Au Nanoparticles Supported on Mn- or/and La-Doped CeO2 Nanorods for One-Step Oxidative Esterification of Methacrolein and Methanol to Methyl Methacrylate
Abstract
1. Introduction
2. Results and Discussion
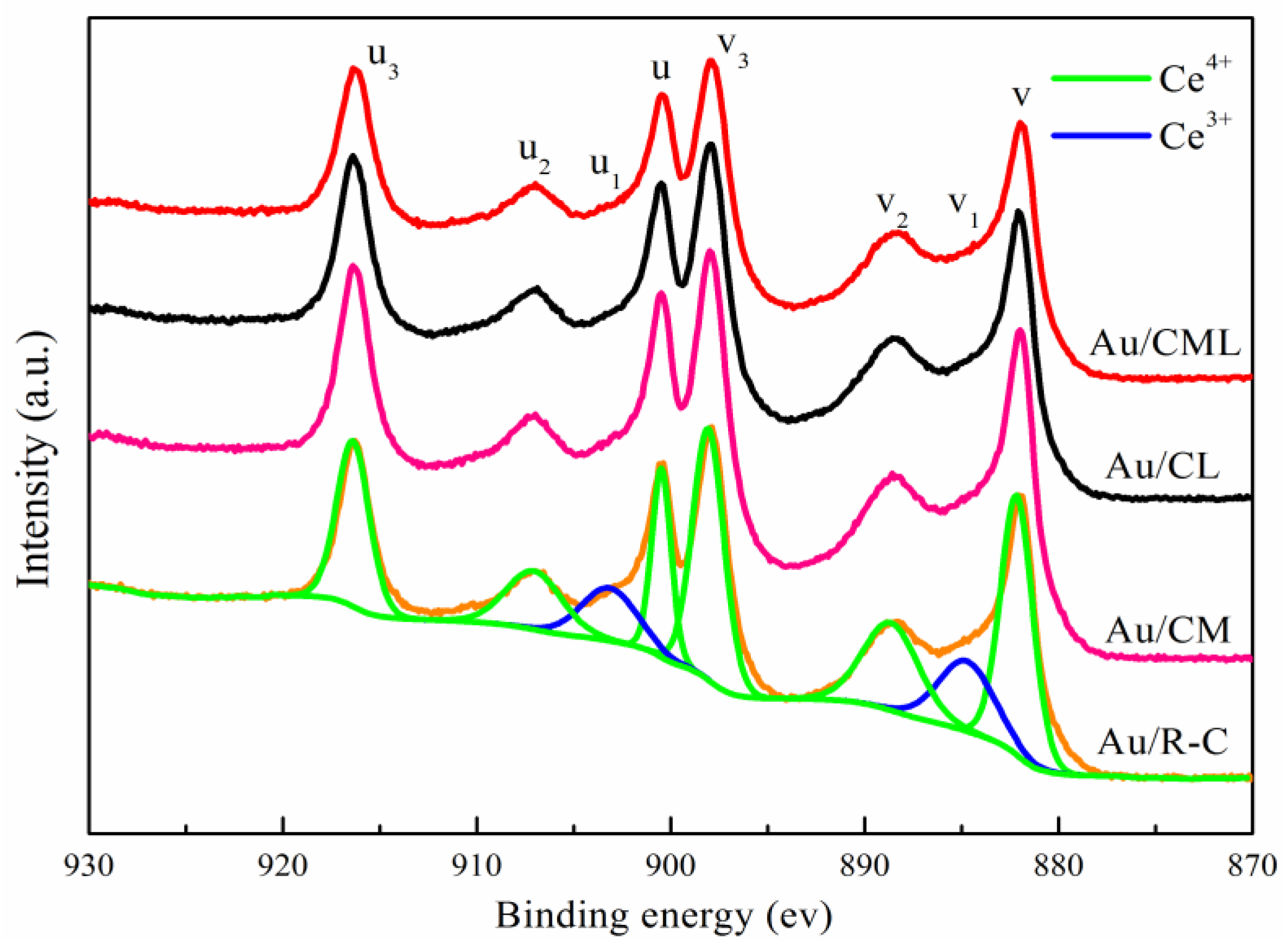
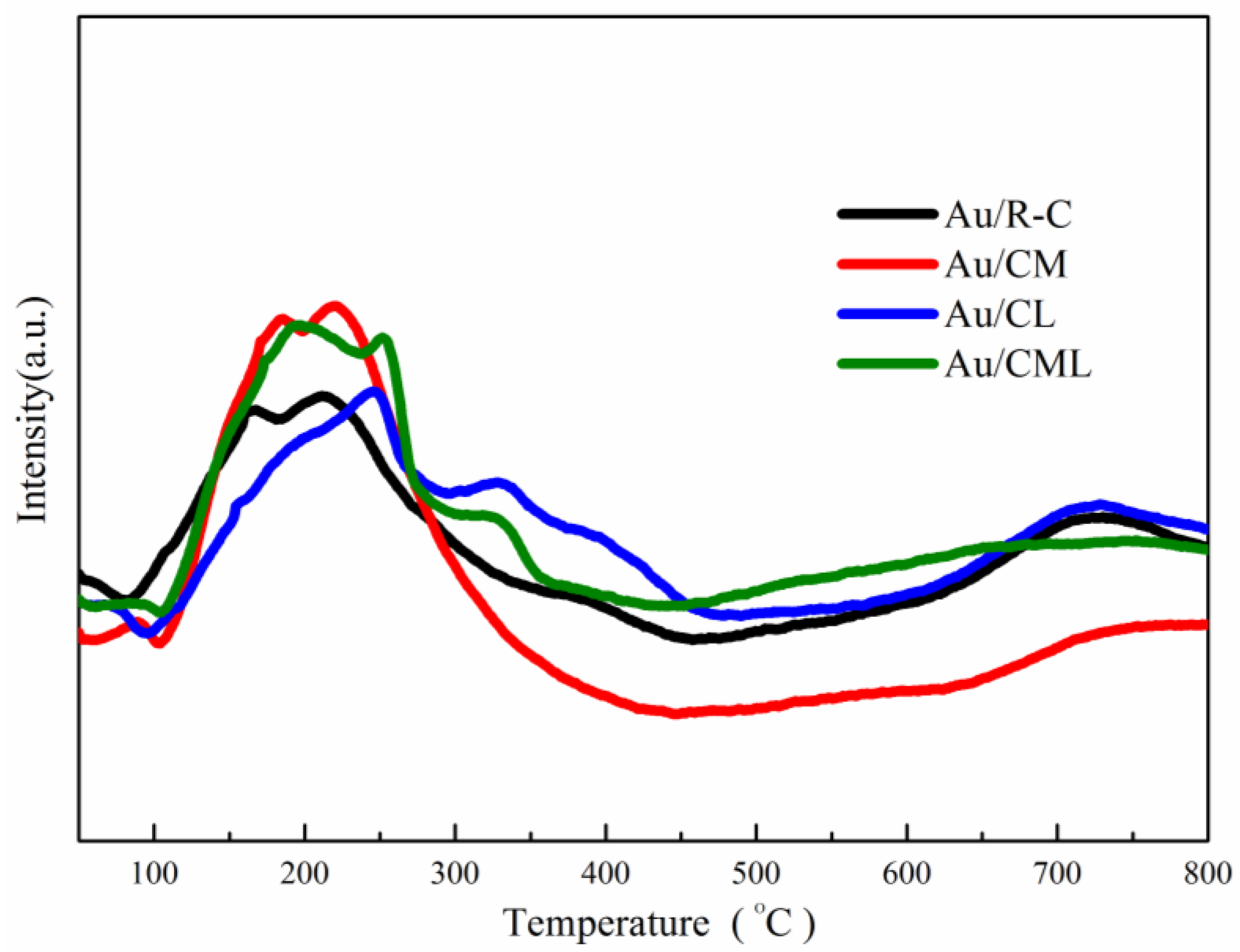
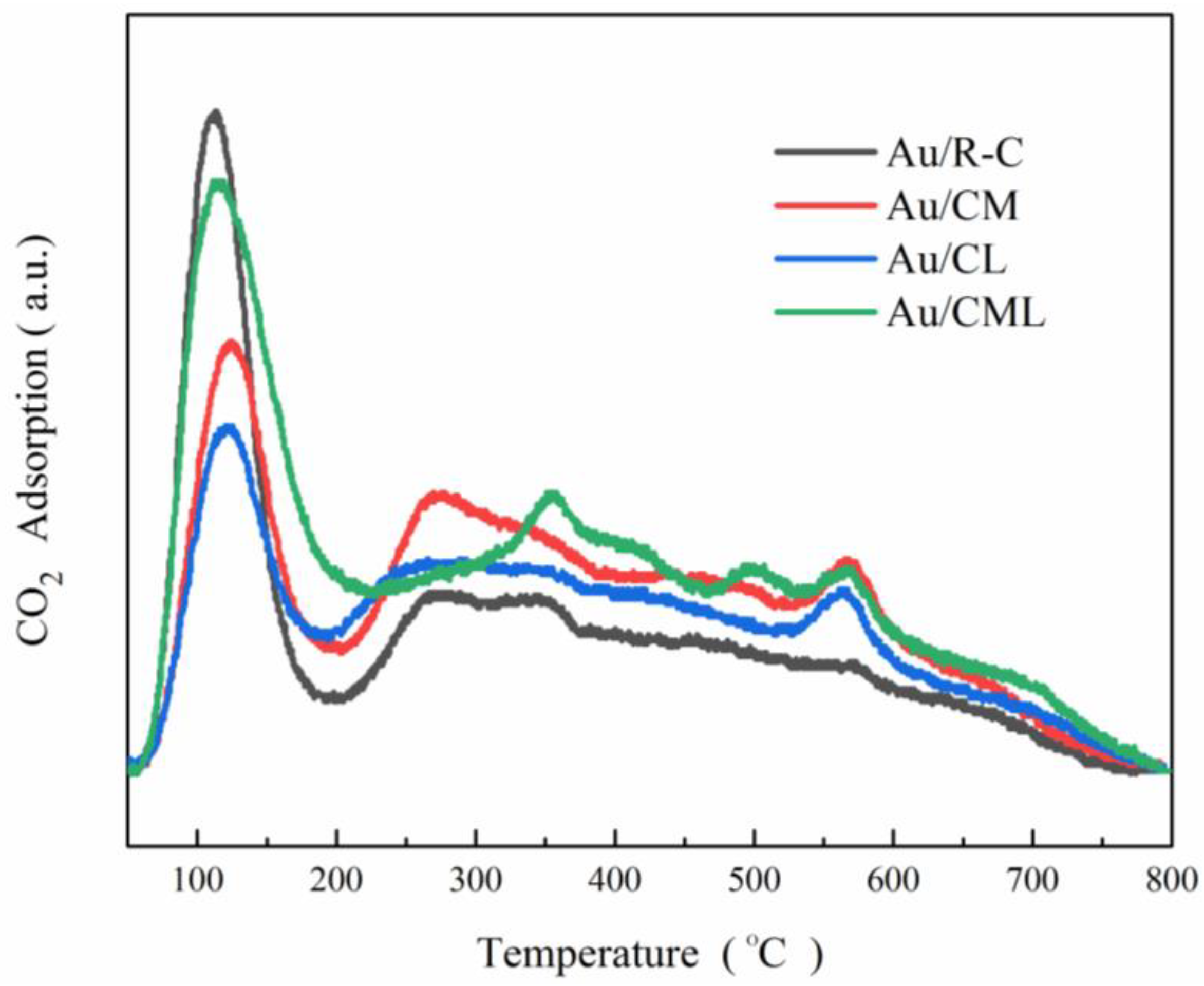
2.1. Catalysts Characterization
2.2. Catalytic Performance
3. Materials and Methods
3.1. Synthesis of Mn- or/and La-Doped CeO2 Nanorods and Supporting Au Catalysts
3.2. Catalysts Characterization
3.3. Catalysts Evaluation
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagai, K. New developments in the production of methyl methacrylate. Appl. Catal. A Gen. 2001, 221, 367–377. [Google Scholar] [CrossRef]
- Yamamatsu, S.; Yamaguchi, T.; Yokota, K.; Nagano, O.; Chono, M.; Aoshima, A. Development of catalyst technology for producing methyl methacrylate (MMA) by direct methyl esterification. Catal. Surv. Asia 2010, 14, 124–131. [Google Scholar] [CrossRef]
- Mahboub, M.J.D.; Dubois, J.L.; Cavani, F.; Rostamizadeh, M.; Patience, G.S. Catalysis for the synthesis of methacrylic acid and methyl methacrylate. Chem. Soc. Rev. 2018, 47, 7703–7738. [Google Scholar]
- Yoshida, Y.; Mikami, Y.; Oh-Kita, M. Process for Producing Carboxylic Acid Esters and Catalyst. EP 0972759, 19 March 1998. [Google Scholar]
- Diao, Y.Y.; Yan, R.Y.; Zhang, S.J.; Yang, P.; Li, Z.X.; Wang, L.; Dong, H.F. Effects of Pb and Mg doping in Al2O3-supported Pd catalyst on direct oxidative esterification of aldehydes with alcohols to esters. J. Mol. Catal. A Chem. 2009, 303, 35–42. [Google Scholar] [CrossRef]
- Jiang, L.; Diao, Y.Y.; Han, J.X.; Yan, R.Y.; Zhang, X.P.; Zhang, S.J. MgO-SBA-15 supported Pd-Pb catalysts for oxidative esterification of methacrolein with methanol to methyl methacrylate. Chin. J. Chem. Eng. 2014, 22, 1098–1104. [Google Scholar] [CrossRef]
- Wang, B.H.; Sun, W.J.; Zhu, J.; Ran, W.L.; Chen, S. Pd-Pb/SDB bimetallic catalysts for the direct oxidative esterification of methacrolein to methyl methacrylate. Ind. Eng. Chem. Res. 2012, 51, 15004–15010. [Google Scholar] [CrossRef]
- Wang, B.H.; Li, H.; Zhu, J.; Sun, W.J.; Chen, S. Preparation and characterization of mono-/multi-metallic hydrophobic catalysts for the oxidative esterification of methacrolein to methyl methacrylate. J. Mol. Catal. A Chem. 2013, 379, 322–326. [Google Scholar] [CrossRef]
- Wang, B.H.; Ran, W.L.; Sun, W.J.; Wang, K. Direct Oxidative Esterification of Aldehyde with Alcohol to Ester over Pd/Styrene-Divinyl Benzene Copolymer Catalyst. Ind. Eng. Chem. Res. 2012, 51, 3932–3938. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, T.; Matsushita, K.; Iitsuka, C.; Miura, J.; Akaogi, T.; Ishida, H. Aerobic Oxidative Esterification of Aldehydes with Alcohols by Gold-Nickel Oxide Nanoparticle Catalysts with a Core-Shell Structure. ACS Catal. 2013, 3, 1845–1849. [Google Scholar] [CrossRef]
- Costa, V.V.; Estrada, M.; Demidova, Y.; Prosvirin, I.; Kriventsov, V.; Cotta, R.F.; Fuentes, S.; Simakov, A.; Gusevskaya, E.V. Gold Nanoparticles Supported on Magnesium Oxide as Catalysts for the Aerobic Oxidation of Alcohols under Alkali-Free Conditions. J. Catal. 2012, 292, 148–156. [Google Scholar] [CrossRef]
- Wan, X.; Deng, W.; Zhang, Q.; Wang, Y. Magnesia-Supported Gold Nanoparticles as Efficient Catalysts for Oxidative Esterification of Aldehydes or Alcohols with Methanol to Methyl Esters. Catal. Today 2014, 233, 147–154. [Google Scholar] [CrossRef]
- Gao, J.; Fan, G.L.; Yang, L.; Cao, X.Z.; Zhang, P.; Li, F. Oxidative esterification of methacrolein to methyl methacrylate over gold nanoparticles on hydroxyapatite. ChemCatChem 2017, 9, 1230–1241. [Google Scholar] [CrossRef]
- Li, J.; Li, H.Y.; Liu, Z.Y.; Akri, M.; Tan, Y.; Kang, L.L.; Chi, J.; Qiao, B.T.; Ding, Y.J. Synergic effect between gold and vanadate substituted hydroxyapatite support for synthesis of methyl methacrylate by one-step oxidative esterification. Chem. Eng. J. 2022, 431, 133207–133219. [Google Scholar] [CrossRef]
- Paul, B.; Khatun, R.; Sharma, S.K.; Adak, S.; Singh, G.; Das, D.; Siddiqui, N.; Bhandari, S.; Joshi, V.; Sasaki, T.; et al. Fabrication of Au nanoparticles supported on one-dimensional (1D) La2O3 nanorods for selective Esterification of Methacrolein to Methyl Methacrylate with Molecular Oxygen. ACS Sustinable Chem. Eng. 2019, 7, 3982–3994. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, L.; Yan, R.Y.; Han, J.X.; Zhang, S.J. Promoting effects of MgO, (NH4)2SO4 or MoO3 modification in oxidative esterification of methacrolein over Au/Ce0.6Zr0.4O2-based catalysts. Catal. Sci. Technol. 2016, 6, 5453–5463. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.C.; Zuo, C.C.; Yin, D.F.; Wang, L.; Zheng, Y.X.; Huang, H.F.; Fu, Z.J.; Wang, M. Ionic-Liquid-Modified Porous Au/CeMnOx Nanorods for Methyl Methacrylate (MMA) Synthesis via Direct Oxidative Esterification. ChemNanoMat 2019, 5, 1361–1366. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.C.; Zheng, Y.X.; Wang, M.; Zuo, C.C.; Huang, H.F.; Yin, D.F.; Fu, Z.J.; Tan, J.; Zhou, Z.C. Nano-Au/MCeOx Catalysts for the Direct Oxidative Esterification of Methylacrolein to Methyl Esters. Ind. Eng. Chem. Res. 2019, 58, 19397–19405. [Google Scholar] [CrossRef]
- Li, Y.C.; Tian, Y.X.; Ge, T.T.; Fu, Z.J.; Jiao, T.T.; Wang, M.; Huang, H.F.; Zuo, C.C. Direct oxidation esterification of methacrolein with methanol: Oxygen vacancy promotion of Zr-doped Au/CeO2 nanorods. Can. J. Chem. Eng. 2020, 3, 767–774. [Google Scholar] [CrossRef]
- Lim, S.; Kwon, S.; Kim, N.; Na, K. A Multifunctional Au/CeO2-Mg(OH)2 Catalyst for One-Pot Aerobic Oxidative Esterification of Aldehydes with Alcohols to Alkyl Esters. Nanomaterials 2021, 11, 1536. [Google Scholar] [CrossRef]
- Lei, T.Q.; Guo, H.Y.; Miao, C.X.; Hua, W.M.; Yue, Y.H.; Gao, Z. Mn-doped CeO2 Nanorod Supported Au Catalysts for Dehydrogenation of Ethane with CO2. Catalysts 2019, 9, 119. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Jampaiah, D.; Mukherjee, D.; Aniz, C.U.; Reddy, B.M. Mn-doped ceria solid solutions for CO oxidation at lower temperatures. Catal. Lett. 2016, 146, 2105–2118. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Gandhi, H.S.; Graham, G.W.; McCabe, E.W. Automotive exhaust catalysis. J. Catal. 2003, 216, 433–442. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Grazini, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today. 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Bueno-López, A.; Krishna, K.; Makkee, M.; Moulijn, J.A. Enhanced soot oxidation by lattice oxygen via La3+-doped CeO2. J. Catal. 2005, 230, 237–248. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Ji, S.; Li, Y.T.; Hou, F.; Zhang, H.C.; Zhang, X.W.; Wang, L.; Li, G.Z. Tuning oxygen vacancies on mesoporous ceria nanorods by metal doping: Controllable magnetic property. Appl. Surf. Sci. 2018, 455, 1037–1044. [Google Scholar] [CrossRef]
- Manzoli, M.; Menegazzo, F.; Signoretto, M.; Cruciani, G.; Pinna, F. Effects of synthetic parameters on the catalytic performance of Au/CeO2 for furfural oxidative esterification. J. Catal. 2015, 330, 465–473. [Google Scholar] [CrossRef]
- Xu, B.J.; Liu, X.Y.; Haubrich, J.; Friend, C.M. Vapour-phase gold-surface-mediated coupling of aldehydes with methanol. Nat. Chem. 2010, 2, 61–65. [Google Scholar] [CrossRef]
- Katta, L.; Sudarsanam, P.; Mallesham, B.; Reddy, B.M. Preparation of silica supported ceria-lanthana solid solutions useful for synthesis of 4-methylpent-1-ene and dehydroacetic acid. Catal. Sci. Technol. 2012, 2, 995–1004. [Google Scholar] [CrossRef]
- Burroughs, A.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalton. Trans. 1976, 17, 1686–1698. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.Q.; Wu, Y.N.; Wang, Y.J.; Luo, M. UV and Visible Raman Studies of Oxygen Vacancies in Rare-Earth-Doped Ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef]
- Salvatore, S.; Simona, M.; Carmelo, C.; Cristina, S.; Alessandro, P. Catalytic combustion of volatile organic compounds on gold/cerium oxide catalysts. Appl. Catal. B-Environ. 2003, 40, 43–49. [Google Scholar]
- Jia, L.S.; Gao, J.; Fang, W.P. Carbon dioxide hydrogenation to methanol over the pre-reduced LaCr0.5Cu0.5O3 catalyst. Catal. Commun. 2009, 10, 2000–2003. [Google Scholar] [CrossRef]
- Azzouz, A.; Nistor, D.; Miron, D.; Ursu, A.V.; Sajin, T.; Monette, F.; Niquette, P.; Hausler, R. Assessment of acid–base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements. Thermochim. Acta 2006, 449, 27–34. [Google Scholar] [CrossRef]
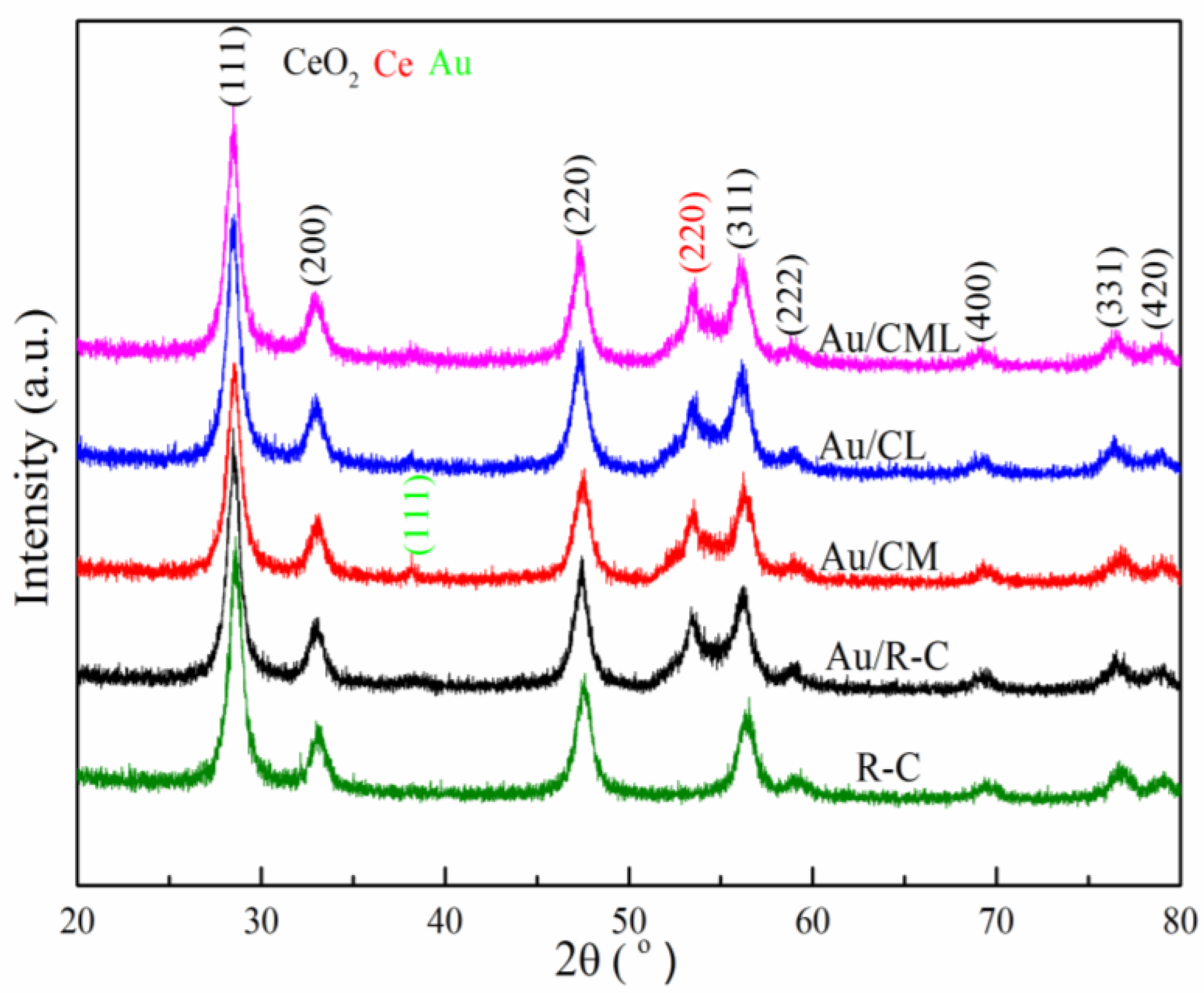
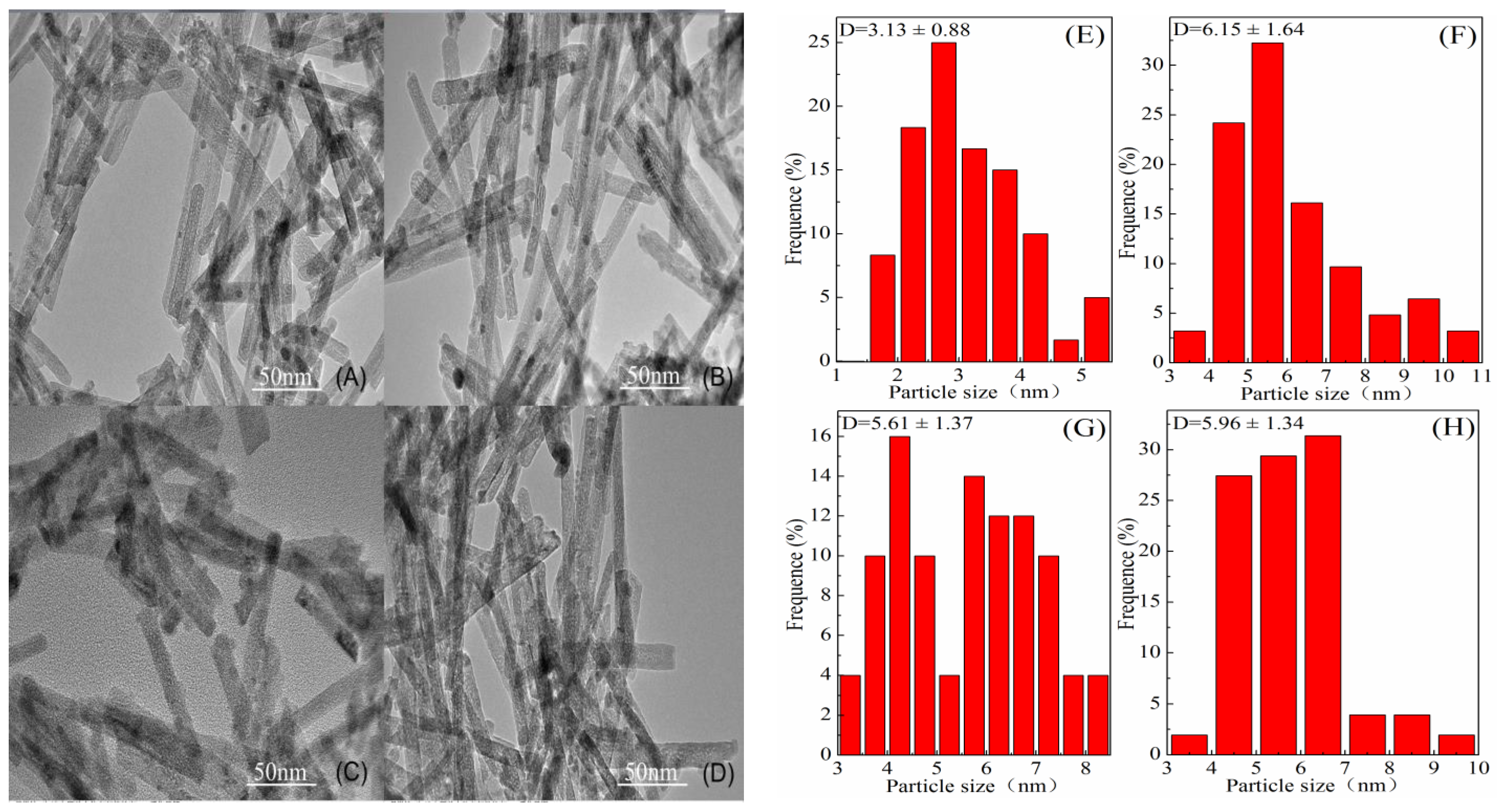
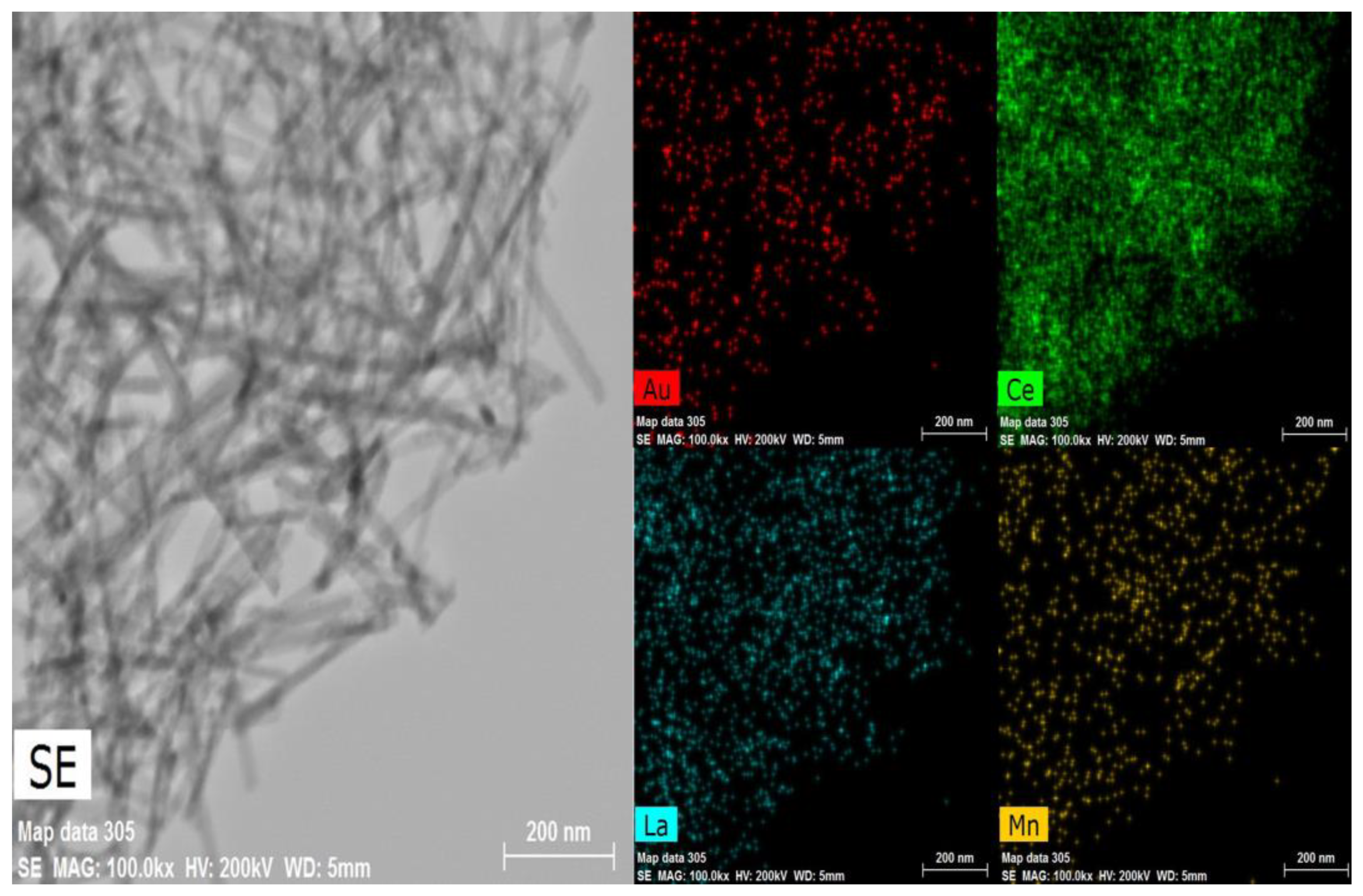
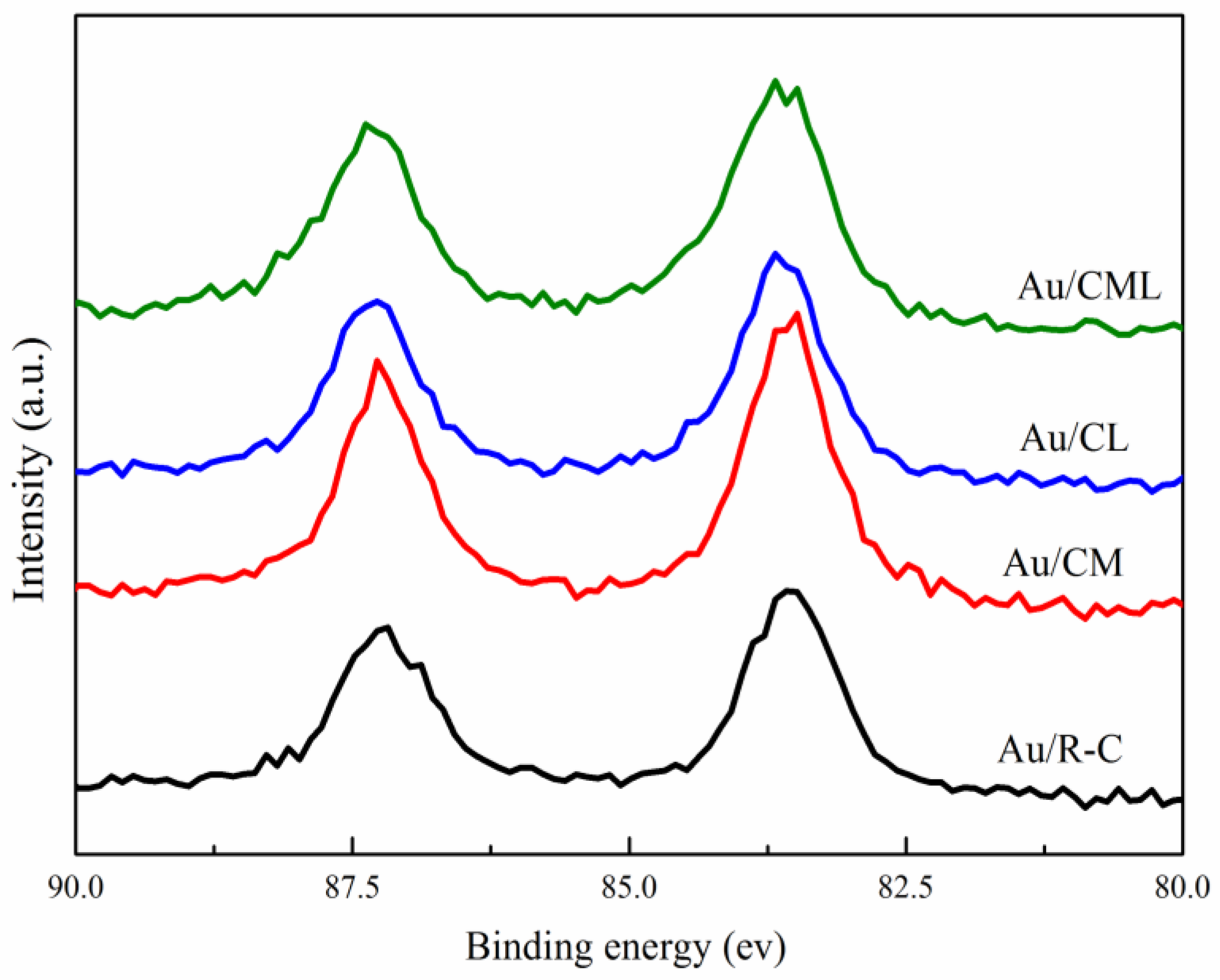
| Catalysts | Surface Area m2/g | Pore Volume cm3/g | Average Pore Size nm | Au Content a wt(%) | Crystallite Size of Support b nm |
|---|---|---|---|---|---|
| Au/R-C | 90 | 0.50 | 23 | 1.44 | 24.2 |
| Au/CM | 103 | 0.49 | 19 | 1.47 | 21.9 |
| Au/CL | 103 | 0.45 | 17 | 1.49 | 21.4 |
| Au/CML | 96 | 0.39 | 15 | 1.44 | 23.8 |
| Catalysts | Au/R-C | Au/CM | Au/CL | Au/CML | |
|---|---|---|---|---|---|
| Au species | Au 4f5/2 (ev) | 87.20 | 87.23 | 87.29 | 87.33 |
| Au 4f7/2 (ev) | 83.50 | 83.53 | 83.62 | 83.65 | |
| Ce species | Ce3+ (%) | 7.97 | 12.72 | 9.04 | 12.4 |
| Ce4+ (%) | 92.03 | 87.28 | 90.96 | 87.6 | |
| R(Ce3+:Ce4+) (%) | 8.66 | 14.57 | 9.94 | 14.16 |
| Catalysts | Total Basicity (mmol CO2/g) |
|---|---|
| Au/R-C | 0.692 |
| Au/CM | 0.817 |
| Au/CL | 0.737 |
| Au/CML | 0.989 |
| Catalysts | MAL Conversion (%) | Selectivity b (%) | MMA Yield (%) | |
|---|---|---|---|---|
| MMA | Others | |||
| Au/R-C | 71.1 | 91.6 | 8.4 | 65.1 |
| Au/CM | 98.6 | 97.5 | 2.5 | 96.1 |
| Au/CL | 91.5 | 98.0 | 2.0 | 89.7 |
| Au/CML | 92.6 | 98.9 | 1.1 | 91.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H. Au Nanoparticles Supported on Mn- or/and La-Doped CeO2 Nanorods for One-Step Oxidative Esterification of Methacrolein and Methanol to Methyl Methacrylate. Catalysts 2023, 13, 767. https://doi.org/10.3390/catal13040767
Zhang H. Au Nanoparticles Supported on Mn- or/and La-Doped CeO2 Nanorods for One-Step Oxidative Esterification of Methacrolein and Methanol to Methyl Methacrylate. Catalysts. 2023; 13(4):767. https://doi.org/10.3390/catal13040767
Chicago/Turabian StyleZhang, Haojian. 2023. "Au Nanoparticles Supported on Mn- or/and La-Doped CeO2 Nanorods for One-Step Oxidative Esterification of Methacrolein and Methanol to Methyl Methacrylate" Catalysts 13, no. 4: 767. https://doi.org/10.3390/catal13040767
APA StyleZhang, H. (2023). Au Nanoparticles Supported on Mn- or/and La-Doped CeO2 Nanorods for One-Step Oxidative Esterification of Methacrolein and Methanol to Methyl Methacrylate. Catalysts, 13(4), 767. https://doi.org/10.3390/catal13040767







