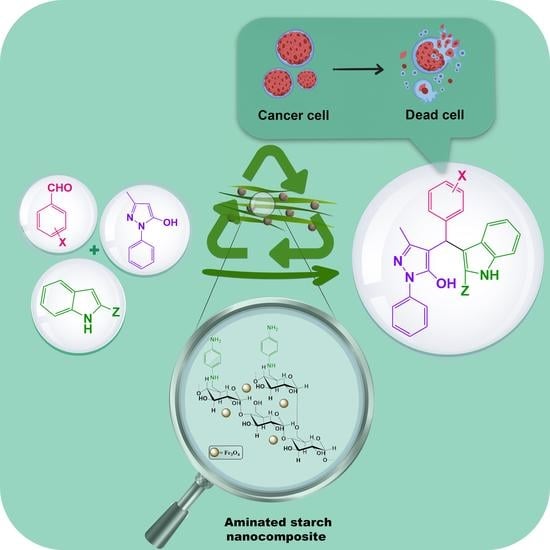
Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst
Abstract
1. Introduction
2. Results and Discussions
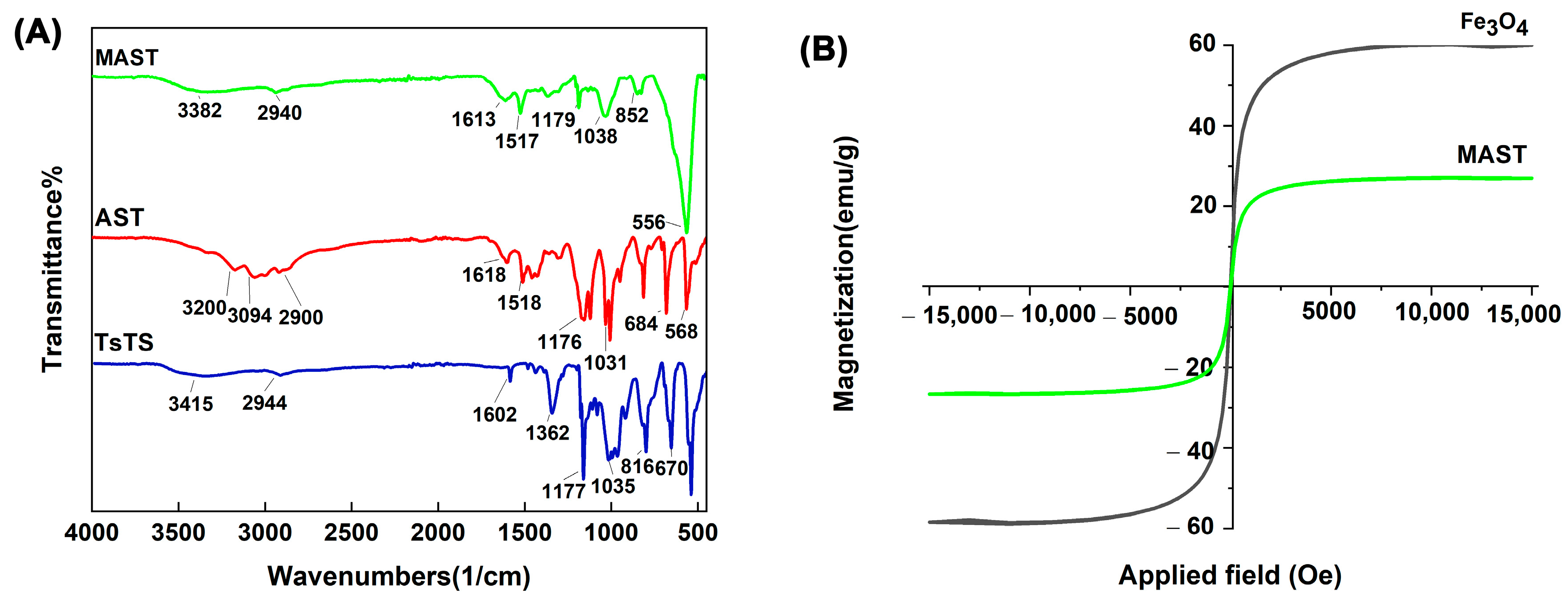
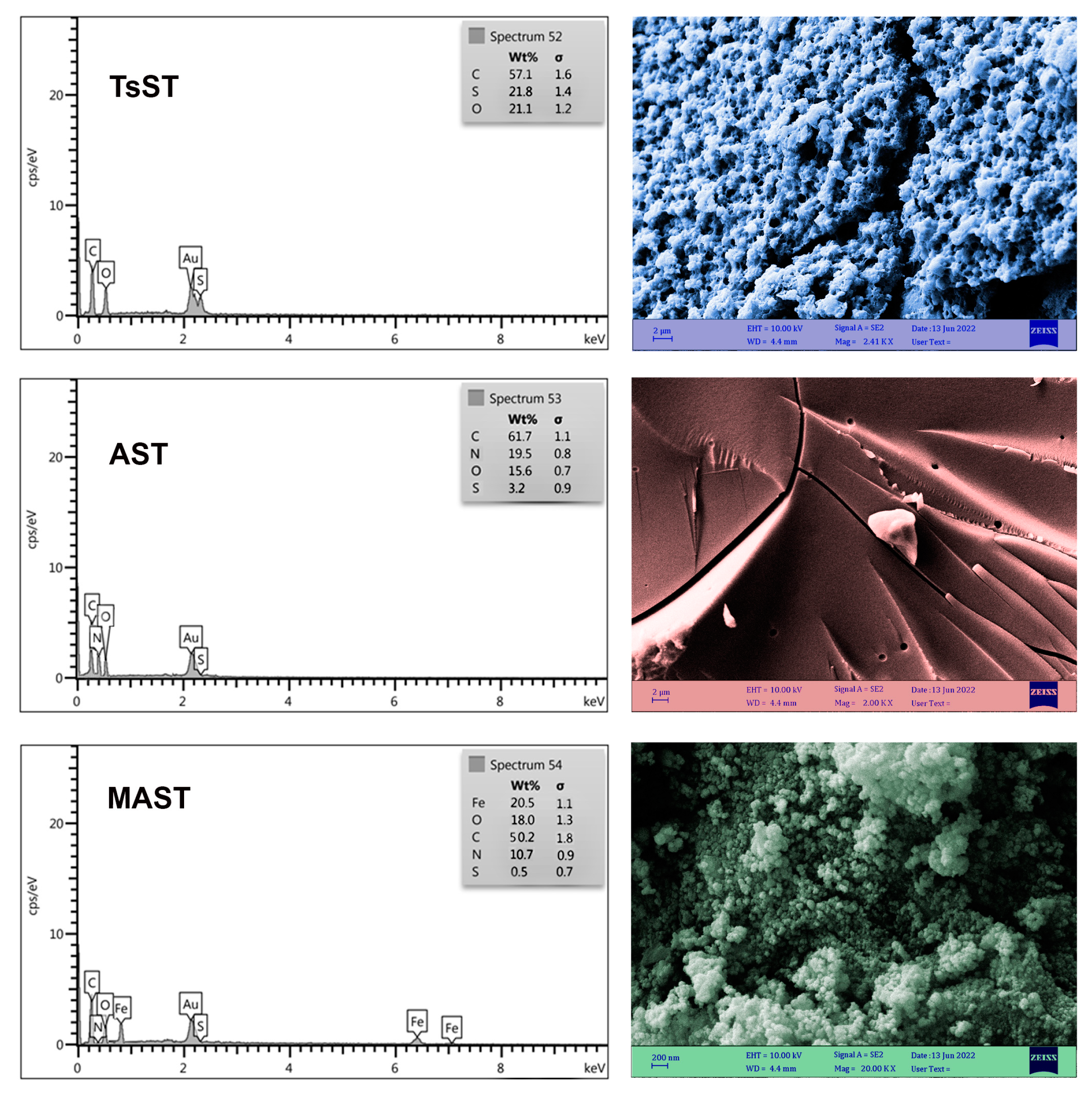
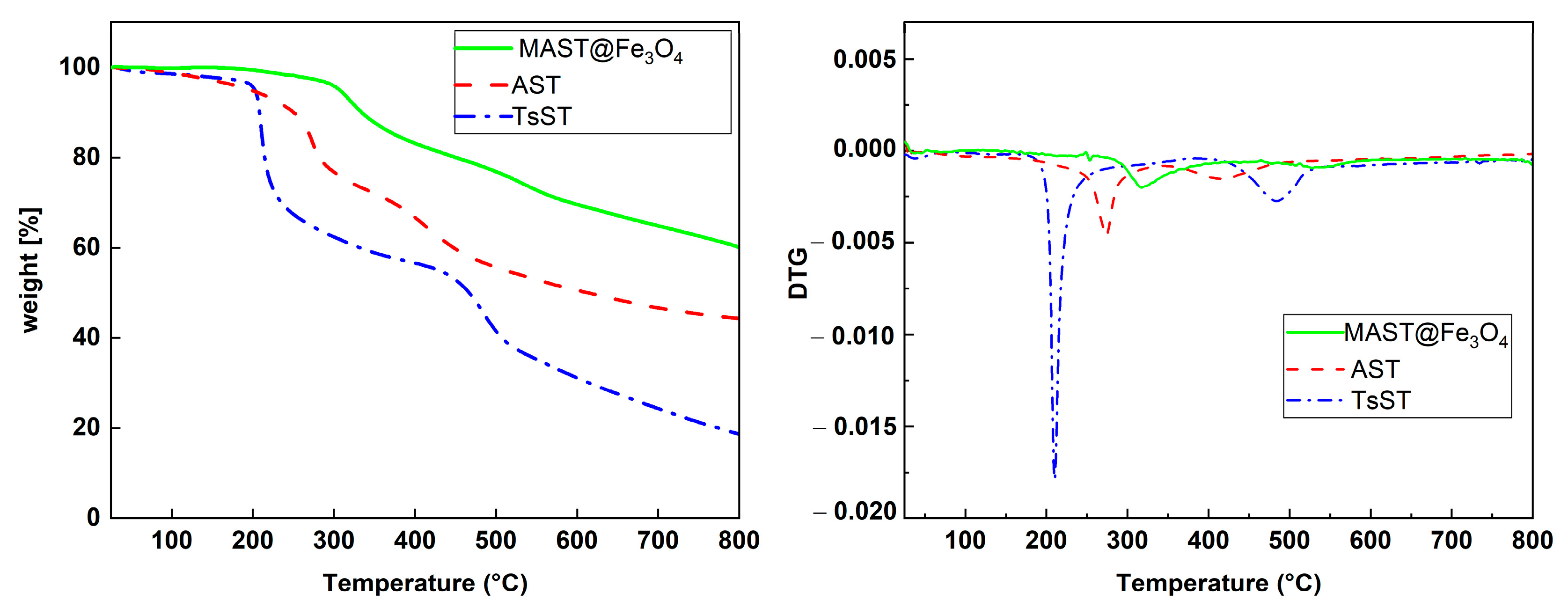
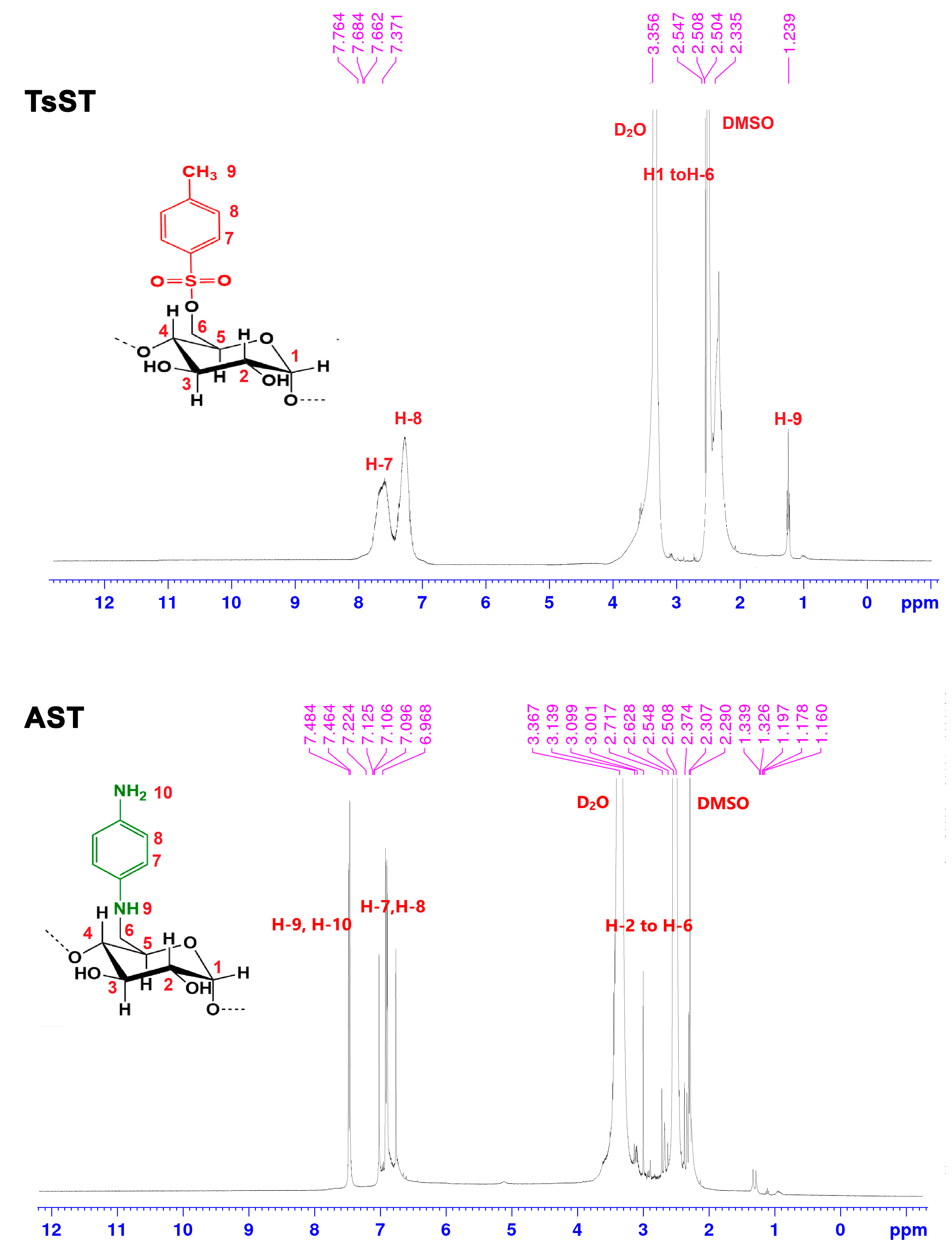
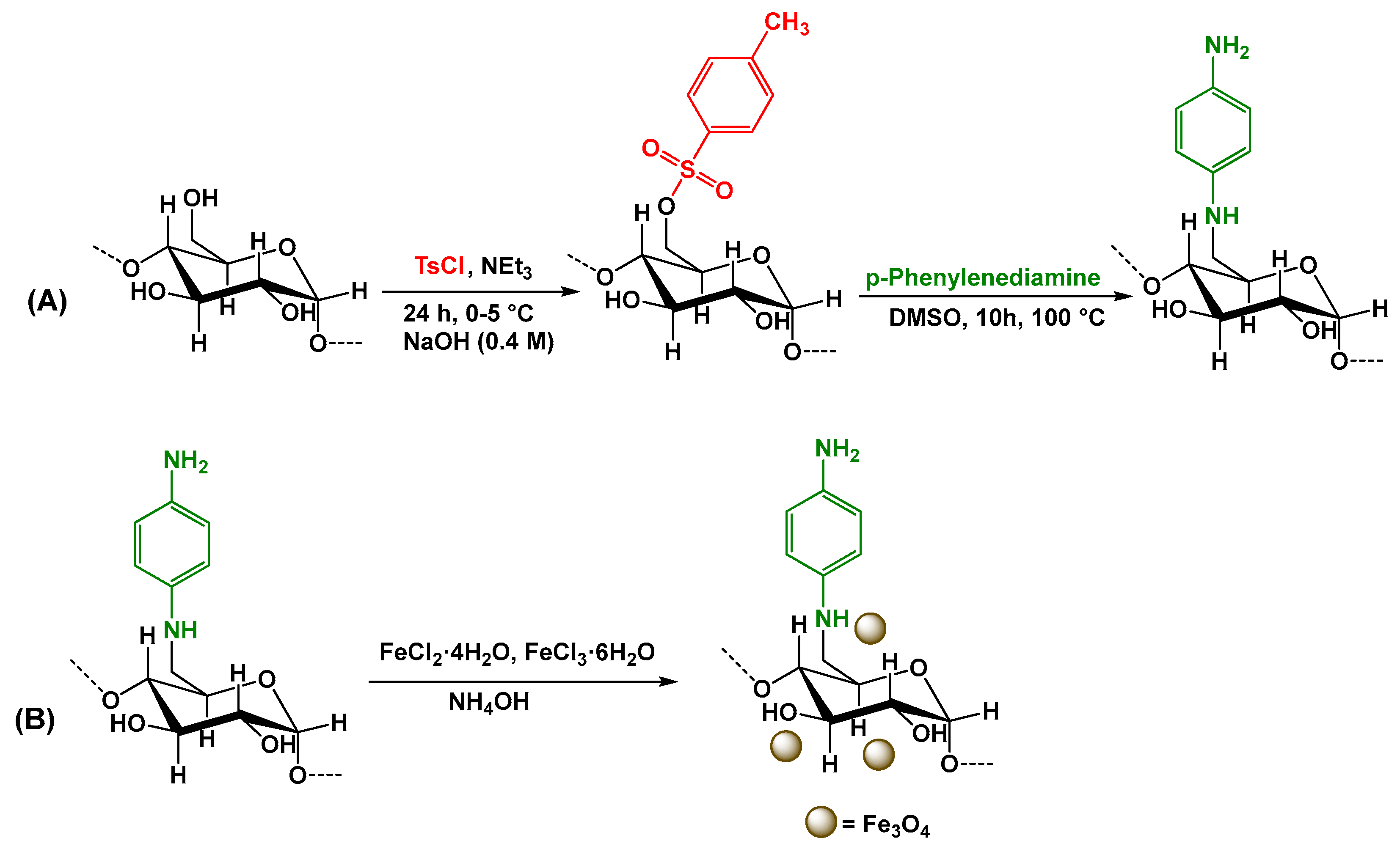
2.1. Characterization of Biocatalyst
2.2. Investigation of the Catalytic Activity of the MAST
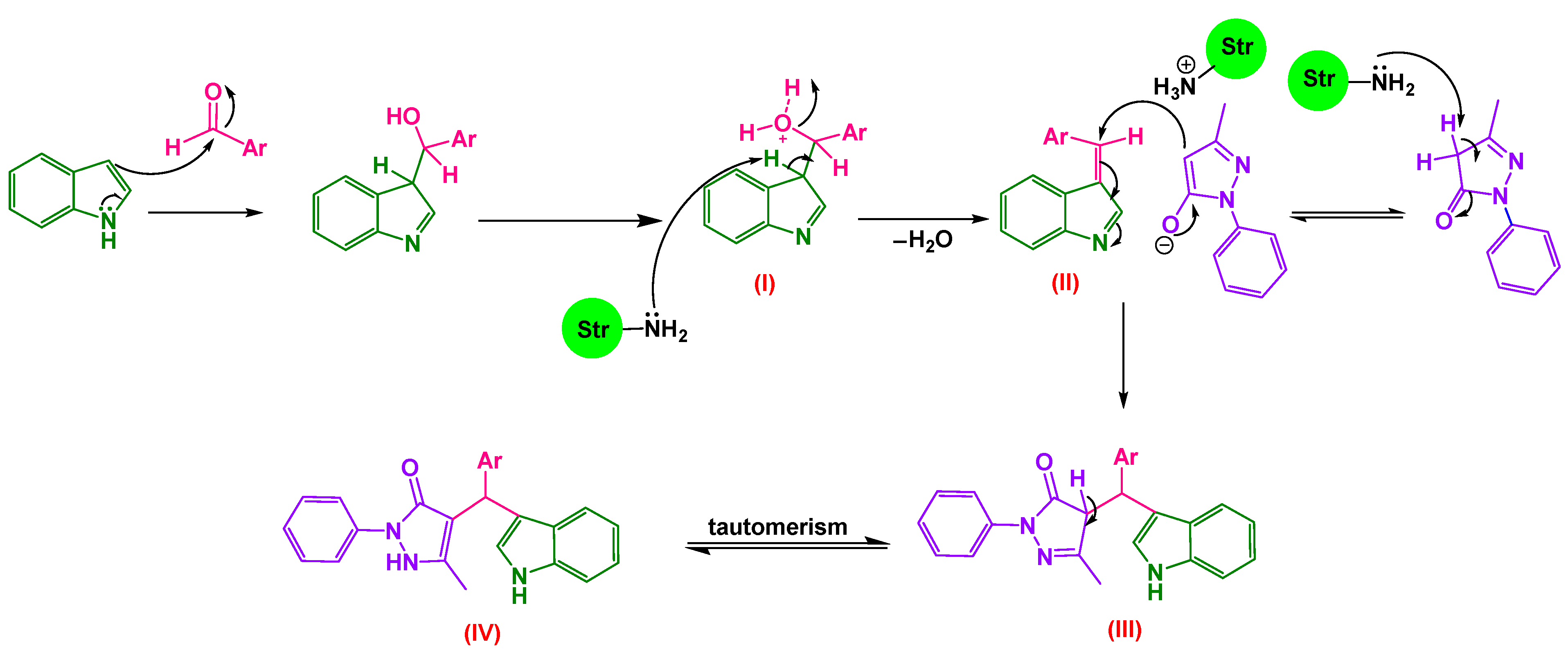
2.2.1. Proposed Mechanism
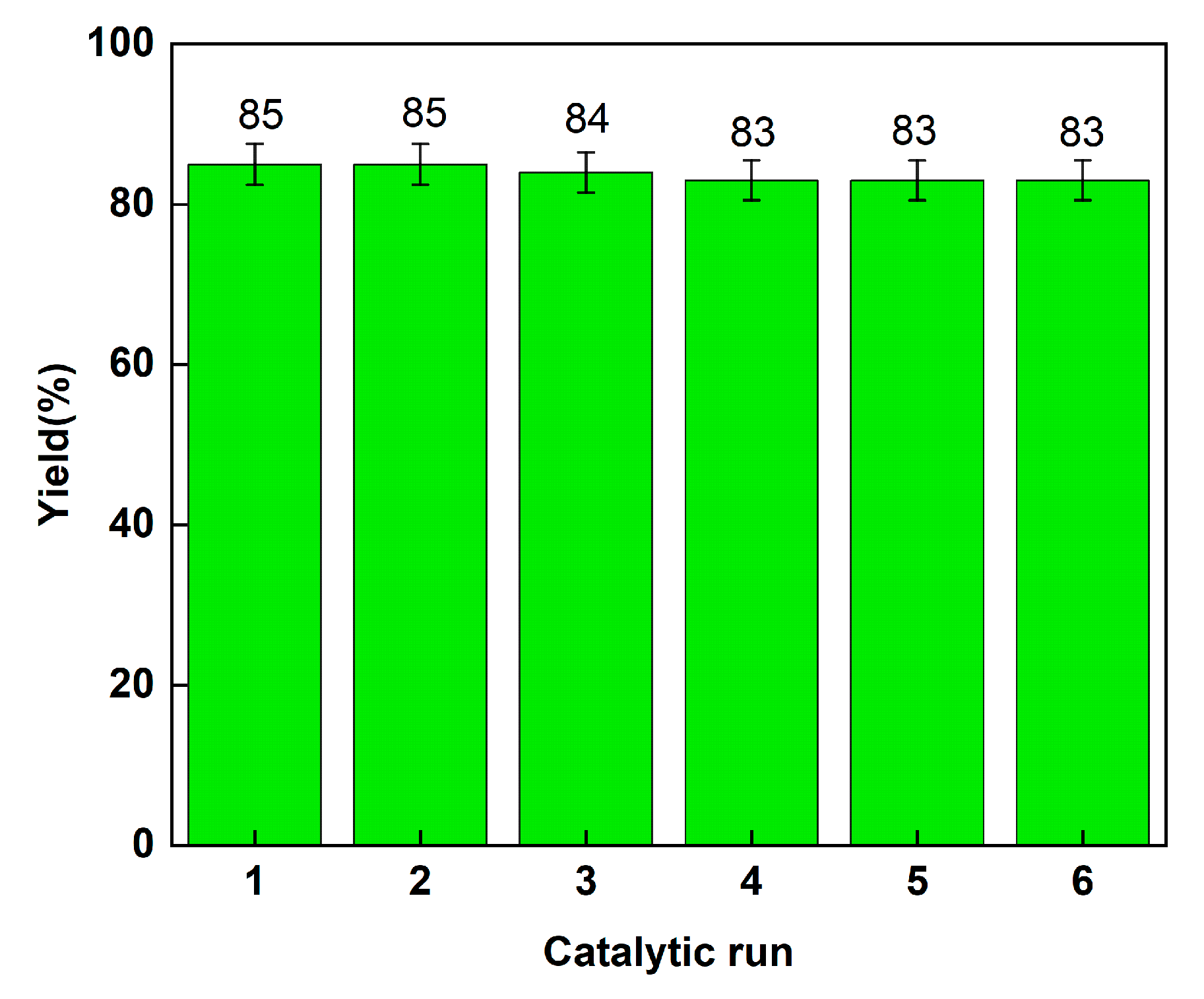
2.2.2. Recovery
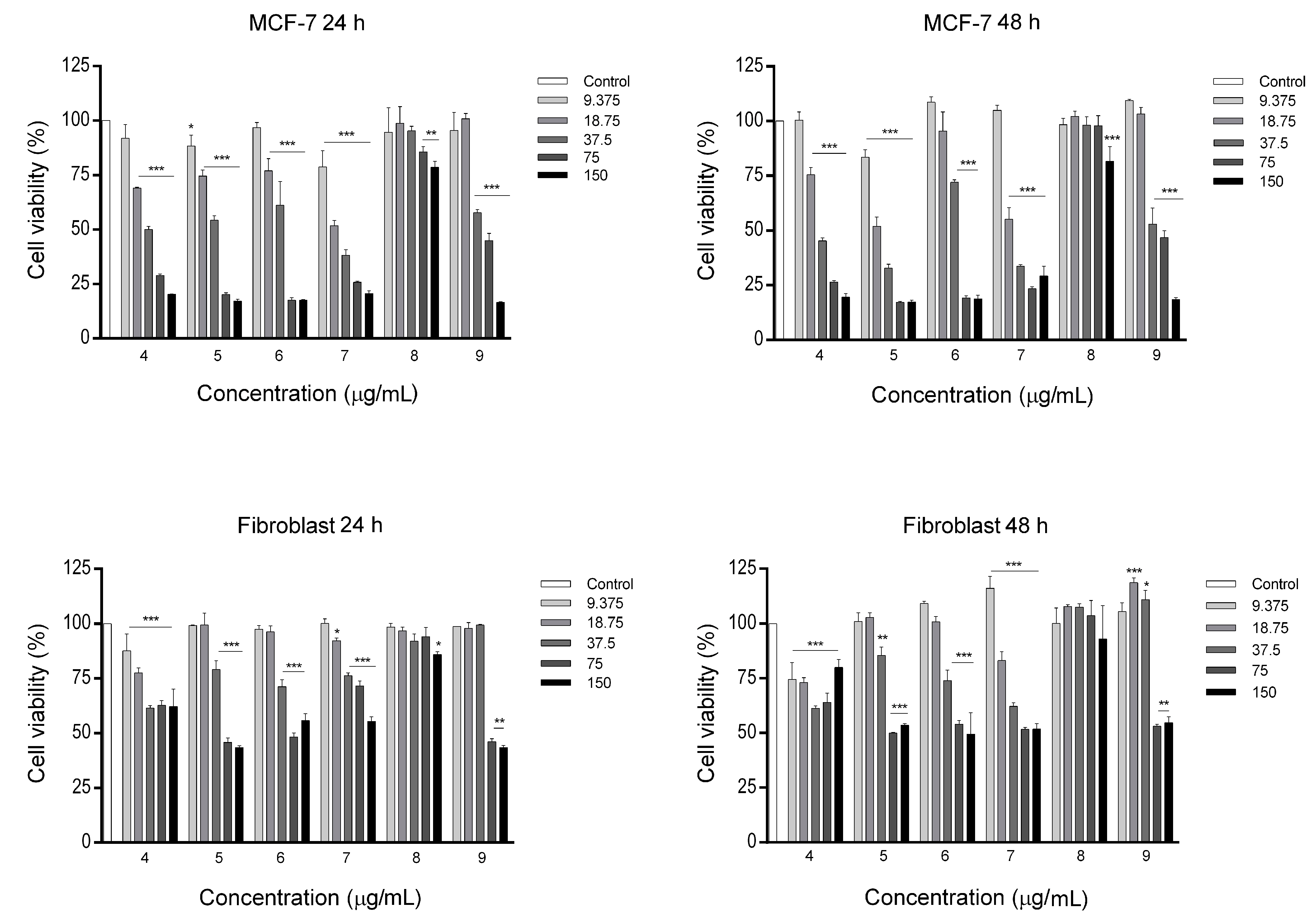
2.3. In-Vitro Anticancer Study
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Aminated Starch
3.3. Preparation of Magnetic Aminated Starch
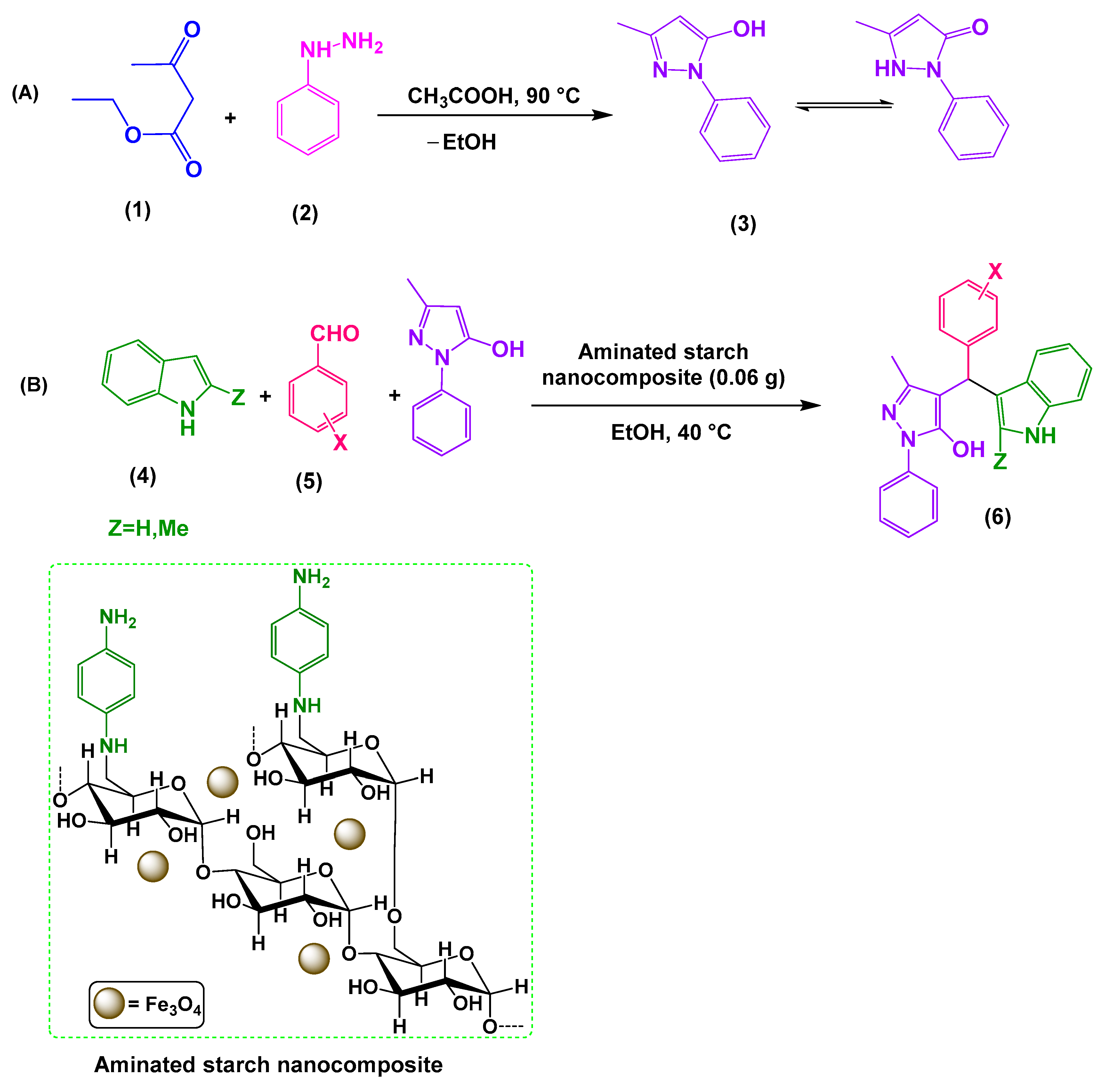
3.4. Synthesis of 3-Methyl-1-phenyl-1H-pyrazole5-ol: A General Procedure
3.5. Synthesis of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolones
3.6. In Vitro Cell Viability Assay
4. Conclusions
5. Spectroscopic Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heidarizadeh, F.; Rahimi, E. Synthesis of 3-substituted Indoles through Yonemitsu Reaction with Copper Benzene-1,3,5-tricarboxylate acid Catalyst. Mater. Chem. Horizons 2022, 1, 169–176. [Google Scholar] [CrossRef]
- Asadi Nasr, M.; Deinavizadeh, M.; Kiasat, A.R. Fe3O4@SiO2/DABCO(OH) Core-Shell Hybrid Nanocomposite: Efficient Nanomagnetic and Basic Reusable Catalyst in the One-pot Synthesis of Trithiocarbonate Derivatives. Mater. Chem. Horizons. 2023; in press. [Google Scholar] [CrossRef]
- Reddy, K.R.; Rajgopal, K.; Kantam, M.L. Copper-alginates: A biopolymer supported Cu(II) catalyst for 1,3-dipolar cycloaddition of alkynes with azides and oxidative coupling of 2-naphthols and phenols in water. Catal. Letters 2007, 114, 36–40. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Motamedi, A.; Hosseini, S.H.; Nazari, M. Magnetic starch nanocomposite as a green heterogeneous support for immobilization of large amounts of copper ions: Heterogeneous catalyst for click synthesis of 1,2,3-triazoles. RSC Adv. 2016, 6, 19128–19135. [Google Scholar] [CrossRef]
- Zareh, E.N.; Moghadam, P.N.; Azariyan, E.; Sharifian, I. Conductive and biodegradable polyaniline/starch blends and their composites with polystyrene. Iran. Polym. J. 2011, 20, 319–328. [Google Scholar]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Alizadeh, E.; Baseri, H. Photocatalytic degradation of Sumatriptan Succinate by ZnO, Fe doped ZnO and TiO2-ZnO nanocatalysts. Mater. Chem. Horizons 2022, 1, 7–21. [Google Scholar] [CrossRef]
- Heidari, G.; Hassanpour, M.; Nejaddehbashi, F.; Sarfjoo, M.R.; Yousefiasl, S.; Sharifi, E.; Bigham, A.; Agarwal, T.; Borzacchiello, A.; Lagreca, E.; et al. Biosynthesized Nanomaterials with Antioxidant and Antimicrobial Properties. Mater. Chem. Horizons 2022, 1, 35–48. [Google Scholar] [CrossRef]
- Ghorbanipour, F.; Nezhad, S.M.; Pourmousavi, S.A.; Zare, E.N.; Heidari, G. Superparamagnetic polymer nanocomposite as a catalyst for the synthesis of pyrano[3,2-c]chromene, pyrano[2,3-c]pyrazole, and benzylpyrazolyl coumarin. Inorg. Chem. Commun. 2023, 147, 110271. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ohyama, R.; Kimata, A.; Suzuki, T.; Miyata, N. Hydroxyl radical scavenging by edaravone derivatives: Efficient scavenging by 3-methyl-1-(pyridin-2-yl)-5-pyrazolone with an intramolecular base. Bioorg. Med. Chem. Lett. 2006, 16, 5939–5942. [Google Scholar] [CrossRef]
- Alam, M.A. Antibacterial pyrazoles: Tackling resistant bacteria. Future Med. Chem. 2022, 14, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Vijesh, A.M.; Isloor, A.M.; Isloor, S.; Shivananda, K.N.; Shyma, P.C.; Arulmoli, T. Synthesis of some new pyrazolone derivatives as potent antimicrobial agents. Der Pharma Chem. 2011, 3, 454–463. [Google Scholar]
- Ramajayam, R.; Tan, K.P.; Liu, H.G.; Liang, P.H. Synthesis and evaluation of pyrazolone compounds as SARS-coronavirus 3C-like protease inhibitors. Bioorg. Med. Chem. 2010, 18, 7849–7854. [Google Scholar] [CrossRef]
- Marković, V.; Erić, S.; Stanojković, T.; Gligorijević, N.; Aranelović, S.; Todorović, N.; Trifunović, S.; Manojlović, N.; Jelić, R.; Joksović, M.D. Antiproliferative activity and QSAR studies of a series of new 4-aminomethylidene derivatives of some pyrazol-5-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4416–4421. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, P.; Perumal, S.; Yogeeswari, P.; Sriram, D. A facile four-component sequential protocol in the expedient synthesis of novel 2-aryl-5-methyl-2,3-dihydro-1H-3-pyrazolones in water and their antitubercular evaluation. Eur. J. Med. Chem. 2011, 46, 4530–4536. [Google Scholar] [CrossRef]
- Ragab, F.A.F.; Abdel-Gawad, N.M.; Georgey, H.H.; Said, M.F. Pyrazolone derivatives: Synthesis, anti-inflammatory, analgesic quantitative structure-activity relationship and in vitro studies. Chem. Pharm. Bull. 2013, 61, 834–845. [Google Scholar] [CrossRef]
- Rao, D.J.; Nagaraju, K.; Maddila, S. Microwave irradiated mild, rapid, one-pot and multi-component synthesis of isoxazole-5(4H)-ones. Chem. Data Collect. 2021, 32, 100669. [Google Scholar] [CrossRef]
- Sivakumar, K.K.; Rajasekaran, A.; Senthilkumar, P.; Wattamwar, P.P. Conventional and microwave assisted synthesis of pyrazolone Mannich bases possessing anti-inflammatory, analgesic, ulcerogenic effect and antimicrobial properties. Bioorg. Med. Chem. Lett. 2014, 24, 2940–2944. [Google Scholar] [CrossRef]
- Sarfjoo, M.R.; Shad, A.; Hassanpour, M.; Varma, R. An Overview on New Anticancer Drugs Approved by Food and Drug Administration: Impending Economic and Environmental Challenges. Mater. Chem. Horizons 2022, 1, 189–198. [Google Scholar] [CrossRef]
- Ma, R.; Zhu, J.; Liu, J.; Chen, L.; Shen, X.; Jiang, H.; Li, J. Microwave-assisted one-pot synthesis of pyrazolone derivatives under solvent-free conditions. Molecules 2010, 15, 3593–3601. [Google Scholar] [CrossRef]
- Malle, A.; Yehualawork, A. This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Int. J. Spec. Educ. 2015, 30, 70–84. [Google Scholar]
- Mousa, S.A.S.; Ishak, E.A.; Bakheet, M.E.M.; Abu-shanab, F.A. New Route for the Synthesis of Pyrazolone Derivatives. Elixir Org. Chem. 2015, 89, 36854–36859. [Google Scholar]
- Yang, Z.; Wang, Z.; Bai, S.; Liu, X.; Lin, L.; Feng, X. Asymmetric α-amination of 4-substituted pyrazolones catalyzed by a chiral Gd(OTf)3/ N,N′-dioxide complex: Highly enantioselective synthesis of 4-amino-5-pyrazolone derivatives. Org. Lett. 2011, 13, 596–599. [Google Scholar] [CrossRef]
- Isaad, J.; El Achari, A. Synthesis and spectroscopic characterization of azoic dyes based on pyrazolone derivatives catalyzed by an acidic ionic liquid supported on silica-coated magnetite nanoparticle. J. Mol. Struct. 2018, 1154, 557–564. [Google Scholar] [CrossRef]
- Ekbote, S.S.; Gadge, S.T.; Bhanage, B.M. Synthesis of Pyrazole by Using Polyvinylsulfonic Acid (PVSA) as a Novel Bronsted Acid Catalyst. Curr. Catal. 2016, 5, 4–10. [Google Scholar] [CrossRef]
- Mojtahedi, M.M.; Javadpour, M.; Abaee, M.S. Convenient ultrasound mediated synthesis of substituted pyrazolones under solvent-free conditions. Ultrason. Sonochem. 2008, 15, 828–832. [Google Scholar] [CrossRef]
- Nouri, A.; Jelkmann, M.; Khoee, S.; Bernkop-Schnürch, A. Diaminated Starch: A Competitor of Chitosan with Highly Mucoadhesive Properties due to Increased Local Cationic Charge Density. Biomacromolecules 2020, 21, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Lu, Y. Solid-state synthesis of 4-[(indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5- pyrazolones by catalysis of molecular iodine. Synth. Commun. 2006, 36, 2389–2399. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, Y.M.; Tian, B.; Matsuura, T.; Meng, J. Ben The Solid-State Michael Addition of 3-Methyl-1-phenyl-5-pyrazolone. J. Heterocycl. Chem. 1998, 35, 129–134. [Google Scholar] [CrossRef]
- Nezhad, S.M.; Pourmousavi, S.A.; Zare, E.N.; Heidari, G.; Makvandi, P. Magnetic sulfonated melamine-Formaldehyde Resin as an efficient catalyst for the synthesis of antioxidant and antimicrobial pyrazolone derivatives. Catalysts 2022, 12, 626. [Google Scholar] [CrossRef]
- Yusuf, H.; Satria, D.; Suryawati, S.; Fahriani, M. Combination therapy of eurycomanone and doxorubicin as anticancer on T47D and MCF-7 Cell Lines. Syst. Rev. Pharm. 2020, 11, 335–341. [Google Scholar]
- Fang, X.J.; Jiang, H.; Zhu, Y.Q.; Zhang, L.Y.; Fan, Q.H.; Tian, Y. Doxorubicin induces drug resistance and expression of the novel CD44st via NF-κB in human breast cancer MCF-7 cells. Oncol. Rep. 2014, 31, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Rouibah, H.; Kebsa, W.; Lahouel, M.; Zihlif, M.; Ahram, M.; Aburmaileh, B.; Mansour Al Shhab, M.A.; Al-Ameer, H.J.; Mustafa, E. Algerian propolis: Between protection of normal cells and potentialisation of the anticancer effects of doxorubicin against breast cancer cells via P-glycoprotein inhibition and cell cycle arrest in the S phase. J. Physiol. Pharm. 2021, 72, 10–26402. [Google Scholar]
| Entry | Solvent | Catalyst (g) | Temp. (°C) | Time (Min.) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | EtOH/H2O | 0.04 | 40 | 120 | 55 |
| 2 | H2O | 0.04 | 40 | 180 | 30 |
| 3 | THF | 0.04 | 40 | 180 | 30 |
| 4 | EtOH | 0.04 | 40 | 90 | 75 |
| 5 | MeOH | 0.04 | 40 | 90 | 70 |
| 5 | CHCl3 | 0.04 | 40 | 180 | 30 |
| 6 | Hexane | 0.04 | 40 | 180 | 10 |
| 7 | Solvent-free | 0.04 | 40 | 180 | 30 |
| 8 | EtOH | 0.04 | r.t | 100 | 60 |
| 9 | EtOH | 0.04 | 60 | 100 | 55 |
| 10 | EtOH | 0.04 | 80 | 100 | 30 |
| 11 | EtOH | 0.06 | 40 | 80 | 87 |
| 12 | EtOH | 0.08 | 40 | 80 | 88 |
| 13 | EtOH | - | 40 | 120 | 45 |
| Entry | Product | Code | Time (min.) | Yield (%) b | M.P. (°C) | Ref. | |
|---|---|---|---|---|---|---|---|
| Observed | Reported | ||||||
| 1 |  | 6a | 80 | 87 | 233–234 | 235–236 | [28] |
| 2 | 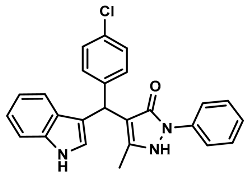 | 6b | 60 | 90 | 178–179 | 173–175 | [28] |
| 3 |  | 6c | 70 | 87 | 182–183 | 180–182 | [28] |
| 4 | 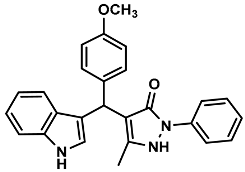 | 6d | 65 | 88 | 170–173 | 170–171 | [28] |
| 5 |  | 6e | 35 | 93 | 184–186 | 184–186 | [28] |
| 6 |  | 6f | 40 | 90 | 239–241 | 242–244 | [28] |
| 7 | 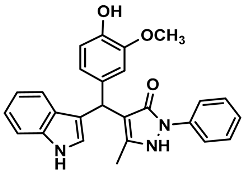 | 6g | 65 | 89 | 195–197 | 206 | [29] |
| 8 | 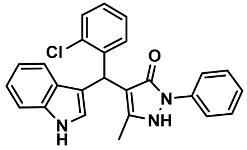 | 6h | 70 | 89 | 162–164 | 161–163 | [28] |
| 9 | 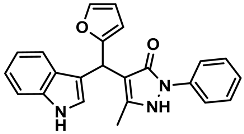 | 6i | 60 | 91 | 194–195 | 191–193 | [28] |
| 10 |  | 6j | 50 | 90 | 241–243 | 242–244 | [30] |
| 11 |  | 6k | 90 | 85 | 245–246 | 246 | [29] |
| 12 |  | 6l | 80 | 86 | 149–151 | NR | |
| 13 |  | 6m | 70 | 90 | 213–215 | NR | |
| 14 | 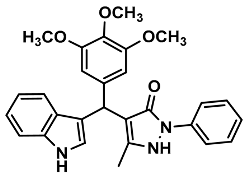 | 6n | 90 | 88 | 187–190 | NR | |
| 15 | 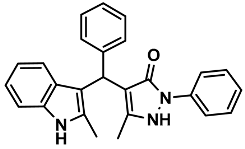 | 6o | 70 | 87 | 211–213 | 210–212 | [30] |
| 16 | 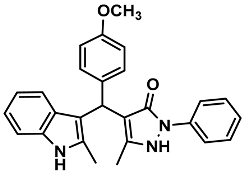 | 6p | 50 | 85 | 195–196 | 193–195 | [30] |
| 17 | 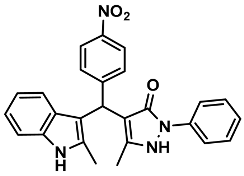 | 6q | 30 | 90 | 232–234 | 231–233 | [30] |
| 18 | 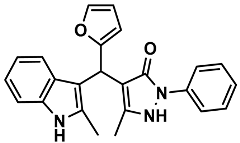 | 6r | 40 | 91 | 187–188 | 185–187 | [30] |
| 19 | 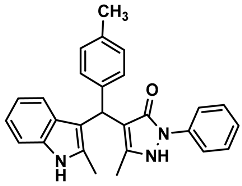 | 6s | 50 | 85 | 188–189 | 185–187 | [30] |
| 20 | 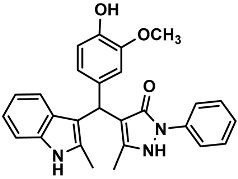 | 6t | 65 | 86 | 182–185 | NR | |
| 21 | 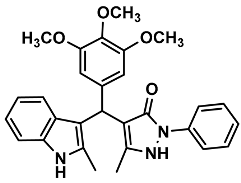 | 6u | 90 | 89 | 194–197 | NR | |
| 22 |  | 6v | 35 | 92 | 180–182 | NR | |
| 23 | 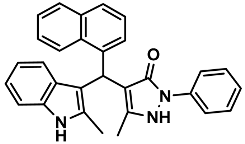 | 6w | 70 | 89 | 200–203 | NR | |
| 24 | 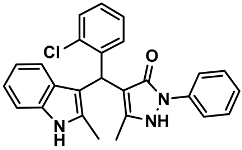 | 6x | 60 | 90 | 161–163 | NR | |
| 25 |  | 6y | 50 | 92 | 225–228 | NR | |
| Sample | Cell | Time (h) | |
|---|---|---|---|
| 24 | 48 | ||
| 6l | MCF-7 | 39.2 | 41.3 |
| Fibroblast | 392.1 | 409.4 | |
| 6p | MCF-7 | 38.2 | 23.1 |
| Fibroblast | 70.07 | 150.1 | |
| 6r | MCF-7 | 34.6 | 52.5 |
| Fibroblast | 81.9 | 115.4 | |
| 6n | MCF-7 | 25.8 | 21.3 |
| Fibroblast | 226.8 | 96.6 | |
| 6j | MCF-7 | 1660.5 | 11,647.4 |
| Fibroblast | 498,041.6 | 597,030.5 | |
| 6y | MCF-7 | 48.5 | 52.0 |
| Fibroblast | 92.5 | 135.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramshini, A.; Nezhad, S.M.; Pourmousavi, S.A.; Nazarzadeh Zare, E.; Pourjafar, M.; Sharifi, E. Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst. Catalysts 2023, 13, 908. https://doi.org/10.3390/catal13050908
Ramshini A, Nezhad SM, Pourmousavi SA, Nazarzadeh Zare E, Pourjafar M, Sharifi E. Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst. Catalysts. 2023; 13(5):908. https://doi.org/10.3390/catal13050908
Chicago/Turabian StyleRamshini, Ali, Shefa Mirani Nezhad, Seied Ali Pourmousavi, Ehsan Nazarzadeh Zare, Mona Pourjafar, and Esmaeel Sharifi. 2023. "Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst" Catalysts 13, no. 5: 908. https://doi.org/10.3390/catal13050908
APA StyleRamshini, A., Nezhad, S. M., Pourmousavi, S. A., Nazarzadeh Zare, E., Pourjafar, M., & Sharifi, E. (2023). Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst. Catalysts, 13(5), 908. https://doi.org/10.3390/catal13050908