Mesoporous Chromium Catalysts Templated on Halloysite Nanotubes and Aluminosilicate Core/Shell Composites for Oxidative Dehydrogenation of Propane with CO2
Abstract
1. Introduction
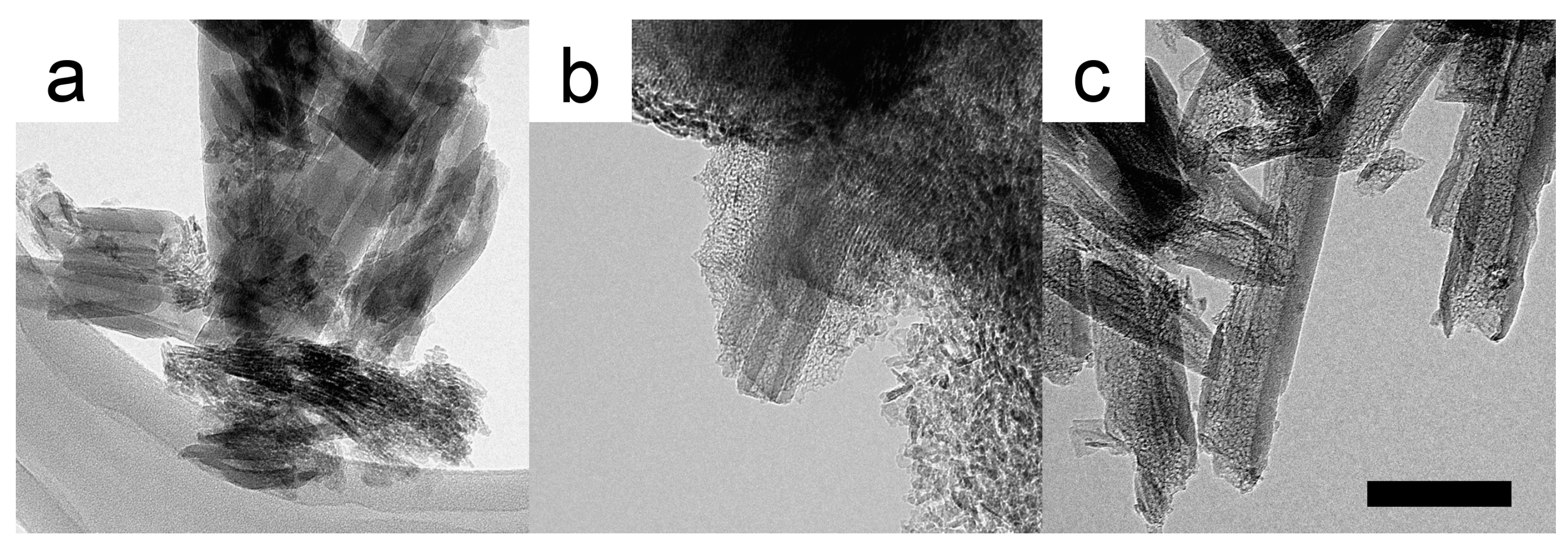
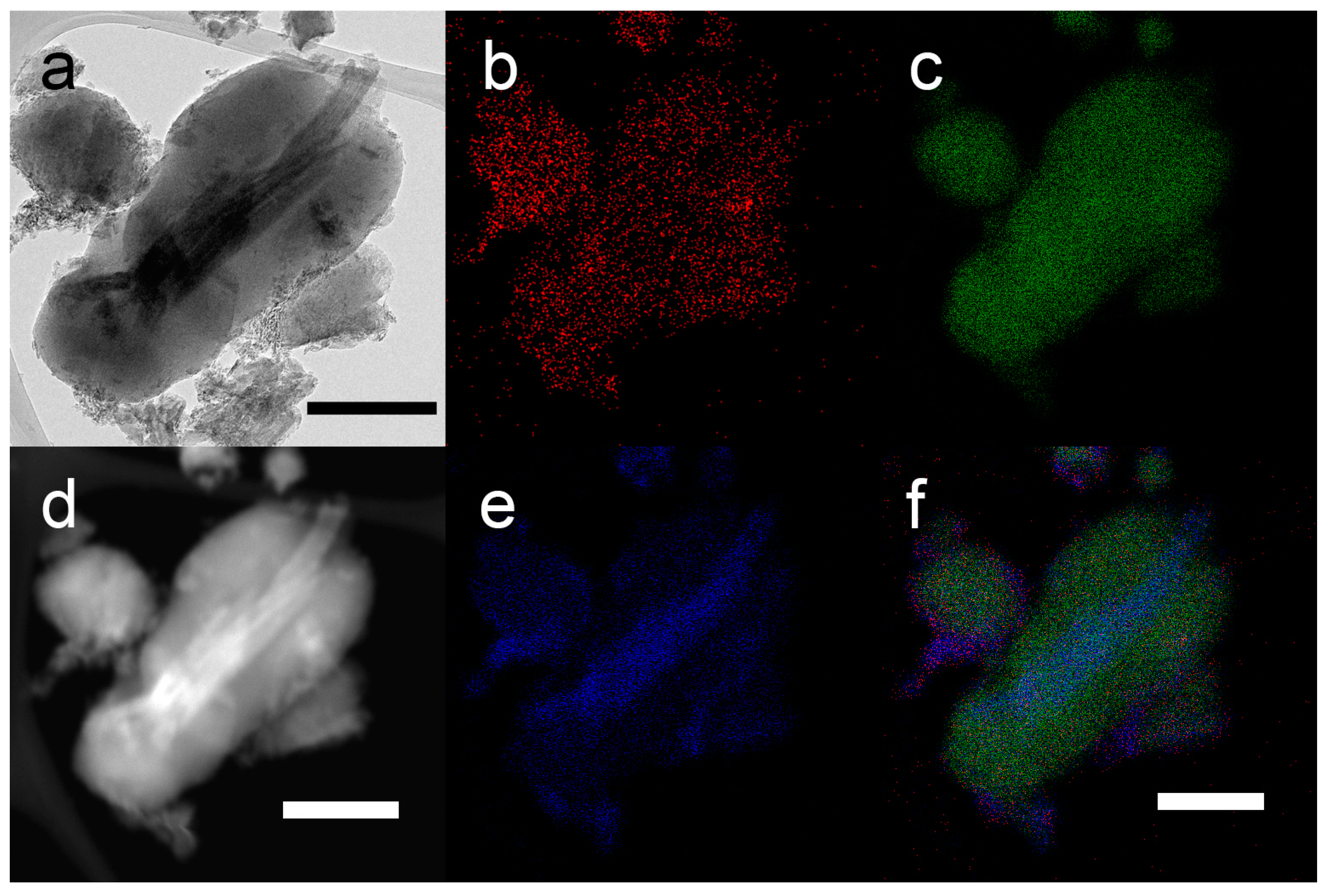
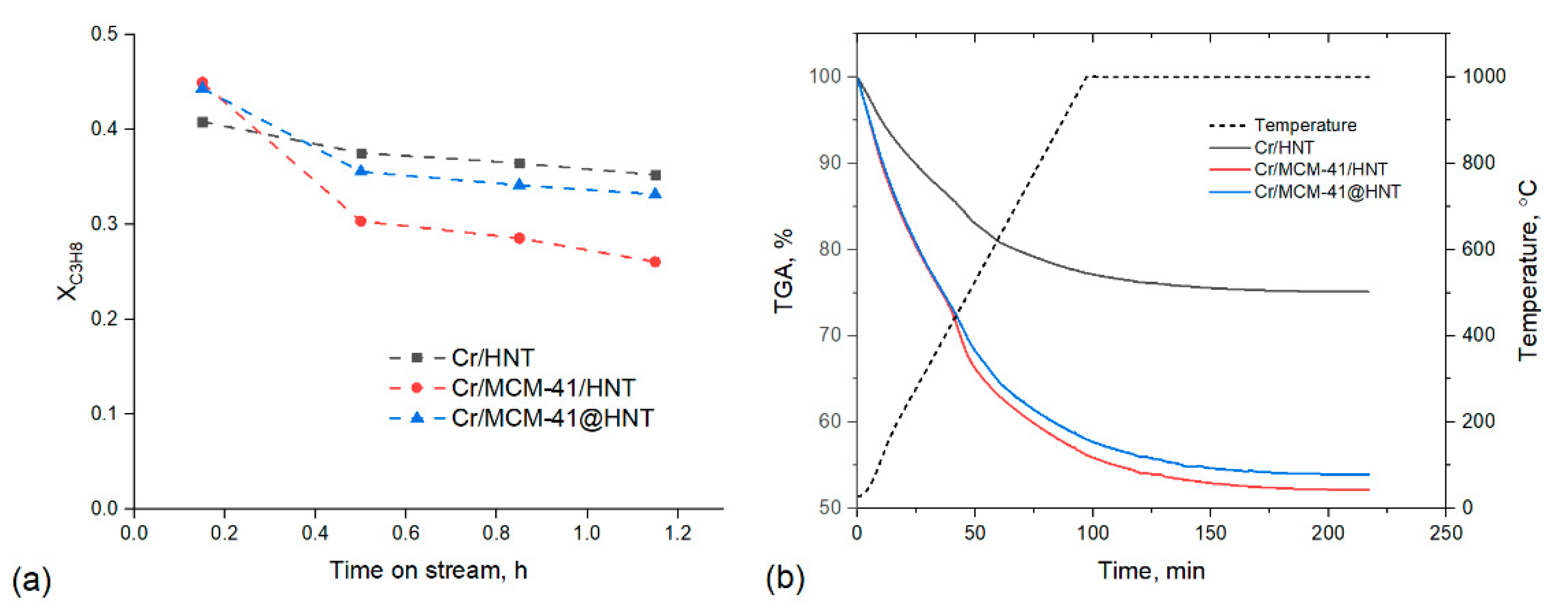
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Functional Materials
3.3. Catalyst Preparation
3.4. Catalyst Characterization
3.5. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eisele, P.; Killpack, R. Propene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Volume 16, pp. 75–81. [Google Scholar]
- Searles, K.; Chan, K.W.; Mendes Burak, J.A.; Zemlyanov, D.; Safonova, O.; Copéret, C. Highly Productive Propane Dehydrogenation Catalyst Using Silica-Supported Ga-Pt Nanoparticles Generated from Single-Sites. J. Am. Chem. Soc. 2018, 140, 11674–11679. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.H.; Bere, T.; Pitchers, J.R.; Hewes, D.G.; Vandegehuchte, B.D.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Direct and Oxidative Dehydrogenation of Propane: From Catalyst Design to Industrial Application. Green Chem. 2021, 23, 9747–9799. [Google Scholar] [CrossRef]
- Monai, M.; Gambino, M.; Wannakao, S.; Weckhuysen, B.M. Propane to Olefins Tandem Catalysis: A Selective Route towards Light Olefins Production. Chem. Soc. Rev. 2021, 50, 11503–11529. [Google Scholar] [CrossRef] [PubMed]
- Akah, A.; Al-Ghrami, M. Maximizing Propylene Production via FCC Technology. Appl. Petrochem. Res. 2015, 5, 377–392. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane Dehydrogenation: Catalyst Development, New Chemistry, and Emerging Technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef]
- Zimmermann, H.; Walzl, R. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Volume 13, pp. 465–529. [Google Scholar]
- Melnikov, D.P.; Novikov, A.A.; Glotov, A.P.; Reshetina, M.V.; Smirnova, E.M.; Wang, H.Q.; Vinokurov, V.A. Dehydrogenation of Light Alkanes (A Review). Pet. Chem. 2022, 62, 1027–1046. [Google Scholar] [CrossRef]
- Caspary, K.J.; Gehrke, H.; Heinritz-Adrian, M.; Schwefer, M. Dehydrogenation of Alkanes. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 3206–3229. [Google Scholar]
- Atanga, M.A.; Rezaei, F.; Jawad, A.; Fitch, M.; Rownaghi, A.A. Oxidative Dehydrogenation of Propane to Propylene with Carbon Dioxide. Appl. Catal. B Environ. 2018, 220, 429–445. [Google Scholar] [CrossRef]
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current Status and Perspectives in Oxidative, Non-Oxidative and CO2-Mediated Dehydrogenation of Propane and Isobutane over Metal Oxide Catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef]
- Rigamonti, M.G.; Shah, M.; Gambu, T.G.; Saeys, M.; Dusselier, M. Reshaping the Role of CO2 in Propane Dehydrogenation: From Waste Gas to Platform Chemical. ACS Catal. 2022, 12, 9339–9358. [Google Scholar] [CrossRef]
- Huš, M.; Kopač, D.; Bajec, D.; Likozar, B. Effect of Surface Oxidation on Oxidative Propane Dehydrogenation over Chromia: An Ab Initio Multiscale Kinetic Study. ACS Catal. 2021, 11, 11233–11247. [Google Scholar] [CrossRef] [PubMed]
- Routray, K.; Reddy, K.R.S.K.; Deo, G. Oxidative Dehydrogenation of Propane on V2O5/Al2O3 and V2O5/TiO2 Catalysts: Understanding the Effect of Support by Parameter Estimation. Appl. Catal. A Gen. 2004, 265, 103–113. [Google Scholar] [CrossRef]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Leonov, A.V.; Dunaev, S.F. Dehydrogenation of Propane with CO2 on Supported CrOx/SiO2 Catalysts. Russ. J. Phys. Chem. A 2018, 92, 2403–2407. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Kustov, A.L.; Stolbov, D.N.; Tedeeva, M.A.; Isaikina, O.Y.; Maslakov, K.I.; Usol’tseva, N.V.; Savilov, S.V. Chromium Catalysts Supported on Carbon Nanotubes and Graphene Nanoflakes for CO2-Assisted Oxidative Dehydrogenation of Propane. Appl. Surf. Sci. 2022, 578, 152099. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Evdokimenko, N.D.; Sarkar, B.; Kustov, L.M. Dehydrogenation of Propane in the Presence of CO2 on Cr(3%)/SiO2 Catalyst under Supercritical Conditions. Mendeleev Commun. 2020, 30, 195–197. [Google Scholar] [CrossRef]
- Nakagawa, K.; Okamura, M.; Ikenaga, N.; Suzuki, T.; Kobayashi, T. Dehydrogenation of Ethane over Gallium Oxide in the Presence of Carbon Dioxide. Chem. Commun. 1998, 3, 1025–1026. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Strekalova, A.A.; Kalmykov, K.B.; Dunaev, S.F.; Kustov, L.M. Dehydrogenation of Propane in the Presence of CO2 on Supported Monometallic MOy/SiO2 and CrOxMOy/SiO2 (M = Fe, Co, and Ni) Bimetallic Catalysts. Russ. J. Phys. Chem. A 2021, 95, 55–62. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Kapustin, G.I.; Leonov, A.V.; Tkachenko, O.P.; Tursunov, O.B.; Evdokimenko, N.D.; Kustov, L.M. Dehydrogenation of Propane in the Presence of CO2 on GaOx/SiO2 Catalyst: Influence of the Texture Characteristics of the Support. Fuel 2022, 313, 122698. [Google Scholar] [CrossRef]
- Mitchell, P.C.H.; Wass, S.A. Propane Dehydrogenation over Molybdenum Hydrotalcite Catalysts. Appl. Catal. A Gen. 2002, 225, 153–165. [Google Scholar] [CrossRef]
- Koc, S.N.; Dayioglu, K.; Ozdemir, H. Oxidative Dehydrogenation of Propane with K-MoO3/MgAl2O4 Catalysts. J. Chem. Sci. 2016, 128, 67–71. [Google Scholar] [CrossRef]
- Castro-Fernández, P.; Mance, D.; Liu, C.; Moroz, I.B.; Abdala, P.M.; Pidko, E.A.; Copéret, C.; Fedorov, A.; Müller, C.R. Propane Dehydrogenation on Ga2O3-Based Catalysts: Contrasting Performance with Coordination Environment and Acidity of Surface Sites. ACS Catal. 2021, 11, 907–924. [Google Scholar] [CrossRef]
- Ascoop, I.; Galvita, V.V.; Alexopoulos, K.; Reyniers, M.-F.; Van Der Voort, P.; Bliznuk, V.; Marin, G.B. The Role of CO2 in the Dehydrogenation of Propane over WOx–VOx/SiO2. J. Catal. 2016, 335, 1–10. [Google Scholar] [CrossRef]
- Searles, K.; Siddiqi, G.; Safonova, O.V.; Copéret, C. Silica-Supported Isolated Gallium Sites as Highly Active, Selective and Stable Propane Dehydrogenation Catalysts. Chem. Sci. 2017, 8, 2661–2666. [Google Scholar] [CrossRef] [PubMed]
- Nazimov, D.A.; Klimov, O.V.; Danilova, I.G.; Trukhan, S.N.; Saiko, A.V.; Cherepanova, S.V.; Chesalov, Y.A.; Martyanov, O.N.; Noskov, A.S. Effect of Alumina Polymorph on the Dehydrogenation Activity of Supported Chromia/Alumina Catalysts. J. Catal. 2020, 391, 35–47. [Google Scholar] [CrossRef]
- Michorczyk, P.; Pietrzyk, P.; Ogonowski, J. Preparation and Characterization of SBA-1-Supported Chromium Oxide Catalysts for CO2 Assisted Dehydrogenation of Propane. Microporous Mesoporous Mater. 2012, 161, 56–66. [Google Scholar] [CrossRef]
- Mitran, G.; Ahmed, R.; Iro, E.; Hajimirzaee, S.; Hodgson, S.; Urdă, A.; Olea, M.; Marcu, I.C. Propane Oxidative Dehydrogenation over VOx/SBA-15 Catalysts. Catal. Today 2018, 306, 260–267. [Google Scholar] [CrossRef]
- Asghari, S.; Haghighi, M.; Taghavinezhad, P. Plasma-Enhanced Dispersion of Cr2O3 over Ceria-Doped MCM-41 Nanostructured Catalyst Used in CO2 Oxidative Dehydrogenation of Ethane to Ethylene. Microporous Mesoporous Mater. 2019, 279, 165–177. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhao, Z.; Gao, M.; Kong, L.; Liu, J.; Wei, Y. Design, Synthesis and Catalytic Performance of Vanadium-Incorporated Mesoporous Silica KIT-6 Catalysts for the Oxidative Dehydrogenation of Propane to Propylene. Catal. Sci. Technol. 2016, 6, 5927–5941. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, R.; Yue, Y.; Yang, W.; Gu, S.; Miao, C.; Hua, W.; Gao, Z. Chromium Oxide Supported on ZSM-5 as a Novel Efficient Catalyst for Dehydrogenation of Propane with CO2. Microporous Mesoporous Mater. 2011, 145, 194–199. [Google Scholar] [CrossRef]
- Guo, H.; He, H.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. CO2 Assisted Ethane Dehydrogenation over Co-Exchanged ZSM-5 and ZSM-11 Zeolites: Effect of Al Spatial Distribution. Appl. Catal. A Gen. 2022, 635, 118569. [Google Scholar] [CrossRef]
- Yu, J.; Shi, J.-L.; Wang, L.-Z.; Ruan, M.-L.; Yan, D.-S. Preparation of High Thermal Stability MCM-41 in the Low Surfactant/Silicon Molar Ratio Synthesis Systems. Mater. Lett. 2001, 48, 112–116. [Google Scholar] [CrossRef]
- Chakraborty, B.; Viswanathan, B. Surface Acidity of MCM-41 by in Situ IR Studies of Pyridine Adsorption. Catal. Today 1999, 49, 253–260. [Google Scholar] [CrossRef]
- Orlyk, S.; Kyriienko, P.; Kapran, A.; Chedryk, V.; Balakin, D.; Gurgul, J.; Zimowska, M.; Millot, Y.; Dzwigaj, S. CO2-Assisted Dehydrogenation of Propane to Propene over Zn-BEA Zeolites: Impact of Acid–Base Characteristics on Catalytic Performance. Catalysts 2023, 13, 681. [Google Scholar] [CrossRef]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional Mesoporous Silica Nanomaterials for Catalysis and Environmental Applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Giraldo, L.F.; López, B.L.; Pérez, L.; Urrego, S.; Sierra, L.; Mesa, M. Mesoporous Silica Applications. Macromol. Symp. 2007, 258, 129–141. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Y.; Gao, Z. Chromium Oxide Supported on Mesoporous SBA-15 as Propane Dehydrogenation and Oxidative Dehydrogenation Catalysts. Catal. Lett. 2002, 83, 19–25. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, L.; Wu, Z.; Wang, F.; Jiang, X. Mesoporous Silica SBA-15 Supported Pt-Ga Nanoalloys as an Active and Stable Catalyst for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2022, 61, 7799–7809. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; El-Toni, A.M.; Alhoshan, M.; Khan, A.; Labis, J.P.; Al-Fatesh, A.; Abasaeed, A.E.; Al-Zahrani, S.M. Impact of Precursor Sequence of Addition for One-Pot Synthesis of Cr-MCM-41 Catalyst Nanoparticles to Enhance Ethane Oxidative Dehydrogenation with Carbon Dioxide. Ceram. Int. 2019, 45, 1125–1134. [Google Scholar] [CrossRef]
- Baek, J.; Yun, H.J.; Yun, D.; Choi, Y.; Yi, J. Preparation of Highly Dispersed Chromium Oxide Catalysts Supported on Mesoporous Silica for the Oxidative Dehydrogenation of Propane Using CO2: Insight into the Nature of Catalytically Active Chromium Sites. ACS Catal. 2012, 2, 1893–1903. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Niemczyk, M. Investigation of Catalytic Activity of CrSBA-1 Materials Obtained by Direct Method in the Dehydrogenation of Propane with CO2. Appl. Catal. A Gen. 2010, 374, 142–149. [Google Scholar] [CrossRef]
- Chernov, A.N.; Sobolev, V.I.; Gerasimov, E.Y.; Koltunov, K.Y. Propane Dehydrogenation on Co-N-C/SiO2 Catalyst: The Role of Single-Atom Active Sites. Catalysts 2022, 12, 1262. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; El-Toni, A.M.; Labis, J.P.; Khan, A.; Ghaithan, H.; Al-Zahrani, A.A.; Abasaeed, A.E.; Al-Zahrani, S.M. Mesoporous Organo-Silica Supported Chromium Oxide Catalyst for Oxidative Dehydrogenation of Ethane to Ethylene with CO2. Catalysts 2021, 11, 642. [Google Scholar] [CrossRef]
- Mashkin, M.Y.; Tedeeva, M.A.; Fedorova, A.A.; Fatula, E.R.; Egorov, A.V.; Dvoryak, S.V.; Maslakov, K.I.; Knotko, A.V.; Baranchikov, A.E.; Kapustin, G.I.; et al. Synthesis of CexZr1-xO2/SiO2 Supports for Chromium Oxide Catalysts of Oxidative Dehydrogenation of Propane with Carbon Dioxide. J. Chem. Technol. Biotechnol. 2023, 98, 1247–1259. [Google Scholar] [CrossRef]
- Massaro, M.; Noto, R.; Riela, S. Halloysite Nanotubes: Smart Nanomaterials in Catalysis. Catalysts 2022, 12, 149. [Google Scholar] [CrossRef]
- Glotov, A.; Stavitskaya, A.; Chudakov, Y.; Ivanov, E.; Huang, W.; Vinokurov, V.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Mesoporous Metal Catalysts Templated on Clay Nanotubes. Bull. Chem. Soc. Jpn. 2019, 92, 61–69. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Filled with MgO for Paper Reinforcement and Deacidification. Appl. Clay Sci. 2021, 213, 106231. [Google Scholar] [CrossRef]
- Guo, W.; Xu, L.; Feng, P.; Gu, Y.; Shuai, C. In-Situ Growth of Silica Nano-Protrusions on Halloysite Nanotubes for Interfacial Reinforcement in Polymer/Halloysite Scaffolds. Appl. Surf. Sci. 2020, 513, 145772. [Google Scholar] [CrossRef]
- Vinokurov, V.; Novikov, A.; Rodnova, V.; Anikushin, B.; Kotelev, M.; Ivanov, E.; Lvov, Y. Cellulose Nanofibrils and Tubular Halloysite as Enhanced Strength Gelation Agents. Polymers 2019, 11, 919. [Google Scholar] [CrossRef]
- Rozza, R.; Armata, N.; Lazzara, G.; Parisi, F.; Ferrante, F. Halloysite Nanotubes and Metal Corrosion Inhibitors: A Computational and Experimental Study. J. Phys. Chem. C 2019, 123, 10451–10461. [Google Scholar] [CrossRef]
- Novikov, A.A.; Sayfutdinova, A.R.; Gorbachevskii, M.V.; Filatova, S.V.; Filimonova, A.V.; Rodrigues-Filho, U.P.; Fu, Y.; Wang, W.; Wang, H.; Vinokurov, V.A.; et al. Natural Nanoclay-Based Silver–Phosphomolybdic Acid Composite with a Dual Antimicrobial Effect. ACS Omega 2022, 7, 6728–6736. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of Halloysite Clay Nanotube Lumen by Selective Etching of Aluminum Oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Jia, S.; Fan, M. Silanization of Heat-Treated Halloysite Nanotubes Using γ-Aminopropyltriethoxysilane. Appl. Clay Sci. 2019, 180, 105204. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Glotov, A.P.; Ivanov, E.V.; Gushchin, P.A.; Lvov, Y.M.; Maximov, A.L.; Muradov, A.V.; Karakhanov, E.A. Core-Shell Nanoarchitecture: Schiff-Base Assisted Synthesis of Ruthenium in Clay Nanotubes. Pure Appl. Chem. 2018, 90, 825–832. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Vinokurov, V.; Lvov, Y. Clay Nanotube-Metal Core/Shell Catalysts for Hydroprocesses. Chem. Soc. Rev. 2021, 50, 9240–9277. [Google Scholar] [CrossRef]
- Glotov, A.; Novikov, A.; Stavitskaya, A.; Nedolivko, V.; Kopitsyn, D.; Kuchierskaya, A.; Ivanov, E.; Stytsenko, V.; Vinokurov, V.; Lvov, Y. Nanoreactors Based on Hydrophobized Tubular Aluminosilicates Decorated with Ruthenium: Highly Active and Stable Catalysts for Aromatics Hydrogenation. Catal. Today 2021, 378, 33–42. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Ashok Kumar, E.; Ahmed, F. Construction of Nickel Cobalt-Layered Double Hydroxide/Functionalized–Halloysite Nanotubes Composite for Electrochemical Detection of Organophosphate Insecticide. Chem. Eng. J. 2022, 433, 133639. [Google Scholar] [CrossRef]
- Rouster, P.; Dondelinger, M.; Galleni, M.; Nysten, B.; Jonas, A.M.; Glinel, K. Layer-by-Layer Assembly of Enzyme-Loaded Halloysite Nanotubes for the Fabrication of Highly Active Coatings. Colloids Surf. B Biointerfaces 2019, 178, 508–514. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Nedolivko, V.; Zasypalov, G.; Stytsenko, V.; Karakhanov, E.; Vinokurov, V. Ruthenium Catalysts Templated on Mesoporous MCM-41 Type Silica and Natural Clay Nanotubes for Hydrogenation of Benzene to Cyclohexane. Catalysts 2020, 10, 537. [Google Scholar] [CrossRef]
- Glotov, A.; Levshakov, N.; Stavitskaya, A.; Artemova, M.; Gushchin, P.; Ivanov, E.; Vinokurov, V.; Lvov, Y. Templated Self-Assembly of Ordered Mesoporous Silica on Clay Nanotubes. Chem. Commun. 2019, 55, 5507–5510. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A.; Vinokurov, V.; Ivanov, E.; Glotov, A. Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid. Catalysts 2020, 10, 7. [Google Scholar] [CrossRef]
- Fu, L.; Yang, H.; Tang, A.; Hu, Y. Engineering a Tubular Mesoporous Silica Nanocontainer with Well-Preserved Clay Shell from Natural Halloysite. Nano Res. 2017, 10, 2782–2799. [Google Scholar] [CrossRef]
- Glotov, A.; Levshakov, N.; Vutolkina, A.; Lysenko, S.; Karakhanov, E.; Vinokurov, V. Aluminosilicates Supported La-Containing Sulfur Reduction Additives for FCC Catalyst: Correlation between Activity, Support Structure and Acidity. Catal. Today 2019, 329, 135–141. [Google Scholar] [CrossRef]
- Glotov, A.P.; Artemova, M.I.; Demikhova, N.R.; Smirnova, E.M.; Ivanov, E.V.; Gushchin, P.A.; Egazar’yants, S.V.; Vinokurov, V.A. A Study of Platinum Catalysts Based on Ordered Al–MCM-41 Aluminosilicate and Natural Halloysite Nanotubes in Xylene Isomerization. Pet. Chem. 2019, 59, 1226–1234. [Google Scholar] [CrossRef]
- Han, Z.-F.; Xue, X.-L.; Wu, J.-M.; Lang, W.-Z.; Guo, Y.-J. Preparation and Catalytic Properties of Mesoporous NV-MCM-41 for Propane Oxidative Dehydrogenation in the Presence of CO2. Chin. J. Catal. 2018, 39, 1099–1109. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Kuśtrowski, P.; Chmielarz, L. Chromium Oxide Supported on MCM-41 as a Highly Active and Selective Catalyst for Dehydrogenation of Propane with CO2. Appl. Catal. A Gen. 2008, 349, 62–69. [Google Scholar] [CrossRef]
- Wang, Y.; Ohishi, Y.; Shishido, T.; Zhang, Q.; Yang, W.; Guo, Q.; Wan, H.; Takehira, K. Characterizations and Catalytic Properties of Cr-MCM-41 Prepared by Direct Hydrothermal Synthesis and Template-Ion Exchange. J. Catal. 2003, 220, 347–357. [Google Scholar] [CrossRef]
- Zaki, M.I.; Fouad, N.E.; Bond, G.C.; Tahir, S.F. Temperature-Programmed Reduction of Calcined Chromia-Coated Alumina and Silica Catalysts: Probing Chromium (VI)-Oxygen Species. Thermochim. Acta 1996, 285, 167–179. [Google Scholar] [CrossRef]
- Cabrera, F.; Ardissone, D.; Gorriz, O.F. Dehydrogenation of Propane on Chromia/Alumina Catalysts Promoted by Tin. Catal. Today 2008, 133–135, 800–804. [Google Scholar] [CrossRef]
- Dinse, A.; Khennache, S.; Frank, B.; Hess, C.; Herbert, R.; Wrabetz, S.; Schlögl, R.; Schomäcker, R. Oxidative Dehydrogenation of Propane on Silica (SBA-15) Supported Vanadia Catalysts: A Kinetic Investigation. J. Mol. Catal. A Chem. 2009, 307, 43–50. [Google Scholar] [CrossRef]
- Gaydhankar, T.R.; Samuel, V.; Jha, R.K.; Kumar, R.; Joshi, P.N. Room Temperature Synthesis of Si-MCM-41 Using Polymeric Version of Ethyl Silicate as a Source of Silica. Mater. Res. Bull. 2007, 42, 1473–1484. [Google Scholar] [CrossRef]




| Functional Material/Catalyst | SBET, m2/g | Pore Volume *, cm3/g | Pore Diameter **, nm | Cr content ***, %wt. | Acidity, mmol NH3/g |
|---|---|---|---|---|---|
| HNT | 67 | 0.30 | 14 | - | 0.175 |
| MCM-41/HNT | 887 | 0.59 | 2.5; 14 | - | 0.326 |
| MCM-41@HNT | 324 | 0.37 | 2.6; 14 | - | 0.271 |
| 5%Cr/HNT | 53 | 0.29 | 12 | 4.4 | 0.101 |
| 5%Cr/MCM-41/HNT | 558 | 0.32 | 2.4; 12 | 4.9 | 0.217 |
| 5%Cr/MCM-41@HNT | 264 | 0.32 | 2.8; 12 | 4.7 | 0.180 |
| Catalyst | T, °C | XC3H8, % | XCO2, % | S(H2), % | Selectivity to Each Component in Hydrocarbon Gases, % | STY, mol/(kg·h) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H4 | C2H6 | C3H6 | ΣC4+ | ||||||
| 5%Cr/HNT | 550 | 13.8 | 3.9 | 1.4 | 11.4 | 9.3 | 3.1 | 75.6 | 0.6 | 2.8 |
| 600 | 19.3 | 6.8 | 2.6 | 12.5 | 14.6 | 4.6 | 67.7 | 0.6 | 3.5 | |
| 650 | 40.8 | 13.9 | 5.5 | 18.5 | 23.5 | 5.9 | 51.0 | 1.1 | 5.6 | |
| 650 * | 33.3 | - | 7.0 | 12.4 | 14.7 | 7.4 | 64.7 | 0.8 | 5.8 | |
| 700 | 71.7 | 21.5 | 9.9 | 28.6 | 35.8 | 6.9 | 26.9 | 1.8 | 5.2 | |
| 5%Cr/MCM-41/HNT | 550 | 19.4 | 8.7 | 3.7 | 11.1 | 2.6 | 3.1 | 82.6 | 0.6 | 4.3 |
| 600 | 31.2 | 16.2 | 6.3 | 13.2 | 7.4 | 4.9 | 73.7 | 0.8 | 6.2 | |
| 650 | 49.2 | 22.2 | 11.1 | 20.8 | 19.2 | 6.8 | 52.2 | 1.0 | 6.9 | |
| 650 * | 38.5 | - | 10.5 | 14.8 | 17.9 | 6.0 | 60.4 | 0.9 | 6.2 | |
| 700 | 87.6 | 23.9 | 13.5 | 30.4 | 30.5 | 7.8 | 30.3 | 1.0 | 7.1 | |
| 5%Cr/MCM-41@HNT | 550 | 12.0 | 12.4 | 1.3 | 12.5 | 11.9 | 3.5 | 71.3 | 0.8 | 2.3 |
| 600 | 25.6 | 13.2 | 4.7 | 15.5 | 12.7 | 4.0 | 67.0 | 0.8 | 4.6 | |
| 650 | 44.3 | 19.7 | 9.1 | 22.3 | 24.6 | 6.2 | 46.0 | 0.9 | 5.5 | |
| 650 * | 27.2 | - | 8.5 | 16.1 | 22.3 | 4.6 | 55.0 | 2.0 | 4.0 | |
| 700 | 71.7 | 33.3 | 14.3 | 31.4 | 35.2 | 8.5 | 23.5 | 1.4 | 4.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikov, D.; Smirnova, E.; Reshetina, M.; Novikov, A.; Wang, H.; Ivanov, E.; Vinokurov, V.; Glotov, A. Mesoporous Chromium Catalysts Templated on Halloysite Nanotubes and Aluminosilicate Core/Shell Composites for Oxidative Dehydrogenation of Propane with CO2. Catalysts 2023, 13, 882. https://doi.org/10.3390/catal13050882
Melnikov D, Smirnova E, Reshetina M, Novikov A, Wang H, Ivanov E, Vinokurov V, Glotov A. Mesoporous Chromium Catalysts Templated on Halloysite Nanotubes and Aluminosilicate Core/Shell Composites for Oxidative Dehydrogenation of Propane with CO2. Catalysts. 2023; 13(5):882. https://doi.org/10.3390/catal13050882
Chicago/Turabian StyleMelnikov, Dmitry, Ekaterina Smirnova, Marina Reshetina, Andrei Novikov, Hongqiang Wang, Evgenii Ivanov, Vladimir Vinokurov, and Aleksandr Glotov. 2023. "Mesoporous Chromium Catalysts Templated on Halloysite Nanotubes and Aluminosilicate Core/Shell Composites for Oxidative Dehydrogenation of Propane with CO2" Catalysts 13, no. 5: 882. https://doi.org/10.3390/catal13050882
APA StyleMelnikov, D., Smirnova, E., Reshetina, M., Novikov, A., Wang, H., Ivanov, E., Vinokurov, V., & Glotov, A. (2023). Mesoporous Chromium Catalysts Templated on Halloysite Nanotubes and Aluminosilicate Core/Shell Composites for Oxidative Dehydrogenation of Propane with CO2. Catalysts, 13(5), 882. https://doi.org/10.3390/catal13050882





