From Fenton and ORR 2e−-Type Catalysts to Bifunctional Electrodes for Environmental Remediation Using the Electro-Fenton Process
Abstract
1. Environmental Problems
- Primary treatment by physicochemical operations;
- Secondary treatment by biological processes;
- Tertiary treatment by additional processes.
2. Fenton Reaction
| Catalyst | Catalyst Concentration | H2O2 Concentration | Pollutant | Pollutant Concentration | Solution pH | Percentage of Elimination | Ref. |
|---|---|---|---|---|---|---|---|
| LaCu0.5Mn0.5O3 | 0.6 g L−1 | 0.7 g L−1 | Carbamazepine (CZP) | 15 mg L−1 | 5.5 | 100% | [65] |
| ZnO dopped Fe3O4@S | 0.25 g L−1 | 5 mL L−1 | Ofloxacin (OFX) | 10 mg L−1 | 5.2–9.0 | 100% | [77] |
| FeSO4·7H2O | 50 mg L−1 | 26.4 mM | Industrial wastewater (DQO) | 850 mg L−1 (DQO) | 3 | 72% | [53] |
| FeSO4·7H2O | 40 mg L−1 | 23 mL L−1 | Hydroxyethylpolydialyldimethylammonium-acrylamide-acrylic-acrylate (PDM) | 200 mg L−1 | 2 | 69.3% | [54] |
| Siderite (FeCO3) | 6 g L−1 | 100 mMol L−1 | sodium sulfadiazine | 50 mg L−1 | 9 | 99% | [78] |
| Goethite | - | 460 mg L−1 | Amoxicillin | 105 mg L−1 | 6.5–7 | 83% | [68] |
| MnFe2O4@C-NH2 | 1 g L−1 | 3 mL L−1 | Amoxicillin | 30 mg L−1 | 3.0 | 99.1% | [56] |
| Fe2(SO4)3·xH2O | 30 mg L−1 | - | Tetracycline | 50 mg L−1 | 6.0 | 90% | [69] |
| Fe2(SO4)·7H2O | 0.095 mg L−1 | 2.35 mg L−1 | Amoxicillin | 0.45 mg L−1 | 3.5–4.5 | 100% | [62] |
| rGO–Fe3O4 | 0.23 g L−1 | - | Ofloxacin | 20 mg L−1 | 7.0–4.0 | 99.9% | [76] |
| MoS2-Fe | 150 mg L−1 | 10 mMol L−1 | Methylene blue | 25 mg L−1 | 3 | 65% | [55] |
| CNTs–Fe3O4 | 0.5 g L−1 | 24.5 mM | Sulfamerazine | 40 mg L−1 | 3 | 70% | [57] |
| Dissolved iron from zero valence iron nanoparticles (nZVI) | 9 mg L−1 | 34 mg L−1 | Sulfathiazole | 0.5 mg L−1 | 3 | >96% | [58] |
| Fe2(SO4)·7H2O with hydroxylamine | 10 μM de Fe2+ | 1.0 mM | Norfloxacin | 10 mg L−1 | 5 | 100% | [66] |
| CuS@Fe3O4/Pt | - | - | Tetracycline | 40 mg L−1 | - | 78% | [79] |
| Single-atom iron fixed in porous carbon (Fe-ISA@CN) | 100 mg L−1 | 10 mM | Sulfadiazine | 2 mg L−1 | 6.5 | 96% | [70] |
| LDH–CuMgFe–CO3 | 0.5 g L−1 | 4 mMol L−1 | Sulfathiazole | 0.15 mg L−1 | 7.5 | 100% | [71] |
| rGO@nFe/Pd | 200 mg L−1 | 167 mMol | Rifampicin | 50 mg L−1 | 6.14 | 94.6% | [72] |
| MnFe2O4–HS | 1 g L−1 | 29.4 mM | Azithromycin | 0.1 mg L−1 | 3.0 | 92.6% | [59] |
| MagFePC (Hybrid) | 250 mg L−1 | 5 mL L−1 | Tetracycline | - | 6.0 | 100% | [80] |
| Magnetic pullulan hydrogels | 1.25 g L−1 | 1.25 mL L−1 | Tetracycline hydrochloride | 20 mg L−1 | 4.0 | 91.3% | [63] |
| Magnetic biocarbon | 0.2 g L−1 | 50 mM | Metronidazole | 20 mg L−1 | 6.0 | 97.4% | [73] |
| CoFe2O4–S | 500 mg L−1 | 5 mMol L−1 | Tetracycline | 0.1 M | 7.0 | 90% | [74] |
| COF (Photo-Fenton) | 167 mg L−1 | 50 mL L−1 | Rhodamine B | 150 mg L−1 | 4.4 | 41.2% | [81] |
| Fe3O4/Schwettmann/Carbon | 50 mg | 45 μL | Norfloxacin | 50 mg L−1 | 3.0 | 100% | [60] |
| δ-Fe3OOH/MWCNTs | 235 mg L−1 | 20.6 mMol | Ciprofloxacin | 10 mg L−1 | 5.3 | 86.9% | [67] |
| Fe2+ | 5.0 mMol L−1 de Fe2+ | 10 mmol L−1 | Antibiotic resistance gene (ARG) | 11.53 Log10(copies) g−1 dry sludge | 3 | 66.86% | [82] |
| Fe–MPC | 0.02 g L−1 | 1.0 mM | Tetracycline | 40 mg L−1 | 4.3 | 83% | [64] |
| Fresh powder from Fe0 | - | - | Sulfamethoxazole | 10 μg L−1 | 3 | 100% | [61] |
| LaCu0.8Mn0.2O3 | 0.2 g L−1 | 13.8 × 10−3 mol L−1 | Paracetamol | 50 mg L−1 | 6.7 | 90% | [75] |
3. Electro-Fenton Process
- Electrogeneration of H2O2 (cathodic reduction of O2).
- Production of OH• (Fenton reaction).
- Promotion of the formation of OH• physisorbed on the surface of the electrode.
- Regeneration of Fe2+ through the direct reduction of Fe3+ at the cathode.
- Direct aeration in solution.
- Aeration through a gas diffusing electrode (GDE).
- The increase in the mass transfer of O2 improves the contact of the gas with the catalyst and, therefore, the active sites for the ORR.
- The increase in the mass transfer is not only in reference to O2 but also between the catalyst and electrolyte, which promotes the release of H2O2 and active sites, improving the formation of the oxidizing species.
4. Promising New Fenton Catalysts: Spinel Ferrites and Perovskites
- Chemical coprecipitation
4.1. Sol–Gel Method
4.2. Hydrothermal/Solvothermal
5. Oxygen Reduction Reaction through Two Electrons (ORR 2e−)
- Oxygen molecules that are converted directly into water (4e−);
- Oxygen molecules that are initially reduced to peroxide and later to water (2e−).
- Being electro-reduced to H2O at the cathode (usually at porous cathodes);
- Being oxidized to O2 at the anode via HO2· as an intermediate;
- Disproportionate to O2 and H2O in a non-electrochemical reaction.
- Lateral adsorption leads to a longer bond length and therefore weakens the O=O bond, giving as a product, H2O (Figure 12).
- Adsorption at the end, where oxygen is adsorbed in the form of OOH, which can produce H2O2 and H2O (Figure 12).
6. Bifunctional Electro-Fenton Catalyst for Direct •OH Formation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Zhao, X.; Liu, D.; Song, K.; Liu, Q.; He, Y. Occurrence and ecological risk assessment of PPCPs in typical inflow rivers of Taihu lake, China. J. Environ. Manag. 2021, 285, 112176. [Google Scholar] [CrossRef]
- Archana, G.; Dhodapkar, R.; Kumar, A. Ecotoxicological risk assessment and seasonal variation of some pharmaceuticals and personal care products in the sewage treatment plant and surface water bodies (lakes). Environ. Monit. Assess. 2017, 189, 446. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Emerging Pollutants in Water and Wastewater. 2020. Available online: https://en.unesco.org/emergingpollutantsinwaterandwastewater (accessed on 1 July 2022).
- Molina, C.B.; Sanz-Santos, E.; Boukhemkhem, A.; Bedia, J.; Belver, C.; Rodriguez, J.J. Removal of emerging pollutants in aqueous phase by heterogeneous Fenton and photo-Fenton with Fe2O3-TiO2-clay heterostructures. Environ. Sci. Pollut. Res. 2020, 27, 38434–38445. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef]
- González-Mira, A.; Torreblanca, A.; Hontoria, F.; Navarro, J.C.; Mañanós, E.; Varó, I. Effects of ibuprofen and carbamazepine on the ion transport system and fatty acid metabolism of temperature conditioned juveniles of Solea senegalensis. Ecotoxicol. Environ. Saf. 2018, 148, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, J.; Villar-Navarro, M.; Ramos-Payán, M.; Aranda-Merino, N.; Román-Hidalgo, C.; Bello-López, M.Á.; Fernández-Torres, R. Monitoring of pharmaceuticals in aquatic biota (Procambarus clarkii) of the Doñana National Park (Spain). J. Environ. Manag. 2021, 297, 113314. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Rashid, S.S.; Liu, Y.Q. Comparison of life cycle toxicity assessment methods for municipal wastewater treatment with the inclusion of direct emissions of metals, PPCPs and EDCs. Sci. Total Environ. 2021, 756, 143849. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef]
- An, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Wang, B.; Yu, G. Pollution characterization of pharmaceutically active compounds (PhACs) in the northwest of Tai Lake Basin, China: Occurrence, temporal changes, riverine flux and risk assessment. J. Hazard. Mater. 2022, 422, 126889. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Gelband, H.; Miller-Petrie, M.; Pant, S.; Gandra, S.; Levinson, J.; Barter, D.; White, A.; Laxminarayan, R. The State of the World’s Antibiotics 2015. In Justice A Distance; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2015; pp. 1–30. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yan, D.; Mo, K.; Chen, Q.; Zhang, J.; Chen, Y.; Wang, Z. Antibiotics along an alpine river and in the receiving lake with a catchment dominated by grazing husbandry. J. Environ. Sci. 2022, 115, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Wilke, C. Many of the World’s Rivers are Flush with Dangerous Levels of Antibiotics 2019. Science News, 14 June 2019. [Google Scholar]
- Gros, M.; Catalán, N.; Mas-Pla, J.; Čelić, M.; Petrović, M.; Farré, M.J. Groundwater antibiotic pollution and its relationship with dissolved organic matter: Identification and environmental implications. Environ. Pollut. 2021, 289, 117927. [Google Scholar] [CrossRef]
- Wang, K.; Zhuang, T.; Su, Z.; Chi, M.; Wang, H. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: Occurrence, removal and environmental impacts. Sci. Total Environ. 2021, 788, 147811. [Google Scholar] [CrossRef]
- Qian, M.; Yang, L.; Chen, X.; Li, K.; Xue, W.; Li, Y.; Zhao, H.; Cao, G.; Guan, X.; Shen, G. The treatment of veterinary antibiotics in swine wastewater by biodegradation and Fenton-like oxidation. Sci. Total Environ. 2020, 710, 136299. [Google Scholar] [CrossRef]
- Jafarisani, M.; Cheshme Khavar, A.H.; Mahjoub, A.R.; Luque, R.; Rodríguez-Padrón, D.; Satari, M.; Gharravi, A.M.; Khastar, H.; Kazemi, S.S.; Masoumikarimi, M. Enhanced visible-light-driven photocatalytic degradation of emerging water contaminants by a modified zinc oxide-based photocatalyst; In-vivo and in-vitro toxicity evaluation of wastewater and PCO-treated water. Sep. Purif. Technol. 2020, 243, 116430. [Google Scholar] [CrossRef]
- Ata, R.; Yıldız Töre, G. Characterization and removal of antibiotic residues by NFC-doped photocatalytic oxidation from domestic and industrial secondary treated wastewaters in Meric-Ergene Basin and reuse assessment for irrigation. J. Environ. Manag. 2019, 233, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Cheng, Y.; Gao, Y.; Chen, Z.; Megharaj, M.; Naidu, R. Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem. Eng. J. 2014, 255, 141–148. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, Y.; Han, S.; Guo, C.; Wen, Z.; Tian, X.; Li, J. Electro-Fenton oxidation of a β-lactam antibiotic cefoperazone: Mineralization, biodegradability and degradation mechanism. Chemosphere 2021, 270, 129486. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, C.Y.; Liao, G.Y. Degradation of antibiotic tetracycline by ultrafine-bubble ozonation process. J. Water Process Eng. 2020, 37, 101463. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Jajko, M.; Sobczak, A. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere 2018, 194, 381–389. [Google Scholar] [CrossRef]
- Salas, G. Tratamiento por Oxidación Avanzada (Reacción Fenton) de Aguas Residuales de la Industria Textil. Rev. Peru. Quim. Ing. Quim. 2010, 12, 30–38. [Google Scholar]
- Fenton, H.J. Oxidation of Tartaric Acid in presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Liu, S.; Li, C.; Yuan, S. Glucose oxidase modified Fenton reactions for in-situ ROS generation and potential application in groundwater remediation. Chemosphere 2020, 253, 126648. [Google Scholar] [CrossRef]
- Ramos, J.M.P.; Pereira-Queiroz, N.M.; Santos, D.H.S.; Nascimento, J.R.; de Carvalho, C.M.; Tonholo, J.; Zanta, C.L.P.S. Printing ink effluent remediation: A comparison between electrochemical and Fenton treatments. J. Water Process Eng. 2019, 31, 100803. [Google Scholar] [CrossRef]
- Usman, M.; Ho, Y.S. A bibliometric study of the Fenton oxidation for soil and water remediation. J. Environ. Manag. 2020, 270, 110886. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.T.; Jang, S.B.; Choong, C.E.; won Kang, C.; Lee, G.C.; Song, J.Y.; Yoon, Y.; Jang, M. In situ Fenton remediation for diesel contaminated clayey zone assisted by thermal plasma blasting: Synergism and cost estimation. Chemosphere 2022, 286, 131574. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Sharma, M.; Mohapatra, T.; Ghosh, P. Hydrodynamics, mass and heat transfer study for emerging heterogeneous Fenton process in multiphase fluidized-bed reactor system for wastewater treatment—A review. Chem. Eng. Res. Des. 2021, 171, 48–62. [Google Scholar] [CrossRef]
- Yu, G.H.; Kuzyakov, Y. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth-Sci. Rev. 2021, 214, 103525. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef] [PubMed]
- Amir, J.; Rojas, A.; Fernando, L.; Giraldo, G.; Trujillo, J.M. Empleo del reactivo de Fenton para la degradación del colorante Tartrazina. Lasallista Investig. 2009, 6, 27–34. [Google Scholar]
- Zhang, X.; Zhang, Y.; Yu, Z.; Wei, X.; Wu, W.D.; Wang, X.; Wu, Z. Facile synthesis of mesoporous anatase/rutile/hematite triple heterojunctions for superior heterogeneous photo-Fenton catalysis. Appl. Catal. B Environ. 2020, 263, 118335. [Google Scholar] [CrossRef]
- Song, W.; Cheng, M.; Ma, J.; Ma, W.; Chen, C.; Zhao, J. Decomposition of hydrogen peroxide driven by photochemical cycling of iron species in clay. Environ. Sci. Technol. 2006, 40, 4782–4787. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Pimentel-Moral, S.; Verardo, V.; Martinez-Ferez, A. A focus on advanced physico-chemical processes for olive mill wastewater treatment. Sep. Purif. Technol. 2017, 179, 161–174. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; González-Rodríguez, J.; Gamallo, M.; Vargas-Osorio, Z.; Vázquez-Vázquez, C.; Piñeiro, Y.; Rivas, J.; Feijoo, G.; Moreira, M.T. Iron oxide-mediated photo-Fenton catalysis in the inactivation of enteric bacteria present in wastewater effluents at neutral pH. Environ. Pollut. 2020, 266, 115181. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Qian, J. Graphene aerogel-based catalysts in Fenton-like reactions for water decontamination: A short review. Chem. Eng. J. Adv. 2021, 8, 100171. [Google Scholar] [CrossRef]
- Dinçer, A.R.; Çifçi, D.İ.; Cinkaya, D.D.; Dülger, E.; Karaca, F. Treatment of organic peroxide containing wastewater and water recovery by fenton-adsorption and fenton-nanofiltration processes. J. Environ. Manag. 2021, 299, 113557. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Huang, D.; Zeng, G.; Cheng, M.; Gong, X.; Wang, R.; Xue, W.; Hu, Z.; Liu, Y. The combination of Fenton process and Phanerochaete chrysosporium for the removal of bisphenol A in river sediments: Mechanism related to extracellular enzyme, organic acid and iron. Chem. Eng. J. 2018, 338, 432–439. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel ferrites nanoparticles: Synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants—A review. Appl. Surf. Sci. Adv. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Rao, S.N.; Shrivastava, S. Treatment of complex recalcitrant wastewater using Fenton process. Mater. Today Proc. 2020, 29, 1161–1165. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Ma, H.; Ma, J.; Gao, D. Effective removal of polymer quaternary ammonium salt by biodegradation and a subsequent Fenton oxidation process. Ecotoxicol. Environ. Saf. 2020, 188, 109919. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yi, H.; Yang, B.; Jia, F.; Song, S. Activation of Fenton reaction by controllable oxygen incorporation in MoS2-Fe under visible light irradiation. Appl. Surf. Sci. 2021, 566, 150674. [Google Scholar] [CrossRef]
- Qin, H.; Cheng, H.; Li, H.; Wang, Y. Degradation of ofloxacin, amoxicillin and tetracycline antibiotics using magnetic core–shell MnFe2O4@C-NH2 as a heterogeneous Fenton catalyst. Chem. Eng. J. 2020, 396, 125304. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Deng, J.; Liu, Y. A novel CNTs-Fe3O4 synthetized via a ball-milling strategy as efficient fenton-like catalyst for degradation of sulfonamides. Chemosphere 2021, 277, 130305. [Google Scholar] [CrossRef]
- Fornazaria, A.L.; Labriola, V.F.; Da Silva, B.F.; Castro, L.F.; Perussi, J.R.; Vieira, E.M.; Azevedo, E.B. Coupling Zero-Valent Iron and Fenton processes for degrading sulfamethazine, sulfathiazole, and norfloxacin. J. Environ. Chem. Eng. 2021, 9, 105761. [Google Scholar] [CrossRef]
- Qin, H.; Yang, Y.; Shi, W.; She, Y.; Wu, S. Heterogeneous Fenton degradation of azithromycin antibiotic in water catalyzed by amino/thiol-functionalized MnFe2O4 magnetic nanocatalysts. J. Environ. Chem. Eng. 2021, 9, 106184. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Wang, X.; Liang, J.; Zhou, L. Modifying organic carbon in Fe3O4-loaded schwertmannite to improve heterogeneous Fenton activity through accelerating Fe(II) generation. Appl. Catal. B Environ. 2021, 285, 119830. [Google Scholar] [CrossRef]
- Goswami, A.; Jiang, J.Q.; Petri, M. Treatability of five micro-pollutants using modified Fenton reaction catalysed by zero-valent iron powder (Fe(0)). J. Environ. Chem. Eng. 2021, 9, 105393. [Google Scholar] [CrossRef]
- Homem, V.; Alves, A.; Santos, L. Amoxicillin degradation at ppb levels by Fenton’s oxidation using design of experiments. Sci. Total Environ. 2010, 408, 6272–6280. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, C.; Li, J.; Pan, X.; Zhai, X.; Jiao, Y.; Li, Y.; Dong, W.; Qi, X. Highly efficient removal of antibiotic from biomedical wastewater using Fenton-like catalyst magnetic pullulan hydrogels. Carbohydr. Polym. 2021, 262, 117951. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Del Álamo, A.C.; González, C.; Pariente, M.I.; Molina, R.; Martínez, F. Fenton-like catalyst based on a reticulated porous perovskite material: Activity and stability for the on-site removal of pharmaceutical micropollutans in a hospital wastewater. Chem. Eng. J. 2020, 401, 126113. [Google Scholar] [CrossRef]
- Wang, C.; Yu, G.; Chen, H.; Wang, J. Degradation of norfloxacin by hydroxylamine enhanced fenton system: Kinetics, mechanism and degradation pathway. Chemosphere 2021, 270, 129408. [Google Scholar] [CrossRef]
- Salari, M.; Rakhshandehroo, G.R.; Nikoo, M.R.; Zerafat, M.M.; Mooselu, M.G. Optimal degradation of Ciprofloxacin in a heterogeneous Fenton-like process using (δ-FeOOH)/MWCNTs nanocomposite. Environ. Technol. Innov. 2021, 23, 101625. [Google Scholar] [CrossRef]
- Karimnezhad, H.; Navarchian, A.H.; Tavakoli Gheinani, T.; Zinadini, S. Amoxicillin removal by Fe-based nanoparticles immobilized on polyacrylonitrile membrane: Individual nanofiltration or Fenton reaction, vs. engineered combined process. Chem. Eng. Res. Des. 2020, 153, 187–200. [Google Scholar] [CrossRef]
- Chen, P.; Sun, F.; Wang, W.; Tan, F.; Wang, X.; Qiao, X. Facile one-pot fabrication of ZnO2 particles for the efficient Fenton-like degradation of tetracycline. J. Alloys Compd. 2020, 834, 155220. [Google Scholar] [CrossRef]
- Yang, W.; Hong, P.; Yang, D.; Yang, Y.; Wu, Z.; Xie, C.; He, J.; Zhang, K.; Kong, L.; Liu, J. Enhanced Fenton-like degradation of sulfadiazine by single atom iron materials fixed on nitrogen-doped porous carbon. J. Colloid Interface Sci. 2021, 597, 56–65. [Google Scholar] [CrossRef]
- de Melo Costa-Serge, N.; Gonçalves, R.G.L.; Rojas-Mantilla, H.D.; Santilli, C.V.; Hammer, P.; Nogueira, R.F.P. Fenton-like degradation of sulfathiazole using copper-modified MgFe-CO3 layered double hydroxide. J. Hazard. Mater. 2021, 413, 125388. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Q.; Owens, G.; Chen, Z. Fenton-oxidation of rifampicin via a green synthesized rGO@nFe/Pd nanocomposite. J. Hazard. Mater. 2021, 402, 123544. [Google Scholar] [CrossRef]
- Yi, Y.; Luo, J.; Fang, Z. Magnetic biochar derived from Eichhornia crassipes for highly efficient Fenton-like degradation of antibiotics: Mechanism and contributions. J. Environ. Chem. Eng. 2021, 9, 106258. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.; Liu, J.; Lin, P.; Zhang, J.; Huang, T.; Liu, K. Mesoporous sulfur-doped CoFe2O4 as a new Fenton catalyst for the highly efficient pollutants removal. Appl. Catal. B Environ. 2021, 295, 120273. [Google Scholar] [CrossRef]
- Carrasco-Díaz, M.R.; Castillejos-López, E.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Efficient removal of paracetamol using LaCu1−xMxO3 (M = Mn, Ti) perovskites as heterogeneous Fenton-like catalysts. Chem. Eng. J. 2016, 304, 408–418. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Xie, Z.; Song, J.; Xiang, L.; Zhou, L.; Li, C.; Liao, L.; Li, J.; Wang, H. Accelerated Fenton reaction for antibiotic ofloxacin degradation in discharge plasma system based on graphene-Fe3O4 nanocomposites. Vacuum 2021, 185, 110022. [Google Scholar] [CrossRef]
- Wang, X.; Jin, H.; Wu, D.; Nie, Y.; Tian, X.; Yang, C.; Zhou, Z.; Li, Y. Fe3O4@S-doped ZnO: A magnetic, recoverable, and reusable Fenton-like catalyst for efficient degradation of ofloxacin under alkaline conditions. Environ. Res. 2020, 186, 109626. [Google Scholar] [CrossRef]
- Sun, F.; Liu, H.; Wang, H.; Shu, D.; Chen, T.; Zou, X.; Huang, F.; Chen, D. A novel discovery of a heterogeneous Fenton-like system based on natural siderite: A wide range of pH values from 3 to 9. Sci. Total Environ. 2020, 698, 134293. [Google Scholar] [CrossRef]
- Ma, E.; Wang, K.; Hu, Z.; Wang, H. Dual-stimuli-responsive CuS-based micromotors for efficient photo-Fenton degradation of antibiotics. J. Colloid Interface Sci. 2021, 603, 685–694. [Google Scholar] [CrossRef]
- Gu, W.; Huang, X.; Tian, Y.; Cao, M.; Zhou, L.; Zhou, Y.; Lu, J.; Lei, J.; Zhou, Y.; Wang, L.; et al. High-efficiency adsorption of tetracycline by cooperation of carbon and iron in a magnetic Fe/porous carbon hybrid with effective Fenton regeneration. Appl. Surf. Sci. 2021, 538, 147813. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, D.; Ke, C.; Zhang, Y.; Han, Q.; Zhang, Y.; Xi, K. Metal-free Fenton-like photocatalysts based on covalent organic frameworks. Appl. Catal. B Environ. 2021, 298, 120548. [Google Scholar] [CrossRef]
- Luo, L.; Wang, G.; Wang, Z.; Ma, J.; He, Y.; He, J.; Wang, L.; Liu, Y.; Xiao, H.; Xiao, Y.; et al. Optimization of Fenton process on removing antibiotic resistance genes from excess sludge by single-factor experiment and response surface methodology. Sci. Total Environ. 2021, 788, 147889. [Google Scholar] [CrossRef]
- Teel, A.L.; Warberg, C.R.; Atkinson, D.A.; Watts, R.J. Comparison of mineral and soluble iron Fenton’s catalysts for the treatment of trichloroethylene. Water Res. 2001, 35, 977–984. [Google Scholar] [CrossRef]
- Lewis, S.; Lynch, A.; Bachas, L.; Hampson, S.; Ormsbee, L.; Bhattacharyya, D. Chelate-modified fenton reaction for the degradation of trichloroethylene in aqueous and two-phase systems. Environ. Eng. Sci. 2009, 26, 849–859. [Google Scholar] [CrossRef]
- Bello, M.M.; Abdul Raman, A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, C.; Huang, D.; Wang, D.; Tian, S.; Wang, R.; Yang, Y.; Wang, W.; Qin, F. Influence of surface functionalities of pyrogenic carbonaceous materials on the generation of reactive species towards organic contaminants: A review. Chem. Eng. J. 2021, 404, 127066. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Huber, G.W.; Boudart, M. Principles of Heterogeneous Catalysis. In Handbook of Heterogeneous Catalysis; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Expósito, E.; Sánchez-Sánchez, C.M.; Montiel, V. Mineral Iron Oxides as Iron Source in Electro-Fenton and Photoelectro-Fenton Mineralization Processes. J. Electrochem. Soc. 2007, 154, E116. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, Q.; Wang, J. Degradation of sulfamethazine antibiotics in Fenton-like system using Fe3O4 magnetic nanoparticles as catalyst. Environ. Prog. Sustain. Energy 2017, 36, 1743–1753. [Google Scholar] [CrossRef]
- Xie, W.; Huang, Z.; Zhou, F.; Li, Y.; Bi, X.; Bian, Q.; Sun, S. Heterogeneous fenton-like degradation of amoxicillin using MOF-derived Fe0 embedded in mesoporous carbon as an effective catalyst. J. Clean. Prod. 2021, 313, 127754. [Google Scholar] [CrossRef]
- Furia, F.; Minella, M.; Gosetti, F.; Turci, F.; Sabatino, R.; Di Cesare, A.; Corno, G.; Vione, D. Elimination from wastewater of antibiotics reserved for hospital settings, with a Fenton process based on zero-valent iron. Chemosphere 2021, 283, 131170. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, J.; Ochando-Pulido, J.M.; Martinez-Ferez, A. The effect of pH in tannery wastewater by Fenton vs. Heterogeneous Fenton process. Chem. Eng. Trans. 2019, 73, 205–210. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Yu, H.; Jin, R.; Zhou, J. Acceleration of goethite-catalyzed Fenton-like oxidation of ofloxacin by biochar. J. Hazard. Mater. 2020, 397, 122783. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Zhao, J.; Zhang, L. Hematite facet confined ferrous ions as high efficient Fenton catalysts to degrade organic contaminants by lowering H2O2 decomposition energetic span. Appl. Catal. B Environ. 2016, 181, 127–137. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, Y.; Ge, Y.; Zhang, S. Heterogeneous Fenton oxidation of nitric oxide by magnetite: Kinetics and mechanism. Mater. Lett. 2018, 218, 257–261. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Liu, D.; Liu, S.; Liu, X.; Xiao, S. Enhancing electronic transfer by magnetic iron materials and metal-organic framework via heterogeneous Fenton-like process and photocatalysis. Mater. Sci. Semicond. Process 2021, 135, 106096. [Google Scholar] [CrossRef]
- Hassani, A.; Karaca, M.; Karaca, S.; Khataee, A.; Açışlı, Ö.; Yılmaz, B. Preparation of magnetite nanoparticles by high-energy planetary ball mill and its application for ciprofloxacin degradation through heterogeneous Fenton process. J. Environ. Manag. 2018, 211, 53–62. [Google Scholar] [CrossRef]
- Gholizadeh, A.M.; Zarei, M.; Ebratkhahan, M.; Hasanzadeh, A. Phenazopyridine degradation by electro-Fenton process with magnetite nanoparticles-activated carbon cathode, artificial neural networks modeling. J. Environ. Chem. Eng. 2021, 9, 104999. [Google Scholar] [CrossRef]
- Gonçalves, R.G.L.; Lopes, P.A.; Resende, J.A.; Pinto, F.G.; Tronto, J.; Guerreiro, M.C.; de Oliveira, L.C.A.; de Castro Nunes, W.; Neto, J.L. Performance of magnetite/layered double hydroxide composite for dye removal via adsorption, Fenton and photo-Fenton processes. Appl. Clay Sci. 2019, 179, 105152. [Google Scholar] [CrossRef]
- Rial, J.B.; Ferreira, M.L. Challenges of dye removal treatments based on IONzymes: Beyond heterogeneous Fenton. J. Water Process Eng. 2021, 41, 102065. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, A.; Shan, C.; Gao, G.; Pan, B. Enhanced Fe(III)-mediated Fenton oxidation of atrazine in the presence of functionalized multi-walled carbon nanotubes. Water Res. 2018, 137, 37–46. [Google Scholar] [CrossRef]
- Seo, J.; Lee, H.J.; Lee, H.; Kim, H.E.; Lee, J.Y.; Kim, H.S.; Lee, C. Enhanced production of reactive oxidants by Fenton-like reactions in the presence of carbon materials. Chem. Eng. J. 2015, 273, 502–508. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, L.; An, T. Hydrothermal Carbon-Mediated Fenton-like Reaction Mechanism in the Degradation of Alachlor: Direct Electron Transfer from Hydrothermal Carbon to Fe(III). ACS Appl. Mater. Interfaces 2017, 9, 17115–17124. [Google Scholar] [CrossRef]
- Bach, A.; Semiat, R. The role of activated carbon as a catalyst in GAC/iron oxide/H2O2 oxidation process. Desalination 2011, 273, 57–63. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Sun, L.; Yao, Y.; Wang, L.; Mao, Y.; Huang, Z.; Yao, D.; Lu, W.; Chen, W. Efficient removal of dyes using activated carbon fibers coupled with 8-hydroxyquinoline ferric as a reusable Fenton-like catalyst. Chem. Eng. J. 2014, 240, 413–419. [Google Scholar] [CrossRef]
- Yao, T.; Qi, Y.; Mei, Y.; Yang, Y.; Aleisa, R.; Tong, X.; Wu, J. One-step preparation of reduced graphene oxide aerogel loaded with mesoporous copper ferrite nanocubes: A highly efficient catalyst in microwave-assisted Fenton reaction. J. Hazard. Mater. 2019, 378, 105. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Kiciński, W.; Norek, M.; Polański, M.; Budner, B. Oxidative and adsorptive removal of chlorophenols over Fe-, N- and S-multi-doped carbon xerogels. J. Environ. Chem. Eng. 2021, 9, 105568. [Google Scholar] [CrossRef]
- Cruz, D.R.S.; de Jesus, G.K.; Santos, C.A.; Silva, W.R.; Wisniewski, A.; Cunha, G.C.; Romão, L.P.C. Magnetic nanostructured material as heterogeneous catalyst for degradation of AB210 dye in tannery wastewater by electro-Fenton process. Chemosphere 2021, 280, 130675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Shi, S.; Liu, E.; Li, T.; Wei, S.; Li, Y.; Li, Y.; Sun, G.; Zhao, Z. Efficient and stable iron-copper montmorillonite heterogeneous Fenton catalyst for removing Rhodamine B. Chem. Phys. Lett. 2021, 776, 138673. [Google Scholar] [CrossRef]
- Li, X.; Tian, Z.; Liang, K.; Wang, Y. Enhanced photo-Fenton degradation performance over multi-metal co-supported SAPO-18 zeolites by promoted active species yield. J. Photochem. Photobiol. A Chem. 2019, 379, 79–87. [Google Scholar] [CrossRef]
- Xu, X.; Chen, W.; Zong, S.; Ren, X.; Liu, D. Magnetic clay as catalyst applied to organics degradation in a combined adsorption and Fenton-like process. Chem. Eng. J. 2019, 373, 140–149. [Google Scholar] [CrossRef]
- Ferroudj, N.; Beaunier, P.; Davidson, A.; Abramson, S. Fe3+-doped ordered mesoporous γ-Fe2O3/SiO2 microspheres as a highly efficient magnetically separable heterogeneous Fenton catalyst. Microporous Mesoporous Mater. 2021, 326, 111373. [Google Scholar] [CrossRef]
- Wei, K.; Liu, X.; Cao, S.; Cui, H.; Zhang, Y.; Ai, Z. Fe2O3@FeB composites facilitate heterogeneous Fenton process by efficient Fe(III)/Fe(II) cycle and in-situ H2O2 generation. Chem. Eng. J. Adv. 2021, 8, 100165. [Google Scholar] [CrossRef]
- Carrasco-Díaz, M.R.; Castillejos-López, E.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. On the textural and crystalline properties of Fe-carbon xerogels. Application as Fenton-like catalysts in the oxidation of paracetamol by H2O2. Microporous Mesoporous Mater. 2017, 237, 282–293. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Yazici Guvenc, S.; Dincer, K.; Varank, G. Performance of electrocoagulation and electro-Fenton processes for treatment of nanofiltration concentrate of biologically stabilized landfill leachate. J. Water Process Eng. 2019, 31, 100863. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, M.; Pan, Y.; Cai, J.; Ren, G. Pre-magnetized Fe0 as heterogeneous electro-Fenton catalyst for the degradation of p-nitrophenol at neutral pH. Chemosphere 2020, 240, 124962. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Z.; Zhu, M.; Liu, Z.; Wang, Z.; Hansen, H.C.B. Single sheet iron oxide: An efficient heterogeneous electro-Fenton catalyst at neutral pH. J. Hazard. Mater. 2019, 364, 39–47. [Google Scholar] [CrossRef]
- Midassi, S.; Bedoui, A.; Bensalah, N. Efficient degradation of chloroquine drug by electro-Fenton oxidation: Effects of operating conditions and degradation mechanism. Chemosphere 2020, 260, 127558. [Google Scholar] [CrossRef]
- Elbatea, A.A.; Nosier, S.A.; Zatout, A.A.; Hassan, I.; Sedahmed, G.H.; Abdel-Aziz, M.H.; El-Naggar, M.A. Removal of reactive red 195 from dyeing wastewater using electro-Fenton process in a cell with oxygen sparged fixed bed electrodes. J. Water Process Eng. 2021, 41, 102042. [Google Scholar] [CrossRef]
- Senthilnathan, J.; Younis, S.A.; Kwon, E.E.; Surenjan, A.; Kim, K.H.; Yoshimura, M. An efficient system for electro-Fenton oxidation of pesticide by a reduced graphene oxide-aminopyrazine@3DNi foam gas diffusion electrode. J. Hazard. Mater. 2020, 400, 123323. [Google Scholar] [CrossRef]
- McQuillan, R.V.; Stevens, G.W.; Mumford, K.A. Assessment of the electro-Fenton pathway for the removal of naphthalene from contaminated waters in remote regions. Sci. Total Environ. 2021, 762, 143155. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M.Á. Current advances and trends in electro-Fenton process using heterogeneous catalysts—A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Matyszczak, G.; Krzyczkowska, K.; Fidler, A. A novel, two-electron catalysts for the electro-Fenton process. J. Water Process Eng. 2020, 36, 101242. [Google Scholar] [CrossRef]
- Liang, J.; Xiang, Q.; Lei, W.; Zhang, Y.; Sun, J.; Zhu, H.; Wang, S. Ferric iron reduction reaction electro-Fenton with gas diffusion device: A novel strategy for improvement of comprehensive efficiency in electro-Fenton. J. Hazard. Mater. 2021, 412, 125195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, M.; Yu, X.; Ma, L.; Yu, F. Modified iron-carbon as heterogeneous electro-Fenton catalyst for organic pollutant degradation in near neutral pH condition: Characterization, degradation activity and stability. Electrochim. Acta 2015, 160, 254–262. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Gao, Y.; Wang, C.; Zhang, J.; Bai, H.; Huang, T. A new method to fabricate the cathode by cyclic voltammetric electrodeposition for electro-Fenton application. Electrochim. Acta 2020, 349, 136415. [Google Scholar] [CrossRef]
- Ganzenko, O.; Trellu, C.; Oturan, N.; Huguenot, D.; Péchaud, Y.; van Hullebusch, E.D.; Oturan, M.A. Electro-Fenton treatment of a complex pharmaceutical mixture: Mineralization efficiency and biodegradability enhancement. Chemosphere 2020, 253, 126659. [Google Scholar] [CrossRef]
- Robles, I.; Becerra, E.; Barrios, J.A.; Maya, C.; Jiménez, B.; Rodríguez-Valadez, F.J.; Rivera, F.; García-Espinoza, J.D.; Godínez, L.A. Inactivation of helminth eggs in an electro-Fenton reactor: Towards full electrochemical disinfection of human waste using activated carbon. Chemosphere 2020, 250, 126260. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, T.; Song, S.; Gui, X.; Liu, H.; Tsiakaras, P. Dimethyl phthalate degradation at novel and efficient electro-Fenton cathode. Appl. Catal. B Environ. 2014, 156–157, 1–7. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X.; Chen, S.; Yu, H.; Zhang, Y.; Zhao, H. Enhanced electro-Fenton performance by fluorine-doped porous carbon for removal of organic pollutants in wastewater. Chem. Eng. J. 2018, 354, 606–615. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H.; Zhao, H.; Zhang, Y. Efficient Mineralization of Perfluorooctanoate by Electro-Fenton with H2O2 Electro-generated on hierarchically Porous Carbon. Environ. Sci. Technol. 2015, 49, 12528–12533. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Duan, X.; Zhan, M.; Ji, L.; Li, X.; Wang, S.; Yan, J. Biochar cathode: Reinforcing electro-Fenton pathway against four-electron reduction by controlled carbonization and surface chemistry. Sci. Total Environ. 2021, 754, 142136. [Google Scholar] [CrossRef] [PubMed]
- Ergan, B.T.; Gengec, E. Dye degradation and kinetics of online Electro-Fenton system with thermally activated carbon fiber cathodes. J. Environ. Chem. Eng. 2020, 8, 104217. [Google Scholar] [CrossRef]
- Ramírez-Pereda, B.; Álvarez-Gallegos, A.; Rangel-Peraza, J.G.; Bustos-Terrones, Y.A. Kinetics of Acid Orange 7 oxidation by using carbon fiber and reticulated vitreous carbon in an electro-Fenton process. J. Environ. Manag. 2018, 213, 279–287. [Google Scholar] [CrossRef]
- Xia, G.; Lu, Y.; Xu, H. An energy-saving production of hydrogen peroxide via oxygen reduction for electro-Fenton using electrochemically modified polyacrylonitrile-based carbon fiber brush cathode. Sep. Purif. Technol. 2015, 156, 553–560. [Google Scholar] [CrossRef]
- Xia, G.; Lu, Y.; Xueli, G.; Gao, C.; Xu, H. Electro-Fenton Degradation of Methylene Blue Using Polyacrylonitrile-Based Carbon Fiber Brush Cathode. CLEAN Soil Ai Water 2014, 43, 229–236. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.; Li, Y.; Li, J.; Yun, S.; Huang, T. Novel porous carbon felt cathode modified by cyclic voltammetric electrodeposited polypyrrole and anthraquinone 2-sulfonate for an efficient electro-Fenton process. Int. J. Hydrogen Energy 2021, 46, 9707–9717. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Feng, H.; Zhou, Y.; Zeng, G.; Lu, Y.; Yu, J.; Ren, X.; Peng, B.; Liu, X. Carbon felt cathodes for electro-Fenton process to remove tetracycline via synergistic adsorption and degradation. Sci. Total Environ. 2019, 670, 921–931. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, K.; Song, S.; Tsiakaras, P.; Liu, H. Preparation and characterization of a novel KOH activated graphite felt cathode for the electro-Fenton process. Appl. Catal. B Environ. 2015, 165, 360–368. [Google Scholar] [CrossRef]
- Want, Y.-T.; Tu, C.-H.; Lin, Y.-S. Application of Graphene and Carbon Nanotubes on Carbon Felt Electrodes for the Electro-Fenton System. Materials 2019, 12, 1698. [Google Scholar] [CrossRef]
- Özcan, A.; Şahin, Y.; Savaş Koparal, A.; Oturan, M.A. Carbon sponge as a new cathode material for the electro-Fenton process: Comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J. Electroanal. Chem. 2008, 616, 71–78. [Google Scholar] [CrossRef]
- Sopaj, F.; Oturan, N.; Pinson, J.; Podvorica, F.I.; Oturan, M.A. Effect of cathode material on electro-Fenton process efficiency for electrocatalytic mineralization of the antibiotic sulfamethazine. Chem. Eng. J. 2020, 384, 123249. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; de Araújo, M.J.G.; de Araújo Costa, E.C.T.; Santos, J.E.L.; dos Santos, E.V.; Martínez-Huitle, C.A.; Pergher, S.B.C. Design of highly efficient porous carbon foam cathode for electro-Fenton degradation of antimicrobial sulfanilamide. Appl. Catal. B Environ. 2021, 283, 119652. [Google Scholar] [CrossRef]
- Ye, Z.; Brillas, E.; Centellas, F.; Cabot, P.L.; Sirés, I. Electro-Fenton process at mild pH using Fe(III)-EDDS as soluble catalyst and carbon felt as cathode. Appl. Catal. B Environ. 2019, 257, 117907. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Qian, Y.; Wang, W.J.; Deng, X.L. A dual-cathode electro-Fenton oxidation coupled with anodic oxidation system used for 4-nitrophenol degradation. J. Hazard. Mater. 2012, 199–200, 179–185. [Google Scholar] [CrossRef]
- Chu, Y.; Su, H.; Lv, R.; Zhang, X. Enhanced electro-reduction of Fe3+ to Fe2+ by acidified carbon nanotube-modified graphite cathode and its application in a novel Fenton process for p-nitrophenol degradation. J. Water Process Eng. 2021, 40, 101912. [Google Scholar] [CrossRef]
- Zarei, M.; Beheshti Nahand, F.; Khataee, A.; Hasanzadeh, A. Removal of nalidixic acid from aqueous solutions using a cathode containing three-dimensional graphene. J. Water Process Eng. 2019, 32, 100978. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Rajic, L.; Xue, Y.; Chen, S.; Ding, Y.; Kou, K.; Wang, Y.; Gao, J.; Qin, Y.; et al. “Floating” cathode for efficient H2O2 electrogeneration applied to degradation of ibuprofen as a model pollutant. Electrochem. Commun. 2018, 96, 37–41. [Google Scholar] [CrossRef]
- Liu, T.; Wang, K.; Song, S.; Brouzgou, A.; Tsiakaras, P.; Wang, Y. New Electro-Fenton Gas Diffusion Cathode based on Nitrogen-doped Graphene@Carbon Nanotube Composite Materials. Electrochim. Acta 2016, 194, 228–238. [Google Scholar] [CrossRef]
- Chu, Y.; Su, H.; Liu, C.; Zheng, X. Fabrication of sandwich-like super-hydrophobic cathode for the electro-Fenton degradation of cefepime: H2O2 electro-generation, degradation performance, pathway and biodegradability improvement. Chemosphere 2022, 286, 131669. [Google Scholar] [CrossRef]
- Karatas, O.; Gengec, N.A.; Gengec, E.; Khataee, A.; Kobya, M. High-performance carbon black electrode for oxygen reduction reaction and oxidation of atrazine by electro-Fenton process. Chemosphere 2021, 287, 132370. [Google Scholar] [CrossRef]
- Huang, A.; Zhi, D.; Zhou, Y. A novel modified Fe–Mn binary oxide graphite felt (FMBO-GF) cathode in a neutral electro-Fenton system for ciprofloxacin degradation. Environ. Pollut. 2021, 286, 117310. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Lv, W.; Li, J.; Yang, C.; Tang, Q.; Wang, D. Promotion removal of aniline with electro-Fenton processes utilizing carbon nanotube 3D morphology modification of an Ag-loaded copper foam cathode. J. Water Process Eng. 2021, 43, 102295. [Google Scholar] [CrossRef]
- Dung, N.T.; Duong, L.T.; Hoa, N.T.; Thao, V.D.; Ngan, L.V.; Huy, N.N. A comprehensive study on the heterogeneous electro-Fenton degradation of tartrazine in water using CoFe2O4/carbon felt cathode. Chemosphere 2022, 287, 132141. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.C.; Aveiro, L.R.; Pinheiro, V.S.; Souza, F.M.; Lima, V.B.; Silva, F.L.; Hammer, P.; Lanza, M.R.V.; Santos, M.C. Evaluation of H2O2 electrogeneration and decolorization of Orange II azo dye using tungsten oxide nanoparticle-modified carbon. Appl. Catal. B Environ. 2018, 232, 436–445. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Acevedo-García, V.; Pazos, M.; Sanromán, M.Á.; Rosales, E. Iron-doped cathodes for electro-Fenton implementation: Application for pymetrozine degradation. Electrochim. Acta 2020, 338, 135768. [Google Scholar] [CrossRef]
- Li, L.; Hu, H.; Teng, X.; Yu, Y.; Zhu, Y.; Su, X. Electrogeneration of H2O2 using a porous hydrophobic acetylene black cathode for electro-Fenton process. Chem. Eng. Process. Process Intensif. 2018, 133, 34–39. [Google Scholar] [CrossRef]
- Yu, F.; Tao, L.; Cao, T. High yield of hydrogen peroxide on modified graphite felt electrode with nitrogen-doped porous carbon carbonized by zeolitic imidazolate framework-8 (ZIF-8) nanocrystals. Environ. Pollut. 2019, 255, 113119. [Google Scholar] [CrossRef]
- Cordeiro-Junior, P.J.; Kronka, M.S.; Goulart, L.A.; Veríssimo, N.C.; Mascaro, L.H.; dos Santos, M.C.; Bertazzoli, R.; de Vasconcelos Lanza, M.R. Catalysis of oxygen reduction reaction for H2O2 electrogeneration: The impact of different conductive carbon matrices and their physicochemical properties. J. Catal. 2020, 392, 56–68. [Google Scholar] [CrossRef]
- An, J.; Li, N.; Zhao, Q.; Qiao, Y.; Wang, S.; Liao, C.; Zhou, L.; Li, T.; Wang, X.; Feng, Y. Highly efficient electro-generation of H2O2 by adjusting liquid-gas-solid three phase interfaces of porous carbonaceous cathode during oxygen reduction reaction. Water Res. 2019, 164, 114933. [Google Scholar] [CrossRef] [PubMed]
- Ridruejo, C.; Alcaide, F.; Álvarez, G.; Brillas, E.; Sirés, I. On-site H2O2 electrogeneration at a CoS2-based air-diffusion cathode for the electrochemical degradation of organic pollutants. J. Electroanal. Chem. 2018, 808, 364–371. [Google Scholar] [CrossRef]
- Li, D.; Zheng, T.; Liu, Y.; Hou, D.; Yao, K.K.; Zhang, W.; Song, H.; He, H.; Shi, W.; Wang, L.; et al. A novel Electro-Fenton process characterized by aeration from inside a graphite felt electrode with enhanced electrogeneration of H2O2 and cycle of Fe3+/Fe2+. J. Hazard. Mater. 2020, 396, 122591. [Google Scholar] [CrossRef] [PubMed]
- Kubannek, F.; Turek, T.; Krewer, U. Modeling Oxygen Gas Diffusion Electrodesfor Various Technical Applications. Chem. Ing. Tech. 2019, 91, 720–733. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Rauf, M.; Luo, H.; Sun, X.; Jiang, Y. Gas diffusion electrodes for H2O2 production and their applications for electrochemical degradation of organic pollutants in water: A review. Sci. Total Environ. 2021, 759, 143459. [Google Scholar] [CrossRef]
- Murawski, E.; Kananizadeh, N.; Lindsay, S.; Rao, A.M.; Popat, S.C. Decreased gas-diffusion electrode porosity due to increased electrocatalyst loading leads to diffusional limitations in cathodic H2O2 electrosynthesis. J. Power Sources 2021, 481, 228992. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, H.C.; Yang, S.Y.; Horng, H.E.; Hung, J.C.; Chen, Y.C.; Hong, C.Y. Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. J. Magn. Magn. Mater. 2004, 283, 210–214. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lai, Y.-P.; Wu, K.-Y. A high-productivity process for mass-producing Fe3O4 nanoparticles by co-precipitation in a rotating packed bed. Powder Technol. 2021, 395, 369–376. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, C.Y. Adsorption of ciprofloxacin in water using Fe3O4 nanoparticles formed at low temperature and high reactant concentrations in a rotating packed bed with co-precipitation. Mater. Chem. Phys. 2020, 240, 122049. [Google Scholar] [CrossRef]
- Rao, M.N.; Sultana, R.; Kota, S.H. Hazardous Waste. Solid Hazard. Waste Manag. 2017, 1, 159–207. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles—Synthesized via sol—Gel route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Dubey, R.S. Temperature-dependent phase transformation of TiO2 nanoparticles synthesized by sol-gel method. Mater. Lett. 2018, 215, 312–317. [Google Scholar] [CrossRef]
- Zanurin, A.; Johari, N.A.; Alias, J.; Ayu, H.M.; Redzuan, N.; Izman, S. Research progress of sol-gel ceramic coating: A review. Mater. Today Proc. 2021, 48, 1849–1854. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal and hydrothermal processes: The main physico-chemical factors involved and new trends. Res. Chem. Intermed. 2010, 37, 107–123. [Google Scholar] [CrossRef]
- Krajian, H.; Abdallah, B.; Kakhia, M.; Alkafri, N. Hydrothermal growth method for the deposition of ZnO films: Structural, chemical and optical studies. Microelectron. Reliab. 2021, 125, 114352. [Google Scholar] [CrossRef]
- Dem, L.N.; Lyutin, V.I. Status of hydrothermal growth of bulk ZnO: Latest issues and advantages. J. Cryst. Growth 2008, 310, 993–999. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A. Importance and challenges of hydrothermal technique for synthesis of transition metal oxides and composites as supercapacitor electrode materials. J. Energy Storage 2021, 44, 103295. [Google Scholar] [CrossRef]
- Guo, X.; Wang, K.; Xu, Y. Tartaric acid enhanced CuFe2O4-catalyzed heterogeneous photo-Fenton-like degradation of methylene blue. Mater. Sci. Eng. B 2019, 245, 75–84. [Google Scholar] [CrossRef]
- He, H.; Lu, J. Highly photocatalytic activities of magnetically separable reduced graphene oxide-CoFe2O4 hybrid nanostructures in dye photodegradation. Sep. Purif. Technol. 2017, 172, 374–381. [Google Scholar] [CrossRef]
- Boepple, M.; Zhu, Z.; Hu, X.; Weimar, U.; Barsan, N. Impact of heterostructures on hydrogen sulfidesensing: Example of core- shell CuO/CuFe2O4 nanostructures. Sens. Actuators B Chem. 2020, 321, 128523. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Akinwolemiwa, B.; Hu, D.; Wu, T.; Peng, C. Low-crystalline transition metal oxide / hydroxide on MWCNT by Fenton-reaction-inspired green synthesis for lithium ion battery and OER electrocatalysis. Electrochim. Acta 2021, 387, 138559. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, S.; Thang, T.; Gao, X.; Luo, S.; Guo, M. Biochar loaded on MnFe2O4 as Fenton catalyst for Rhodamine B removal: Characterizations, catalytic performance, process optimization and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127651. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, X.; Zhai, T.; Ma, K.; Zhang, H. Fabrication of novel three-dimensional Fe3O4 -based particles electrodes with enhanced electrocatalytic activity for Berberine removal. Chemosphere 2022, 287, 132397. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Peng, Q.; Yu, B.; Shen, Y.; Cong, H. Yolk-shell Fe3O4@MOF-5 nanocomposites as a heterogeneous Fenton-like catalyst for organic dye removal. Sep. Purif. Technol. 2021, 267, 118620. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Zou, G.; Chen, Q.; Guo, Y.; Liang, S.; Hu, L.; North, M.; Xie, H. Carboxylcellulose hydrogel confined-Fe3O4 nanoparticles catalyst for Fenton-like degradation of Rhodamine B. Int. J. Biol. Macromol. 2021, 180, 792–803. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Maltha, A.; Reinjtjes, J.; Drimal, J.; Ponec, V. Brongersm The surface of catalytically active spinels. Catalysis 1994, 147, 294–300. [Google Scholar] [CrossRef]
- So, H.L.; Lin, K.Y.; Chu, W. Triclosan removal by heterogeneous Fenton-like process: Studying the kinetics and surface chemistry of Fe3O4 as catalyst. J. Environ. Chem. Eng. 2019, 7, 103432. [Google Scholar] [CrossRef]
- Miller, A. Distribution of Cations in Spinels. Appl. Phys. 1959, 30, S24–S25. [Google Scholar] [CrossRef]
- Han, X.; Liu, S.; Huo, X.; Cheng, F.; Zhang, M.; Guo, M. Facile and large-scale fabrication of (Mg,Ni)(Fe,Al)2O4 heterogeneous photo-Fenton-like catalyst from saprolite laterite ore for effective removal of organic contaminants. J. Hazard. Mater. 2020, 392, 122295. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, D.; Chu, W.; Li, M.; Lu, X. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Amulya, M.A.S.; Nagaswarupa, H.P.; Kumar, M.R.A.; Ravikumar, C.R.; Kusuma, K.B.; Prashantha, S.C. Evaluation of bifunctional applications of CuFe2O4 nanoparticles synthesized by a sonochemical method. J. Phys. Chem. Solids 2021, 148, 109756. [Google Scholar] [CrossRef]
- Gao, J.; Wu, S.; Han, Y.; Tan, F.; Shi, Y.; Liu, M.; Li, X. 3D mesoporous CuFe2O4 as a catalyst for photo-Fenton removal of sulfonamide antibiotics at near neutral pH. J. Colloid Interface Sci. 2018, 524, 409–416. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Ramachandran, S.P.; Ravi, G.; Ganesh, V.; Guduru, R.K.; Yuvakkumar, R. Electrochemical performances of monodispersed spherical CuFe2O4 nanoparticles for pseudocapacitive applications. Vacuum 2019, 168, 108798. [Google Scholar] [CrossRef]
- Wu, X.; Xia, F.; Nan, Z. Facile synthesis of double-mesoporous-shelled hollow spheres of Cu–CuFe2O4/SiO2 composite as excellent Fenton catalyst. Mater. Chem. Phys. 2020, 242, 122490. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Ji, F.; Lai, B. Heterogeneous catalytic oxidation for the degradation of p-nitrophenol in aqueous solution by persulfate activated with CuFe2O4 magnetic nano-particles. Chem. Eng. J. 2017, 324, 63–73. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.A.; Moreno-Castilla, C.; López-Ramón, M.V.; Álvarez, M.A. Mixed iron oxides as Fenton catalysts for gallic acid removal from aqueous solutions. Appl. Catal. B Environ. 2016, 196, 207–215. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, C.; Shih, K. Copper-promoted circumneutral activation of H2O2 by magnetic CuFe2O4 spinel nanoparticles: Mechanism, stoichiometric efficiency, and pathway of degrading sulfanilamide. Chemosphere 2016, 154, 573–582. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croue, J. Production of Sulfate Radical from Peroxymonosulfate Induced by a Magnetically Separable CuFe2O4 Spinel in Water: Efficiency, Stability, and Mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef]
- Suraj, P.; Kumar, V.; Thakur, C.; Ghosh, P. Taguchi optimization of COD removal by heterogeneous Fenton process using copper ferro spinel catalyst in a fixed bed reactor—RTD, kinetic and thermodynamic study. J. Environ. Chem. Eng. 2019, 7, 102859. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; He, C.; Wang, Y.; Liu, X.; Zhou, G.; Mu, Y. Oxygen vacancy on hollow sphere CuFe2O4 as an efficient Fenton-like catalysis for organic pollutant degradation over a wide pH range. Appl. Catal. B Environ. 2021, 291, 120069. [Google Scholar] [CrossRef]
- Yu, D.; Ni, H.; Wang, L.; Wu, M.; Yang, X. Nanoscale-con fined precursor of CuFe2O4 mediated by hyperbranched polyamide as an unusual heterogeneous Fenton catalyst for efficient dye degradation. J. Clean. Prod. 2018, 186, 146–154. [Google Scholar] [CrossRef]
- López-Ramón, M.V.; Álvarez, M.A.; Moreno-Castilla, C.; Fontecha-Cámara, M.A.; Yebra-Rodríguez, Á.; Bailón-García, E. Effect of calcination temperature of a copper ferrite synthesized by a sol-gel method on its structural characteristics and performance as Fenton catalyst to remove gallic acid from water. J. Colloid Interface Sci. 2018, 511, 193–202. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Yan, Y.; Zhao, X.; Yan, J.; Zhang, Y.; Lai, B. Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles. Chem. Eng. J. 2019, 369, 403–413. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Zhao, S. Fast degradation of Acid Orange II by bicarbonate-activated hydrogen peroxide with a magnetic S-modified CoFe2O4 catalyst. Taiwan Inst. Chem. Eng. 2015, 55, 90–100. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Z.; Zhang, D.; Peng, W.; Sun, H.; Wang, S. Magnetic CoFe2O4-Graphene Hybrids: Facile Synthesis, Characterization, and Catalytic Properties. Ind. Eng. Chem. Res. 2012, 51, 6044–6051. [Google Scholar] [CrossRef]
- Hong, P.; Li, Y.; He, J.; Saeed, A.; Zhang, K. Rapid degradation of aqueous doxycycline by surface CoFe2O4/H2O2 system: Behaviors, mechanisms, pathways and DFT calculation. Appl. Surf. Sci. 2020, 526, 146557. [Google Scholar] [CrossRef]
- Feng, X.; Mao, G.Y.; Bu, F.X.; Cheng, X.L.; Jiang, D.M.; Jiang, J.S. Controlled synthesis of monodisperse CoFe2O4 nanoparticles by the phase transfer method and their catalytic activity on methylene blue discoloration with H2O2. Magn. Magn. Mater. 2013, 343, 126–132. [Google Scholar] [CrossRef]
- Singh, S.; Singhal, S. Transition metal doped cobalt ferrite nanoparticles: Efficient photocatalyst for photodegradation of textile dye. Mater. Today Proc. 2019, 14, 453–460. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Das, S.J. Investigation on the role of pH for the structural, optical and magnetic properties of cobalt ferrite nanoparticles and its effect on the photo-fenton activity. Mater. Today Proc. 2018, 5, 8662–8671. [Google Scholar] [CrossRef]
- Kachi, W.; Al-Shammari, A.M.; Zainal, I.G. Cobalt Ferrite Nanoparticles: Preparation, characterization and salinized with 3-aminopropyl triethoxysilane. Energy Procedia 2019, 157, 1353–1365. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, X.; Dong, X.; Zhang, X.; Tan, Y.; Yuan, F.; Zheng, S.; Li, C. Synergistic activation of peroxymonosulfate via in situ growth FeCo2O4 nanoparticles on natural rectorite: Role of transition metal ions and hydroxyl groups. Chemosphere 2021, 263, 127965. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Tsai, Y.Q.; Chen, C.J.; Tsai, Y.T.; Hung, T.F.; Chang, W.S.; Liu, R.S. Ternary Spinel MCo2O4 (M = Mn, Fe, Ni, and Zn) Porous Nanorods as Bifunctional Cathode Materials for Lithium-O2 Batteries. Appl. Mater. Interfaces 2015, 7, 12038–12046. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kulkarni, S.B. Synthesis of multifunctional FeCo2O4 electrode using ultrasonic treatment for photocatalysis and energy storage applications. Ultrason. Sonochem. 2019, 58, 104663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, Q.; Zhou, M.; Li, C.; Sun, J.; Shi, W.; Ai, S. Degradation of methylene blue by a heterogeneous Fenton reaction catalyzed by FeCo2O4-N-C nanocomposites derived by ZIFs. Powder Technol. 2021, 383, 212–219. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, D.; Ma, L.; Feng, X.; Ding, H. An efficient heterogeneous catalyst of FeCo2O4/g-C3N4 composite for catalytic peroxymonosulfate oxidation of organic pollutants under visible light. Colloids Surf. A 2021, 610, 125725. [Google Scholar] [CrossRef]
- Shan, Z.; Ma, F.; You, S.; Shan, L.; Kong, D.; Guo, H.; Cui, C. Enhanced visible light photo-Fenton catalysis by lanthanum-doping BiFeO3 for norfloxacin degradation. Environ. Res. 2023, 216, 114588. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, J.; Wang, Z.; Sun, W.; Sun, K. Layered perovskites with exsolved Co-Fe nanoalloy as highly active and stable anodes for direct carbon solid oxide fuel cells. J. Alloys Compd. 2023, 940, 168872. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.-S.; Illán-Gómez, M.J. Exploring the effect of using carbon black in the sol-gel synthesis of BaMnO3 and BaMn0.7Cu0.3O3 perovskite catalysts for CO oxidation. Catal. Today, 2023; in press. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Phung, V.D.; Mittova, V.O.; Ngo, H.D.; Vo, T.N.; Le Thi, M.L.; Nguyen, V.H.; Mittova, I.Y.; Le, M.L.P.; Ahn, Y.N.; et al. Fabricating nanostructured HoFeO3 perovskite for lithium-ion battery anodes via co-precipitation. Scr. Mater. 2022, 207, 114259. [Google Scholar] [CrossRef]
- Alrozi, R.; Zubir, N.A.; Bakar, N.F.A.; Ladewig, B.P.; Motuzas, J.; Bakar, N.H.H.A.; Wang, D.K.; da Costa, J.C.D. Functional role of B-site substitution on the reactivity of CaMFeO3 (M = Cu, Mo, Co) perovskite catalysts in heterogeneous Fenton-like degradation of organic pollutant. J. Taiwan Inst. Chem. Eng. 2023, 143, 104675. [Google Scholar] [CrossRef]
- Li, J.; Ma, W.; Zhong, D.; Li, K.; Ma, J.; Zhang, S.; Du, X. Oxygen vacancy concentration modulation of perovskite-based heterogeneous catalysts for Fenton-like oxidation of tetracycline. J. Clean. Prod. 2022, 362, 132469. [Google Scholar] [CrossRef]
- Xie, L.; Liu, X.; Chang, J.; Zhang, C.; Li, Y.; Zhang, H.; Zhan, S.; Hu, W. Enhanced redox activity and oxygen vacancies of perovskite triggered by copper incorporation for the improvement of electro-Fenton activity. Chem. Eng. J. 2022, 428, 131352. [Google Scholar] [CrossRef]
- Rusevova, K.; Köferstein, R.; Rosell, M.; Richnow, H.H.; Kopinke, F.D.; Georgi, A. LaFeO3 and BiFeO3 perovskites as nanocatalysts for contaminant degradation in heterogeneous Fenton-like reactions. Chem. Eng. J. 2014, 239, 322–331. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, J.; Lv, H.; Wang, Y.; Zhao, G. 3D nano-scale perovskite-based composite as Fenton-like system for efficient oxidative degradation of ketoprofen. Catal. Commun. 2013, 41, 87–90. [Google Scholar] [CrossRef]
- Ho, J.H.; Li, Y.; Dai, Y.; Kim, T.; Wang, J.; Ren, J.; Yun, H.S.; Liu, X. Ionothermal synthesis of N-doped carbon supported CoMn2O4 nanoparticles as ORR catalyst in direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2021, 46, 20503–20515. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Guadarrama-Pérez, O.; Silva-Martínez, S.; Cuevas-Arteaga, C.; Guadarrama-Pérez, V.H. Oxygen reduction reaction (ORR) electrocatalysts in constructed wetland-microbial fuel cells: Effect of different carbon-based catalyst biocathode during bioelectricity production. Electrochim. Acta 2021, 370, 137745. [Google Scholar] [CrossRef]
- Gao, X.; He, L.; Yu, H.; Xie, F.; Yang, Y.; Shao, Z. The non-precious metal ORR catalysts for the anion exchange membrane fuel cells application: A numerical simulation and experimental study. Int. J. Hydrogen Energy 2020, 45, 23353–23367. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.H.; Wang, X.Y.; Zhang, J.; Han, B.H. N-doped graphitic carbon shell-encapsulated FeCo alloy derived from metal–polyphenol network and melamine sponge for oxygen reduction, oxygen evolution, and hydrogen evolution reactions in alkaline media. J. Colloid Interface Sci. 2021, 581, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Meng, X.; Gao, J.; Alshawabkeh, A.N. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: A state-of-the-art review. Chemosphere 2019, 225, 588–607. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P.; Prato, M. The Rise of Hydrogen Peroxide as the Main Product by Metal-Free Catalysis in Oxygen Reductions. Adv. Mater. 2019, 31, 1802920. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Ma, H. Enhancing the yield of H2O2 from oxygen reduction reaction performance by hierarchically porous carbon modified active carbon fiber as an effective cathode used in electro-Fenton. J. Electroanal. Chem. 2019, 838, 57–65. [Google Scholar] [CrossRef]
- Zhao, Q.; An, J.; Wang, X.; Li, N. In-situ hydrogen peroxide synthesis with environmental applications in bioelectrochemical systems: A state-of-the-art review. Int. J. Hydrogen Energy 2020, 46, 3204–3219. [Google Scholar] [CrossRef]
- Candia-Onfray, C.; Bollo, S.; Yáñez, C.; Escalona, N.; Marco, J.F.; Menéndez, N.; Salazar, R.; Recio, F.J. Nanostructured Fe-N-C pyrolyzed catalyst for the H2O2 electrochemical sensing. Electrochim. Acta 2021, 387, 138468. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, X.; Fan, X.; Wang, H.; Chen, S. High-yield electrosynthesis of hydrogen peroxide from oxygen reduction by hierarchically porous carbon. Angew. Chem. Int. Ed. 2015, 54, 6837–6841. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Ge, X.; Huang, N.; Zhou, S.; Zhang, J.; Xuan, J. Polyethylene oxide-polypropylene oxide -polyethylene oxide derived porous carbon materials with different molecular weights as ORR catalyst in alkaline electrolytes. Int. J. Hydrogen Energy 2021, 46, 2952–2959. [Google Scholar] [CrossRef]
- Inozemtseva, A.I.; Kataev, E.Y.; Frolov, A.S.; Amati, M.; Gregoratti, L.; Beranová, K.; Dieste, V.P.; Escudero, C.; Fedorov, A.; Tarasov, A.V.; et al. On the catalytic and degradative role of oxygen-containing groups on carbon electrode in non-aqueous ORR. Carbon N. Y. 2021, 176, 632–641. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef]
- Eisenberg, D.; Stroek, W.; Geels, N.J.; Tanase, S.; Ferbinteanu, M.; Teat, S.J.; Mettraux, P.; Yan, N.; Rothenberg, G. A rational synthesis of hierarchically porous, N-doped carbon from Mg-based MOFs: Understanding the link between nitrogen content and oxygen reduction electrocatalysis. Phys. Chem. Chem. Phys. 2016, 18, 20778–20783. [Google Scholar] [CrossRef]
- Shibuya, R.; Kondo, T.; Nakamura, J. Active sites in nitrogen-doped carbon materials for oxygen reduction reaction. Carbon-Based Met. Catal. Des. Appl. 2018, 1–2, 227–249. [Google Scholar] [CrossRef]
- Zhou, W.; Rajic, L.; Meng, X.; Nazari, R.; Zhao, Y.; Wang, Y.; Gao, J.; Qin, Y.; Alshawabkeh, A.N. Efficient H2O2 electrogeneration at graphite felt modified via electrode polarity reversal: Utilization for organic pollutants degradation. Chem. Eng. J. 2019, 364, 428–439. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, X.; Zheng, K.; Li, F.; Ma, G.; Yao, H.; Wang, X.; Chen, Y. KOH-treated reduced graphene oxide: 100% selectivity for H2O2 electroproduction. Carbon 2019, 153, 6–11. [Google Scholar] [CrossRef]
- Wu, K.H.; Wang, D.; Lu, X.; Zhang, X.; Xie, Z.; Liu, Y.; Su, B.J.; Chen, J.M.; Su, D.S.; Qi, W.; et al. Highly Selective Hydrogen Peroxide Electrosynthesis on Carbon: In Situ Interface Engineering with Surfactants. Chem 2020, 6, 1443–1458. [Google Scholar] [CrossRef]
- Xu, H.; Guo, H.; Chai, C.; Li, N.; Lin, X.; Xu, W. Anodized graphite felt as an efficient cathode for in-situ hydrogen peroxide production and Electro-Fenton degradation of rhodamine B. Chemosphere 2022, 286, 131936. [Google Scholar] [CrossRef]
- Zahoor, A.; Christy, M.; Hwang, Y.J.; Lim, Y.R.; Kim, P.; Nahm, K.S. Improved electrocatalytic activity of carbon materials by nitrogen doping. Appl. Catal. B Environ. 2014, 147, 633–641. [Google Scholar] [CrossRef]
- Okamoto, Y. First-principles molecular dynamics simulation of O2 reduction on nitrogen-doped carbon. Appl. Surf. Sci. 2009, 256, 335–341. [Google Scholar] [CrossRef]
- Legarreta-Mendoza, A.; Flores-Holguín, N.; Lardizabal-Gutiérrez, D. A proposal based on quantum phenomena for the ORR mechanism on nitrogen-doped carbon-based electrocatalysts. Int. J. Hydrogen Energy 2019, 44, 12374–12380. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, S.; Deng, F.; Ma, F.; Zheng, Y. Degradation of sulfathiazole by electro-Fenton using a nitrogen-doped cathode and a BDD anode: Insight into the H2O2 generation and radical oxidation. Sci. Total Environ. 2020, 722, 137853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, M.; Wang, K.; Song, S. Enhanced electrocatalytic activity for H2O2 production by the oxygen reduction reaction: Rational control of the structure and composition of multi-walled carbon nanotubes. Chin. J. Catal. 2019, 40, 523–533. [Google Scholar] [CrossRef]
- Yu, Y.; You, S.; Du, J.; Zhang, P.; Dai, Y.; Liu, M.; Jiang, B.; Ren, N.; Zou, J. Ti3+-self-doped TiO2 with multiple crystal-phases anchored on acid-pickled ZIF-67-derived Co3O4@N-doped graphitized-carbon as a durable catalyst for oxygen reduction in alkaline and acid media. Chem. Eng. J. 2021, 403, 126441. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Zhou, M.; He, B.; Ren, W.; Chen, L.; Xu, W.; Hou, Z.; Chen, Y. In-situ self-templated preparation of porous core–shell Fe1−xS@N, S co-doped carbon architecture for highly efficient oxygen reduction reaction. J. Energy Chem. 2021, 54, 310–317. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, F.; Qiu, S.; Ma, F.; Zheng, Y.; Lian, R. Enhanced electro-Fenton degradation of sulfonamides using the N, S co-doped cathode: Mechanism for H2O2 formation and pollutants decay. J. Hazard. Mater. 2021, 403, 123950. [Google Scholar] [CrossRef]
- Soo, L.T.; Loh, K.S.; Mohamad, A.B.; Daud, W.R.W.; Wong, W.Y. An overview of the electrochemical performance of modified graphene used as an electrocatalyst and as a catalyst support in fuel cells. Appl. Catal. A Gen. 2015, 497, 198–210. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Su, P.; Zhou, M.; Lu, X.; Yang, W.; Ren, G.; Cai, J. Electrochemical catalytic mechanism of N-doped graphene for enhanced H2O2 yield and in-situ degradation of organic pollutant. Appl. Catal. B Environ. 2019, 245, 583–595. [Google Scholar] [CrossRef]
- Su, P.; Zhou, M.; Song, G.; Du, X.; Lu, X. Efficient H2O2 generation and spontaneous [rad]OH conversion for in-situ phenol degradation on nitrogen-doped graphene: Pyrolysis temperature regulation and catalyst regeneration mechanism. J. Hazard. Mater. 2020, 397, 122681. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Fan, S.; Wang, L.; Gan, G.; Wang, X.; Cheng, J.; Hao, Z.; Li, X. Oxygen and nitrogen co-doped ordered mesoporous carbon materials enhanced the electrochemical selectivity of O2 reduction to H2O2. J. Colloid Interface Sci. 2020, 562, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, Y.; Huang, Z.; Zhang, J.; Jia, X.; Wang, X.S.; Ye, J. Two types of cooperative nitrogen vacancies in polymeric carbon nitride for efficient solar-driven H2O2 evolution. Appl. Catal. B Environ. 2020, 265, 118581. [Google Scholar] [CrossRef]
- Zhou, W.; Xie, L.; Gao, J.; Nazari, R.; Zhao, H.; Meng, X.; Sun, F.; Zhao, G.; Ma, J. Selective H2O2 electrosynthesis by O-doped and transition-metal-O-doped carbon cathodes via O2 electroreduction: A critical review. Chem. Eng. J. 2021, 410, 128368. [Google Scholar] [CrossRef]
- Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M.; et al. N-Doped Graphitized Carbon Nanohorns as a Forefront Electrocatalyst in Highly Selective O2 Reduction to H2O2. Chem 2018, 4, 106–123. [Google Scholar] [CrossRef]
- Chen, J.Y.; Li, N.; Zhao, L. Three-dimensional electrode microbial fuel cell for hydrogen peroxide synthesis coupled to wastewater treatment. J. Power Sources 2014, 254, 316–322. [Google Scholar] [CrossRef]
- He, W.; Jiang, C.; Wang, J.; Lu, L. High-Rate Oxygen Electroreduction over Graphitic-N Species Exposed on 3D Hierarchically Porous Nitrogen-Doped Carbons. Angew. Chem. 2014, 53, 9503–9507. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Wang, Z.; Wei, Z.; Liu, J.; Gong, X. Transfer of molecular oxygen and electrons improved by the regulation of C-N/C=O for highly efficient 2e-ORR. Chem. Eng. J. 2022, 433, 133591. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, J.; Mu, X.; Cheng, R.; Li, W.; Liu, S.; Pu, Z.; Lin, C.; Mu, S. Nitrogen-Doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and zn-air batteries. Appl. Catal. B Environ. 2020, 268, 118729. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.; Wang, C.; Zhao, X.; Shu, M.; Zhang, J.; Bai, H. Enhancement of oxygen reduction on a newly fabricated cathode and its application in the electro-Fenton process. Electrochim. Acta 2020, 330, 135206. [Google Scholar] [CrossRef]
- Teng, Z.; Cai, W.; Sim, W.; Zhang, Q.; Wang, C.; Su, C.; Ohno, T. Applied Catalysis B: Environmental Photoexcited single metal atom catalysts for heterogeneous photocatalytic H2O2 production: Pragmatic guidelines for predicting charge separation. Appl. Catal. B Environ. 2021, 282, 119589. [Google Scholar] [CrossRef]
- Yeager, E. Dioxygen electrocatalysis: Mechanisms in relation to catalyst structure. J. Mol. Catal. 1986, 38, 5–25. [Google Scholar] [CrossRef]
- Chan, A.W.; Hoffmann, R.; Ho, W. Theoretical aspects of photoinitiated chemisorption, dissociation, and desorption of O2 on Pt(111). Langmuir 1992, 8, 1111–1119. [Google Scholar] [CrossRef]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Norskov, J. Unifying the 2e− and 4e− reduction of oxygen on metal surfaces. J. Phys. Chem. Lett. 2012, 3, 2948–2951. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.T.; Joo, S.H. Electrocatalyst design for promoting two-electron oxygen reduction reaction: Isolation of active site atoms. Curr. Opin. Electrochem. 2020, 21, 109–116. [Google Scholar] [CrossRef]
- Choi, C.H.; Kwon, H.C.; Yook, S.; Shin, H.; Kim, H.; Choi, M. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated pt surface. J. Phys. Chem. C 2014, 118, 30063–30070. [Google Scholar] [CrossRef]
- Perry, S.C.; Mavrikis, S.; Wang, L.; León, C.P. De Future perspectives for the advancement of electrochemical hydrogen peroxide production. Curr. Opin. Electrochem. 2021, 30, 100792. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Kan, S.; Liu, P.; Hao, R.; Hu, H.; Zhang, J.; Liu, H.; Liu, M.; Liu, K. Tuning morphology and structure of Fe–N–C catalyst for ultra-high oxygen reduction reaction activity. Int. J. Hydrogen Energy 2020, 45, 6380–6390. [Google Scholar] [CrossRef]
- Daniel, G.; Kosmala, T.; Dalconi, M.C.; Nodari, L.; Badocco, D.; Pastore, P.; Lorenzetti, A.; Granozzi, G.; Durante, C. Upcycling of polyurethane into iron-nitrogen-carbon electrocatalysts active for oxygen reduction reaction. Electrochim. Acta 2020, 362, 137200. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.H.; Zhao, C.F.; Yin, X.P.; Zhao, Y.F. Co, N co-doped porous carbons as high-performance oxygen reduction electrocatalysts. Xinxing Tan Cailiao/N. Carbon Mater. 2021, 36, 209–218. [Google Scholar] [CrossRef]
- Chen, E.; Bevilacqua, M.; Tavagnacco, C.; Montini, T.; Yang, C.M.; Fornasiero, P. High surface area N/O co-doped carbon materials: Selective electrocatalysts for O2 reduction to H2O2. Catal. Today 2020, 356, 132–140. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Mao, J.; Liu, C.; Chen, Y.; Lu, Z.; Feng, S.P. Bimetallic Ag–Cu nanosheets assembled flower-like structure for oxygen reduction reaction. J. Alloys Compd. 2021, 856, 157379. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wu, J.; Zhu, J.; Cheng, S.; Wang, Y.; Hao, Y.; Zeng, S. Electrocatalytic selectivity to H2O2 enabled by two-electron pathway on Cu-deficient Au@Cu2-xS-CNTs electrocatalysts. Chem. Eng. J. 2023, 454, 140317. [Google Scholar] [CrossRef]
- Wang, B.; Jin, C.; Shao, S.; Yue, Y.; Zhang, Y.; Wang, S.; Chang, R.; Zhang, H.; Zhao, J.; Li, X. Electron-deficient Cu site catalyzed acetylene hydrochlorination. Green Energy Environ. 2022; 1–13, in press. [Google Scholar] [CrossRef]
- Noh, S.H.; Seo, M.H.; Ye, X.; Makinose, Y.; Okajima, T.; Matsushita, N.; Han, B.; Ohsaka, T. Design of an active and durable catalyst for oxygen reduction reactions using encapsulated Cu with N-doped carbon shells (Cu@N-C) activated by CO2 treatment. J. Mater. Chem. A 2015, 3, 22031–22034. [Google Scholar] [CrossRef]
- Lenarda, A.; Bevilacqua, M.; Tavagnacco, C.; Nasi, L.; Criado, A.; Vizza, F.; Melchionna, M.; Prato, M.; Fornasiero, P. Selective Electrocatalytic H2O2 Generation by Cobalt@N-Doped Graphitic Carbon Core–Shell Nanohybrids. ChemSusChem 2019, 12, 1664–1672. [Google Scholar] [CrossRef]
- Gao, J.; Yang, H.; Gao, J.; Yang, H.; Huang, X.; Hung, S.; Cai, W.; Jia, C. Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst. Chem 2019, 6, 658–674. [Google Scholar] [CrossRef]
- Cao, K.W.; Huang, H.; Li, F.M.; Yao, H.C.; Bai, J.; Chen, P.; Jin, P.J.; Deng, Z.W.; Zeng, J.H.; Chen, Y. Co nanoparticles supported on three-dimensionally N-doped holey graphene aerogels for electrocatalytic oxygen reduction. J. Colloid Interface Sci. 2020, 559, 143–151. [Google Scholar] [CrossRef]
- Coliman, J.P.; Denisevich, P.; Konai, Y.; Marrocco, M.; Koval, C.; Anson, F.C. Electrode Catalysis of the Four-Electron Reduction of Oxygen to Water by Dicobalt Face-to-Face Porphyrins. J. Am. Chem. Soc. 1980, 102, 6027–6036. [Google Scholar] [CrossRef]
- Ferrara, M.; Bevilacqua, M.; Melchionna, M.; Criado, A.; Crosera, M.; Tavagnacco, C.; Vizza, F.; Fornasiero, P. Exploration of cobalt@N-doped carbon nanocomposites toward hydrogen peroxide (H2O2) electrosynthesis: A two level investigation through the RRDE analysis and a polymer-based electrolyzer implementation. Electrochim. Acta 2020, 364, 137287. [Google Scholar] [CrossRef]
- Cheng, X.; Dou, S.; Qin, G.; Wang, B.; Yan, P.; Taylor, T.; Yang, X. Rational design of highly selective nitrogen-doped Fe2O3-CNTs catalyst towards H2O2 generation in alkaline media. Int. J. Hydrogen Energy 2020, 45, 6128–6137. [Google Scholar] [CrossRef]
- Yue, D.; Qian, X.; Kan, M.; Fang, M.; Jia, J.; Yang, X.; Zhao, Y. A metal-free visible light active photo-electro-Fenton-like cell for organic pollutants degradation. Appl. Catal. B Environ. 2018, 229, 211–217. [Google Scholar] [CrossRef]
- Hartmann, M.; Kullmann, S.; Keller, H. Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J. Mater. Chem. 2010, 20, 9002–9017. [Google Scholar] [CrossRef]
- Li, Y.; Cao, W.X.; Zuo, X.J. O- and F-doped porous carbon bifunctional catalyst derived from polyvinylidene fluoride for sulfamerazine removal in the metal-free electro-Fenton process. Environ. Res. 2022, 212, 113508. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Oturan, N.; Li, Y.; Su, P.; Oturan, M.A. Enhanced activation of hydrogen peroxide using nitrogen doped graphene for effective removal of herbicide 2,4-D from water by iron-free electrochemical advanced oxidation. Electrochim. Acta 2019, 297, 582–592. [Google Scholar] [CrossRef]
- Haider, M.R.; Jiang, W.L.; Han, J.L.; Sharif, H.M.A.; Ding, Y.C.; Cheng, H.Y.; Wang, A.J. In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants. Appl. Catal. B Environ. 2019, 256, 117774. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, K.; Quan, X.; Cao, P.; Chen, S.; Yu, H. Highly efficient metal-free electro-Fenton degradation of organic contaminants on a bifunctional catalyst. J. Hazard. Mater. 2021, 416, 125859. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Sun, W.; Yang, Z.; Jin, J.; You, D.; Liu, G. Enhanced electrochemical advanced oxidation on boride activated carbon: The influences of boron groups. Electrochim. Acta 2021, 400, 139462. [Google Scholar] [CrossRef]
- Xie, F.; Gao, Y.; Zhang, J.; Bai, H.; Zhang, J.; Li, Z.; Zhu, W. A novel bifunctional cathode for the generation and activation of H2O2 in electro-Fenton: Characteristics and mechanism. Electrochim. Acta 2022, 430, 141099. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Wang, S.G.; Zhou, W.; Sang, Y.; Wang, X.H. Integrated electro-Fenton process enabled by a rotating Fe3O4/gas diffusion cathode for simultaneous generation and activation of H2O2. Electrochim. Acta 2017, 231, 694–704. [Google Scholar] [CrossRef]
- Cui, L.; Huang, H.; Ding, P.; Zhu, S.; Jing, W.; Gu, X. Cogeneration of H2O2 and [rad]OH via a novel Fe3O4/MWCNTs composite cathode in a dual-compartment electro-Fenton membrane reactor. Sep. Purif. Technol. 2020, 237, 116380. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Yu, J.; Nie, W.; Sun, J.; Wang, S. Duet Fe3C and FeNxSites for H2O2Generation and Activation toward Enhanced Electro-Fenton Performance in Wastewater Treatment. Environ. Sci. Technol. 2021, 55, 1260–1269. [Google Scholar] [CrossRef]
- Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Niu, J. Selective electrochemical H2O2 generation and activation on a bifunctional catalyst for heterogeneous electro-Fenton catalysis. J. Hazard. Mater. 2020, 382, 121102. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C.; Hassani, A.; Orooji, Y. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manag. 2020, 267, 110629. [Google Scholar] [CrossRef]
- Cui, L.; Li, Z.; Li, Q.; Chen, M.; Jing, W.; Gu, X. Cu/CuFe2O4 integrated graphite felt as a stable bifunctional cathode for high-performance heterogeneous electro-Fenton oxidation. Chem. Eng. J. 2020, 127666. [Google Scholar] [CrossRef]
- Luo, T.; Feng, H.; Tang, L.; Lu, Y.; Tang, W.; Chen, S.; Yu, J.; Xie, Q.; Ouyang, X.; Chen, Z. Efficient degradation of tetracycline by heterogeneous electro-Fenton process using Cu-doped Fe@Fe2O3: Mechanism and degradation pathway. Chem. Eng. J. 2020, 382, 122970. [Google Scholar] [CrossRef]
- Sun, X.; Qi, H.; Sun, Z. Bifunctional nickel foam composite cathode co-modified with CoFe@NC and CNTs for electrocatalytic degradation of atrazine over wide pH range. Chemosphere 2022, 286, 131972. [Google Scholar] [CrossRef]
- Yu, D.; He, J.; Wang, Z.; Pang, H.; Li, L.; Zheng, Y.; Chen, Y.; Zhang, J. Mineralization of norfloxacin in a CoFe–LDH/CF cathode-based heterogeneous electro-fenton system: Preparation parameter optimization of the cathode and conversion mechanisms of H2O2 to ·OH. Chem. Eng. J. 2021, 417, 129240. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Pan, S.; Zhao, X.; Liu, N. Mn doping improves in-situ H2O2 generation and activation in electro-Fenton process by Fe/Mn@CC cathode using high-temperature shock technique. Chemosphere 2022, 307, 136074. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, Z.; Fan, J.; Majima, T.; Zhao, H.; Zhao, G. Selective Electrocatalytic Reduction of Oxygen to Hydroxyl Radicals via 3-Electron Pathway with FeCo Alloy Encapsulated Carbon Aerogel for Fast and Complete Removing Pollutants. Angew. Chem. Int. Ed. 2021, 60, 10375–10383. [Google Scholar] [CrossRef] [PubMed]
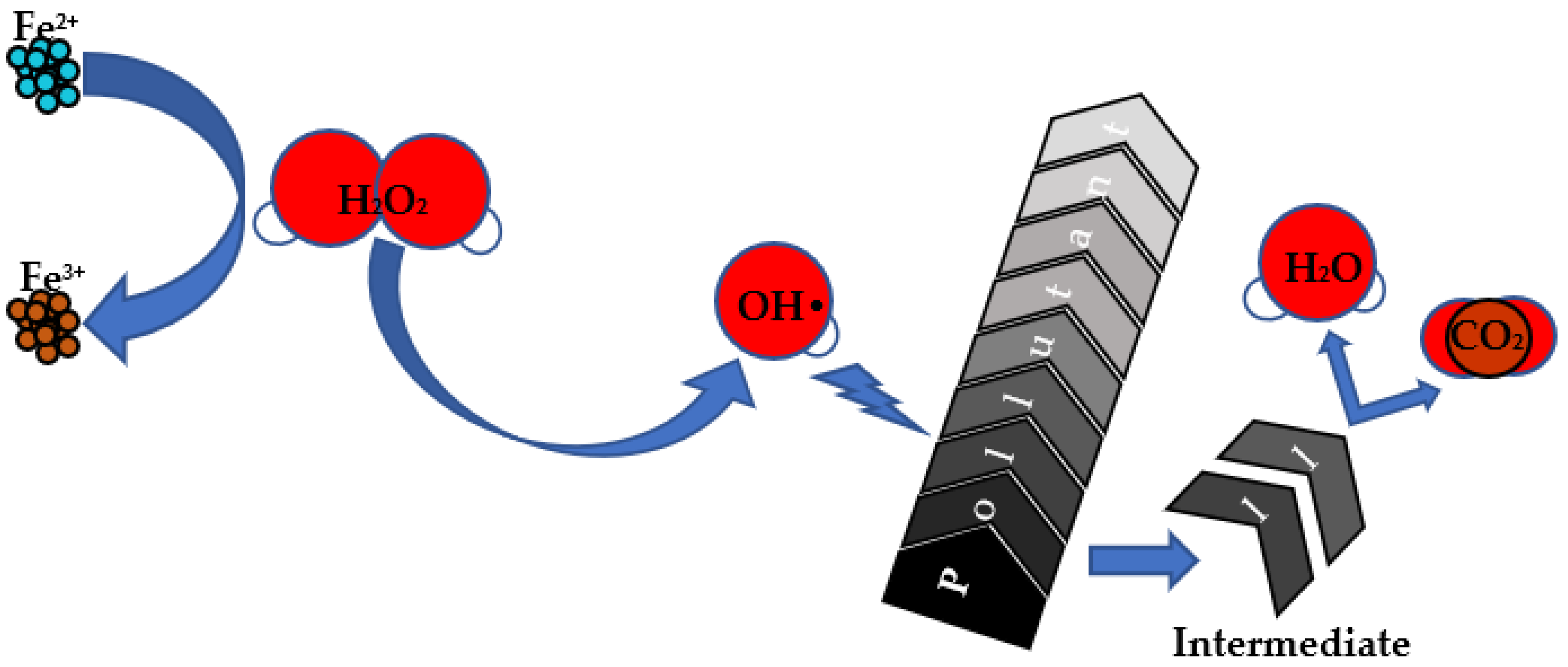
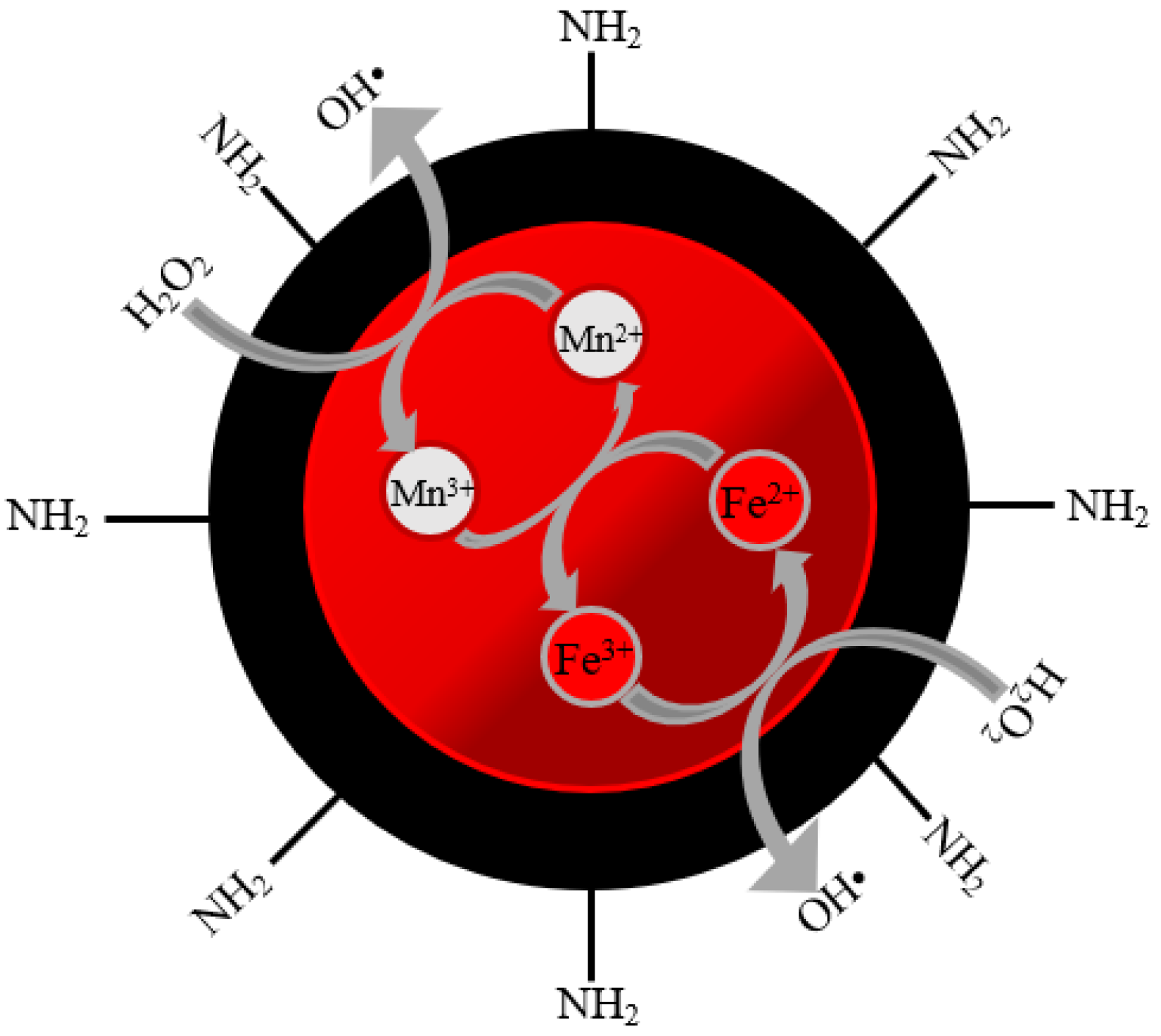

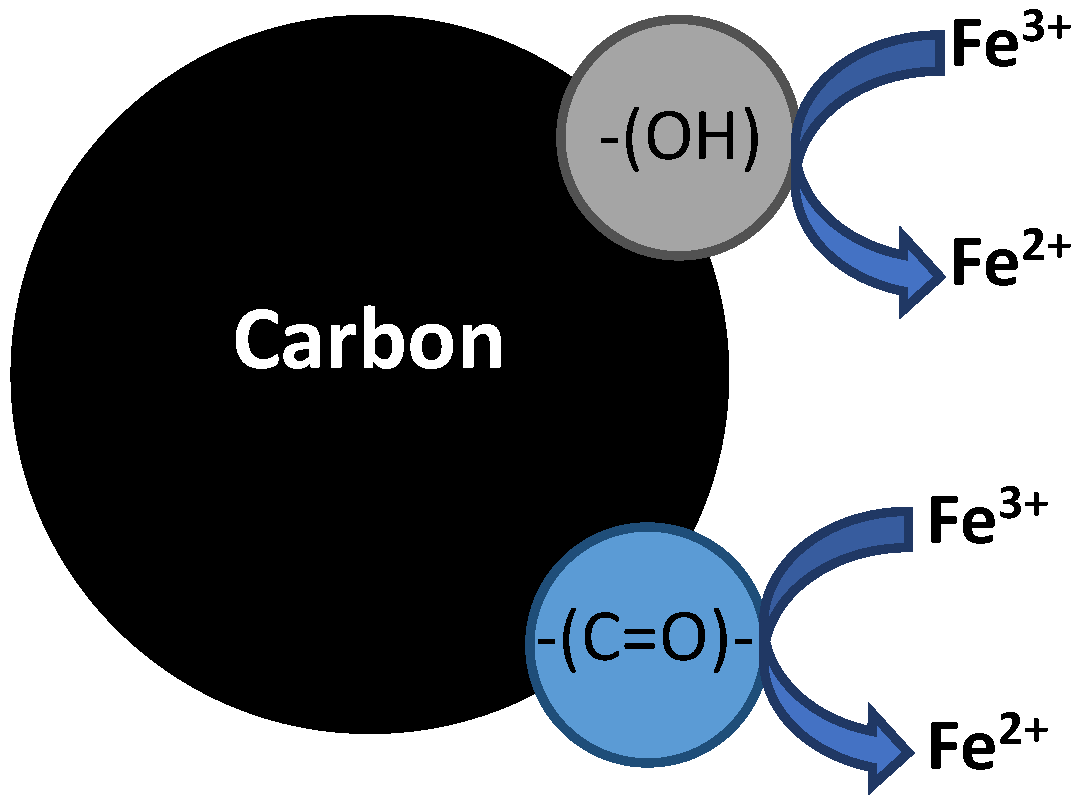
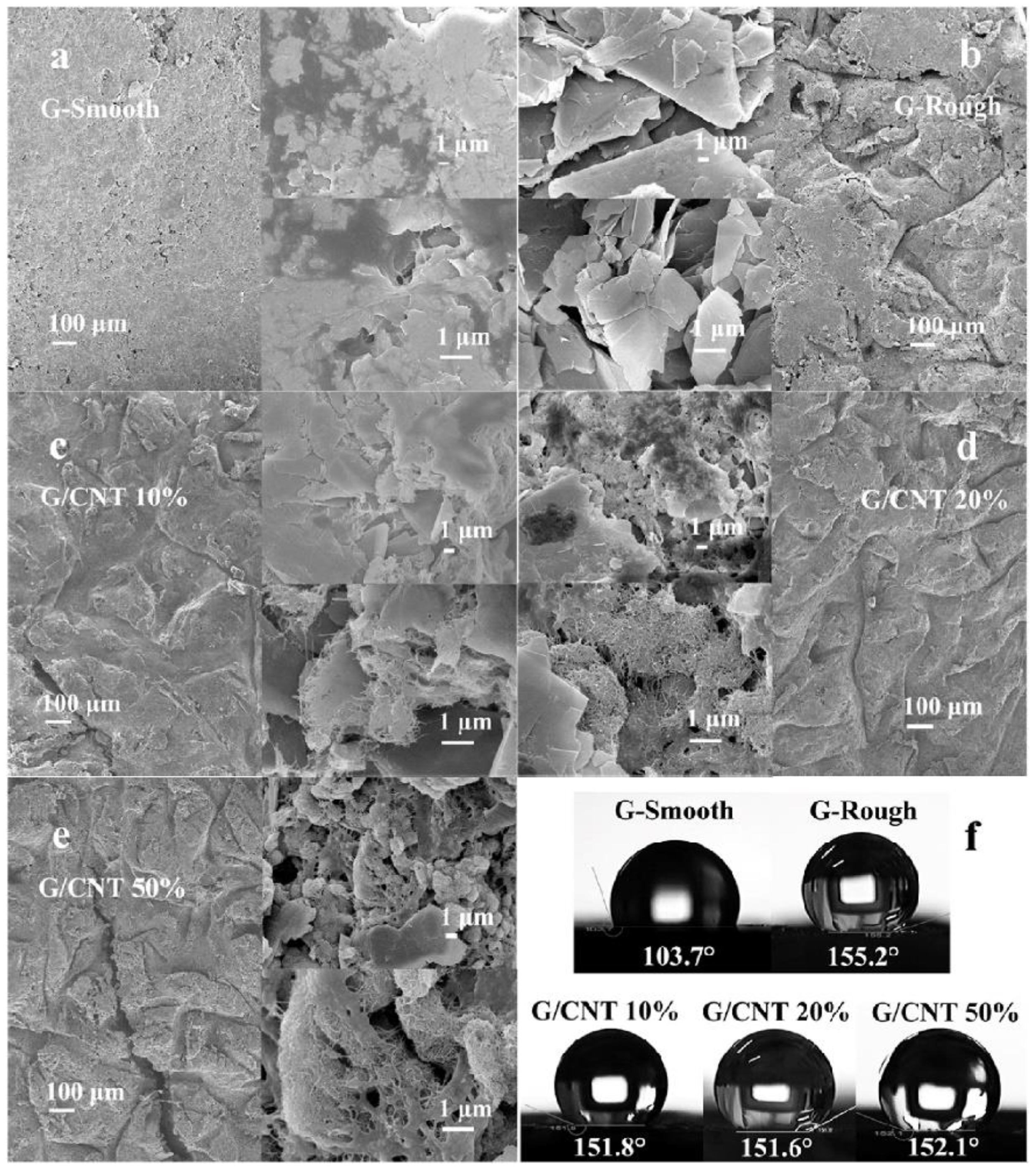
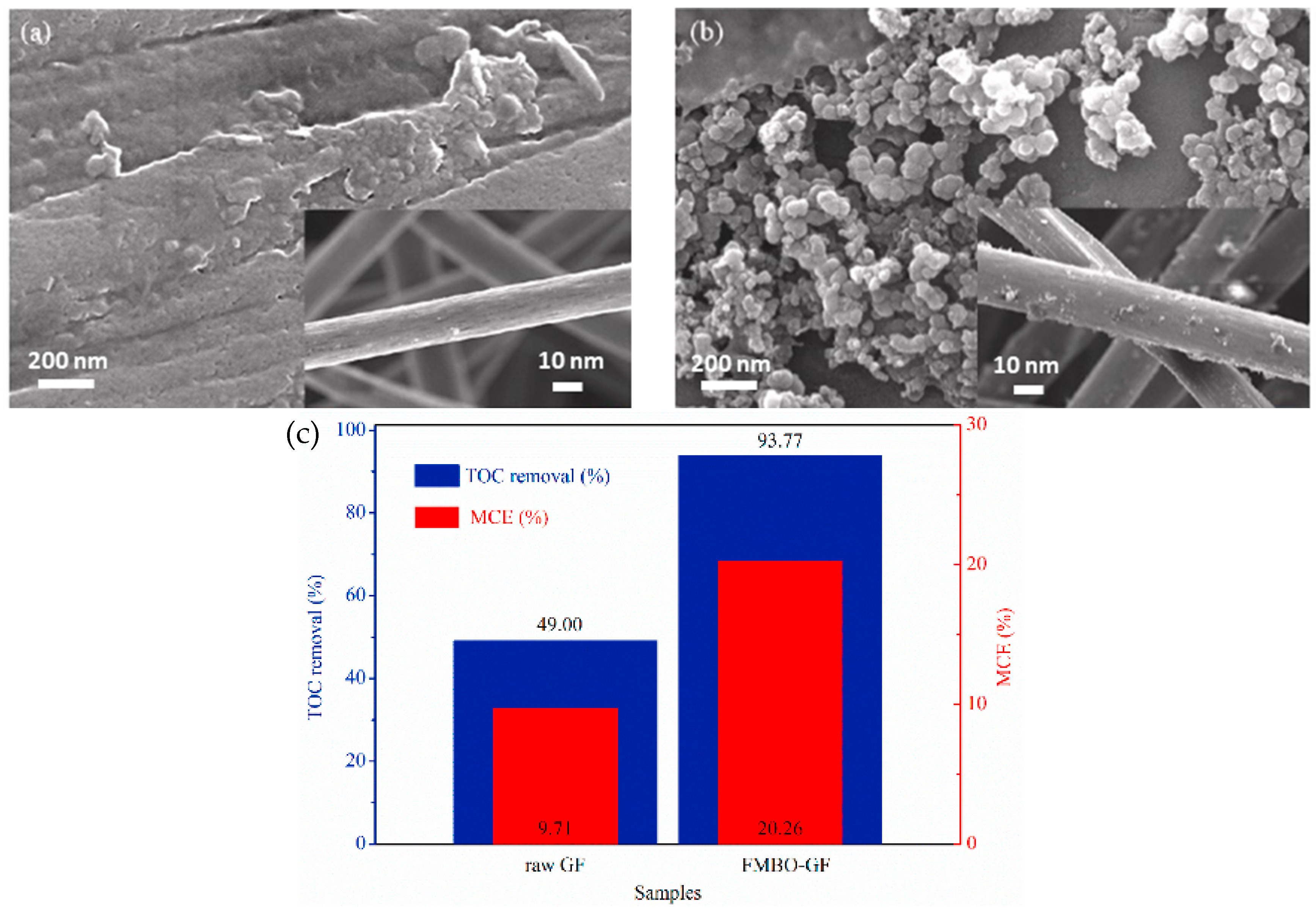
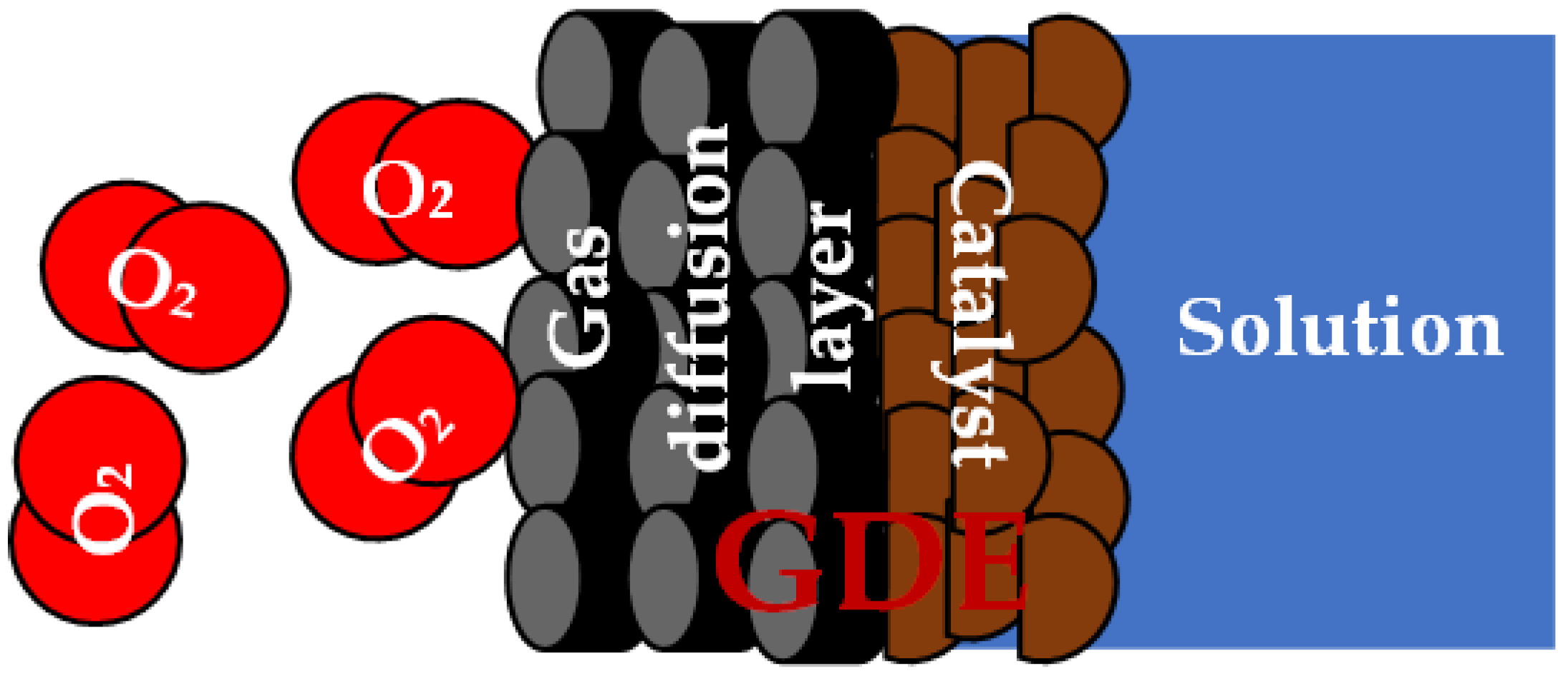
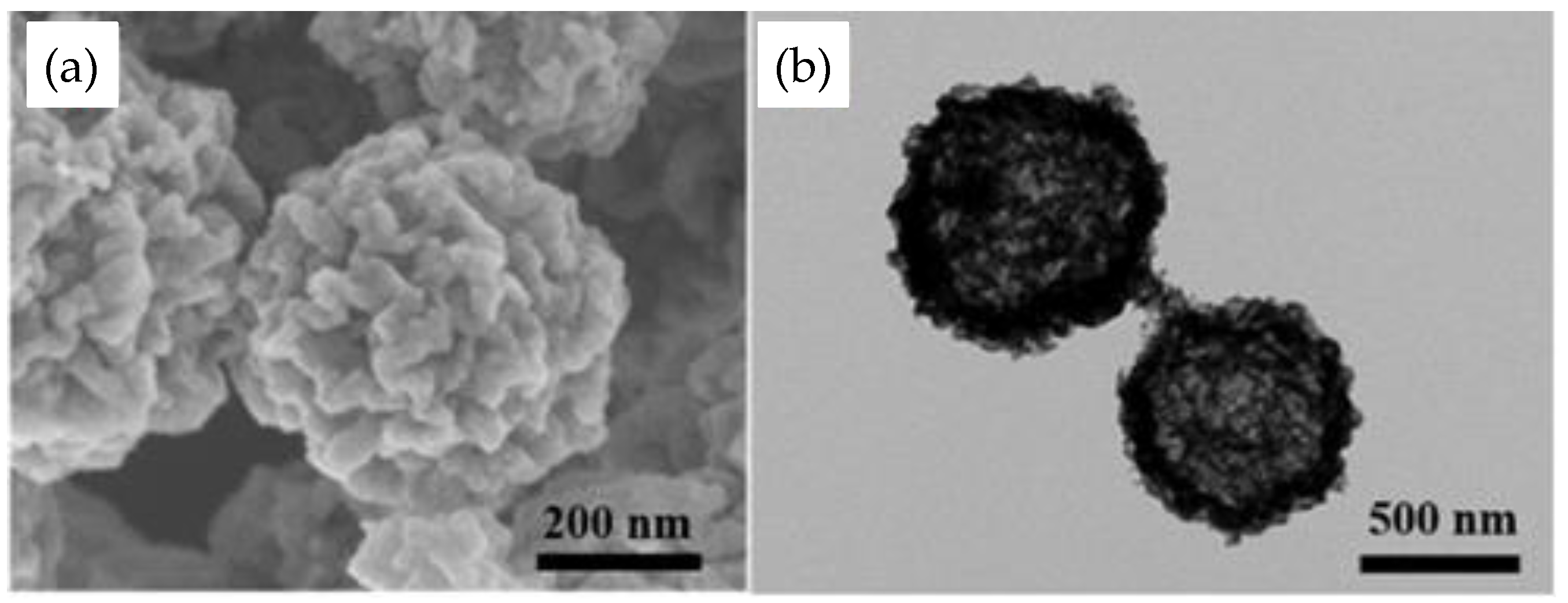
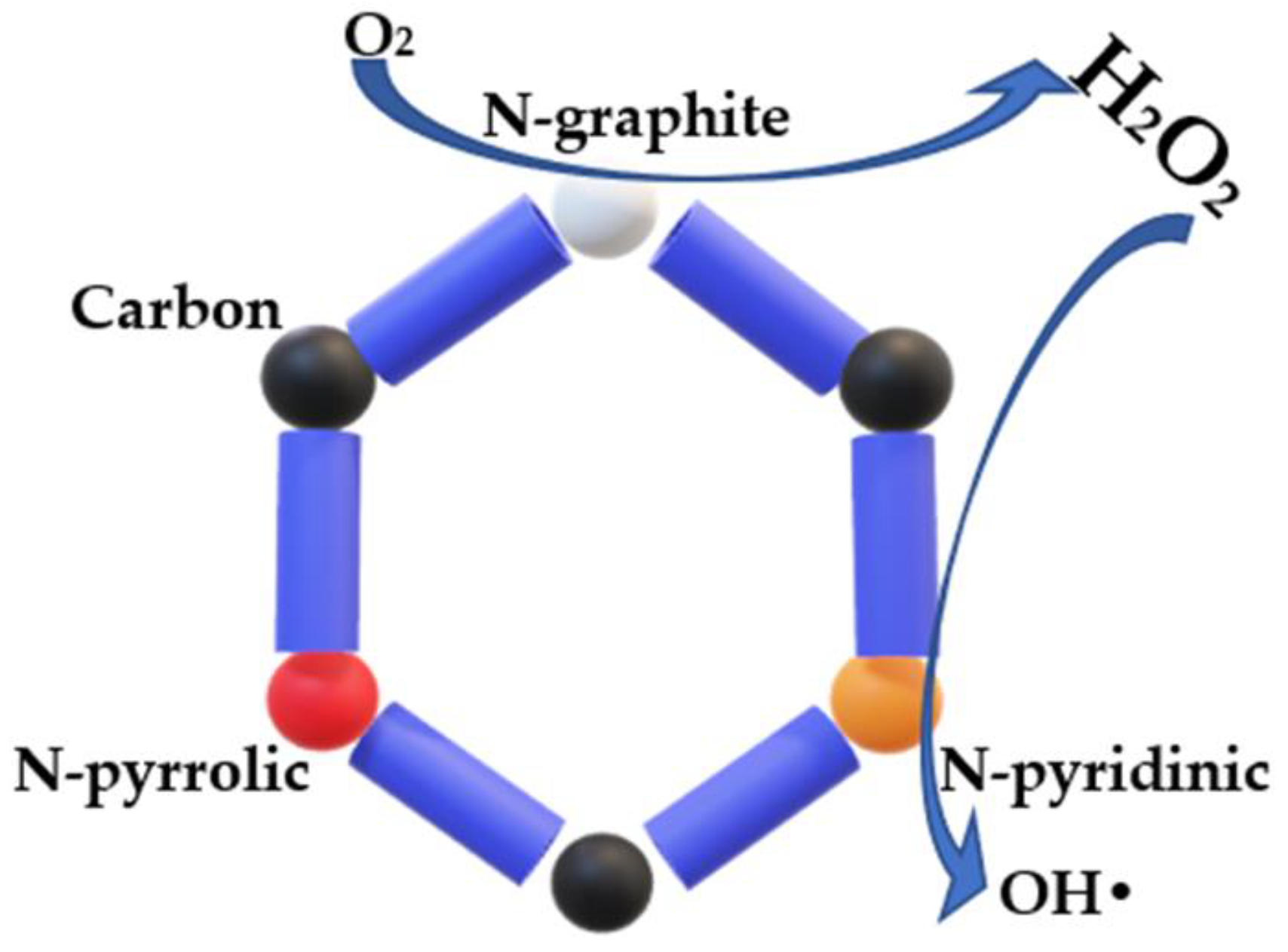

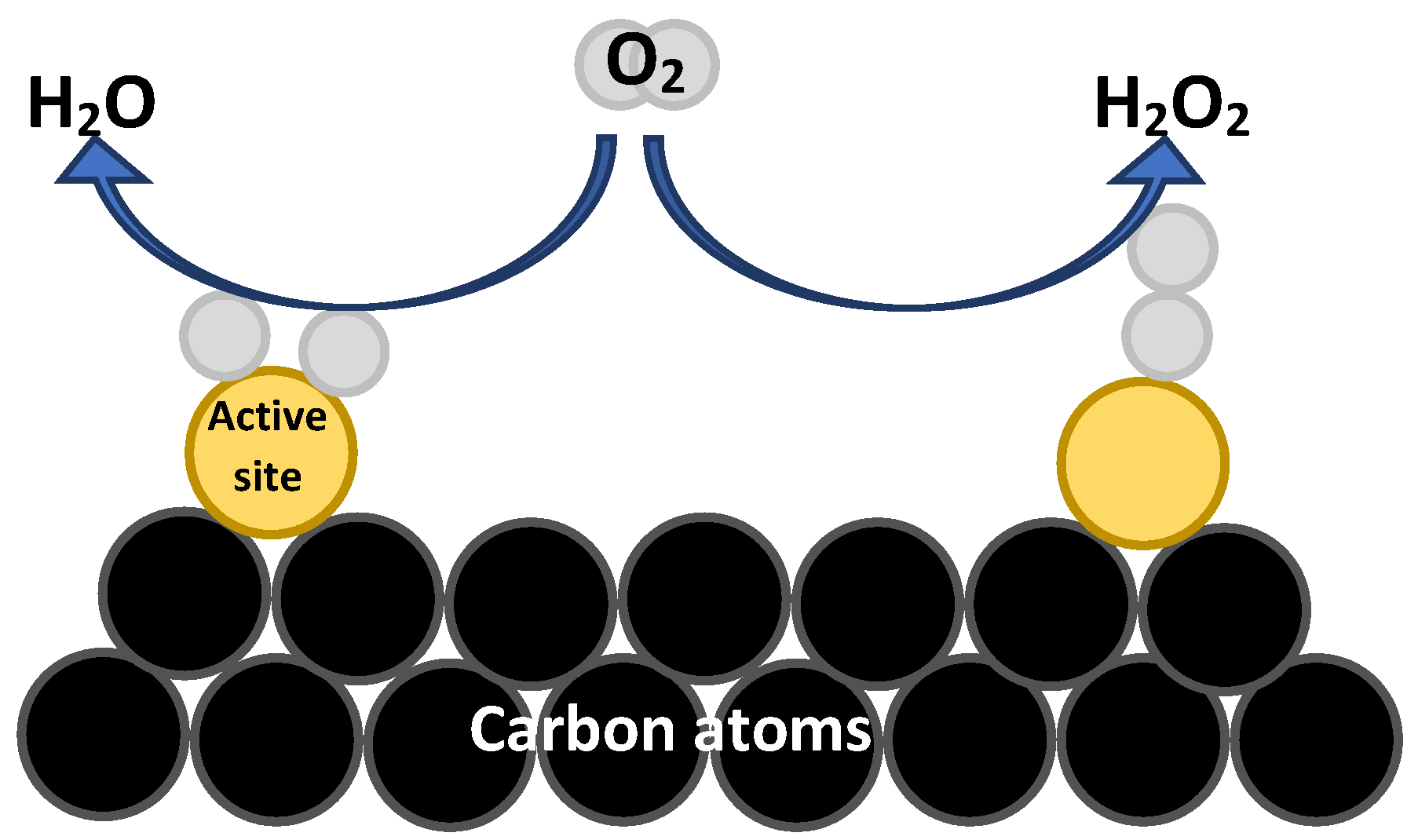
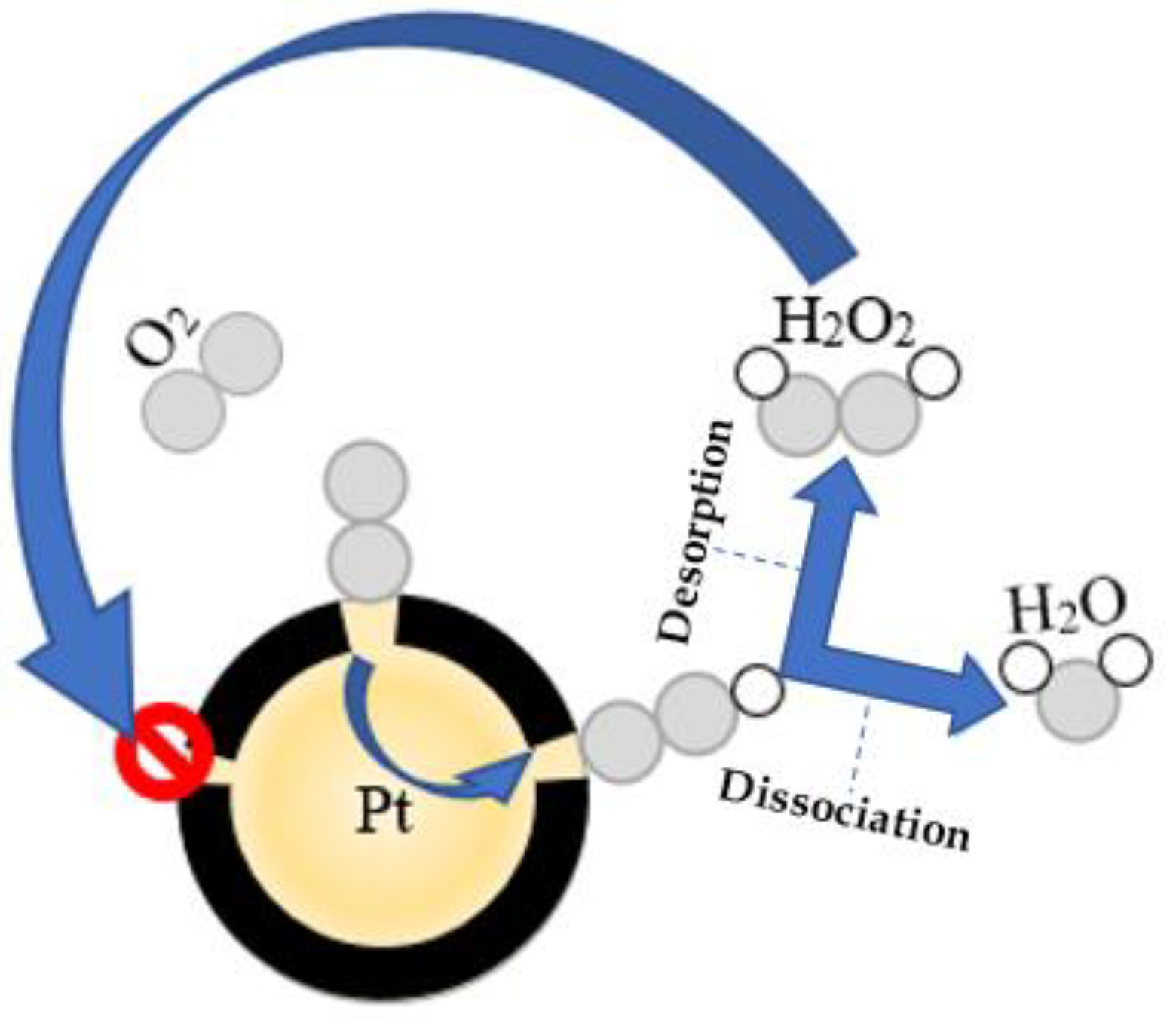
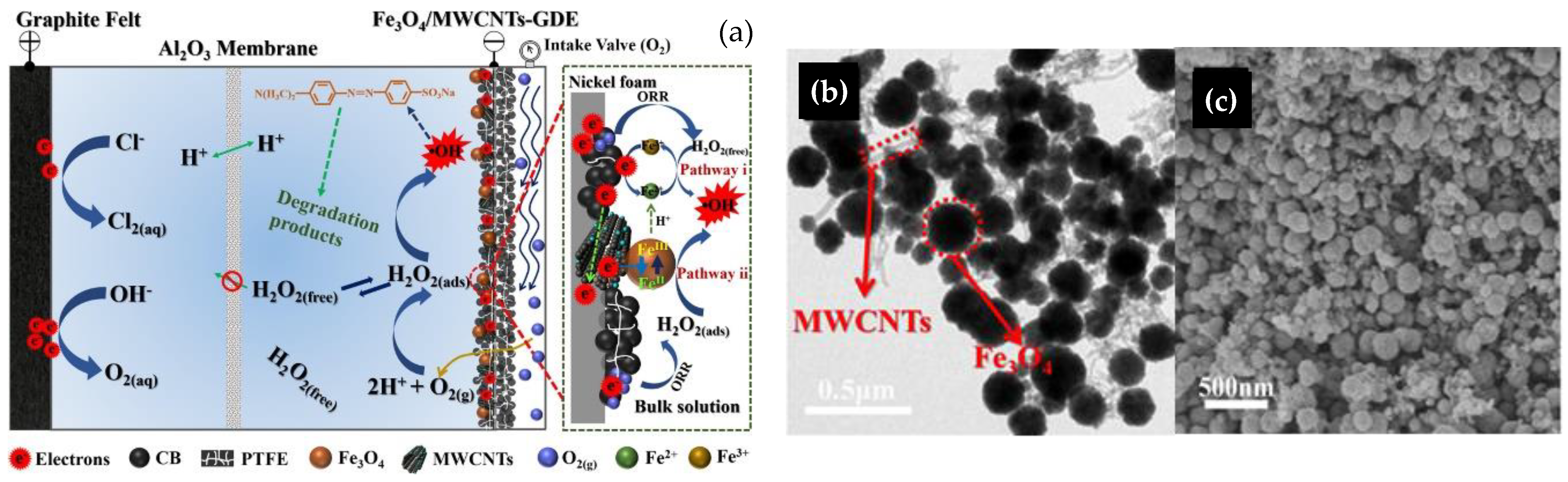
| Catalyst | Strategy | Improvement | Ref. |
|---|---|---|---|
| Fe (NO3)3·9H2O | Addition of MWCNT and complexation on the surface of the MWCNT through the surface groups. |
| [102] |
| FeCl3·6H2O | Addition of carbon materials (powdered activated carbon and carbon nanotubes). |
| [103] |
| Fe (NO3)3·9H2O | Addition of hydrothermally prepared carbon. |
| [104] |
| Fe2O3@FeB | FeB coating to Fe2O3 core. |
| [115] |
| FeCl3·6H2O | Addition of granular activated carbon. |
| [105] |
| Ferrihydrite | Addition of biocarbon and S. oneidensis. |
| [106] |
| Montmorillonite (Fe/Cu-MMT) | Preparation of Fe–Cu bimetallic active sites intercalated on montmorillonite. |
| [111] |
| Fe–Mn/SAPO-18 | Fe–Mn bimetallic active sites supported on SAPO-18. |
| [112] |
| Fe3O4–Sep | Sepiolite support for Fe3O4 nanoparticles. |
| [113] |
| γ-Fe2O3–SiO2 | Mesoporosity creation and Fe3+ doping of maghemite/silica microspheres. |
| [114] |
| QuFe@ACF | Active carbon fiber support for ferric hydroxyquinoline. |
| [107] |
| Fe-C | Fe-doped carbon xerogel. |
| [116] |
| CF/rGO | Reduced graphene oxide support for copper ferrite. |
| [108] |
| Fe–N/C | Carbon xerogel support for heteroatoms (Fe, N). |
| [109] |
| CoFe2O4/NOM | Xerogel support from the natural organic matrix (NOM) for CoFe2O4. |
| [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajardo-Puerto, E.; Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. From Fenton and ORR 2e−-Type Catalysts to Bifunctional Electrodes for Environmental Remediation Using the Electro-Fenton Process. Catalysts 2023, 13, 674. https://doi.org/10.3390/catal13040674
Fajardo-Puerto E, Elmouwahidi A, Bailón-García E, Pérez-Cadenas AF, Carrasco-Marín F. From Fenton and ORR 2e−-Type Catalysts to Bifunctional Electrodes for Environmental Remediation Using the Electro-Fenton Process. Catalysts. 2023; 13(4):674. https://doi.org/10.3390/catal13040674
Chicago/Turabian StyleFajardo-Puerto, Edgar, Abdelhakim Elmouwahidi, Esther Bailón-García, Agustín Francisco Pérez-Cadenas, and Francisco Carrasco-Marín. 2023. "From Fenton and ORR 2e−-Type Catalysts to Bifunctional Electrodes for Environmental Remediation Using the Electro-Fenton Process" Catalysts 13, no. 4: 674. https://doi.org/10.3390/catal13040674
APA StyleFajardo-Puerto, E., Elmouwahidi, A., Bailón-García, E., Pérez-Cadenas, A. F., & Carrasco-Marín, F. (2023). From Fenton and ORR 2e−-Type Catalysts to Bifunctional Electrodes for Environmental Remediation Using the Electro-Fenton Process. Catalysts, 13(4), 674. https://doi.org/10.3390/catal13040674










