Synthesis of Ce and Sm Co-Doped TiO2 Nanoparticles with Enhanced Photocatalytic Activity for Rhodamine B Dye Degradation
Abstract
1. Introduction
2. Results and Discussions
2.1. Phase Analysis
2.2. Morphological Observations
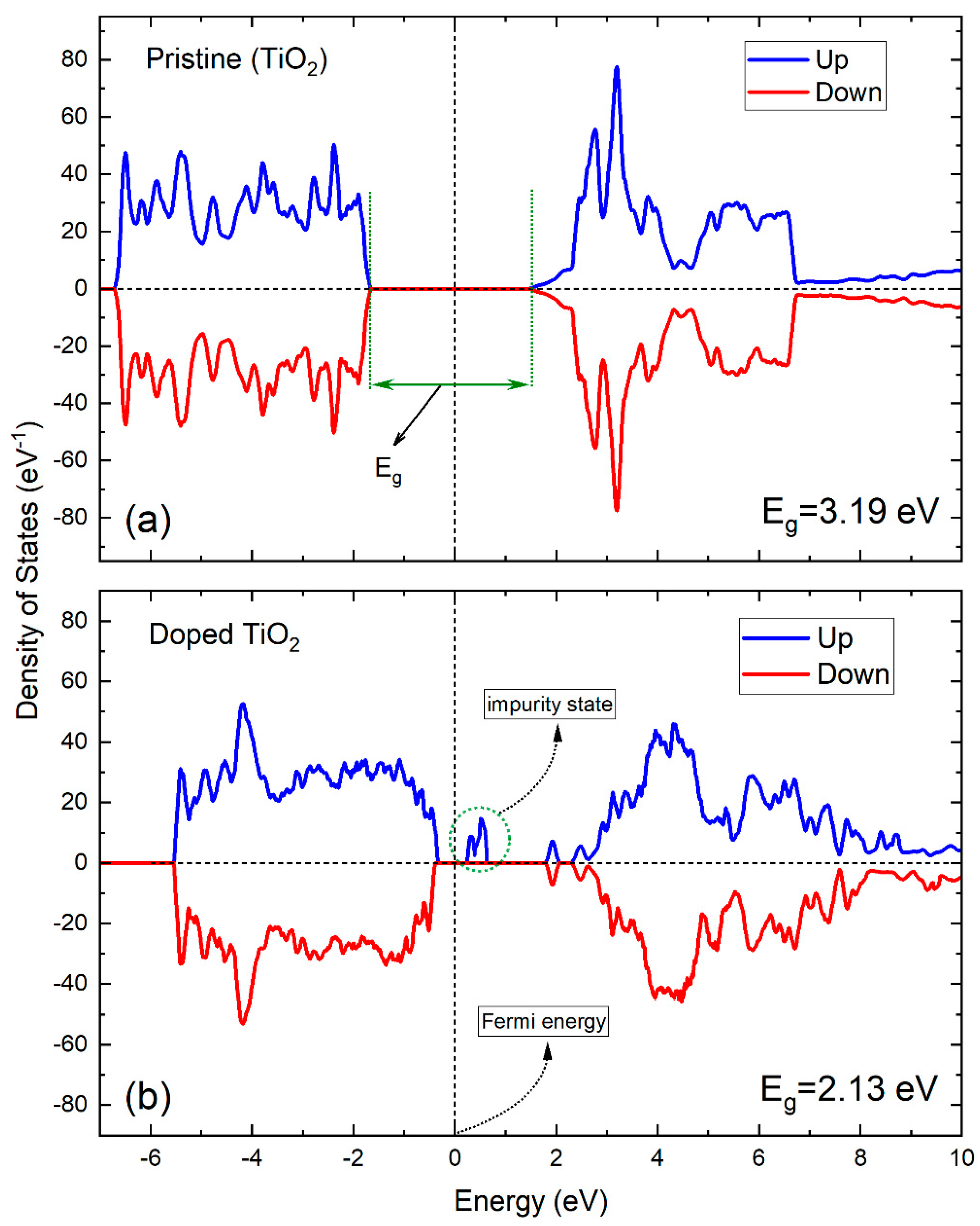
2.3. Electronic Structure Properties
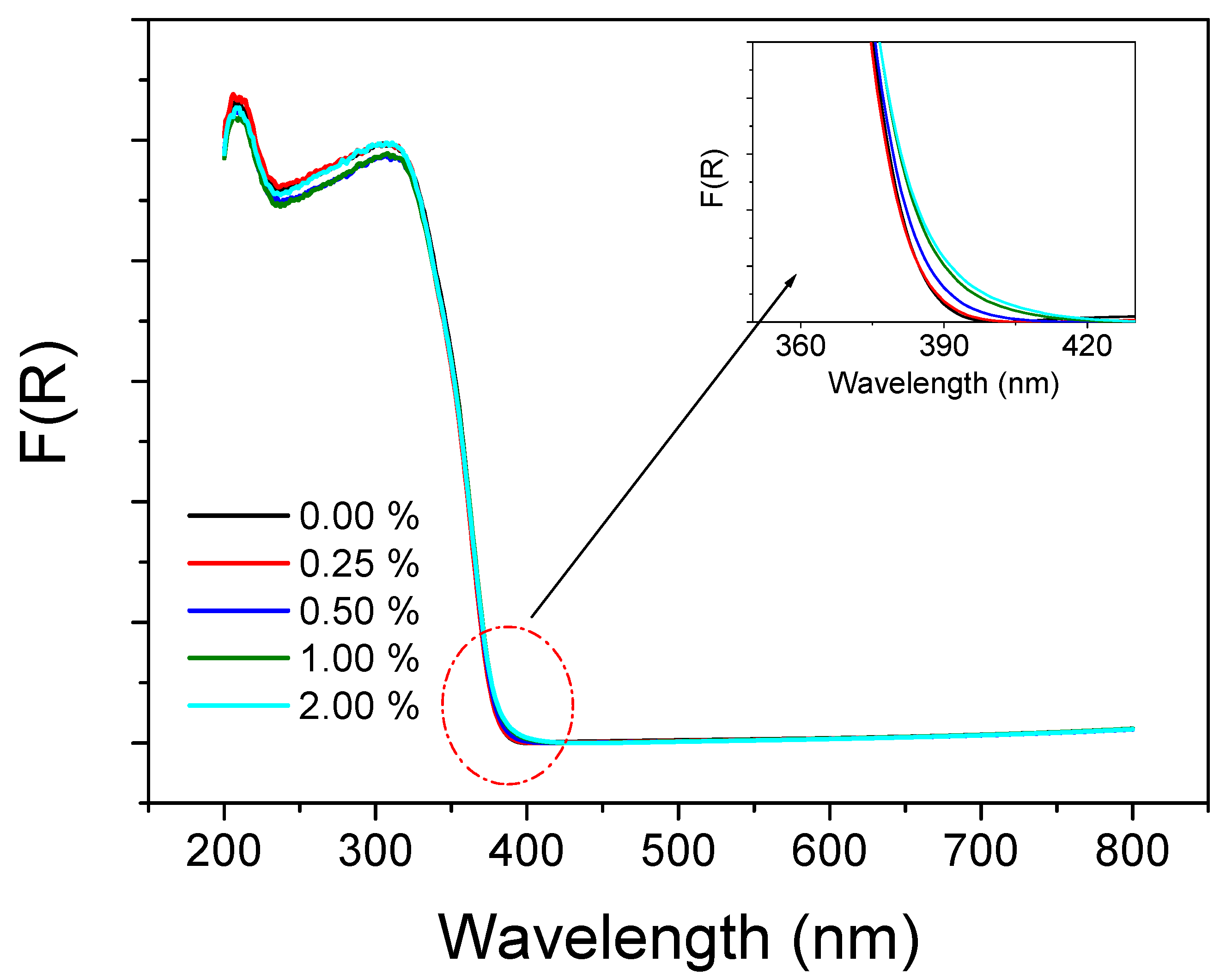
2.4. UV/Visible Spectra Investigation
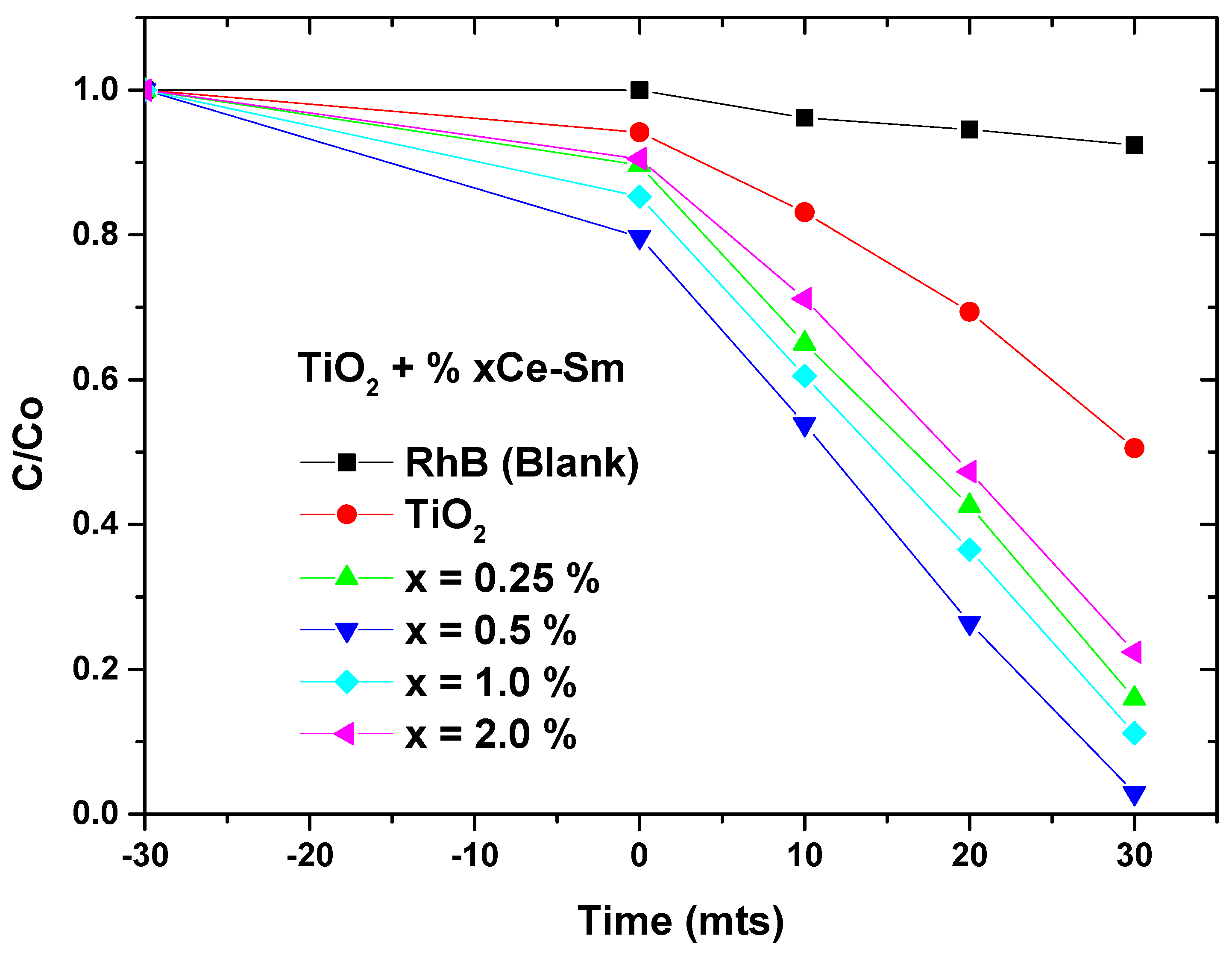
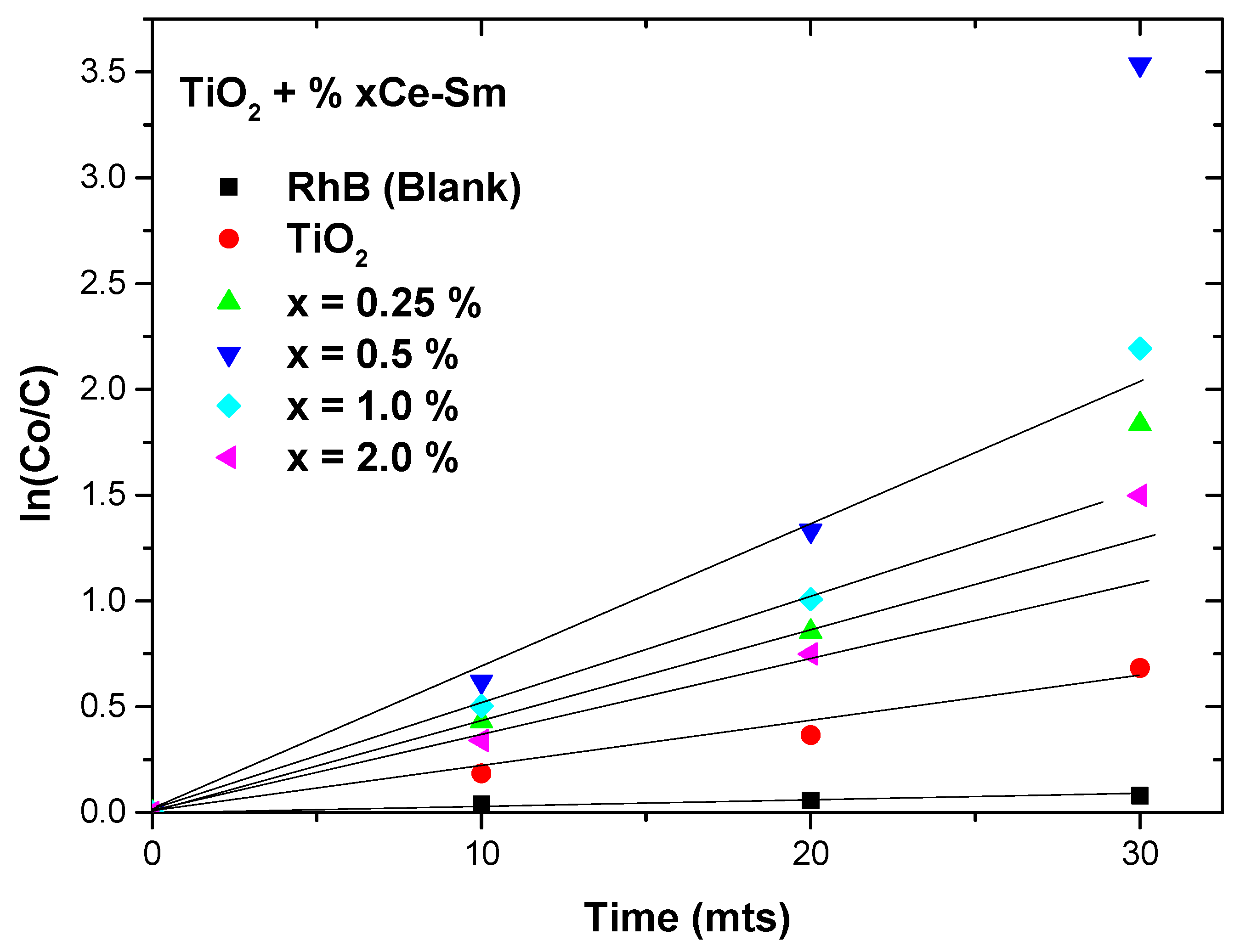
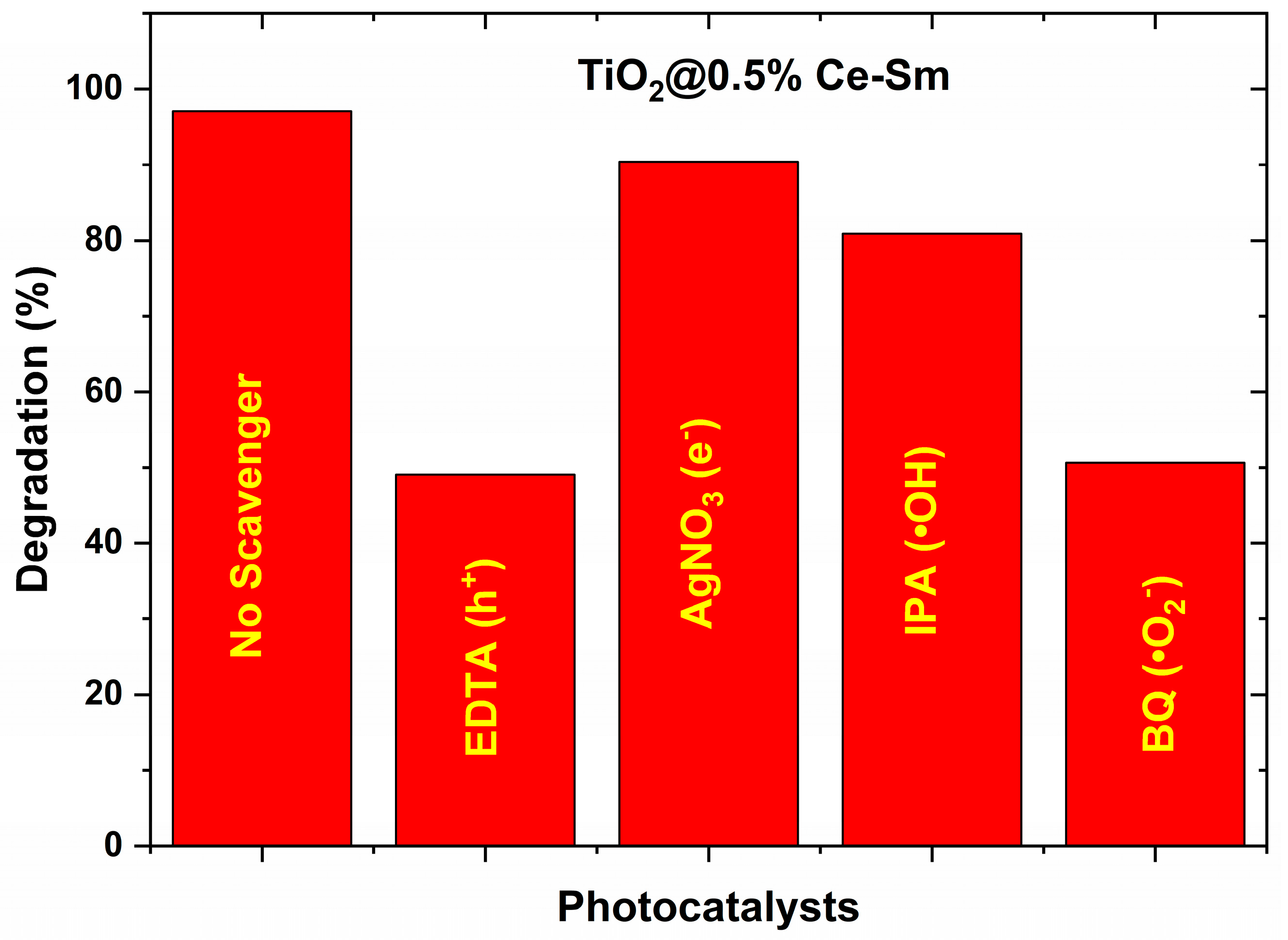
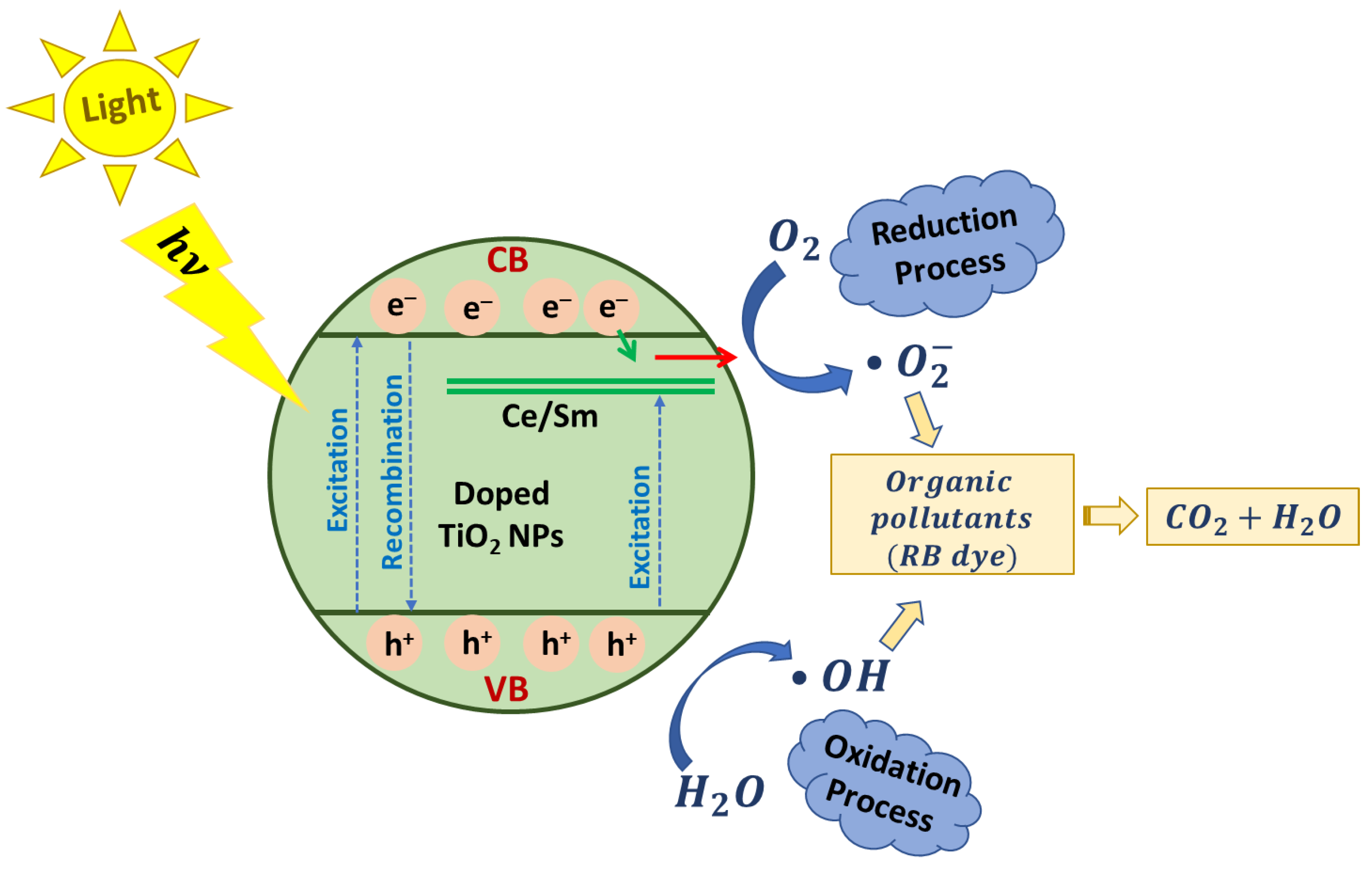
2.5. Photocatalytic Degradation
3. Experimental Details
3.1. Materials
3.2. Synthesis of Pristine and Ce–Sm Co-Doped TiO2 NPs
3.3. Characterization Techniques
3.4. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannachi, E.; Slimani, Y.; Nawaz, M.; Trabelsi, Z.; Yasin, G.; Bilal, M.; Almessiere, M.A.; Baykal, A.; Thakur, A.; Thakur, P. Synthesis, characterization, and evaluation of the photocatalytic properties of zinc oxide co-doped with lanthanides elements. J. Phys. Chem. Solids 2022, 170, 110910. [Google Scholar] [CrossRef]
- Rizwan, K.; Bilal, M.; Slimani, Y.; Show, P.L.; Rtimi, S.; Roy, A.; Iqbal, H.M.N. Hydrogen-based sono-hybrid catalytic degradation and mitigation of industrially-originated dye-based pollutants. Int. J. Hydrogen Energy 2023, 48, 6597–6612. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.M.J.; Shenoy, U.S.; Bhat, D.K. NiWO4-ZnO-NRGO ternary nanocomposite as an efficient photocatalyst for degradation of methylene blue and reduction of 4-nitro phenol. J. Phys. Chem. Solids 2017, 109, 124–133. [Google Scholar] [CrossRef]
- Da Silva, W.L.; Hamilton, J.W.J.; Sharma, P.K.; Dunlop, P.S.M.; Byrne, J.A.; Dos Santos, J.H.Z. Agro and industrial residues: Potential raw materials for photocatalyst development. J. Photochem. Photobiol. A 2021, 411, 113184. [Google Scholar] [CrossRef]
- Muraro, P.C.L.; Wouters, R.D.; Pavoski, G.; Espinosa, D.C.R.; Ruiz, Y.P.M.; Galembeck, A.; Rech, V.C.; da Silva, W.L. Ag/TiNPS nanocatalyst: Biosynthesis, characterization and photocatalytic activity. J. Photochem. Photobiol. A 2023, 439, 114598. [Google Scholar] [CrossRef]
- Wouters, R.D.M.; Muraro, P.C.L.; Druzian, D.M.; Viana, A.R.; de Oliveira Pinto, E.; da Silva, J.K.L.; Vizzotto, B.S.; Ruiz, Y.P.M.; Galembeck, A.; Pavoski, G.; et al. Zinc oxide nanoparticles: Biosynthesis, characterization, biological activity and photocatalytic degradation for tartrazine yellow dye. J. Mol. Liq. 2023, 371, 121090. [Google Scholar] [CrossRef]
- Gote, Y.M.; Sinhmar, P.S.; Gogate, P.R. Sonocatalytic Degradation of Chrysoidine R Dye Using Ultrasonically Synthesized NiFe2O4 Catalyst. Catalysts 2023, 13, 597. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Pang, C.K.; Affandi, N.A.; Maruja, S.N.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. Chem. Eng. 2022, 6, 58. [Google Scholar] [CrossRef]
- Ghafoor, A.; Bibi, I.; Ata, S.; Majid, F.; Kamal, S.; Rehman, F.; Iqbal, S.; Aamir, M.; Slimani, Y.; Iqbal, M.; et al. Synthesis and characterization of magnetically separable La1−xBixCr1−yFeyO3 and photocatalytic activity evaluation under visible light. Z. Für. Phys. Chem. 2021, 235, 1413–1431. [Google Scholar] [CrossRef]
- Aroh, A.O.; Gimba, C.E.; Omoniyi, K.I.; Abba, H.; Yilleng, M.T. Comparison of photocatalytic degradation of 4-chlorophenol and 3-chlorophenol using silver/palladium nanoparticles doped on TiO2. Int. J. Acad. Res. Bus. Soc. Sci. 2019, 1, 232–254. [Google Scholar]
- Aamir, M.; Bibi, I.; Ata, S.; Majid, F.; Alwadai, N.; Almuqrin, A.H.; Albalawi, H.; Slimani, Y.; Bashir, M.; Iqbal, M. Micro-emulsion approach for the fabrication of La1−xGdxCr1−yFeyO3: Magnetic, dielectric and photocatalytic activity evaluation under visible light irradiation. Results Phys. 2021, 23, 104023. [Google Scholar] [CrossRef]
- Ajeesha, T.; Ashwini, A.; George, M.; Manikandan, A.; Mary, J.A.; Slimani, Y.; Almessiere, M.A.; Baykal, A. Nickel substituted MgFe2O4 nanoparticles via co-precipitation method for photocatalytic applications. Phys. B 2021, 606, 412660. [Google Scholar] [CrossRef]
- Mathiarasu, R.R.; Manikandan, A.; Panneerselvam, K.; George, M.; Raja, K.K.; Almessiere, M.A.; Slimani, Y.; Baykal, A.; Asirii, A.M.; Kamal, T.; et al. Photocatalytic degradation of reactive anionic dyes RB5, RR198 and RY145 via Rare earth element (REE) Lanthanum substituted CaTiO3 perovskite catalysts. J. Mater. Res. Technol. 2021, 15, 5936–5947. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Hannachi, E.; Slimani, Y.; Nawaz, M.; Sivakumar, R.; Trabelsi, Z.; Vignesh, R.; Akhtar, S.; Almessiere, M.A.; Baykal, A.; Yasin, G. Preparation of cerium and yttrium doped ZnO nanoparticles and tracking their structural, optical, and photocatalytic performances. J. Rare Earths 2022. [Google Scholar] [CrossRef]
- Geetha, G.V.; Sivakumar, R.; Slimani, Y.; Sanjeeviraja, C.; Kannapiran, E. Rare earth (RE: La and Ce) elements doped ZnWO4 nanoparticles for enhanced photocatalytic removal of methylene blue dye from aquatic environment. Phys. B 2022, 239, 414028. [Google Scholar] [CrossRef]
- Police, A.K.R.; Vattikuti, S.V.P.; Mandari, K.K.; Chennaiahgari, M.; Sharma, P.; Valluri, D.K.; Byon, C. Bismuth oxide cocatalyst and copper oxide sensitizer in Cu2O/TiO2/Bi2O3 ternary photocatalyst for efficient hydrogen production under solar light irradiation. Ceram. Int. 2018, 44, 11783–11791. [Google Scholar] [CrossRef]
- Chand, K.; Cao, D.; Fouad, D.E.; Lakhan, M.N.; Dayo, A.Q.; Sagar, H.J.; Zhu, K.; Mohamed, A.M.A. Photocatalytic and antimicrobial activity of biosynthesized silver and titanium dioxide nanoparticles: A comparative study. J. Mol. Liq. 2020, 316, 113821. [Google Scholar] [CrossRef]
- Al Jitan, S.; Palmisano, G.; Garlisi, C. Synthesis and surface modification of TiO2-based photocatalysts for the conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar]
- Police, A.K.R.; Vattikuti, S.V.P.; Baik, Y.J.; Chan, B. Eco-friendly, hydrogen fluoride-free, morphology-oriented synthesis of TiO2 with exposed (001) facets. Ceram. Int. 2019, 45, 2178–2184. [Google Scholar]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic degradation of sulfamethoxazole using TiO2-based materials–Perspectives for the development of a sustainable water treatment technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.M.J.; Shenoy, U.S.; Bhat, D.K. Novel NRGO-CoWO4-Fe2O3 nanocomposite as an efficient catalyst for dye degradation and reduction of 4-nitrophenol. Mater. Chem. Phys. 2018, 208, 112–122. [Google Scholar]
- Galstyan, V.; Macak, J.M.; Djenizian, T. Anodic TiO2 nanotubes: A promising material for energy conversion and storage. Appl. Mater. Today 2022, 29, 101613. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Devarayapalli, K.C.; Nallabala, N.K.R.; Nguyen, T.N.; Dang, N.N.; Shim, J. Onion-ring-like carbon and nitrogen from ZIF-8 on TiO2/Fe2O3 nanostructure for overall electrochemical water splitting. J. Phys. Chem. Lett. 2021, 12, 5909–5918. [Google Scholar] [CrossRef]
- Jeon, J.P.; Kweon, D.H.; Jang, B.J.; Ju, M.J.; Baek, J.B. Enhancing the Photocatalytic Activity of TiO2 Catalysts. Adv. Sustain. Syst. 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2 based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Mikolajczyk, A.; Sizochenko, N.; Mulkiewicz, E.; Malankowska, A.; Nischk, M.; Jurczak, P.; Hirano, S.; Nowaczyk, G.; Zaleska-Medynska, A.; Leszczynski, J.; et al. Evaluating the toxicity of TiO2-based nanoparticles to Chinese hamster ovary cells and Escherichia coli: A complementary experimental and computational approach. Beilstein J. Nanotechnol. 2017, 8, 2171–2180. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C.D.N. Neodymium doped TiO2 nanoparticles by sol-gel method for antibacterial and photocatalytic activity. Mater. Sci. Semicond. Process. 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Sathiyan, K.; Bar-Ziv, R.; Mendelson, O.; Zidki, T. Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 2020, 126, 110842. [Google Scholar]
- Khade, G.V.; Gavade, N.L.; Suwarnkar, M.B.; Dhanavade, M.J.; Sonawane, K.D.; Garadkar, K.M. Enhanced photocatalytic activity of europium doped TiO2 under sunlight for the degradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 11002–11011. [Google Scholar]
- Prakash, J.; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Xiu, Z.; Xing, Z.; Li, Z.; Wu, X.; Yan, X.; Hu, M.; Cao, Y.; Yang, S.; Zhou, W. Ti3+-TiO2/Ce3+-CeO2 Nanosheet heterojunctions as efficient visible-light-driven photocatalysts. Mater. Res. Bull. 2018, 100, 191–197. [Google Scholar] [CrossRef]
- Khade, G.V.; Suwarnkar, M.B.; Gavade, N.L.; Garadkar, K.M. Sol-gel microwave assisted synthesis of Sm-doped TiO2 nanoparticles and their photocatalytic activity for the degradation of Methyl Orange under sunlight. J. Mater. Sci. Mater. Electron. 2016, 27, 6425–6432. [Google Scholar] [CrossRef]
- Peng, H.; Guo, R.; Lin, H. Photocatalytic reduction of CO2 over Sm-doped TiO2 nanoparticles. J. Rare Earths 2020, 38, 1297–1304. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Chaudhary, R.P. Structural and optical characterization of Zn doped TiO2 nanoparticles prepared by sol–gel method. J. Sol-Gel Sci. Technol. 2012, 61, 585–591. [Google Scholar] [CrossRef]
- Tsotetsi, D.; Dhlamini, M.; Mbule, P. Sol-gel synthesis and characterization of Ho3+ doped TiO2 nanoparticles: Evaluation of absorption efficiency and electrical conductivity for possible application in perovskite solar cells. Opt. Mater. 2022, 130, 112569. [Google Scholar] [CrossRef]
- Ouardi, Y.E.; Aissouq, A.E.; Chennah, A.; Ouammou, A.; Laatikainen, K. Synthesis, characterization, and DFT investigation of rhodamine B dye removal by activated carbon produced from argan nutshell. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- QuantumATK Version S-2021.06, Synopsys. Available online: www.synopsys.com/silicon/quantumatk.html (accessed on 30 September 2022).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Ney, V.; Ye, S.; Kammermeier, T.; Ollefs, K.; Wilhelm, F.; Rogalev, A.; Lebègue, S.; da Rosa, A.L.; Ney, A. Structural and magnetic analysis of epitaxial films of Gd-doped ZnO. Phys. Rev. B 2012, 85, 235203. [Google Scholar] [CrossRef]
- Fabris, S.; de Gironcoli, S.; Baroni, S.; Vicario, G.; Balducci, G. Taming multiple valency with density functionals: A case study of defective ceria. Phys. Rev. B 2005, 71, 041102(R). [Google Scholar] [CrossRef]
- van Setten, M.J.; Giantomassi, M.; Bousquet, E.; Verstraete, M.J.; Hamann, D.R.; Gonze, X.; Rignanese, G.M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Jafari, A.; Khademi, S.; Farahmandjou, M.; Darudi, A.; Rasuli, R. Structural and Optical Properties of Ce3+-Doped TiO2 Nanocrystals Prepared by Sol–Gel Precursors. J. Electron. Mater. 2018, 47, 6901. [Google Scholar] [CrossRef]
- Ho, C.H.; Tsai, M.C.; Wong, M.S. Characterization of indirect and direct interband transitions of anatase TiO2 by thermoreflectance spectroscopy. App. Phys. Lett. 2008, 93, 081904. [Google Scholar] [CrossRef]
- Calandra, P.; Ruggirello, A.; Pistone, A.; Liveri, V.T. Structural and optical properties of novel surfactant coated TiO2–Ag based nanoparticles. J. Clust. Sci. 2010, 21, 767–778. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Choudhury, B.; Borah, B.; Choudhury, A. Ce–Nd codoping effect on the structural and optical properties of TiO2 nanoparticles. Mater. Sci. Eng. B 2013, 178, 239–247. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Revathy, V.R.; Rosmin, P.; Thrivedu, B.; Elsa, K.M.; Nimmymol, J.; Balakrishna, K.M.; Varghese, T. Influence of La doping on structural and optical properties of TiO2 nanocrystals. Mater. Charact. 2016, 113, 144–151. [Google Scholar] [CrossRef]
- Fu, C.; Li, T.; Qi, J.; Pan, J.; Chen, S.; Cheng, C. Theoretical study on the electronic and optical properties of Ce3+-doped TiO2 photocatalysts. Chem. Phys. Lett. 2010, 494, 117–122. [Google Scholar] [CrossRef]
- Ahmetović, S.; Vasiljević, Z.Ž.; Rajić, V.; Bartolić, D.; Novaković, M.; Tadić, N.B.; Cvjetićanin, N.; Nikolić, M.V. Examination of the doping effects of samarium (Sm3+) and zirconium (Zr4+) on the photocatalytic activity of TiO2 nanofibers. J. Alloys Compd. 2023, 930, 167423. [Google Scholar] [CrossRef]
- Singh, K.; Harish, S.; Kristy, A.P.; Shivani, V.; Archana, J.; Navaneethan, M.; Shimomura, M.; Hayakawa, Y. Erbium doped TiO2 interconnected mesoporous spheres as an efficient visible light catalyst for photocatalytic applications. Appl. Surf. Sci. 2018, 449, 755–763. [Google Scholar] [CrossRef]
- Fan, Z.; Meng, F.; Gong, J.; Li, H.; Ding, Z.; Ding, B. One-step hydrothermal synthesis of mesoporous Ce-doped anatase TiO2 nanoparticles with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 11866–11872. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumara, R.; Ravi, G.; Al-Sehemi, A.G.; Velauthapillai, D. Investigation of optimum Mn dopant level on TiO2 for dye degradation. Chemosphere 2022, 306, 135574. [Google Scholar] [CrossRef] [PubMed]
- El Mragui, A.; Zegaoui, O.; Esteves da Silva, J.C.G. Elucidation of the photocatalytic degradation mechanism of an azo dye under visible light in the presence of cobalt doped TiO2 nanomaterials. Chemosphere 2021, 266, 128931. [Google Scholar] [CrossRef]
- Arasi, S.E.; Madhavan, J.; Raj, M.V.A. Effect of samarium (Sm3+) doping on structural, optical properties and photocatalytic activity of titanium dioxide nanoparticles. J. Taibah Univ. Sci. 2018, 12, 186–190. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible light activity of rare earth metal doped (Er3+, Yb3+ or Er3+/Yb3+) titania photocatalysts. Appl. Catal. B 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Song, K.; Wang, X.; Yu, K.; Liang, C. Enhanced photocatalytic activities of Nd-doped TiO2 under visible light using a facile sol-gel method. J. Rare Earths 2020, 38, 148–156. [Google Scholar] [CrossRef]
- Bao, R.; Li, R.; Chen, C.; Wu, H.; Xia, J.; Long, C.; Li, H. Biotemplated synthesis of 3D rare earth–doped TiO2 hollow spheres for photocatalytic application. J. Phys. Chem. Solids 2019, 126, 78–84. [Google Scholar] [CrossRef]
- Singh, I.; Birajdar, B. Synthesis, characterization and photocatalytic activity of mesoporous Na-doped TiO2 nano-powder prepared via a solvent-controlled non-aqueous sol–gel route. RSC Adv. 2017, 7, 54053–54062. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Ma, C.Y.; Yi, X.Y.; Yuan, F.; Xie, Y.; Hu, J.M.; Hu, B.C.; Zhang, Q.Y. Structural, morphological, optical and photocatalytic properties of Gd-doped TiO2 films. Thin Solid Film. 2016, 615, 13–18. [Google Scholar] [CrossRef]
- Irshad, A.; Zulfiqar, M.; Ali, H.M.; Shahzadi, N.; Abd El-Gawad, H.H.; Chokejaroenrat, C.; Sakulthaew, C.; Anjum, F.; Suleman, M. Co-substituted Mg–Zn spinel nanocrystalline ferrites: Synthesis, characterization and evaluation of catalytic degradation efficiency for colored and colorless compounds. Ceram. Int. 2022, 48, 29805–29815. [Google Scholar] [CrossRef]
- Sadiq, M.M.J.; Shenoy, U.S.; Bhat, D.K. Novel RGO–ZnWO4–Fe3O4 nanocomposite as high performance visible light photocatalyst. RSC Adv. 2016, 6, 61821–61829. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, J.; Li, X.; Guo, L.; Zhang, Q.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Fu, Y.; et al. Rational design of graphite carbon nitride-decorated zinc oxide nanoarrays on three-dimensional nickel foam for the efficient production of reactive oxygen species through stirring-promoted piezo–photocatalysis. J. Colloid Interface Sci. 2023, 632, 271–284. [Google Scholar] [CrossRef]
- Sadiq, M.M.J.; Shenoy, U.S.; Bhat, D.K. Synthesis of BaWO4/NRGO–g-C3N4 nanocomposites with excellent multifunctional catalytic performance via microwave approach. Front. Mater. Sci. 2018, 12, 247–263. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Guo, B.L.; Han, P.; Guo, L.C.; Cao, Y.Q.; Li, A.D.; Kong, J.Z.; Zhai, H.F.; Wu, D. The Antibacterial Activity of Ta-doped ZnO nanoparticles. Nanoscale Res. Lett. 2015, 10, 336. [Google Scholar] [CrossRef]
- Rajoriya, S.; Bargole, S.; George, S.; Saharan, V.K.; Gogate, P.R.; Pandit, A.B. Synthesis and characterization of Samarium and Nitrogen doped TiO2 photo-catalysts for photo-degradation of 4-Acetamidophenol in combination with hydrodynamic and acoustic cavitation. Sep. Purif. Technol. 2019, 209, 254–269. [Google Scholar] [CrossRef]
- Hannachi, E.; Almessiere, M.A.; Slimani, Y.; Baykal, A.; Ben Azzouz, F. AC susceptibility investigation of YBCO superconductor added by carbon nanotubes. J. Alloys Compd. 2020, 812, 152150. [Google Scholar] [CrossRef]
- Slimani, Y.; Sivakumar, R.; Meena, S.S.; Vignesh, R.; Yasin, G.; Hannachi, E.; Almessiere, M.A.; Trabelsi, Z.; Batoo, K.M.; Baykal, A.; et al. BaTiO3/(Co0.8Ni0.1Mn0.1Fe1. 9Ce0.1O4)x composites: Analysis of the effect of Co0.8Ni0.1Mn0.1Fe1.9Ce0.1O4 doping at different concentrations on the structural, morphological, optical, magnetic, and magnetoelectric coupling properties of BaTiO3. Ceram. Int. 2022, 48, 30499–30509. [Google Scholar] [CrossRef]
- Slimani, Y.; Shirsath, S.E.; Hannachi, E.; Almessiere, M.A.; Aouna, M.M.; Aldossary, N.E.; Yasin, G.; Baykal, A.; Ozçelik, B.; Ercan, I. (BaTiO3)1−x+(Co0.5Ni0.5Nb0.06Fe1.94O4)x nanocomposites: Structure, morphology, magnetic and dielectric properties. J. Amer. Ceram. Soc. 2021, 104, 5648–5658. [Google Scholar] [CrossRef]
- George, M.; Ajeesha, T.L.; Manikandan, A.; Anantharaman, A.; Jansi, R.S.; Kumar, E.R.; Slimani, Y.; Almessiere, M.A.; Baykal, A. Evaluation of Cu–MgFe2O4 spinel nanoparticles for photocatalytic and antimicrobial activates. J. Phys. Chem. Solids 2021, 153, 110010. [Google Scholar] [CrossRef]
- Dinesh, A.; Raja, K.K.; Manikandan, A.; Almessiere, M.A.; Slimani, Y.; Baykal, A.; Alorfi, H.S.; Hussein, M.A.; Khan, A. Sol–gel combustion synthesis and photocatalytic dye degradation studies of rare earth element Ce substituted Mn–Zn ferrite nanoparticles. J. Mater. Res. Technol. 2022, 18, 5280–5289. [Google Scholar] [CrossRef]
- Sadiq, M.M.J.; Shenoy, U.S.; Bhat, D.K. Enhanced photocatalytic performance of N-doped RGO-FeWO4/Fe3O4 ternary nanocomposite in environmental applications. Mater. Today Chem. 2017, 4, 133–141. [Google Scholar] [CrossRef]

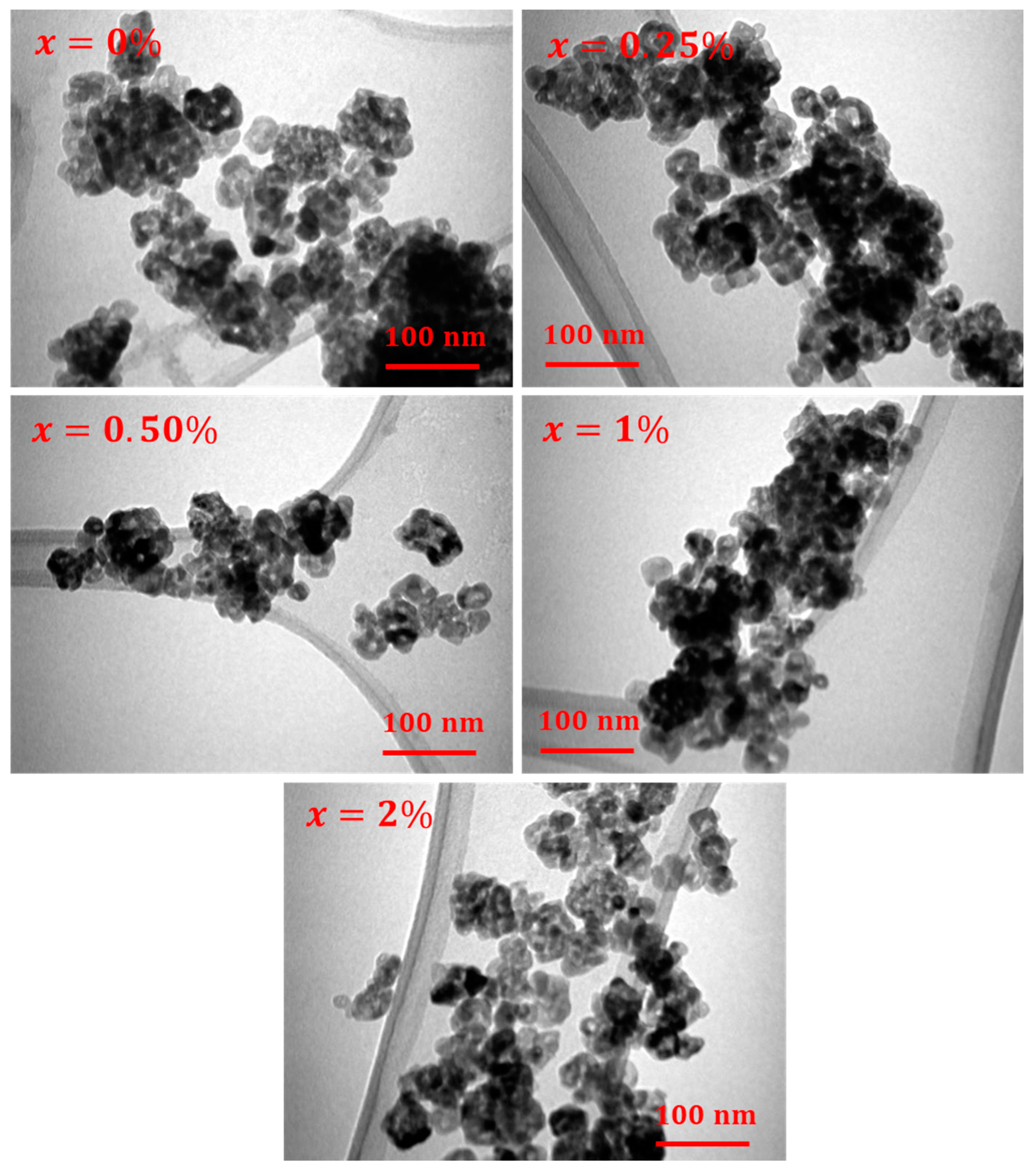
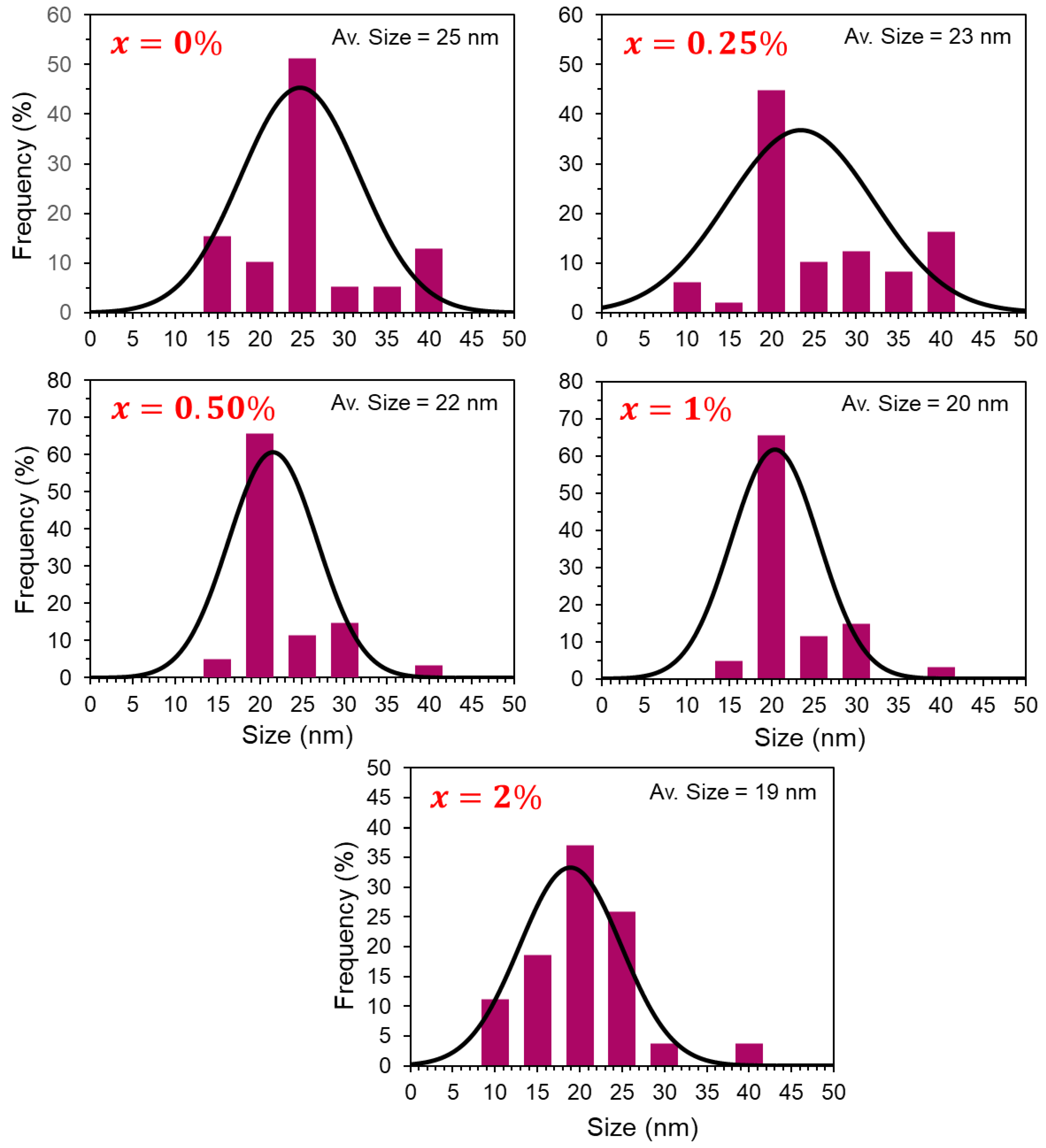






| x (%) | a = b (Å) | c (Å) | V (Å3) | DXRD (nm) | RBragg | χ2 (chi2) |
|---|---|---|---|---|---|---|
| 0.00 | 3.7856 | 9.5034 | 136.19 | 17.8 | 11.3 | 1.3 |
| 0.25 | 3.7853 | 9.5036 | 136.17 | 16.6 | 22.3 | 4.4 |
| 0.50 | 3.7846 | 9.5027 | 136.11 | 15.7 | 26.0 | 3.7 |
| 1.00 | 3.7851 | 9.4974 | 136.06 | 15.5 | 23.4 | 3.4 |
| 2.00 | 3.7856 | 9.5035 | 136.19 | 15.1 | 23.6 | 3.8 |
| Materials | Irradiation Source | Dyes | Degradation % | Duration (min) | Ref. |
|---|---|---|---|---|---|
| Ce–Sm co-doped TiO2 NPs | Xenon lamp (250 W, λ ≤ 400 nm) | RB | 98 | 30 | This work |
| Ce-doped TiO2 nanosheets | UV light (15 W, 365 nm) | RB | 95 | 120 | [54] |
| Mn-doped TiO2 | Tungten halogen lamp (300 W, 500 nm) | Malachite green (MG) | 96 | 105 | [55] |
| Cobalt-doped TiO2 | Fluorescent lamp (23 W, 6400 K, 1311 Lumens) | Methyl orange (MO) | 34.7 | 360 | [56] |
| Sm-doped TiO2 NPs | UV light (365 nm) | MO | 96 | 120 | [34] |
| Sm-doped TiO2 NPs | UV source (125 W Hg lamp) | RB | 95.7 | 120 | [57] |
| Er3+-doped TiO2 NPs | Xe lamp (400 W, λ > 450 nm) | Phenol | 75 | 180 | [58] |
| Nd-doped TiO2 NPs | Xe lamp (350 W, λ > 420 nm) | MO | 96.5 | 60 | [59] |
| La–Gd co-doped TiO2 hollow spheres | Xe lamp (300 W, PLS-SXE 300/300UV) | MO | 97 | 150 | [60] |
| Na-doped TiO2 | UV lamp (300 W, 365 nm) | Methylene blue (MB) | 92.5 | 60 | [61] |
| Gd-doped TiO2 films | UV source (500 W high-voltage mercury lamp) | MO | 41 | 28 | [62] |
| x (%) | Titanium Isopropoxide (mL) | Cerium Nitrate Hexahydrate (mg) | Samarium Nitrate Hexahydtrate (mg) |
|---|---|---|---|
| 0.00 | 12.9 | -- | -- |
| 0.25 | 12.9 | 49.3 | 50.5 |
| 0.50 | 12.9 | 98.6 | 101 |
| 1.00 | 12.9 | 197.2 | 202 |
| 2.00 | 12.9 | 394.4 | 404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slimani, Y.; Almessiere, M.A.; Mohamed, M.J.S.; Hannachi, E.; Caliskan, S.; Akhtar, S.; Baykal, A.; Gondal, M.A. Synthesis of Ce and Sm Co-Doped TiO2 Nanoparticles with Enhanced Photocatalytic Activity for Rhodamine B Dye Degradation. Catalysts 2023, 13, 668. https://doi.org/10.3390/catal13040668
Slimani Y, Almessiere MA, Mohamed MJS, Hannachi E, Caliskan S, Akhtar S, Baykal A, Gondal MA. Synthesis of Ce and Sm Co-Doped TiO2 Nanoparticles with Enhanced Photocatalytic Activity for Rhodamine B Dye Degradation. Catalysts. 2023; 13(4):668. https://doi.org/10.3390/catal13040668
Chicago/Turabian StyleSlimani, Yassine, Munirah A. Almessiere, Mohamed J. S. Mohamed, Essia Hannachi, Serkan Caliskan, Sultan Akhtar, Abdulhadi Baykal, and Mohammed A. Gondal. 2023. "Synthesis of Ce and Sm Co-Doped TiO2 Nanoparticles with Enhanced Photocatalytic Activity for Rhodamine B Dye Degradation" Catalysts 13, no. 4: 668. https://doi.org/10.3390/catal13040668
APA StyleSlimani, Y., Almessiere, M. A., Mohamed, M. J. S., Hannachi, E., Caliskan, S., Akhtar, S., Baykal, A., & Gondal, M. A. (2023). Synthesis of Ce and Sm Co-Doped TiO2 Nanoparticles with Enhanced Photocatalytic Activity for Rhodamine B Dye Degradation. Catalysts, 13(4), 668. https://doi.org/10.3390/catal13040668










