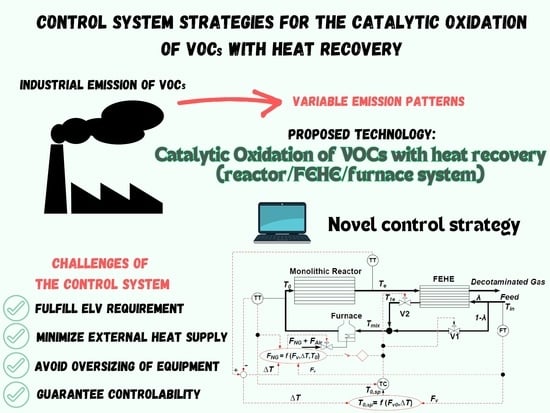
Novel Control System Strategy for the Catalytic Oxidation of VOCs with Heat Recovery
Abstract
:1. Introduction
2. Results and Discussion
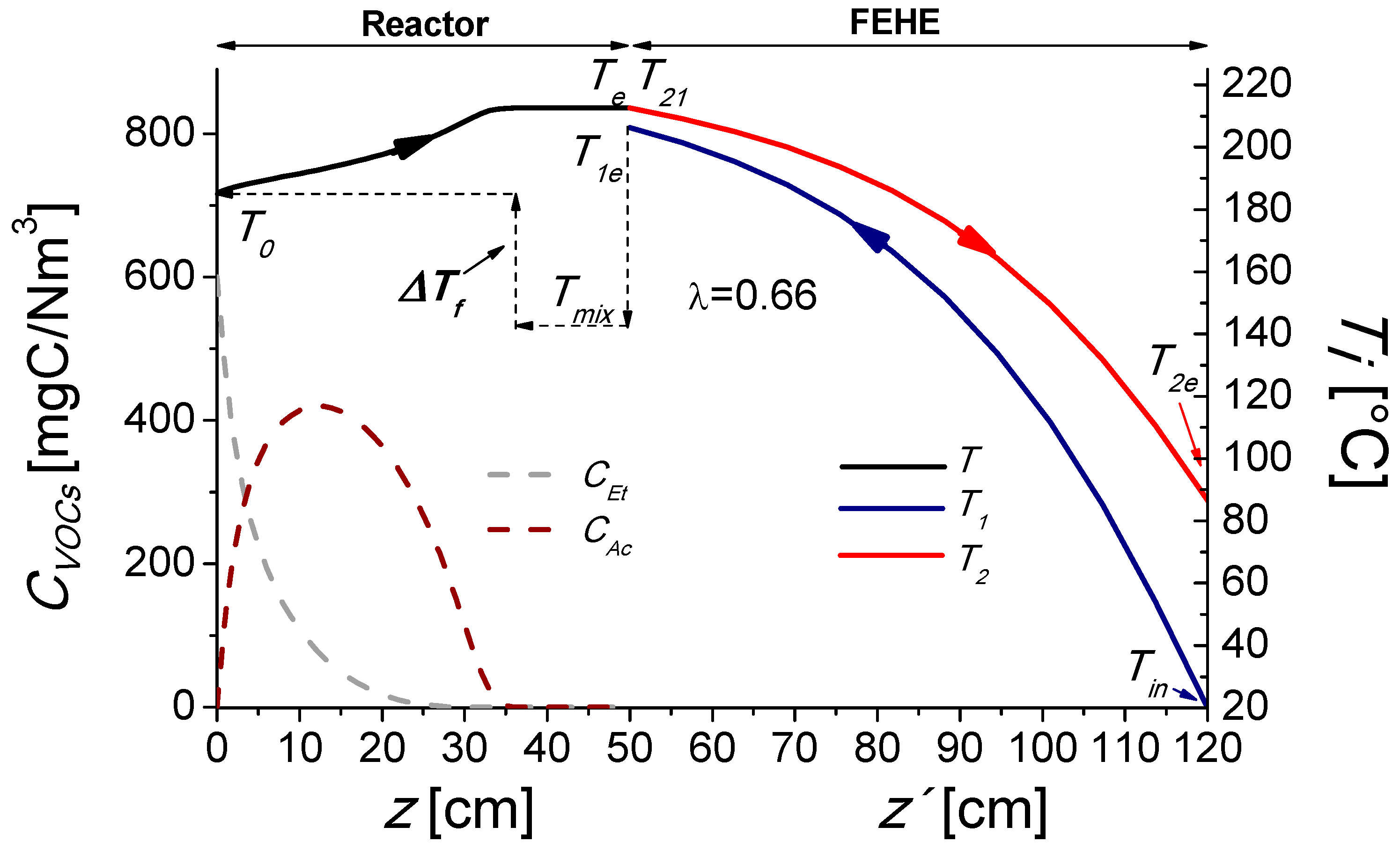
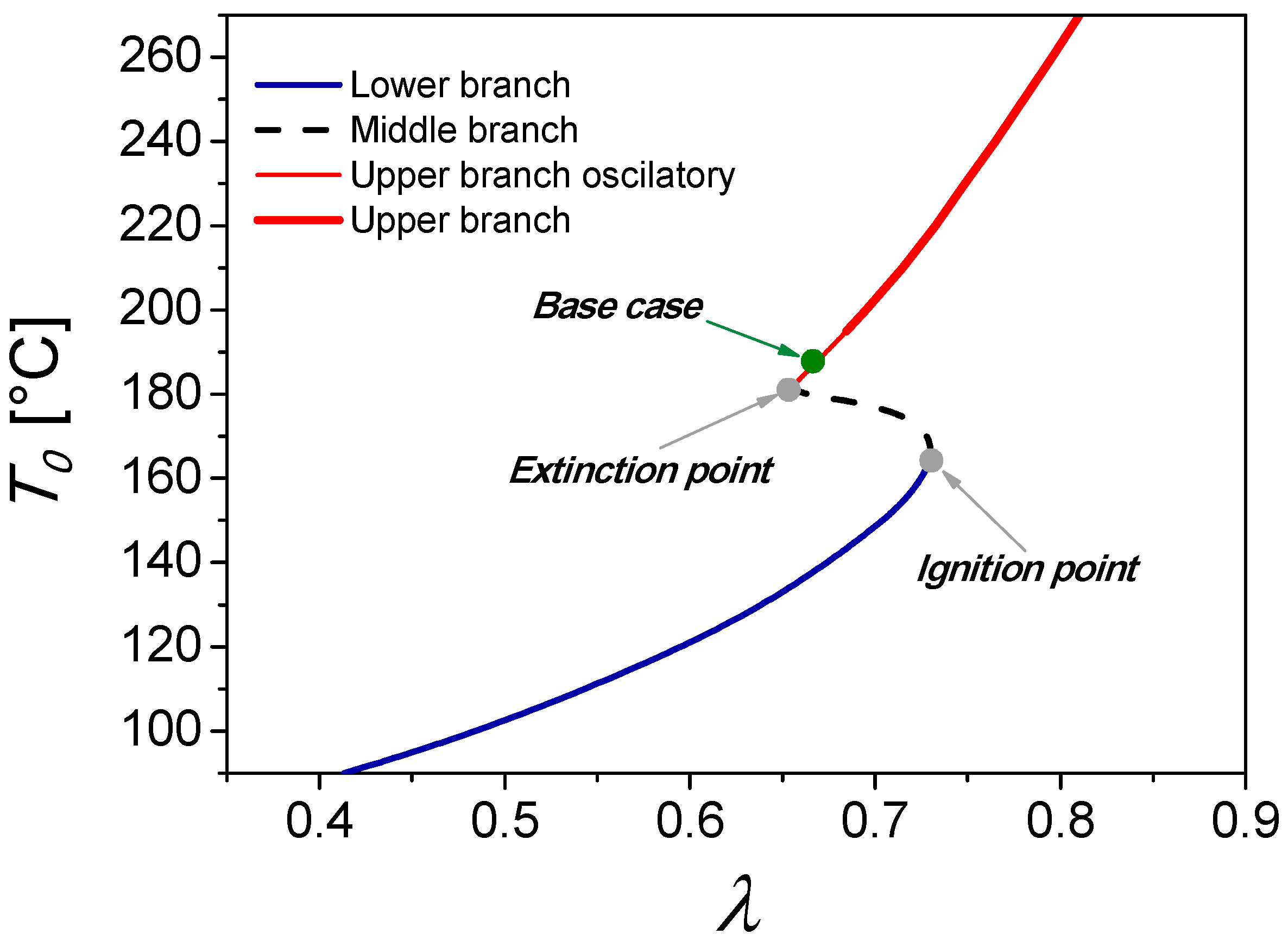
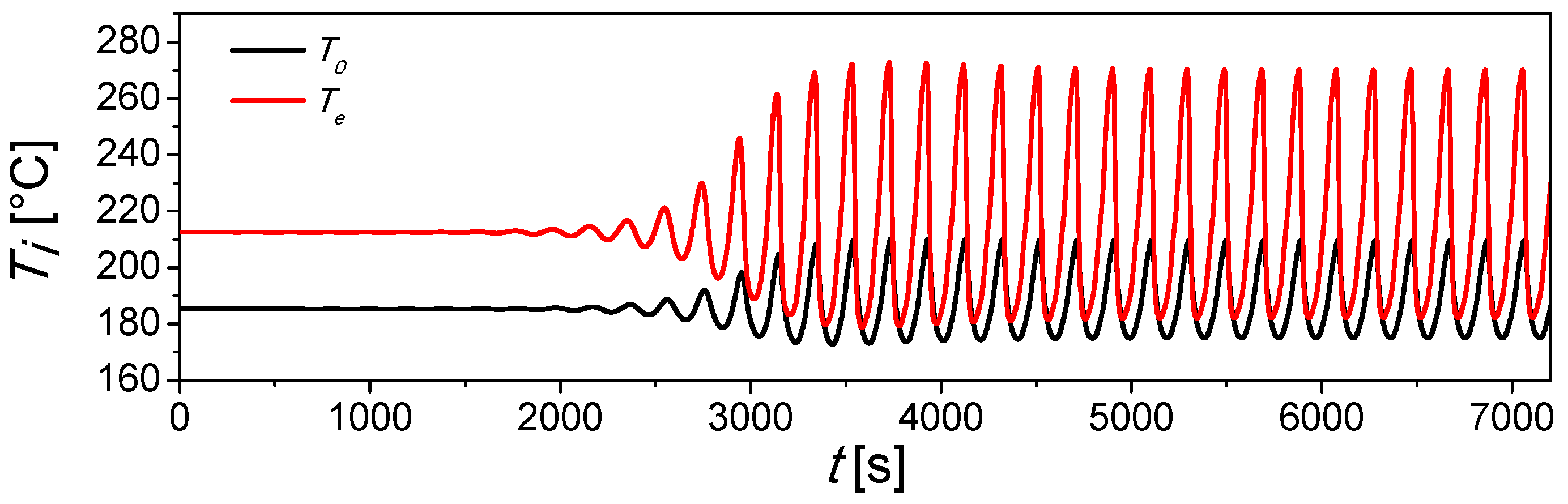
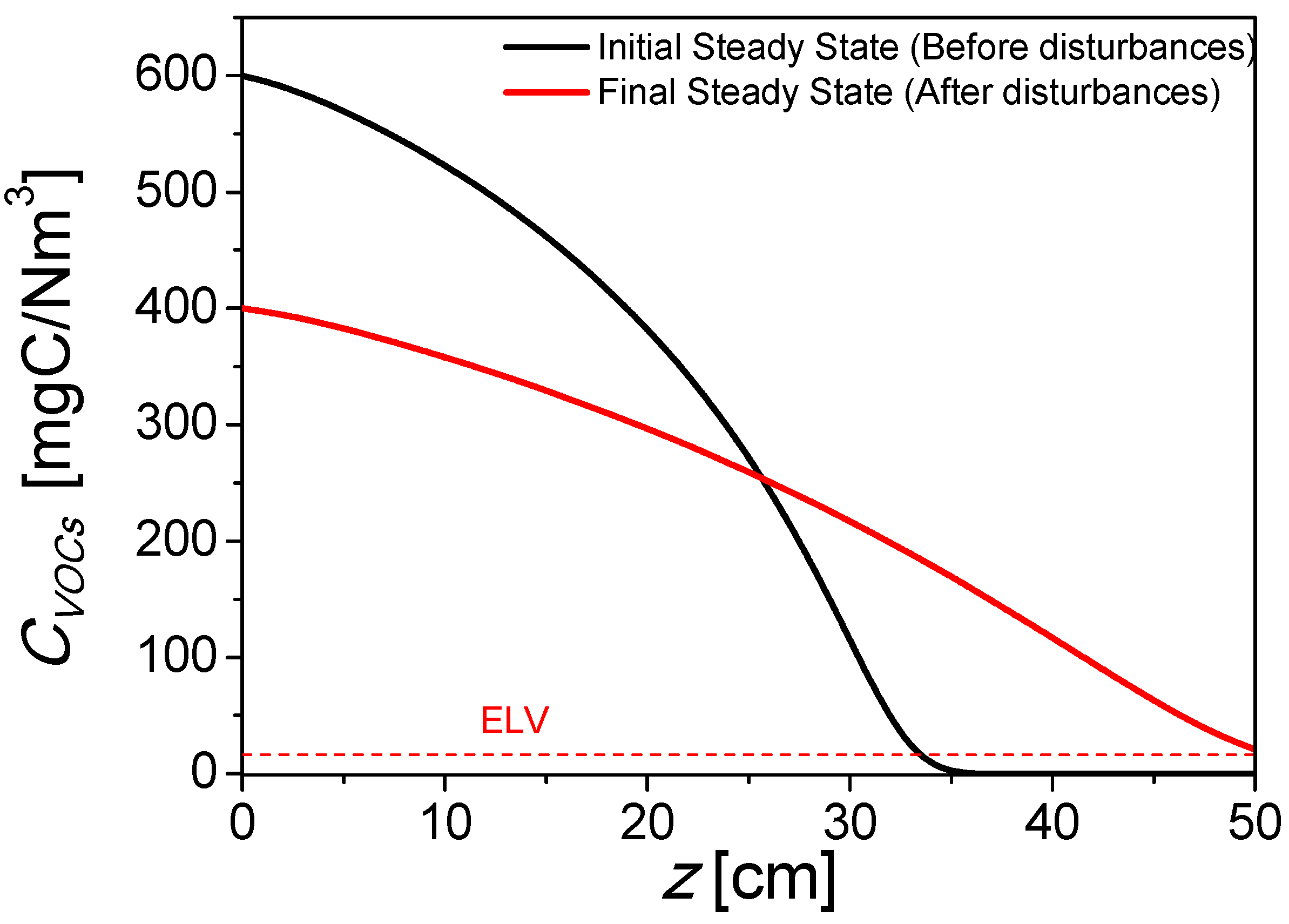
2.1. Base Case
2.2. Control Strategy Results
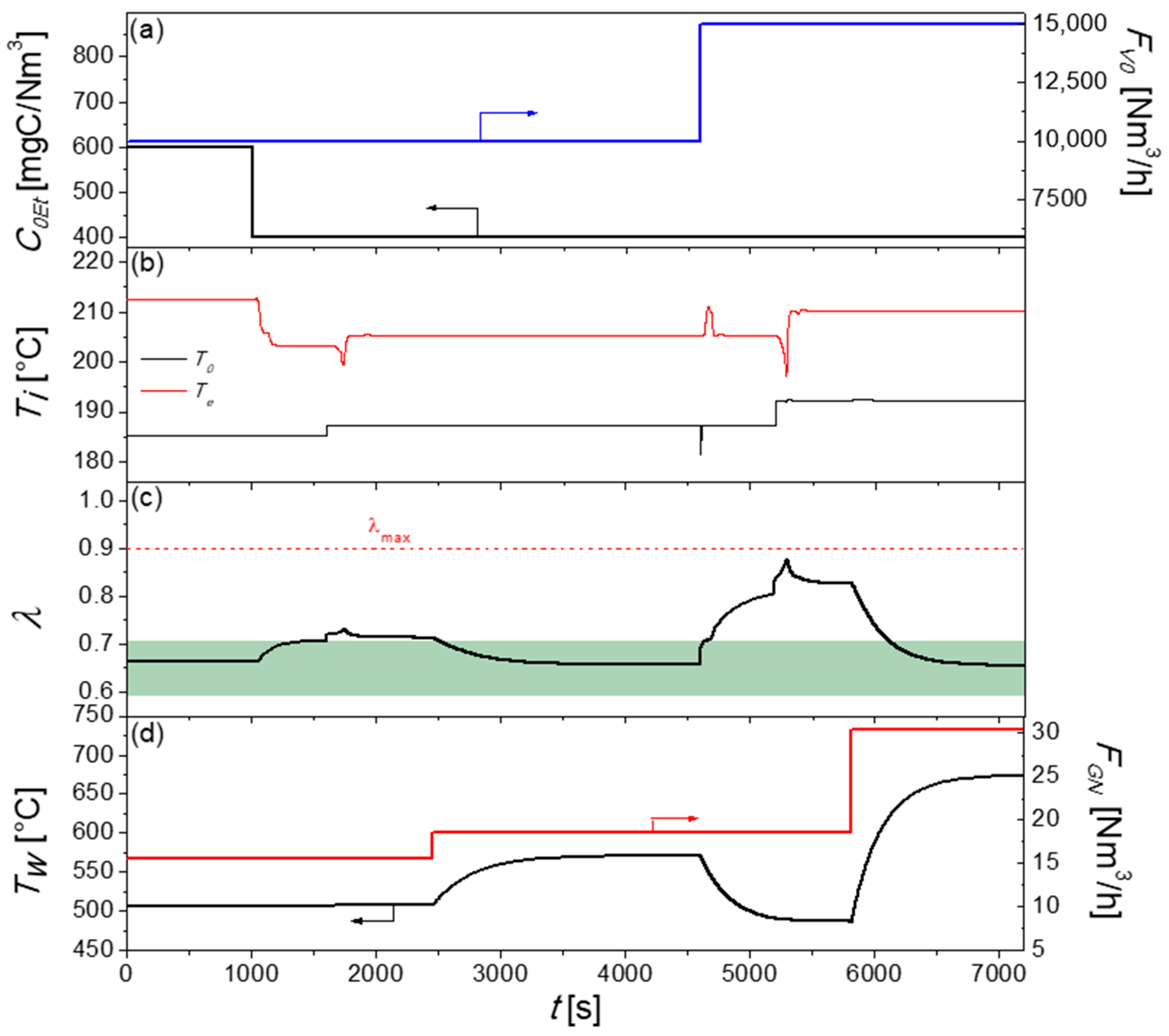
2.2.1. Control Strategy One: Single-Loop Feedback Control
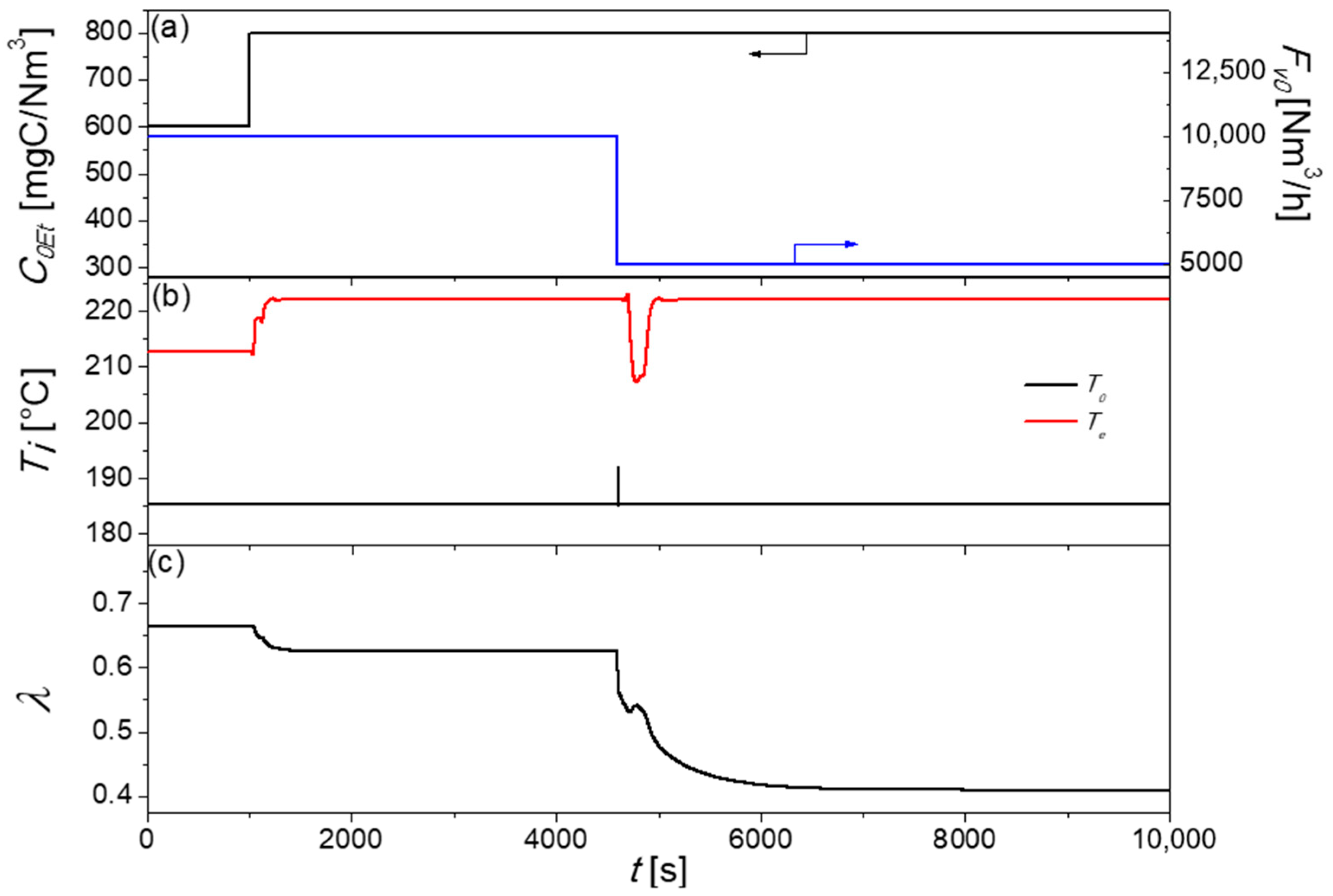
2.2.2. Control Strategy Two: Single-Loop Feedback System with Servomechanism Changes in T0
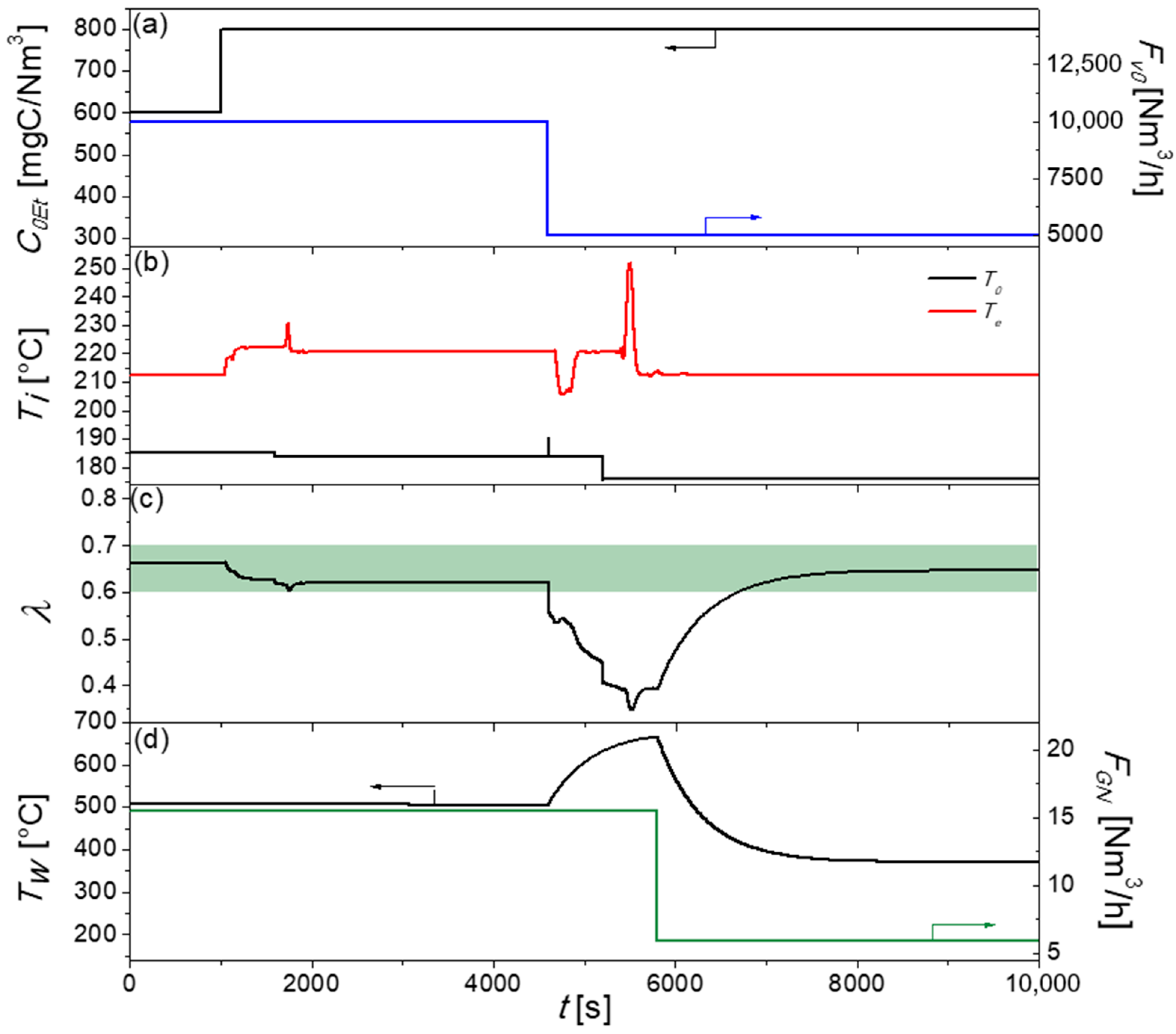
2.2.3. Control Strategy Three: Sequential Control System
2.3. Comparison of Control Strategies: Energy Savings
3. Mathematical Model
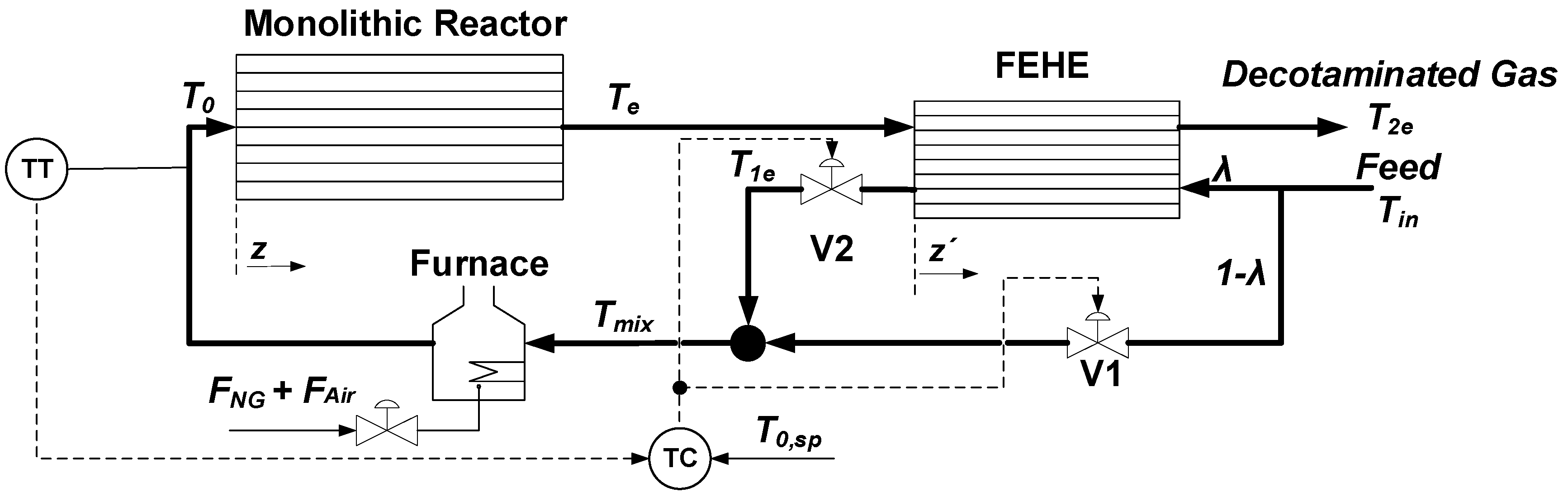
3.1. Description of the Process and Control Strategies
3.1.1. Single-Loop Feedback Control
3.1.2. Single-Loop Feedback System with Servomechanism Changes in T0.
3.1.3. Sequential Control System
3.2. Reactor, FEHE, and Furnace
| Parameter | Value |
|---|---|
| Feed VOC concentration, CEt0 | 400–800 mgC/Nm3 |
| Volumetric feed flowrate, FV0 | 5000–15,000 Nm3/h |
| Pressure, P | 101.325 kPa |
| Feed temperature, TIn | 20 °C |
| Reactor gas hourly space velocity, GHSV | 5.93 × 103–1.798 × 104 1/h |
| Flow fraction through FEHE, λ | 0.1–0.9 |
| Furnace heat, Qf | 50–300 kW |
| Parameter | Value |
|---|---|
| Reactor | |
| Channel length, L | 0.5 m |
| Channel width = height, b | 1115 um |
| Cell density | 400 cpsi |
| Channel number | 13,924 × 102 |
| Monolithic material | Cordierite (2MgO-2Al2O3-5SiO2) |
| Catalytic material | Mn-Cu |
| Average washcoat thickness | 20 µm |
| Washcoat density, ρw | 4030 kg/m3 |
| Reactor weight (washcoat + cordierite) | 682 kg |
| Heat exchanger [25] | |
| Type | Plate fin heat exchanger (PFHE) |
| Flow configuration | Counter-current |
| Plate length, L’ | 0.7 m |
| Plate width | 0.7 m |
| Channel height | 6.35 mm |
| Plate thickness | 0.4 mm |
| Plates number | 220 |
| Fin thickness | 0.154 mm |
| Fin density | 437 fins/m |
| FEHE weight | 903 kg |
| Heat exchanger material | Stainless steel (AISI 316) |
| Furnace | |
| Type | Indirect fired |
| Tube arrangement | Horizontal |
| Tube number | 98 + chamber |
| Tube length | 1000 mm |
| Tube/chamber diameters | 25.4 mm/400 mm |
| Fuel | Natural gas |
| Furnace weight | 260 kg |
| Efficiency, ԑf | 0.9 |
3.3. Controller
3.3.1. Control Strategy One: Single-Loop Feedback System
3.3.2. Control Strategy Two: Single-Loop Feedback System with Servomechanism Changes in T0
3.3.3. Control Strategy Three: Advanced Control System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| b | Channel width=height, mm |
| Cj | Concentration of component j, molj/m3 or mg C/Nm3 |
| er | Error, dimensionless |
| Ei | Activation energy of reaction i, J/mol |
| ELV | Emission limit value, 20 mg C/Nm3 (total VOC emissions under normal conditions) |
| FNG | Natural gas flow under normal conditions, Nm3/h |
| FV0 | Volumetric feed flowrate, Nm3/h |
| FAir | Volumetric air flowrate, Nm3/h |
| GHSV | Gas hourly space velocity, 1/h |
| kref,1 | Kinetic constant for reaction one, 1/s |
| kref,2 | Kinetic constant for reaction two, mol/(m3 s) |
| Kcj | Adsorption constant for component j, m3/mol |
| Kc | Proportional action parameter |
| L | Reactor channel length, m |
| L’ | Heat exchanger length, m |
| P | Pressure, kPa |
| Qf | Furnace heat supply, kW |
| Reaction rate for reaction i, mol/(mg3s) | |
| t | Time, s |
| T | Reactor temperature, °C |
| Te | Reactor outlet temperature = hot stream inlet temperature (heat exchanger), °C |
| Tk | Temperature of stream k in the heat exchanger, °C |
| Tw | Temperature of furnace tube wall |
| V1 | Split-range valve to manipulate the bypass flowrate |
| V2 | Split-range valve to manipulate the flowrate through the heat exchanger |
| Xj | Conversion of component j, dimensionless |
| XVOCs | VOC conversion |
| Z | Reactor axial coordinate, m |
| z’ | Heat exchanger axial coordinate, m |
| Compounds/acronyms | |
| VOC | Volatile organic compound |
| Greek letters | |
| ∆Hri° | Heat of reaction i under standard conditions, J/mol |
| ∆T | Temperature difference between the reactor outlet and the reactor inlet |
| ∆Tad | Adiabatic temperature gradient, °C |
| ∆Tf | Temperature rise caused by the furnace, °C |
| ∆C0Et | Concentration disturbance, mg C/Nm3 |
| ԑ1 | Degree of advancement of reaction one |
| ԑ2 | Degree of advancement of reaction two |
| ԑf | Furnace thermal efficiency |
| λ | Fraction of stream through the heat exchanger |
| 1 − λ | Fraction of stream through bypass |
| Integral action parameter | |
| Subscripts | |
| Ac | Acetaldehyde |
| e | Exit |
| Et | Ethanol |
| f | Furnace |
| w | Furnace tube wall |
| HE | Heat exchanger |
| i | Reaction |
| j | Component |
| mix | Mixing point |
| R | Reactor |
| sp | Set point (reference value) |
| VOCs | Volatile Organic Compounds (ethanol + acetaldehyde) |
| T | Total |
| 1 | Heat exchanger cold stream |
| 2 | Heat exchanger hot stream |
References
- United States Environmental Protection Agency (EPA). Available online: http://www.epw.senate.gov (accessed on 15 April 2023).
- The Paints Directive 2004/42/EC—European Commission. Off. J. L 2004, 143, 0087–0096. Available online: http://eur-lex.europa.eu (accessed on 17 April 2023).
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef]
- Luyben, W.L.; Tyreus, B.D.; Luyben, M.L. Plantwide Process Control; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Morud, J.; Skogestad, S. Effects of recycle on dynamics and control of chemical processing plants. Comput. Chem. Eng. 1994, 18, S529–S534. [Google Scholar] [CrossRef]
- Denn, M.; Lavie, R. Dynamics of plants with recycle. Chem. Eng. J. 1982, 24, 55–59. [Google Scholar] [CrossRef]
- Luyben, W.L. Snowball effects in reactor/separator processes with recycle. Ind. Eng. Chem. Res. 1994, 33, 299–305. [Google Scholar] [CrossRef]
- Morud, J.; Skogestad, S. Dynamic behaviour of integrated plants. J. Process Control 1996, 6, 145–156. [Google Scholar] [CrossRef]
- Bildea, C.S.; Dimian, A.C.; Cruz, S.C.; Iedema, P.D. Design of tubular reactors in recycle systems. Comput. Chem. Eng. 2004, 28, 63–72. [Google Scholar] [CrossRef]
- Morud, J.C.; Skogestad, S. Analysis of instability in an industrial ammonia reactor. AIChE J. 1998, 44, 888–895. [Google Scholar] [CrossRef]
- Luyben, W.L. Dynamics and control of recycle systems. 1. Simple open-loop and closed-loop systems. Ind. Eng. Chem. Res. 1993, 32, 466–475. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Chapter 7—Process Synthesis by the Hierarchical Approach. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 35, pp. 253–300. [Google Scholar] [CrossRef]
- Bildea, C.S.; Dimian, A.C. Stability and multiplicity approach to the design of heat-integrated PFR. AIChE J. 1998, 44, 2703–2712. [Google Scholar] [CrossRef]
- Silverstein, J.L.; Shinnar, R. Effect of design on the stability and control of fixed bed catalytic reactors with heat feedback. 1. Concepts. Ind. Eng. Chem. Process. Des. Dev. 1982, 21, 241–256. [Google Scholar] [CrossRef]
- Terrill, D.L.; Douglas, J.M. Heat-exchanger network analysis. 2. Steady-state operability evaluation. Ind. Eng. Chem. Res. 1987, 26, 691–696. [Google Scholar] [CrossRef]
- Luyben, W.L. New Control Structure for Feed-Effluent Heat Exchanger/Reactor Systems. Ind. Eng. Chem. Res. 2012, 51, 8566–8574. [Google Scholar] [CrossRef]
- Tyreus, B.D.; Luyben, W.L. Dynamics and control of recycle systems. 4. Ternary systems with one or two recycle streams. Ind. Eng. Chem. Res. 1993, 32, 1154–1162. [Google Scholar] [CrossRef]
- Miranda, A.F.; Rodríguez, M.L.; Borio, D.O. Open-loop dynamic analysis of the catalytic oxidation of vocs with heat recovery. Chem. Eng. Res. Des. 2023, 193, 432–446. [Google Scholar] [CrossRef]
- Miranda, Á.; Rodríguez, M.; Cadús, L.; Borio, D. Stability of a Feed-Effluent Heat Exchanger/Reactor system for catalytic combustion of VOCs: Influence of variable emission patterns. J. Environ. Chem. Eng. 2021, 9, 105597. [Google Scholar] [CrossRef]
- Luyben, W.L. Control structures for process piping systems. Chem. Eng. Res. Des. 2020, 162, 28–37. [Google Scholar] [CrossRef]
- Rodríguez, M.; Cadús, L.; Borio, D. VOCs abatement in adiabatic monolithic reactors: Heat effects, transport limitations and design considerations. Chem. Eng. J. 2016, 306, 86–98. [Google Scholar] [CrossRef]
- Campesi, M.A.; Mariani, N.J.; Pramparo, M.C.; Barbero, B.P.; Cadús, L.E.; Martínez, O.M.; Barreto, G.F. Combustion of volatile organic compounds on a MnCu catalyst: A kinetic study. Catal. Today 2011, 176, 225–228. [Google Scholar] [CrossRef]
- Miranda, A.F.; Rodriguez, M.L.; Borio, D.O. Obtaining Effective Reaction Rates in Monolithic Reactors for Ethanol Combustion. IV RITeQ, Carlos Paz, Cordoba, Junio. 2018. Available online: https://drive.google.com/file/d/17HuZ89LsrHVHZIiFIn7hNXdZ9bnuCMo3/view (accessed on 17 April 2023). (In Spanish).
- Shampine, L.F.; Gear, C.W. A User’s View of Solving Stiff Ordinary Differential Equations. SIAM Rev. 1979, 21, 1–17. [Google Scholar] [CrossRef]
- Kays, W.M.; London, A.L. Compact Heat Exchangers, 3rd ed.; Scientific International: New Delhi, India, 2018; ISBN 978-93-87938-03-8. [Google Scholar]






| Reaction System | Kinetic Expression | |
|---|---|---|
| C2H6O + (1/2) O2 → C2H4O + H2O | (1) | |
| C2H4O + (5/2)O2 → 2CO2 + 2H2O | (2) |
| Parameter | Value |
|---|---|
| kref,1 | 1.71 × 103 s−1 |
| kref,2 | 1.8 × 10−1 mol s−1 m−3 |
| E1 | 1.1124 × 105 J mol−1 |
| E2 | 1.62 × 105 J mol−1 |
| KcAc | 6.75 × 102 m3 mol−1 |
| KcEt | ~0 |
| ∆H0r1 | −1.73 × 105 J/mol |
| ∆H0r2 | −1.10 x 106 J/mol |
| Controller Parameter | Value |
|---|---|
| 0.003 | |
| 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, A.F.; Rodríguez, M.L.; Serra, F.M.; Borio, D.O. Novel Control System Strategy for the Catalytic Oxidation of VOCs with Heat Recovery. Catalysts 2023, 13, 897. https://doi.org/10.3390/catal13050897
Miranda AF, Rodríguez ML, Serra FM, Borio DO. Novel Control System Strategy for the Catalytic Oxidation of VOCs with Heat Recovery. Catalysts. 2023; 13(5):897. https://doi.org/10.3390/catal13050897
Chicago/Turabian StyleMiranda, Angel Federico, María Laura Rodríguez, Federico Martin Serra, and Daniel Oscar Borio. 2023. "Novel Control System Strategy for the Catalytic Oxidation of VOCs with Heat Recovery" Catalysts 13, no. 5: 897. https://doi.org/10.3390/catal13050897
APA StyleMiranda, A. F., Rodríguez, M. L., Serra, F. M., & Borio, D. O. (2023). Novel Control System Strategy for the Catalytic Oxidation of VOCs with Heat Recovery. Catalysts, 13(5), 897. https://doi.org/10.3390/catal13050897