In Situ Entrapment of Catalase within Macroporous Cryogel Matrix for Ethanol Oxidation: Flow-through Mode versus Batch Reactor
Abstract
1. Introduction
2. Results and Discussion
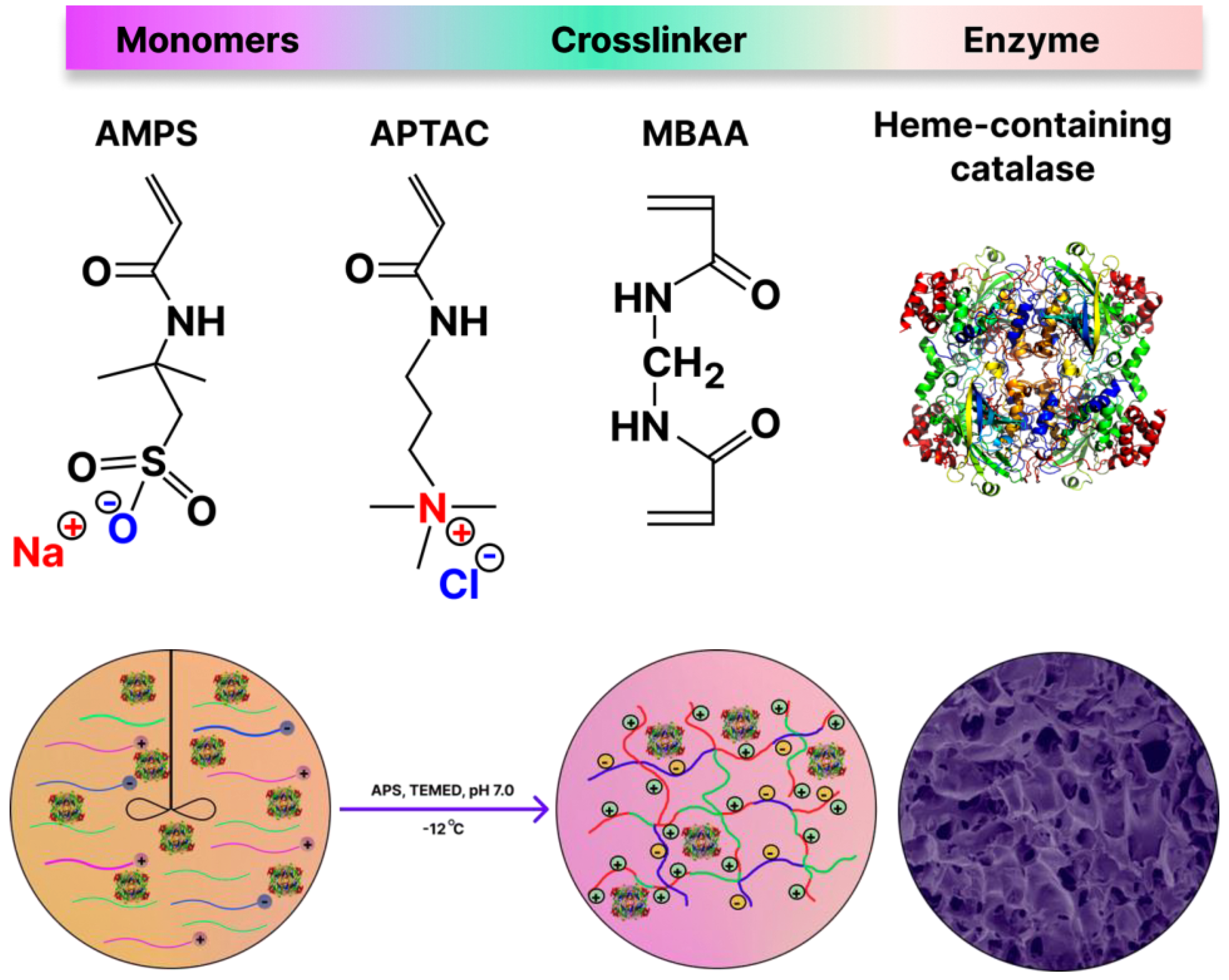
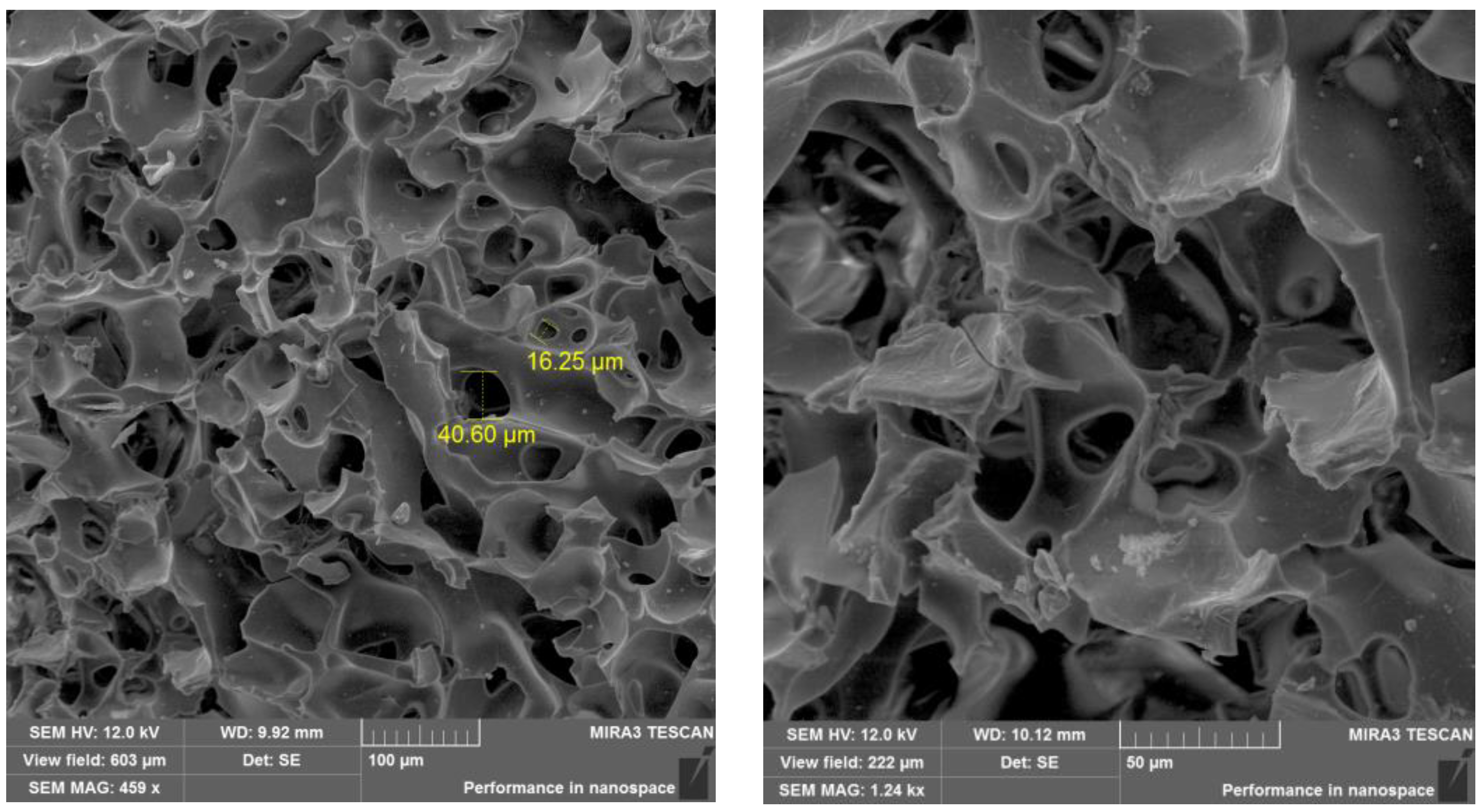
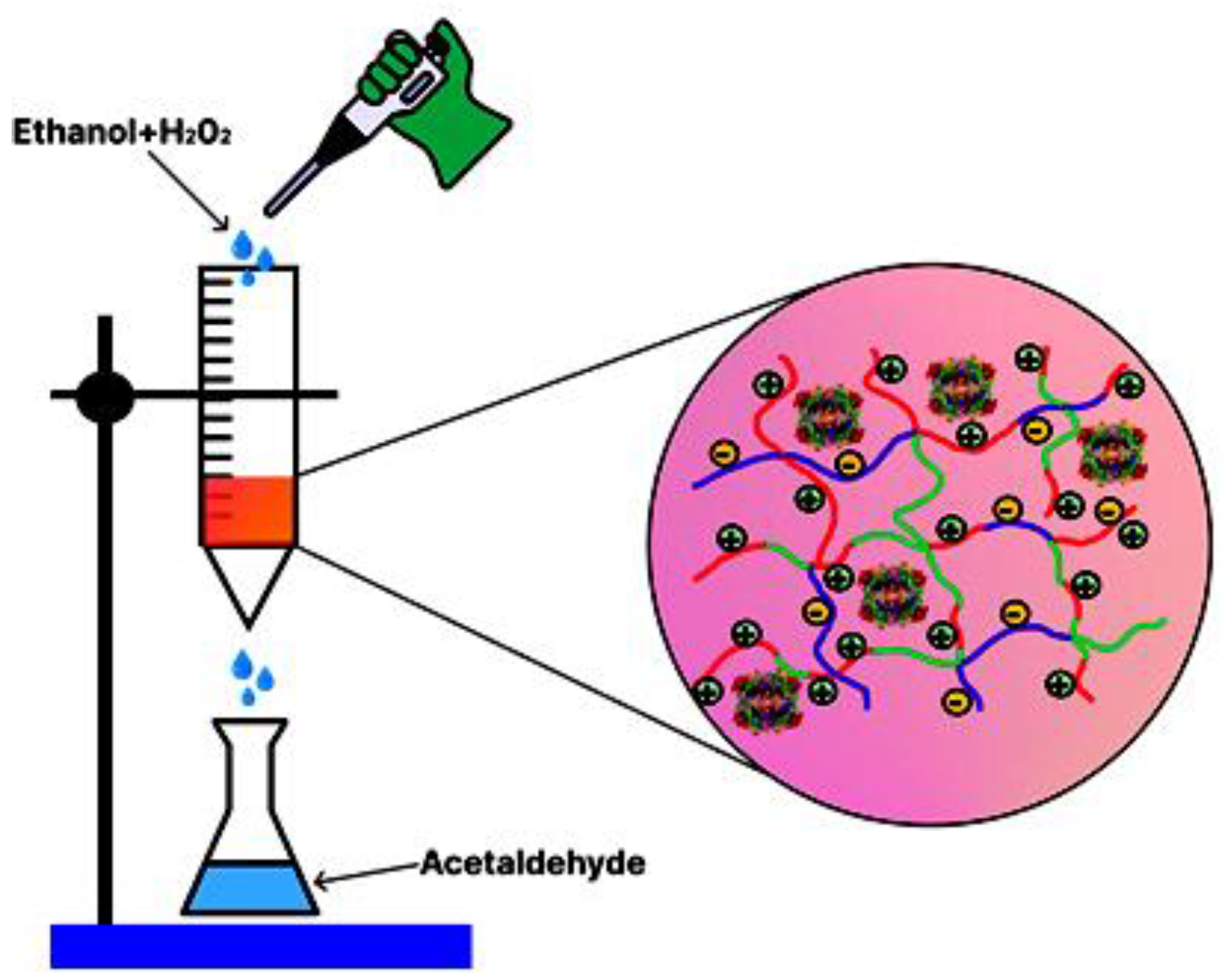
2.1. Preparation and Characterization of Biocatalyst
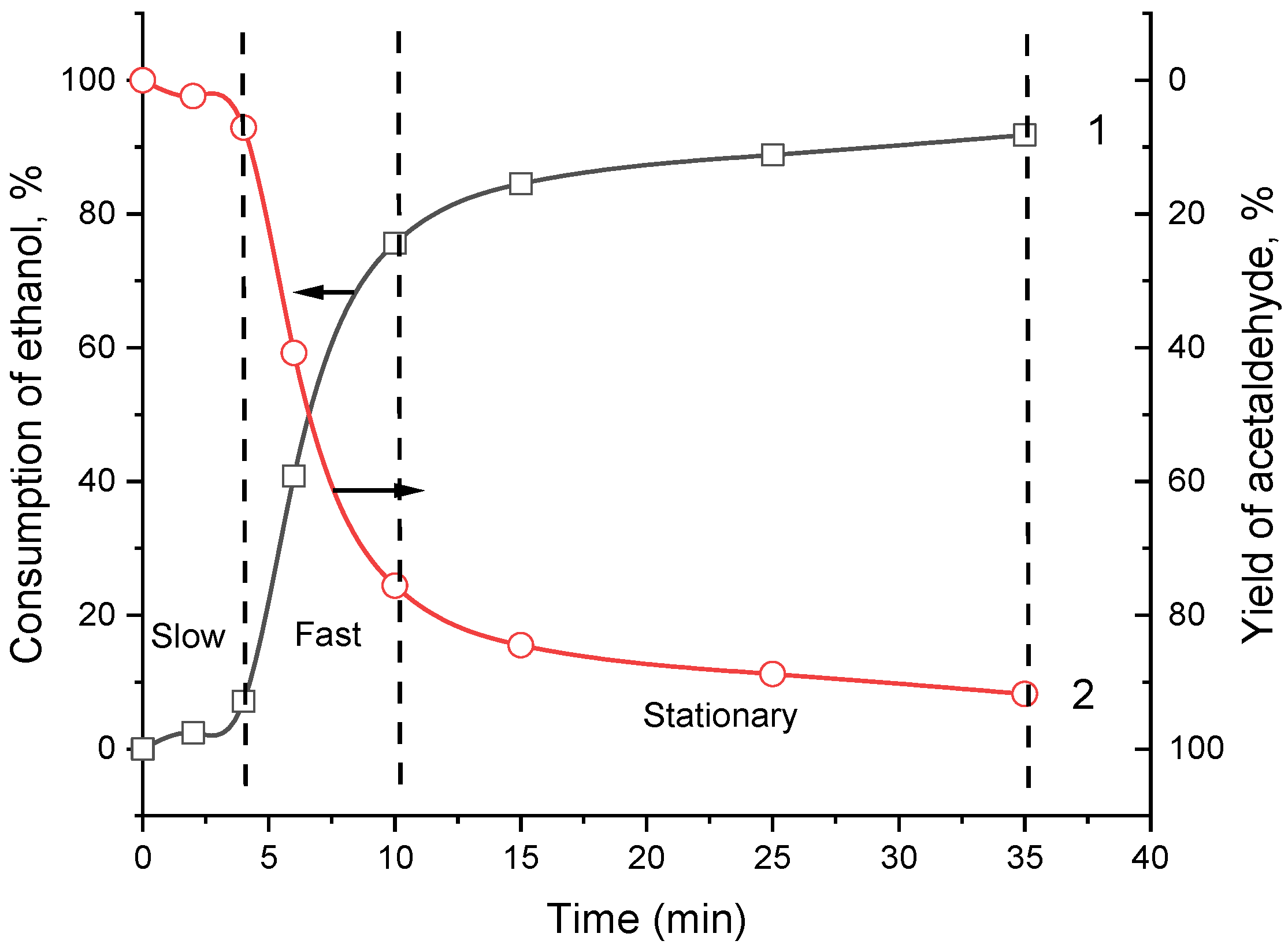
2.2. Catalytic Oxidation of Ethanol Using Catalase Immobilized within the Monolithic Cryogel p(APTAC-co-AMPS) in a Flow-through Catalytic Reactor
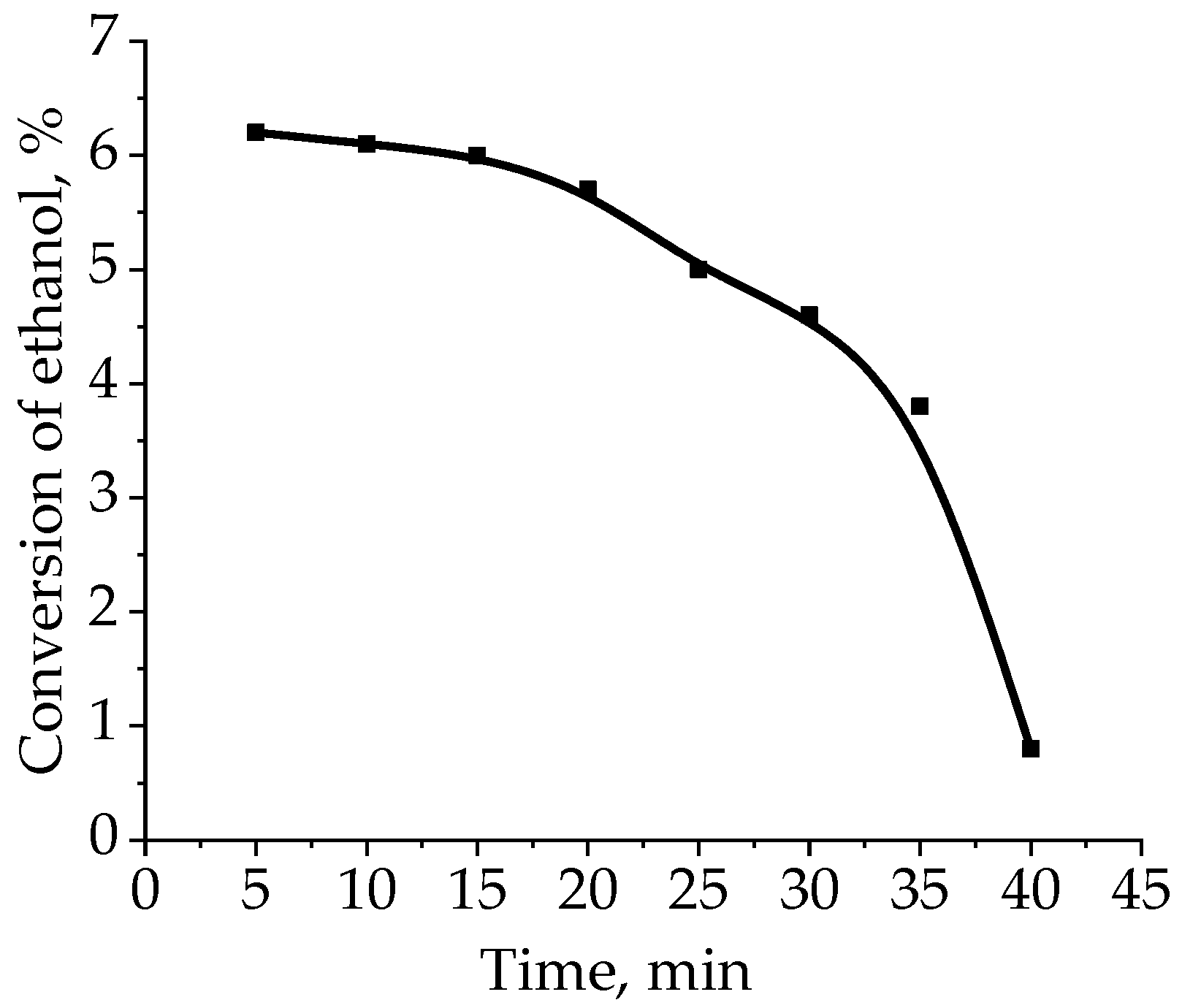
2.3. Oxidation of Ethanol by Cryogel-Immobilized Enzyme—Catalase in Batch-Type Catalytic Reactor
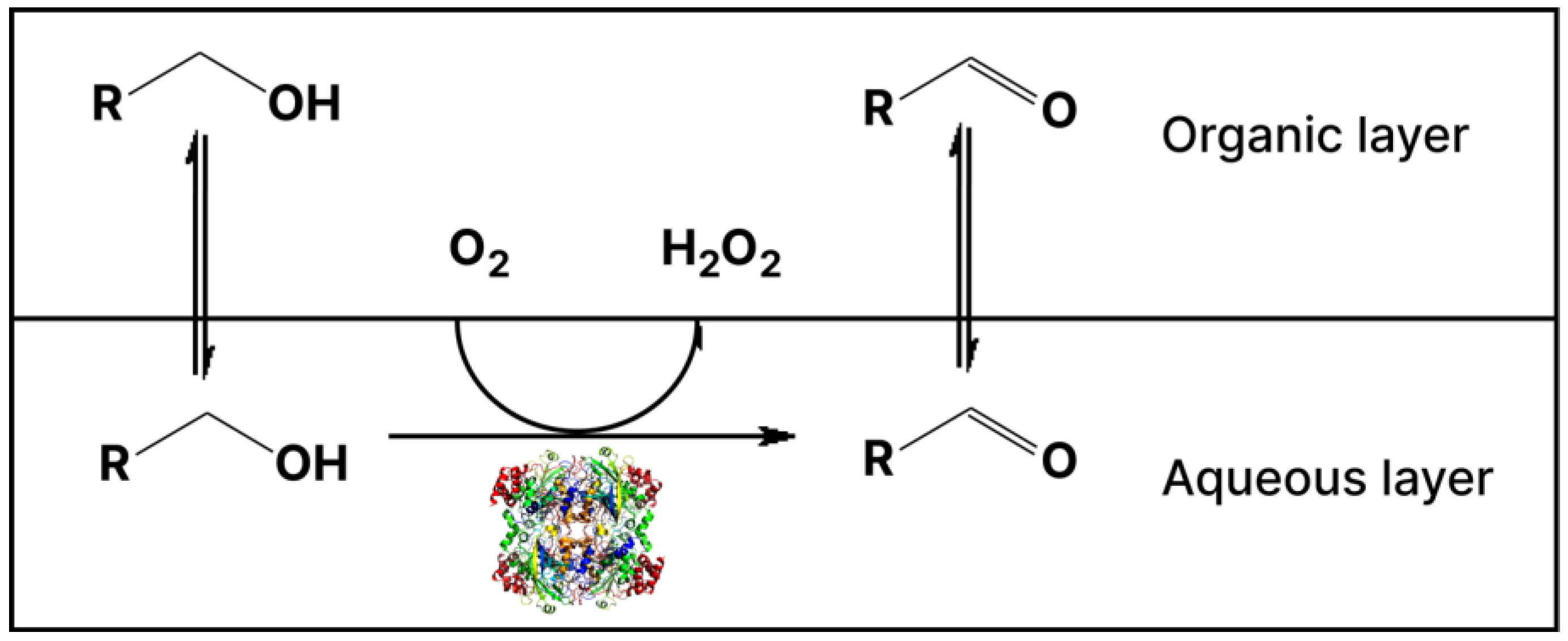
2.4. Mechanistic Aspects of Ethanol Oxidation
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Catalase@p(APTAC-co-AMPS) Cryogel
3.3. Enzymatic Activity Determination of Catalase
3.4. Calculation of the Immobilization Yield of Catalase in Cryogel Samples
3.5. Oxidation of Ethanol in Flow-through and Batch-Type Catalytic Reactors
3.5.1. Flow-through Reactor
3.5.2. Batch-Type Reactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jira, R. Oxidations. In Applied Homogeneous Catalysis with Organometallic Compounds; Cornils, B., Herrmann, W.A., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Chapter 2.4.; pp. 386–467. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Kühn, F.E. Basic aqueous chemistry: Section 2.2. In Aqueous-Phase Organometallic Catalysis: Concepts and Applications; Cornils, B., Herrmann, W.A., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 44–56. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Shaikh, B.Y.; Borhade, A.S.; Chavhan, S.W.; Dhondge, A.P.; Gaikwad, D.D.; Desai, M.P.; Birhade, D.R.; Dhatrak, N.R. Greening the Wacker process. Tetrahedron Lett. 2013, 54, 2293–2295. [Google Scholar] [CrossRef]
- Sapi, A.; Liu, F.; Cai, X.; Thompson, C.M.; Wang, H.; An, K.; Krier, J.M.; Somorjai, G.A. Comparing the catalytic oxidation of ethanol at the solid-gas and solid-liquid interfaces over size-controlled Pt nanoparticles: Striking differences in kinetics and mechanism. Nano Lett. 2014, 14, 6727–6730. [Google Scholar] [CrossRef]
- Amrollahi, R.; Wenderich, K.; Mul, G. Room temperature oxidation of ethanol to acetaldehyde over Pt/WO3. Adv. Mater. Interfaces 2016, 3, 160026. [Google Scholar] [CrossRef]
- Guo, J.; Chen, R.; Zhu, F.-C.; Sun, S.-G.; Villullas, H.M. New understandings of ethanol oxidation reaction mechanism on Pd/C and Pd2Ru/C catalysts in alkaline direct ethanol fuel cells. Appl. Catal. B Environ. 2018, 224, 602–611. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P. Photocatalytic synthesis of acetaldehyde by selective oxidation of ethanol on RuOx−VOx/TiO2. Chem. Eng. Trans. 2013, 32, 625–630. [Google Scholar] [CrossRef]
- Guan, Y.; Hensen, E.J.M. Selective oxidation of ethanol to acetaldehyde by Au-Ir catalysts. J. Catal. 2013, 305, 135–145. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E.J.M. Highly efficient and robust Au/MgCuCr2O4 catalyst for gas-phase oxidation of ethanol to acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef]
- Redina, E.A.; Greish, A.A.; Mishin, I.V.; Kapustin, G.I.; Tkachenko, O.P.; Kirichenko, O.A.; Kustov, L.M. Selective oxidation of ethanol to acetaldehyde over Au-Cu catalysts prepared by a redox method. Catal. Today 2014, 241, 246–254. [Google Scholar] [CrossRef]
- Neramittagapong, A.; Attaphaiboon, W.; Neramittagapong, S. Acetaldehyde production from ethanol over Ni-based catalysts. Chiang Mai J. Sci. 2008, 35, 171–177. [Google Scholar]
- Arends, I.W.C.E.; Sheldon, R.A. Modern oxidation of alcohols using environmentally benign oxidants. In Modern Oxidation Methods; Bäckvall, J.-E., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 147–185. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green oxidation in water. In Handbook of Green Chemistry; Li, C.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2010; Volume 5, pp. 75–103. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Catalytic oxidation of alcohols: Recent advances. In Advances in Organometallic Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 63, Chapter 3; pp. 1–157. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Keilin, D.; Hartree, E.F. Catalase, peroxidase and metmyoglobin as catalysts of coupled peroxidatic reactions. Biochem. J. 1955, 60, 310–325. [Google Scholar] [CrossRef]
- Gandolfi, R.; Ferrara, N.; Molinari, F. An easy and efficient method for the production of carboxylic acids and aldehydes by microbial oxidation of primary alcohols. Tetrahedron Lett. 2001, 42, 513–514. [Google Scholar] [CrossRef]
- Villa, R.; Romano, A.; Gandolfi, R.; Sinisterra Gago, J.V.; Molinari, F. Chemoselective oxidation of primary alcohols to aldehydes with Gluconobacter oxydans. Tetrahedron Lett. 2002, 43, 6059–6061. [Google Scholar] [CrossRef]
- Ukeda, H.; Ishii, T.; Sawamura, M.; Isobe, K. Glycolaldehyde production from ethylene glycol with immobilized alcohol oxidase and catalase. Biosci. Biotech. Biochem. 1998, 62, 1589–1591. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P.; Macchitella, D. An oxidation of alcohols by oxygen with the enzyme laccase and mediation by TEMPO. Tetrahedron Lett. 2001, 42, 7551–7553. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B. Introduction to polymeric gels. In Polymeric Gels. Characterization, Properties and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 1; pp. 3–27. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels based on natural and synthetic polymers: Preparation, properties and applications. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Mattiasson, B.; Kumar, A.; Galeaev, I.Y. (Eds.) Macroporous Polymers: Production, Properties and Biotechnological/Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2017; p. 530. [Google Scholar]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef]
- Sahiner, N.; Seven, F. The use of superporous p(AAc (acrylic acid)) cryogels as support for Co and Ni nanoparticle preparation and as reactor in H2 production from sodium borohydride hydrolysis. Energy 2014, 71, 170–179. [Google Scholar] [CrossRef]
- Sahiner, N.; Yildiz, S. Preparation of superporous poly(4-vinyl pyridine) cryogel and their templated metal nanoparticle composites for H2 production via hydrolysis reactions. Fuel Process. Technol. 2014, 126, 324–331. [Google Scholar] [CrossRef]
- Sahiner, N.; Seven, F. Energy and environmental usage of super porous poly(2-acrylamido-2-methyl-1-propan sulfonic acid) cryogel support. RSC Adv. 2014, 4, 23886–23897. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, M.; Yilmaz, S.; Karadag, E.; Sahiner, N. Enhanced enzymatic activity and stability by in situ entrapment of α-Glucosidase within super porous p(HEMA) cryogels during synthesis. Biotechnol. Rep. 2020, 28, e00534. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, N. Superporous neutral, anionic, and cationic cryogel reactors to improved enzymatic activity and stability of α-Glucosidase enzyme via entrapment method. Chem. Eng. J. 2021, 409, 128233. [Google Scholar] [CrossRef]
- Altunbas, C.; Aslan, A.; Kusat, K.; Sahiner, M.; Akgöl, S.; Sahiner, N. Synthesis and characterization of a new cryogel matrix for covalent immobilization of catalase. Gels 2022, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Coloma, J.; Guiavarc’h, Y.; Hagedoorn, P.L.; Hanefeld, U. Immobilisation and flow chemistry: Tools for implementing biocatalysis. Chem. Commun. 2021, 57, 11416–11428. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- Horn, A.; Kumar, S.; Liese, A.; Kragl, U. Reactions on immobilized biocatalysts. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knozinger, H., Schuth, F., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 2010; Chapter 16; pp. 75–103. [Google Scholar] [CrossRef]
- Smagulova, I.; Tatykhanova, G.; Shakhvorostov, A.; Akbayeva, D.; Kudaibergenov, S. Oxidation of iso-propanol and n-butanol by catalase encapsulated within macroporous polyampholyte cryogel matrix. Polym. Adv. Technol. 2021, 32, 3817–3826. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E. Physicochemical complexation and catalytic properties of polyampholyte cryogels. Gels 2019, 5, 8. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Dzhardimalieva, G.I. Flow-through catalytic reactors based on metal nanoparticles immobilized within porous polymeric gels and surfaces/hollows of polymeric membranes. Polymers 2020, 12, 572. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Zharmagambetova, A.K.; Kudaibergenov, S.E.; Uflyand, I.E. Polymer-immobilized clusters and metal nanoparticles in catalysis. Kinet. Catal. 2020, 61, 198–223. [Google Scholar] [CrossRef]
- Dorfman, Y.A.; Abdreimova, R.R. Oxidative alkoxylation of tetraphosphorus. Russ. J. Gen. Chem. 1993, 63, 289–303. [Google Scholar]
- Dorfman, Y.A.; Abdreimova, R.R.; Akbayeva, D.N. Kinetics and mechanism of oxidative alkoxylation of tetraphosphorus in the presence of Cu(II) sulfates and carboxylates. Kinet. Catal. 1995, 36, 103–110. [Google Scholar]
- Akbayeva, D.N.; Faizova, F.C.; Abdreimova, R.R.; Peruzzini, M. Oxidation of white phosphorus by peroxides in aqueous and alcoholic solutions: Mechanistic aspects and catalytic studies. J. Mol. Catal. A Chem. 2007, 267, 181–193. [Google Scholar] [CrossRef]
- Akbayeva, D.N.; Bakirova, B.S.; Seilkhanova, G.A.; Sitzmann, H. Oxidation of octene-1 in the presence of palladium-polyvinylpyrrolidone complex. Bull. Chem. React. Eng. Catal. 2018, 13, 560–572. [Google Scholar] [CrossRef]
- Bektenova, G.A.; Kudaibergenov, S.E.; Bekturov, E.A. Interaction of catalase with cationic hydrogels: Influence of pH, kinetics of process and isotherms of adsorption. Polym. Adv. Technol. 1999, 10, 141–145. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow bioreactors as complementary tools for biocatalytic process intensification. Trends Biotech. 2018, 36, 73–88. [Google Scholar] [CrossRef]
- Nikeshin, V.V.; Klinov, A.V.; Razinov, A.I.; Nikeshina, Y.M. Hydrodynamics, heat and mass transfer processes, energy. Description of diffusion of liquid mixtures ethanol water, methanol water. Bull. Kazan Technol. Univ. 2014, 17, 160–161. (In Russian) [Google Scholar]
- Popa, M.; Bajan, N.; Popa, A.A.; Verestiuc, L. The preparation, characterization and properties of catalase immobilized on crosslinked gellan. J. Macromol. Sci. Part A Pure Appl. Chem. 2006, 43, 355–367. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Öktem, H.A.; Öktem, Z.; Tuncel, S.A. Immobilization of catalase in poly(isopropylacrylamide-co-hydroxyethylmethacrylate) thermally reversible hydrogels. Polym. Int. 1999, 48, 879–884. [Google Scholar] [CrossRef]
- Trawczynska, I. New method of determining kinetic parameters for decomposition of hydrogen peroxide by catalase. Catalysts 2020, 10, 323. [Google Scholar] [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Braun Azeredo, J.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. Adv. Inorg. Chem. 2000, 51, 51–106. [Google Scholar] [CrossRef]
- Puetz, H.; Puchlova, E.; Vrankova, K.; Hollmann, F. Biocatalytic oxidation of alcohols. Catalysts 2020, 10, 952. [Google Scholar] [CrossRef]
- Kroutil, W.; Mang, H.; Edegger, K.; Faber, K. Biocatalytic oxidation of primary and secondary alcohols. Adv. Synth. Catal. 2004, 346, 125–142. [Google Scholar] [CrossRef]
- Ling, W.; Huiping, Z.; Ying, Y.; Xinya, Z. Total oxidation of isopropanol over manganese oxide modified ZSM-5 zeolite membrane catalysts. RSC Adv. 2015, 5, 29482. [Google Scholar] [CrossRef]
- Asatiani, V.S. Fermentative Methods of Analysis; Nauka: Moscow, Russia, 1969; p. 740. [Google Scholar]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]




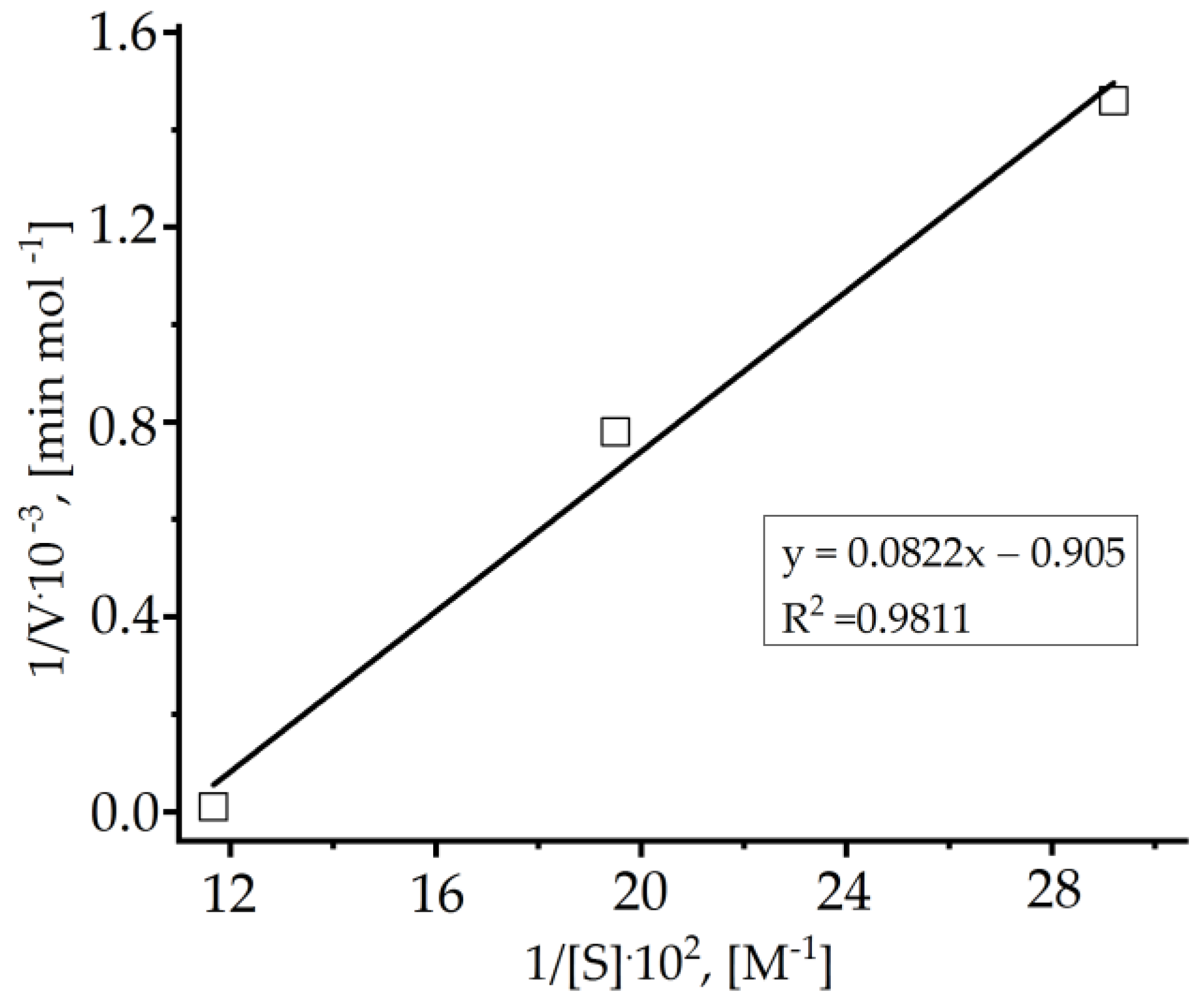
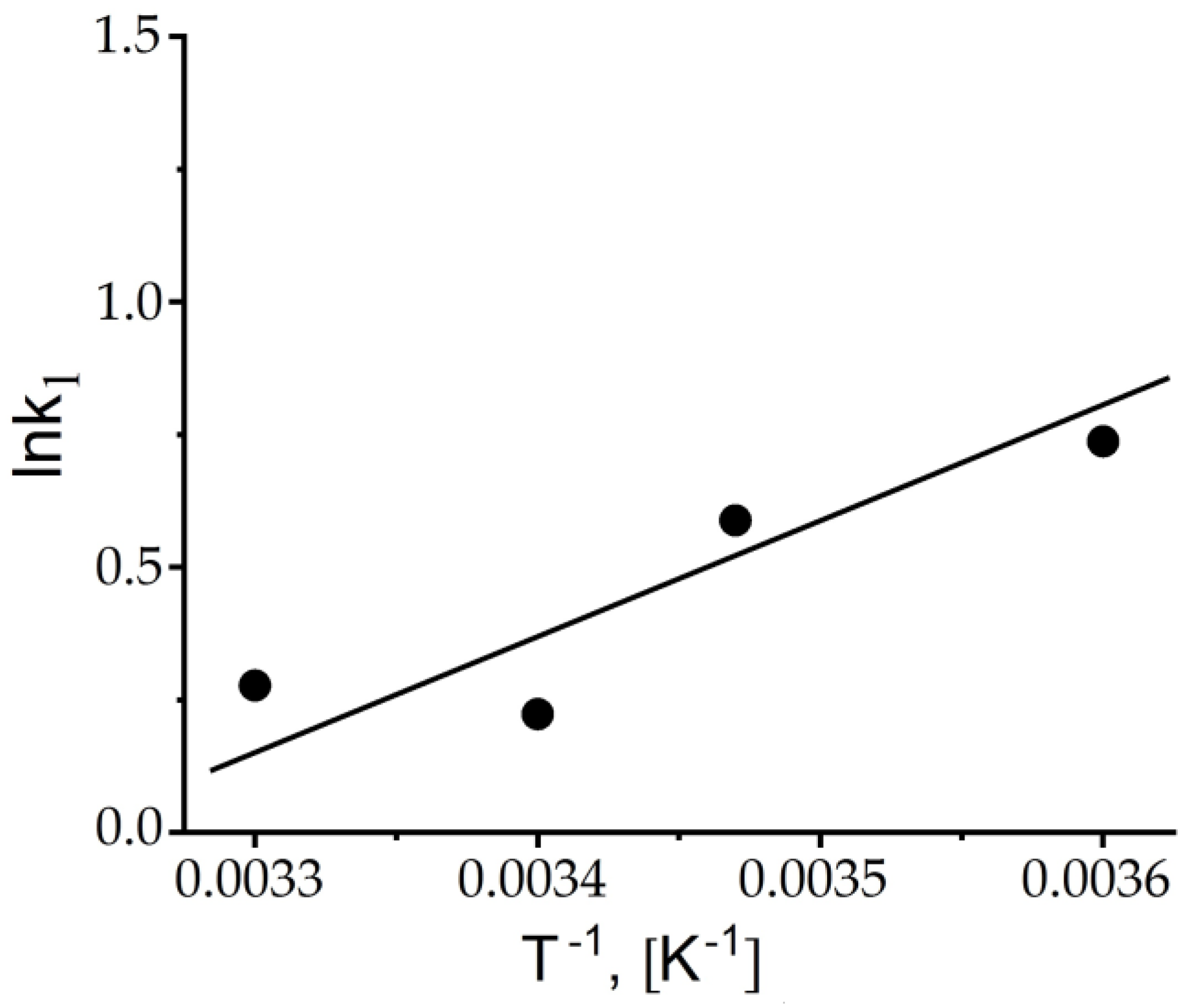

| Cryogels | p(APTAC) | p(AMPS) | p(APTAC-co-AMPS) | p(APTAC-co-AMPS) |
|---|---|---|---|---|
| Composition (mol.%) | 100 | 100 | 50:50 | 25:75 |
| Ethanol conversion (%) | 6.7 | 7.0 | 7.3 | 7.4 |
| Runs | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Throughput rate, mL·min−1 | 5.0 | 5.0 | 3.0 | 2.0 | 2.0 |
| Contact time, min | 2.0 | 4.0 | 9.0 | 20.0 | 25.0 |
| Conversion, % | 91.8 | 85.2 | 14.5 | 0.7 | 0.3 |
| [EtOH]:[H2O2], mL | 1:9 | 2:8 | 3:7 | 4:6 | 5:5 | 6:4 | 7:3 | 8:2 | 9:1 |
|---|---|---|---|---|---|---|---|---|---|
| TON·10−7 [a] | 0 | 0.14 | 0.23 | 6.75 | 16.86 | 3.23 | 0.45 | 0.48 | 0.10 |
| TOF·10−7 [b] | 0 | 0.15 | 0.28 | 9.00 | 22.48 | 4.31 | 0.89 | 1.07 | 0.51 |
| Reaction Time, min (Volume of Absorbed O2) | Yield of Acetaldehyde, % | Mass of Sample, mg | ||||||
|---|---|---|---|---|---|---|---|---|
| Cryogel Monolith | Cryogel Powder | Cryogel Monolith | Cryogel Powder | |||||
| H2O2 | O2 | Air | H2O2 | O2 | Air | |||
| 30 | 97.0 | - | - | 95.2 | - | - | 98.8 | 86.0 |
| 30 (13 mL) | - | 97.7 | - | - | - | - | 95.2 | - |
| 30 (11.8 mL) | - | - | - | 96.7 | - | - | 95.6 | |
| 30 | - | - | 59.8 | - | - | 60.5 | 95.2 | 93.2 |
| 5 | 96.7 | - | - | - | - | - | 89.6 | - |
| Runs | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Contact time, min | 40.0 | 30.0 | 30.0 | 20.0 | 15.0 |
| Conversion, % | 65.8 | 63.8 | 60.4 | 57.9 | 57.3 |
| Oxidant | H2O2 | O2 | Air | ||||
|---|---|---|---|---|---|---|---|
| Cryogel Monolith | Cryogel Powder | Cryogel Monolith | Cryogel Powder | Cryogel Monolith | Cryogel Powder | ||
| TON·10−7 [b] | 18.3 | 18.2 | 17.9 | 18.4 | 18.2 | 11.3 | 11.4 |
| TOF·10−7 [c] | 0.6 | 3.7 | 0.6 | 0.6 | 0.6 | 0.4 | 0.4 |
| Parameter | Flow | Batch | ||
|---|---|---|---|---|
| Oxidant | H2O2 | H2O2 | O2 | air |
| [EtOH], M | 8.6 | 8.6 | 8.6 | 8.6 |
| Conversion, % | 89.5 | 96.7 | 97.7 | 59.8 |
| tres, min | 0.75 | 5 | 30 | 30 |
| TON·10−7 | 16.9 | 18.2 | 18.4 | 11.3 |
| TOF·10−7 | 22.5 | 3.7 | 0.6 | 0.4 |
| S.T.Y.·10−2, g·L−1·h−1 | 48.6 | 8.8 | 8.9 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbayeva, D.N.; Smagulova, I.A.; Maksotova, K.S.; Bakirova, B.S.; Tatykhanova, G.S.; Kudaibergenov, S.E. In Situ Entrapment of Catalase within Macroporous Cryogel Matrix for Ethanol Oxidation: Flow-through Mode versus Batch Reactor. Catalysts 2023, 13, 1075. https://doi.org/10.3390/catal13071075
Akbayeva DN, Smagulova IA, Maksotova KS, Bakirova BS, Tatykhanova GS, Kudaibergenov SE. In Situ Entrapment of Catalase within Macroporous Cryogel Matrix for Ethanol Oxidation: Flow-through Mode versus Batch Reactor. Catalysts. 2023; 13(7):1075. https://doi.org/10.3390/catal13071075
Chicago/Turabian StyleAkbayeva, Dina N., Indira A. Smagulova, Kuralay S. Maksotova, Botagoz S. Bakirova, Gulnur S. Tatykhanova, and Sarkyt E. Kudaibergenov. 2023. "In Situ Entrapment of Catalase within Macroporous Cryogel Matrix for Ethanol Oxidation: Flow-through Mode versus Batch Reactor" Catalysts 13, no. 7: 1075. https://doi.org/10.3390/catal13071075
APA StyleAkbayeva, D. N., Smagulova, I. A., Maksotova, K. S., Bakirova, B. S., Tatykhanova, G. S., & Kudaibergenov, S. E. (2023). In Situ Entrapment of Catalase within Macroporous Cryogel Matrix for Ethanol Oxidation: Flow-through Mode versus Batch Reactor. Catalysts, 13(7), 1075. https://doi.org/10.3390/catal13071075








