Abstract
The electrochemical reduction of CO2 to value-added chemicals renewable electricity is a promising and ecofriendly strategy to achieve the national strategic goal of “carbon peak and carbon neutrality” and solve the greenhouse effect. Due to the variety of products in CO2 electroreduction (CO2ER), catalytic selectivity has become a key factor in the design of electrode structure. Herein, a systematic investigation of CO2ER on the nanoporous gold films with different thicknesses prepared by the self-deposition method developed by ourselves. Mass transfer effects are found to play an important role in determining product selectivity and activity. The specific activity for CO evolution (jCO) with exponential declination has more dramatic tendency than the specific activity for hydrogen evolution (jH2) with linear decay with increasing nanoporous gold film thickness. Different from the behaviors within the mesoporous structures in previous studies, the retarded transport of HCO3− ions within the nanoscale pores is more sensitive than that of protons. This phenomenon implies the necessity of considering mass transfer effects in the design of outstanding electrocatalysts for CO2ER as well as for understanding the geometrical infrastructure-performance relationships.
1. Introduction
Since the industrial revolution, the concentrations of carbon dioxide (CO2) in the atmosphere is constantly increasing due to the excessive consumption of fossil fuels [1,2]. The excessive emission of CO2 leads to a series of severe environmental issues (e.g., global warming, melting glaciers, ocean acidification, etc.), which seriously threaten the survival of human society [3]. In order mitigate or even reverse this trend, one of the most urgent tasks for mankind is how to effectively transform and utilize CO2 for the sustainable development. Of all the transformation methods, CO2 electroreduction (CO2ER) has attracted more and more attention [4]. Since it not only can be implemented under room temperature and atmospheric pressure, but also can be combined with renewable energy harvesting devices, such as wind, water and solar energy [5,6]. However, there are several challenges to reduce CO2 to the desirable product, such as high overpotential results from the activation of CO2 molecules, low Faradaic efficiency (FE) due to the competition with hydrogen evolution reaction (HER) in aqueous environment [7].
The efficiency of CO2ER are main influenced by the intrinsic properties of the catalytic materials. Early studies have mainly focused on polycrystalline bulk metal. The results show that the difference in product selectivity is due to the binding energy of intermediates which can be optimized by tuning electronic structure of catalyst surface [8,9]. Recent studies have demonstrated that nanostructure can significantly changed the electronic properties and improve the performance of catalysts [10]. Nano-sized metal catalysts (e.g., nanowires [11], nanoparticles (NPs) [12], nanosheets [13]) are prepared for CO2ER. For example, the current density of Ag nanosheet was 37 times (vs. 17 times increased in the surface area) larger than that of polycrystalline Ag at −0.6 V, and reached a high CO Faradaic efficiency (FECO) of 95% at an overpotential as low as 0.29 V. In addition, catalytic efficiency have pioneered breakouts in the intrinsic catalytic nature of electrocatalysts with different design approaches, such as alloying [14], particle size [15], and morphology [16], and so forth. For example, bimetallic PdAg3 exhibits CO selectivity with a low overpotential and a much high Faradaic efficiency (FE) (96.2% at −0.8 V) compared to Pd [17]. Furthermore, nanoporous metals have received considerable attention due to the large internal surface area, excellently conductive skeletons, and interconnected channels for ion transportation, including nanoporous Cu, Ag and Au, and so forth [18,19,20]. For example, the FECO value for nanoporous gold can reach up to 98% at an overpotential of 390 mV [21].
Furthermore, recent studies show that mass transport also play an important role in determining product selectivity [22,23,24]. For example, considering CO2ER catalyzed by Au or Ag, which predominantly generates CO and H2 as following reactions:
CO2 + 2H+ + 2e− → CO + H2O
2H+ + 2e− → H2
The specific activity for hydrogen evolution diminishes by 10-fold with increasing thickness of mesostructure gold films with the same surfaced structure, while CO evolution activity is unchanged [25]. Additionally, the specific activity for catalyzed CO evolution systematically rises by three-fold and the specific activity for catalyzed hydrogen evolution systematically declines by ten-fold with increasing thickness of similar mesostructure silver films [26]. Because mass transport within the mesoporous channels significantly has the potential to optimize catalytic selectivity, pore refinement of metallic electrocatalyst might improve the catalysis. However, the effect of ion transport within nanoscale pores on the catalysis is hardly reported in experiment so far. Herein, we demonstrate that the nanoscale channels of hierarchically porous gold film will retard the transport of the protons and HCO3− ions as well, thus their kinetics can make the positive contribution to the selective CO2ER.
2. Results
Hollow nanoporous Au shells (NASs) in this study were synthesized via a galvanic replacement reaction using Ag NPs as sacrificial templates, as illustrated in Figure 1a [27]. The chemical reaction between Ag NP and HAuCl4 in aqueous solution can be written as
3Ag(s) + AuCl4−(aq) → Au(s) + 3Ag(aq) + 4Cl−(aq)
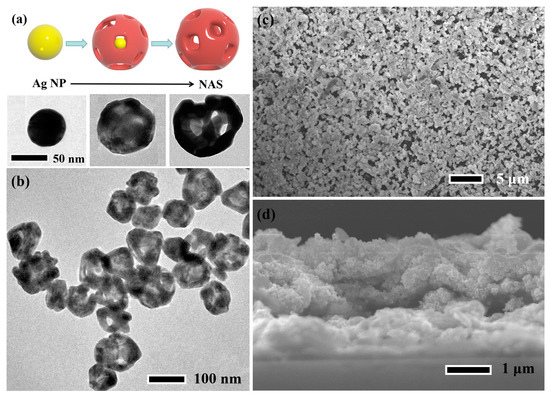
Figure 1.
(a) Schematic illustration of the procedure to fabricate the NAS, and its microstructure evolution, (b) TEM image of the NASs, (c) plane-view and (d) cross section of the NAS film.
The hollow interior results from the asymmetrical diffusion between Ag core and Au shell due to Kirkendall mechanism [28]. TEM observations indicate that outer diameters of NASs shown in Figure 1b are ~70–130 nm, and HRTEM is shown in Figure S1. The average value is about 102 nm according to statistical analysis (shown in Figure S2). The average thickness of the NAS shell is ~10 nm, and pore size on the shell is 9–29 nm. The energy dispersive X-ray spectroscopy (EDX) in Figure S3 shows that the Au concentration is ~82%, and the residual Ag atoms is ~18% in the NASs. Additionally, the detailed elemental mapping shown in Figure S4 and the X-ray diffraction (XRD) pattern shown in Figure S5 indicate that there is only one phase in the NASs. The NAS films with the thickness of 0.6, 1.3 and 2.6 µm (they are denoted to NAS-06, NAS-13 and NAS-26, respectively) are synthesized by drop casting method [29]. The plane-view and cross-sectional images of NAS-26 in Figure 1c,d and Figure S6 show the uniform morphology of the NAS film.
To quantitatively characterize crystal facets on the NAS films, underpotential deposition of lead (Pb-upd) is employed (shown in Figure S7a–c). The voltammetric profiles exhibit four peaks at −0.63, −0.57, −0.47 and −0.42 V vs. Hg/HgO, which correspond to step sites, {111}, {100}, and {110} facets, respectively. Since the surface areas of crystal facets are proportional to the peak intensity, the fractions of difffferent facets are calculated by deconvolution of the stripping waves. As displayed in Figure S7d, the fractions of step sites and {111}, {100}, and {110} facets in NAS films are estimated. Noticeably, NAS-06 shows more step sites than the other films, which may contribute to high electrocatalytic activity.
Gold catalysts usually produce CO and H2 during the CO2ER [2]. To evaluate the effect of mass transport on the product selectivity, we investigate electrochemical catalysis of the NAS films with different thicknesses in CO2-saturated 0.1 M KHCO3 (pH 6.8) electrolyte. Figure 2 shows the CO and H2 FEs of the NAS films with the thicknesses of 0.6, 1.3 and 2.6 μm during the CO2ER at various potentials. For the NAS-13 and NAS-26 catalysts, the FE for CO production (FECO) in Figure 2a increases toward 80% with the potential shifting from −0.45 V to −0.7 V. Thereafter, the FECO holds a minor declination toward 76.2% at −0.85 V, which shows the comparable tendency on most Au catalysts during the CO2ER [2]. For the NAS-06 catalyst, the FECO increases toward 84% with the potential shifting from −0.45 V to −0.85 V, following by a slight drop at −0.95 V. Obviously, maximum FECO on the NAS-06 is higher than the other two catalysts. In comparison with the FECO, the variation of H2 Faradaic efficiency (FEH2) with the potential shows the reverse tendency on all the NASs in Figure 2b. The FEH2 on the NAS-06 shows the lowest level of 13% at −0.85 V, where the maximum CO2-to-CO selectivity is obtained.
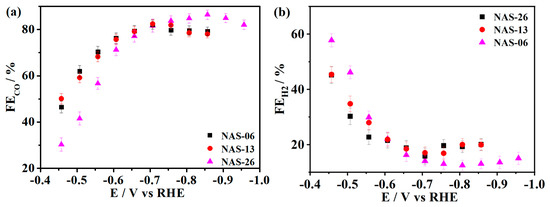
Figure 2.
FE for CO (a) and H2 (b) on the NAS films with the thickness of 0.6, 1.3 and 2.6 μm in CO2-saturated 0.1 M KHCO3 electrolyte, pH 6.8.
To understand intrinsic mechanism on the thickness dependence of catalytic selectivity, we evaluate the specific activity of the NAS films at various potentials (Figure 3). Specific activity is defined as partial current density corresponding to CO and H2 productions that is normalized by the electrochemical active surface area (ECSA), and the ECSA is examined by Pb UPD [19,26]. For all the NASs, current density of CO production (jCO) exhibits sharp increment with a potential shifting from −0.45 V to −0.7 V, and almost reach a saturation at −0.85 V (Figure 3a). All the NASs show a comparable jCO at a potential higher than −0.6 V. When the potential is lower than −0.6 V, the jCO of the NAS-26 is 1.9~3 times smaller than the NAS-06. The NAS-06 exhibits the highest jCO of 1.33 mA/cm2 at −0.95 V. Besides potential dependence, thickness dependence of the jCO is also observed. Quantitative variation of the jCO with film thickness is shown in Figure 3b. Obviously, an exponential attenuation with a factor of ~3.7–5.2 is obtained at −0.65, −0.75, −0.85 V. In comparison with the jCO, the specific current densities of H2 (jH2) of the NASs represents a monotonous increment with decreasing potential (Figure 3c). However, no saturation of the jH2 appears even if the potential is as low as −0.95 V. Besides potential effect, the jH2 also indicates the thickness dependence in Figure 3d. A linear attenuation with a factor of ~2.4–3.1 is obtained.

Figure 3.
Specific current density for CO (a) and H2 (b) evolution as a function of electrochemical potential for the different NASs films. Specific current density at different potentials for (c) H2 and (d) CO productions as a function of thickness. All data were collected in CO2-saturated 0.1 M KHCO3 electrolyte.
Porous metals with micron scale channels exhibit the significant thickness dependence of selectivity by considering the kinetics of mass transport [30,31]. In principle, both CO2ER and HER consume protons in the electrolyte. However, the HER needs large quantity of protons in comparison with the CO2ER, and this phenomenon results in proton depletion within the mesoporous catalysts. Therefore, proton concentration within the micron pores is the key parameter for product selectivity. Different from mesoporous metals, nanoporous Au films in our work contains large number of tiny pores (9–29 nm) on the shell and relatively large pores (~80 nm) inside the shell. So significant thickness dependence in Figure 3 can be experimentally demonstrated in both jCO and jH2, implying that these hollow channels affect mass transports of the HCO3− and protons dramatically. The jH2 in Figure 3d holds the linear dependence on film thickness at −0.65 V, −0.75 V and −0.85 V, implying coherent gradient of proton concentration at different depths within hollow channels. Different from the jH2, the jCO exhibits the exponential dependence on film thickness in the same condition. This non-linear behavior indicates that the restricted transport of HCO3− ion makes an additional contribution to the conversion of CO2 into CO besides the gradient of proton concentration.
To clarify the mechanism of mass transport in nanoporous films, electrochemical impedance spectroscopy (EIS) in Figure 4 was performed in 0.1 M KHCO3 [32]. The EIS of the NAS-06 shows a semicircle at high-frequency region of 2 × 104–106 Hz, in favor of estimating electrode resistance Re, and charge-transfer resistance Rct. The Re and Rct on the NAS-06 are 14.5 Ω and 69.2 Ω, respectively. The slope of the oblique line at low frequency is ~79.7°, implying that electrochemical impedance is primarily dominated by the capacitance on the porous electrode. By comparison, the Re on the NAS-26 is almost the same as that on the NAS-06, indicating that these two metallic catalysts are conductive. Different from the NAS-06, two semicircles on the NAS-26 indicates the distinct behavior of charge transfer at the catalyst/electrolyte interfaces. One semicircle at high-frequency region (2 × 104–106 Hz) that is similar to the NAS-06 corresponds to the Rct1 (72.3 Ω). The other at middle-frequency region (560–21,530 Hz) corresponds to the Rct2 (19 Ω). Dual impedances corresponding to Rct1 and Rct2 may be associated with the interacted ion transports within hierarchical pores including the larger pores inside the shell and the smaller pores on the shell. The slope of the oblique line at low frequency is ~62°, implying that electrochemical impedance is primarily dominated by ion transfer at the electrode/electrolyte interface for the NAS-26.
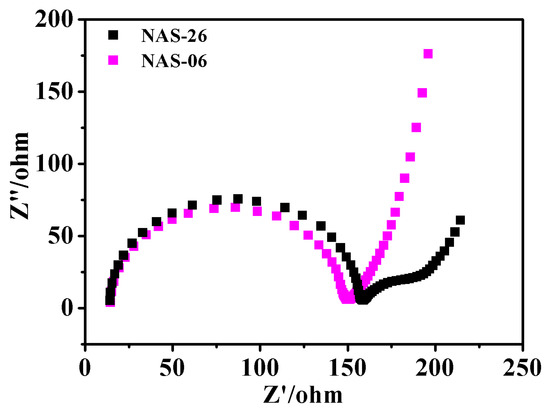
Figure 4.
The Nyquist plots of the NASs films with different thicknesses are collected in 0.1 M KHCO3 solution.
3. Discussion
Table S1 summarizes the CO2ER performances of Au and Ag nanostructures with different morphologies reported in previous studies. NASs exhibits medium performance in comparison with other investigations. Maximum FECO and the corresponding current density of NASs is similar to nanoporous Au film (88%, 1.06 mA cm−2) and 80 nm NPAS12 (86.4%, 0.69 mA cm−2) [22,27]. Maximum FECO of the NASs (88%) 11.6% lower than hierarchically nanoporous gold (99.6%) and 12.6% higher than Au NPs (75.4%) [12,33]. While the corresponding current density of NASs (1.1 mA cm−2) is 3.6 and 3.4 times and as much as hierarchically nanoporous gold (0.3 mA cm−2) and Au NPs (0.32 mA cm−2). In other words, the nanoporous structure has better catalytic performance than the solid structure. These can be attributed to the high surface-to-volume ratios for sufficient active sites and the nanoporous structure which promotes mass transport.
In addition, both the CO2ER and HER consume the protons in the pores, with a subsequent high OH− concentration that is localized nearby the catalyst surface [34,35]. Thus, high pH value localized will inhibit the HER and enhance CO2ER which are verified in Cu nanowire arrays with different lengths [11]. Based on this principle, proton transport from the bulk electrolyte to the hollow channels was timely for the upper layer of the NAS film. By contrast, proton transport within the bottom layer should be restricted, resulting in the better CO selectivity due to the rising pH value localized in the hollow channels. However, the thicker NAS film represent the worse catalysis for CO production relative to the thin NAS film, and the tendency can be identified by the exponential reduction of the jCO with film thickening. The thickness dependence of the jCO observed here is significantly different from the behavior on mesoporous Au, and in later case no thickness dependence of the jCO can be observed even if proton transport is inhibited [25]. This distinct phenomenons imply that proton transport does not make the dominate contribution to the production selectivity in the NAS. Besides proton transport, the inhibited transport of the HCO3− ions should be the other parameter to affect the kinetics of CO2ER. Especially, more HCO3− ions must be consumed at low potential (smaller than −0.6 V). Therefore, the jCO in Figure 3a shows a saturation with the decreasing potential. By comparison, the jH2 in Figure 3c does not show the saturation with decreasing potential, even if toward −0.85 V. The completely distinct behaviors between the jCO and jH2 indicate that different gradients of proton and HCO3− ion within the hollow channels of the NAS film. Meanwhile, the restricted transport of HCO3− ion within the thicker NAS film is also supported by its dual Rct values during the EIS measurement as shown in Figure 4. In general, the transport of HCO3− ion within nanoscale pores is different from that within the micron pores in the previous investigations [25,26]. Therefore, understanding the kinetic mechanism of mass transport within the nanoscale pores is important to rationally design the hierarchical structures with the outstanding catalysis and selectivity in the future [36].
4. Materials and Methods
4.1. Synthesis of Nanoporous Au Shells (NASs)
The detailed procedure of synthesizing NASs are described in our previous investigation [27]. First, 100 mL AgNO3 aqueous solution with a concentration of 1 mM was heated to boiling temperature. 1 mL sodium citrate solution (1% by weight) was added to the solution with vigorous stirring. The mixture was kept boiling for 1 h. The product is Ag NPs with the diameter of ~60 nm. Then, 1.8 mL of a 50mM HAuCl4 solution was added quickly, the mixture was kept boiling for 1 h under continuous stirring. Finally, the purplish red ANSs were obtained.
4.2. Microstructural Characterizations
The phase information was obtained via X-ray diffraction (XRD, Philips PW-1830, JEOL, Tokyo, Japan) with Cu Kα radiation. The microstructures of NASs were characterized by using a fieldemission scanning electron microscope (SEM, JSM-7600F, JEOL, Tokyo, Japan) and a JEOL JEM-2100F transmission electron microscope (TEM, JEOL, Tokyo, Japan) with an acceleration voltage of 200 kV.
4.3. Electrode Fabrications
Six milliliters NASs were centrifuged (4000 rpm, 10 min) and re-dispersed in 0.5 mL water. The reactant was added to 0.5 mL nafion (0.5 wt%). Different volumes (15 μL, 30 μL, 60 μL, respectively) of the above mixture were deposited on a polished glassy carbon electrode (GCE) with 3 mm diameter, leading to the NASs films with the thickness of 0.6, 1.3 and 2.6 µm. The NASs films were carefully rinsed with distilled water several times to remove the residual impurity.
4.4. Electrochemical Measurements
Electrochemical measurements were performed at ambient temperature (~23 °C) using a CHI760E electrochemical workstation in a gas-tight H-shaped cell with two compartments and a three-electrode configuration. Every compartment contained 25 mL of the 0.1 M KHCO3 electrolyte. The anodic electrolytes and cathodic electrolytes were separated by the commercial Nafion212 membrane, which conduct protons (H+) from anode to cathode. The counter electrode is a Pt mesh, all potentials were measured versus an Hg/HgCl reference electrode (SCE, saturated KCl, TJ Aida R0232, Tianjin Aida Hengsheng Technology Development Co., LTD, Tianjin, China) and converted to reversible hydrogen electrode (RHE) by the equation E (vs. RHE) = E (vs. SCE) + 0.0591 × pH + 0.244 V. A high-purity CO2 gas was purged into the electrolyte for at least 30 min prior to the CO2ER. During the electrolysis, the KHCO3 electrolyte (pH 6.8) was stirred with a rate of 1200 RPM using a magnetic stirrer. All the reactions were performed for 15 min and repeated three times, i-t curves are shown in Figure S8. Compartment cell with working electrode was connected with the sampling loop of gas chromatograph (GC-9790II, Zhejiang Fuli Analytical Instruments Co., LTD, Wenling, China).
Underpotential depositions of lead (Pb UPD) were carried out in a 0.1 M NaOH + 1 mM Pb(NO3)2 electrolyte at a scan rate of 50 mV/s, which was purged with argon for 30 min. The Hg/HgO (1 M KOH, TJ Aida R0501, Tianjin Aida Hengsheng Technology Development Co., LTD, Tianjin, China) electrode was selected as reference electrode.
Electrochemical impedance spectroscopy (EIS, Tianjin Aida Hengsheng Technology Development Co., LTD, Tianjin, China) was acquired at the open circuit potential (OCP) potentiostatically. The EIS spectrum was collected with an amplitude of 10 mV at the range of 10–106 Hz in CO2-saturated 0.1 M KHCO3 electrolyte.
5. Conclusions
Nanoporous metals are widely utilized as the catalysts for electrochemical reaction because of their large surface-to-volume ratio and high conductivity. Kinetic diffusions of ions and reactant within the hollow channels significantly affect the catalytic activity and selectivity in the aqueous solution. In this work, hierarchical pores in the NASs are demonstrated to dominate the kinetics of mass transport within hollow channel that can be tuned by the electrode thickness. The specific current density for CO evolution (jCO) which is exponential declination has more dramatic tendency than the specific current density for hydrogen evolution (jH2) which is linear decay with increasing nanoporous gold film thickness. In comparison with the restricted diffusion of proton within the mesoporous structures, the transport of HCO3− ions within the nanoscale pores is more sensitive to the thickness of the NASs catalysts film. This understanding clarifies the strategy to consider geometrical infrastructure of hierarchically porous electrocatalysts in favor of the kinetics between outstanding catalysis and selectivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13050883/s1, Figure S1: HRTEM image of the NAS; Figure S2: Statistical distribution for outer diameter of the NASs; Figure S3: EDX spectrum quantitatively indicates the concentration of Au and Ag elements in the NASs; Figure S4: The morphology of an isolated NAS and its elemental mapping; Figure S5: XRD patterns of the NASs, AuNPs and Ag NPs, indicate that there is only one phase existed in the NASs; Figure S6. SEM image of the NAS-26; Figure S7: Pb-upd for (a) NAS-06, (b) NAS-13, (c) NAS-26 in a 0.1 M NaOH + 1 mM Pb(NO3)2 electrolyte, and (d) the percentages of different facets can be calculated by integrating the corresponding peak areas; Figure S8: i-t Curves for (a) NAS-06, (b) NAS-13, (c) NAS-26 at various potentials; Table S1. Catalytic performances of the NASs in our work, and the performances of Au nanostructures reported in the references are included [12,20,22,33,37,38,39,40,41,42,43].
Author Contributions
Conceptualization, L.Q.; methodology, R.Z. and L.Q.; formal analysis, W.Y., D.Y., R.Z., X.L. and L.Q.; investigation, W.Y. and X.L.; resources, W.Y. and L.Q.; data curation, W.Y.; writing—original draft preparation, W.Y.; writing—review and editing, X.L., D.Y., R.Z. and L.Q.; supervision, R.Z. and L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52201215, 91545131, 51771078 and 51371084.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Chen, Q.; Tsiakaras, P.; Shen, P. Electrochemical Reduction of Carbon Dioxide: Recent Advances on Au-Based Nanocatalysts. Catalysts 2022, 12, 1348. [Google Scholar] [CrossRef]
- Wang, J.-J.; Li, X.-P.; Cui, B.-F.; Zhang, Z.; Hu, X.-F.; Ding, J.; Deng, Y.-D.; Han, X.-P.; Hu, W.-B. A review of non-noble metal-based electrocatalysts for CO2 electroreduction. Rare Met. 2021, 40, 3019–3037. [Google Scholar] [CrossRef]
- Farooqi, S.A.; Farooqi, A.S.; Sajjad, S.; Yan, C.; Victor, A.B. Electrochemical reduction of carbon dioxide into valuable chemicals: A review. Environ. Chem. Lett. 2023, 21, 1515–1553. [Google Scholar] [CrossRef]
- Talukdar, B.; Mendiratta, S.; Huang, M.H.; Kuo, C.H. Recent Advances in Bimetallic Cu-Based Nanocrystals for Electrocatalytic CO2 Conversion. Chem. Asian J. 2021, 16, 2168–2184. [Google Scholar] [CrossRef]
- Han, N.; Ding, P.; He, L.; Li, Y.; Li, Y. Promises of Main Group Metal–Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate. Adv. Energy Mater. 2019, 10, 1902338. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, C.; Wang, D. Metallic nanocatalysts for electrochemical CO2 reduction in aqueous solutions. J. Colloid Interface Sci. 2018, 527, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Michalsky, R.; Metin, O.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.B.; Dinh, C.T.; Li, Y.; Kim, D.; De Luna, P.; Sargent, E.H.; Yang, P. Tunable Cu Enrichment Enables Designer Syngas Electrosynthesis from CO2. J. Am. Chem. Soc. 2017, 139, 9359–9363. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ma, M.; Wu, L.; Valenti, M.; Cardenas-Morcoso, D.; Hofmann, J.P.; Bisquert, J.; Gimenez, S.; Smith, W.A. Electronic Effects Determine the Selectivity of Planar Au-Cu Bimetallic Thin Films for Electrochemical CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 16546–16555. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, K.; Liu, S.; Liu, M.; Peng, F.; An, P.; Qin, B.; Zhou, H.; Li, H.; He, Z. Low-overpotential selective reduction of CO2 to ethanol on electrodeposited CuxAuy nanowire arrays. J. Energy Chem. 2019, 37, 176–182. [Google Scholar] [CrossRef]
- Mistry, H.; Reske, R.; Zeng, Z.; Zhao, Z.J.; Greeley, J.; Strasser, P.; Cuenya, B.R. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. J. Am. Chem. Soc. 2014, 136, 16473–16476. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Zhao, Y.; Wang, C.; Mitchell, D.R.G.; Wallace, G.G. Rapid formation of self-organised Ag nanosheets with high efficiency and selectivity in CO2 electroreduction to CO. Sustain. Energy Fuels 2017, 1, 1023–1027. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Cho, A.; Kim, S.; Han, J.W.; Lee, J. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO. Appl. Catal. B 2019, 246, 82–88. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size-Dependent Electrocatalytic Reduction of CO2 over Pd Nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef]
- Klinkova, A.; De Luna, P.; Dinh, C.-T.; Voznyy, O.; Larin, E.M.; Kumacheva, E.; Sargent, E.H. Rational Design of Efficient Palladium Catalysts for Electroreduction of Carbon Dioxide to Formate. ACS Catal. 2016, 6, 8115–8120. [Google Scholar] [CrossRef]
- Gunji, T.; Ochiai, H.; Ohira, T.; Liu, Y.; Nakajima, Y.; Matsumoto, F. Preparation of Various Pd-Based Alloys for Electrocatalytic CO2 Reduction Reaction—Selectivity Depending on Secondary Elements. Chem. Mater. 2020, 32, 6855–6863. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical Reduction of CO2 at Copper Nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, T.; Wang, H.; Qian, L.; Lei, P. Electrochemical Fabrication and Reactivation of Nanoporous Gold with Abundant Surface Steps for CO2 Reduction. ACS Catal. 2020, 10, 8860–8869. [Google Scholar] [CrossRef]
- Hossain, M.N.; Liu, Z.; Wen, J.; Chen, A. Enhanced catalytic activity of nanoporous Au for the efficient electrochemical reduction of carbon dioxide. Appl. Catal. B 2018, 236, 483–489. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, X.; Chen, Y.; Chen, J.; Yuan, S.; Qian, L.; Lu, X.; Liu, P.; Lei, P.; Li, X. Intrinsic Contribution of Mass Transport within Nanoscale Channels of Nanoporous Gold for CO2 Electrochemical Reduction. Adv. Mater. Interfaces 2022, 9, 2200895. [Google Scholar] [CrossRef]
- Hyun, G.; Song, J.T.; Ahn, C.; Ham, Y.; Cho, D.; Oh, J.; Jeon, S. Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proc. Natl. Acad. Sci. USA 2020, 117, 5680–5685. [Google Scholar] [CrossRef]
- Raciti, D.; Mao, M.; Park, J.H.; Wang, C. Mass transfer effects in CO2 reduction on Cu nanowire electrocatalysts. Catal. Sci. Technol. 2018, 8, 2364–2369. [Google Scholar] [CrossRef]
- Hall, A.S.; Yoon, Y.; Wuttig, A.; Surendranath, Y. Mesostructure-Induced Selectivity in CO2 Reduction Catalysis. J. Am. Chem. Soc. 2015, 137, 14834–14837. [Google Scholar] [CrossRef]
- Yoon, Y.; Hall, A.S.; Surendranath, Y. Tuning of Silver Catalyst Mesostructure Promotes Selective Carbon Dioxide Conversion into Fuels. Angew. Chem. Int. Ed. 2016, 55, 15282–15286. [Google Scholar] [CrossRef]
- Yang, W.; Wu, K.; Yang, W.; Wang, H.; Lv, X.; Qian, L.; Yu, T.; Li, Z.; Zhou, X.; Okumu Barasa, G.; et al. Nanoporous Au-Ag shell with fast kinetics: Integrating chemical and plasmonic catalysis. Nanotechnology 2017, 28, 425704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Z.; Peng, B.; Cao, C.; Zhang, C.; You, H.; Xiong, Q.; Li, Z.; Fang, J. Highly sensitive, uniform, and reproducible surface-enhanced Raman spectroscopy from hollow Au-Ag alloy nanourchins. Adv. Mater. 2014, 26, 2431–2439. [Google Scholar] [CrossRef]
- Strong, V.; Dubin, S.; El-Kady, M.F.; Lech, A.; Wang, Y.; Weiller, B.H.; Kaner, R.B. Patterning and Electronic Tuning of Laser Scribed Graphene for Flexible All-Carbon Devices. ACS Nano 2012, 6, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, B.; Zhong, J.; Cheng, Z. Selective electrochemical CO2 reduction over highly porous gold films. J. Mater. Chem. A 2017, 5, 21955–21964. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Mehio, N.; Jin, X.; Dai, S. Thickness- and Particle-Size-Dependent Electrochemical Reduction of Carbon Dioxide on Thin-Layer Porous Silver Electrodes. ChemSusChem 2016, 9, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4 V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, X.; Chen, Y.; Chen, J.; Yuan, S.; Zhang, Z.; Qian, L.; Li, S. Robust catalysis of hierarchically nanoporous gold for CO2 electrochemical reduction. Electrochim. Acta 2023, 437, 141537. [Google Scholar] [CrossRef]
- Zhang, B.A.; Ozel, T.; Elias, J.S.; Costentin, C.; Nocera, D.G. Interplay of Homogeneous Reactions, Mass Transport, and Kinetics in Determining Selectivity of the Reduction of CO2 on Gold Electrodes. ACS Cent. Sci. 2019, 5, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Suter, S.; Haussener, S. Optimizing mesostructured silver catalysts for selective carbon dioxide conversion into fuels. Energy Environ. Sci. 2019, 12, 1668–1678. [Google Scholar] [CrossRef]
- Wuttig, A.; Yaguchi, M.; Motobayashi, K.; Osawa, M.; Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl. Acad. Sci. USA 2016, 113, E4585–E4593. [Google Scholar] [CrossRef] [PubMed]
- Van Cleve, T.; Gibara, E.; Linic, S. Electrochemical Oxygen Reduction Reaction on Ag Nanoparticles of Different Shapes. ChemCatChem 2016, 8, 256–261. [Google Scholar] [CrossRef]
- Kim, C.; Jeon, H.S.; Eom, T.; Jee, M.S.; Kim, H.; Friend, C.M.; Min, B.K.; Hwang, Y.J. Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850. [Google Scholar] [CrossRef]
- Yang, W.; Qian, L.; Lei, P.; Chen, J.; Lu, X.; Yu, T.; Xiao, H.; Zhou, X.; Kathale, B. Active and selective CO2 electroreduction on a hierarchically nanoporous Au-Ag shell. Chem. Phys. Lett. 2020, 753, 137563. [Google Scholar] [CrossRef]
- Woldu, A.R.; Wang, Y.; Shah, A.H.; Zhang, X.; He, T. Efficient reduction of CO2 to CO over grain boundary rich gold film reconstructed by O2 plasma treatment. Appl. Catal. A Gen. 2021, 625, 118333. [Google Scholar] [CrossRef]
- Qi, Z.; Biener, J.; Biener, M. Surface Oxide-Derived Nanoporous Gold Catalysts for Electrochemical CO2-to-CO Reduction. ACS Appl. Energy Mater. 2019, 2, 7717–7721. [Google Scholar] [CrossRef]
- Abbas, S.A.; Ma, A.; Seo, D.; Lim, Y.J.; Park, J.Y.; Lee, G.; Nam, K.M. Facile and large-scale production of Ag nanoparticles for selective electrochemical CO2 reduction reaction. Bull. Korean Chem. Soc. 2021, 42, 1534–1538. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Senanayake, S.D.; Zhang, Y.; Xu, W.; Polyansky, D.E. Effect of Chloride Anions on the Synthesis and Enhanced Catalytic Activity of Silver Nanocoral Electrodes for CO2 Electroreduction. ACS Catal. 2015, 5, 5349–5356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).