Comprehensive Transformation of Escherichia coli for Nicotinamide Mononucleotide Production
Abstract
1. Introduction
2. Results
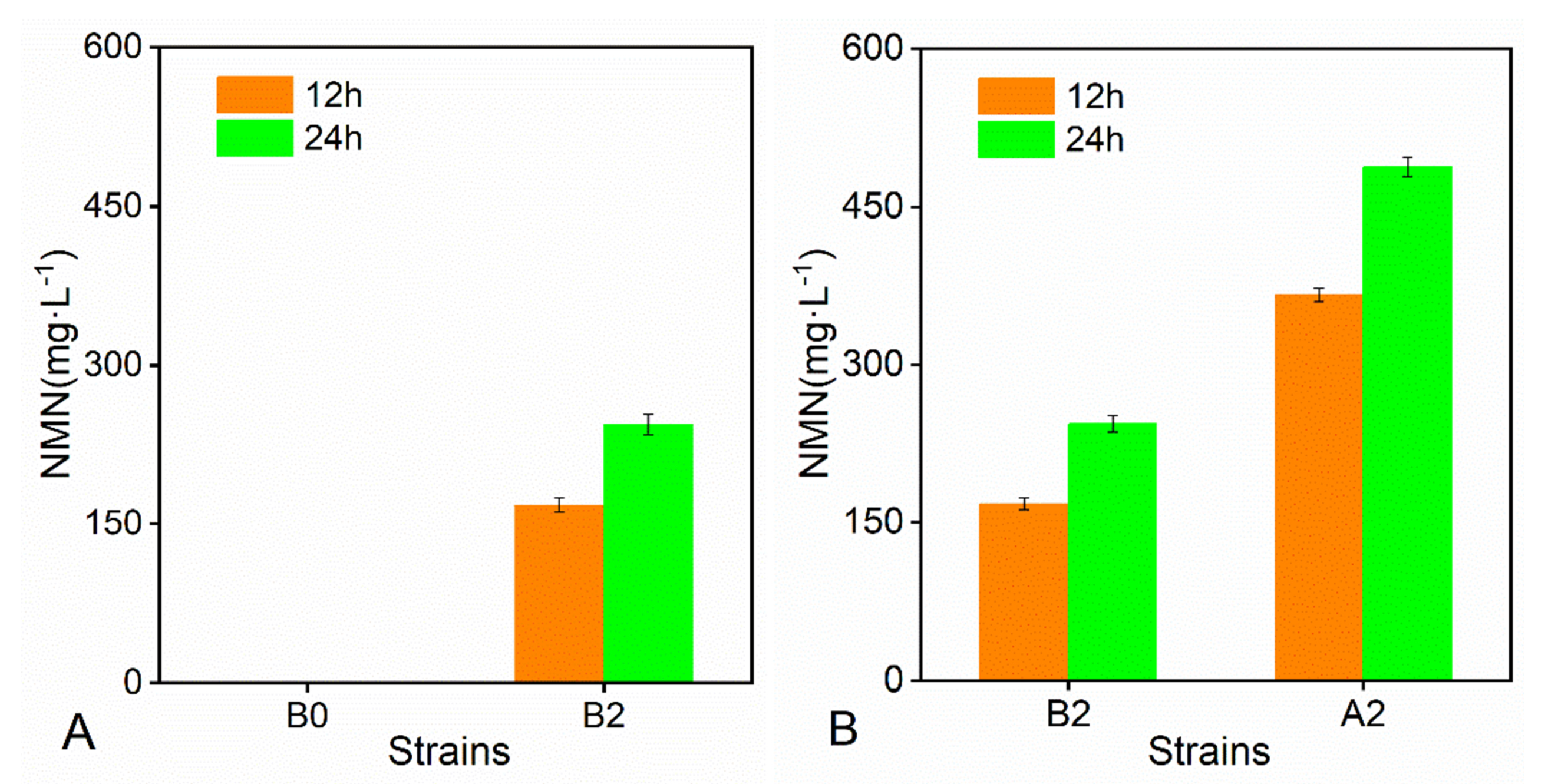
2.1. Introduction of Nampt and PnuC Confers NMN Accumulation Ability
2.2. Improvement in ATP Supply Using CRISPR/Cas9
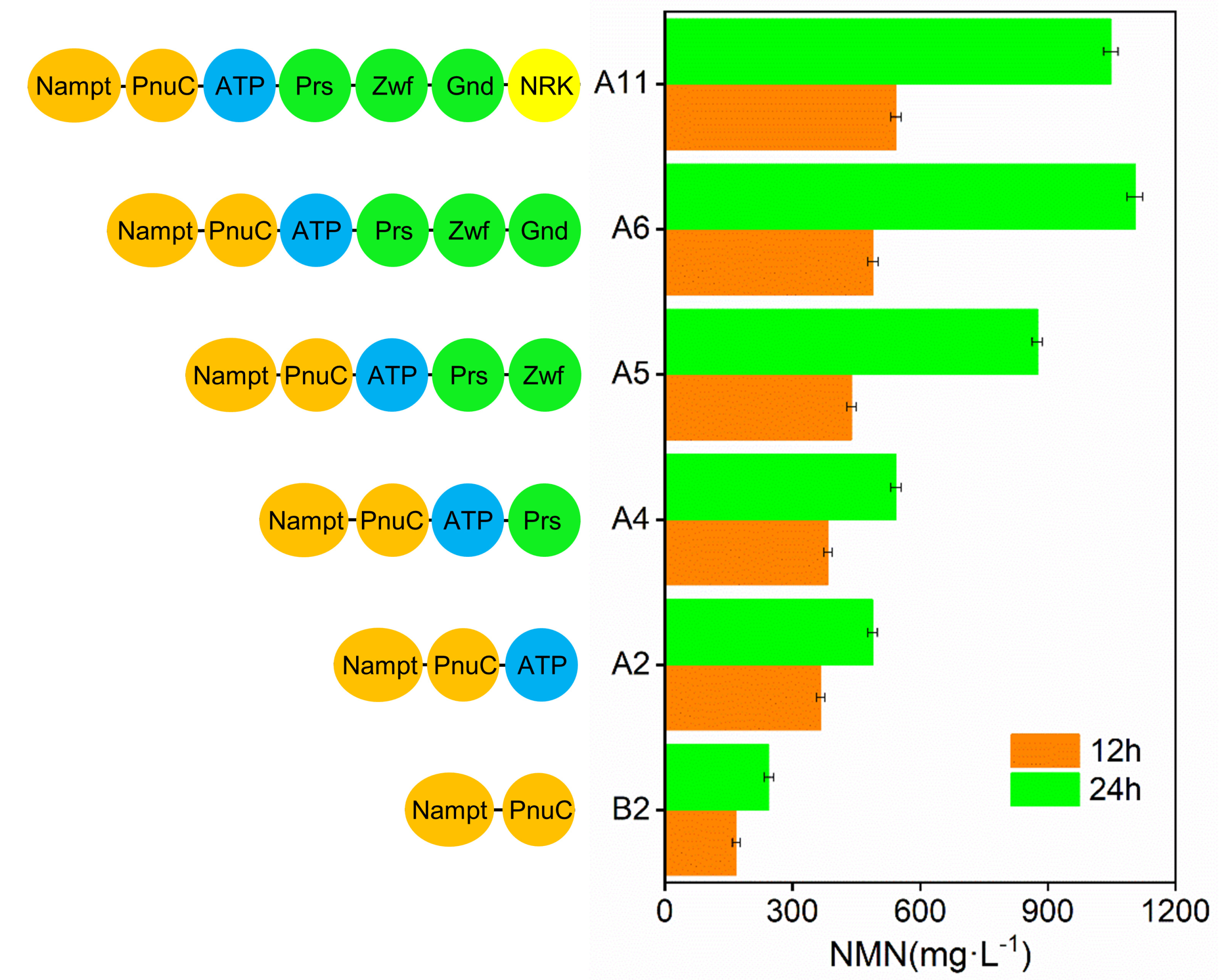
2.3. Strengthening the PRPP Pathway to Increase NMN Production
2.4. Effect of the Introduction of NRK from Kluyveromyces marxianus on NMN Yield
2.5. Multiple Strategies for Improving NMN Production in E. coli
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Strains and Medium
4.3. Construction of Plasmids
4.4. Construction of SgRNAs and DNA Templates
4.5. Production and Transformation of Receptive Cells
4.6. Shake-Flask Culture
4.7. Analytical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, K.; Li, B.; Ma, Y.; Tu, T.; Lin, Q.; Zhu, J.; Zhou, Y.; Liu, N.; Liu, Q. Nicotinamide mononucleotide attenuates HIF-1α activation and fibrosis in hypoxic adipose tissue via NAD+/SIRT1 axis. Front. Endocrinol. 2023, 14, 1099134. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Balasubramanian, P.; Valcarcel-Ares, M.N.; Tarantini, S.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Reglodi, D.; Zhang, X.A.; Bari, F.; et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: A potential mechanism for the prevention of vascular cognitive impairment. GeroScience 2019, 41, 619–630. [Google Scholar] [CrossRef]
- Rashid, M.A.; Oliveros, A.; Kim, Y.S.; Jang, M.-H. Nicotinamide mononucleotide prevents cisplatin-induced mitochondrial defects in cortical neurons derived from human induced pluripotent stem cells. Brain Plast. 2022, 8, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xu, T.; Sun, J.; Shi, J.; Li, F.; Yin, Y.; Wang, Z.; Liu, Y. Nicotinamide mononucleotide ameliorates sleep deprivation-induced gut microbiota dysbiosis and restores colonization resistance against intestinal infections. Adv. Sci. 2023, 10, 2207170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Li, Z.; Miller, E.S. Vibrio phage KVP40 encodes a functional NAD+ salvage pathway. J. Bacteriol. 2017, 199, e00855-16. [Google Scholar] [CrossRef]
- Bossé, J.T.; Durham, A.L.; Rycroft, A.N.; Kroll, J.S.; Langford, P.R. New plasmid tools for genetic analysis of Actinobacillus pleuropneumoniae and other Pasteurellaceae. Appl. Environ. Microbiol. 2009, 75, 6124–6131. [Google Scholar] [CrossRef]
- Marinescu, G.C.; Popescu, R.-G.; Stoian, G.; Dinischiotu, A. β-nicotinamide mononucleotide (NMN) production in Escherichia coli. Sci. Rep. 2018, 8, 12278. [Google Scholar] [CrossRef]
- Sorci, L.; Blaby, I.; De Ingeniis, J.; Gerdes, S.; Raffaelli, N.; De Crécy Lagard, V.; Osterman, A. Genomics-driven reconstruction of Acinetobacter NAD metabolism. J. Biol. Chem. 2010, 285, 39490–39499. [Google Scholar] [CrossRef]
- Shoji, S.; Yamaji, T.; Makino, H.; Ishii, J.; Kondo, A. Metabolic design for selective production of nicotinamide mononucleotide from glucose and nicotinamide. Metab. Eng. 2021, 65, 167–177. [Google Scholar] [CrossRef]
- Black, W.B.; Aspacio, D.; Bever, D.; King, E.; Zhang, L.; Li, H. Metabolic engineering of Escherichia coli for optimized biosynthesis of nicotinamide mononucleotide, a noncanonical redox cofactor. Microb. Cell Factories 2020, 19, 150. [Google Scholar] [CrossRef]
- Sorci, L.; Martynowski, D.; Rodionov, D.A.; Eyobo, Y.; Zogaj, X.; Klose, K.E.; Nikolaev, E.V.; Magni, G.; Zhang, H.; Osterman, A.L. Nicotinamide mononucleotide synthetase is the key enzyme for an alternative route of NAD biosynthesis in Francisella tularensis. Proc. Natl. Acad. Sci. USA 2009, 106, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, B. Metabolic engineering of Escherichia coli for biosynthesis of β-nicotinamide mononucleotide from nicotinamide. Microb. Biotechnol. 2021, 14, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-R.; Sun, C.-C.; Zhu, G.; Hu, S.-H.; Xiang, L.-X.; Shao, J.-Z. New function for Escherichia coli xanthosine phophorylase (xapA): Genetic and biochemical evidences on its participation in NAD+ salvage from nicotinamide. BMC Microbiol. 2014, 14, 29. [Google Scholar] [CrossRef]
- Huang, Z.; Li, N.; Yu, S.; Zhang, W.; Zhang, T.; Zhou, J. Systematic engineering of Escherichia coli for efficient production of nicotinamide mononucleotide from nicotinamide. ACS Synth. Biol. 2022, 11, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Xie, X.; Xu, Q.; Zhang, C.; Chen, N. Enhancement of cytidine production by coexpression of gnd, zwf, and prs genes in recombinant Escherichia coli CYT15. Biotechnol. Lett. 2013, 35, 245–251. [Google Scholar] [CrossRef]
- Willemoës, M.; Hove-Jensen, B.; Larsen, S. Steady state kinetic model for the binding of substrates and allosteric effectors to Escherichia coli phosphoribosyl-diphosphate synthase. J. Biol. Chem. 2000, 275, 35408–35412. [Google Scholar] [CrossRef]
- Yang, L.; Mu, X.; Nie, Y.; Xu, Y. Improving the production of NAD+ via multi-strategy metabolic engineering in Escherichia coli. Metab. Eng. 2021, 64, 122–133. [Google Scholar] [CrossRef]
- Pontrelli, S.; Chiu, T.-Y.; Lan, E.I.; Chen, F.Y.H.; Chang, P.; Liao, J.C. Escherichia coli as a host for metabolic engineering. Metab. Eng. 2018, 50, 16–46. [Google Scholar] [CrossRef]
- Pinson, B.; Ceschin, J.; Saint-Marc, C.; Daignan-Fornier, B. Dual control of NAD+ synthesis by purine metabolites in yeast. eLife 2019, 8, e43808. [Google Scholar] [CrossRef]
- Ji, X.-J.; Xia, Z.-F.; Fu, N.-H.; Nie, Z.-K.; Shen, M.-Q.; Tian, Q.-Q.; Huang, H. Cofactor engineering through heterologous expression of an NADH oxidase and its impact on metabolic flux redistribution in Klebsiella pneumoniae. Biotechnol. Biofuels 2013, 6, 7. [Google Scholar] [CrossRef]
- San, K.-Y.; Bennett, G.N.; Berríos-Rivera, S.J.; Vadali, R.V.; Yang, Y.-T.; Horton, E.; Rudolph, F.B.; Sariyar, B.; Blackwood, K. Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab. Eng. 2002, 4, 182–192. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, L.; Shi, Z.; Du, G.; Chen, J. ATP in current biotechnology: Regulation, applications and perspectives. Biotechnol. Adv. 2009, 27, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Leibach, T.K.; Spiess, G.I.; Neudecker, T.J.; Peschke, G.J.; Puchwein, G.; Hartmann, G.R. Purification and properties of adenosine kinase from dried brewer’s Yeast. Hoppe-Seyler’s Z. Physiol. Chem. 1971, 352, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Elaskalani, O.; Falasca, M.; Moran, N.; Berndt, M.C.; Metharom, P. The role of platelet-derived ADP and ATP in promoting pancreatic cancer cell survival and gemcitabine resistance. Cancers 2017, 9, 142. [Google Scholar] [CrossRef]
- Chapman, A.G.; Fall, L.; Atkinson, D.E. Adenylate energy charge in Escherichia coli during growth and starvation. J. Bacteriol. 1971, 108, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Ataullakhanov, F.I.; Vitvitsky, V.M. What determines the intracellular ATP concentration. Biosci. Rep. 2002, 22, 501–511. [Google Scholar] [CrossRef]
- Manfredi, J.P.; Holmes, E.W. Purine salvage pathways in myocardium. Annu. Rev. Physiol. 1985, 47, 691–705. [Google Scholar] [CrossRef]
- Thalmann, R.; Marcus, N.Y.; Thalmann, I. Adenylate energy charge, energy status, and phosphorylation state of stria vascularis under metabolic stress. Laryngoscope 1978, 88, 1985–1998. [Google Scholar] [CrossRef]
- Jung, J.; Lim, J.H.; Kim, S.Y.; Im, D.-K.; Seok, J.Y.; Lee, S.-J.V.; Oh, M.-K.; Jung, G.Y. Precise precursor rebalancing for isoprenoids production by fine control of gapA expression in Escherichia coli. Metab. Eng. 2016, 38, 401–408. [Google Scholar] [CrossRef]
- Qian, X.-L.; Dai, Y.-S.; Li, C.-X.; Pan, J.; Xu, J.-H.; Mu, B. Enzymatic synthesis of high-titer nicotinamide mononucleotide with a new nicotinamide riboside kinase and an efficient ATP regeneration system. Bioresour. Bioprocess. 2022, 9, 26. [Google Scholar] [CrossRef]
- Ngivprom, U.; Lasin, P.; Khunnonkwao, P.; Worakaensai, S.; Jantama, K.; Kamkaew, A.; Lai, R.Y. Synthesis of nicotinamide mononucleotide from xylose via coupling engineered Escherichia coli and a biocatalytic cascade. Chembiochem 2022, 23, e202200071. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Pérez, R.; García-Saura, A.G.; Scantlebery, A.M.; Schomakers, B.V.; Rabadán-Ros, R.; van Weeghel, M.; Houtkooper, R.H.; Sánchez-Ferrer, Á. Biotechnological production of reduced and oxidized NAD+ precursors. Food Res. Int. 2023, 165, 112560. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.T. Cloning Techniques. BioScience 1983, 33, 721–722. [Google Scholar] [CrossRef]



| Strains or Plasmids | Description | Sources |
|---|---|---|
| Plasmids | ||
| pCas | repA101(Ts)kan pCas-cas9 ParaB–Red lacIq Ptrc-sgRNA-pMB1 | Lab stock |
| pTargetF | pMB1 aadA sgRNA | Lab stock |
| pTargetF-add | pMB1 aadA sgRNA-add | This study |
| pTargetF-amn | pMB1 aadA sgRNA-amn | This study |
| pET-28a | pBR322 origin lacI, T7lac, KanR | Lab stock |
| pET-28a-N-P | pET-28a containing Niap-SD-AS-PnuC gene, KanR | This study |
| pET-28a-Nampt | pET-28a containing Nampt gene, KanR | This study |
| pET-28a-NRK | pET-28a containing NRK gene, KanR | This study |
| pET-28a-Ado1 | pET-28a containing Ado1 gene, KanR | This study |
| pACYCDuet | This study | |
| pACYCDuet-Nampt | pACYCDuet containing Nampt gene, CmR | This study |
| pACYCDuet-N-P | pACYCDuet containing Nampt-SD-AS-PnuC gene, CmR | This study |
| pACYCDuet-N-N | pACYCDuet containing Nampt-SD-AS-PnuC-SD-AS-NRK gene, CmR | This study |
| pCDFDuet | This study | |
| pCDFDuet-Prs | pCDFDuet containing Prs gene, SmR | This study |
| pCDFDuet-PZ | pCDFDuet containing Prs-SD-AS-Zwf gene, SmR | This study |
| pCDFDuet-PGZ | pCDFDuet containing Prs-SD-AS-Gnd-SD-AS-Zwf gene, SmR | This study |
| Strains | ||
| E. coli DH5α | the cloning host | Lab stock |
| B0 | E. coli BL21(DE3) | Lab stock |
| B1 | B0 with pACYCDuet-Nampt | This study |
| B2 | B0 with pACYCDuet-Nampt-SD-AS-PnuC | This study |
| B3 | B0 with pET-28a-Niap-SD-AS-PnuC | This study |
| B4 | B0 with pET-28a-NRK | This study |
| B5 | B0 with pCDFDuet-Prs | This study |
| B6 | B0 with pCDFDuet-Prs-Zwf | This study |
| B7 | B0 with pCDFDuet-Prs-Zwf-Gnd | This study |
| A0 | B0△amn,△add::Ado1, gene Ado1 with PT7 | This study |
| A1 | A0 with pACYCDuet-Nampt | This study |
| A2 | A0 with pACYCDuet-Nampt-SD-AS-PnuC | This study |
| A3 | A1 with pET-28a-Niap-SD-AS-PnuC | This study |
| A4 | A2 with pCDFDuet-Prs | This study |
| A5 | A2 with pCDFDuet-Prs-SD-AS-Zwf | This study |
| A6 | A2 with pCDFDuet-Prs-SD-AS-Zwf-SD-AS-Gnd | This study |
| A7 | A0 with pET-28a-NRK | This study |
| A8 | A7 with pET-28a-Niap-SD-AS-PnuC | This study |
| A9 | A7 with pACYCDuet-Nampt-SD-AS-PnuC | This study |
| A10 | A0 with pCDFDuet-Prs-SD-AS-Zwf-SD-AS-Gnd | This study |
| A11 | A10 with pACYCDuet-Nampt-SD-AS-PnuC-SD-AS-NRK | This study |
| Primers | Sequences (5′-3′) |
|---|---|
| Nampt-F | gccatcaccatcatcaccacatgaccaaagaaaacctgattctg |
| Nampt-R | attcggatcctggctttagatggttgcgtttttacggatctgctcaaagc |
| pac-F | gtaaaaacgcaaccatctaaagccaggatccgaattcgagctcg |
| pac-R | atcaggttttctttggtcatgtggtgatgatggtgatggctgctgc |
| PnuC-F | tctaaagaaggagatatacaatggttcgtagtccgctgtttctgct |
| PnuC-R | tcattgtatatctccttctttagatgtagttgttcacgcgttcacgttct |
| NRK-F | tctaaagaaggagatatacaatgacgacaactaaagtcaaactgattgcg |
| NRK-R | tcgaattcggatcctggctctaattcgcgtctaagtgcgacacgatataa |
| amn-F1 | cagaatatggggctaccgcgcgaactt |
| amn-R1 | ataagaaggttcagaacttagtgtgtctcctgttccatac |
| amn-F2 | gtatggaacaggagacacactaagttctgaaccttcttat |
| amn-R2 | gtttcatctccgccgcctttggctttatc |
| Ado1-F | cttactctaaatagctcgagtaagttctgaaccttcttatcaga |
| Ado1-R | gtgagtcgtattaatttcgcgtgtgtctcctgttccatacaatt |
| add-F | cgcggatccatgaccgcaccattggtagtatt |
| add-R | ccgctcgagctatttagagtaagatattttttcggaagggtaagag |
| Prs-F | cagccaggatccgaattcgtgcctgatatgaagctttttgctggtaacgc |
| Prs-R | tgcggccgcaagcttttagtgttcgaacatggcagagatcgattcttcgt |
| pcd-F | cgaacactaaaagcttgcggccgcataatgcttaag |
| pcd-R | aaaaagcttcatatcaggcacgaattcggatcctggctgtggtgatgatg |
| Gnd-F | gaacactaaagaaggagatatacaatgtccaagcaacagatcggcgtagt |
| Gnd-R | ttgtatatctccttctttaatccagccattcggtatggaacacaccttct |
| Zwf-F | attaaagaaggagatatacaatggcggtaacgcaaacagccc |
| Zwf-R | tatgcggccgcaagcttttactcaaactcattccaggaacgaccatcacg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, T.; Wu, T.; Yang, L.; Xu, Y.; Mu, X. Comprehensive Transformation of Escherichia coli for Nicotinamide Mononucleotide Production. Catalysts 2023, 13, 815. https://doi.org/10.3390/catal13050815
Bi T, Wu T, Yang L, Xu Y, Mu X. Comprehensive Transformation of Escherichia coli for Nicotinamide Mononucleotide Production. Catalysts. 2023; 13(5):815. https://doi.org/10.3390/catal13050815
Chicago/Turabian StyleBi, Tianjiao, Tao Wu, Linyan Yang, Yan Xu, and Xiaoqing Mu. 2023. "Comprehensive Transformation of Escherichia coli for Nicotinamide Mononucleotide Production" Catalysts 13, no. 5: 815. https://doi.org/10.3390/catal13050815
APA StyleBi, T., Wu, T., Yang, L., Xu, Y., & Mu, X. (2023). Comprehensive Transformation of Escherichia coli for Nicotinamide Mononucleotide Production. Catalysts, 13(5), 815. https://doi.org/10.3390/catal13050815






