Laser-Induced Nitrogen-Doped Graphene Composite Iron–Cobalt Hydroxide for Methylene Blue Degradation via Electrocatalytic Activation of Peroxymonosulfate
Abstract
1. Introduction
2. Results and Discussion
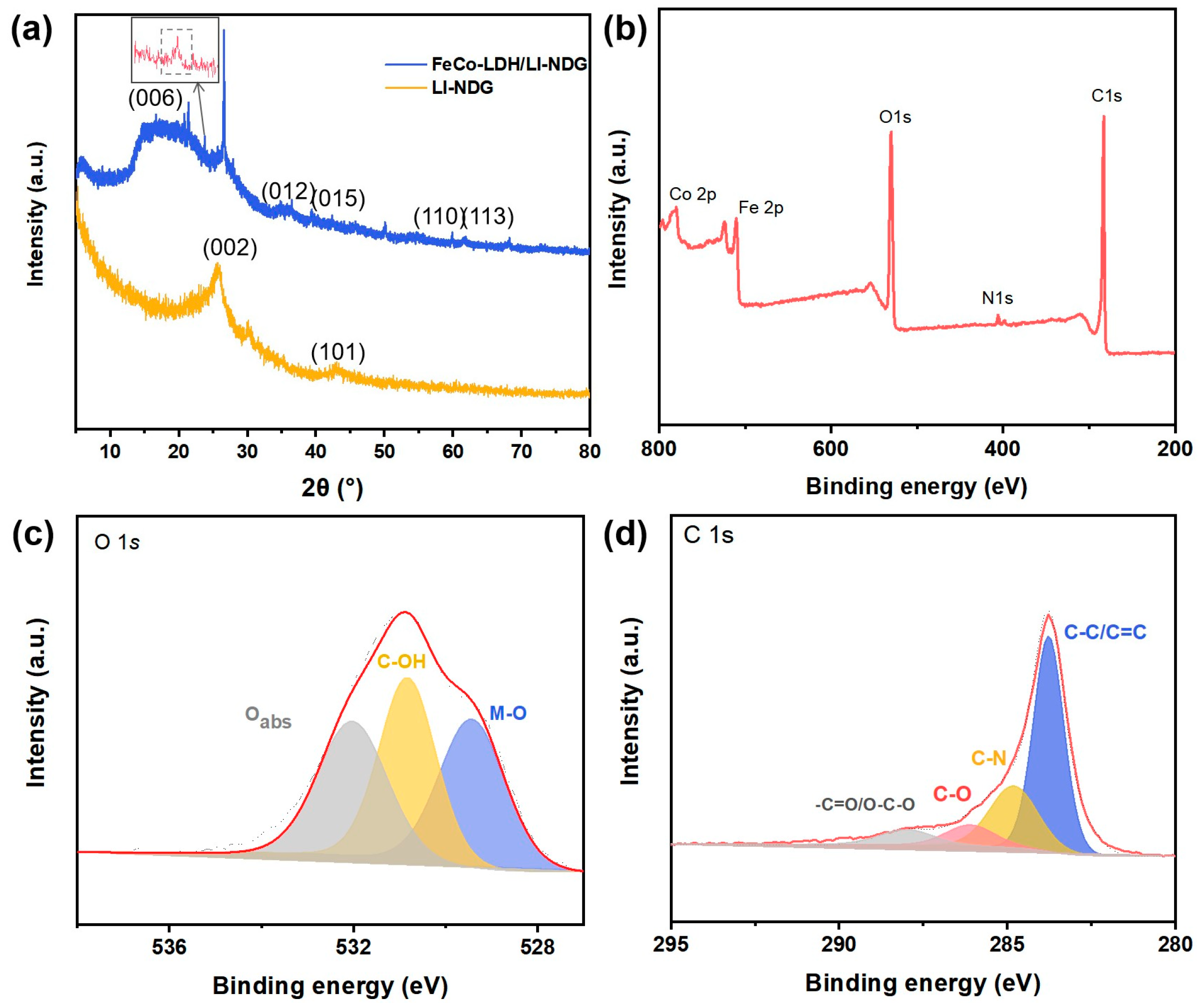
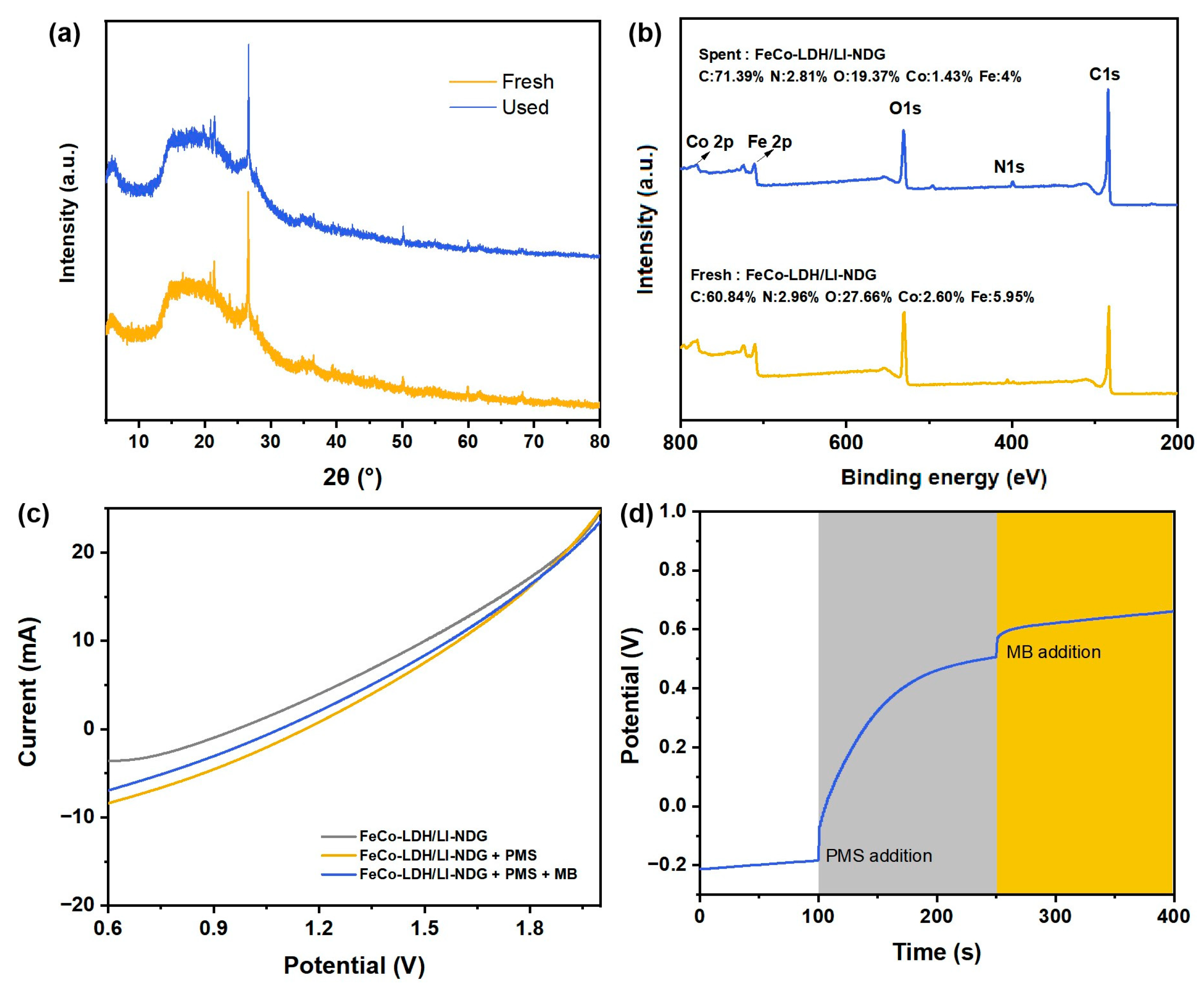
2.1. Characterizations
2.2. Catalytic Activity
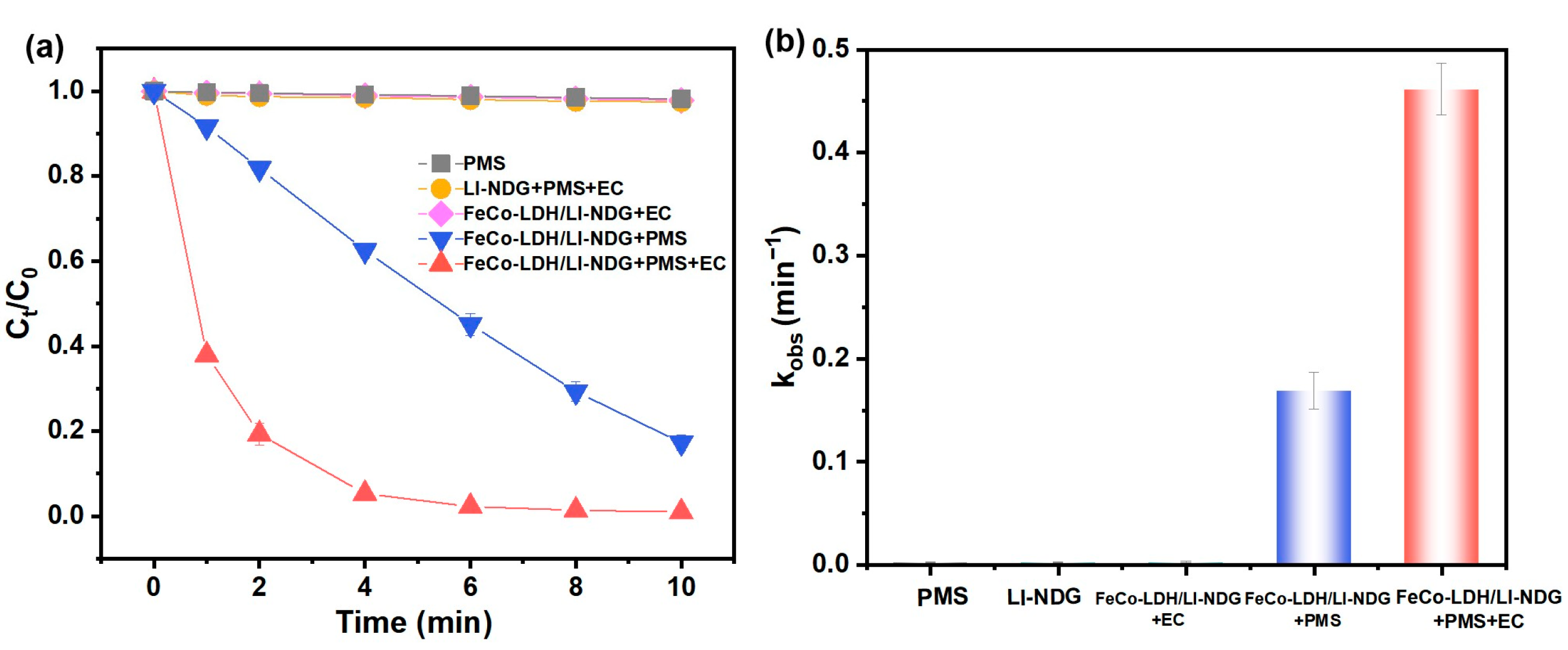
2.2.1. Electrochemical Performance
2.2.2. Catalytic Performance
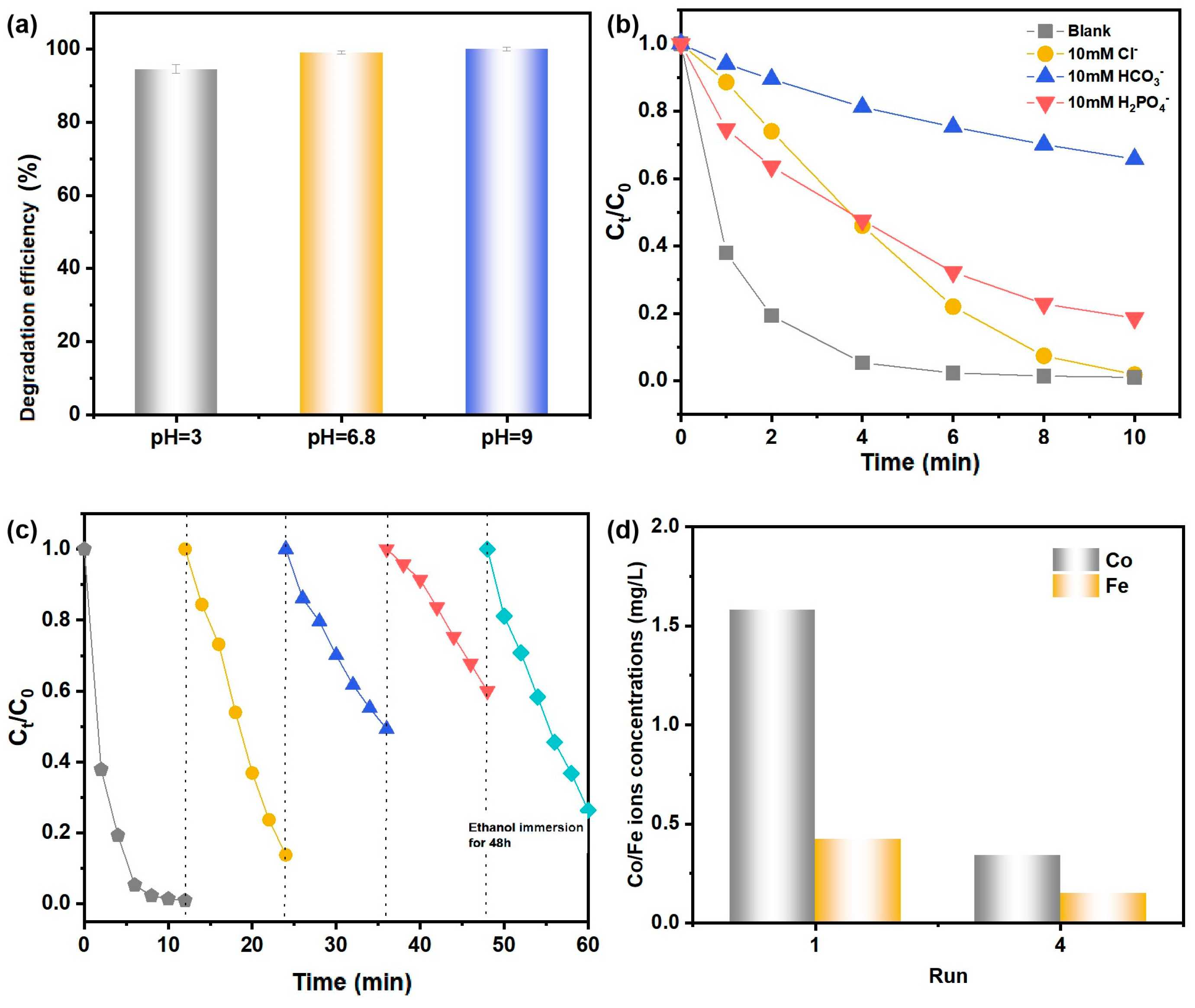
2.2.3. The Effect of Reaction Parameters
2.2.4. Interference Resistance and Universality
2.2.5. Reusability and Stability
2.3. Activation Mechanism
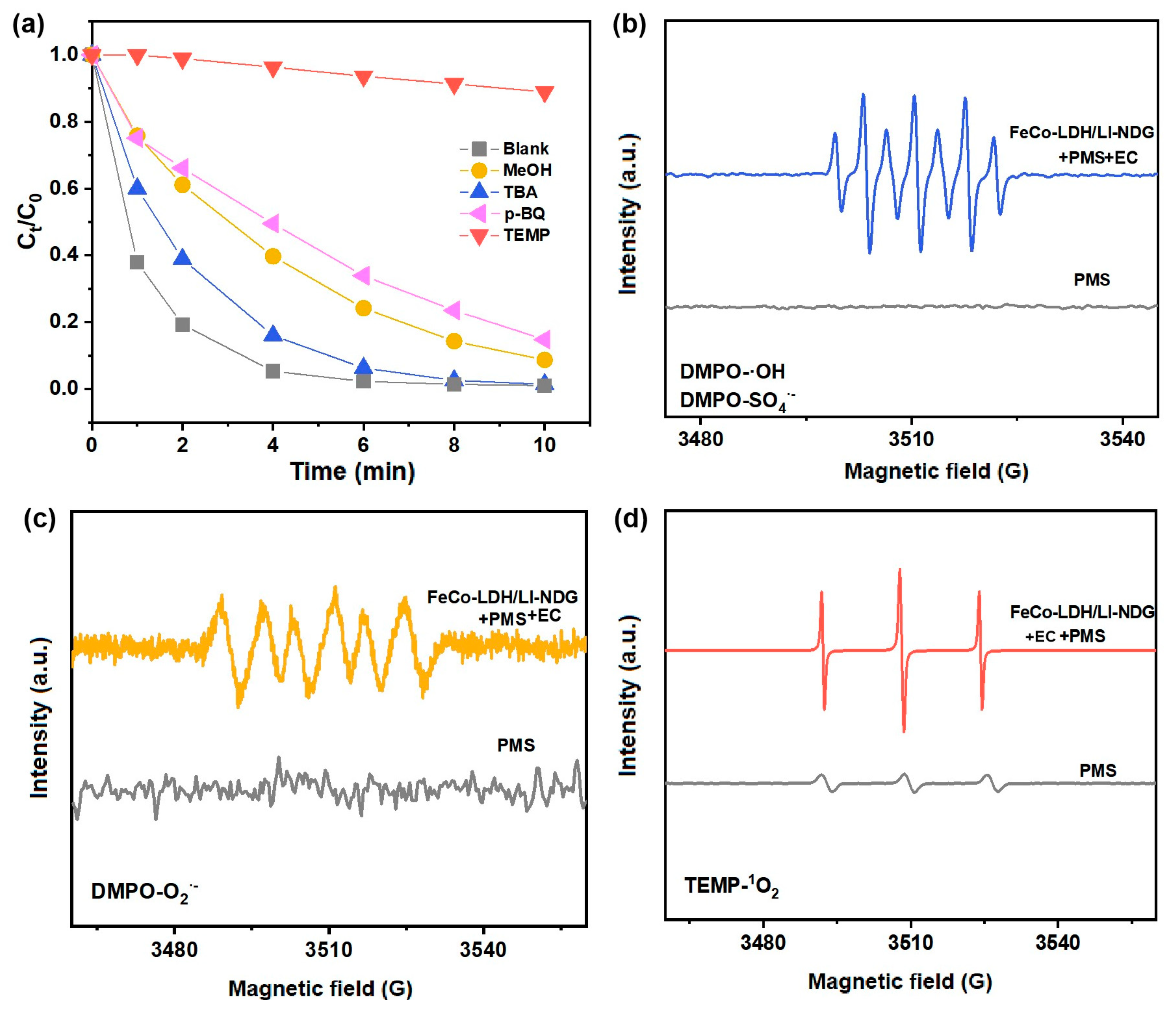
2.3.1. Reactive Oxygen Species
2.3.2. Feasible Activation Mechanism
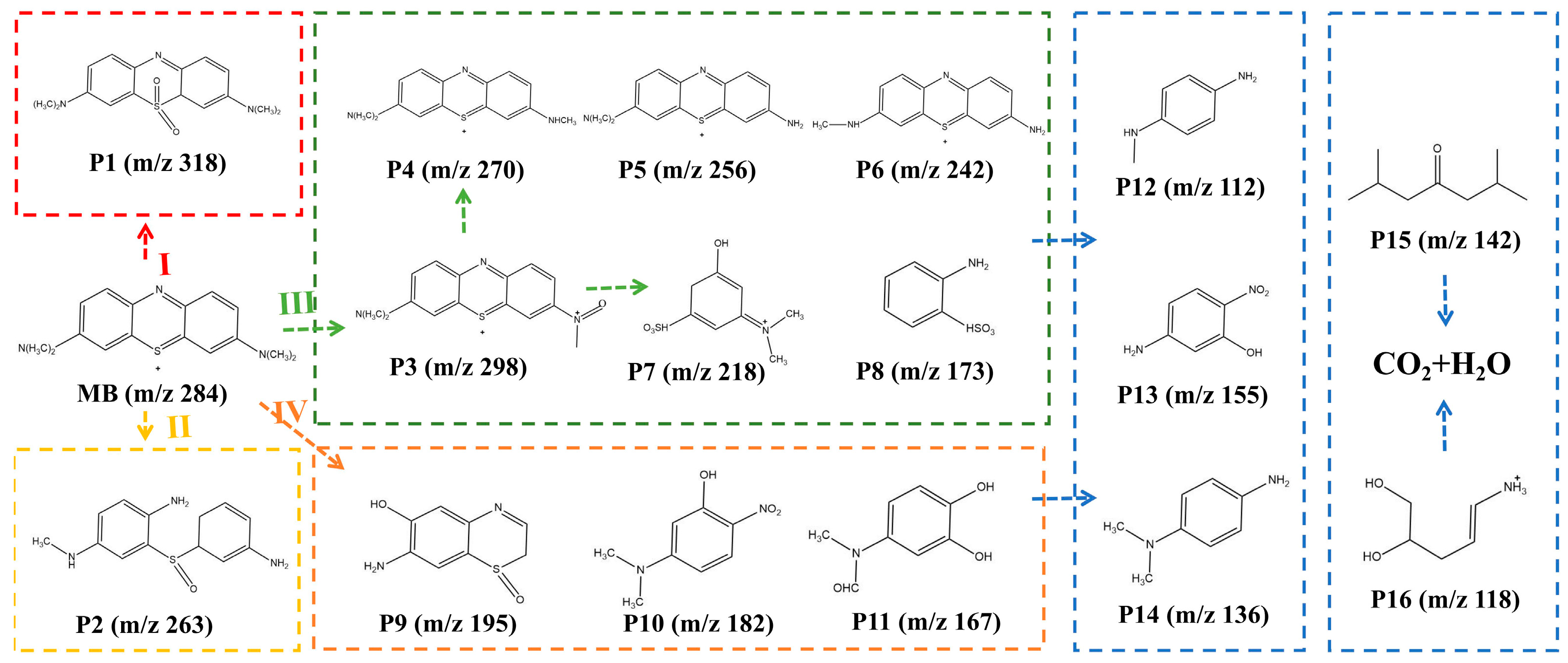
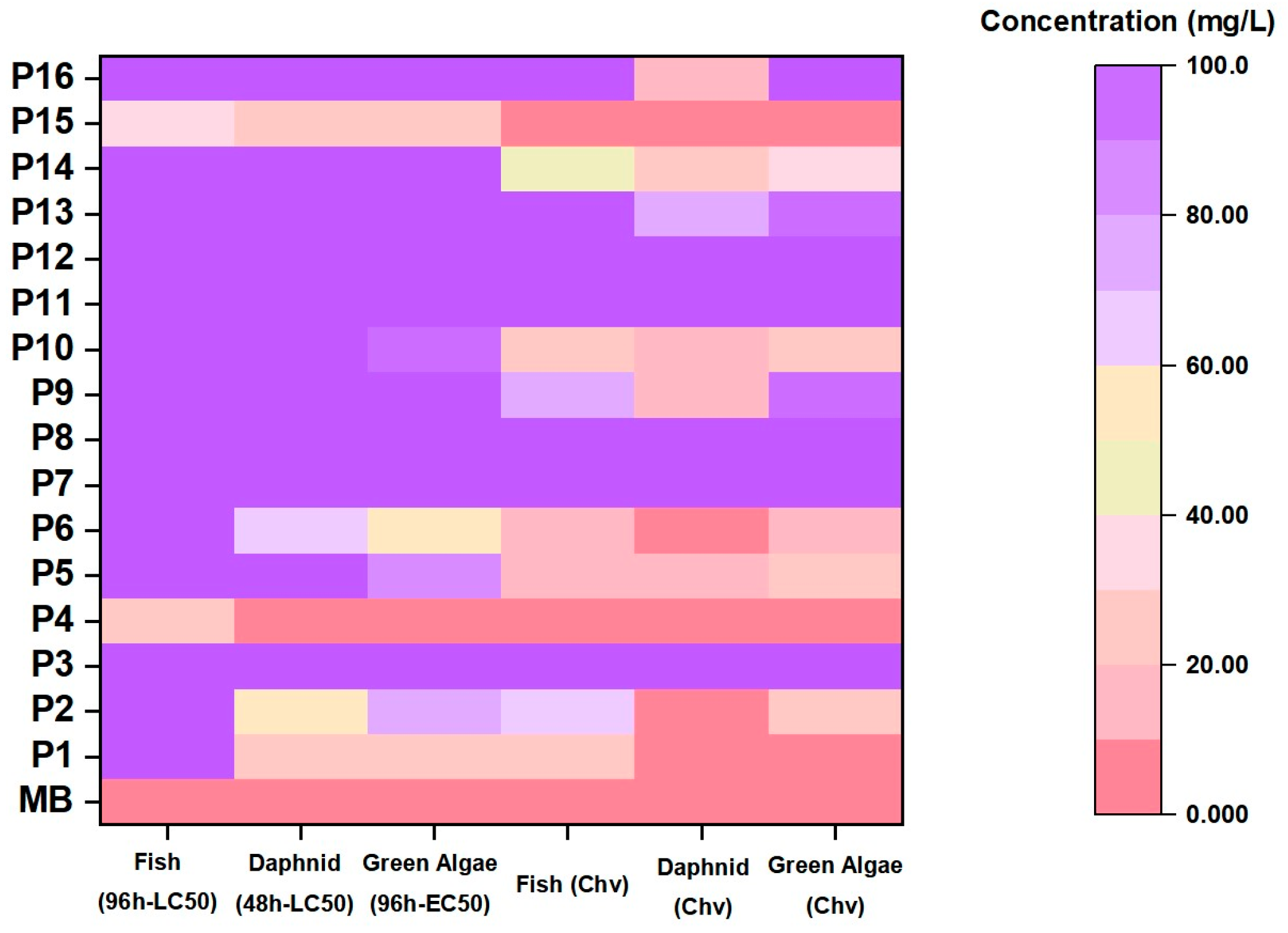
2.4. Degradation Intermediate Product Identification and Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of the Precursor FeCo-LDH/LI-NDG
3.2.2. Catalytic Degradation Experiments
3.2.3. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Meng, L.; Chu, H.; Wang, J.F.; Wang, T.; Ma, Y.; Wang, C.C. Ultrafast degradation of emerging organic pollutants via activation of peroxymonosulfate over Fe3C/Fe@N-C-x: Singlet oxygen evolution and electron-transfer mechanisms. Appl. Catal. B 2023, 321, 122034. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.-T.; Teng, Y.; Zhao, J.; Kuang, L.; Sun, X. Activation of persulfate by core–shell structured Fe3O4@C/CDs-Ag nanocomposite for the efficient degradation of penicillin. Sep. Purif. Technol. 2021, 254, 117617. [Google Scholar] [CrossRef]
- Hu, T.; Deng, F.; Feng, H.; Zhang, J.; Shao, B.; Feng, C.; Tang, W.; Tang, L. Fe/Co bimetallic nanoparticles embedded in MOF-derived nitrogen-doped porous carbon rods as efficient heterogeneous electro-Fenton catalysts for degradation of organic pollutants. Appl. Mater. Today 2021, 24, 101161. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Hou, Z.; Chen, P.; Zhou, X.; Wang, W.; Tan, F.; Wang, X.; Qiao, X. Improvement of carbonyl groups and surface defects in carbon nanotubes to activate peroxydisulfate for tetracycline degradation. Nanomaterials 2023, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Meijide, J.; Dunlop, P.S.M.; Pazos, M.; Sanromán, M.A. Heterogeneous electro-fenton as “green” technology for pharmaceutical removal: A review. Catalysts 2021, 11, 85. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.T.; Teng, Y.; Zhao, J.; Sun, X. Heterogeneous activation of persulfate by carbon nanofiber supported Fe3O4@carbon composites for efficient ibuprofen degradation. J. Hazard. Mater. 2021, 401, 123428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Guo, Z.; Zheng, X.; Guo, P.; Xu, J.; Lei, Y. The decomplexation of Cu-EDTA by electro-assisted heterogeneous activation of persulfate via acceleration of Fe(II)/Fe(III) redox cycle on Fe-MOF catalyst. Chem. Eng. J. 2021, 430, 133025. [Google Scholar] [CrossRef]
- Qi, F.; Zeng, Z.; Wen, Q.; Huang, Z. Enhanced organics degradation by three-dimensional (3D) electrochemical activation of persulfate using sulfur-doped carbon particle electrode: The role of thiophene sulfur functional group and specific capacitance. J. Hazard. Mater. 2021, 416, 125810. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Le, T.S.D.; Phan, H.P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.W.; et al. Recent advances in laser-induced graphene: Mechanism, fabrication, properties, and applications in flexible electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Wang, W.; Lu, L.; Xie, Y.; Yuan, W.; Wan, Z.; Tang, Y.; Teh, K.S. A highly stretchable microsupercapacitor using laser-induced graphene/NiO/Co3O4 electrodes on a biodegradable waterborne polyurethane substrate. Adv. Mater. Technol. 2020, 5, 1900903. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Tour, J.M. Laser-induced graphene hybrid catalysts for rechargeable Zn-air batteries. ACS Appl. Energy Mater. 2019, 2, 1460–1468. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, J.; Wang, Z.; Song, W.; Cao, G. Recent advances in preparation and application of laser-induced graphene in energy storage devices. Mater. Today Energy 2020, 18, 100569. [Google Scholar] [CrossRef]
- Yan, B.; Deng, H.; Wei, H.; Chen, L.; Liu, H.; Song, T.; Yu, X. Performance and kinetics of BPA degradation initiated by powdered iron (or ferrous sulfate) and persulfate in aqueous solutions. Catalysts 2022, 13, 36. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Xing, L.; Li, J.; Xu, M.; Pan, G.; Li, J. Superoxide radical mediated persulfate activation by nitrogen doped bimetallic MOF (FeCo/N-MOF) for efficient tetracycline degradation. Sep. Purif. Technol. 2021, 282, 120124. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Guo, Q.; Wang, X.; Zhang, L.; Zhu, W.; Luo, Y. Activation of peroxymonosulfate by the CoFe/ZSM-5 for efficient sulfamethoxazole degradation. J. Environ. Chem. Eng. 2022, 10, 107012. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, H.; Ye, D.; Duan, H.; Tang, Y.; He, T.; Shah, L.A.; Zhang, J. High efficiency UOR electrocatalyst based on crossed nanosheet structured FeCo-LDH for hydrogen production. Appl. Catal. A-Gen. 2022, 643, 118745. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Jiang, Y.; Liu, X.; Shen, W.; Li, M.; He, R. Interface engineering of FeCo LDH@NiCoP nanowire heterostructures for highly efficient and stable overall water splitting. Chin. Chem. Lett. 2022, 33, 4003–4007. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zeng, G.; Liu, Y.; Liang, Q.; He, Q.; Wu, T.; Pan, Y.; Huang, J.; Peng, Z.; et al. Synthesis of 2D/2D CoAl-LDHs/Ti3C2Tx schottky-junction with enhanced interfacial charge transfer and visible-light photocatalytic performance. Appl. Catal. B 2021, 286, 119867. [Google Scholar] [CrossRef]
- Min, K.; Hwang, M.; Shim, S.E.; Lim, D.; Baeck, S.-H. Defect-rich Fe-doped Co3O4 derived from bimetallic-organic framework as an enhanced electrocatalyst for oxygen evolution reaction. Chem. Eng. J. 2021, 424, 130400. [Google Scholar] [CrossRef]
- Kong, X.; Gao, Q.; Bu, S.; Xu, Z.; Shen, D.; Liu, B.; Lee, C.-S.; Zhang, W. Plasma-assisted synthesis of nickel-cobalt nitride–oxide hybrids for high-efficiency electrochemical hydrogen evolution. Mater. Today Energy 2021, 21, 100784. [Google Scholar] [CrossRef]
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P.V. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Feng, H.; Yu, J.; Tang, J.; Tang, L.; Liu, Y.; Lu, Y.; Wang, J.; Ni, T.; Yang, Y.; Yi, Y. Enhanced electro-oxidation performance of FeCoLDH to organic pollutants using hydrophilic structure. J. Hazard. Mater. 2022, 430, 128464. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Wang, J. Peroxymonosulfate activation by Fe-Co-O-codoped graphite carbon nitride for degradation of sulfamethoxazole. Environ. Sci. Technol. 2020, 54, 10361–10369. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, X.; Dong, X.; Zhang, X.; Tan, Y.; Yuan, F.; Zheng, S.; Li, C. Synergistic activation of peroxymonosulfate via in situ growth FeCo2O4 nanoparticles on natural rectorite: Role of transition metal ions and hydroxyl groups. Chemosphere 2021, 263, 127965. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Q.; Zhou, M.; Li, C.; Sun, J.; Shi, W.; Ai, S. Degradation of methylene blue by a heterogeneous Fenton reaction catalyzed by FeCo2O4-N-C nanocomposites derived by ZIFs. Powder Technol. 2021, 383, 212–219. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, L.; Ma, J.; Yang, J.; Feng, J.; Fan, Z. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B 2015, 165, 572–578. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Zhou, Y. Efficiently activate peroxymonosulfate by Fe3O4@MoS2 for rapid degradation of sulfonamides. Chem. Eng. J. 2021, 422, 130126. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Zhang, Y.; Lai, Y.; Fang, Q.; Duan, Y. Activation of peroxymonosulfate by CuFe2O4-CoFe2O4 composite catalyst for efficient bisphenol a degradation: Synthesis, catalytic mechanism and products toxicity assessment. Chem. Eng. J. 2021, 423, 130093. [Google Scholar] [CrossRef]
- Cai, C.; Kang, S.; Xie, X.; Liao, C.; Duan, X.; Dionysiou, D.D. Efficient degradation of bisphenol A in water by heterogeneous activation of peroxymonosulfate using highly active cobalt ferrite nanoparticles. J. Hazard. Mater. 2020, 399, 122979. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Hao, X.; Wang, J.; Yang, Z.; Yang, Q. Green synthesis of stable structure spindle FeCo-LDH through Fe-MOF template for efficient degradation of 2,4-D. J. Water Process. Eng. 2022, 46, 102602. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhao, X.; Cao, D.; Guo, T.; Yang, B. Insights into heterogeneous catalysis of peroxymonosulfate activation by boron-doped ordered mesoporous carbon. Carbon 2018, 135, 238–247. [Google Scholar] [CrossRef]
- You, J.; Zhang, C.; Wu, Z.; Ao, Z.; Sun, W.; Xiong, Z.; Su, S.; Yao, G.; Lai, B. N-doped graphite encapsulated metal nanoparticles catalyst for removal of Bisphenol A via activation of peroxymonosulfate: A singlet oxygen-dominated oxidation process. Chem. Eng. J. 2021, 415, 128890. [Google Scholar] [CrossRef]
- Liu, L.; Mi, H.; Zhang, M.; Sun, F.; Zhan, R.; Zhao, H.; He, S.; Zhou, L. Efficient moxifloxacin degradation by CoFe2O4 magnetic nanoparticles activated peroxymonosulfate: Kinetics, pathways and mechanisms. Chem. Eng. J. 2021, 407, 127201. [Google Scholar] [CrossRef]
- Peng, W.; Liao, J.; Chen, L.; Wu, X.; Zhang, X.; Sun, W.; Ge, C. Constructing a 3D interconnected “trap-zap” β-CDPs/Fe-g-C3N4 catalyst for efficient sulfamethoxazole degradation via peroxymonosulfate activation: Performance, mechanism, intermediates and toxicity. Chemosphere 2022, 294, 133780. [Google Scholar] [CrossRef]
- Ye, J.; Yang, D.; Dai, J.; Li, C.; Yan, Y. Confinement of ultrafine Co3O4 nanoparticles in nitrogen-doped graphene-supported macroscopic microspheres for ultrafast catalytic oxidation: Role of oxygen vacancy and ultrasmall size effect. Sep. Purif. Technol. 2022, 281, 119963. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. N-doped graphene from metal–Organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism. ACS Sustain. Chem. Eng. 2017, 5, 2693–2701. [Google Scholar] [CrossRef]
- Zeng, H.; Deng, L.; Yang, K.; Huang, B.; Zhang, H.; Shi, Z.; Zhang, W. Degradation of sulfamethoxazole using peroxymonosulfate activated by self-sacrificed synthesized CoAl-LDH@CoFe-PBA nanosheet: Reactive oxygen species generation routes at acidic and alkaline pH. Sep. Purif. Technol. 2021, 268, 118654. [Google Scholar] [CrossRef]
- Ye, J.; Dai, J.; Li, C.; Yan, Y. Lawn-like Co3O4@N-doped carbon-based catalytic self-cleaning membrane with peroxymonosulfate activation: A highly efficient singlet oxygen dominated process for sulfamethoxazole degradation. Chem. Eng. J. 2021, 421, 127805. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Y.; Yi, Q.; Zhou, L.; Lei, J.; Wang, L.; Xing, M.; Liu, Y.; Zhang, J. Singlet oxygen mediated Fe2+/peroxymonosulfate photo-Fenton-like reaction driven by inverse opal WO3 with enhanced photogenerated charges. Chem. Eng. J. 2021, 425, 128644. [Google Scholar] [CrossRef]
- Su, P.; Fu, W.; Du, X.; Song, G.; Zhou, M. Confined Fe0@CNTs for highly efficient and super stable activation of persulfate in wide pH ranges: Radicals and non-radical co-catalytic mechanism. Chem. Eng. J. 2021, 420, 129446. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Yan, X.; Zhang, H.; Xiao, C.; Qi, J.; Zhu, Z.; Zhou, Y.; Sun, X.; Duan, X.; et al. Rational regulation of Co-N-C coordination for high-efficiency generation of 1O2 toward nearly 100% selective degradation of organic pollutants. Environ. Sci. Technol. 2022, 56, 8833–8843. [Google Scholar] [CrossRef]
- Ma, S.; Yang, D.; Guan, Y.; Yang, Y.; Zhu, Y.; Zhang, Y.; Wu, J.; Sheng, L.; Liu, L.; Yao, T. Maximally exploiting active sites on yolk@shell nanoreactor: Nearly 100% PMS activation efficiency and outstanding performance over full pH range in fenton-like reaction. Appl. Catal. B 2022, 316, 121594. [Google Scholar] [CrossRef]
- Mi, X.; Wang, P.; Xu, S.; Su, L.; Zhong, H.; Wang, H.; Li, Y.; Zhan, S. Almost 100 % peroxymonosulfate conversion to singlet oxygen on single-atom CoN2+2 Sites. Angew. Chem. Int. Ed. 2021, 60, 4588–4593. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R.-A.; Huang, C.P.; Chen, C.-W.; Dong, C.-D. Activation of persulfate by CoO nanoparticles loaded on 3D mesoporous carbon nitride (CoO@meso-CN) for the degradation of methylene blue (MB). Sci. Total Environ. 2019, 675, 531–541. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Cao, W.; Qiao, L.; Peng, X.; Yu, D.; Wang, S.; Li, C.; Wang, C. Degradation of methylene blue by ellipsoidal β-FeOOH@MnO2 core-shell catalyst: Performance and mechanism. Appl. Surf. Sci. 2023, 619, 156667. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W.; Sun, W.; Yang, C.; Lu, Z.; Li, C. Mechanisms and toxicity evaluation of the degradation of sulfamethoxazole by MPUV/PMS process. Chemosphere 2018, 212, 365–375. [Google Scholar] [CrossRef]
- Nashat, M.; Mossad, M.; El-Etriby, H.K.; Gar Alalm, M. Optimization of electrochemical activation of persulfate by BDD electrodes for rapid removal of sulfamethazine. Chemosphere 2022, 286, 131579. [Google Scholar] [CrossRef] [PubMed]
- Thamaraiselvan, C.; Bandyopadhyay, D.; Powell, C.D.; Arnusch, C.J. Electrochemical degradation of emerging pollutants via laser-induced graphene electrodes. Chem. Eng. J. Adv. 2021, 8, 100195. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Thakur, A.K.; Gupta, A.; Arnusch, C.J. Electrochemical Removal of Organic and Inorganic Pollutants Using Robust Laser-Induced Graphene Membranes. ACS Appl. Mater. Interfaces 2021, 13, 1452–1462. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liao, J.; Li, C.; Xu, Y.; Ge, C.; Xu, W.; He, X.; Liu, W. Laser-Induced Nitrogen-Doped Graphene Composite Iron–Cobalt Hydroxide for Methylene Blue Degradation via Electrocatalytic Activation of Peroxymonosulfate. Catalysts 2023, 13, 922. https://doi.org/10.3390/catal13060922
Chen L, Liao J, Li C, Xu Y, Ge C, Xu W, He X, Liu W. Laser-Induced Nitrogen-Doped Graphene Composite Iron–Cobalt Hydroxide for Methylene Blue Degradation via Electrocatalytic Activation of Peroxymonosulfate. Catalysts. 2023; 13(6):922. https://doi.org/10.3390/catal13060922
Chicago/Turabian StyleChen, Liqin, Jianjun Liao, Chen Li, Yandong Xu, Chengjun Ge, Wen Xu, Xiong He, and Wenyu Liu. 2023. "Laser-Induced Nitrogen-Doped Graphene Composite Iron–Cobalt Hydroxide for Methylene Blue Degradation via Electrocatalytic Activation of Peroxymonosulfate" Catalysts 13, no. 6: 922. https://doi.org/10.3390/catal13060922
APA StyleChen, L., Liao, J., Li, C., Xu, Y., Ge, C., Xu, W., He, X., & Liu, W. (2023). Laser-Induced Nitrogen-Doped Graphene Composite Iron–Cobalt Hydroxide for Methylene Blue Degradation via Electrocatalytic Activation of Peroxymonosulfate. Catalysts, 13(6), 922. https://doi.org/10.3390/catal13060922





