Preparation-Height-Specific Surface Area of Flower-like ZnO for Norfloxacin Removal in Dialysis Wastewater
Abstract
1. Introduction
2. Results and Discussion
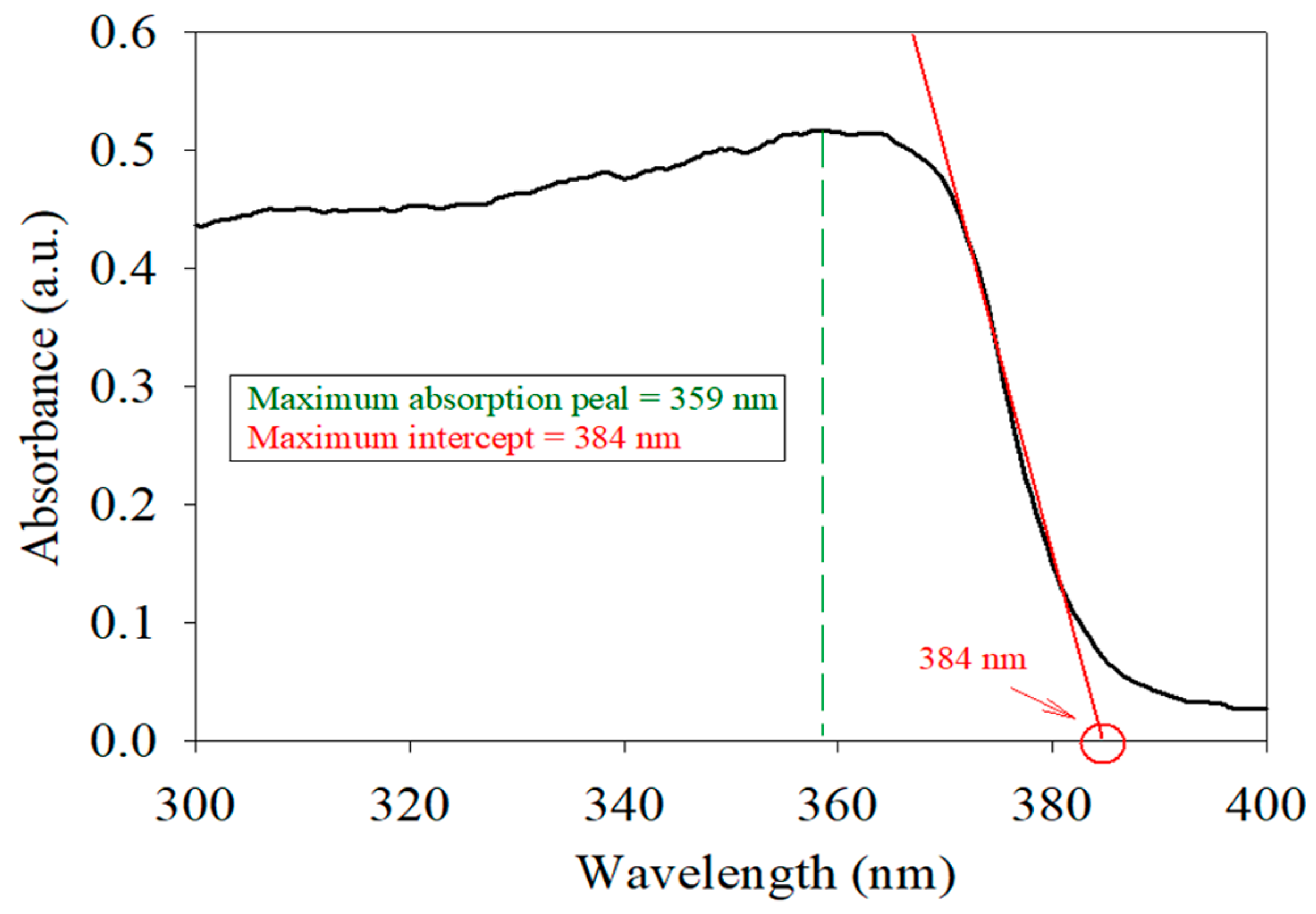
2.1. Characterization of Materials
2.2. Effect of Catalytic Dosage
2.3. Effect of Initial NF Concentration
2.4. Effect of pH Values on Degradation Efficiency
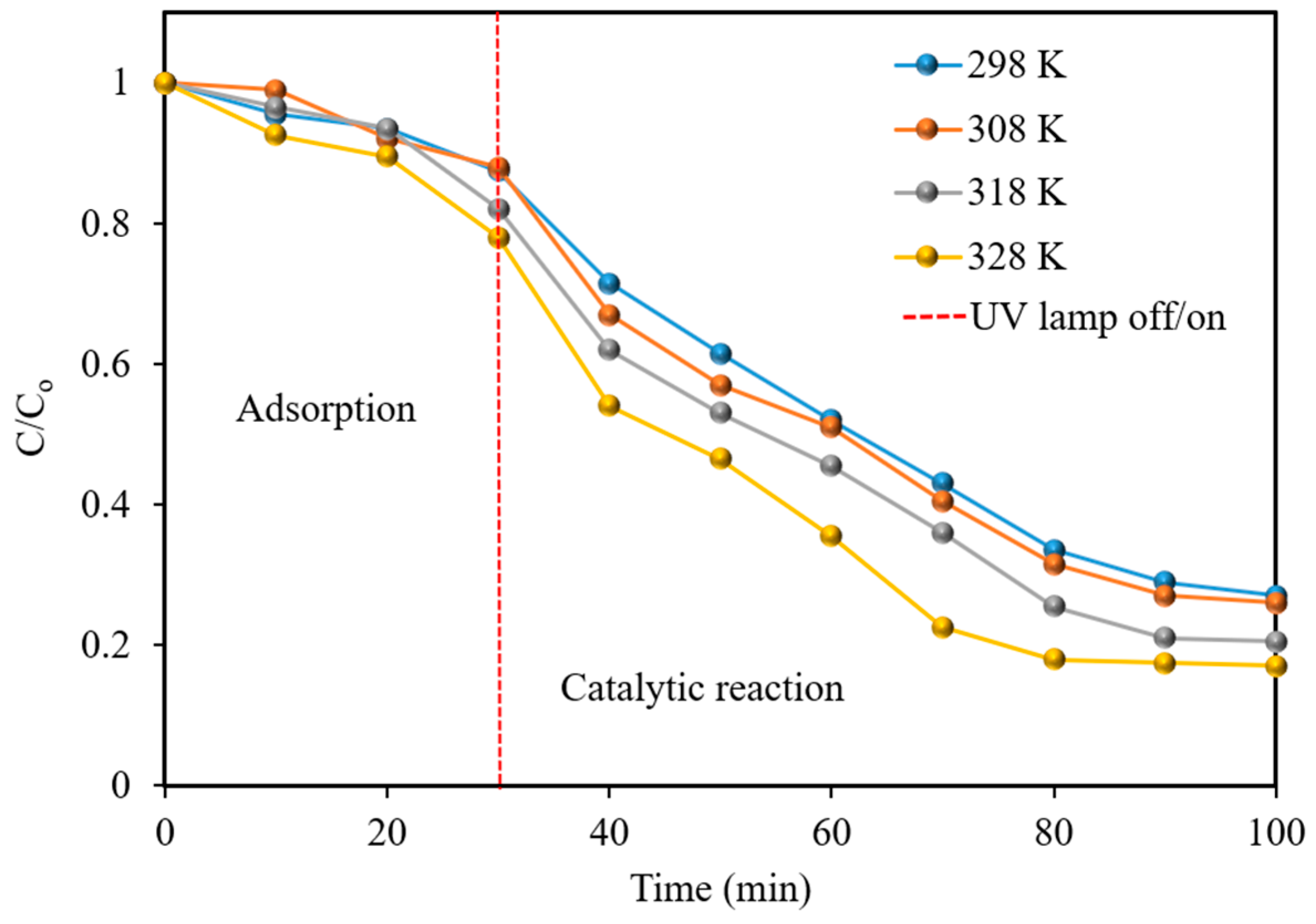
2.5. Effect of Different Temperature on NF Removal Efficiency
2.6. Kinetic Model Analysis Results
3. Materials and Methods
3.1. Chemicals and Starting Materials
3.2. Preparation of Flower-like ZnO
3.3. Materials Characterization
3.4. Performance Assessment
3.5. Kinetic Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- End Stage Renal Disease: Chapter 11. Available online: https://usrds-adr.niddk.nih.gov/2022/end-stage-renal-disease/11-international-comparisons (accessed on 15 April 2023).
- 2021 Annual Report Kidney Disease in Taiwan. Available online: https://www.tsn.org.tw/twrds.html (accessed on 13 April 2023).
- Ghodsi, J.; Rafati, A.A.; Shoja, Y. First report on electrocatalytic oxidation of oxytetracycline by horse radish peroxidase: Application in developing a biosensor to oxytetracycline determination. Sens. Actuators B Chem. 2016, 224, 692–699. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, H.; Li, Y.; Liu, Z. Photodegradation of oxytetracycline in aqueous by 5A and 13X loaded with TiO2 under UV irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of pH on photolytic and photocatalytic degradation of antibiotic oxytetracycline in aqueous solution under visible/solar light: Kinetics and mechanism studies. Appl. Catal. B 2013, 134, 83–92. [Google Scholar] [CrossRef]
- Zheng, S.; Qiu, X.; Chen, B.; Yu, X.; Liu, Z.; Zhong, G.; Li, H.; Chen, M.; Sun, G.; Huang, H.; et al. Antibiotics pollution in Jiulong River estuary: Source, distribution and bacterial resistance. Chemosphere 2011, 84, 1677–1685. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere 2011, 83, 1284–1289. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef]
- Hsiao, S.S.; Wang, P.H.; Nguyen, N.T.; Le, T.T.; Chen, C.K.; Chen, S.S.; Chang, C.T. Aqueous oxytetracycline and norfloxacin sonocatalytic degradation with multilayer sheet-like ZnO in the presence of peroxydisulfate. J. Nanosci. Nanotechnol. 2021, 21, 1653–1658. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, Y.; Gao, Y.; Tian, Z.; Yang, M. Anaerobic treatment of antibiotic production wastewater pretreated with enhanced hydrolysis: Simultaneous reduction of COD and ARGs. Water Res. 2017, 110, 211–217. [Google Scholar] [CrossRef]
- Zhou, S.L.; Zhang, S.; Liu, F.; Liu, J.J.; Xue, J.J.; Yang, D.J.; Chang, C.T. ZnO nanoflowers photocatalysis of norfloxacin: Effect of triangular silver nanoplates and water matrix on degradation rates. J. Photochem. Photobiol. A 2016, 328, 97–104. [Google Scholar] [CrossRef]
- Tao, H.; Liang, X.; Zhang, Q.; Chang, C.T. Enhanced photo activity of graphene/titanium dioxide nanotubes for removal of Acetaminophen. Appl. Surf. Sci. 2015, 324, 258–264. [Google Scholar] [CrossRef]
- Souza Santos, L.V.; Meireles, A.M.; Lange, L.C. Degradation of antibiotics norfloxacin by Fenton, UV and UV/H2O2. J. Environ. Manag. 2015, 154, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, X.; Pan, Z.; Ma, S.; Li, L. Synthesis of MnOx/SBA-15 for norfloxacin degradation by catalytic ozonation. Sep. Purif. Technol. 2017, 173, 99–104. [Google Scholar] [CrossRef]
- Novoa-Luna, K.A.; Mendoza-Zepeda, A.; Natividad, R.; Romero, R.; Galar-Martínez, M.; Gómez-Oliván, L.M. Biological hazard evaluation of a pharmaceutical effluent before and after a photo Fenton treatment. Sci. Total Environ. 2016, 569–570, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Gao, N.Y.; Deng, Y.; Gu, J.; Gu, Y.L.; Zhang, D. Factor affecting sonolytic degradation of sulfamethazine in water. Ultrason. Sonochem. 2013, 20, 1401–1407. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Wei, C.; Li, G.; Wu, Q. Assisted sonocatalytic degradation of pethidine hydrochloride (dolantin) with some inorganic oxidants caused by CdS coated ZrO2 composite. Sep. Purif. Technol. 2017, 172, 202–210. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ. Chem. Lett. 2016, 14, 259–290. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Chen, Y.; She, J.; Deng, S.; Xu, N.; Chen, J. Controllable preparation of 1-D and dendritic ZnO nanowires and their large area field-emission properties. J. Alloys Compd. 2017, 690, 304–314. [Google Scholar] [CrossRef]
- Anju, S.G.; Yesodharan, S.; Yesodharan, E.P. Zinc oxide mediated sonophotocatalytic degradation of phenol in water. Chem. Engin. J. 2012, 189–190, 84–93. [Google Scholar] [CrossRef]
- Bedi, P.S.; Kaur, A. An overview on uses of zinc oxide nanoparticles. World J. Pharm. Pharm. Sci. 2015, 4, 1177–1196. [Google Scholar]
- Wang, J.; Jiang, Z.; Zhang, L.; Kang, P.; Xie, Y.; Lv, Y.; Xu, R.; Zhang, X. Sonocatalytic degradation of some dyestuffs and comparison of catalytic activities of nano-sized TiO2, nano-sized ZnO and composite TiO2/ZnO powders under ultrasonic irradiation. Ultrason. Sonochem. 2009, 16, 225–231. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhang, Z.; Liu, X.; Jia, H.; Xu, B. Synthesis of spherical Ag/ZnO heterostructural composites with excellent photocatalytic activity under visible light and UV irradiation. Appl. Surf. Sci. 2015, 355, 644–652. [Google Scholar] [CrossRef]
- Wang, C.; Tan, X.; Yan, J.; Chai, B.; Li, J.; Chen, S. Electrospinning direct synthesis of magnetic ZnFe2O4/ZnO multi-porous nanotubes with enhanced photocatalytic activity. Appl. Surf. Sci. 2017, 396, 780–790. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, S.L.; Zhang, L.N.; Wu, D.H.; Du, S.; Chang, C.T. Facile synthesis of triangular silver nanoplate-coated power-like ZnO nanostructures. Mater. Lett. 2016, 171, 263–267. [Google Scholar] [CrossRef]
- Hui, A.; Ma, J.; Liu, J.; Bao, Y.; Zhang, J. Morphological evolution of Fe doped sea urchin-shaped ZnO nanoparticles with enhanced photocatalytic activity. J. Alloys Compd. 2017, 696, 639–647. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Engin. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Bao, Y.; Gao, L.; Feng, C.; Ma, J.; Liu, C.; Zhang, W. A solvent-dependent fabrication of flower-like and hexagonally ring-like ZnO architectures in one minute. Arab. J. Chem. 2020, 13, 4035–4042. [Google Scholar] [CrossRef]
- Liao, F.; Han, X.; Zhang, Y.; Xu, C.; Chen, H. Hydrothermal synthesis of flower-like zinc oxide microstructures with large specific surface area. J. Mater. Sci. Mater. Electron. 2017, 28, 16855–16860. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Preparation of flower-like ZnO hierarchical structures for photodegradation of phenol under UV irradiation. Res. Chem. Intermed. 2015, 41, 2489–2502. [Google Scholar] [CrossRef]
- Luo, X.; Lou, Z.; Wang, L.; Zheng, X.; Zhang, T. Fabrication of flower-like ZnO nanosheet and nanorod-assembled hierarchical structures and their enhanced performance in gas sensors. New J. Chem. 2014, 38, 84–89. [Google Scholar] [CrossRef]
- Wang, P.; Du, H.; Shen, S.; Zhang, M.; Liu, B. Preparation and characterization of ZnO microcantilever for nanoactuation. Nanoscale Res. Lett. 2012, 7, 176. [Google Scholar] [CrossRef]
- Noureddine, B.H.T.; Radhouane, B.H.T.; Abdelhamid, B.S.; André, S. Effects of individual layer thickness on the microstructure and optoelectronic properties of sol–gel-derived zinc oxide thin films. J. Am. Ceram. Soc. 2008, 91, 846–851. [Google Scholar]
- Koao, L.F.; Dejene, B.F.; Hone, F.G.; Swart, H.C.; Motloung, S.V.; Motaung, T.E.; Pawade, V.B. Effects of octadecylammine molar concentration on the structure, morphology and optical properties of ZnO nanostructure prepared by homogeneous precipitation method. J. Lumin. 2018, 200, 206–215. [Google Scholar] [CrossRef]
- Raji, R.; Sibi, K.S.; Gopchandran, K.G. ZnO:Ag nanorods as efficient photocatalysts: Sunlight driven photocatalytic degradation of sulforhodamine B. Appl. Surf. Sci. 2018, 427, 863–875. [Google Scholar]
- Ungula, J.; Dejene, B.F.; Swart, H.C. Band gap engineering, enhanced morphology and photoluminescence of un-doped, Ga and/or Al-doped ZnO nanoparticles by reflux precipitation method. J. Lumin. 2018, 195, 54–60. [Google Scholar] [CrossRef]
- Chen, M.; Chu, W. Degradation of antibiotic norfloxacin in aqueous solution by visible-light-mediated C-TiO2 photocatalysis. J. Hazard. Mater. 2012, 219–220, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalin. Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Sobana, N.; Swaminathan, M. Combination effect of ZnO and activated carbon for solar assisted photocatalytic degradation of Direct Blue 53. Sol. Energy Mater Sol. Cells 2007, 91, 727–734. [Google Scholar] [CrossRef]
- Guo, H.; Gao, N.; Yang, Y.; Zhang, Y. Kinetics and transformation pathways on oxidation of fluoroquinolones with thermally activated persulfate. Chem. Eng. J. 2016, 292, 82–91. [Google Scholar] [CrossRef]
- Lei, J.H. Screening of Various Persulfate Activations for Remediating Persistent Organic Compounds. Master’s Thesis, National Chung Hsing University, Taichung City, Taiwan, 2011. [Google Scholar]
- Li, S.; Fang, L.; Ye, M.; Zhang, Y. Enhanced adsorption of norfloxacin on modified TiO2 particles prepared via surface molecular imprinting technique. Desalination Water Treat. 2016, 57, 408–418. [Google Scholar]
- Zhang, L.; Li, Y.; Guo, J.; Kan, Z.; Jia, Y. Catalytic ozonation mechanisms of norfloxacin using Cu–CuFe2O4. Environ. Res. 2023, 216, 114521. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Kou, F.; Ouyang, Q.; Chen, J.; Fang, Z. Removal of norfloxacin by surface Fenton system (MnFe2O4/H2O2): Kinetics, mechanism and degradation pathway. Chem. Engin. J. 2018, 351, 747–755. [Google Scholar] [CrossRef]
- Shiue, A.; Kang, Y.H.; Hu, S.C.; Jou, G.T.; Lin, C.H.; Hu, M.C.; Lin, S.I. Vapor adsorption characteristics of toluene in an activated carbon adsorbent-loaded nonwoven fabric media for chemical filters applied to cleanrooms. Build. Environ. 2010, 45, 2123–2131. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.H. Synthesis of MCM-41 from coal fly ash by a green approach: Influence of synthesis pH. J. Hazard. Mater. 2006, 137, 1135–1148. [Google Scholar] [CrossRef] [PubMed]









| Material | Surface Area (m2 g−1) | Ref. |
|---|---|---|
| Commercial ZnO | 3.5 | [27] |
| Flower-like ZnO | 14.15 | [28] |
| Hexagonally ring-like ZnO | 17.12 | [28] |
| Flower-like ZnO | 35.01 | [29] |
| Flower-like ZnO | 18.7 | [30] |
| Flower-like ZnO | 46.45 | This research |
| Critical Wavelength λ (nm) | Band Gap Energy (eV) | Reference |
|---|---|---|
| 380 | 3.29 | [34] |
| 389 | 3.18 | [35] |
| 390 | 3.18 | [36] |
| 384 | 3.23 | This study |
| Method | Material | Removal Eff. (%) | Advantages or Disadvantages | Ref. |
|---|---|---|---|---|
| Sonocatalysis | Multilayer-sheet-like ZnO | 64 | Low cost | [9] |
| Advanced oxidation | UV-H2O2 | 60 | Low cost | [13] |
| Photodegradation | TiO2 | 79 | Low cost | [42] |
| Catalytic ozonation | CuFe2O4 | 82 | Hight cost | [43] |
| Fenton | MnFe2O4/H2O2 | 91 | Hight cost | [44] |
| Photodegradation | Flower-like ZnO | 83 | Low cost | This research |
| Concentration (ppm) | Correlation Coefficient Squared (Rc2) | Reaction Rate Constant k1 (min−1) |
|---|---|---|
| 2 | 0.88 | 0.0042 |
| 4 | 0.98 | 0.0015 |
| 8 | 0.99 | 0.0008 |
| 12 | 0.98 | 0.0007 |
| Concentration (ppm) | Correlation Coefficient Squared (Rc2) | Reaction Rate Constant k2 (L mg−1 min−1) |
|---|---|---|
| 2 | 0.90 | 0.00460 |
| 4 | 0.98 | 0.00070 |
| 8 | 0.99 | 0.00010 |
| 12 | 0.98 | 0.00009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-S.; Nguyen, T.-M.-P.; Wang, L.-P.; Hong, G.-B.; Cheng, Y.-C.; Wang, P.-H.; Duong, C.-C.; Yang, C.-J. Preparation-Height-Specific Surface Area of Flower-like ZnO for Norfloxacin Removal in Dialysis Wastewater. Catalysts 2023, 13, 979. https://doi.org/10.3390/catal13060979
Hsiao S-S, Nguyen T-M-P, Wang L-P, Hong G-B, Cheng Y-C, Wang P-H, Duong C-C, Yang C-J. Preparation-Height-Specific Surface Area of Flower-like ZnO for Norfloxacin Removal in Dialysis Wastewater. Catalysts. 2023; 13(6):979. https://doi.org/10.3390/catal13060979
Chicago/Turabian StyleHsiao, Shui-Shu, Thi-Minh-Phuong Nguyen, Li-Pang Wang, Gui-Bing Hong, Yu-Chen Cheng, Pei-Hua Wang, Cong-Chinh Duong, and Chia-Jui Yang. 2023. "Preparation-Height-Specific Surface Area of Flower-like ZnO for Norfloxacin Removal in Dialysis Wastewater" Catalysts 13, no. 6: 979. https://doi.org/10.3390/catal13060979
APA StyleHsiao, S.-S., Nguyen, T.-M.-P., Wang, L.-P., Hong, G.-B., Cheng, Y.-C., Wang, P.-H., Duong, C.-C., & Yang, C.-J. (2023). Preparation-Height-Specific Surface Area of Flower-like ZnO for Norfloxacin Removal in Dialysis Wastewater. Catalysts, 13(6), 979. https://doi.org/10.3390/catal13060979








