Abstract
Asymmetric enamine base activation of carbonyl compounds is a well-known and widely used strategy for providing functionalization of organic compounds in an efficient way. The use of solely organic substances, which in most cases are commercially available primary or secondary amines that are easy to obtain, avoids the use of hazardous substances or metal traces, making this type of catalysis a highly convenient methodology from a sustainable point of view. In many cases, the reactivity or the stereoselectivity obtained is far from being a practical and advantageous strategy; this can be improved by using a hydrogen bonding co-catalyst that can help during the activation of one species or by using a bifunctional catalyst that can direct the approximation of reagents during the reaction outcome. In this review, we describe the most efficient methodologies that make use of a dual activation of reagents for performing α-functionalization (enamine activation) or remote functionalization (such as dienamine or trienamine activation) of carbonyl compounds.
1. Introduction
In 2000, List, Lerner and Barbas III presented the intermolecular cross aldol reaction between acetone and diversely substituted aldehydes promoted by the simple and natural amino acid (S)-proline [1] (Scheme 1a). This was not the first time that this amino acid was employed as the catalyst in an organic transformation [2,3], but it was presented as a powerful and broad-in-scope catalyst for performing intermolecular cross aldol reactions, also comparable to the best organometallic catalysts for carrying out the same reaction. In this report, based on a previous reaction mechanism of aldolases described by Barbas III [4] and confirmed by computational studies carried out by Houk and List [5], the proposed mechanism included a dual behavior of the catalyst: firstly, it formed an intermediate enamine that increased the nucleophilicity of the α-carbon in acetone, and secondly, it was also involved in the activation of the aldehyde, the second carbonyl unit (Scheme 1b). This catalyst could also provide excellent yields and enantioselectivities using a simple reaction design: stirring the aldehyde and 30 mol% of (S)-proline in a mixture of acetone/DMSO (1:4) for 2–48 h.
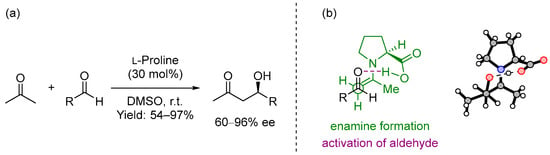
Scheme 1.
(a) Proline-catalyzed asymmetric aldol reaction and (b) proposed transition state.
The use of proline as catalyst, together with the well-known MacMillan imidazolidinone introduced the same year [6,7], represented the starting point in a new research area of great interest: aminocatalysis. This type of catalyst has emerged as a valuable tool for the alleviation of some problems associated with metal catalysis related to trace contamination of those elements. In fact, aminocatalysis [8,9,10,11] and other types of organocatalysis [12,13,14,15,16,17,18] have been recognized by researchers as one of the most important strategies aligned to green and sustainable catalysis [19]. Aminocatalysis also represents the strategy of choice in many asymmetric transformations, especially when carbonyl compounds are involved, offering good results in terms of yields and enantioselectivities.
In this area, numerous examples of successful reactions have made extensive use of the incorporation of an H-bond donor entity either as an external additive or as a component of a bifunctional aminocatalyst [20,21,22,23,24,25,26]. In bifunctional H-bond donor/aminocatalysts, the H-bond donor reagent typically plays a role in the activation of an electrophile that reacts with an enamine-type intermediate. As a result, it becomes a potent stereodirecting component (Scheme 2).
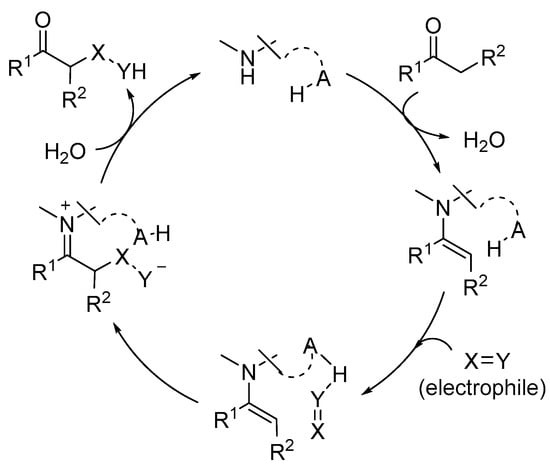
Scheme 2.
Illustration of dual activation of reagents in enamine base catalysis.
Alternately, the H-bond donor moiety can also play an important role in events that occur prior to or after the stereogenic center’s installation, either as part of a bifunctional reagent or as an external cocatalyst. This occurs when a Brønsted acidic cocatalyst or stoichiometric additive is added to the reaction mixture to speed up the rate of catalyst turnover during the hydrolysis step or to make it easier to activate the carbonyl group of the aldehyde or ketone substrate during the condensation with the aminocatalyst in the activation stage. This science is beyond the scope of this paper and will not be examined exhaustively.
2. Dual H-Bonding and Enamine Activation
An excellent illustration of the involvement of an H-bond donor site in the structure of the aminocatalyst during the aldol reaction between the intermediate enamine and the external aldehyde reagent is the pioneering proline-catalyzed aldol reaction (Scheme 3). As a result, outstanding levels of both diastereo- and enantioselectivity are achieved as the reaction progresses through a cyclic chair-like transition state with reduced conformational mobility. This generated H-bond during the C-C bond forming step has been used as a general strategy during experimental and computational studies [5,27,28,29,30,31,32,33,34]. In fact, the conformational restricted transition state does not just record the extremely high facial selectivity; this situation restricts the E diastereoisomer formed in the transient enamine species, as the presence of serious dynamic and thermodynamic restrictions in the less favored Z diastereoisomer (see Scheme 2).
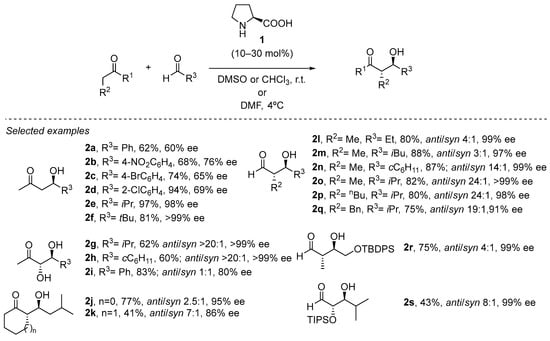
Scheme 3.
General proline-catalyzed cross-aldol reaction.
This transformation is rather wide in scope with respect to the possibility of using different aldehydes in combination with acetone [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], α-hydroxyketones [57,58] or cyclic ketones [59] as the pronucleophile source, including the possibility of performing the reaction at multigram or even kilogram scale. Moreover, the reaction can also be carried out in open air and without the need for degassed or dry solvent, which is an important benefit in terms of operational simplicity. In addition, the cross-aldol reaction between two different aldehydes can also be carried out under slightly modified conditions [60], also enabling the use of polyhydroxylated aldehydes as either pronucleophiles or electrophiles [61], which also opens the possibility for the stereoselective synthesis of sugars through this methodology [62].
The same stereochemical model can be applied to structurally related electrophiles, thus expanding the portfolio of organic transformations in which this approach can be applied [63,64]. In particular, proline has been demonstrated to be an outstanding catalyst for performing the Mannich reaction (Scheme 4) [65,66,67,68,69]. In this case, the availability of a single electron pair at the nitrogen atom available to engage in the H-bonding interaction with the carboxylate group of the proline leads to the formation of the syn diastereoisomer as a consequence of the (E) geometry of the azomethine moiety, in contrast to the anti diastereoselection previously observed in the parent aldol reaction (see Scheme 4). Also, the possibility of performing the challenging cross-Mannich reaction using acetaldehyde as the pronucleophile should be highlighted, providing acceptable yields of the corresponding α-unsubstituted β-aminoaldehydes and minimizing to a great extent the presence of byproducts arising from competitive polymerization through self-aldol condensation [70].
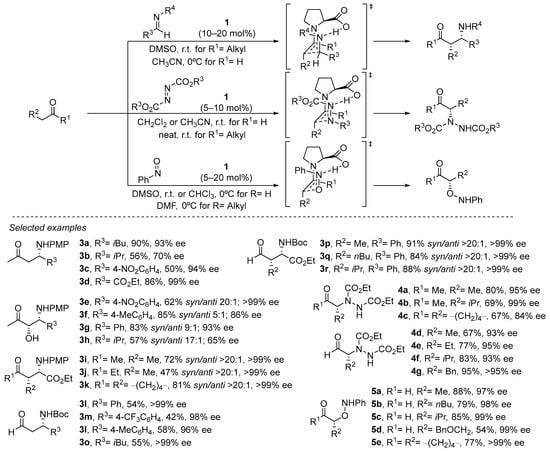
Scheme 4.
Proline-catalyzed α-functionalization reactions.
Other electronically similar electrophiles such as azodicarboxylates or nitrosoarenes also perform excellently in the α-amination [71,72,73,74,75,76] and the α-hydroxylation [77,78,79,80,81,82,83] (or nitroso-aldol reaction) of aldehydes and ketones under proline catalysis, providing an efficient and direct entry to enantioenriched α-aminocarbonyl or α-hydroxycarbonyl compounds or related derivatives (See Scheme 4). In the latter case, the participation of such an H-bonding interaction with the electrophile also conditions the chemoselectivity of the reaction, observing that Jørgensen–Hayashi catalysts that do not contain any H-bond donor motif also efficiently catalyze the reaction between aldehydes and nitrosobenzene but lead to the formation of the opposite N-addition isomer, thus resulting in the α-hydroxyamination of the starting material [84].
There are still a few situations in which the reaction is limited due to either poor enantiocontrol or low conversion, despite the fact that proline performs exceptionally well in many of these reactions and has a rather broad substrate scope. This has been credited by and large to the unfortunate dissolvability of proline in the normal natural solvents utilized for the response. A possible solution has been found through the incorporation of achiral H-bond donor additives able to engage in H-bonding interactions with the carboxylate moiety that can increase the solubility of the catalyst and also contribute to the activation of the electrophile through the H-bonding interactions. This is the case of the combined use of l-proline and chiral BINOL 6, which, used in the correct matched combination and under optimized ratio, provided a remarkable improvement in the aldol reaction between acetone and several aromatic aldehydes compared to the parent reaction without any additive (Scheme 5) [38]. Further research on this behavior has led to improved systems that combine the use of l-proline with an external achiral thiourea cocatalyst [47,85,86,87] as H-bond donor.
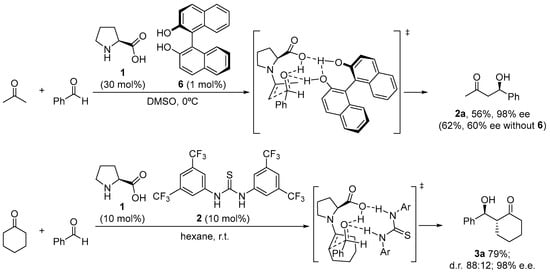
Scheme 5.
Proline/H-bond additive catalyzed asymmetric aldol reaction.
Obviously, proline is not a universal catalyst for this type of transformation, and a wide variety of structurally related catalysts based on the proline scaffold have been studied and employed with different degrees of success in aldol [88], Mannich [89,90,91], α-amination [92] or α-aminoxylation [93] involving the enamine activation of aldehydes and ketones. The logical evolution of the initial system relied on the modification of the hydrogen-bond donor moiety, also tuning its acidity and availability or incorporating multiple additional H-bond donor units within its structure [94,95]. Some selected examples that provide an overall view of the different directions taken in this area are displayed in Figure 1. For instance, simply changing from the carboxylate moiety on proline to the corresponding N-arylamide 7 [96,97,98] or N-sulfonylamide 8 [99,100,101,102,103,104,105] leads to competent catalysts in such transformations. The same applies to the substitution of the carboxylate with other related motifs, as in the case of prolinamide 9 [106,107,108,109] or pyrrolidine-tetrazole catalyst 10 [110,111,112,113,114] or the possibility of using modified versions of trans-4-hydroxyproline as in the case of compound 11 [115,116]. As alternatives, several authors have also surveyed the incorporation of substituents with additional stereogenic elements like α-amino acids [117,118,119,120], sugar moieties [121] or other, more complex alkaloids [122,123,124] (see, for example, catalysts 12, 13 and 14). Another strategy has also relied on incorporating functionalities with additional H-bond donor motifs that lead to the formation of a transition state in which both reagents, the enamine intermediate and the electrophile are connected through a network of H-bonding interactions that turn into reduced conformational mobility. Some examples include the use of aminoalcohol-derived prolinamide 15 [96,125,126] or diamine-based catalysts such as 16 [127,128] or 17 [129,130,131] that also incorporate additional stereogenic elements on the N-substituent or even with a terminal thiourea moiety that provides enhanced H-bond donor ability [132,133,134,135,136] (see catalyst 18 for a representative example). Alternatively, dipeptide-type catalysts [137,138,139] with a terminal secondary amide moiety (like in catalysts 19 [140] or 20 [141]) or more complex polypeptidic scaffolds (see catalysts 21 [142] and 22 [143] as examples), which are based on either natural or unnatural [144] amino acid scaffolds, have also proved to be useful in this type of reaction (23) [145].
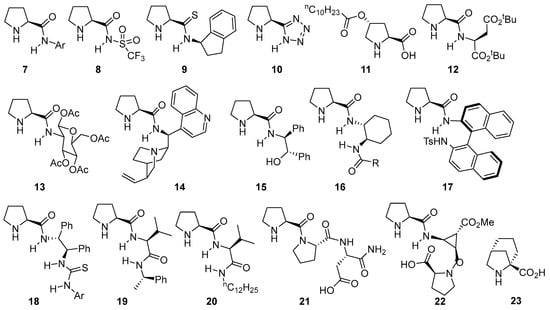
Figure 1.
Selected examples of bifunctional proline-based aminocatalysts incorporating H-bond donor moieties employed in aldol, Mannich and related reactions.
Interestingly, moving the carboxylate group in the pyrrolidine core from the 2-position to the 3-position leads to a huge difference in how the electrophile and the enamine intermediate organize in the transition state. For instance, the use of pyrrolidine-3-carboxylate 24 in the Mannich reaction leads to a complete change in the simple diastereoselection of the reaction, moving from the syn-selectivity reported for the proline-catalyzed reaction (see Scheme 4) to provide the anti Mannich adducts in excellent yield and stereocontrol (Scheme 6) [146]. This behavior was explained through the formation of an H-bonded intermediate in which the chair-like TS operating in the l-proline-catalyzed reaction—in which the H-bonded imine approaches the reactive (E)-s-trans enamine conformer—was no longer operating, thus moving to a situation in which—while the carboxylate still directs the incoming electrophile from the same face of the (E)-enamine intermediate—this enamine has changed its reactive conformation to s-cis, thus exposing the opposite diastereotopic face. A similar behavior has also been described for related catalyst systems based on trans-4-hydroxyproline [147,148] or trans-3-aminoproline [149,150].
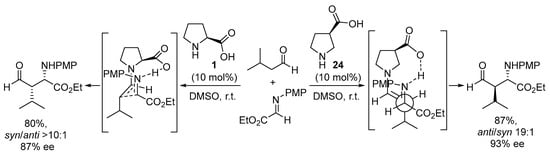
Scheme 6.
The proline- vs. 3-pyrrolidinecarboxylate-catalyzed enantioselective Mannich reactions.
A similar effect in which internal H-bonding changes the reactive conformation of the enamine intermediate in order to provide opposite simple diastereoselection to those observed in the archetypical l-proline-catalyzed aldol or Mannich reactions has been explored by Maruoka and coworkers with bifunctional catalyst 25a based on the binaphthyl core (Scheme 7) [151,152,153,154,155,156]. In this case, an acidic sulfonamide substituent at the 3-position of the binaphthyl core plays the role of the stereodirecting element as an H-bond donor motif.
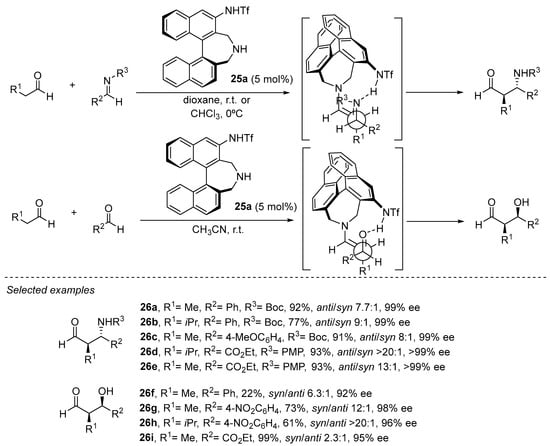
Scheme 7.
Binaphthyl-based chiral catalyst 24a in enantioselective aldol and Mannich reactions.
Primary amines have been demonstrated to perform well in aldol, Mannich, α-amination and α-aminoxylation reactions [133,157,158]. The enamine formed after condensation with a primary amine has a reduced degree of steric congestion around the enamine moiety and, therefore, enables the activation of more sterically demanding substrates such as, for example, acyclic ketones or α,α-disubstituted aldehydes. In addition, the NH moiety of the secondary enamine is able to engage in H-bonding interactions with the incoming electrophile or with additional Lewis basic sites of the catalysts, facilitating the formation of a geometrically defined intermediate and, therefore, a higher degree of stereocontrol. In fact, the smallest natural chiral α-amino acid, L-alanine (27), has demonstrated its proficiency in catalyzing the aldol reaction (Scheme 8) [159], and other reports afterwards demonstrated that several other proteinogenic α-amino acids were also good catalysts for aldol and related reactions [160].
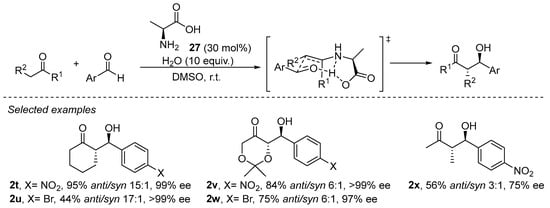
Scheme 8.
The L-alanine-catalyzed enantioselective aldol reaction.
There are also several other bifunctional catalysts involving primary amines that incorporate additional H-bond donor entities reported for these transformations (see Figure 2 for several representative examples), starting with simple modifications of the a-amino acid core like, for example 28 [161]), chiral diamine-based compounds like 29 [162] or 30 [163] and also dipeptides, tripeptides or even longer peptide compounds (illustrative cases: 31 [164], 32 [165,166] and 33 [167]).
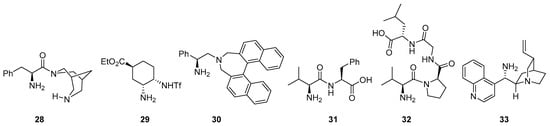
Figure 2.
Selected examples of bifunctional primary amine/H-bond donor catalysts employed in aldol, Mannich and related reactions.
The use of α,β-unsaturated carbonyl compounds as electrophiles in the Michael reaction with aldehydes and ketones under enamine catalysis deserves special attention [168]. The fact that the Lewis basic site at the Michael acceptor that has to interact with the H-bond donor motif of the catalyst is placed at a longer distance in comparison with the previously discussed electrophiles entails that most of the bifunctional hydrogen bond donor/aminocatalysts that perform well in the aldol, Mannich, α-hydrazination or α-aminoxylation tend to provide poor results in the Michael reaction. For example, the l-proline-catalyzed Michael reaction between cyclohexanone and β-nitrostyrene proceeds and provides the corresponding Michael adduct in excellent yield and diastereoselectivity but with poor enantiocontrol [169], and the same applies to tetrazole analogue 10 [170], but increasing the distance between the secondary amine moiety and the H-bond donor site, as in homoproline/tetrazole catalyst 34, led to a significant improvement in the enantioselectivity (Scheme 9) [171]. In this sense, the well-known ability of thioureas to establish persistent interactions through double H-bonding events with the nitro group also has been applied to the design of efficient bifunctional pyrrolidine/thiourea catalysts such as 35 [172,173], which also performs well in the Michael reaction using nitroalkenes as electrophiles [174,175,176,177,178].
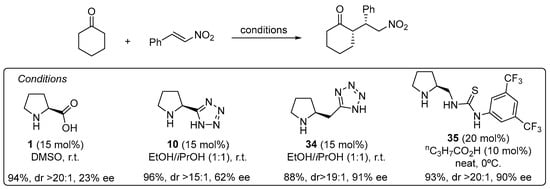
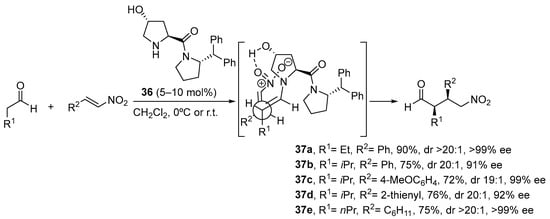

Scheme 9.
The Michael reaction between cyclohexanone and β-nitrostyrene catalyzed by proline and tetrazolylpyrrolidine vs. functionalized pyrrolidines 34 and 35. As an alternative, trans-4-hydoxy-l-prolinamide 36 has also been described to promote very efficiently the enantioselective Michael reaction between aldehydes and nitroalkenes (Scheme 10) [179]. In this case, the facial selectivity is reversed with respect to the reactions catalyzed by l-proline or the related derivatives shown in the previous scheme, which results from the internal activation of the nitroalkene by the 4-OH moiety of the enamine intermediate. The bulky amide substituents also contribute to enhance the enantioselectivity of the reaction through favoring the selective formation of the (E)-s-trans conformer in which steric interactions are minimized.

Scheme 10.
The Michael reaction between aldehydes and nitroalkenes catalyzed by trans-hydroxyprolinamide 36.
On the other hand, tripeptide 38a has been demonstrated to be, up to date, the best performing catalyst in this transformation, being able to catalyze the transformation in a remarkably low 1 mol% catalyst loading (Scheme 11) [180]. The reaction can be carried out on a remarkably wide set of aldehyde donors and nitroalkene Michael acceptors, including the highly challenging nitroethylene [181] and also α,β- and β,β-disubstituted nitroalkenes [182,183]. In addition, the reaction proceeds under almost equimolar amounts of both Michael donor and acceptor, in deep contrast with most reported methodologies that typically required a large excess of the nitroalkene. Several key elements are required for the high performance of this catalyst. On one hand, the presence of the secondary pyrrolidine from the initial d-proline residue is crucial for both activity and enantioselectivity, and the configuration of the other two L-amino acid residues has also been recognized as the matched combination that provides the highest enantiocontrol [184]. On the other hand, the terminal carboxylic acid moiety of the final glutamic acid residue was also identified to be key for both activity and enantioselectivity [185]. Interestingly, reducing the size of this final carboxylate-containing side chain (from glutamic acid to aspartic acid) also led to a slight decrease in yield and enantioselectivity. A series of in-depth mechanistic studies [186,187,188,189,190,191,192] indicate that this carboxylic acid moiety is engaged in H-bonding interactions with the nitroalkene during the Michael addition between the intermediate enamine and the nitroalkene, but this acidic moiety is also crucial for promoting the fast protonation of the nitronate intermediate formed after the conjugate addition step. This favors catalyst turnover and leaves the C-C bond-forming step as the stereodefining event in the catalytic cycle [193]. Further studies in this area by other authors have shown that other structurally related tripeptides [194,195,196] or smaller dipeptides [197,198] also can catalyze this reaction with success.
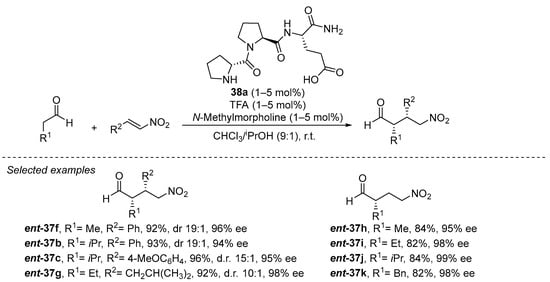
Scheme 11.
The D-Pro-Pro-Glu-NH2 (38a)-catalyzed Michael reaction between aldehydes and nitroalkenes.
As an interesting variant, catalyst 38b, in which steric bulk at C4 of the active proline residue has been increased through the introduction of two methyl substituents, is an outstanding catalyst for the same Michael reaction, providing the opposite anti diastereoisomer with excellent yield and enantioselectivity (Scheme 12) [199]. The basis for this change in diastereoselectivity relies on the change in reactive conformation of the enamine intermediate, which in this case adopts a s-cis conformation in order to avoid steric clash between the alkene moiety and the two methyl substituents.
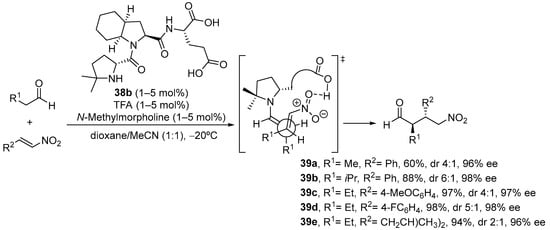
Scheme 12.
The tripeptide 38b-catalyzed Michael reaction between aldehydes and nitroalkenes providing anti diastereoisomers.
The use of acyclic ketones as Michael donors typically makes use of primary amines as catalysts in order to favor the enamine intermediate with a lower degree of steric congestion compared to the situation when secondary amines are used. In this field, bifunctional primary amine/thioureas have gained a prominent position among the different systems reported to promote the Michael reaction between ketones and nitroalkenes. For example, 1,2-diphenylethylenediamine-based thiourea 40a has demonstrated its proficiency in this transformation with both acetone and other substituted acyclic ketones [200,201], in the latter case leading to the diastereoselective formation of the anti γ-nitro ketone diastereoisomer with high enantioselectivity (Scheme 13). This is explained in terms of the preferential participation of the Z enamine intermediate that avoids destabilizing steric interactions between the two alkyl substituents across the C=C bond. Moreover, in this case the reaction was also found to be completely regioselective, providing α-branched adduct 41e and without observing the formation of the potential regioisomer arising through the competitive formation of an unsubstituted enamine intermediate upon condensation of the substrate with the catalyst. Further progress in this field has evolved into a wide variety of structurally related primary amine/thiourea catalysts that also perform well in this transformation [144].
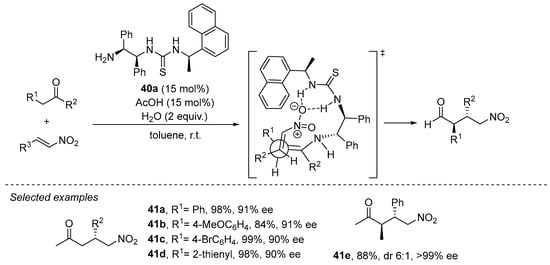
Scheme 13.
The Michael reaction between acyclic ketones and nitroalkenes catalyzed by primary amine/thiourea 40a.
The same situation shows up when α,α-disubstituted aldehydes are to be used as Michael donors [202], in which the problems associated with the high degree of steric congestion around the nucleophilic carbon of the enamine are circumvented through the use of a primary amine catalyst instead of the archetypical pyrrolidine-based secondary amines. A particularly efficient approach comprises the use of bifunctional trans-cyclohexanediamine-derived thiourea 42a (Scheme 14) [203]. As can be seen in this scheme, this reaction performs excellently for a variety of situations, including challenging β-alkyl substituted nitroalkenes and also functionalized aldehydes, providing generally excellent yields and stereocontrol. It should also be pointed out that this catalyst is also particularly efficient in the Michael reaction between acyclic ketones and nitroalkenes [204].
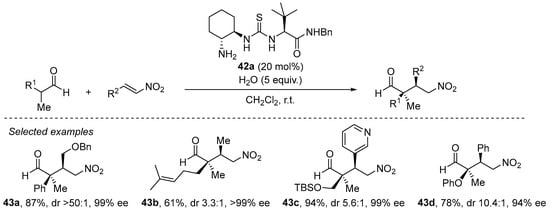
Scheme 14.
The Michael reaction between α,α-disubstituted aldehydes and nitroalkenes catalyzed by primary amine/thiourea 42a.
As can be seen across the different examples of Michael reactions shown before, the weakly nucleophilic nature of the enamine intermediate requires a strongly electrophilic Michael acceptor such as a nitroalkene for the reaction to proceed with high yields. However, there are several reports, although much more limited in number, that disclose the possibility of using other types of highly activated alkenes in this reaction. For instance, maleimines [205,206,207,208], alkylidenemalonates [209,210,211,212] or acrylates and related compounds [213,214] also have been found to react efficiently in several examples of Michael reactions under bifunctional amine/H-bond donor catalysis, as can be seen from the examples shown in Scheme 15, although in many cases the scope of the reaction is rather limited in terms of the potential Michael donor, mostly being effective when cyclohexanone is involved as the pronucleophile.
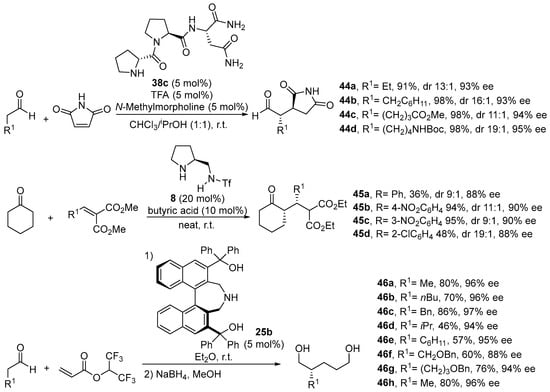
Scheme 15.
Some examples on the use of different electron-poor olefins in the Michael reaction under bifunctional amine/H-bond donor catalysis.
The ability of external H-bond donor cocatalysts to activate the Michael acceptor moiety can be used for the intramolecular Michael reaction shown in Scheme 16 [215] that involves α,β-unsaturated esters, which are typically unreactive electrophiles toward the Michael reaction under enamine activation. In this case, Schreiner thiourea 47 [216,217] is incorporated into the reaction scheme in which O-TMS protected diarylprolinol catalyst 48 is employed for the activation of the aldehyde moiety [218,219,220,221,222,223,224]. This catalyst is known to exert an excellent facial selectivity in many other reactions that involve the a-functionalization of aldehydes through a very effective steric shielding of one of the prostereogenic faces of the enamine intermediate [225,226,227], without being involved in any H-bonding interaction event; therefore, the reaction in the presence only of this catalyst provided only traces of the intramolecular Michael reaction product, while the incorporation of thiourea 47 led to the clean formation of 49 in high yield and diastereoselectivity, also minimizing the presence of side products arising from the competitive intermolecular aldol reaction between two molecules of the substrate. As an illustrative example of the potential of the methodology in synthesis, the authors also accomplished the total synthesis of natural product (−)-yohimbane 50 in only three additional steps from 49 and with an overall 25% yield from the starting material.
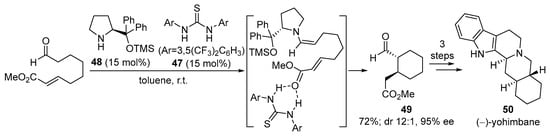
Scheme 16.
Intramolecular Michael reaction catalyzed by diarylprolinol 48/Schreiner thiourea 47 and application to the total synthesis of (−)-yohimbane.
3. Dual H-Bonding and Dienamine Activation
The combination of the enamine activation manifold with the principle of vinylogy [228] allows the extension of nucleophilic reactivity to remote locations of the initial nucleophile [229,230,231]. Following Jørgensen’s pioneering example of the use of dienamine intermediates in the catalytic enantioselective γ-amination of alkenals [232], this methodology has been extended to the use of simple dienamines [233,234] trienamines and tetraenamines. Apart from the possibility of enamine intermediates, this possibility has received considerable attention [235]. However, the fact that new stereocenters are installed away from where the chiral information of the amine catalyst forces a high conformational control of the dienamine, trienamine, or tetraenamine systems. For catalysts endowed with facet selectivity only by steric effects, such as the prototypical Jørgensen–Hayashi type catalysts, the level of steric control can be challenging [218]. Thus, the use of bifunctional amine/H-bond donor catalysts that can interact with the introduced electrophile can provide a useful solution to this problem and lead to a more conformationally restricted reagent during the formation of new stereocenters (see Scheme 17).
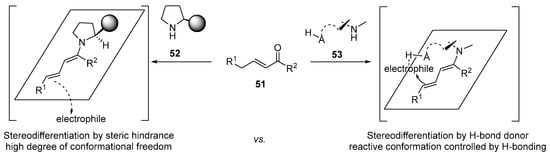
Scheme 17.
Stereochemical outcome of reactions under dienamine activation.
A good example of this strategy is the cycloaddition reaction using bifunctional pyrrolidine/squaramide catalyst 55a developed by Jørgensen, which has been successfully applied to formal enantioselectivity (2 + 2) between nitroalkenes and in situ generated dienes (Scheme 18) [236]. The reaction is a Michel/Michael cascade in which hydrogen-bonding interactions between the nitro substituents of the nitroalkene reagents control the proximity between the reagents. This reaction afforded a large number of tetrasubstituted cyclobutanes in excellent yields as single diastereomers and enantiomers. This catalyst was also later used successfully by the authors in several examples of inverse electron-demanding Diels–Alder cycloaddition reactions involving the remote alkene moiety of a dienamine as the electron-rich dienophile moiety [237,238].
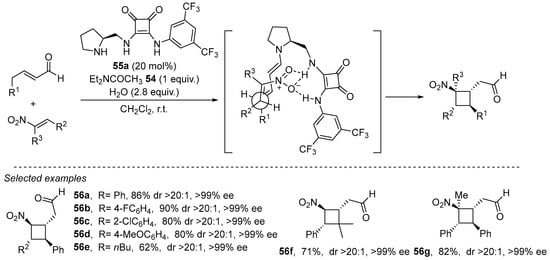
Scheme 18.
Use of bifunctional catalyst 55a in the formal (2 + 2) under dienamine activation.
Furthermore, the same type of catalyst has also been shown to be useful in formal 1,3-dipolar cycloaddition chemistry. In particular, the (5 + 2) cycloaddition between in situ generated pyrylium ylides and enolized enols proceeds via dienamine activation of the latter, also in the presence of pyrrolidine/squaramide 55b with excellent yield and stereocontrol (Scheme 19) [239]. These reactions consist of a stepwise process in which 1,2-addition of the dienamine to the oxonium moiety occurs once the oxidopyrylium reagent is formed via an elimination of AcOH facilitated by DABCO. Due to both H-bonding interactions between the oxidopyrylium reagent and the squaramide moiety and interactions between the former and the (bistrifluoromethyl)benzyl substituent at one of the squaramide’s N-atoms, the initial step is rigid [240]. The use of the Jørgensen–Hayashi catalyst 48, in addition to providing high yields and e.e., failed to provide high diastereoselectivity in relation to the relative arrangement of the two reagents.
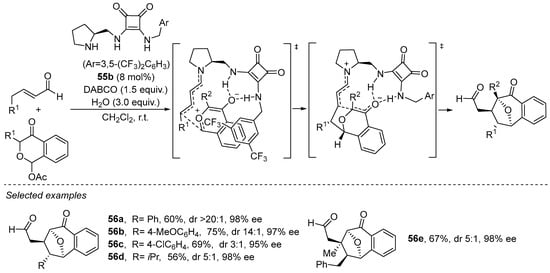
Scheme 19.
Use of bifunctional catalyst 55b in the formal (5 + 2) under dienamine activation.
The bifunctional pyrrolidine/thiourea 34 that is structurally related to this type of squaramide catalysts 55 has been identified to operate through a similar manifold in the cyclocondensation reaction between enolizable aldehydes and α-acetoxymethyl nitrostyrenes (Scheme 20) [241]. This reaction takes place through initial Michael reaction between the terminal position of the nucleophilic dienamine intermediate and the nitroalkene Michael acceptor, in which the bifunctional thiourea establishes a well-defined trajectory for the reagents through the formation of a network of H-bonding interactions. Next, elimination of AcOH regenerates a terminal nitroalkene moiety ready to participate in a second intramolecular Michael reaction that forms the final densely substituted cyclohexenecarbaldehyde adducts. Initially, the reaction produced the cyclocondensation adducts 57 with high enantioselectivities but as mixtures of diastereoisomers with respect to the NO2-containing stereocenter. This could be solved by adding an external Brønsted base such as Na2CO3, which provided the thermodynamic products 57 as single diastereoisomers, also indicating that the bifunctional catalyst had performed excellently in providing a high degree of diastereo- and enantiocontrol in the first Michael reaction.
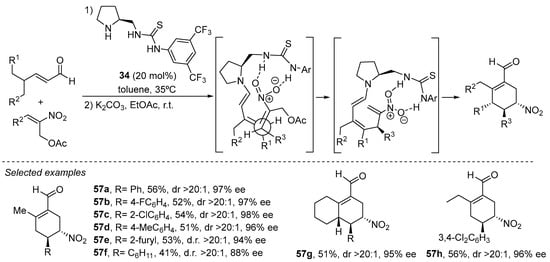
Scheme 20.
Use of bifunctional catalyst 34 in the cyclocondensation of enals with α-acetoxymethylnitroalkenes.
There are also several examples in which external H-bond donor additives are incorporated together with the aminocatalyst in order to interact with the electrophile with the aim of increasing its reactivity and/or modulating the stereochemical outcome of the reaction. For example, the incorporation of Schreiner thiourea 47 as cocatalyst in combination with diphenylprolinol derivative 48 is key for the success of the formal (2 + 2) cycloaddition between a-hydroxymethyl-substituted nitroalkenes and enals (Scheme 21) [242]. This reaction, which is closely related to the reaction displayed in Scheme 18 in which a bifunctional catalyst was employed, provided direct access to the 3-oxabicyclo [4.2.0]octane scaffold in a single step after intramolecular hemiacetalization with the pendant hydroxymethyl substituent of the nitroalkene reagent. This final hemiacetal formation step contributes to the stabilization of the final product and provides an additional thermodynamic driving force for the overall process. Remarkably, poor yields were typically observed for a variety of conditions tested in the absence of this cocatalyst 47, which shows the key role played by the H-bond donor additive in both the activation of the nitroalkene electrophile and in the stabilization of the nitronate intermediate formed after the initial Michael reaction step.
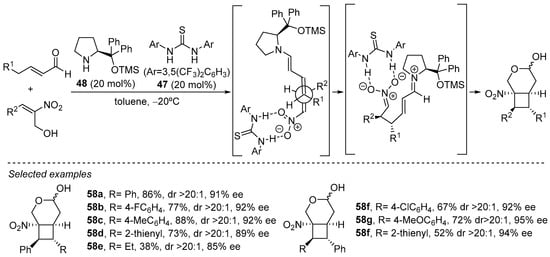
Scheme 21.
Combined use of diarylprolinol catalyst 48 and thiourea 47 in the formal (2 + 2) cycloaddition reaction between enals and α-hydroxymethylnitroalkenes.
Several authors have explored the simple γ-functionalization of enones or enals that does not involve formal cycloaddition chemistry and in which bifunctional aminocatalysts containing H-bond donor units provide a solution for solving stereoselectivity issues. A representative example is shown in Scheme 22, in which quinidine-based primary amine 59 has been identified as a good catalyst for the γ-alkylation of α-substituted enals with benzhydryl alcohols through SN1 reaction [243]. Key features of this reaction involve the incorporation of a BINOL-based phosphoric acid for the generation of the required carbocation via dehydration and the presence of a 6′-hydroxy substituent at the quinolone moiety of the catalyst. This phenolic H-bond donor site is proposed to be involved in H-bonding with the phosphate counterion of the carbocationic intermediate, thus directing the approach of the electrophile to the γ-position of the dienamine and leading to high enantiocontrol. For this reason, the matched combination between the two chiral components of the catalyst salt employed had to be identified, observing that the combination of quinidine-derived catalyst 59 with (S)-BINOL-based phosphoric acid 60 resulted in the highest enantiocontrol. A similar combination between the same types of phenolic cinchona alkaloid-based primary amine catalysts and achiral Brønsted acids has also been employed in the γ-functionalization of enones through Michael reaction with nitroalkenes [244,245,246,247,248,249].
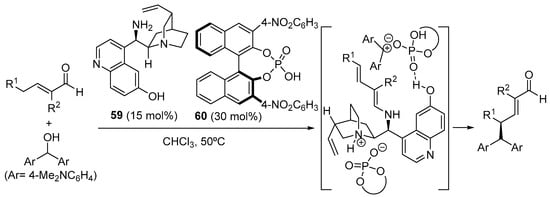
Scheme 22.
γ-Alkylation of α-substituted enals through SN1 reaction under cooperative primary amine Brønsted acid catalysis.
In another example of the ability of this type of reactivity to achieve selective g-functionalization of α,β-unsaturated carbonyl compounds, bifunctional primary amine/thiourea 50b emerges as an excellent catalyst for the aldol reaction between β-methylcyclohexenones and α-ketoesters [250]. This reaction takes place with complete γ-selectivity and with an excellent degree of enantiocontrol, directly related to the ability of the thiourea moiety to interact with the electrophilic ketone moiety, directing its approach to the, on the other hand, highly conformationally rigid exocyclic dienamine intermediate (Scheme 23).
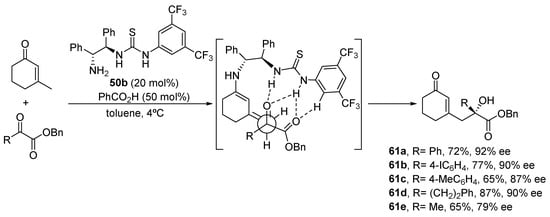
Scheme 23.
γ-Functionalization of β-methylcyclohexenone through enantioselective aldol reaction.
The reactivity of this type of cyclic enone toward dienamine activation can also be directed to the formation of an endocyclic dienamine intermediate that enables the functionalization of the cyclic scaffold at the 4-position. In this case, diamine 61a has been identified as an excellent catalyst for the formal vinylogous Michael reaction between cyclopentenones and cyclohexenones with maleimides, resulting in an interesting methodology for the formation of densely functionalized carbocycles (Scheme 24) [245]. Even though the formation of the products can be envisaged to take place at first sight through direct conjugate addition of the dienamine intermediate at the terminal nucleophilic position with the maleimide reagent, detailed mechanistic studies showed that the true mechanism of the reaction involved a Diels–Alder cycloaddition followed by retro-Mannich reaction. This provided a plausible explanation for the fact that when α-substituted maleimides were employed as substrates, the reaction delivered the regioisomer with a quaternary stereocenter at the maleimide moiety.
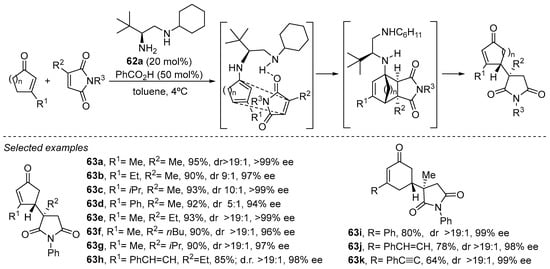
Scheme 24.
Cascade Diels–Alder/retro-Mannich reaction between cycloalkenones and maleimides catalyzed by diamine 62a.
Another possibility to generate this type of dienamine intermediate relies on the use of α,β-unsaturated ketones as the starting materials, which, upon condensation with the aminocatalyst, generate exactly the same dienamine intermediate that is formed when the parent α,β-unsaturated ketone is employed. This strategy has the evident benefit of the higher reactivity of the unconjugated starting material toward the condensation with the catalyst in the initial step of the reaction [251,252]. Scheme 25 shows the use of this strategy for the enantioselective synthesis of 3,4-disubstituted cyclohexenones, in the presence of bifunctional catalyst 63 [253]. The H-bond donor element directs the approach of the Brønsted acid during the selective g-protonation of the dienamine intermediate, forming an α,β-unsaturated iminium ion that, upon hydrolysis, releases the catalyst and the final conjugated product, which was obtained as highly enantioenriched material. This reaction was applied as the key step in the total synthesis of natural product (−)-isoacanthodoral.
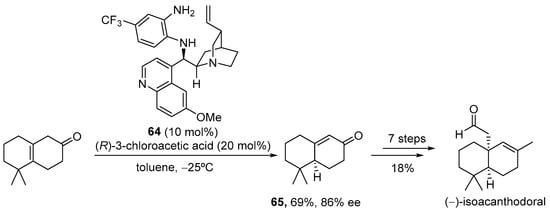
Scheme 25.
Enantioselective isomerization of 3,4-disubstituted cyclohex-3-en-1-ones through protonation of dienamine intermediates and application to the total synthesis of (−)-isoacanthodoral.
However, when enones are used as the substrate with a primary or secondary amine catalyst, they can offer the possibility to form cross-conjugated dienamine intermediates instead of the linear dienamines that are generated when enals are employed. This is the typical behavior observed when cyclohexanones are employed, which, when not biased toward the formation of linear dienamines (as in the case of β-methylcyclohexenone shown in Scheme 23), can form a 2-aminodiene intermediate with potential to participate as electron-rich diene in Diels–Alder-type reactivity. In those cases, bifunctional catalysts with H-bond donor moieties able to interact with the dienophile containing Lewis basic sites can be used to unveil interesting reactivity. A good example of this behavior is shown in Scheme 26, in which the reaction of cyclohexenones with nitrosobenzene in the presence of pyrrolidine-tetrazole catalyst 10 provides a variety of bicyclic adducts with excellent yield and enantioselectivity [50,104,254]. Despite the mentioned possibility for a Diels–Alder [4 + 2] pericyclic reaction to occur, further studies have demonstrated that this reaction is a stepwise process that consists of an initial highly enantioselective O-nitroso-aldol reaction controlled by the H-bond donor ability of the tetrazole stereodirecting element followed by intramolecular conjugate addition [255].
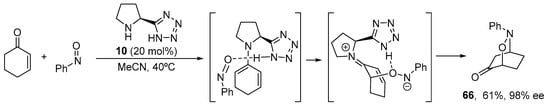
Scheme 26.
The pyrolidine-tetrazole 10-catalyzed nitroso-Diels–Alder reaction.
This type of reactivity is amenable to being further extended to electronically related electrophiles with potential to undergo the same type of cascade a-functionalization followed by intramolecular conjugate addition. For instance, in situ-generated formaldehyde N-arylimines also undergo formal aza-Diels–Alder reaction with cyclohexenones and cycloheptenone in the presence of l-proline 1, providing the corresponding bicyclic adducts in excellent yields and enantioselectivity (Scheme 27a) [256,257,258,259]. The use of cyclopentenone as substrate, which requires the formation of a strained cyclic dienamine intermediate, did not provide any formal cycloaddition product. In addition, 4,4-disubstituted cyclohexenones also react efficiently with nitroalkenes, providing bicyclic adducts 69 in high yield, single endo-diastereoisomers and excellent enantiomeric excess (Scheme 27b), in this case using the pyridinium salt of compound 68 as the best performing catalyst. Interestingly, this reaction afforded the highest performance when it was carried out in brine [260,261,262,263].
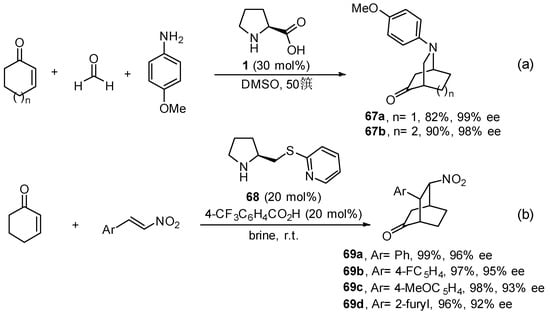
Scheme 27.
Comparison between (a) proline and (b) pyrolidine-tetrazole 10 in the nitroso-Diels–Alder reaction.
Alternatively, and as happened in the reactions proceeding through enal-derived dienamines, there are also some examples involving 2-aminodiene-type intermediates in which the H-bond donor is added as an external additive or cocatalyst instead of being part of a bifunctional catalyst. This is the situation shown in Scheme 28, in which, again, Schreiner thiourea 47 is added as an external additive to assist in the formal (4 + 2) cycloaddition between enones and isatins catalyzed by quinine-derived primary amine 70, and with the H-bond donor ability of 47 contributing to enhance the reactivity of the heterodienophile [264]. The reaction also proceeds more efficiently when a modified threonine derivative is incorporated as Brønsted acid additive, which is proposed to participate in the formation of a carboxylate salt with the quinuclidine scaffold of the catalyst, enhancing its ability to exert stereodifferentiation between the two prostereogenic faces of the 2-aminodiene intermediate.
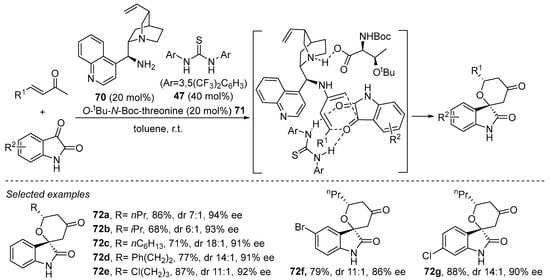
Scheme 28.
Enantioselective hetero-Diels–Alder reaction between enones and isatins catalyzed by a primary amine in combination with Schreiner thiourea.
Finally, it should also be mentioned that, in addition to the ability of the H-bond donor group to govern the stereoselectivity of the process, this type of element incorporated as part of a bifunctional catalyst can also be employed to direct the regioselectivity of the reaction, taking into account the polydentate nucleophilic nature of the dienamine intermediate. For instance, l-proline 1 catalyzes the Mannich reaction between enolizable enals and imines (Scheme 29), providing clean α-addition products that, upon isomerizaton of the C=C bond in the presence of one equivalent of a Brønsted base such as imidazole, provide a direct entry to aza-Morita-Baylis-Hilman-type adducts 72 [265,266,267]. Other authors have extended this reaction platform to the use of aldehydes as the electrophilic counterparts using proline as catalyst [115] and also employing a bifunctional primary amine/thiourea in the intramolecular Rauhut Currier reaction [268].
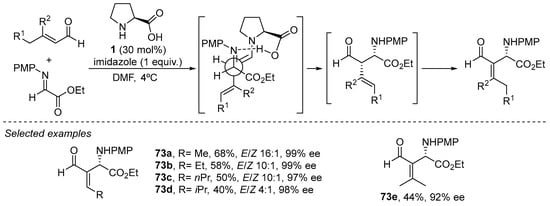
Scheme 29.
The proline-catalyzed Mannich reaction between enals and imines under dienamine activation.
4. Dual H-Bonding and Trienamine/Tetraenamine Activation
The combination of the principle of vinylogy with the enamine activation manifold can be further applied to extended conjugated systems, enabling functionalization of far more remote positions [210]. However, in this case, in addition to the stereochemical issues to be solved, the polydentate nucleophilic nature of this extended polyconjugate enamine system entails an additional challenge with respect to regioselectivity control. As a consequence, the use of a bifunctional amine/H-bond donor catalysts can provide useful and very effective solutions to many of these problems. A good example of this behavior can be found in the Diels–Alder reaction between chromones and enolizable α,β,γ,δ-unsaturated aldehydes (Scheme 30), in which the H-bond donor squaramide moiety plays a crucial role in facilitating a stereodefined trienamine intermediate through establishing a selective H-bonding interaction with the nitrile substituent that activates the dienophile [269]. The [4 + 2] cycloaddition involving the terminal diene moiety of the trienamine that adopts a preferential reactive s-cis/s-cis conformation across the triene scaffold takes place with excellent endo-selectivity and provides a family of tetrahydroxanthones in excellent yields and enantioselectivities. This reaction design has been applied in other, related [4 + 2] cycloadditions between dienals and other dieophiles with success [270,271].
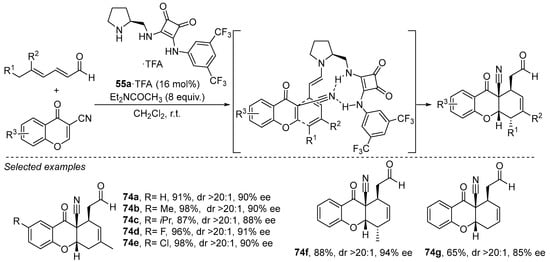
Scheme 30.
Enantioselective [4 + 2] cycloaddition between dienals and chromones catalyzed by pyrrolidine/squaramide 55a under trienamine activation.
In addition, the same catalyst has been found to perform excellently in the activation of anthracen-9-yl-acetaldehydes toward Diels–Alder cycloaddition through the formation of a linear trienamine intermediate (Scheme 31) [272]. This transformation takes place together with the dearomatization of the anthracene core and using a rather low catalyst loading in comparison with other methods reported using this type of organocatalytic activation manifold. The stereodirecting ability of the squaramide moiety via H-bonding interactions with the nitroalkene dienophile was key to obtaining good face selectivity, observing that the archetypical diarylprolinol-based catalyst 48, while being also able to catalyze this transformation, only furnished the corresponding cycloadducts in 60% e.e. In the presence of catalyst 55a. the reaction provides a single diastereoisomer with excellent enantioselectivity, and it was also found to be remarkably wide in scope with respect to the possibility of using differently substituted nitroalkenes, including β-alkyl substituted ones.
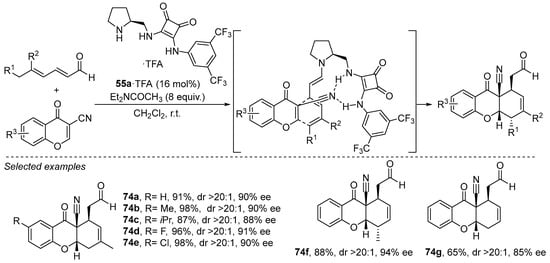
Scheme 31.
Enantioselective [4 + 2] cycloaddition between dienals and chromones catalyzed by pyrrolidine/squaramide 55a under trienamine activation.
Finally, there are also some examples of reactions involving even more extended tetraenamine intermediates in which a bifunctional secondary amine/H-bond donor has been employed as the catalyst of choice to achieve high stereocontrol. This reactivity typically involves the use of 5-allylfurfurals as convenient substrates to undergo condensation with a secondary amine that generates a somewhat conformationally rigid tetraenamine intermediate in which the aromaticity of the furan moiety has been removed. In particular, pyrrolidine/thiourea 34 has been identified to be able to catalyze the hetero-Diels–Alder reaction of these substrates using highly activated alkylideneoxindoles as the oxodiene counterpart reacting with the tetraenamine intermediate in which the terminal alkene is participating as the dienophile (Scheme 32) [273]. A related report has also shown the possibility of carrying out the functionalization at the terminal carbon of this polyunsaturated system through Michael reaction [274].
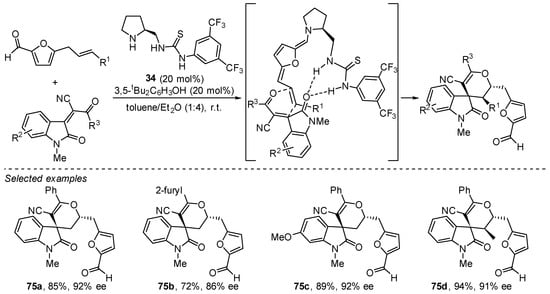
Scheme 32.
Enantioselective Diels–Alder reaction between anthracen-9-yl-acetaldehydes and nitroalkenes under trienamine activation.
5. Conclusions
As demonstrated by the aforementioned examples, enamine activation is a useful strategy for the functionalization of carbonyl compounds. In this sense, the use of a cocatalyst that can interact with the reagent used for functionalizing the carbonyl compound can provide better conversion and yields and also better stereoselectivities. This can be attributed to a more rigid transition state in which the approximation of this reagent to the enamine (or dienamine, trienamine, etc.) is controlled by secondary interactions.
Author Contributions
The manuscript was written through contributions of all authors. Writing, L.P., L.C. and U.U.; writing and review, E.R. and J.L.V. All authors have read and agreed to the published version of the manuscript.
Funding
PID2020-118422GB-I00 funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in your future” are gratefully acknowledged together with the Basque Government (Grupos IT1558-22) and the University of the Basque Country (UPV/EHU).
Data Availability Statement
Not necessary.
Conflicts of Interest
The authors declare no conflict of interest.
References
- List, B.; Lerner, R.A.; Barbas, C.F., III. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Hajos, Z.G.; Parrish, D.R. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974, 39, 1615–1621. [Google Scholar] [CrossRef]
- Eder, U.; Sauer, G.; Wiecert, R. New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures. Angew. Chem. Int. Ed. 1971, 10, 496–497. [Google Scholar] [CrossRef]
- Wagner, J.; Lerner, R.A.; Barbas, C.F., III. Efficient Aldolase Catalytic Antibodies That Use the Enamine Mechanism of Natural Enzymes. Science 1995, 270, 1797–1800. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Houk, K.N.; Martin, H.J.; List, B. Quantum Mechanical Predictions of the Stereoselectivities of Proline-Catalyzed Asymmetric Intermolecular Aldol Reactions. J. Am. Chem. Soc. 2003, 125, 2475–2479. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels-Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Jen, W.S.; Wiener, J.J.M.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Enantioselective Organocatalytic 1,3-Dipolar Cycloaddition. J. Am. Chem. Soc. 2000, 122, 9874–9875. [Google Scholar] [CrossRef]
- Mukherjee, S.; Yang, J.W.; Hoffmann, S.; List, B. Asymmetric Enamine Catalysis. Chem. Rev. 2007, 107, 5471–5569. [Google Scholar] [CrossRef]
- Sulzer-Mossé, S.; Alexakis, A. Chiral amines as organocatalysts for asymmetric conjugate addition to nitroolefins and vinyl sulfonesviaenamine activation. Chem. Commun. 2007, 30, 3123–3135. [Google Scholar] [CrossRef]
- Kano, T.; Maruoka, K. Design of chiral bifunctional secondary amine catalysts for asymmetric enamine catalysis. Chem. Commun. 2008, 43, 5465–5473. [Google Scholar] [CrossRef]
- Melchiorre, P. Cinchona-based Primary Amine Catalysis in the Asymmetric Functionalization of Carbonyl Compounds. Angew. Chem. Int. Ed. 2012, 51, 9748–9770. [Google Scholar] [CrossRef]
- Xiang, S.-H.; Tan, B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 2020, 11, 3786. [Google Scholar] [CrossRef]
- List, B.; Maruoka, K. (Eds.) Science of Synthesis, Asymmetric Organocatalysis; Georg Thieme Verlag: Stuttgart, Germany, 2012. [Google Scholar]
- Pellissier, H. (Ed.) Recent Developments in Asymmetric Organocatalysis; RSC Publishing: Cambridge, UK, 2011. [Google Scholar]
- Jacobsen, E.N.; MacMillan, D.W.C. Organocatalysis. Proc. Natl. Acad. Sci. USA 2010, 107, 20618–20619. [Google Scholar] [CrossRef]
- Bertelsen, S.; Jørgensen, K.A. Organocatalysis-after the gold rush. Chem. Soc. Rev. 2009, 38, 2178–2189. [Google Scholar] [CrossRef]
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef]
- Holland, M.C.; Gilmour, R. Deconstructing Covalent Organocatalysis. Angew. Chem. Int. Ed. 2015, 54, 3862–3871. [Google Scholar] [CrossRef]
- Sihtmae, M.; Silm, M.; Kriis, K.; Kahru, A.; Kanger, T. Aminocatalysts are More Environmentally Friendly than Hydrogen-Bonding Catalysts. ChemSusChem 2022, 15, e202201045. [Google Scholar] [CrossRef]
- Alemán, J.; Parra, A.; Jiang, H.; Jørgensen, K.A. Squaramides: Bridging from Molecular Recognition to Bifunctional Organocatalysis. Chem. Eur. J. 2011, 17, 6890–6899. [Google Scholar] [CrossRef]
- Matos Paz, B.; Jiang, H.; Jørgensen, K.A. Aminocatalysis: Beyond Steric Shielding and Hydrogen-Bonding. Chem. Eur. J. 2015, 21, 1846–1853. [Google Scholar] [CrossRef]
- Doyle, A.G.; Jacobsen, E.N. Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev. 2007, 107, 5713–5743. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Hong, L.; Sun, W.; Yang, D.; Li, G.; Wang, R. Additive Effects on Asymmetric Catalysis. Chem. Rev. 2016, 116, 4006–4123. [Google Scholar] [CrossRef]
- Anebouselvy, K.; Shruthi, K.S.; Ramachary, D.B. Asymmetric Supramolecular Organocatalysis: A Complementary Upgrade to Organocatalysis. Eur. J. Org. Chem. 2017, 2017, 5460–5483. [Google Scholar] [CrossRef]
- Meeuwissen, J.; Reek, J.N.H. Supramolecular catalysis beyond enzyme mimics. Nature Chem. 2010, 2, 615–621. [Google Scholar] [CrossRef]
- Allemann, C.; Gordillo, R.; Clemente, F.R.; Cheong, P.H.-Y.; Houk, K.N. Theory of Asymmetric Organocatalysis of Aldol and Related Reactions: Rationalizations and Predictions. Acc. Chem. Res. 2004, 37, 558–569. [Google Scholar] [CrossRef]
- Hoang, L.; Bahmanyar, S.; Houk, K.N.; List, B. Kinetic and Stereochemical Evidence for the Involvement of Only One Proline Molecule in the Transition States of Proline-Catalyzed Intra- and Intermolecular Aldol Reactions. J. Am. Chem. Soc. 2003, 125, 16–17. [Google Scholar] [CrossRef]
- List, B.; Hoang, L.; Martin, H.J. New mechanistic studies on the proline-catalyzed aldol reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 5839–5842. [Google Scholar] [CrossRef]
- Orlandi, M.; Ceotto, M.; Benaglia, M. Kinetics versus thermodynamics in the proline catalyzed aldol reaction. Chem. Sci. 2016, 7, 5421–5427. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sunoj, R.B. Enamine versus Oxazolidinone: What Controls Stereoselectivity in Proline-Catalyzed Asymmetric Aldol Reactions? Angew. Chem. Int. Ed. 2010, 49, 6373–6377. [Google Scholar] [CrossRef]
- Schmid, M.B.; Zeitler, K.; Gschwind, R.M. The Elusive Enamine Intermediate in Proline-Catalyzed Aldol Reactions: NMR Detection, Formation Pathway, and Stabilization Trends. Angew. Chem. Int. Ed. 2010, 49, 4997–5003. [Google Scholar] [CrossRef]
- Clemente, F.R.; Houk, K.N. Computational Evidence for the Enamine Mechanism of Intramolecular Aldol Reactions Catalyzed by Proline. Angew. Chem. Int. Ed. 2004, 43, 5766–5768. [Google Scholar] [CrossRef]
- Haindl, M.H.; Hioe, J.; Gschwind, R.M. The Proline Enamine Formation Pathway Revisited in Dimethyl Sulfoxide: Rate Constants Determined via NMR. J. Am. Chem. Soc. 2015, 137, 12835–12842. [Google Scholar] [CrossRef]
- Martínez, A.; Zumbansen, K.; Döhring, A.; van Gemmeren, M.; List, B. Improved Conditions for the Proline-Catalyzed Aldol Reaction of Acetone with Aliphatic Aldehydes. Synlett 2014, 25, 932–934. [Google Scholar] [CrossRef]
- Martínez-Castañeda, A.; Poladura, B.; Rodríguez-Solla, H.; Concellón, C.; del Amo, V. Direct Aldol Reactions Catalyzed by a Heterogeneous Guanidinium Salt/Proline System under Solvent-Free Conditions. Org. Lett. 2011, 13, 3032–3035. [Google Scholar] [CrossRef]
- Doyagüez, E.G.; Calderón, F.; Sánchez, F.; Fernández-Mayoralas, A. Asymmetric Aldol Reaction Catalyzed by a Heterogenized Proline on a Mesoporous Support. The Role of the Nature of Solvents. J. Org. Chem. 2007, 72, 9353–9356. [Google Scholar] [CrossRef]
- Zhou, Y.; Shan, Z. Chiral Diols: A New Class of Additives for Direct Aldol Reaction Catalyzed by l-Proline. J. Org. Chem. 2006, 71, 9510–9512. [Google Scholar] [CrossRef]
- Pan, Q.; Zou, B.; Wang, Y.; Ma, D. Diastereoselective Aldol Reaction of N,N-Dibenzyl-α-amino Aldehydes with Ketones Catalyzed by Proline. Org. Lett. 2004, 6, 1009–1012. [Google Scholar] [CrossRef]
- Itoh, T.; Yokoya, M.; Miyauchi, K.; Nagata, K.; Ohsawa, A. Proline-Catalyzed Asymmetric Addition Reaction of 9-Tosyl-3,4-dihydro-β-carboline with Ketones. Org. Lett. 2003, 5, 4301–4304. [Google Scholar] [CrossRef]
- Cordova, A.; Notz, W.; Barbas , C.F., III. Proline-Catalyzed One-Step Asymmetric Synthesis of 5-Hydroxy-(2E)-hexenal from Acetaldehyde. J. Org. Chem. 2002, 67, 301–303. [Google Scholar] [CrossRef]
- Villano, R.; Rosaria Acocella, M.; Scettri, A. Influence of a remote sulfinyl group on l-proline-catalyzed direct asymmetric aldol addition of acetone. Tetrahedron 2016, 72, 5414–5419. [Google Scholar] [CrossRef]
- Llanes, P.; Sayalero, S.; Rodríguez-Escrich, C.; Pericàs, M.A. Asymmetric cross- and self-aldol reactions of aldehydes in water with a polystyrene-supported triazolylproline organocatalyst. Green Chem. 2016, 18, 3507–3512. [Google Scholar] [CrossRef]
- Demir, A.S.; Basceken, S. Study of asymmetric aldol and Mannich reactions catalyzed by proline–thiourea host–guest complexes in nonpolar solvents. Tetrahedron Asymmetry 2013, 24, 515–525. [Google Scholar] [CrossRef]
- Montroni, E.; Sanap, S.P.; Lombardo, M.; Quintavalla, A.; Trombini, C.; Dhavale, D.D. A New Robust and Efficient Ion-Tagged Proline Catalyst Carrying an Amide Spacer for the Asymmetric Aldol Reaction. Adv. Synth. Catal. 2011, 353, 3234–3240. [Google Scholar] [CrossRef]
- Karmakar, A.; Maji, T.; Wittmann, S.; Reiser, O. l-Proline/CoCl2-Catalyzed Highly Diastereo- and Enantioselective Direct Aldol Reactions. Chem. Eur. J. 2011, 17, 11024–11029. [Google Scholar] [CrossRef]
- El-Hamdouni, N.; Companyó, X.; Rios, R.; Moyano, A. Substrate-Dependent Nonlinear Effects in Proline–Thiourea-Catalyzed Aldol Reactions: Unraveling the Role of the Thiourea Co-Catalyst. Chem. Eur. J. 2010, 16, 1142–1148. [Google Scholar] [CrossRef]
- Shah, J.; Blumenthal, H.; Yacob, Z.; Liebscher, J. Proline-Catalyzed Asymmetric Aldol Reaction in Guanidine- Derived Ionic Liquids. Adv. Synth. Catal. 2008, 350, 1267–1270. [Google Scholar] [CrossRef]
- Pihko, P.M.; Laurikainen, K.M.; Usano, A.; Nyberg, A.I.; Kaavi, J.A. Effect of additives on the proline-catalyzed ketone–aldehyde aldol reactions. Tetrahedron 2006, 62, 317–328. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamaguchi, J.; Hibino, K.; Sumiya, T.; Urushima, T.; Shoji, M.; Hashizume, D.; Koshino, H. A Highly Active 4-Siloxyproline Catalyst for Asymmetric Synthesis. Adv. Synth. Catal. 2004, 346, 1435–1439. [Google Scholar] [CrossRef]
- Pihko, P.M.; Erkkilä, A. Enantioselective synthesis of prelactone B using a proline-catalyzed crossed-aldol reaction. Tetrahedron Lett. 2003, 44, 7607–7609. [Google Scholar] [CrossRef]
- Kotrusz, P.; Kmentová, I.; Gotov, B.; Toma, S.; Solčániová, E. Proline-catalysed asymmetric aldol reaction in the room temperature ionic liquid [bmim]PF6. Chem. Commun. 2002, 8, 2510–2511. [Google Scholar] [CrossRef]
- Pidathala, C.; Hoang, L.; Vignola, N.; List, B. Direct Catalytic Asymmetric Enolexo Aldolizations. Angew. Chem. Int. Ed. 2003, 42, 2785–2788. [Google Scholar] [CrossRef]
- Chandler, C.L.; List, B. Catalytic, Asymmetric Transannular Aldolizations: Total Synthesis of (+)-Hirsutene. J. Am. Chem. Soc. 2008, 130, 6737–6739. [Google Scholar] [CrossRef]
- Cordova, A.; Notz, W.; Barbas, C.F., III. Direct organocatalytic aldol reactions in buffered aqueous media. Chem. Commun. 2002, 24, 3024–3025. [Google Scholar] [CrossRef]
- Hayashi, Y.; Aratake, S.; Itoh, T.; Okano, T.; Sumiya, T.; Shoji, M. Dry and wet prolines for asymmetric organic solvent-free aldehyde–aldehyde and aldehyde–ketone aldol reactions. Chem. Commun. 2007, 9, 957–959. [Google Scholar] [CrossRef]
- Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C.F., III. Amino Acid Catalyzed Direct Asymmetric Aldol Reactions: A Bioorganic Approach to Catalytic Asymmetric Carbon−Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 2001, 123, 5260–5267. [Google Scholar] [CrossRef]
- Notz, W.; List, B. Catalytic Asymmetric Synthesis of anti-1,2-Diols. J. Am. Chem. Soc. 2000, 122, 7386–7387. [Google Scholar] [CrossRef]
- List, B.; Pojarliev, P.; Castello, C. Proline-Catalyzed Asymmetric Aldol Reactions between Ketones and α-Unsubstituted Aldehydes. Org. Lett. 2001, 3, 573–575. [Google Scholar] [CrossRef]
- Northrup, A.B.; MacMillan, D.W.C. The First Direct and Enantioselective Cross-Aldol Reaction of Aldehydes. J. Am. Chem. Soc. 2002, 124, 6798–6799. [Google Scholar] [CrossRef]
- Northrup, A.B.; Mangion, F.H.; MacMillan, D.W.C. Enantioselective Organocatalytic Direct Aldol Reactions of α-Oxyaldehydes: Step One in a Two-Step Synthesis of Carbohydrates. Angew. Chem. Int. Ed. 2004, 43, 2152–2154. [Google Scholar] [CrossRef]
- Northrup, A.B.; MacMillan, D.W.C. Two-step synthesis of carbohydrates by selective aldol reactions. Science 2004, 305, 1752–1755. [Google Scholar] [CrossRef]
- Notz, W.; Tanaka, F.; Barbas, C.F., III. Enamine-Based Organocatalysis with Proline and Diamines: The Development of Direct Catalytic Asymmetric Aldol, Mannich, Michael, and Diels−Alder Reactions. Acc. Chem. Res. 2004, 37, 580–591. [Google Scholar] [CrossRef]
- List, B. Enamine Catalysis Is a Powerful Strategy for the Catalytic Generation and Use of Carbanion Equivalents. Acc. Chem. Res. 2004, 37, 548–557. [Google Scholar] [CrossRef]
- Cordova, A.; Notz, W.; Zhong, G.; Betancort, J.; Barbas, C.F., III. A Highly Enantioselective Amino Acid-Catalyzed Route to Functionalized α-Amino Acids. J. Am. Chem. Soc. 2002, 124, 1842–1843. [Google Scholar] [CrossRef]
- List, B.; Pojarliev, P.; Biller, W.T.; Martin, H.J. The Proline-Catalyzed Direct Asymmetric Three-Component Mannich Reaction: Scope, Optimization, and Application to the Highly Enantioselective Synthesis of 1,2-Amino Alcohols. J. Am. Chem. Soc. 2002, 124, 827–833. [Google Scholar] [CrossRef]
- List, B. The Direct Catalytic Asymmetric Three-Component Mannich Reaction. J. Am. Chem. Soc. 2000, 122, 9336–9337. [Google Scholar] [CrossRef]
- Yang, J.W.; Stadler, M.; List, B. Proline-Catalyzed Mannich Reaction of Aldehydes with N-Boc-Imines. Angew. Chem. Int. Ed. 2007, 46, 609–611. [Google Scholar] [CrossRef]
- Hayashi, Y.; Urushima, T.; Tsuboi, W.; Shoji, M. l-Proline-catalyzed enantioselective one-pot cross-Mannich reaction of aldehydes. Nat. Protoc. 2007, 2, 113–118. [Google Scholar] [CrossRef]
- Yang, J.; Chandler, C.; Stadler, M.; Kempen, D.; List, B. Proline-catalysed Mannich reactions of acetaldehyde. Nature 2008, 452, 453–455. [Google Scholar] [CrossRef]
- Bøgevig, A.; Juhl, K.; Kumaragurubaran, N.; Zhuang, W.; Jørgensen, K.A. Direct Organo-Catalytic Asymmetric α-Amination of Aldehydes—A Simple Approach to Optically Active α-Amino Aldehydes, α-Amino Alcohols, and α-Amino Acids. Angew. Chem. Int. Ed. 2002, 41, 1790–1793. [Google Scholar] [CrossRef]
- List, B. Direct Catalytic Asymmetric α-Amination of Aldehydes. J. Am. Chem. Soc. 2002, 124, 5656–5657. [Google Scholar] [CrossRef]
- Kumaragurubaran, N.; Juhl, K.; Zhuang, W.; Bøgevig, A.; Jørgensen, K.A. Direct l-Proline-Catalyzed Asymmetric α-Amination of Ketones. J. Am. Chem. Soc. 2002, 124, 6254–6255. [Google Scholar] [CrossRef]
- Juhl, K.; Jørgensen, K.A. Catalytic Asymmetric Direct α-Amination Reactions of 2-Keto Esters: A Simple Synthetic Approach to Optically Active syn-β-Amino-α-hydroxy Esters. J. Am. Chem Soc. 2002, 124, 2420–2421. [Google Scholar] [CrossRef]
- Ashley, M.A.; Hirschi, J.S.; Izzo, J.A.; Vetticatt, M.J. Isotope Effects Reveal the Mechanism of Enamine Formation in l-Proline-Catalyzed α-Amination of Aldehydes. J. Am. Chem. Soc. 2016, 138, 1756–1759. [Google Scholar] [CrossRef]
- Kanzian, T.; Lakhdar, S.; Mayr, H. Kinetic Evidence for the Formation of Oxazolidinones in the Stereogenic Step of Proline-Catalyzed Reactions. Angew. Chem. Int. Ed. 2010, 49, 9526–9529. [Google Scholar] [CrossRef]
- Brown, S.P.; Brochu, M.P.; Sinz, C.J.; MacMillan, D.W.C. The Direct and Enantioselective Organocatalytic α-Oxidation of Aldehydes. J. Am. Chem. Soc. 2003, 125, 10808–10809. [Google Scholar] [CrossRef]
- Zhong, G. A Facile and Rapid Route to Highly Enantiopure 1,2-Diols by Novel Catalytic Asymmetric α-Aminoxylation of Aldehydes. Angew. Chem. Int. Ed. 2003, 42, 4247–4250. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamaguchi, J.; Sumiya, T.; Shoji, M. Direct Proline-Catalyzed Asymmetric α-Aminoxylation of Ketones. Angew. Chem. Int. Ed. 2004, 43, 1112–1115. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamaguchi, J.; Sumiya, T.; Hibino, K.; Shoji, M. Direct Proline-Catalyzed Asymmetric α-Aminoxylation of Aldehydes and Ketones. J. Org. Chem. 2004, 69, 5966–5973. [Google Scholar] [CrossRef]
- Bogevig, A.; Sunden, H.; Cordova, A. Direct Catalytic Enantioselective α-Aminoxylation of Ketones: A Stereoselective Synthesis of α-Hydroxy and α,α′-Dihydroxy Ketones. Angew. Chem. Int. Ed. 2004, 43, 1129–1132. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamaguchi, J.; Hibino, K.; Shoji, M. Direct proline catalyzed asymmetric α-aminooxylation of aldehydes. Tetrahedron Lett. 2003, 44, 8293–8296. [Google Scholar] [CrossRef]
- Kumar, P.; Dwivedi, N. Proline Catalyzed α-Aminoxylation Reaction in the Synthesis of Biologically Active Compounds. Acc. Chem. Res. 2013, 46, 289–299. [Google Scholar] [CrossRef]
- Palomo, C.; Vera, S.; Velilla, I.; Mielgo, A.; Gomez-Bengoa, E. Regio- and Enantioselective Direct Oxyamination Reaction of Aldehydes Catalyzed by α,α-Diphenylprolinol Trimethylsilyl Ether. Angew. Chem. Int. Ed. 2007, 46, 8054–8056. [Google Scholar] [CrossRef]
- Companyó, X.; Valero, G.; Crovetto, L.; Moyano, A.; Rios, R. Highly Enantio- and Diastereoselective Organocatalytic Desymmetrization of Prochiral Cyclohexanones by Simple Direct Aldol Reaction Catalyzed by Proline. Chem. Eur. J. 2009, 15, 6564–6568. [Google Scholar] [CrossRef]
- Reis, O.; Eymur, S.; Reis, B.; Demir, A.S. Direct enantioselectivealdol reactions catalyzed by a proline–thiourea host–guest complex. Chem. Commun. 2009, 9, 1088–1090. [Google Scholar] [CrossRef]
- Poe, S.L.; Bogdan, A.R.; Mason, B.P.; Steinbacher, J.L.; Opalka, S.M.; McQuade, D.T. Use of Bifunctional Ureas to Increase the Rate of Proline-Catalyzed α-Aminoxylations. J. Org. Chem. 2009, 74, 1574–1580. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yasukawa, T.; Yoo, W.-J.; Kitanosono, T.; Kobayashi, S. Catalytic enantioselective aldol reactions. Chem. Soc. Rev. 2018, 47, 4388–4480. [Google Scholar] [CrossRef]
- Gómez Arrayás, R.; Carretero, J.C. Catalytic asymmetric direct Mannich reaction: A powerful tool for the synthesis of α,β-diamino acids. Chem. Soc. Rev. 2009, 38, 1940–1948. [Google Scholar] [CrossRef]
- Verkade, J.M.M.; van Hemert, L.J.C.; Quaedfliegb, P.J.L.M.; Rutjes, F.P.J.T. Organocatalysed asymmetric Mannich reactions. Chem. Soc. Rev. 2008, 37, 29–41. [Google Scholar] [CrossRef]
- Cai, X.-H.; Guo, H.; Bing, X. Recent progress in the asymmetric Mannich reaction. Eur. J. Chem. 2012, 3, 258–266. [Google Scholar] [CrossRef]
- Duthaler, R.O. Proline-Catalyzed Asymmetric α-Amination of Aldehydes and Ketones—An Astonishingly Simple Access to Optically Active α-Hydrazino Carbonyl Compounds. Angew. Chem. Int. Ed. 2003, 42, 975–978. [Google Scholar] [CrossRef]
- Merino, P.; Tejero, T. Organocatalyzed Asymmetric α-Aminoxylation of Aldehydes and Ketones—An Efficient Access to Enantiomerically Pure α-Hydroxycarbonyl Compounds, Diols, and Even Amino Alcohols. Angew. Chem. Int. Ed. 2004, 43, 2995–2997. [Google Scholar] [CrossRef]
- Albrecht, Ł.; Jiang, H.; Jørgensen, K.A. Hydrogen-Bonding in Aminocatalysis: From Proline and Beyond. Chem. Eur. J. 2014, 20, 358–368. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Feng, X. Amide-based bifunctional organocatalysts in asymmetric reactions. Chem. Commun. 2009, 41, 6145–6158. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, F.; Yu, L.T.; Cui, X.; Gong, L.Z.; Mi, A.Q.; Jiang, Y.Z. Novel Small Organic Molecules for a Highly Enantioselective Direct Aldol Reaction. J. Am. Chem. Soc. 2003, 125, 5262–5263. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, F.; Cui, X.; Gong, L.-Z.; Mi, A.-Q.; Jiang, Y.-Z.; Wu, Y.-D. Enantioselective direct aldol reactions catalyzed by l-prolinamide derivatives. Proc. Natl. Acad. Sci. USA 2004, 101, 5755–5760. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.H.; Chen, X.H.; Cun, L.F.; Mi, A.Q.; Jiang, Y.Z.; Gong, L.Z. A Highly Efficient Organocatalyst for Direct Aldol Reactions of Ketones with Aldedydes. J. Am. Chem. Soc. 2005, 127, 9285–9289. [Google Scholar] [CrossRef]
- Yang, H.; Carter, R.G. Proline Sulfonamide Based Organocatalysis: Better Late than Never. Synlett 2010, 19, 2827–2838. [Google Scholar] [CrossRef]
- Berkessel, A.; Koch, B.; Lex, J. Proline-Derived N-Sulfonylcarboxamides: Readily Available, Highly Enantioselective and Versatile Catalysts for Direct Aldol Reactions. Adv. Synth. Catal. 2004, 346, 1141–1146. [Google Scholar] [CrossRef]
- Cobb, A.J.A.; Shaw, D.M.; Longbottom, D.A.; Gold, J.B.; Ley, S.V. Organocatalysis with proline derivatives: Improved catalysts for the asymmetric Mannich, nitro-Michael and aldol reactions. Org. Biomol. Chem. 2005, 3, 84–96. [Google Scholar] [CrossRef]
- Bellis, E.; Kokotos, G. 4-Substituted prolines as organocatalysts for aldol reactions. Tetrahedron 2005, 61, 8669–8676. [Google Scholar] [CrossRef]
- Silva, F.; Sawicki, M.; Gouverneur, V. Enantioselective Organocatalytic Aldol Reaction of Ynones and Its Synthetic Applications. Org. Lett. 2006, 8, 5417–5419. [Google Scholar] [CrossRef]
- Sundén, H.; Dahlin, N.; Ibrahem, I.; Adolfsson, H.; Cordova, A. Novel organic catalysts for the direct enantioselective α-oxidation of carbonyl compounds. Tetrahedron Lett. 2005, 46, 3385–3389. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Li, H. A Simple and Efficient l-Prolinamide-Catalyzed α-Selenenylation Reaction of Aldehydes. Org. Lett. 2004, 6, 2817–2820. [Google Scholar] [CrossRef]
- Gryko, D.; Chromiński, M.; Pielacińska, D.J. Prolinethioamides versus Prolinamides in Organocatalyzed Aldol Reactions—A Comparative Study. Symmetry 2011, 3, 265–282. [Google Scholar] [CrossRef]
- Gryko, D.; Lipinski, R. l-Prolinethioamides—Efficient Organocatalysts for the Direct Asymmetric Aldol Reaction. Adv. Synth. Catal. 2005, 347, 1948–1952. [Google Scholar] [CrossRef]
- Almaşi, D.; Alonso, D.A.; Nájera, C. Prolinamides versus Prolinethioamides as Recyclable Catalysts in the Enantioselective Solvent-Free Inter- and Intramolecular Aldol Reactions. Adv. Synth. Catal. 2008, 350, 2467–2472. [Google Scholar] [CrossRef]
- Wang, B.; Chen, G.; Liu, L.; Chang, W.; Li, J. A Novel Proline-Valinol Thioamide Small Organic Molecule for a Highly Enantioselective Direct Aldol Reaction. Adv. Synth. Catal. 2009, 351, 2441–2448. [Google Scholar] [CrossRef]
- Torii, H.; Nakadai, M.; Ishihara, K.; Saito, S.; Yamamoto, H. Asymmetric direct aldol reaction assisted by water and a proline-derived tetrazole catalyst. Angew. Chem. Int. Ed. 2004, 43, 1983–1986. [Google Scholar] [CrossRef]
- Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H. O-nitroso aldol synthesis: Catalytic enantioselective route to α-aminooxy carbonyl compounds via enamine intermediate. Proc. Natl. Acad. Sci. USA 2004, 101, 5374–5378. [Google Scholar] [CrossRef]
- Cobb, A.J.A.; Shaw, D.M.; Ley, S.V. 5-Pyrrolidin-2-yltetrazole: A New, Catalytic, More Soluble Alternative to Proline in an Organocatalytic Asymmetric Mannich-type Reaction. Synlett 2004, 3, 558–560. [Google Scholar] [CrossRef]
- Hartikka, A.; Arvidsson, P.I. 5-(Pyrrolidine-2-yl)tetrazole: Rationale for the Increased Reactivity of the Tetrazole Analogue of Proline in Organocatalyzed Aldol Reactions. Eur. J. Org. Chem. 2005, 2005, 4287–4295. [Google Scholar] [CrossRef]
- Maji, B.; Yamamoto, H. Proline-Tetrazole-Catalyzed Enantioselective N-Nitroso Aldol Reaction of Aldehydes with In Situ Generated Nitrosocarbonyl Compounds. Angew. Chem. Int. Ed. 2014, 53, 8714. [Google Scholar] [CrossRef]
- Hayashi, Y.; Aratake, S.; Okano, T.; Takahashi, J.; Sumiya, T.; Shoji, M. Combined Proline–Surfactant Organocatalyst for the Highly Diastereo- and Enantioselective Aqueous Direct Cross-Aldol Reaction of Aldehydes. Angew. Chem. Int. Ed. 2006, 45, 5527–5529. [Google Scholar] [CrossRef]
- Zlotin, S.G. Hydroxyproline Derivatives as Asymmetric Organocatalysts. In Sustainable Catalysis: Without Metals or Other Endangered Elements, Part 1; Chapter 10; RSC Publishing: Cambridge, UK, 2015; p. 236. [Google Scholar]
- Martin, H.J.; List, B. Mining Sequence Space for Asymmetric Aminocatalysis: N-Terminal Prolyl-Peptides Efficiently Catalyze Enantioselective Aldol and Michael Reactions. Synlett 2003, 12, 1901–1902. [Google Scholar] [CrossRef]
- Hernandez, J.G.; Juaristi, E. Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Dipeptides: Improved Stereoinduction under Solvent-Free Conditions. J. Org. Chem. 2011, 76, 1464–1467. [Google Scholar] [CrossRef]
- Bisticha, A.; Triandafillidi, I.; Kokotos, C.G. tert-Butyl esters of peptides as organocatalysts for the asymmetric aldol reaction. Tetrahedron Asymmetry 2015, 26, 102–108. [Google Scholar] [CrossRef]
- Lei, M.; Shi, L.; Li, G.; Chen, S.; Fang, W.; Ge, Z.; Cheng, T.; Li, R. Dipeptide-catalyzed direct asymmetric aldol reactions in the presence of water. Tetrahedron 2007, 63, 7892–7898. [Google Scholar] [CrossRef]
- Agarwal, J. Progress in aminosugar derived asymmetric organocatalysis. Org. Biomol. Chem. 2016, 14, 10747–10762. [Google Scholar] [CrossRef]
- Yeboah, E.M.O.; Yeboah, S.O.; Singh, G.S. Recent applications of Cinchona alkaloids and their derivatives as catalysts in metal-free asymmetric synthesis. Tetrahedron 2011, 67, 1725–1762. [Google Scholar] [CrossRef]
- Chen, J.-R.; An, X.-L.; Zhu, X.-Y.; Wang, X.-F.; Xiao, W.-J. Rational Combination of Two Privileged Chiral Backbones: Highly Efficient Organocatalysts for Asymmetric Direct Aldol Reactions between Aromatic Aldehydes and Acylic Ketones. J. Org. Chem. 2008, 73, 6006–6009. [Google Scholar] [CrossRef]
- Barrulas, P.; Benaglia, M.; Burke, A.J. Synthesis of novel cinchona-amino acid hybrid organocatalysts for asymmetric catalysis. Tetrahedron Asymmetry 2014, 25, 923–935. [Google Scholar] [CrossRef]
- Guo, H.-M.; Cheng, L.; Cun, L.-F.; Gong, L.-Z.; Mi, A.-Q.; Jiang, Y.-Z. l-Prolinamide-catalyzed direct nitroso aldol reactions of α-branched aldehydes: A distinct regioselectivity from that with l-proline. Chem. Commun. 2006, 429–431. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, H. Design of Acid−Base Catalysis for the Asymmetric Direct Aldol Reaction. Acc. Chem. Res. 2004, 37, 570–579. [Google Scholar] [CrossRef]
- Chen, J.R.; Lu, H.H.; Li, X.Y.; Cheng, L.; Wan, J.; Xiao, W.J. Readily Tunable and Bifunctional l-Prolinamide Derivatives: Design and Application in the Direct Enantioselective Aldol Reactions. Org. Lett. 2005, 7, 4543–4545. [Google Scholar] [CrossRef]
- Gandhi, S.; Singh, V.K. Synthesis of Chiral Organocatalysts derived from Aziridines: Application in Asymmetric Aldol Reaction. J. Org. Chem. 2008, 73, 9411–9416. [Google Scholar] [CrossRef]
- Banon-Caballero, A.; Guillena, G.; Nájera, C. Solvent-free direct enantioselective aldol reaction using polystyrene-supported N-sulfonyl-(Ra)-binam-d-prolinamide as a catalyst. Green Chem. 2010, 12, 1599–1606. [Google Scholar] [CrossRef]
- Bradshaw, B.; Etxebarria-Jardi, G.; Bonjoch, J.; Viozquez, S.F.; Guillena, G.; Nájera, C. Efficient Solvent-Free Robinson Annulation Protocols for the Highly Enantioselective Synthesis of the Wieland–Miescher Ketone and Analogues. Adv. Synth. Catal. 2009, 351, 2482–2490. [Google Scholar] [CrossRef]
- Viozquez, S.F.; Guillena, G.; Nájera, C.; Bradshaw, B.; Etxebarria-Jardi, G.; Bonjoch, J. (Sa,S)-N-[2‘-(4-Methylphenylsulfonamido)-1,1’-Binaphthyl-2-Yl]pyrrolidine-2-Carboxamide: An Organocatalyst for the Direct Aldol Reaction. Org. Synth. 2011, 88, 317–329. [Google Scholar] [CrossRef]
- Connon, S.J. Organocatalysis Mediated by (Thio)urea Derivatives. Chem. Eur. J. 2006, 12, 5418–5427. [Google Scholar] [CrossRef]
- Tsakos, M.; Kokotos, C.G. Primary and secondary amine-(thio)ureas and squaramides and their applications in asymmetric organocatalysis. Tetrahedron 2013, 69, 10199–10222. [Google Scholar] [CrossRef]
- Fotaras, S.; Kokotos, C.G.; Tsandi, E.; Kokotos, G. Prolinamides Bearing Thiourea Groups as Catalysts for Asymmetric Aldol Reactions. Eur. J. Org. Chem. 2011, 2011, 1310–1317. [Google Scholar] [CrossRef]
- Kokotos, C.G. Construction of Tertiary Alcohols Bearing Perfluoroalkyl Chains Catalyzed by Prolinamide-Thioureas. J. Org. Chem. 2012, 77, 1131–1135. [Google Scholar] [CrossRef]
- Narayaperumal, S.; Rivera, D.G.; Silva, R.C.; Paixao, M.W. Terpene-Derived Bifunctional Thioureas in Asymmetric Organocatalysis. ChemCatChem. 2013, 5, 2756–2773. [Google Scholar] [CrossRef]
- Metrano, A.J.; Chinn, A.J.; Shugrue, C.R.; Stone, A.; Kim, B.; Miller, S.J. Asymmetric Catalysis Mediated by Synthetic Peptides, Version 2.0: Expansion of Scope and Mechanisms. Chem. Rev. 2020, 120, 11479–11615. [Google Scholar] [CrossRef]
- Wennemers, H. Asymmetric catalysis with peptides. Chem. Commun. 2011, 47, 12036–12041. [Google Scholar] [CrossRef]
- Davie, E.A.C.; Mennen, S.M.; Xu, Y.; Miller, S.J. Asymmetric Catalysis Mediated by Synthetic Peptides. Chem. Rev. 2007, 107, 5759–5812. [Google Scholar] [CrossRef]
- Rodriguez-Llansola, F.; Miravet, J.F.; Escuder, B. A supramolecular hydrogel as a reusable heterogeneous catalyst for the direct aldol reaction. Chem. Commun. 2009, 47, 7303–7305. [Google Scholar] [CrossRef]
- Hu, X.-M.; Zhang, D.-X.; Zhang, S.-Y.; Wang, P.-A. Highly modular dipeptide-like organocatalysts for direct asymmetric aldol reactions in brine. RSC Adv. 2015, 5, 39557–39564. [Google Scholar] [CrossRef]
- Krattiger, P.; Kovasy, R.; Revell, J.D.; Ivan, S.; Wennemers, H. Increased Structural Complexity Leads to Higher Activity: Peptides as Efficient and Versatile Catalysts for Asymmetric Aldol Reactions. Org. Lett. 2005, 7, 1101–1103. [Google Scholar] [CrossRef]
- D’Elia, V.; Zwicknagl, H.; Reiser, O. Short α/β-Peptides as Catalysts for Intra- and Intermolecular Aldol Reactions. J. Org. Chem. 2008, 73, 3262–3265. [Google Scholar] [CrossRef]
- Agirre, M.; Arrieta, A.; Arrastia, I.; Cossio, F.P. Organocatalysts Derived from Unnatural α-Amino Acids: Scope and Applications. Chem. Asian J. 2019, 14, 44–66. [Google Scholar] [CrossRef]
- List, B.; Coric, I.; Grygornko, O.O.; Kaib, P.S.J.; Komarov, I.; Lee, A.; Leutzsch, M.; Pan, S.C.; Tymtsunik, A.V.; van Gemmeren, M. The Catalytic Asymmetric α-Benzylation of Aldehydes. Angew. Chem. Int. Ed. 2014, 53, 282–285. [Google Scholar] [CrossRef]
- Zhang, H.; Mitsumori, S.; Utsumi, N.; Imai, M.; Garcia-Delgado, N.; Mifsud, M.; Albertshofer, K.; Cheong, P.H.-Y.; Houk, K.N.F.; Tanaka, F.; et al. Catalysis of 3-Pyrrolidinecarboxylic Acid and Related Pyrrolidine Derivatives in Enantioselective anti-Mannich-Type Reactions: Importance of the 3-Acid Group on Pyrrolidine for Stereocontrol. J. Am. Chem. Soc. 2008, 130, 875–886. [Google Scholar] [CrossRef]
- Gómez-Bengoa, E.; Maestro, M.; Mielgo, A.; Otazo, I.; Palomo, C.; Velilla, I. A 4-Hydroxypyrrolidine-Catalyzed Mannich Reaction of Aldehydes: Control of anti-Selectivity by Hydrogen Bonding Assisted by Brønsted Acids. Chem. Eur. J. 2010, 16, 5333–5342. [Google Scholar] [CrossRef]
- Martín-Rapún, R.; Fan, X.; Sayalero, S.; Bahramnejad, M.; Cuevas, F.; Pericàs, M.A. Highly Active Organocatalysts for Asymmetric anti-Mannich Reactions. Chem. Eur. J. 2011, 17, 8780–8783. [Google Scholar] [CrossRef]
- Chuan, Y.-M.; Chen, G.-H.; Gao, J.-Z.; Zhang, H.; Peng, Y.-G. A facile direct anti-selective catalytic asymmetric Mannich reaction of aldehydes with preformed N-Boc and N-Cbz imines. Chem. Commun. 2011, 47, 3260–3262. [Google Scholar] [CrossRef]
- Gao, J.; Chuan, Y.; Li, J.; Xie, F.; Peng, Y. A convenient and mild chromatography-free method for the purification of the products of Wittig and Appel reactions. Org. Biomol. Chem. 2012, 10, 3730–3737. [Google Scholar] [CrossRef]
- Kano, T.; Maruoka, K. Unique properties of chiral biaryl-based secondary aminecatalysts for asymmetric enamine catalysis. Chem. Sci. 2013, 4, 907–915. [Google Scholar] [CrossRef]
- Kano, T.; Yamaguchi, Y.; Tokuda, O.; Maruoka, K. anti-Selective Direct Asymmetric Mannich Reactions Catalyzed by Axially Chiral Amino Sulfonamide as an Organocatalyst. J. Am. Chem. Soc. 2005, 127, 14609–16408. [Google Scholar] [CrossRef]
- Kano, T.; Yamaguchi, Y.; Maruoka, K. A Designer Axially Chiral Amino Sulfonamide as an Efficient Organocatalyst for Direct Asymmetric Mannich Reactions of N-Boc-Protected Imines. Angew. Chem. Int. Ed. 2009, 48, 1838–1840. [Google Scholar] [CrossRef]
- Kano, T.; Yamaguchi, Y.; Tanaka, Y.; Maruoka, K. syn-Selective and Enantioselective Direct Cross-Aldol Reactions between Aldehydes Catalyzed by an Axially Chiral Amino Sulfonamide. Angew. Chem. Int. Ed. 2007, 46, 1768–1770. [Google Scholar] [CrossRef]
- Kano, T.; Takai, J.; Tokuda, O.; Maruoka, K. Design of an Axially Chiral Amino Acid with a Binaphthyl Backbone as an Organocatalyst for a Direct Asymmetric Aldol Reaction. Angew. Chem. Int. Ed. 2005, 44, 3055–3057. [Google Scholar] [CrossRef]
- Kano, T.; Yamaguchi, Y.; Maruoka, K. A Designer Axially Chiral Amino Sulfonamide as an Efficient Organocatalyst for Direct Asymmetric anti-Selective Mannich Reactions and syn-Selective Cross-Aldol Reactions. Chem. Eur. J. 2009, 15, 6678–6687. [Google Scholar] [CrossRef]
- Xu, L.-W.; Luo, J.; Lu, Y. Asymmetric catalysis with chiral primary amine-based organocatalysts. Chem. Commun. 2009, 14, 1807–1821. [Google Scholar] [CrossRef]
- Serdyuk, O.V.; Heckel, C.M.; Tsogoeva, S.B. Bifunctional primary amine-thioureas in asymmetric organocatalysis. Org. Biomol. Chem. 2013, 11, 7051–7071. [Google Scholar] [CrossRef]
- Cordova, A.; Zou, W.; Ibrahem, I.; Reyes, E.; Engqvist, M.; Liao, W.-W. Acyclic amino acid-catalyzed direct asymmetric aldol reactions: Alanine, the simplest stereoselective organocatalyst. Chem. Commun. 2005, 28, 3586–3588. [Google Scholar] [CrossRef]
- Xu, L.-W.; Lu, Y. Primary amino acids: Privileged catalysts in enantioselective organocatalysis. Org. Biomol. Chem. 2008, 6, 2047–2053. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Wang, Z.; Wang, F.; Chen, X.; Liu, X.; Feng, X.; Su, Z.; Hu, C. Asymmetric Direct Aldol Reaction of Functionalized Ketones Catalyzed by Amine Organocatalysts Based on Bispidine. J. Am. Chem. Soc. 2008, 130, 5654–5655. [Google Scholar] [CrossRef]
- Nakayama, K.; Maruoka, K. Complete Switch of Product Selectivity in Asymmetric Direct Aldol Reaction with Two Different Chiral Organocatalysts from a Common Chiral Source. J. Am. Chem. Soc. 2008, 130, 17666–17667. [Google Scholar] [CrossRef]
- Deng, Y.H.; Chen, J.-Q.; He, L.; Kang, T.-R.; Liu, Q.-Z.; Luo, S.-W.; Yuan, W.-C. Highly Enantioselective Aldol Reactions between Acetaldehyde and Activated Acyclic Ketones Catalyzed by Chiral Primary Amines. Chem. Eur. J. 2013, 19, 7143–7150. [Google Scholar] [CrossRef]
- Dziedzic, P.; Zou, W.; Hafren, J.; Cordova, A. The small peptide-catalyzed direct asymmetric aldol reaction in water. Org. Biomol. Chem. 2006, 4, 38–40. [Google Scholar] [CrossRef]
- Wu, F.-C.; Da, C.-S.; Du, Z.-X.; Guo, Q.-P.; Li, W.-P.; Yi, L.; Jia, Y.-N.; Ma, X. N-Primary-Amine-Terminal β-Turn Tetrapeptides as Organocatalysts for Highly Enantioselective Aldol Reaction. J. Org. Chem. 2009, 74, 4812–4818. [Google Scholar] [CrossRef]
- Du, Z.-H.; Tao, B.-X.; Yuan, M.; Qin, W.-J.; Xu, Y.-L.; Wang, P.; Da, C.-S. Peptide-Catalyzed Highly Asymmetric Cross-Aldol Reaction of Aldehydes to Biomimetically Synthesize 1,4-Dicarbonyls. Org. Lett. 2020, 22, 4444–4450. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Cui, H.-L.; Jiang, K.; Du, W.; He, Z.-Q.; Chen, Y.-C. Organocatalytic and Highly Enantioselective Direct α-Amination of Aromatic Ketones. Org. Lett. 2007, 9, 3671–3674. [Google Scholar] [CrossRef]
- Reyes, E.; Uria, U.; Carrillo, L.; Vicario, J.L. The Catalytic Enantioselective Michael reaction. In Organic Reactions; Denmark, S.E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 1–898. [Google Scholar]
- List, B.; Pojarliev, P.; Martin, H.J. Efficient Proline-Catalyzed Michael Additions of Unmodified Ketones to Nitro Olefins. Org. Lett. 2001, 3, 2423–2425. [Google Scholar] [CrossRef]
- Cobb, A.J.A.; Longbottom, D.A.; Shaw, D.M.; Ley, S.V. 5-Pyrrolidin-2-yltetrazole as an asymmetric organocatalyst for the addition of ketones to nitro-olefins. Chem. Commun. 2004, 16, 1808–1809. [Google Scholar] [CrossRef]
- Mitchell, C.E.T.; Cobb, A.J.A.; Ley, S.V. A Homo-Proline Tetrazole as an Improved Organocatalyst for the Asymmetric Michael Addition of Carbonyl Compounds to Nitro-Olefins. Synlett 2005, 4, 611–614. [Google Scholar] [CrossRef]
- Cao, C.-L.; Ye, M.-C.; Sun, X.-L.; Tang, Y. Pyrrolidine−Thiourea as a Bifunctional Organocatalyst: Highly Enantioselective Michael Addition of Cyclohexanone to Nitroolefins. Org. Lett. 2006, 8, 2901–2904. [Google Scholar] [CrossRef]
- Kokotos, C.G.; Limnios, D.; Triggidou, D.; Trifonidou, M.; Kokotos, G. Novel pyrrolidine-thiohydantoins/thioxotetrahydropyrimidinones as highly effective catalysts for the asymmetric Michael addition. Org. Biomol. Chem. 2011, 9, 3386–3395. [Google Scholar] [CrossRef]
- Mahato, C.K.; Mukherjee, S.; Kundu, M.; Pramanik, A. Pyrrolidine-Oxadiazolone Conjugates as Organocatalysts in Asymmetric Michael Reaction. J. Org. Chem. 2019, 84, 1053–1063. [Google Scholar] [CrossRef]
- Kucherenko, A.S.; Lisnyak, V.G.; Kostenko, A.A.; Kochetkov, S.V.; Zlotin, S.G. C 2-Symmetric pyrrolidine-derived squaramides as recyclable organocatalysts for asymmetric Michael reactions. Org. Biomol. Chem. 2016, 14, 9751–9759. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, K.N.; Sharma, E.; Rani, S.P.; Sharma, S.K. Pyrrolidine-carbamate based new and efficient chiral organocatalyst for asymmetric Michael addition of ketones to nitroolefins. Tetrahedron 2018, 74, 6137–6143. [Google Scholar] [CrossRef]
- Reyes-Rangel, G.; Vargas-Caporali, J.; Juaristi, E. Asymmetric Michael addition reaction organocatalyzed by stereoisomeric pyrrolidine sulfinamides under neat conditions. A brief study of self-disproportionation of enantiomers. Tetrahedron 2017, 73, 4707–4718. [Google Scholar] [CrossRef]
- Cruz-Hernández, C.; Martínez-Martínez, E.; Hernández-González, P.E.; Juaristi, E. Synthesis of a New N-Diaminophosphoryl-N′-[(2S)-2-pyrrolidinylmethyl]thiourea as a Chiral Organocatalyst for the Stereoselective Michael Addition of Cyclohexanone to Nitrostyrenes and Chalcones—Application in Cascade Processes for the Synthesis of Polycyclic Systems. Eur. J. Org. Chem. 2018, 2018, 6890–6900. [Google Scholar] [CrossRef]
- Palomo, C.; Vera, S.; Mielgo, A.; Gomez-Bengoa, E. Highly Efficient Asymmetric Michael Addition of Aldehydes to Nitroalkenes Catalyzed by a Simple trans-4-Hydroxyprolylamide. Angew. Chem. Int. Ed. 2006, 45, 5984–5987. [Google Scholar] [CrossRef]
- Wiesner, M.; Upert, G.; Angelici, G.; Wennemers, H. Enamine Catalysis with Low Catalyst Loadings—High Efficiency via Kinetic Studies. J. Am. Chem. Soc. 2010, 132, 6–7. [Google Scholar] [CrossRef]
- Wiesner, M.; Revell, J.D.; Tonazzi, S.; Wennemers, H. Peptide Catalyzed Asymmetric Conjugate Addition Reactions of Aldehydes to Nitroethylene—A Convenient Entry into γ2-Amino Acids. J. Am. Chem. Soc. 2008, 130, 5610–5611. [Google Scholar] [CrossRef]
- Duschmale, J.; Wennemers, H. Adapting to Substrate Challenges: Peptides as Catalysts for Conjugate Addition Reactions of Aldehydes to α,β-Disubstituted Nitroolefins. Chem. Eur. J. 2012, 18, 1111–1120. [Google Scholar] [CrossRef]
- Kastl, R.; Wennemers, H. Peptide-Catalyzed Stereoselective Conjugate Addition Reactions Generating All-Carbon Quaternary Stereogenic Centers. Angew. Chem. Int. Ed. 2013, 52, 7228–7232. [Google Scholar] [CrossRef]
- Wiesner, M.; Revell, J.D.; Wennemers, H. Tripeptides as Efficient Asymmetric Catalysts for 1,4-Addition Reactions of Aldehydes to Nitroolefins–A Rational Approach. Angew. Chem. Int. Ed. 2008, 47, 1871–1874. [Google Scholar] [CrossRef]
- Wiesner, M.; Neuburger, M.; Wennemers, H. Tripeptides of the Type H-D-Pro-Pro-Xaa-NH2 as Catalysts for Asymmetric 1,4-Addition Reactions: Structural Requirements for High Catalytic Efficiency. Chem. Eur. J. 2009, 15, 10103–10109. [Google Scholar] [CrossRef]
- Bachle, F.; Duschmale, J.; Ebner, C.; Pfaltz, A.; Wennemers, H. Organocatalytic Asymmetric Conjugate Addition of Aldehydes to Nitroolefins: Identification of Catalytic Intermediates and the Stereoselectivity-Determining Step by ESI-MS. Angew. Chem. Int. Ed. 2013, 52, 12619–12623. [Google Scholar] [CrossRef]
- Schnitzer, T.; Wennemers, H. Influence of the Trans/Cis Conformer Ratio on the Stereoselectivity of Peptidic Catalysts. J. Am. Chem. Soc. 2017, 139, 15356–15362. [Google Scholar] [CrossRef]
- Schnitzer, T.; Wennemers, H. Effect of γ-Substituted Proline Derivatives on the Performance of the Peptidic Catalyst H-dPro-Pro-Glu-NH2. Synthesis 2018, 50, 4377–4382. [Google Scholar] [CrossRef]
- Siebler, C.; Maryasin, B.; Kuemin, M.; Erdmann, R.S.; Rigling, C.; Grunenfelder, C.; Ochsenfeld, C.; Wennemers, H. Importance of dipole moments and ambient polarity for the conformation of Xaa–Pro moieties—A combined experimental and theoretical study. Chem. Sci. 2015, 6, 6725–6730. [Google Scholar] [CrossRef]
- Rigling, C.; Kisunzu, J.K.; Duschmale, J.; Haussinger, D.; Wiesner, M.; Ebert, M.-O.; Wennemers, H. Conformational Properties of a Peptidic Catalyst: Insights from NMR Spectroscopic Studies. J. Am. Chem. Soc. 2018, 140, 10829–10838. [Google Scholar] [CrossRef]
- Duschmale, J.; Wiest, J.; Wiesner, M.; Wennemers, H. Effects of internal and external carboxylic acids on the reaction pathway of organocatalytic 1,4-addition reactions between aldehydes and nitroolefins. Chem. Sci. 2013, 4, 1312–1318. [Google Scholar] [CrossRef]
- Schnitzer, T.; Mohler, J.S.; Wennemers, H. Effect of the enamine pyramidalization direction on the reactivity of secondary amine organocatalysts. Chem. Sci. 2020, 11, 1943–1947. [Google Scholar] [CrossRef]
- Bures, J.; Armstrong, A.; Blackmond, D.G. Explaining Anomalies in Enamine Catalysis: “Downstream Species” as a New Paradigm for Stereocontrol. Acc. Chem. Res. 2016, 49, 214–222. [Google Scholar] [CrossRef]
- Znabet, A.; Ruijter, E.; de Kanter, F.J.J.; Köhler, V.; Helliwell, M.; Turner, N.J.; Orru, R.V.A. Highly Stereoselective Synthesis of Substituted Prolyl Peptides Using a Combination of Biocatalytic Desymmetrization and Multicomponent Reactions. Angew. Chem. Int. Ed. 2010, 49, 5289–5292. [Google Scholar] [CrossRef]
- Cortes-Clerget, M.; Gager, O.; Monteil, M.; Pirat, J.-L.; Migianu-Griffoni, E.; Deschamp, J.; Lecouvey, M. Novel Easily Recyclable Bifunctional Phosphonic Acid Carrying Tripeptides for the Stereoselective Michael Addition of Aldehydes with Nitroalkenes. Adv. Synth. Catal. 2016, 358, 34–40. [Google Scholar] [CrossRef]
- Durini, M.; Sahr, F.A.; Kuhn, M.; Civera, M.; Gennari, C.; Piarulli, U. Bifunctional 2,5-Diketopiperazines as Efficient Organocatalysts for the Enantioselective Conjugate Addition of Aldehydes to Nitroolefins. Eur. J. Org. Chem. 2011, 2011, 5599–5607. [Google Scholar] [CrossRef]
- Tsogoeva, S.B.; Jagtap, S. Dual Catalyst Control in the Chiral Diamine-Dipeptide-Catalyzed Asymmetric Michael Addition. Synlett 2004, 14, 2624–2626. [Google Scholar] [CrossRef]
- de la Torre, A.F.; Rivera, D.G.; Ferreira, M.A.B.; Correia, A.G.; Paixao, M.W. Multicomponent Combinatorial Development and Conformational Analysis of Prolyl Peptide–Peptoid Hybrid Catalysts: Application in the Direct Asymmetric Michael Addition. J. Org. Chem. 2013, 78, 10221–10232. [Google Scholar] [CrossRef]
- Schnitzer, T.; Budinská, A.; Wennemers, H. Organocatalysed conjugate addition reactions of aldehydes to nitroolefins with anti selectivity. Nat. Catal. 2020, 3, 143–147. [Google Scholar] [CrossRef]
- Tsogoeva, S.B.; Wei, S. Highly enantioselective addition of ketones to nitroolefins catalyzed by new thiourea–amine bifunctional organocatalysts. Chem. Commun. 2006, 13, 1451–1453. [Google Scholar] [CrossRef]
- Yalalov, D.A.; Tsogoeva, S.B.; Schmatz, S. Chiral Thiourea-Based Bifunctional Organocatalysts in the Asymmetric Nitro-Michael Addition: A Joint Experimental-Theoretical Study. Adv. Synth. Catal. 2006, 348, 826–832. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Coeffard, V.; Moreau, X.; Greck, C. Asymmetric organocatalytic functionalization of α,α-disubstituted aldehydes through enamine activation. Tetrahedron 2014, 70, 2491–2513. [Google Scholar] [CrossRef]
- Lalonde, M.P.; Chen, Y.; Jacobsen, E.N. A Chiral Primary Amine Thiourea Catalyst for the Highly Enantioselective Direct Conjugate Addition of α,α-Disubstituted Aldehydes to Nitroalkenes. Angew. Chem. Int. Ed. 2006, 45, 6366–6370. [Google Scholar] [CrossRef]
- Huang, H.; Jacobsen, E.N. Highly Enantioselective Direct Conjugate Addition of Ketones to Nitroalkenes Promoted by A Chiral Primary Amine−Thiourea Catalyst. J. Am. Chem. Soc. 2006, 128, 7170–7171. [Google Scholar] [CrossRef]
- Grînenfelder, C.E.; Kisunzu, J.K.; Wennemers, H. Peptide-Catalyzed Stereoselective Conjugate Addition Reactions of Aldehydes to Maleimide. Angew. Chem. Int. Ed. 2016, 55, 8571–8574. [Google Scholar] [CrossRef]
- Xue, F.; Liu, L.; Zhang, S.; Duan, W.; Wang, W. A Simple Primary Amine Thiourea Catalyzed Highly Enantioselective Conjugate Addition of α,α-Disubstituted Aldehydes to Maleimides. Chem. Eur. J. 2010, 16, 7979–7982. [Google Scholar] [CrossRef]
- Sunden, E.; Eriksson, L.; Sayah, M.; Cordova, A. Organocatalytic enantioselective conjugate addition of aldehydes to maleimides. Chem. Commun. 2007, 7, 734–735. [Google Scholar] [CrossRef]
- Kokotos, C.G. An Asymmetric Michael Addition of α,α-Disubstituted Aldehydes to Maleimides Leading to a One-Pot Enantioselective Synthesis of Lactones Catalyzed by Amino Acids. Org. Lett. 2013, 15, 2406–2409. [Google Scholar] [CrossRef]
- Cao, C.-L.; Sun, X.-L.; Zhou, J.-L.; Tang, Y. Enantioselectively Organocatalytic Michael Addition of Ketones to Alkylidene Malonates. J. Org. Chem. 2007, 72, 4073–4076. [Google Scholar] [CrossRef]
- Faísca Phillips, A.M.; Barros, M.T. Synthesis of geminal bisphosphonates via organocatalyzed enantioselective Michael additions of cyclic ketones and 4-piperidones. Org. Biomol. Chem. 2012, 10, 404–412. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Liu, X.; Wang, Z.; Liu, Y.; Bai, S.; Lin, L.; Feng, X. Organocatalyzed highly stereoselective Michael addition of ketones to alkylidene malonates and nitroolefins using chiral primary-secondary diamine catalysts based on bispidine. Org. Biomol. Chem. 2009, 7, 4120–4127. [Google Scholar] [CrossRef]
- Zhao, G.-L.; Vesely, J.; Sun, J.; Christensen, K.E.; Bonneau, C.; Cordova, A. Organocatalytic Highly Enantioselective Conjugate Addition of Aldehydes to Alkylidine Malonates. Adv. Synth. Catal. 2008, 350, 657–661. [Google Scholar] [CrossRef]
- Kano, T.; Shirozu, F.; Akakura, M.; Maruoka, K. Powerful Amino Diol Catalyst for Effecting the Direct Asymmetric Conjugate Addition of Aldehydes to Acrylates. J. Am. Chem. Soc. 2012, 134, 16068–16073. [Google Scholar] [CrossRef]
- Kang, J.Y.; Carter, R.G. Primary Amine, Thiourea-Based Dual Catalysis Motif for Synthesis of Stereogenic, All-Carbon Quaternary Center-Containing Cycloalkanones. Org. Lett. 2002, 14, 3178–3181. [Google Scholar] [CrossRef]
- Girvin, Z.C.; Lampkin, P.P.; Liu, X.; Gellman, S.H. Catalytic Intramolecular Conjugate Additions of Aldehyde-Derived Enamines to α,β-Unsaturated Esters. Org. Lett. 2020, 22, 4568–4573. [Google Scholar] [CrossRef]
- Schreiner, P.R. Metal-free organocatalysis through explicit hydrogen bonding interactions. Chem. Soc. Rev. 2003, 32, 289–296. [Google Scholar] [CrossRef]
- Zhang, Z.; Schreiner, P.R. (Thio)urea organocatalysis—What can be learnt from anion recognition? Chem. Soc. Rev. 2009, 38, 1187–1198. [Google Scholar] [CrossRef]
- Gotoh, H.; Hayashi, Y. Diarylprolinol silyl ethers: Development and application as organocatalysts. In Sustainable Catalysis; Dunn, P.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 287–316. [Google Scholar]
- Donslund, B.S.; Johansen, T.K.; Poulsen, P.H.; Halskov, K.S.; Jørgensen, K.A. The Diarylprolinol Silyl Ethers: Ten Years After. Angew. Chem. Int. Ed. 2015, 54, 13860–13874. [Google Scholar] [CrossRef]
- Meninno, S.; Volpe, C.; Lattanzi, A. Diaryl Prolinols in Stereoselective Catalysis and Synthesis: An Update. ChemCatChem 2019, 11, 3716–3729. [Google Scholar] [CrossRef]
- Jensen, K.L.; Dickmeiss, G.; Jiang, H.; Albrecht, Ł.; Jørgensen, K.A. The Diarylprolinol Silyl Ether System: A General Organocatalyst. Acc. Chem. Res. 2012, 45, 248–264. [Google Scholar] [CrossRef]
- Palomo, C.; Mielgo, A. Diarylprolinol Ethers: Expanding the Potential of Enamine/Iminium-Ion Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7876–7880. [Google Scholar] [CrossRef]
- Vega-Peñaloza, A.; Paria, S.; Bonchio, M.; Dell’Amico, L.; Companyó, X. Profiling the Privileges of Pyrrolidine-Based Catalysts in Asymmetric Synthesis: From Polar to Light-Driven Radical Chemistry. ACS Catal. 2019, 9, 6058–6072. [Google Scholar] [CrossRef]
- Reyes-Rodríguez, G.J.; Rezayee, N.M.; Vidal-Albalat, A.; Jørgensen, K.A. Prevalence of Diarylprolinol Silyl Ethers as Catalysts in Total Synthesis and Patents. Chem. Rev. 2019, 119, 4221–4260. [Google Scholar] [CrossRef]
- Nielsen, M.; Worgull, D.; Zweifel, T.; Gschwend, B.; Bertelsen, S.; Jørgensen, K.A. Mechanisms in aminocatalysis. Chem. Commun. 2011, 47, 632–649. [Google Scholar] [CrossRef]
- Halskov, K.S.; Donslund, B.S.; Paz, B.M.; Jørgensen, K.A. Computational Approach to Diarylprolinol-Silyl Ethers in Aminocatalysis. Acc. Chem. Res. 2016, 49, 974–986. [Google Scholar] [CrossRef]
- Renzi, P.; Hioe, J.; Gschwind, R.M. Enamine/Dienamine and Brønsted Acid Catalysis: Elusive Intermediates, Reaction Mechanisms, and Stereoinduction Modes Based on in Situ NMR Spectroscopy and Computational Studies. Acc. Chem. Res. 2017, 50, 2936–2948. [Google Scholar] [CrossRef]
- Fuson, R.C. The Principle of Vinylogy. Chem. Rev. 1935, 16, 1–27. [Google Scholar] [CrossRef]
- Arceo, E.; Melchiorre, P. Extending the Aminocatalytic HOMO-Raising Activation Strategy: Where Is the Limit? Angew. Chem. Int. Ed. 2012, 51, 5290–5292. [Google Scholar] [CrossRef]
- Jurberg, I.D.; Chatterjee, I.; Tannert, R.; Melchiorre, P. When asymmetric aminocatalysis meets the vinylogy principle. Chem. Commun. 2013, 49, 4869–4883. [Google Scholar] [CrossRef]
- Jiang, H.; Albrecht, Ł.; Jørgensen, K.A. Aminocatalytic remote functionalization strategies. Chem. Sci. 2013, 4, 2287–2300. [Google Scholar] [CrossRef]
- Bertelsen, S.; Marigo, M.; Brandes, S.; Diner, P.; Jørgensen, K.A. Dienamine Catalysis: Organocatalytic Asymmetric γ-Amination of α,β-Unsaturated Aldehydes. J. Am. Chem. Soc. 2006, 128, 12973–12980. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Reddy, Y.V. Dienamine Catalysis: An Emerging Technology in Organic Synthesis. Eur. J. Org. Chem. 2012, 2012, 865–887. [Google Scholar] [CrossRef]
- Marcos, V.; Alemán, J. Old tricks, new dogs: Organocatalytic dienamine activation of α,β-unsaturated aldehydes. Chem. Soc. Rev. 2016, 45, 6812–6832. [Google Scholar] [CrossRef]
- Kumar, I.; Ramaraju, P.; Mir, N.A. Asymmetric trienamine catalysis: New opportunities in amine catalysis. Org. Biomol. Chem. 2013, 11, 709–716. [Google Scholar] [CrossRef]
- Albrecht, Ł.; Dickmeiss, G.; Cruz Acosta, F.; Rodriguez-Escrich, C.; Davis, R.L.; Jørgensen, K.A. Asymmetric Organocatalytic Formal [2 + 2]-Cycloadditions via Bifunctional H-Bond Directing Dienamine Catalysis. J. Am. Chem. Soc. 2012, 134, 2543–2546. [Google Scholar] [CrossRef]
- Albrecht, Ł.; Dickmeiss, G.; Weise, C.F.; Rodriguez-Escrich, C.; Jørgensen, K.A. Dienamine-Mediated Inverse-Electron-Demand Hetero-Diels–Alder Reaction by Using an Enantioselective H-Bond-Directing Strategy. Angew. Chem. Int. Ed. 2012, 51, 13109–13113. [Google Scholar] [CrossRef]
- Weise, C.F.; Lauridsen, V.H.; Rambo, R.S.; Iversen, E.H.; Olsen, M.-L.; Jørgensen, K.A. Organocatalytic Access to Enantioenriched Dihydropyran Phosphonates via an Inverse-Electron-Demand Hetero-Diels–Alder Reaction. J. Org. Chem. 2014, 79, 3537–3546. [Google Scholar] [CrossRef]
- Orue, A.; Uria, U.; Reyes, E.; Carrillo, L.; Vicario, J.L. Catalytic Enantioselective [5+2] Cycloaddition between Oxidopyrylium Ylides and Enals under Dienamine Activation. Angew. Chem. Int. Ed. 2015, 54, 3043–3046. [Google Scholar] [CrossRef]
- Roca Lopez, D.; Uria, U.; Reyes, E.; Carrillo, L.; Jørgensen, K.A.; Vicario, J.L.; Merino, P. Mechanistic Insights into the Mode of Action of Bifunctional Pyrrolidine-Squaramide-Derived Organocatalysts. Chem. Eur. J. 2016, 22, 884–889. [Google Scholar] [CrossRef]
- Xiao, W.; Yin, X.; Zhou, Z.; Du, W.; Chen, Y.-C. Asymmetric α,γ-Regioselective [3 + 3] Formal Cycloadditions of α,β-Unsaturated Aldehydes via Cascade Dienamine–Dienamine Catalysis. Org. Lett. 2016, 18, 116–119. [Google Scholar] [CrossRef]
- Talavera, G.; Reyes, E.; Vicario, J.L.; Carrillo, L. Cooperative Dienamine/Hydrogen-Bonding Catalysis: Enantioselective Formal [2+2] Cycloaddition of Enals with Nitroalkenes. Angew. Chem. Int. Ed. 2012, 51, 4104–4107. [Google Scholar] [CrossRef]
- Bergonzini, G.; Vera, S.; Melchiorre, P. Cooperative Organocatalysis for the Asymmetric γ Alkylation of α-Branched Enals. Angew. Chem. Int. Ed. 2010, 49, 9685–9688. [Google Scholar] [CrossRef]
- Bencivenni, G.; Galzerano, P.; Mazzanti, A.; Bartoli, G.; Melchiorre, P. Direct asymmetric vinylogous Michael addition of cyclic enones to nitroalkenes via dienamine catalysis. Proc. Natl. Acad. Sci. USA 2010, 107, 20642–20647. [Google Scholar] [CrossRef]
- Zou, C.; Zeng, C.; Liu, Z.; Lu, M.; Sun, X.; Ye, J. γ′-Selective Functionalization of Cyclic Enones: Construction of a Chiral Quaternary Carbon Center by [4+2] Cycloaddition/Retro-Mannich Reaction with 3-Substituted Maleimides. Angew. Chem. Int. Ed. 2016, 55, 14257–14261. [Google Scholar] [CrossRef]
- Tian, X.; Melchiorre, P. Control of Remote Stereochemistry in the Synthesis of Spirocyclic Oxindoles: Vinylogous Organocascade Catalysis. Angew. Chem. Int. Ed. 2013, 52, 5360–5363. [Google Scholar] [CrossRef]
- Dilorio, D.; Righi, P.; Mazzanti, A.; Mancinelli, M.; Ciogli, A.; Bencivenni, G. Remote Control of Axial Chirality: Aminocatalytic Desymmetrization of N-Arylmaleimides via Vinylogous Michael Addition. J. Am. Chem. Soc. 2014, 136, 10250–10253. [Google Scholar] [CrossRef]
- Gu, X.; Guo, T.; Dai, Y.; Franchino, A.; Fei, J.; Zou, C.; Dixon, D.J.; Ye, J. Direct Catalytic Asymmetric Doubly Vinylogous Michael Addition of α,β-Unsaturated γ-Butyrolactams to Dienones. Angew. Chem. Int. Ed. 2015, 54, 10249–10253. [Google Scholar] [CrossRef]
- Zhan, G.; He, Q.; Yuan, X.; Chen, Y.-C. Asymmetric Direct Vinylogous Michael Additions of Allyl Alkyl Ketones to Maleimides through Dienamine Catalysis. Org. Lett. 2014, 16, 6000–6003. [Google Scholar] [CrossRef]
- Bastida, D.; Liu, Y.; Tian, X.; Escuder-Adan, E.; Melchiorre, P. Asymmetric Vinylogous Aldol Reaction via H-Bond-Directing Dienamine Catalysis. Org. Lett. 2013, 15, 220–223. [Google Scholar] [CrossRef]
- Jia, Z.-J.; Zhou, Q.; Zhou, Q.-Q.; Chen, P.-Q.; Chen, Y.-C. exo-Selective Asymmetric Diels–Alder Reaction of 2,4-Dienals and Nitroalkenes by Trienamine Catalysis. Angew. Chem. Int. Ed. 2011, 50, 8638–8641. [Google Scholar] [CrossRef]
- Prieto, L.; Talavera, G.; Uria, U.; Reyes, E.; Vicario, J.L.; Carrillo, L. Favoring Trienamine Activation through Unconjugated Dienals: Organocatalytic Enantioselective Remote Functionalization of Alkenes. Chem. Eur. J. 2014, 20, 2145–2148. [Google Scholar] [CrossRef]
- Lee, J.H.; Deng, L. Asymmetric Approach toward Chiral Cyclohex-2-enones from Anisoles via an Enantioselective Isomerization by a New Chiral Diamine Catalyst. J. Am. Chem. Soc. 2012, 134, 18209–18212. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Momiyama, N.; Yamamoto, H. Enantioselective Tandem O-Nitroso Aldol/Michael Reaction. J. Am. Chem. Soc. 2004, 126, 5962–5963. [Google Scholar] [CrossRef]
- Momiyama, N.; Yamamoto, Y.; Yamamoto, H. Diastereo- and Enantioselective Synthesis of Nitroso Diels−Alder-Type Bicycloketones Using Dienamine: Mechanistic Insight into Sequential Nitroso Aldol/Michael Reaction and Application for Optically Pure 1-Amino-3,4-diol Synthesis. J. Am. Chem. Soc. 2007, 129, 1190–1195. [Google Scholar] [CrossRef]
- Sundén, H.; Ibrahem, I.; Eriksson, L.; Cordova, A. Direct Catalytic Enantioselective Aza-Diels–Alder Reactions. Angew. Chem. Int. Ed. 2005, 44, 4877–4880. [Google Scholar] [CrossRef]
- Itoh, T.; Yokoya, M.; Miyauchi, K.; Nagata, K.; Ohsawa, A. Total Synthesis of ent-Dihydrocorynantheol by Using a Proline-Catalyzed Asymmetric Addition Reaction. Org. Lett. 2006, 8, 1533–1535. [Google Scholar] [CrossRef]
- Yadav, J.; Pawar, A.P.; Nagare, Y.K.; Iype, E.; Rangan, K.; Ohshita, J.; Kumar, D.; Kumar, I. Direct Amine-Catalyzed Enantioselective Synthesis of Pentacyclic Dibenzo[b,f][1,4]oxazepine/Thiazepine-Fused Isoquinuclidines along with DFT Calculations. J. Org. Chem. 2020, 85, 14094–14108. [Google Scholar] [CrossRef]
- Yang, H.; Carter, R.C. Asymmetric Construction of Nitrogen-Containing [2.2.2] Bicyclic Scaffolds Using N-(p-Dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide. J. Org. Chem. 2009, 74, 5151–5156. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Xia, A.-B.; Luo, S.-P.; Tang, J.; Zhang, S.; Jiang, J.-R.; Xu, Z.-Y. In Situ Enamine Activation in Aqueous Salt Solutions: Highly Efficient Asymmetric Organocatalytic Diels–Alder Reaction of Cyclohexenones with Nitroolefins. Angew. Chem. Int. Ed. 2009, 48, 3821–3824. [Google Scholar] [CrossRef]
- Sundén, H.; Rios, R.; Xu, Y.; Eriksson, L.; Cordova, A. Direct Enantioselective Synthesis of Bicyclic Diels–Alder Products. Adv. Synth. Catal. 2007, 349, 2549–2555. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, Z.; Zhou, R.; Zhou, Q.-Q.; Dong, L.; Chen, Y.-C. Stereodivergence in Amine-Catalyzed Regioselective [4 + 2] Cycloadditions of β-Substituted Cyclic Enones and Polyconjugated Malononitriles. J. Am. Chem. Soc. 2012, 134, 19942–19947. [Google Scholar] [CrossRef]
- Xia, A.-B.; Xu, D.-Q.; Wu, C.; Zhao, L.; Xu, Z.-Y. Organocatalytic Diels–Alder Reactions Catalysed by Supramolecular Self-Assemblies Formed from Chiral Amines and Poly(alkene glycol)s. Chem. Eur. J. 2012, 18, 1055–1059. [Google Scholar] [CrossRef]
- Cui, H.-L.; Tanaka, F. Catalytic Enantioselective Formal Hetero-Diels–Alder Reactions of Enones with Isatins to Give Spirooxindole Tetrahydropyranones. Chem. Eur. J. 2013, 19, 6213–6216. [Google Scholar] [CrossRef]
- Utsumi, N.; Zhang, H.; Tanaka, F.; Barbas, C.F., III. A Way to Highly Enantiomerically Enriched aza-Morita–Baylis–Hillman–Type Products. Angew. Chem. Int. Ed. 2007, 46, 1878–1880. [Google Scholar] [CrossRef]
- Vesely, J.; Dziedzic, P.; Cordova, A. Aza-Morita–Baylis–Hillman-type reactions: Highly enantioselective organocatalytic addition of unmodified α,β-unsaturated aldehydes to N-Boc protected imines. Tetrahedron Lett. 2007, 48, 6900–6904. [Google Scholar] [CrossRef]
- Číhalová, S.; Dziedzic, P.; Cordova, A.; Veselý, J. Asymmetric Aza-Morita–Baylis–Hillman-Type Reactions: The Highly Enantioselective Reaction between Unmodified α,β- Unsaturated Aldehydes and N-Acylimines by Organo-co-catalysis. Adv. Synth. Catal. 2011, 353, 1096–1108. [Google Scholar] [CrossRef]
- Maity, S.; Sar, S.; Ghorai, P. Primary Aminothiourea-Catalyzed Enantioselective Synthesis of Rauhut–Currier Adducts of 3-Arylcyclohexenone with a Tethered Enone on the Aryl Moiety at the Ortho-Position. Org. Lett. 2018, 20, 1707–1711. [Google Scholar] [CrossRef]
- Albrecht, Ł.; Acosta, F.C.; Fraile, A.; Albrecht, A.; Christensen, J.; Jørgensen, K.A. Enantioselective H-Bond-Directing Approach for Trienamine-mediated Reactions in Asymmetric Synthesis. Angew. Chem. Int. Ed. 2012, 51, 9088–9092. [Google Scholar] [CrossRef]
- Albrecht, A.; Skrzynska, A.; Pietrzak, A.; Bojanowski, J.; Albrecht, Ł. Asymmetric Aminocatalysis in the Synthesis of δ-Lactone Derivatives. Asian J. Org. Chem. 2016, 5, 1115–1119. [Google Scholar] [CrossRef]
- Monleon, A.; Glaus, F.; Vergura, S.; Jørgensen, K.A. Organocatalytic Strategy for the Enantioselective Cycloaddition to Trisubstituted Nitroolefins to Create Spirocyclohexene-Oxetane Scaffolds. Angew. Chem. Int. Ed. 2016, 55, 2478–2482. [Google Scholar] [CrossRef]
- Jiang, H.; Rodriguez-Escrich, C.; Johansen, T.K.; Davis, R.L.; Jørgensen, K.A. Organocatalytic Activation of Polycyclic Aromatic Compounds for Asymmetric Diels–Alder Reactions. Angew. Chem. Int. Ed. 2012, 51, 10271–10274. [Google Scholar] [CrossRef]
- He, X.-L.; Zhao, H.-R.; Duan, C.-Q.; Du, W.; Chen, Y.-C. Remote Asymmetric Oxa-Diels–Alder Reaction of 5-Allylic Furfurals via Dearomatizative Tetraenamine Catalysis. Org. Lett. 2018, 20, 804–807. [Google Scholar] [CrossRef]
- Xu, C.-J.; Li, H.-W.; He, X.-L.; Du, W.; Chen, Y.-C. Asymmetric Direct Remote Michael Addition Reactions of Allyl Furfurals via Dearomative Trienamine and Tetraenamine Catalysis. Asian J. Org. Chem. 2019, 8, 1037–1040. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).