Synthesis of a Series of Methyl Benzoates through Esterification with a Zr/Ti Solid Acid Catalyst
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Catalyst
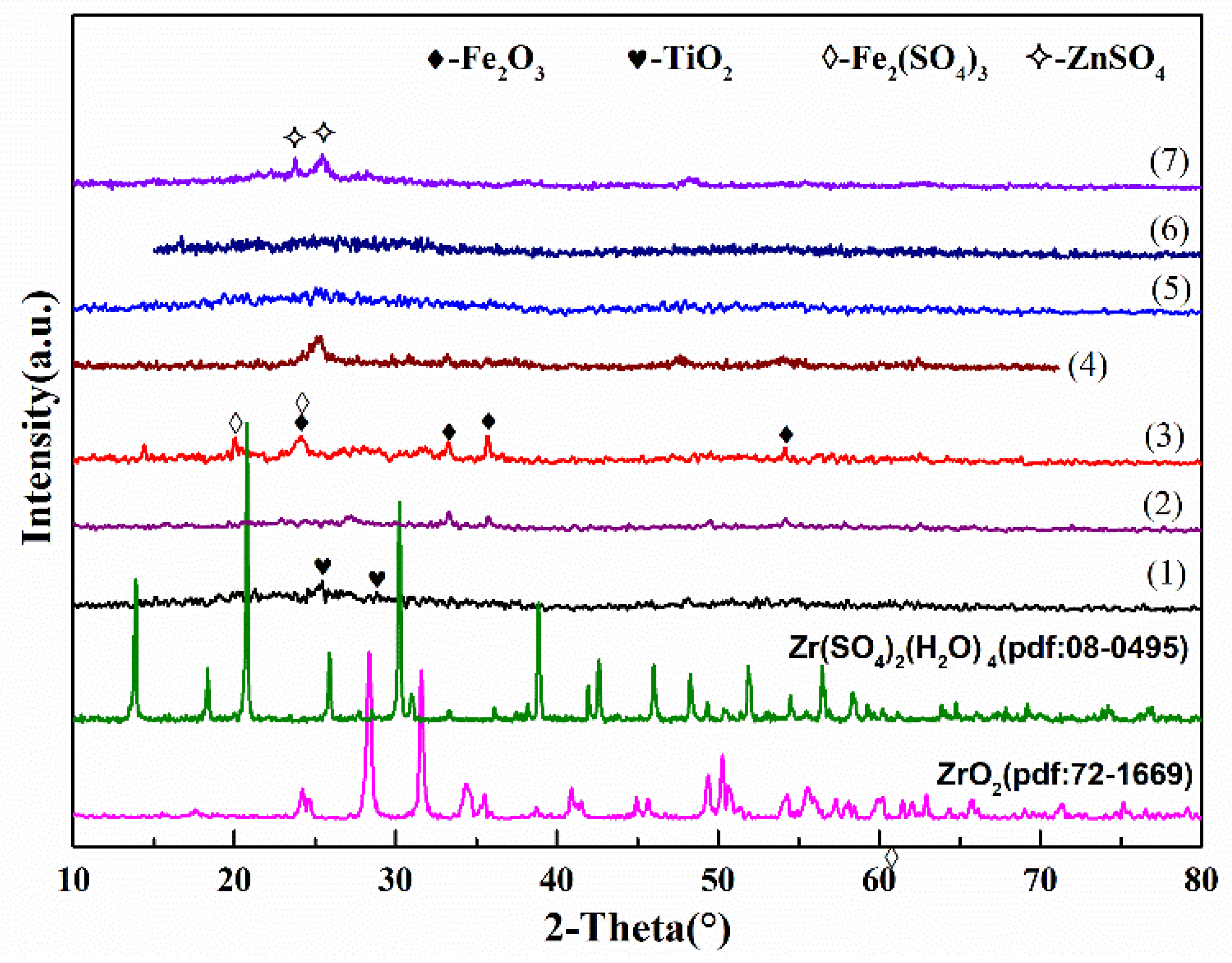
2.1.1. XRD
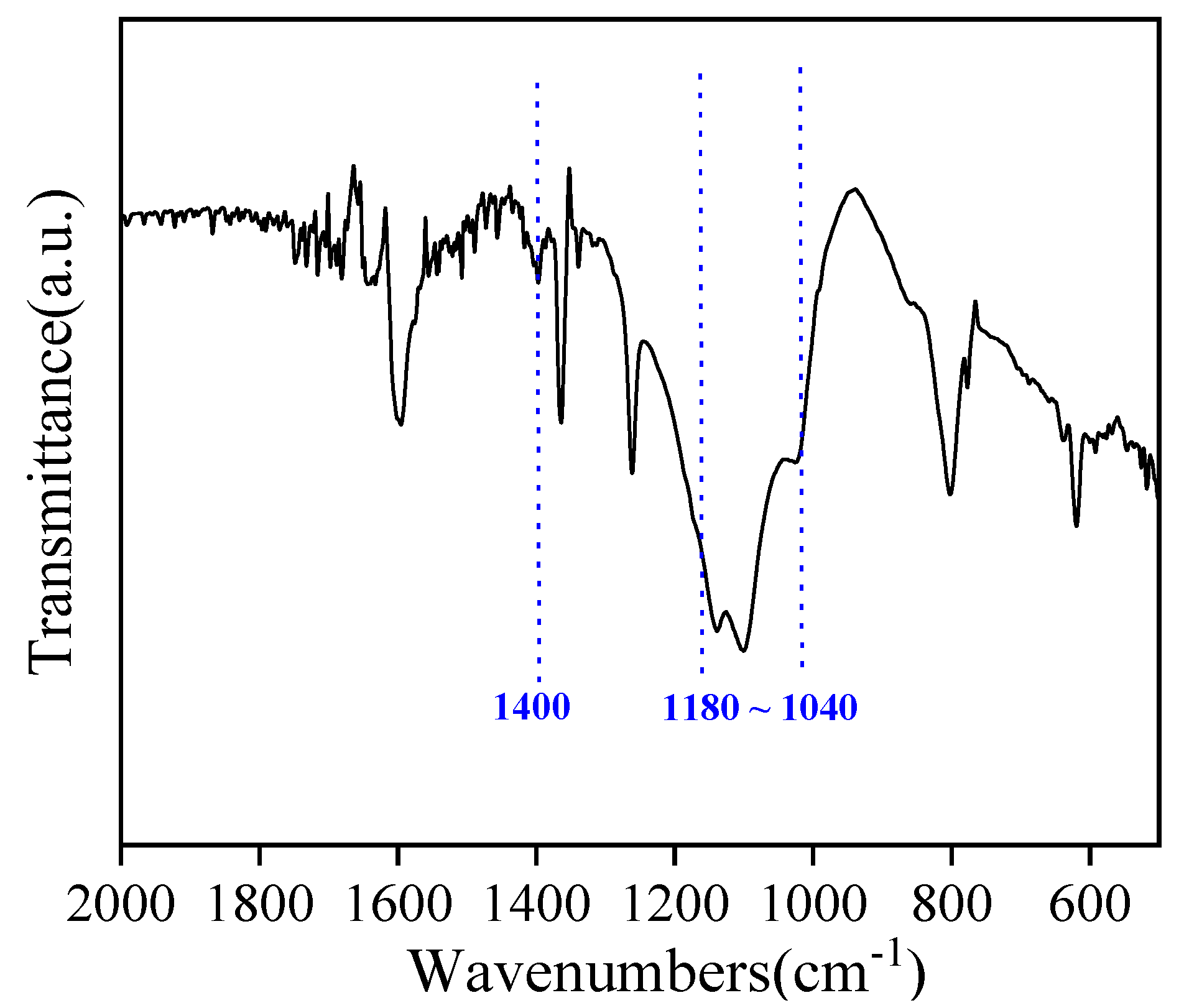
2.1.2. FT-IR
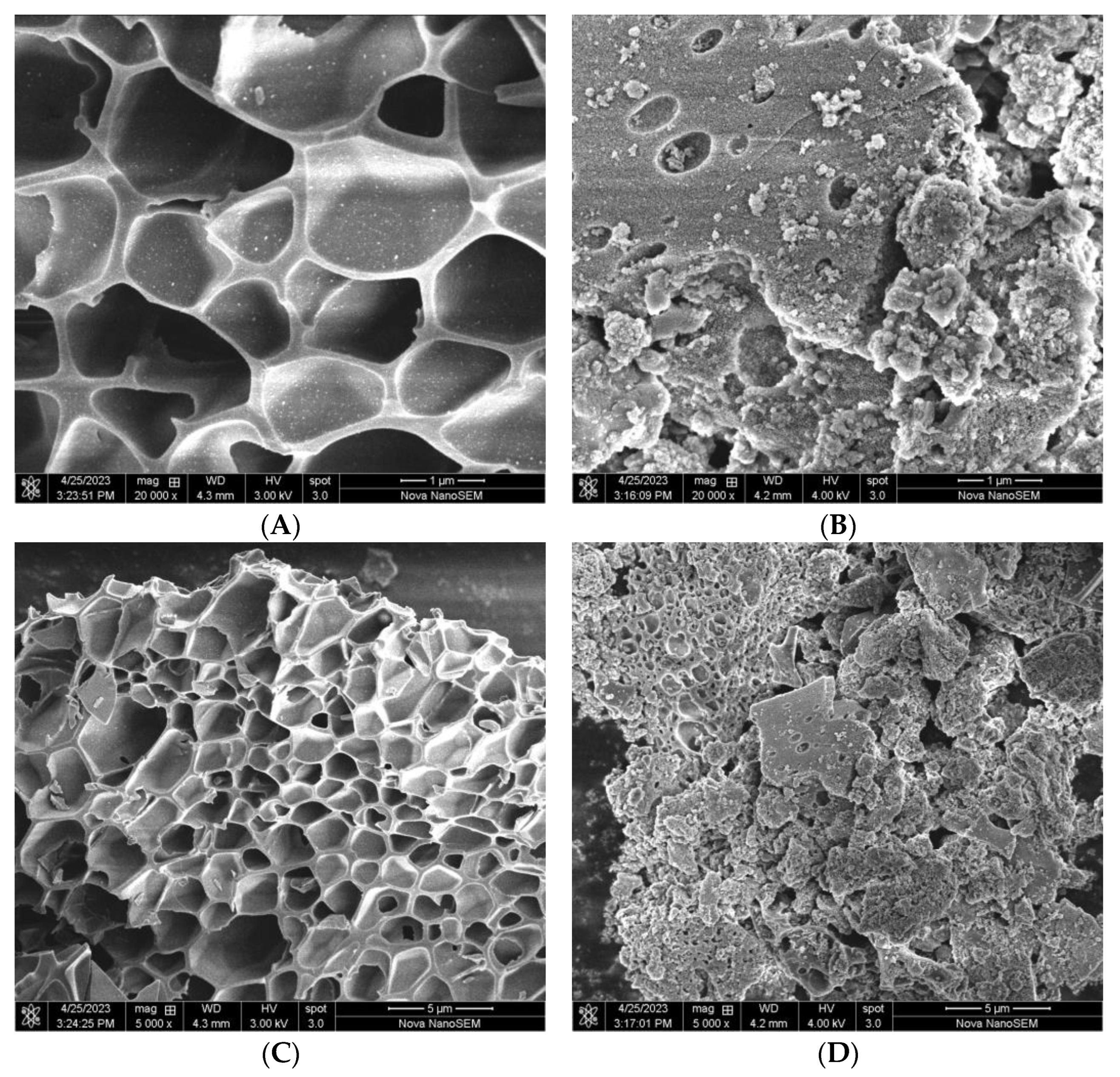
2.1.3. SEM
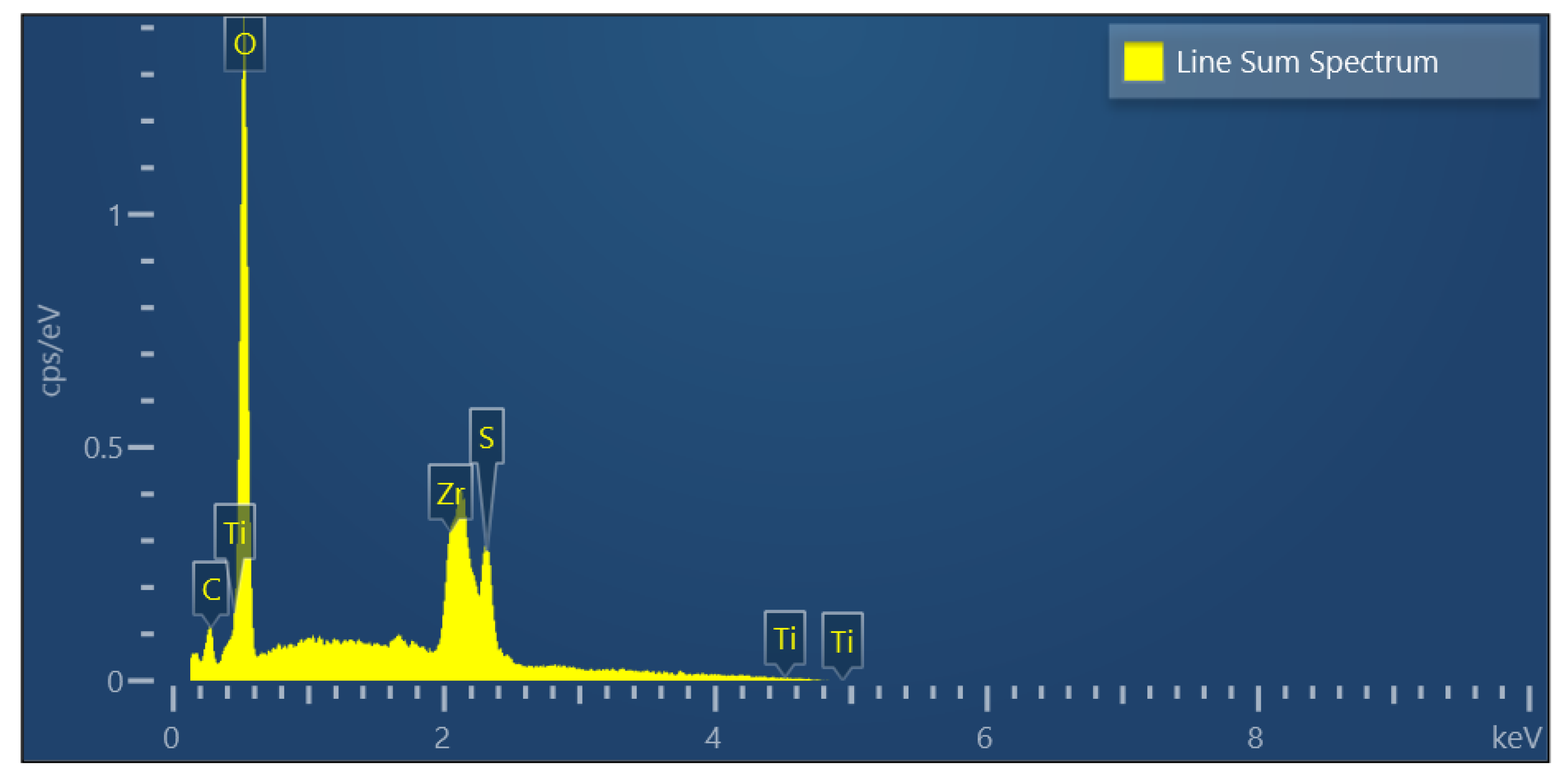
2.1.4. EDS
2.2. Comparison of Catalyst Activity
2.3. Properties and Activity of Preferred Catalyst
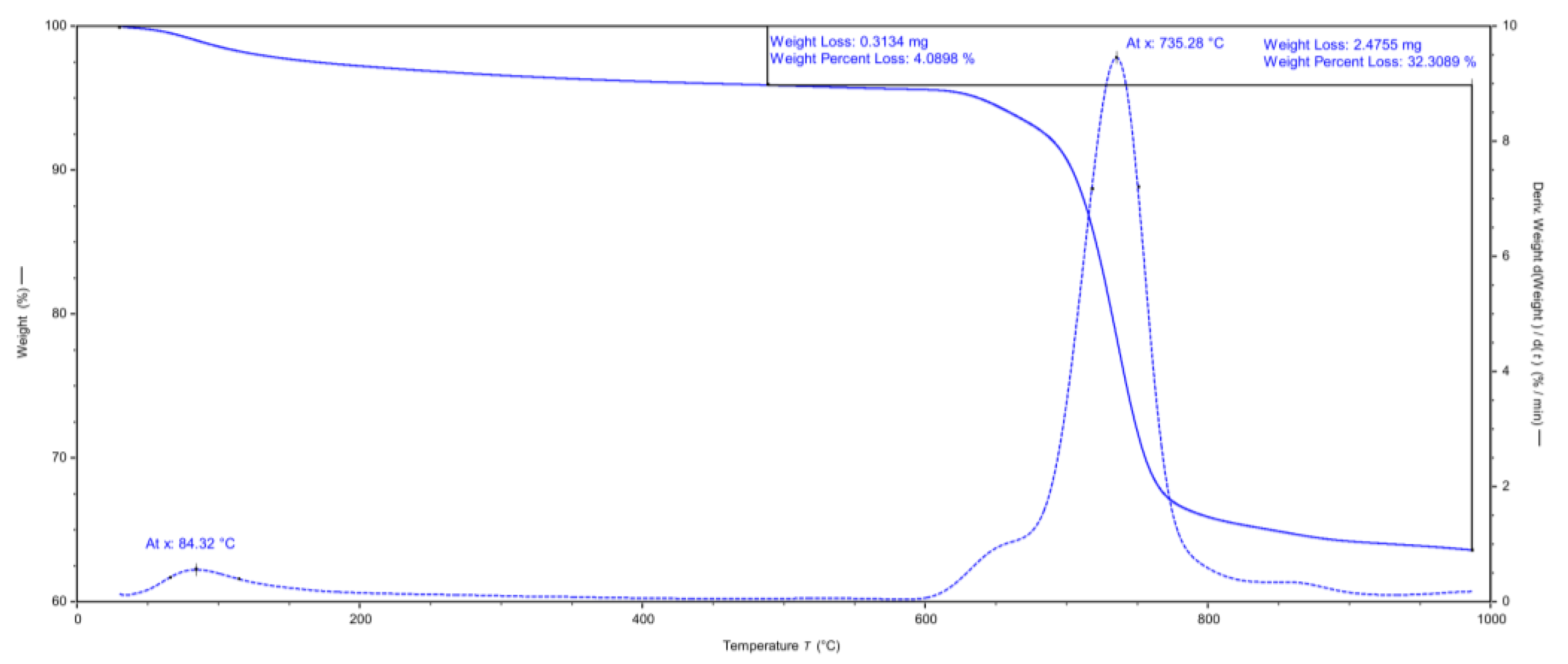
2.4. Properties and Activity of Recovered Catalyst
3. Materials and Methods
3.1. Synthesis of the Catalyst
3.2. General Catalytic Process
3.3. Recovery Catalyst Catalytic Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kapoor, D.; Bhardwaj, S.; Sharma, N.R. Fragrance Stimulation Mechanisms of Flowers and their Regulation under Environmental Constraints. J. Plant Growth Regul. 2023, 42, 60–82. [Google Scholar] [CrossRef]
- Tian, Y.-F.; Chen, Y.-X.; Tong, X.; Hou, S.; Zhao, M.-M.; Feng, Y.-Z. Flavor differences of soybean and defatted soybean fermented soy sauce and its correlation with the enzyme profiles of the kojis. J. Sci. Food Agric. 2023, 103, 606–615. [Google Scholar] [CrossRef]
- El Mansouri, A.-E.; Lachhab, S.; Oubella, A.; Mehdi, A.; Neyts, J.; Jochmans, D.; Chiu, W.; Vangeel, L.; De Jonghe, S.; Morjani, H.; et al. Synthesis, characterization, molecular docking, and anticancer activities of new 1,3,4-oxadiazole-5-fluorocytosine hybrid derivatives. J. Mol. Struct. 2023, 1272, 134135. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Zheng, L.; Gao, Q.; Shi, L.; Liu, Y.; Sun, C.; Zhang, Z.; Xiang, J. New electrotriggers: P-methoxycarbonylbenzyl (pMCB) as an electroremovable protecting group for carboxylic acids, phosphoric acids and alcohols. Green Chem. 2022, 24, 5632–5636. [Google Scholar] [CrossRef]
- Li, J.-G.; Peng, Y.-Q. Efficient Methyl Esterification Using Methoxyl Silica Gel as a Novel Dehydrating Reagent. J. Chin. Chem. Soc. 2010, 57, 305–308. [Google Scholar] [CrossRef]
- Travis, B.R.; Sivakumar, M.; Hollist, G.O.; Borhan, B. Facile Oxidation of Aldehydes to Acids and Esters with Oxone. Org. Lett. 2003, 5, 1031–1034. [Google Scholar] [CrossRef]
- Talukdar, D.; Sharma, K.; Bharadwaj, S.K.; Thakur, A.J. VO (acac)2: An Efficient Catalyst for the Oxidation of Aldehydes to the Corresponding Acids in the Presence of Aqueous H2O2. Synlett 2013, 24, 963–966. [Google Scholar] [CrossRef]
- Han, J.; Gu, F.; Li, Y. N-Doped Sub-3 nm Co Nanoparticles as Highly Efficient and Durable Aerobic Oxidative Coupling Catalysts. Chem.-Asian J. 2016, 11, 2594–2601. [Google Scholar] [CrossRef]
- Lerebours, R.; Wolf, C. Chemoselective Nucleophilic Arylation and Single-Step Oxidative Esterification of Aldehydes Using Siloxanes and a Palladium−Phosphinous Acid as a Reaction Switch. J. Am. Chem. Soc. 2006, 128, 13052–13053. [Google Scholar] [CrossRef]
- Subramanian, K.; Yedage, S.L.; Bhanage, B.M. An Electrochemical Method for Carboxylic Ester Synthesis from N-Alkoxyamides. J. Org. Chem. 2017, 82, 10025–10032. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Yan, J.; Liu, S.; Liu, H.; Liu, Z.; Wang, W.; He, Z.; Han, B. Aerobic Oxidative Cleavage and Esterification of C(OH)–C Bonds. Chem 2020, 6, 3288–3296. [Google Scholar] [CrossRef]
- Mao, F.; Qi, Z.; Fan, H.; Sui, D.; Chen, R.; Huang, J. Heterogeneous cobalt catalysts for selective oxygenation of alcohols to aldehydes, esters and nitriles. RSC Adv. 2017, 7, 1498–1503. [Google Scholar] [CrossRef]
- Chng, L.L.; Yang, J.; Ying, J.Y. Efficient Synthesis of Amides and Esters from Alcohols under Aerobic Ambient Conditions Catalyzed by a Au/Mesoporous Al2O3 Nanocatalyst. ChemSusChem 2015, 8, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Meng, L.; Deng, Y.; Li, Y.; Lei, A. Palladium-Catalyzed Aerobic Oxidative Direct Esterification of Alcohols. Angew. Chem. Int. Ed. 2011, 50, 5144–5148. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Wang, J. Palladium-Catalyzed Cyclizative Borylation of Allenyl Ketones through Carbene Boryl Migratory Insertion: Access to Densely Substituted Furyl Boronates. Chem.-A Eur. J. 2023, 29, e2022036972023. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, S.; Zhu, J.; Niu, Y.; Xiong, W.; Chen, F. Identification of key aromas of grapefruit juice and study of their contributions to the enhancement of sweetness perception. Eur. Food Res. Technol. 2023, 249, 537–551. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; He, L.; Li, C.; Tao, H.; Wang, X.; Liu, L.; Zeng, X.; Ran, G. Effects of perilla seed oil addition on the physicochemical properties, sensory, and volatile compounds of potato blueberry flavored yogurt and its shelf-life prediction. LWT 2023, 173, 114383. [Google Scholar] [CrossRef]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria fermentation. Food Biosci. 2023, 51, 102337. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Cai, S.X. New reagents for photoaffinity labeling: Synthesis and photolysis of functionalized perfluorophenyl azides. J. Org. Chem. 1990, 55, 3640–3647. [Google Scholar] [CrossRef]
- De Oliveira, C.S.; Lira, B.F.; Dos Santos Falcão-Silva, V.; Siqueira-Junior, J.P.; Barbosa-Filho, J.M.; De Athayde-Filho, P.F. Synthesis, Molecular Properties Prediction, and Anti-staphylococcal Activity of N-Acylhydrazones and New 1,3,4-Oxadiazole Derivatives. Molecules 2012, 17, 5095–5107. [Google Scholar] [CrossRef] [PubMed]
- Štefane, B.; Kočevar, M.; Polanc, S. Ceric (IV) Ammonium nitrate mediated transesterification and esterification. Synth. Commun. 2002, 32, 1703–1707. [Google Scholar] [CrossRef]
- Martínez, R.; Zamudio, G.J.N.; Pretelin-Castillo, G.; Torres-Ochoa, R.O.; Medina-Franco, J.L.; Pinzón, C.I.E.; Miranda, M.S.; Hernández, E.; Alanís-Garza, B. Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl)carboxamides. Heterocycl. Commun. 2019, 25, 52–59. [Google Scholar] [CrossRef]
- Roy, S.; Samanta, D.; Kumar, P.; Maji, T.K. Pure white light emission and charge transfer in organogels of symmetrical and unsymmetrical π-chromophoric oligo-p-(phenyleneethynylene) bola-amphiphiles. Chem. Commun. 2018, 54, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.D.; Yodsanit, N.; Melander, C. Potentiation of the fosmidomycin analogue FR 900098 with substituted 2-oxazolines against Francisella novicida. MedChemComm 2016, 7, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Gianni, J.; Pirovano, V.; Abbiati, G. Silver triflate/p-TSA co-catalysed synthesis of 3-substituted isocoumarins from 2-alkynylbenzoates. Org. Biomol. Chem. 2018, 16, 3213–3219. [Google Scholar] [CrossRef]
- Sekewael, S.J.; Pratika, R.A.; Hauli, L.; Amin, A.K.; Utami, M.; Wijaya, K. Recent Progress on Sulfated Nanozirconia as a Solid Acid Catalyst in the Hydrocracking Reaction. Catalysts 2022, 12, 191. [Google Scholar] [CrossRef]
- He, X.; Guo, X.; Xia, G.; Xu, R.; Wu, Y.; Luan, X. A Green Route to Methyl Formate from CO2-Derived Formamides over Solid Base Catalysts. Catalysts 2023, 13, 487. [Google Scholar] [CrossRef]
- Saravanan, K.; Tyagi, B.; Shukla, R.S.; Bajaj, H.C. Esterification of palmitic acid with methanol over template-assisted mesoporous sulfated zirconia solid acid catalyst. Appl. Catal. B Environ. 2015, 172, 108–115. [Google Scholar] [CrossRef]
- Saravanan, K.; Tyagi, B.; Shukla, R.S.; Bajaj, H.C. Solvent free synthesis of methyl palmitate over sulfated zirconia solid acid catalyst. Fuel 2016, 165, 298–305. [Google Scholar] [CrossRef]
- Ameen, M.; Zafar, M.; Ramadan, M.F.; Ahmad, M.; Makhkamov, T.; Bokhari, A.; Mubashir, M.; Chuah, L.F.; Show, P.L. Conversion of novel non-edible Bischofia javanica seed oil into methyl ester via recyclable zirconia-based phyto-nanocatalyst: A circular bioeconomy approach for eco-sustenance. Environ. Technol. Innov. 2023, 30, 103101. [Google Scholar] [CrossRef]
- Sharghi, H.; Sarvari, M.H. Al2O3/MeSO3H (AMA) as a new reagent with high selective ability for monoesterification of diols. Tetrahedron 2003, 59, 3627–3633. [Google Scholar] [CrossRef]
- Xu, Q.-H.; Liu, W.-Y.; Chen, B.-H.; Ma, Y.-X. An improved method for the esterification of aromatic acids with ethanol and methanol. Synth. Commun. 2001, 31, 2113–2117. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Huang, J.; Tong, J.; Liu, X.; Wang, Y.; Qiao, W.; Han, J. Theoretical and experimental insight into plasma-catalytic degradation of aqueous p-nitrophenol with graphene-ZnO nanoparticles. Sep. Purif. Technol. 2022, 295, 121362. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yao, X.; Zhang, Y.; Li, Z.; Pan, S.; Han, J.; Xu, L.; Qiao, W.; Li, J.; et al. A comprehensive insight into plasma-catalytic removal of antibiotic oxytetracycline based on graphene-TiO2-Fe3O4 nanocomposites. Chem. Eng. J. 2021, 425, 130614. [Google Scholar] [CrossRef]
- Chavan, S.P.; Dantale, S.W.; Govande, C.A.; Venkatraman, M.S.; Praveen, C. Titanosilicate (TS-1) Catalyzed Oxidation of Aromatic Aldehydes to Esters. Synlett 2002, 2, 0267–0268. [Google Scholar] [CrossRef]
- Jaoui, M.; Kleindienst, T.E.; Lewandowski, M.; Edney, E.O. Identification and Quantification of Aerosol Polar Oxygenated Compounds Bearing Carboxylic or Hydroxyl Groups. 1. Method Development. Anal. Chem. 2004, 76, 4765–4778. [Google Scholar] [CrossRef]
- Gopinath, R.; Barkakaty, B.; Talukdar, B.; Patel, B.K. Peroxovanadium-Catalyzed Oxidative Esterification of Aldehydes. J. Org. Chem. 2003, 68, 2944–2947. [Google Scholar] [CrossRef]
- El-Damasy, A.K.; Jin, H.; Seo, S.H.; Bang, E.-K.; Keum, G. Design, synthesis, and biological evaluations of novel 3-amino-4-ethynyl indazole derivatives as Bcr-Abl kinase inhibitors with potent cellular antileukemic activity. Eur. J. Med. Chem. 2020, 207, 112710. [Google Scholar] [CrossRef]
- Jiang, Y.; Pan, S.; Zhang, Y.; Yu, J.; Liu, H. Copper-Catalyzed Decarboxylative Methylation of AromaticCarboxylic Acids with PhI(OAc)2. Eur. J. Org. Chem. 2014, 10, 2027–2031. [Google Scholar] [CrossRef]
- Senaweera, S.M.; Singh, A.; Weaver, J.D. Photocatalytic Hydrodefluorination: Facile Access to Partially Fluorinated Aromatics. J. Am. Chem. Soc. 2014, 136, 3002–3005. [Google Scholar] [CrossRef]
- Ross, J.P.; Couture, P.; Warkentin, J. Nucleophilic aromatic substitution with dialkoxycarbenes. Can. J. Chem. 1997, 75, 1331–1335. [Google Scholar] [CrossRef]
| No. | Raw Material/g | Raw Material/g | ||
|---|---|---|---|---|
| 1 | ZrOCl2·8H2O | 10 g | (C4H9O)4Ti | 10.56 g |
| 2 | ZrOCl2·8H2O | 10 g | CdCl2 | 5.69 g |
| 3 | ZrOCl2·8H2O | 10 g | FeCl3 | 5.03 g |
| 4 | ZrOCl2·8H2O | 10 g | - | - |
| 5 | ZrOCl2·8H2O | 10 g | AlCl3 | 4.14 g |
| 6 | ZrOCl2·8H2O | 10 g | (C2H5O)4Si | 6.46 g |
| 7 | ZrOCl2·8H2O | 10 g | ZnCl2 | 4.23 g |
| Entry | Zr:C c | Molar Ratio | 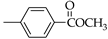 | 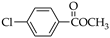 |
|---|---|---|---|---|
| 1 | Zr:Ti | 1:1 | 55.9% | 52.7% |
| 2 | Zr:Cd | 1:1 | 49.9% | 35.8% |
| 3 | Zr:Fe | 1:1 | 50.4% | 50.4% |
| 4 | Zr | - | 10.0% | 7.2% |
| 5 | Zr:Al | 1:1 | 9.0% | 14.6% |
| 6 | Zr:Si | 1:1 | 19.9% | 21.5% |
| 7 | Zr:Zn | 1:1 | 51.2% | 50.1% |
| 8 | Zr:Ti | 1:0.5 | 50.4% | 50.3% |
| 9 | Zr:Ti | 0.5:1 | 43.1% | 45.5% |
| 10 | Zr:Ti | 1.5:1 | 66.1% | 68.1% |
| 11 | Zr:Ti | 1.2:1 | 60.3% | 62.1% |
| 12 | Zr:Ti | 2:1 | 40.5% | 45.2% |
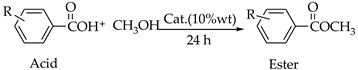 | |||||
|---|---|---|---|---|---|
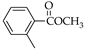 | 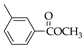 | 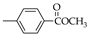 |  | 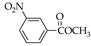 | 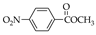 |
| 1 85.2% | 2 87.8% | 3 91.2% | 19 20.1% | 20 34.6% | 21 45.2% |
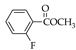 | 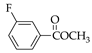 | 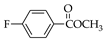 | 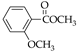 | 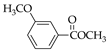 | 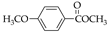 |
| 4 84.2% | 5 87.8% | 6 91.1% | 22 88.2% | 23 86.5% | 24 95.7% |
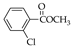 | 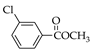 | 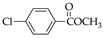 | 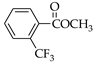 | 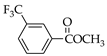 | 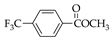 |
| 7 83.1% | 8 83.4% | 9 86.3% | 25 29.1% | 26 33.4% | 27 55.0% |
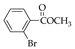 | 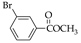 |  | 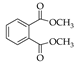 |  | 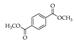 |
| 10 70.1% | 11 79.3% | 12 83.9% | 28 34.2% | 29 45.6% | 30 60.3% |
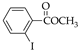 | 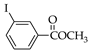 | 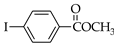 | 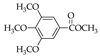 |  | |
| 13 70.0% | 14 74.2% | 15 79.3% | 31 50.2% | 32 75.6% | |
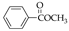 | 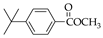 | 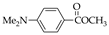 | |||
| 16 90.5% | 17 91.7% | 18 93.3% | |||
| Samples | Surface Area (m2/g) | Pore Volume (cm−3g−1) | Pore Diameter (nm) |
|---|---|---|---|
| Catalyst (zirconium: titanium = 1.2:1) | 242 | 0.210 | 2.32 |
| Regenerated Catalyst (zirconium: titanium = 1.2:1) | 248 | 0.205 | 2.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Shi, C.; Cheng, Y.; Zhu, Y.; Song, R.; Hu, S. Synthesis of a Series of Methyl Benzoates through Esterification with a Zr/Ti Solid Acid Catalyst. Catalysts 2023, 13, 915. https://doi.org/10.3390/catal13050915
Yu X, Shi C, Cheng Y, Zhu Y, Song R, Hu S. Synthesis of a Series of Methyl Benzoates through Esterification with a Zr/Ti Solid Acid Catalyst. Catalysts. 2023; 13(5):915. https://doi.org/10.3390/catal13050915
Chicago/Turabian StyleYu, Xiaofeng, Chunjie Shi, Yueling Cheng, Yejing Zhu, Renyuan Song, and Shengfei Hu. 2023. "Synthesis of a Series of Methyl Benzoates through Esterification with a Zr/Ti Solid Acid Catalyst" Catalysts 13, no. 5: 915. https://doi.org/10.3390/catal13050915
APA StyleYu, X., Shi, C., Cheng, Y., Zhu, Y., Song, R., & Hu, S. (2023). Synthesis of a Series of Methyl Benzoates through Esterification with a Zr/Ti Solid Acid Catalyst. Catalysts, 13(5), 915. https://doi.org/10.3390/catal13050915









