasticizer production [3,12,13,14,15,16]. FDCA-based polyesters, polyethylene furanoate (PEF), and poly(butylene 2,5-furan dicarboxylate) (PBF) have shown comparable and, is some cases, superior features compared to their petroleum-derived counterparts polyethylene terephthalate (PET) and polybutylene terephthalate (PBT), respectively [17], such as enhanced mechanical and thermal properties and an improved gas barrier [12,18,19,20,21,22]. These characteristics render FDCA-based polymers suitable for film and fiber production, packing materials, and soft drink bottles [14].
FDCA has also been used to prevent corrosion, as a crosslinking agent for polyvinyl alcohols and for the synthesis of different biochemical molecules and pharmaceutical intermediates [14].
The increasing interest toward FDCA has been fostered by Avantium, Dupont and Archer Daniels Midland (ADM), companies actively working the production of FDCA, as well as on the preparation of its methyl ester—2,5-furadicarboxylic acid dimethyl ester (FDME)—at an industrial scale, starting from biomass (mainly cellulose, hemi-cellulose, starch, sucrose, and fructose) [3,23,24,25].
At present, most of the reported synthetic approaches to FDCA use HMF as the substrate, since its alcoholic and aldehydic functionalities can be easily converted into carboxylic acid groups via oxidation, employing as catalysts transition metal materials and nanoparticles, i.e., Ru [26,27], Pt and Pt/Sn [28,29,30], Pd, Au and Au-Pd [31,32,33], FeIII [34], and Cu-MnO2 [35]. FDCA can be also prepared though electrooxidation [36,37,38] and biocatalytic techniques [39,40,41].
The main drawbacks of a HMF-mediated process to prepare FDCA can be ascribed to HMF’s intrinsic instability, together with its difficult separation from the reaction media, which is typically a polar solvent [42]. In a previous investigation, we showed that the latter issue can be solved by performing the D-fructose triple dehydration to HMF by employing a biphasic system constituted by dimethyl carbonate (DMC) and tetraethyl ammonium bromide (TEAB), where DMC acts as a very efficient extracting solvent [4]. In addition, it should be mentioned that HMF, as many other bio-based platform chemicals, is mostly obtained from glucose and fructose, originating from first-generation feedstocks that overlap with food for human consumption [16,43]. The above-mentioned problems, together with the low scale and diluted experimental conditions generally used, hamper FDCA production at the industrial scale, leading to a consequent high market price [6,44].
An alternative promising route to FDCA and its esters involve the utilization of aldaric acids as starting materials [6,45]. These compounds have also been employed as bio-based monomers for polyaldaramides [46,47,48] and polyester production [49,50,51].
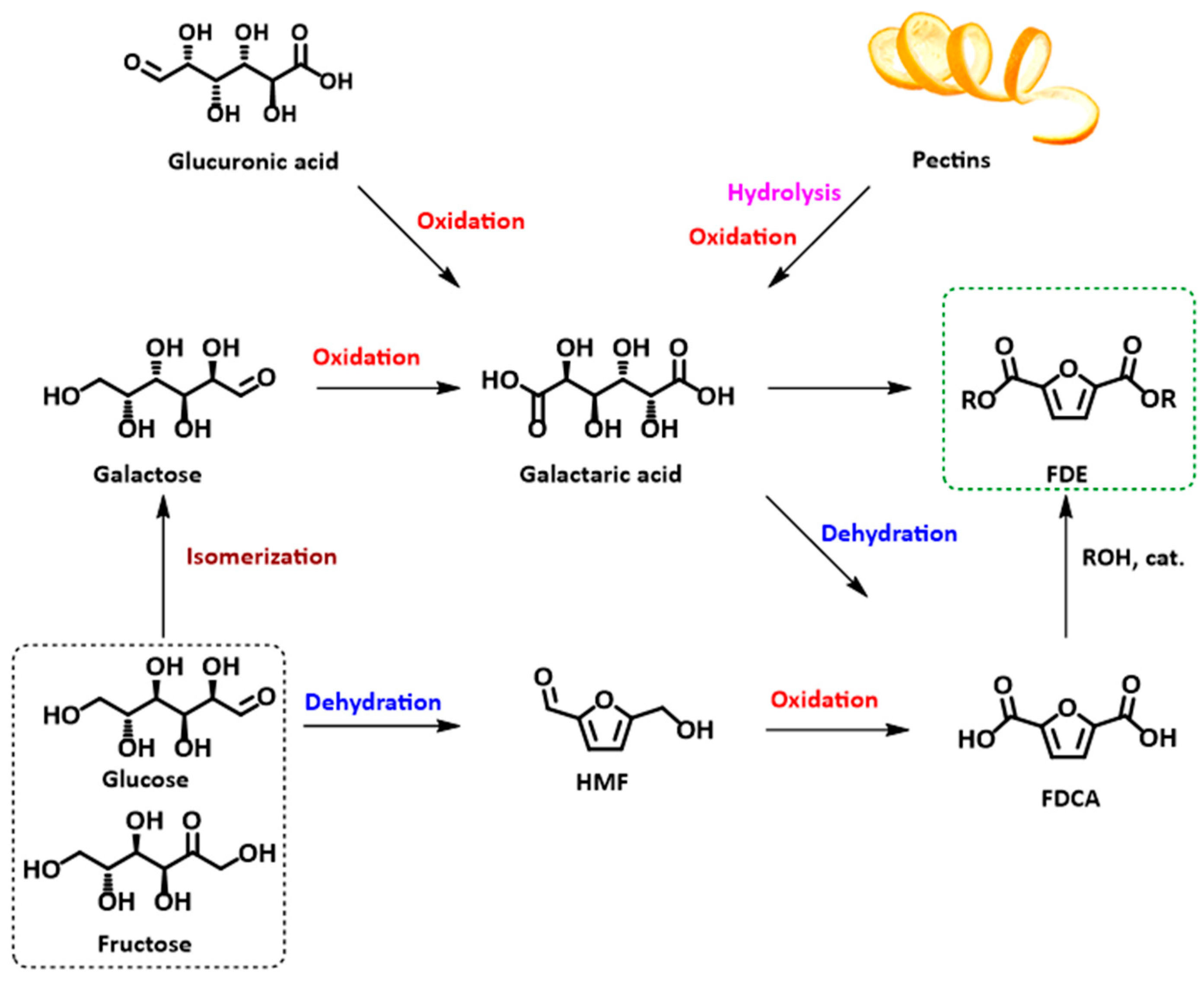
Aldaric acids can be obtained through the oxidation of carbohydrates, such as (i) oxidation of glucose into glucaric acid, one of the most investigated reactions [52], and (ii) uronic acid oxidation. Uronic acids are contained in pectins, heteropolysaccharides found mainly in citrus peels, and in agrifood production residues (sugar beet, chicory pulp, citrus fruit peels, etc.) (Figure 1) [51,53]. It must be mentioned that oxidation of uronic acids into aldaric acids is complicated by the acid-sensitive nature of the former compounds; nevertheless, some examples have been reported in the literature [51,54].
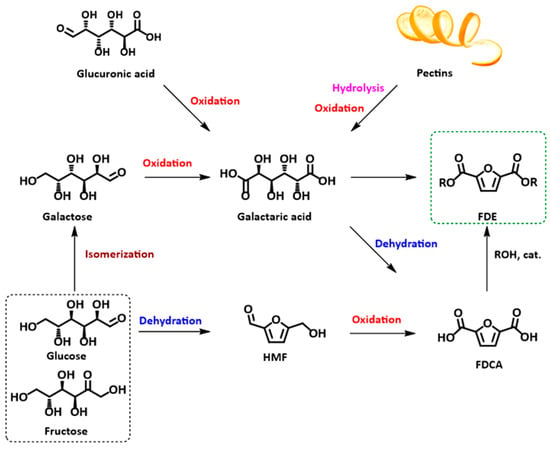
Figure 1.
Synthetic pathways to FDCA and FDME starting from carbohydrates or pectins.
Pioneering works carried out in 1901 and 1956 demonstrated that glucose-derived aldaric acid (glucaric acid) can be converted into FDCA through an HBr catalyzed dehydration in a ca 50% yield [55,56,57], while subsequent trials employing heterogeneous catalysts like Amberlyst-15, MCM-41, and sulphated zirconia have provided discouraging results (<1% yield) [58].
The conversion of a specific aldaric acid, galactaric (mucic) acid, into FDCA was first reported in 1876 by Fittig and Heinzelmann by employing strong acids—H2SO4 or HBr−—under pressure and resulting in an approximately 50% yield [59]. Other trials have since been reported, changing the dehydrating agent but still employing harsh reaction conditions and ultimately leading to similar yield values [60].
Since then, little to no investigations regarding this synthetic approach to FDCA can be found in the literature, both due to the lack of a large-scale production of galactaric acid and to the moderate FDCA yields obtained [15,61].
Some investigations have instead focused on obtaining FDCA esters; interest in these compounds originates from their greater solubility in organic media, which favors their isolation either by distillation or recrystallization [62].
Van der Klis and co-workers reported a three-step conversion of uronic acids into FDCA esters by employing an Au-based catalyst for the oxidation step obtaining an overall yield of 45% of 2,5-furandicarboxylic acid dimethyl ester (FDME) [51].
p-Toluene sulfonic acid (p-TSA) and its silica-supported derivative have proven to be viable catalysts for the dehydration and aromatization of galactaric acid into 2,5-furandicarboxylic acid dibutyl ester (FDBE), obtained in one step at reflux temperature [62] or under two-step autoclave conditions [45]. Nevertheless, the yields did not exceed 56% in the former case, while there is no indication on the isolated yield in the latter.
A one-pot, two-step procedure for the synthesis of 2,5-furandicarboxylic acid diethyl ester (FDEE) starting from galactaric acid was then reported using methanesulfonic acid (MSA) and ethanol with a moderate yield of 29% [63].
Recently, our research group developed a green and simple procedure for producing FDCA esters, particularly FDME, starting from galactaric acid and dimethyl carbonate (DMC) as the solvent and reagent, in the presence of an acidic heterogeneous Amberlyst resin as a catalyst. The reaction was conducted in a stainless steel autoclave under pressure for 2 h at 200 °C, yielding FDME as a white crystalline solid in a 70% yield. The reaction can be performed to a maximum of 3 g of galactaric acid [6]. In fact, one of the main drawbacks of this synthesis is the elevated autogenous pressure generated during the process (ca 100 bar), which hampers further up-scaling of the synthesis. The main reason behind the pressure increase is attributed to DMC decarboxylation into CO2 and MeOH, caused by the acidic catalyst in combination with the high reaction temperature. Another issue connected to the reaction conditions is the impossibility of recycling the catalyst as the temperature required is far above Amberlyst’s mechanical stability. These reasons led us to investigate an alternative synthetic process that can reduce the reaction pressure, thus also favoring the reaction scale-up.
Therefore, in this work, we evaluated the activity of numerous homogeneous and heterogenous acids/bases to be employed for the DMC-mediated synthesis of FDME. Interestingly, iron(III) sulfate demonstrated the best promoter performance as the reaction proceeded at a lower pressure, while maintaining comparable yield values to the Amberlyst-catalyzed reaction. In addition, this newly developed procedure allowed the synthesis of FDME starting from up to 5.0 g of galactaric acid. Recycling iron(III) sulfate was also investigated.
2. Results
Following our previous synthetic approach for 2,5-furandicarboxylic acid dimethyl ester (FDME) production [4], we decided to further investigate this chemical transformation by searching for an alternative, more robust heterogenous acid/base so as to avoid its decomposition during the reaction and evaluate its recycling.
2.1. Screening for an Alternative Reaction Promoter
Different acids/bases were selected and investigated for the one-pot conversion of galactaric acid into FDME; the data collected are reported in Table 1.

Table 1.
Promoter screening for FDME production from galactaric acid a.
In a typical experiment, galactaric acid, dimethyl carbonate (DMC), and the selected acid or base were added to a stainless steel autoclave and the mixture was heated to 200 °C for 2 h under magnetic stirring. At the end of the reaction, the system was allowed to cool down to room temperature and the solution was then vacuum filtered on a Gooch with a celite pad. When a homogeneous system was employed, the vacuum filtration was performed either on silica or basic alumina to remove any traces of the base or acid, respectively. The mixture was then concentrated under a vacuum and further purified through silica gel column chromatography.
Due to the poor solubility of galactaric acid in the reaction media, the determination of its conversion could not be evaluated; therefore, it was not reported in Table 1.
The ability of various heterogeneous (#1–11; Table 1) and homogeneous (#12,13; Table 1) systems to promote FDME formation were tested. Particular attention was given to acidic compounds, since they showed better performance in galactaric acid cyclization and aromatization according to previously published works [6,45,62,63]. In fact, a basic environment did not seem to favor galactaric acid conversion into the desired product. In these trials (#1–3; Table 1), the 1H-NMR spectra of the reaction mixtures mostly showed the presence of furoic acid and its methyl ester derivative. A similar behavior was observed when amphoteric hydrotalcites were employed (#4,5; Table 1). Hydrotalcites KW2000 and KW500 have previously been shown to be efficient catalysts in decarboxylation reactions [64]. Therefore, it is reasonable that they are also able to favor the decarboxylation of FDME into furoic acid or furoic acid methyl ester.
Ce2O-promoted reactions (#6; Table 1) only led to the formation of galactaric acid methyl esters; the NMR spectrum also showed the presence of small aromatic signals ascribable to furoic acid.
Reactions conducted in the presence of acidic alumina (#7; Table 1) mostly achieved furoic derivatives, while FDME was detected and isolated only in a small amount.
Among the Lewis acids tested, Fe2(SO4)3 displayed encouraging results, providing FDME in a 54% yield at a relatively low autogenous pressure that did not exceed 25 bar (#8; Table 1). Pure FDME was easily isolated from the reaction mixture via flash chromatography as a lightly yellow crystalline solid. Following this result, other iron(III)-based Lewis acids were tested, but without obtaining comparable results (#9–11; Table 1).
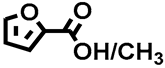
Concerning homogeneous acids, methanesulfonic acid (MSA) showed good selectivity toward FDME and the product could be isolated as a white solid in a 15% yield (#12; Table 1). On the contrary, when p-toluene sulfonic acid (p-TSA) was used (#13; Table 1), despite the detection of the desired product via proton NMR, it was not possible to isolate the pure FDME from the reaction mixture. A possible explanation could be ascribed to the observed methylation of the acidic –OH group of the p-TSA by DMC (Figure S1, Supporting Information), which notoriously acts as a methylating agent at T > 150 °C [65]. The formation of methyl 4-methylbenzenesulfonate inhibited the efficiency of this acid and hampered the isolation of pure FDME due to a similar retention factor to the desired product. As proof, a large amount of black insoluble material was recovered, most likely due to galactaric acid degradation. For this reason, p-TSA was not investigated further. A similar issue probably also occurs when Amberlysts and sulfonated silica are used in this reaction, with the difference being that their heterogeneous nature may be able to prevent the methylation of sulfonic groups. In addition, the solid residue can be easily removed by filtration, thus it is not visible in the proton NMR. Indeed, p-TSA constitutes the active functional group on the surface of these heterogenous acids, which has already shown good performance in other investigations [6,45,62,63].
According to the data reported in Table 1, Fe2(SO4)3 is the most promising promoter; thus, further optimization trials were performed, investigating how the amount of this acid and the reaction temperature influence the FDME yield (Table 2).

Table 2.
Effect of the amount of Fe2(SO4)3 and temperature on FDME formation a.
Employing 50% mol. of Fe2(SO4)3 allowed to isolate FDME in a 54% yield (#3; Table 2), which was the highest yield among the conditions tested. In fact, when a lower acid loading was employed (#1,2; Table 2), the presence of a black oil was observed, most of which was insoluble in the eluent phase used for the purification. This could mean that quantities lower than 50% mol. of Fe2(SO4)3 are not sufficient for converting galactaric acid before its degradation occurs due to the high temperature. Premature reagent degradation, most likely into insoluble humins, could be responsible for the observed yield drop and dark oil formation.
Moreover, a temperature of 200 °C seemed to be optimal for obtaining the highest FDME yield. Either lowering or increasing the reaction temperature to 180 and 220 °C caused a sharp reduction in the FDME yield (#5,7; Table 2). This may be ascribed to a lack of conversion or to a degradation of galactaric acid, respectively. In addition, at 220 °C, the appearance of other aromatic peaks referable to both furoates and 5-(methoxycarbonyl)furan-2-carboxylic acid (ca 15%) were noted. Most likely, these compounds formed as a result of FDME decarboxylation due to the harsher reaction conditions.
Some supplementary trials were then performed, employing tetraethylammonium bromide (TEAB) or DMSO as additional media, as well as using molecular sieves to eliminate the water formed during the reaction. However, in all cases, the isolated yields were very low or the formation of the desired product was not detected (Table S2, Supporting Information).
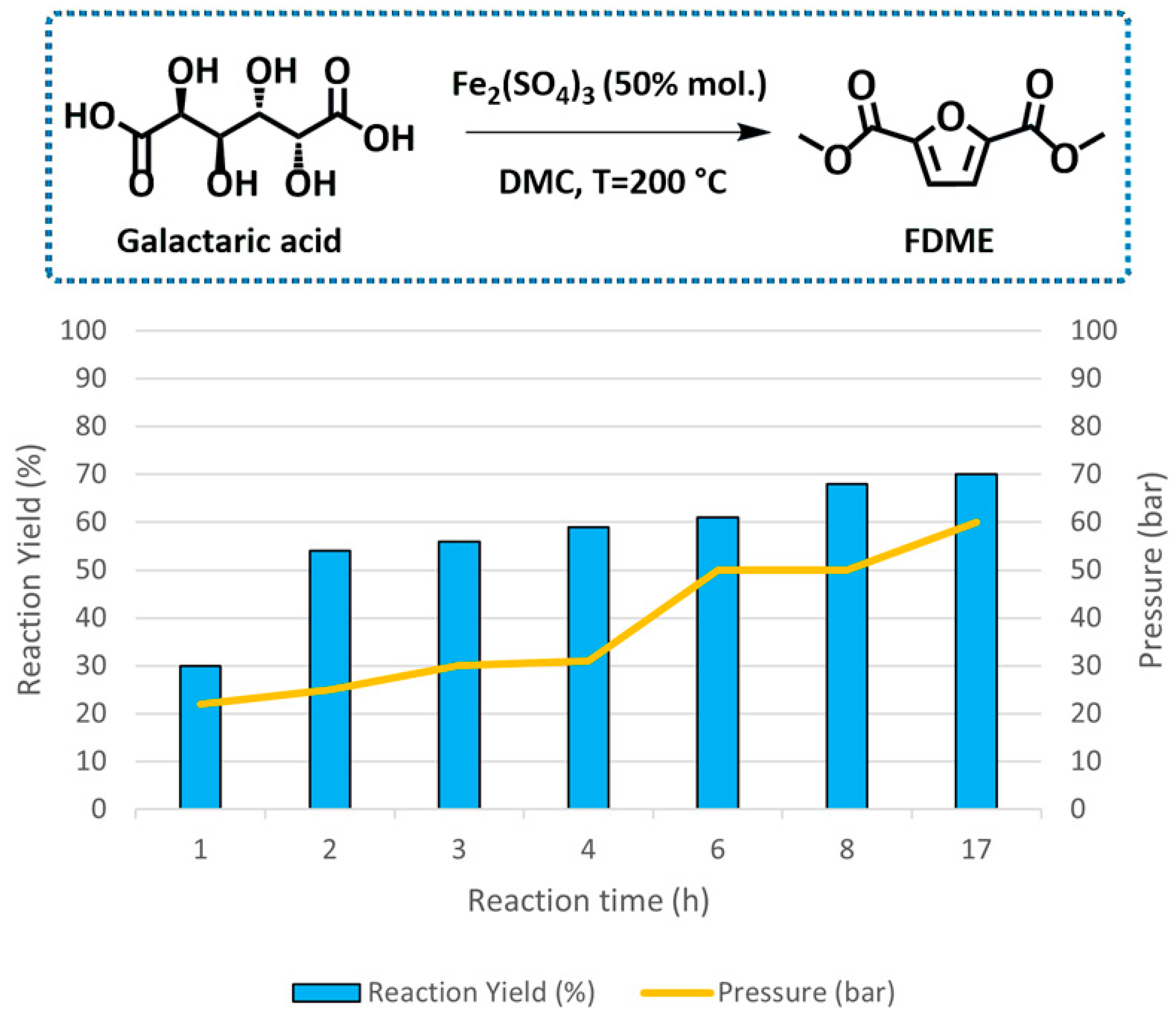
The influence of the reaction time on FDME yield was also evaluated (Figure 2 and Table S1, Supporting Information).
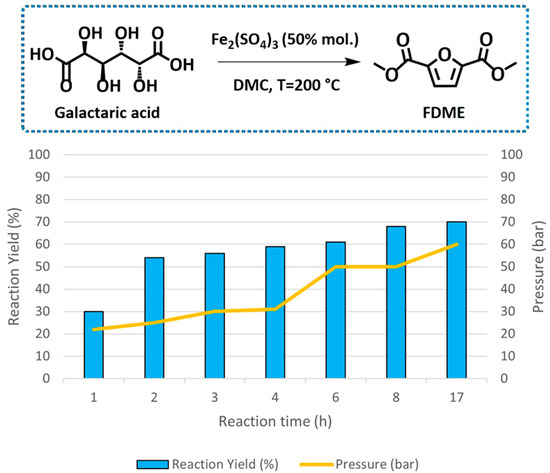
Figure 2.
Effect of the reaction time on FDME formation from galactaric acid. Reaction conditions: 1.0 g of galactaric acid, 0.95 g (50% mol.) of Fe2(SO4)3 in 35 mL of DMC in an autoclave at 200 °C under magnetic stirring (500 rpm). Pressure values refer to the autogenous pressure generated inside the autoclave at the end of the reaction.
When the reaction was performed under the best-found reaction conditions—Fe2(SO4)3 (50% mol. compared to galactaric acid) in 35 mL of DMC at 200 °C—for 17 h, FDME was isolated in 70%, a value comparable to our previous Amberlyst-catalyzed procedure [6]. The same result was achieved when the reaction was stopped after 8 h. Most interestingly, such a high FDME yield was obtained while the autoclave maintained an autogenous pressure of 50 bar, i.e., much lower than that previously observed when using Amberlyst (ca 100 bar). The lower pressure was ascribed to a reduced CO2 and MeOH formation due to a more contained DMC decarboxylation observed in the presence of Fe2(SO4)3. As a result, in these trials, it was possible to recover most of the solvent employed (ca 25 mL out of 35 mL); part of the DMC was inevitably lost due to its additional role as reagent in esterification and possibly carboxymethylation (favoring the cyclization and aromatization) reactions.
Finally, reactions performed for 1–6 h led to reduced FDME yields.
In all of the above reported trials, FDME isolation was efficiently carried out by performing flash silica column chromatography of the dried reaction crude. Some crystallization attempts were also conducted (Table S3, Supporting Information), leading to the recovery of brown crystals when a mixture of H2O/MeOH was employed. However, the lower purity of the recovered FDME led to selecting flash chromatography as the best purification procedure for this synthetic approach.
2.2. Multi-Gram Scale Reaction
The FDME reaction was tested employing 2.0–5.0 g of galactaric acid, maintaining the amount of Fe2(SO4)3 as 50% mol. In the first set of experiments, an amount of 35 mL of DMC (#1–4; Table 3) was kept constant, thus increasing the substrate concentration. In trials employing 4.0–5.0 g of galactaric acid, the amount of DMC was slightly increased (#5–7; Table 3) so as to ensure a higher solubility of the substrate. In fact, maintaining the same substrate concentration would require up to 175 mL of DMC (for 5.0 g of galactaric acid), inevitably leading to a sharp increase in the reaction pressure above the functioning limit of our equipment.

Table 3.
FDME synthesis reactions with 2.0–5.0 g of galactaric acid a.
The reaction conducted employing 2.0 g of galactaric acid in 35 mL of DMC (#1; Table 3), leading to the formation of FDME in a 50% yield. This discrepancy with the previous 1.0 g scale reactions may be ascribed to the higher mixture concentration, which most likely affected the solubility of galactaric acid, consequently hampering its conversion and leading to its degradation. Indeed, a higher amount of black burned material could be found on the inner sides of the autoclave.
As can be noticed from Table 3, when the reaction was performed on 3.0 g of substrate, the reaction temperature was lowered to 180 °C (#2,3; Table 3). In fact, the reaction conducted at 200 °C produced an autogenous pressure that exceeded the safety limit of our apparatus (100 bar). Under these conditions, FDME was then isolated in a 53% yield (#3; Table 3).
Trials performed using 4.0 g of galactaric acid had to be conducted with increasing amounts of DMC (#4,5; Table 3) in order to achieve a higher isolated yield (58%). A further increase in the DMC volume to 70 mL (#6; Table 3) caused a high autogenous pressure, which reached 100 bar after 6 h. Despite the lower reaction time, FDME was isolated with a higher yield compared to more concentrated trials (55% in 6 h vs. 33% in 8 h), confirming the importance of galactaric acid solubilization in DMC for reaction success.
A final attempt was conducted using 5.0 g of galactaric acid in 50 mL of DMC. The reaction had to be stopped after 5 h due to the high autogenous pressure observed; however, also in this case, it was possible to isolate FDME in a good yield (45%).
3. Discussion
Among all of the acids/bases tested, iron(III) sulfate (Fe2(SO4)3) proved to have the best performance for the conversion of galactaric acid into FDME, displaying similar yield values to those obtained employing Amberlyst resins in our previous work (ca 70% in both cases) [6]. Additionally, compared to our previous results, a drastic reduction in the autogenous pressure generated during the reaction was detected (50 bar vs. 100 bar, respectively), even if a longer reaction time was required (8 h vs. 2 h, respectively).
It should be mentioned that to promote the conversion of galactaric acid into FDME, iron(III) sulfate has to promote several reactions, including two esterifications and three dehydrations, that might take place by simple water elimination or via DMC chemistry, i.e., methoxycarbonylation of an hydroxylic moiety followed by either an intramolecular nucleophilic attack (cyclization) or an elimination reaction (aromatization into a furan ring). If we thus consider the complexity of this procedure, it is evident that Fe2(SO4)3 is able to promote several concatenated reactions.
Fe2(SO4)3 and iron salts in general have been reported as efficient catalysts able to promote several reactions, also playing different roles in the same one-pot procedure [66]. Comprehensive literature reviews about iron catalysis in organic synthesis have been published over the years, displaying their wide applications in the production of various chemicals [66,67]. However, the other iron-based compounds tested in the present work were not able to effectively convert galactaric acid, as FDME was detected only in traces in those trials (#9–11; Table 1). This could be ascribed to the pronounced ability of Fe2(SO4)3 to generate H3O+ cations, compared to FeCl3 and other transition metal sulfate salts, i.e., CuSO4, FeSO4, ZnSO4, MnSO4, and NiSO4 (Fe2(SO4)3 pH = −0.06 in a levulinic acid/ethanol solution) [68], which could have led to a more efficient galactaric acid conversion into FDME. Indeed, strong acidic media seem to be required for the conversion of galactaric acid into FDCA and its esters, since, as reported in the Section 1, the most successful reactions employed HBr, H2SO4, or p-TSA-based catalysts [45,55,56,57,59,62].
In addition, the iron(III) cation in Fe2(SO4)3 may be responsible for the interaction with the carbonyl group of DMC, thus polarizing the C=O bond, increasing the electrophilic character of the carbon atom and activating the carbonyl group for the nucleophilic attack by one of the –OH groups of galactaric acid [69]. A similar behavior of Fe2(SO4)3 might be responsible for the high yields reported in the O-acetylation of saccharides with acetic anhydride [70].
It should be mentioned that thermodynamically driven carboxymethylation of galactaric acid hydroxyl groups by DMC may also play a role in favoring the cyclization reaction, as hypothesized in our previous work [6], even if the isolation of any of these intermediates has not been achieved thus far. In addition, both DMC esterification of the substrate’s acidic groups and the carboxymethylation of galactaric acid alcoholic functionalities most likely increase the substrate solubility in the reaction media, thus allowing the subsequent cyclization and aromatization reactions to take place. This statement is in line with the observations reported in Table 3, where a higher mixture concentration led to more evident galactaric acid degradation and an overall lower FDME yield.
Moreover, Fe(III)-based systems seem to be able to promote the dehydration of 5-membered rings, leading to the formation of furanic compounds, as reported by Cao and co-workers by reacting alfa-hydroxy ketones and alkynes [66,71].
Finally, it must be mentioned that, as a result of the cyclization and aromatization of galactaric acid to FDME, three equivalents of water are formed (one for cyclization and two for the aromatization step) for each equivalent of galactaric acid. The sulphate ions may also react with the aqueous media to form dilute sulfuric acid (H2SO4) [72], which could contribute to promote the reaction.
3.1. Iron(III) Sulfate Recycling
In order to overcome the iron(III) sulfate degradation issues found in our previous work using Ambrlyst-36 resin [6], its recycling was also investigated.
Fe2(SO4)3 was therefore filtered off from a standard 1.0 g of galactaric acid reaction, washed with EtOAc, dried in the oven at 100 °C overnight, and finally employed in a second FDME reaction. Unfortunately, the recycled Fe2(SO4)3 was not able to promote the reaction, as only galactaric acid was recovered at the end of the process. This could be ascribed to a changing in iron oxidation state from Fe3+ to Fe2+ during the reaction. This hypothesis was corroborated by previous studies that employed Fe2(SO4)3 for the self-etherification of HMF to OBMF [5]. To verify this claim, X-ray powder diffraction (XRPD) analyses were performed on the iron(III) sulfate before and after the reaction (see Section 3.2 and Figure S2, Supporting Information). A possibility for recycling Fe2(SO4)3 is to use a green oxidant to restore Fe3+ before employing it in a new reaction.
3.2. XRPD Analysis
After performing a standard 1.0 g of galactaric acid reaction, Fe2(SO4)3 was filtered on paper, rinsed with ethyl acetate, and dried overnight. The grey powder obtained was then analyzed via XRPD and its structure compared with the clean and dried Fe2(SO4)3.
The initial structure of the acid was mainly indexed to the Fe2(SO4)3(H2O)7.5 phase (ICSD#10374) of Fe2(SO4)3 material (Figure S2, left, Supporting Information), confirming the presence of Fe3+ as the only inorganic ion. After the reaction, iron(III) sulfate was converted into a hydrated phase of Fe(SO4) (ICSD#759715) without traces of any other impurities (Figure S2, right, Supporting Information). The exhaustion of the oxidizing potential of the acid during the reaction cycle caused the loss of its ability to carry out its promoter functions, as it underwent a complete reduction from Fe3+ to Fe2+.
3.3. Iron(III) Sulfate Leching
The yellowish and sometimes brown color of the FDME obtained in some of the Fe-promoted reactions also after purification with silica filtration or via recrystallization (Table S3, Supporting Information) could be ascribed to an iron leaching coming from Fe2(SO4)3. To investigate this possibility, ICP-EOS analyses of the reaction mixture (brown solid), the recrystallized FDME (brown crystals), and the purified FDME (yellowish solid) were performed. Results are provided in Table 4.

Table 4.
ICP-EOS analyses purified and unpurified FDME.
The data obtained show that some iron traces can be detected in the recrystallized FDME, which could be responsible for the brown color of the crystals. On the contrary, simple flash silica column chromatography was sufficient for removing almost all traces of Fe in the product. However, the insignificant quantity of iron present in the FDME sample may, in some cases, be sufficient to alter its color from white to yellow.
4. Materials and Methods
4.1. General
All reagents and solvents were purchased from Merk KGaA (Darmstadt, Germany). Fe2(SO4)3 was dried in the oven overnight at 100 °C before use. NMR spectra were recorded with a Bruker (Billerica, MA, USA) 300 MHz and 400 MHz spectrometer in MeOD. Reactions were conducted in a stainless steel autoclave (capacity 220 mL) with a thermocouple for temperature control and under magnetic stirring (500 rpm).
4.2. Synthesis of 2,5-Furandicarboxylic Acid Dimethyl Ester (FDME)
In a typical reaction, galactaric acid (1.0 g, 4.76 mmol), 50% mol. eq of iron(III) sulfate hydrate, H2O content ≤ 24% at r.t. (Fe2(SO4)3, 0.95 g, 2.38 mmol), and 35 mL of DMC (415.7 mmol, 87.3 mol. eq) were added to a stainless steel autoclave and heated at 200 °C for 8 h. The autogenous pressure reached a maximum of 50 bar. After cooling, the crude was vacuum filtered on a Gooch packed with celite. Both the autoclave and the Gooch were rinsed with ethyl acetate. The solution was then concentrated at the rotavapor and dried under vacuum to provide a brown solid (1.14 g) containing almost exclusively FDME (Figure S3, Supporting Information). The compound was further purified via flash silica column chromatography with a 7:3 mixture of hexane and ethyl acetate as the eluent phase. FDME was isolated as a white solid in a 70% yield.
1H-NMR (400 MHz; MeOD) δ (ppm): 7.32 (2H, s), 3.93 (6H, s).
4.3. XRPD Analyses
X-ray powder diffraction (XRPD) data were acquired using Malvern Panalytical equipment comprising a diffractometer (Empyrean) with Bragg–Brentano geometry (Malvern, UK). Nickel-filtered Cu Kα radiation and a step-by-step technique were employed with steps of 0.05° and a collection time of 60 s.
4.4. Iron(III) Sulfate Leching Analyses
ICP-MS (NexION 350X, Perkin Elmer-Waltham, MA, USA) analyses were performed via microwave-assisted acid mineralization (Ethos UP, Milestone-Sorisole (BG), Italy). The mineralization was conducted by adding 4 mL of ultrapure nitric acid and 2 mL of ultrapure hydrogen peroxide to 25 mg of the powered sample in a closed vessel. The mineralization was conducted in 30 min, reaching a temperature of 220 °C in 15 min and holding it for a further 15 min. The samples were diluted to 50 mL with ultrapure water and directly analyzed. The quantification of residual Fe was conducted using an external calibration from 1 to 50 ug/L of Fe, considering a wavelength at 259.939 nm.
5. Conclusions
An alternative synthetic procedure was reported for the synthesis of 2,5-furandicarboxylic acid dimethyl ester (FDME) starting from galactaric acid and using Fe2(SO4)3 as an inexpensive Lewis acid and dimethyl carbonate (DMC) as green media. According to the best-found reaction conditions, the synthesis was conducted in an autoclave for 8 h at 200 °C, allowing the isolation of FDME in up to a 70% yield. Compared to our previous work, this procedure (1.0 g of galactaric acid) generated a lower autogenous pressure (50 bar), allowing both the recovery of Fe2(SO4)3 and of part of the solvent. However, despite our best efforts, Fe2(SO4)3 could not be reused in further trials due to its reduction to Fe2+ observed via XRPD analyses and, as already noted in the previously investigated HMF, self-etherification to OBMF. In addition, several trials to employ greener solvents (i.e., DMC, EtOAc, acetone, 2-Me-THF, CPME, and MIBK) for the reaction work-up were performed. Nevertheless, flash silica column chromatography was required to isolate FDME as a white solid.
Further trials allowed to employ a maximum of 4.0 g of the starting aldaric acid, with comparable yield values. A subsequent increase to 5.0 g of galactaric acid caused the pressure to exceed 100 bar; thus, the reaction had to be stopped after 3 h. However, in this trial, FDME could be retrieved in good yield (45%). A suitable DMC volume (at least 50 mL for 4.0 g of galactaric acid) proved to be essential for favoring galactaric acid solubilization, facilitating its conversion into FDME and preventing its degradation into humins.
Purification of the final product was achieved either through flash chromatography on silica or by recrystallization of the reaction crude with a mixture of water and methanol, even if lower yields and a darker FDME color were obtained in the latter case. Nevertheless, Fe2(SO4)3 leaching analyses via ICP-MS showed, in both cases, a negligible iron contamination of FDME, especially after silica purification. Iron(III) sulfate was the most efficient among the iron-based Lewis acids tested, probably due to its synergistic action with DMC in both the cyclization and aromatization steps. Further studies on this topic are required to better understand the mechanism behind the interaction between galactaric acid and DMC, with particular efforts directed to the isolation of possible reaction intermediates and the use of greener solvents in the reaction work-up.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13071114/s1, Table S1: Effect of reaction time on FDME formation; Table S2: Additional reactions for FDME synthesis: TEAB, molecular sieves, and the addition of DMSO; Table S3: FDME crystallization trials; Figure S1: 1H-NMR (MeOD) spectra of the reaction conducted employing p-TSA; Figure S2: X-ray powder diffraction (XRPD) analyses of Iron(III) sulfate before and after the reaction; Figure S3: 1H-NMR (MeOD) spectra of the reaction crude after filtration of Fe2(SO4)3; Figure S4: 1H-NMR (MeOD) spectra of the purified FDME (white crystals).
Author Contributions
Conceptualization, F.A. and G.T.; methodology, G.T.; investigation, B.C., T.G., and G.T.; writing—original draft preparation, G.T.; writing—review and editing, F.A.; XRPD analyses, D.R.-B.; supervision, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge Istituto Nazionale della Previdenza Sociale (INPS) for funding the Ph.D. fellowship of Giacomo Trapasso.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This article is also within the frame of COST Action FUR4Sustain (CA18220-European network of FURan based chemicals and materials FOR a Sustainable development), supported by COST (European Cooperation in Science and Technology).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kühlborn, J.; Groß, J.; Opatz, T. Making Natural Products from Renewable Feedstocks: Back to the Roots? Nat. Prod. Rep. 2020, 37, 380–424. [Google Scholar] [CrossRef] [PubMed]
- Funk, M.; Ash, C. A Cleaner, Greener Future for Chemicals. Science 2020, 367, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery-Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Trapasso, G.; Mazzi, G.; Chícharo, B.; Annatelli, M.; Dalla Torre, D.; Aricò, F. Multigram Synthesis of Pure HMF and BHMF. Org. Process Res. Dev. 2022, 26, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Annatelli, M.; Trapasso, G.; Torre, D.D.; Pietrobon, L.; Redolfi-Bristol, D.; Aricò, F. A Green Synthesis of 5,5′-[Oxybis(Methylene)]Bis-2-Furfural: From By-Product to Attractive Bio-Based Platform Chemical. Adv. Sustain. Syst. 2022, 6, 2200297. [Google Scholar] [CrossRef]
- Trapasso, G.; Annatelli, M.; Dalla Torre, D.; Aricò, F. Synthesis of 2,5-Furandicarboxylic Acid Dimethyl Ester from Galactaric Acid via Dimethyl Carbonate Chemistry. Green Chem. 2022, 24, 2766–2771. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Sathicq, A.G.; Annatelli, M.; Abdullah, I.; Romanelli, G.; Aricò, F. Alkyl Carbonate Derivatives of Furanics: A Family of Bio-Based Stable Compounds. Sustain. Chem. Pharm. 2021, 19, 100352. [Google Scholar] [CrossRef]
- Karlinskii, B.Y.; Ananikov, V.P. Catalytic C−H Functionalization of Unreactive Furan Cores in Bio-Derived Platform Chemicals. ChemSusChem 2021, 14, 558–568. [Google Scholar] [CrossRef]
- Musolino, M.; Andraos, J.; Aricò, F. An Easy Scalable Approach to HMF Employing DMC as Reaction Media: Reaction Optimization and Comparative Environmental Assessment. ChemistrySelect 2018, 3, 2359–2365. [Google Scholar] [CrossRef]
- Musolino, M.; Ginés-Molina, M.J.; Moreno-Tost, R.; Aricò, F. Purolite-Catalyzed Etherification of 2,5-Bis(Hydroxymethyl)Furan: A Systematic Study. ACS Sustain. Chem. Eng. 2019, 7, 10221–10226. [Google Scholar] [CrossRef]
- Dick, G.R.; Frankhouser, A.D.; Banerjee, A.; Kanan, M.W. A Scalable Carboxylation Route to Furan-2,5-Dicarboxylic Acid. Green Chem. 2017, 19, 2966–2972. [Google Scholar] [CrossRef]
- Stadler, B.M.; Wulf, C.; Werner, T.; Tin, S.; de Vries, J.G. Catalytic Approaches to Monomers for Polymers Based on Renewables. ACS Catal. 2019, 9, 8012–8067. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-Furandicarboxylic Acid (FDCA) from 5-Hydroxymethylfurfural (HMF): Recent Progress Focusing on the Chemical-Catalytic Routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Sakuta, R.; Nakamura, N. Production of Hexaric Acids from Biomass. Int. J. Mol. Sci. 2019, 20, 3660. [Google Scholar] [CrossRef]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts 2020, 10, 743. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Xie, W.; Chen, P.-H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(Butylene 2,5-Furan Dicarboxylate), a Biobased Alternative to PBT: Synthesis, Physical Properties, and Crystal Structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing Fossil Based PET with Biobased PEF; Process Analysis, Energy and GHG Balance. Energy Environ. Sci. 2012, 5, 6407. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(Ethylene Furanoate) Compared to Poly(Ethylene Terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon Dioxide Sorption and Transport in Amorphous Poly(Ethylene Furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Chen, G.; van Straalen, N.M.; Roelofs, D. The Ecotoxicogenomic Assessment of Soil Toxicity Associated with the Production Chain of 2,5-Furandicarboxylic Acid (FDCA), a Candidate Bio-Based Green Chemical Building Block. Green Chem. 2016, 18, 4420–4431. [Google Scholar] [CrossRef]
- Joshi, A.S.; Alipourasiabi, N.; Kim, Y.-W.; Coleman, M.R.; Lawrence, J.G. Role of Enhanced Solubility in Esterification of 2,5-Furandicarboxylic Acid with Ethylene Glycol at Reduced Temperatures: Energy Efficient Synthesis of Poly(Ethylene 2,5-Furandicarboxylate). React. Chem. Eng. 2018, 3, 447–453. [Google Scholar] [CrossRef]
- De Jong, E.; Visser, H. (Roy) A.; Dias, A.S.; Harvey, C.; Gruter, G.-J.M. The Road to Bring FDCA and PEF to the Market. Polymers 2022, 14, 943. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.J.M. Furandicarboxylic acid (FDCA), a versatile building block for a very interesting class of polyesters. In Biobased Monomers, Polymers and Materials; Smith, P.B., Gross, R., Eds.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2012; Volume 1105, pp. 1–13. [Google Scholar] [CrossRef]
- DuPont, ADM Eye Biobased Polyester. CEN Glob. Enterp. 2016, 94, 6. [CrossRef]
- Gorbanev, Y.Y.; Kegnæs, S.; Riisager, A. Selective Aerobic Oxidation of 5-Hydroxymethylfurfural in Water Over Solid Ruthenium Hydroxide Catalysts with Magnesium-Based Supports. Catal. Lett. 2011, 141, 1752–1760. [Google Scholar] [CrossRef]
- Gao, T.; Yin, Y.; Fang, W.; Cao, Q. Highly Dispersed Ruthenium Nanoparticles on Hydroxyapatite as Selective and Reusable Catalyst for Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid under Base-Free Conditions. Mol. Catal. 2018, 450, 55–64. [Google Scholar] [CrossRef]
- Siankevich, S.; Mozzettini, S.; Bobbink, F.; Ding, S.; Fei, Z.; Yan, N.; Dyson, P.J. Influence of the Anion on the Oxidation of 5-Hydroxymethylfurfural by Using Ionic-Polymer-Supported Platinum Nanoparticle Catalysts. ChemPlusChem 2018, 83, 19–23. [Google Scholar] [CrossRef]
- Ait Rass, H.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2,5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2-and ZrO2-Based Supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef]
- Bonincontro, D.; Lolli, A.; Storione, A.; Gasparotto, A.; Berti, B.; Zacchini, S.; Dimitratos, N.; Albonetti, S. Pt and Pt/Sn Carbonyl Clusters as Precursors for the Synthesis of Supported Metal Catalysts for the Base-Free Oxidation of HMF. Appl. Catal. A Gen. 2019, 588, 117279. [Google Scholar] [CrossRef]
- Siyo, B.; Schneider, M.; Radnik, J.; Pohl, M.-M.; Langer, P.; Steinfeldt, N. Influence of Support on the Aerobic Oxidation of HMF into FDCA over Preformed Pd Nanoparticle Based Materials. Appl. Catal. A Gen. 2014, 478, 107–116. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, C.; Chen, J.; Deng, W.; Zhang, Q.; Yang, Y.; Wang, Y. Base-Free Aerobic Oxidation of 5-Hydroxymethyl-Furfural to 2,5-Furandicarboxylic Acid in Water Catalyzed by Functionalized Carbon Nanotube-Supported Au–Pd Alloy Nanoparticles. ACS Catal. 2014, 4, 2175–2185. [Google Scholar] [CrossRef]
- Buonerba, A.; Impemba, S.; Litta, A.D.; Capacchione, C.; Milione, S.; Grassi, A. Aerobic Oxidation and Oxidative Esterification of 5-Hydroxymethylfurfural by Gold Nanoparticles Supported on Nanoporous Polymer Host Matrix. ChemSusChem 2018, 11, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Gupta, D.; Abu-Omar, M.M.; Modak, A.; Bhaumik, A. Porphyrin-Based Porous Organic Polymer-Supported Iron(III) Catalyst for Efficient Aerobic Oxidation of 5-Hydroxymethyl-Furfural into 2,5-Furandicarboxylic Acid. J. Catal. 2013, 299, 316–320. [Google Scholar] [CrossRef]
- Tong, X.; Yu, L.; Chen, H.; Zhuang, X.; Liao, S.; Cui, H. Highly Efficient and Selective Oxidation of 5-Hydroxymethylfurfural by Molecular Oxygen in the Presence of Cu-MnO2 Catalyst. Catal. Commun. 2017, 90, 91–94. [Google Scholar] [CrossRef]
- Lie, W.H.; Deng, C.; Yang, Y.; Tsounis, C.; Wu, K.-H.; Hioe, M.V.C.; Bedford, N.M.; Wang, D.-W. High Yield Electrooxidation of 5-Hydroxymethyl Furfural Catalysed by Unsaturated Metal Sites in CoFe Prussian Blue Analogue Films. Green Chem. 2021, 23, 4333–4337. [Google Scholar] [CrossRef]
- Wei, T.; Liu, W.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. A Dual-Functional Bi-Doped Co3O4 Nanosheet Array towards High Efficiency 5-Hydroxymethylfurfural Oxidation and Hydrogen Production. Chem. Commun. 2023, 59, 442–445. [Google Scholar] [CrossRef]
- Chai, X.; Jiang, K.; Wang, J.; Ren, Z.; Liu, X.; Chen, L.; Zhuang, X.; Wang, T. Efficient Catalytic Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Ruthenium Cluster-Embedded Ni(OH)2 Catalyst. ChemSusChem 2022, 15, e202200863. [Google Scholar] [CrossRef]
- Koopman, F.; Wierckx, N.; de Winde, J.H.; Ruijssenaars, H.J. Efficient Whole-Cell Biotransformation of 5-(Hydroxymethyl)Furfural into FDCA, 2,5-Furandicarboxylic Acid. Bioresour. Technol. 2010, 101, 6291–6296. [Google Scholar] [CrossRef] [PubMed]
- Hossain, G.S.; Yuan, H.; Li, J.; Shin, H.; Wang, M.; Du, G.; Chen, J.; Liu, L. Metabolic Engineering of Raoultella Ornithinolytica BF60 for Production of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural. Appl. Environ. Microbiol. 2017, 83, e02312–e02316. [Google Scholar] [CrossRef]
- Hsu, C.; Kuo, Y.; Liu, Y.; Tsai, S. Green Conversion of 5-hydroxymethylfurfural to Furan-2,5-dicarboxylic Acid by Heterogeneous Expression of 5-hydroxymethylfurfural Oxidase in Pseudomonas Putida S12. Microb. Biotechnol. 2020, 13, 1094–1102. [Google Scholar] [CrossRef]
- Rosenfeld, C.; Konnerth, J.; Sailer-Kronlachner, W.; Solt, P.; Rosenau, T.; Herwijnen, H.W.G. Current Situation of the Challenging Scale-Up Development of Hydroxymethylfurfural Production. ChemSusChem 2020, 13, 3544–3564. [Google Scholar] [CrossRef] [PubMed]
- Senatore, A.; Dalena, F.; Sola, A.; Marino, A.; Valletta, V.; Basile, A. First-Generation Feedstock for Bioenergy Production. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–57. [Google Scholar]
- Kim, M.; Su, Y.; Fukuoka, A.; Hensen, E.J.M.; Nakajima, K. Aerobic Oxidation of 5-(Hydroxymethyl)Furfural Cyclic Acetal Enables Selective Furan-2,5-dicarboxylic Acid Formation with CeO2-Supported Gold Catalyst. Angew. Chem. Int. Ed. 2018, 57, 8235–8239. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, N.; Rautiainen, S.; Asikainen, M.; Thomas, D.A.; Linnekoski, J.; Niemelä, K.; Harlin, A. A Unique Pathway to Platform Chemicals: Aldaric Acids as Stable Intermediates for the Synthesis of Furandicarboxylic Acid Esters. Green Chem. 2020, 22, 8271–8277. [Google Scholar] [CrossRef]
- Kiely, D.E.; Chen, L.; Lin, T.H. Hydroxylated Nylons Based on Unprotected Esterified D-Glucaric Acid by Simple Condensation Reactions. J. Am. Chem. Soc. 1994, 116, 571–578. [Google Scholar] [CrossRef]
- Kiely, D.E.; Chen, L.; Lin, T.H. Synthetic Polyhydroxypolyamides from Galactaric, Xylaric, D-Glucaric, and D-Mannaric Acids and Alkylenediamine Monomers—Some Comparisons. J. Polym. Sci. A Polym. Chem. 2000, 38, 594–603. [Google Scholar] [CrossRef]
- Muñoz-Guerra, S. Carbohydrate-Based Polyamides and Polyesters: An Overview Illustrated with Two Selected Examples. High Perform. Polym. 2012, 24, 9–23. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; de Ilarduya, A.M.; Benito, E.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Carbohydrate-Based Copolyesters Made from Bicyclic Acetalized Galactaric Acid. J. Polym. Sci. A Polym. Chem. 2012, 50, 1591–1604. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; Martínez de Ilarduya, A.; Benito, E.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Carbohydrate-Based Polyesters Made from Bicyclic Acetalized Galactaric Acid. Biomacromolecules 2011, 12, 2642–2652. [Google Scholar] [CrossRef]
- Van der Klis, F.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Waste Not, Want Not: Mild and Selective Catalytic Oxidation of Uronic Acids. ChemSusChem 2013, 6, 1640–1645. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Converting Carbohydrates to Bulk Chemicals and Fine Chemicals over Heterogeneous Catalysts. Green Chem. 2011, 13, 520. [Google Scholar] [CrossRef]
- Mudgil, D. The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 35–59. [Google Scholar]
- Fauvarque, J.F.; Guerin, C.; Petit, S.; De Regis, B. The Prepn of Galactaric Acid from Galacturonic Acid. French Patent 2699937A1, 29 December 1992. [Google Scholar]
- Phelps, I.K.; Hale, W.J. on Dehydromucic Acid and Certain of Its Derivatives. Am. Chem. J. 1901, 25, 445–463. [Google Scholar]
- Cope, A.; Keller, R. Notes—Benzofuran from Saccharic Acid. J. Org. Chem. 1956, 21, 141. [Google Scholar] [CrossRef]
- Gonis, G.; Amstutz, E.D. The Preparation of Furan-2,5-Dicarboxylic Acid1. J. Org. Chem. 1962, 27, 2946–2947. [Google Scholar] [CrossRef]
- Miller, D.J.; Peereboom, L.; Wegener, E.; Gattinger, M. Formation of 2,5-Furan Dicarboxylic Acid from Aldaric Acids. U.S. Patent 9,994,539, 11 July 2017. [Google Scholar]
- Fittig, R.; Heinzelmann, H. Production of 2, 5-Furandicarboxylic Acid by the Reaction of Fuming Hydrobromic Acid with Mucic Acid under Pressure. Chem. Ber. 1876, 9, 1198. [Google Scholar]
- Lewkowski, J. Synthesis, Chemistry and Applications of 5-Hydroxymethylfurfural and Its Derivatives. Arkivoc 2001, 1, 17–54. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Pukin, A.; van Haveren, J.; Lutz, M.; van Es, D.S. Concurrent Formation of Furan-2,5- and Furan-2,4-Dicarboxylic Acid: Unexpected Aspects of the Henkel Reaction. RSC Adv. 2013, 3, 15678–15686. [Google Scholar] [CrossRef]
- Taguchi, Y.; Oishi, A.; Iida, H. One-Step Synthesis of Dibutyl Furandicarboxylates from Galactaric Acid. Chem. Lett. 2008, 37, 50–51. [Google Scholar] [CrossRef]
- Zhao, D.; Delbecq, F.; Len, C. One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters. Molecules 2019, 24, 1030. [Google Scholar] [CrossRef]
- Tundo, P.; Aricò, F.; Rosamilia, A.E.; Memoli, S. Synthesis of Dialkyl Ethers by Decarboxylation of Dialkyl Carbonates. Green Chem. 2008, 10, 1182. [Google Scholar] [CrossRef]
- Tundo, P.; Musolino, M.; Aricò, F. The Reactions of Dimethyl Carbonate and Its Derivatives. Green Chem. 2018, 20, 28–85. [Google Scholar] [CrossRef]
- Vrancken, E.; Campagne, J.-M. Organic Transformations Promoted by Lewis Acid Iron Catalysts. In PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Bauer, I.; Knölker, H.-J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef]
- Martins, F.; Rodrigues, F.; Silva, M. Fe2(SO4)3-Catalyzed Levulinic Acid Esterification: Production of Fuel Bioadditives. Energies 2018, 11, 1263. [Google Scholar] [CrossRef]
- Esposito, R.; Raucci, U.; Cucciolito, M.E.; Di Guida, R.; Scamardella, C.; Rega, N.; Ruffo, F. Iron(III) Complexes for Highly Efficient and Sustainable Ketalization of Glycerol: A Combined Experimental and Theoretical Study. ACS Omega 2019, 4, 688–698. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, G.; Pan, F. Fe2(SO4)3·xH2O-Catalyzed per-O-Acetylation of Sugars Compatible with Acid-Labile Protecting Groups Adopted in Carbohydrate Chemistry. Tetrahedron 2008, 64, 2572–2575. [Google Scholar] [CrossRef]
- Cao, H.; Zhan, H.; Wu, J.; Zhong, H.; Lin, Y.; Zhang, H. An Efficient and General Iron-Catalyzed One-Pot Synthesis of Furans via α-Hydroxy Ketones and Activated Alkynes. Eur. J. Org. Chem. 2012, 2012, 2318–2322. [Google Scholar] [CrossRef]
- Mukherjee, A.; Portillo-Perez, G.; Dumont, M.-J. Synthesis of Hydroxymethylfurfural and Furfural from Hardwood and Softwood Pulp Using Ferric Sulphate as Catalyst. Front. Chem. Sci. Eng. 2019, 13, 531–542. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).