Analysis of the Effectiveness Factor in a Fixed-Bed Tubular Reactor System: Catalytic Dehydrogenation of Cyclohexanol
Abstract
1. Introduction
2. Results
2.1. Regarding the Gas Phase (External Conditions to the Catalyst Particles)
2.2. Regarding the Solid Phase (Conditions inside the Catalyst Particle)
- Molar flux density profile of each component.
- Heat flux density profile.
- Concentration profile of each component.
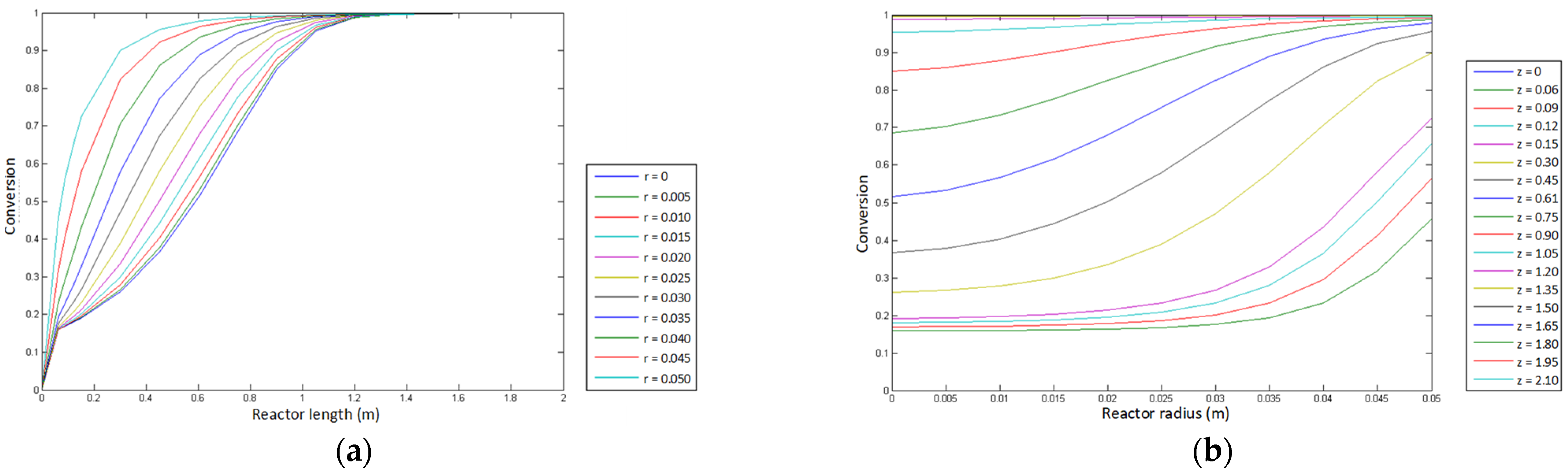
- Conversion profile of cyclohexanol.
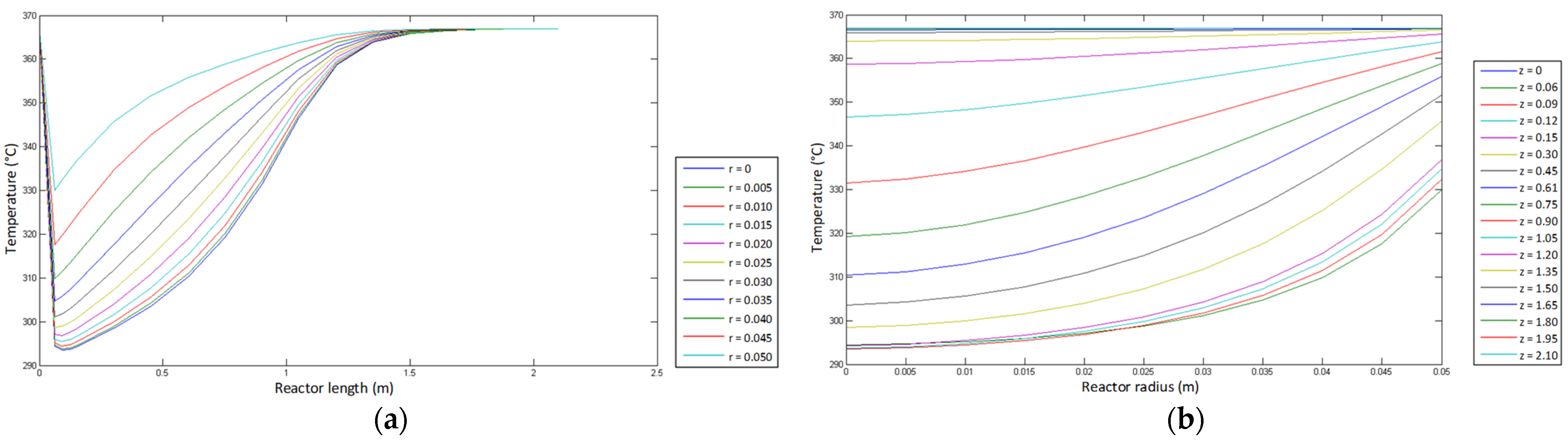
- Temperature profile.
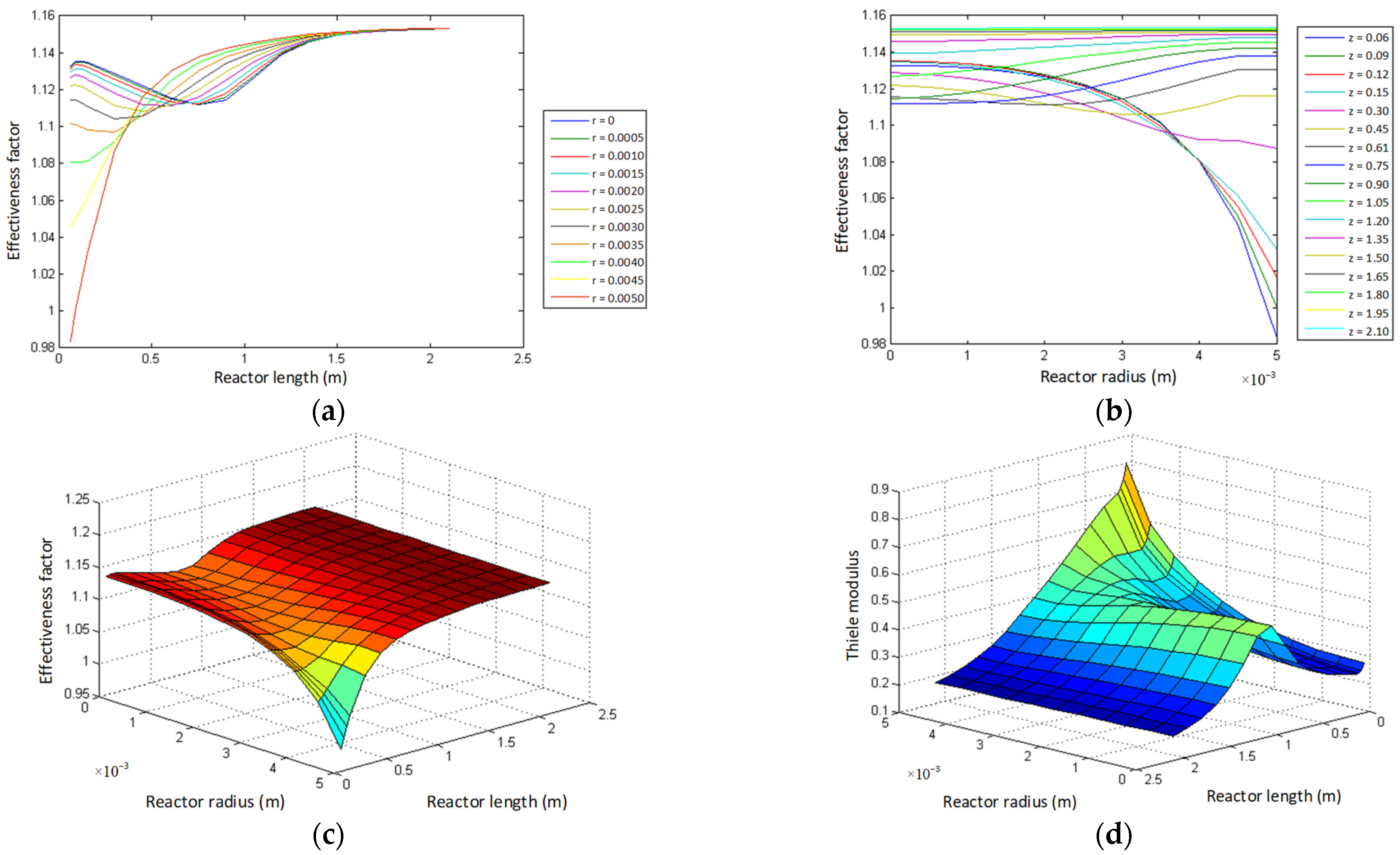
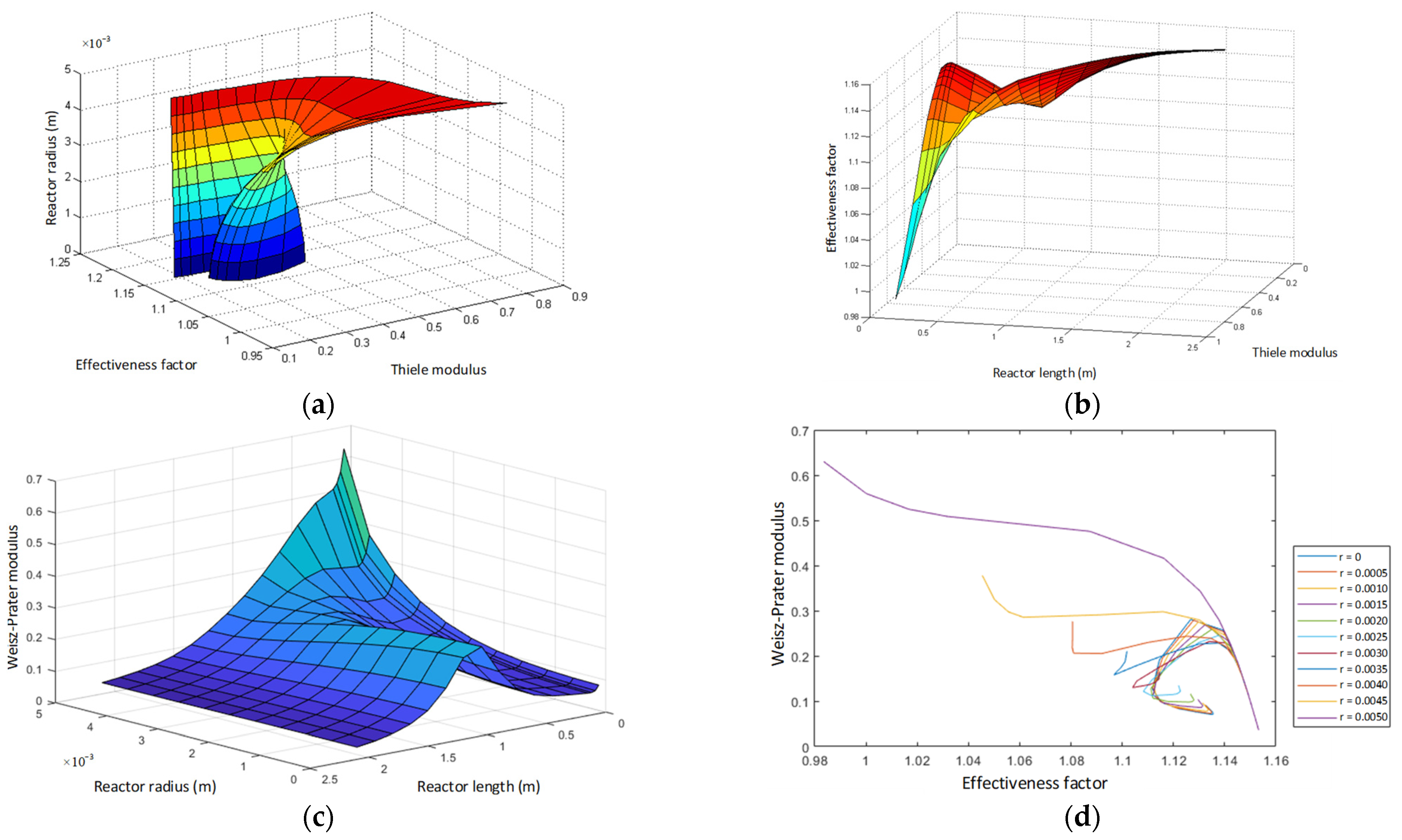
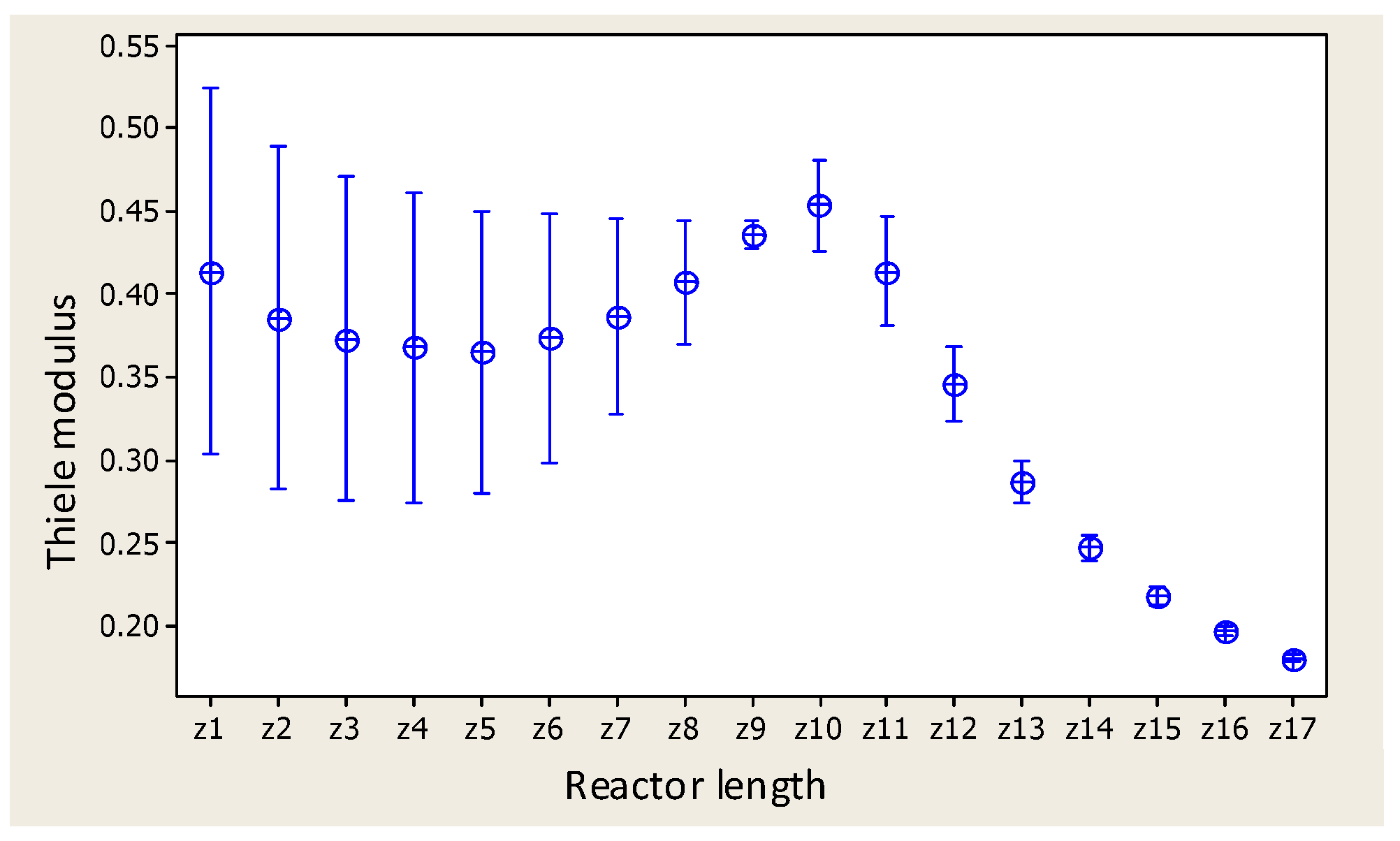
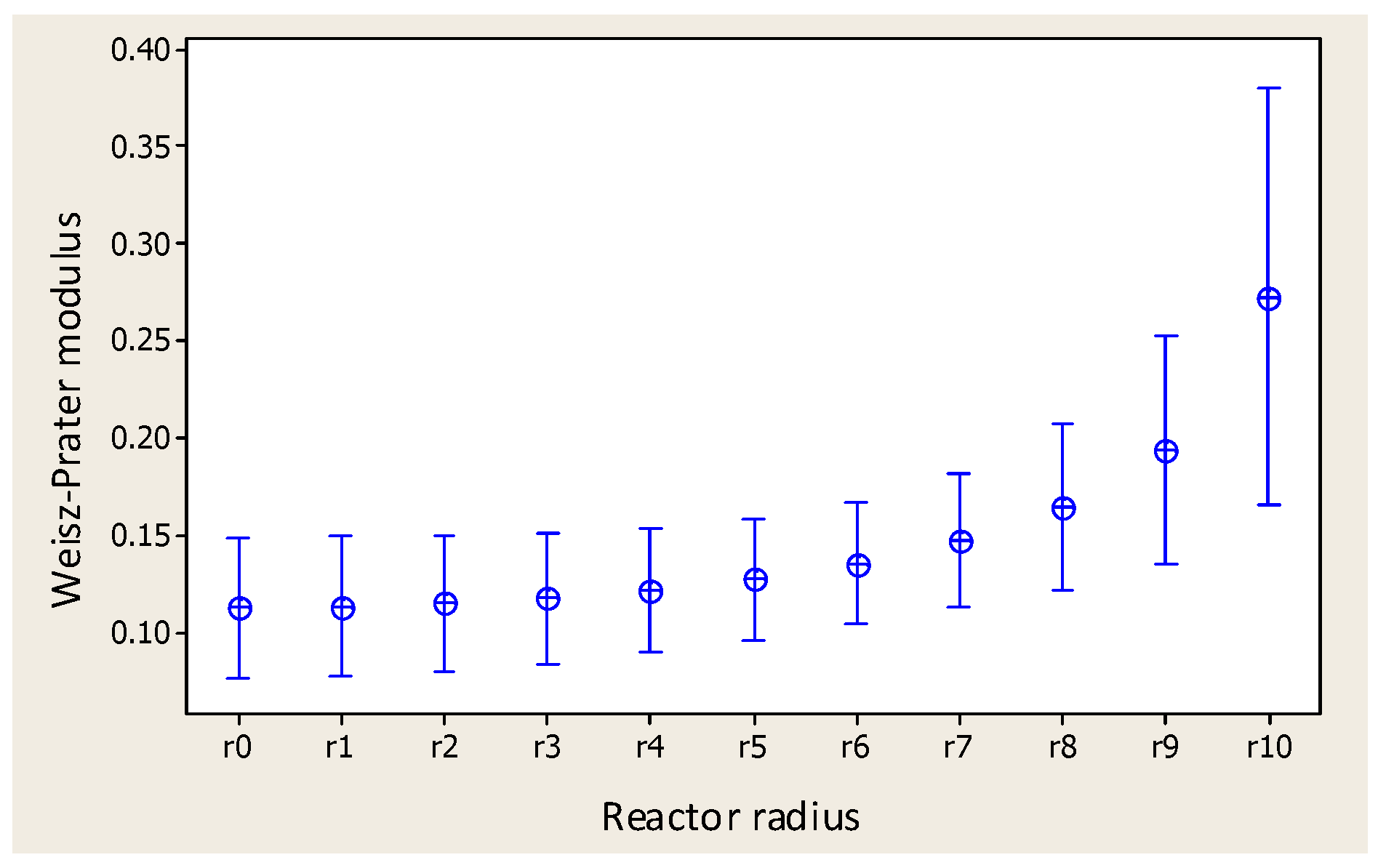
- Thiele modulus profile.
- Effectiveness factor profile.
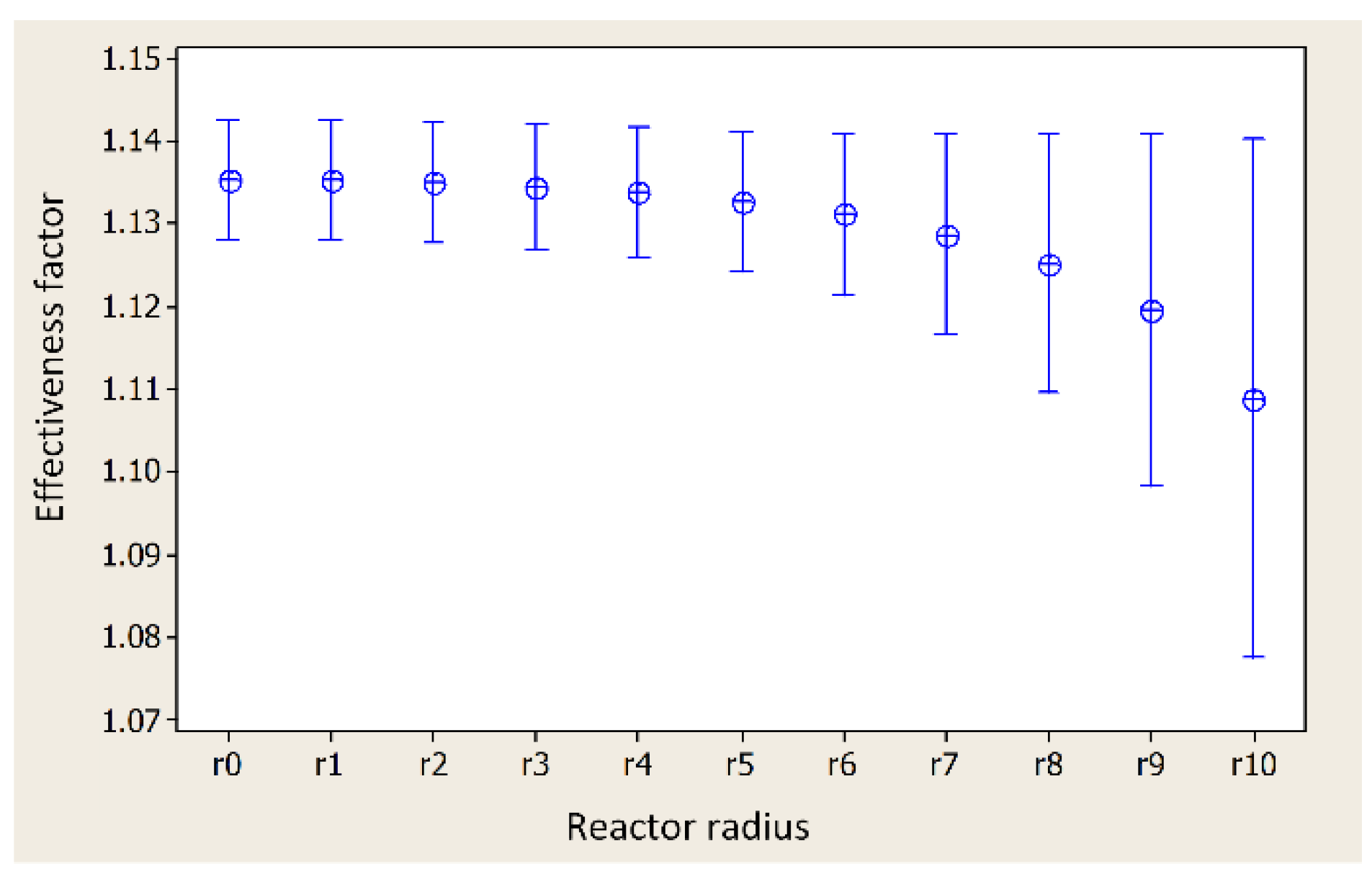
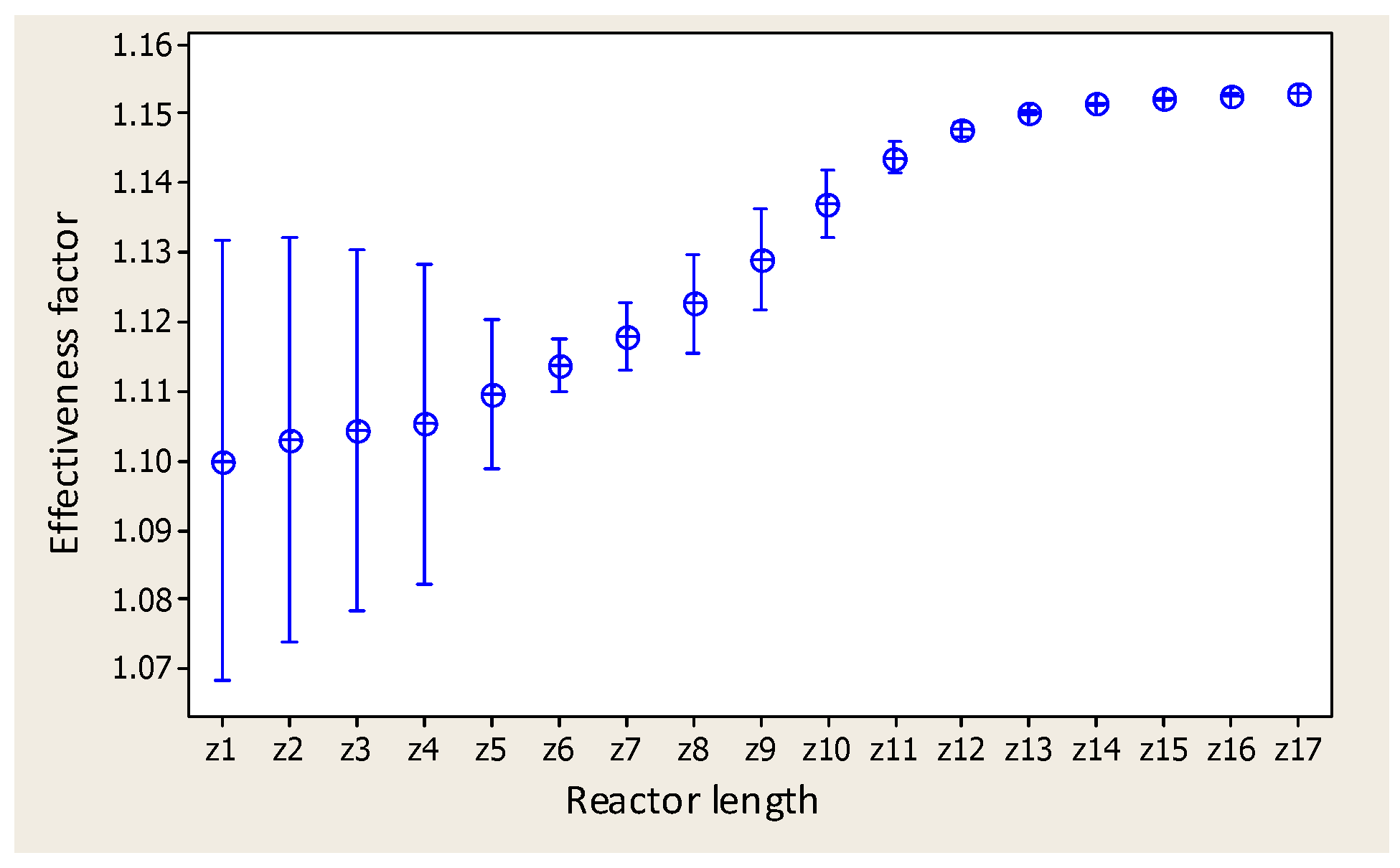
2.3. Variance Analysis
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| : | Reaction rate of cyclohexanol (kmol/kg cat h) |
| : | Partial pressure of cyclohexanol (atm) |
| : | Temperature (K) |
| : | Radial effective diffusion coefficient in the bed (m2/s) |
| : | Cyclohexanol concentration in the reactor (kmol/m3) |
| : | Reactor radius or particle radius (m) |
| : | Total radius of the reactor or total radius of the particle (m) |
| : | Superficial velocity of the reaction mixture (m/s) |
| : | Reactor length (m) |
| : | Total length of the reactor (m) |
| : | Specific heat of the gas mixture (kJ/kg K) |
| : | Temperature (°C) |
| : | Reaction enthalpy (kJ/kmol) |
| : | Cyclohexanol conversion |
| : | Radial effective conductivity in the bed (W/m K) |
| : | Reactor wall conductivity (W/m K) |
| : | Wall temperature (°C) |
| : | Radial mass transfer Peclet number |
| : | Radial heat transfer Peclet number |
| : | Particle diameter (m) |
| : | Initial concentration of cyclohexanol (kmol/m3) |
| : | Mass flow rate (kg/m2 h) |
| : | Biot number on the reactor wall |
| : | Convective heat transfer coefficient (W/m2 K) |
| Resulting parameters as a consequence of the change of variables | |
| DF: | Degrees of freedom |
| SS: | Sum of squares between treatments |
| MS: | Mean square of the factor |
| F: | Fisher’s probability value |
| : | Bed porosity |
| : | Density of the gas mixture (kg/m3) |
| : | Catalyst density (kg/m3) |
| : | Stoichiometric coefficient of cyclohexanol (in this case equal to −1) |
| : | Cyclohexanol molar flux density (kmol/m2 s) |
| : | Cyclohexanone molar flux density (kmol/m2 s) |
| : | Hydrogen molar flux density (kmol/m2 s) |
| : | Heat flux density (W/m2) |
| : | Partial pressure of cyclohexanol (atm) |
| : | Initial pressure of cyclohexanol (atm) |
| : | Total pressure in the reactor (atm) |
| : | Effective diffusion coefficient of cyclohexanol within the particle (m2/s) |
| : | Mole fraction of A in the gas mixture |
| : | Effective conductivity (conductivity of the support plus the conductivity of the gas inside the particle) (W/m K) |
Appendix A
| Reactor Length (m) | Reactor Radius (m) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.000 | 0.005 | 0.010 | 0.015 | 0.020 | 0.025 | 0.030 | 0.035 | 0.040 | 0.045 | 0.050 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.062129 | 0.1592739 | 0.159527 | 0.1601365 | 0.161307 | 0.1634776 | 0.167578 | 0.175717 | 0.193059 | 0.23222 | 0.317604 | 0.4574177 |
| 0.090879 | 0.1696498 | 0.170236 | 0.1715925 | 0.174089 | 0.1785163 | 0.186539 | 0.201841 | 0.232668 | 0.295521 | 0.41169 | 0.565697 |
| 0.121924 | 0.1804467 | 0.181473 | 0.1838163 | 0.188069 | 0.1954962 | 0.208729 | 0.233272 | 0.279959 | 0.365874 | 0.503068 | 0.6566091 |
| 0.152339 | 0.1915655 | 0.193099 | 0.1965869 | 0.20289 | 0.2138352 | 0.233092 | 0.267753 | 0.329882 | 0.434002 | 0.581347 | 0.7258942 |
| 0.300702 | 0.2610684 | 0.266399 | 0.2784496 | 0.299751 | 0.3347199 | 0.389545 | 0.470556 | 0.579469 | 0.705925 | 0.823724 | 0.8999694 |
| 0.452046 | 0.3673999 | 0.378711 | 0.4032108 | 0.443413 | 0.502145 | 0.580381 | 0.674293 | 0.773033 | 0.860621 | 0.923384 | 0.9563314 |
| 0.605338 | 0.5165364 | 0.53306 | 0.566545 | 0.616541 | 0.6807252 | 0.753522 | 0.826096 | 0.888751 | 0.93497 | 0.963766 | 0.9777223 |
| 0.752063 | 0.685826 | 0.702419 | 0.7338076 | 0.776589 | 0.825358 | 0.873549 | 0.915199 | 0.946859 | 0.968216 | 0.980996 | 0.9871432 |
| 0.901171 | 0.8492475 | 0.859636 | 0.878004 | 0.901065 | 0.9249256 | 0.946348 | 0.963503 | 0.976021 | 0.984466 | 0.989673 | 0.9922549 |
| 1.050096 | 0.9532047 | 0.956233 | 0.9614448 | 0.967867 | 0.9744603 | 0.980465 | 0.985481 | 0.989399 | 0.992272 | 0.99419 | 0.9951887 |
| 1.203608 | 0.9881097 | 0.988559 | 0.9893705 | 0.990445 | 0.9916595 | 0.9929 | 0.994077 | 0.995121 | 0.995981 | 0.996607 | 0.9969471 |
| 1.351685 | 0.995467 | 0.99556 | 0.9957348 | 0.995981 | 0.9962793 | 0.996611 | 0.996954 | 0.997284 | 0.997572 | 0.997791 | 0.9979105 |
| 1.504993 | 0.9976047 | 0.997634 | 0.9976913 | 0.997774 | 0.9978767 | 0.997995 | 0.998122 | 0.998246 | 0.998356 | 0.998439 | 0.9984842 |
| 1.651238 | 0.9984105 | 0.998424 | 0.9984504 | 0.998489 | 0.9985368 | 0.998592 | 0.998652 | 0.99871 | 0.99876 | 0.998798 | 0.9988183 |
| 1.802474 | 0.9988338 | 0.998841 | 0.9988552 | 0.998876 | 0.9989013 | 0.998931 | 0.998961 | 0.998991 | 0.999017 | 0.999035 | 0.9990451 |
| 1.952201 | 0.9990802 | 0.999085 | 0.999093 | 0.999105 | 0.9991201 | 0.999137 | 0.999155 | 0.999171 | 0.999185 | 0.999196 | 0.9992009 |
| 2.103393 | 0.9992425 | 0.999245 | 0.9992506 | 0.999258 | 0.9992674 | 0.999278 | 0.999288 | 0.999298 | 0.999307 | 0.999313 | 0.9993156 |
| Reactor Length (m) | Reactor Radius (m) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.000 | 0.005 | 0.010 | 0.015 | 0.020 | 0.025 | 0.030 | 0.035 | 0.040 | 0.045 | 0.050 | |
| 0 | 367 | 367 | 367 | 367 | 367 | 367 | 367 | 367 | 367 | 367 | 367 |
| 0.062129 | 294.4705 | 294.6832 | 295.1432 | 295.9058 | 297.0635 | 298.7549 | 301.1884 | 304.6918 | 309.8205 | 317.5967 | 330.05 |
| 0.090879 | 293.4396 | 293.741 | 294.3732 | 295.3842 | 296.8564 | 298.9188 | 301.7732 | 305.7479 | 311.399 | 319.6975 | 332.3498 |
| 0.121924 | 293.6254 | 293.974 | 294.6994 | 295.8486 | 297.5042 | 299.7981 | 302.9401 | 307.2699 | 313.3428 | 322.0475 | 334.733 |
| 0.152339 | 294.2377 | 294.6157 | 295.4013 | 296.6439 | 298.4309 | 300.9023 | 304.2794 | 308.9115 | 315.3397 | 324.348 | 336.945 |
| 0.300702 | 298.382 | 298.8875 | 299.9401 | 301.6073 | 304.0048 | 307.3039 | 311.741 | 317.608 | 325.1919 | 334.6268 | 345.74 |
| 0.452046 | 303.5895 | 304.25 | 305.6191 | 307.7697 | 310.8142 | 314.8907 | 320.1348 | 326.6176 | 334.2555 | 342.7504 | 351.6789 |
| 0.605338 | 310.376 | 311.203 | 312.8924 | 315.4896 | 319.0458 | 323.5847 | 329.059 | 335.3086 | 342.0588 | 348.9857 | 355.8269 |
| 0.752063 | 319.1652 | 320.1102 | 321.9948 | 324.7987 | 328.4648 | 332.8763 | 337.8481 | 343.1453 | 348.531 | 353.8164 | 358.8892 |
| 0.901171 | 331.5043 | 332.4045 | 334.14 | 336.6168 | 339.6898 | 343.1828 | 346.9125 | 350.7157 | 354.4678 | 358.0873 | 361.5312 |
| 1.050096 | 346.6607 | 347.2101 | 348.2485 | 349.6999 | 351.4642 | 353.4389 | 355.5317 | 357.6669 | 359.7872 | 361.852 | 363.8347 |
| 1.203608 | 358.7358 | 358.9271 | 359.295 | 359.82 | 360.4756 | 361.2322 | 362.0606 | 362.933 | 363.8251 | 364.7157 | 365.5881 |
| 1.351685 | 363.9793 | 364.0418 | 364.1636 | 364.34 | 364.5647 | 364.8292 | 365.1254 | 365.4438 | 365.776 | 366.1135 | 366.4495 |
| 1.504993 | 365.9176 | 365.9387 | 365.98 | 366.0403 | 366.1175 | 366.2096 | 366.3135 | 366.4268 | 366.5463 | 366.6694 | 366.7935 |
| 1.651238 | 366.5505 | 366.5589 | 366.5753 | 366.5993 | 366.6303 | 366.6674 | 366.7097 | 366.7561 | 366.8058 | 366.8575 | 366.9105 |
| 1.802474 | 366.7887 | 366.7925 | 366.7998 | 366.8106 | 366.8246 | 366.8415 | 366.8609 | 366.8824 | 366.9057 | 366.9303 | 366.9559 |
| 1.952201 | 366.8826 | 366.8846 | 366.8885 | 366.8943 | 366.9018 | 366.9108 | 366.9214 | 366.9332 | 366.946 | 366.9599 | 366.9745 |
| 2.103393 | 366.9261 | 366.9273 | 366.9296 | 366.9331 | 366.9377 | 366.9432 | 366.9499 | 366.957 | 366.9653 | 366.9739 | 366.9834 |
| Reactor Length (m) | Reactor Radius (m) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.000 | 0.005 | 0.010 | 0.015 | 0.020 | 0.025 | 0.030 | 0.035 | 0.040 | 0.045 | 0.050 | |
| 0.062129 | 0.2814893 | 0.284617 | 0.2914151 | 0.302809 | 0.3203628 | 0.346392 | 0.38399 | 0.436396 | 0.505734 | 0.601078 | 0.8003052 |
| 0.090879 | 0.2559924 | 0.259889 | 0.2681105 | 0.281403 | 0.3009643 | 0.328387 | 0.365279 | 0.412429 | 0.470954 | 0.555858 | 0.7475899 |
| 0.121924 | 0.2494981 | 0.253635 | 0.2622567 | 0.275962 | 0.2956199 | 0.322152 | 0.35604 | 0.397252 | 0.448979 | 0.530759 | 0.718275 |
| 0.152339 | 0.2492964 | 0.253473 | 0.2621115 | 0.27567 | 0.2947189 | 0.319654 | 0.350425 | 0.387498 | 0.436553 | 0.518604 | 0.7020302 |
| 0.300702 | 0.2594392 | 0.263301 | 0.2709921 | 0.28247 | 0.2977294 | 0.317237 | 0.342913 | 0.379231 | 0.433529 | 0.516189 | 0.6613653 |
| 0.452046 | 0.2724388 | 0.275957 | 0.2829863 | 0.293832 | 0.3093076 | 0.331052 | 0.361465 | 0.40274 | 0.454933 | 0.516589 | 0.6104053 |
| 0.605338 | 0.2947658 | 0.298654 | 0.3067328 | 0.319659 | 0.3383641 | 0.363657 | 0.395299 | 0.430769 | 0.465273 | 0.497164 | 0.551177 |
| 0.752063 | 0.3369915 | 0.341658 | 0.35125 | 0.365814 | 0.3849488 | 0.40714 | 0.429359 | 0.4475507 | 0.458889 | 0.466996 | 0.4960345 |
| 0.901171 | 0.4145082 | 0.4182 | 0.4252674 | 0.434381 | 0.4435528 | 0.450286 | 0.452221 | 0.448102 | 0.439078 | 0.431497 | 0.4439124 |
| 1.050096 | 0.4992492 | 0.497355 | 0.4936709 | 0.48736 | 0.4777709 | 0.464512 | 0.447687 | 0.42816 | 0.408241 | 0.393386 | 0.3950071 |
| 1.203608 | 0.475071 | 0.470923 | 0.4630915 | 0.451737 | 0.4372428 | 0.420092 | 0.400972 | 0.380978 | 0.362153 | 0.348193 | 0.3448642 |
| 1.351685 | 0.3865429 | 0.383879 | 0.3787143 | 0.371165 | 0.3614489 | 0.349884 | 0.336989 | 0.323639 | 0.311289 | 0.302105 | 0.298753 |
| 1.504993 | 0.3113573 | 0.309793 | 0.3067268 | 0.302215 | 0.2963784 | 0.28944 | 0.281758 | 0.273938 | 0.266871 | 0.261675 | 0.2594809 |
| 1.651238 | 0.2625416 | 0.261549 | 0.2595896 | 0.256718 | 0.2530331 | 0.248696 | 0.243974 | 0.239285 | 0.235153 | 0.232162 | 0.2308225 |
| 1.802474 | 0.2279913 | 0.227327 | 0.2260368 | 0.224155 | 0.2217592 | 0.218995 | 0.216057 | 0.21321 | 0.210754 | 0.209008 | 0.2082106 |
| 1.952201 | 0.2036722 | 0.20322 | 0.2023219 | 0.201037 | 0.1994339 | 0.197602 | 0.195707 | 0.193892 | 0.192369 | 0.191288 | 0.1908073 |
| 2.103393 | 0.1853874 | 0.185058 | 0.184433 | 0.183546 | 0.1824422 | 0.181201 | 0.179935 | 0.17875 | 0.177769 | 0.177077 | 0.1767737 |
| Reactor Length (m) | Reactor Radius (m) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.000 | 0.005 | 0.010 | 0.015 | 0.020 | 0.025 | 0.030 | 0.035 | 0.040 | 0.045 | 0.050 | |
| 0.062129 | 1.132799 | 1.132335 | 1.131308 | 1.129526 | 1.126631 | 1.12198 | 1.114412 | 1.101825 | 1.080621 | 1.045399 | 0.9835003 |
| 0.090879 | 1.135408 | 1.134825 | 1.133562 | 1.131417 | 1.128018 | 1.122706 | 1.114352 | 1.101137 | 1.080598 | 1.050146 | 1.000145 |
| 0.121924 | 1.135482 | 1.134825 | 1.133408 | 1.131017 | 1.127264 | 1.121493 | 1.112675 | 1.099428 | 1.08052 | 1.055593 | 1.016718 |
| 0.152339 | 1.13472 | 1.134003 | 1.13246 | 1.129869 | 1.125835 | 1.119733 | 1.110696 | 1.097842 | 1.081029 | 1.06139 | 1.031786 |
| 0.300702 | 1.128806 | 1.127833 | 1.125787 | 1.122498 | 1.117765 | 1.111505 | 1.104079 | 1.096785 | 1.092105 | 1.091813 | 1.087333 |
| 0.452046 | 1.121899 | 1.120851 | 1.118768 | 1.115727 | 1.112003 | 1.108261 | 1.10572 | 1.105991 | 1.110013 | 1.115956 | 1.116296 |
| 0.605338 | 1.1154 | 1.114695 | 1.113454 | 1.112057 | 1.111151 | 1.111615 | 1.114257 | 1.119184 | 1.12524 | 1.130188 | 1.130403 |
| 0.752063 | 1.111761 | 1.11188 | 1.112319 | 1.113512 | 1.115919 | 1.119776 | 1.124788 | 1.13011 | 1.13473 | 1.13777 | 1.137729 |
| 0.901171 | 1.114561 | 1.115708 | 1.117916 | 1.121178 | 1.125261 | 1.129713 | 1.133995 | 1.137685 | 1.140549 | 1.14235 | 1.142343 |
| 1.050096 | 1.126752 | 1.127855 | 1.129788 | 1.132297 | 1.135056 | 1.137785 | 1.140292 | 1.142466 | 1.144217 | 1.14537 | 1.145507 |
| 1.203608 | 1.139359 | 1.139747 | 1.140455 | 1.141418 | 1.142548 | 1.143763 | 1.144984 | 1.146135 | 1.14712 | 1.147805 | 1.147995 |
| 1.351685 | 1.145953 | 1.146092 | 1.146357 | 1.146735 | 1.147204 | 1.147738 | 1.148305 | 1.148861 | 1.14935 | 1.149701 | 1.14983 |
| 1.504993 | 1.149352 | 1.149412 | 1.149527 | 1.149695 | 1.149906 | 1.150152 | 1.150416 | 1.150676 | 1.150904 | 1.151068 | 1.151137 |
| 1.651238 | 1.151039 | 1.15107 | 1.151131 | 1.151218 | 1.151329 | 1.151457 | 1.151594 | 1.151726 | 1.151841 | 1.151923 | 1.15196 |
| 1.802474 | 1.152034 | 1.152052 | 1.152086 | 1.152135 | 1.152197 | 1.152268 | 1.152342 | 1.152413 | 1.152473 | 1.152516 | 1.152535 |
| 1.952201 | 1.152643 | 1.152653 | 1.152674 | 1.152704 | 1.152741 | 1.152783 | 1.152826 | 1.152867 | 1.152901 | 1.152925 | 1.152935 |
| 2.103393 | 1.153052 | 1.153059 | 1.153072 | 1.153091 | 1.153114 | 1.15314 | 1.153166 | 1.153191 | 1.153211 | 1.153225 | 1.153231 |
References
- Gut, G.; Jaeger, R. Kinetics of the Catalytic Dehydrogenation of Cyclohexanol to Cyclohexanone on a Zinc Oxide Catalyst in a Gradientless Reactor. Chem. Eng. Sci. 1982, 37, 319–326. [Google Scholar] [CrossRef]
- Romero, A.; Santos, A.; Escrig, D.; Simón, E. Comparative Dehydrogenation of Cyclohexanol to Cyclohexanone with Commercial Copper Catalysts: Catalytic Activity and Impurities Formed. Appl. Catal. A Gen. 2011, 392, 19–27. [Google Scholar] [CrossRef]
- Lorenzo, D.; Santos, A.; Simón, E.; Romero, A. Kinetics of Alkali-Catalyzed Condensation of Impurities in the Cyclohexanone Purification Process. Ind. Eng. Chem. Res. 2013, 52, 15780–15788. [Google Scholar] [CrossRef]
- Morita, M.; Kawashima, E.; Nomura, K.; Sumi, M.; Hirai, E. Catalytic Dehydrogenation of Cyclohexanol in Gas Phase. Kanazawa Daigaku Kogakubu Kiyo 1970, 5, 389–397. [Google Scholar]
- Athappan, R.; Srivastava, R.D. Kinetics of Parallel Dehydrogenation and Dehydration of Cyclohexanol on NiO-Al2O3 Catalyst Systems. AIChE J. 1980, 26, 517–521. [Google Scholar] [CrossRef]
- Orizarsky, I.V.; Petrov, L.A.; Petrova, V.M. Kinetics of Dehydrogenation of Cyclohexanol to Cyclohexanone on a. Bag Type Copper-Alloy Catalyst. React. Kinet. Catal. Lett. 1981, 17, 427–431. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Li, W.; Guan, N.-J.; Tag, K.-Y. Kinetics Study on Catalytic Dehydrogenation of Cyclohexanol. Chem. J. Chin. Univ. 2002, 23, 861. [Google Scholar]
- Patil, K.N.; Manikanta, P.; Nikam, R.R.; Srinivasappa, P.M.; Jadhav, A.H.; Aytam, H.P.; Rama Rao, K.S.; Nagaraja, B.M. Effect of Precipitating Agents on Activity of Co-Precipitated Cu–MgO Catalysts towards Selective Furfural Hydrogenation and Cyclohexanol Dehydrogenation Reactions. Results Eng. 2023, 17, 100851. [Google Scholar] [CrossRef]
- Joseph, H.M.; Sugunan, S. Copper Loaded HPfCNT/TiO2 Ternary Nanohybrids as Green and Robust Catalysts for Dehydrogenation of Cyclohexanol under Visible Light. Mater. Sci. Semicond. Process. 2021, 129, 105784. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Borrachero, C.; Molina, G.; Romero, A. Simulación de Reactores de Lecho Fijo Por El Modelo de Dos Dimensiones: I-Reacciones Simples. An. De Química 1991, 88, 180–190. [Google Scholar]
- Stull, D.R.; Westrum, E.F.; Sinke, G.C. The Chemical Thermodynamics of Organic Compounds; J. Wiley: New York, NY, USA, 1969. [Google Scholar]
- Cubberley, A.H.; Mueller, M.B. Equilibrium Studies on the Dehydrogenation of Primary and Secondary Alcohols. II. Cyclohexanols. J. Am. Chem. Soc. 1947, 69, 1535–1536. [Google Scholar] [CrossRef]
- Carrasco Venegas, L.A. Modelamiento de Los Fenómenos de Transporte, 1st ed.; Macro EIRL: Lima, Peru, 2018. [Google Scholar]
- Davis, M.E.; Davis, R.J. Effects of Transport Limitations on Rates of Solid-Catalyzed Reactions. In Fundamentals of Chemical Reaction Engineering; McGraw-Hill: New York, NY, USA, 2003; pp. 184–239. [Google Scholar]
- Azimi, A.; Azimi, M. Investigation on Reaction Diffusion Process Inside a Porous Bio-Catalyst Using DTM. J. Bioequivalence Bioavailab. 2015, 7, 123–126. [Google Scholar] [CrossRef]
- Fogler, H.S. Elementos de Ingeniería de Las Reacciones Químicas, 3rd ed.; Pearson: Mexico City, Mexico, 2006. [Google Scholar]
- Kimura, S.; Nakagawa, J.; Tone, S.; Otake, T.; Nakagawa, J. Non-Isothermal Behavior of Gas-Solid Reactions Based on the Volume Reaction Model. J. Chem. Eng. Jpn. 1982, 15, 115–121. [Google Scholar] [CrossRef]
- de Silva, E.C.L.; Bamunusingha, B.A.N.N.; Gunasekera, M.Y. Heterogeneous Kinetic Study for Esterification of Acetic Acid with Ethanol. J. Inst. Eng. 2014, 47, 15. [Google Scholar] [CrossRef]
- Iordanidis, A.A. Mathematical Modeling of Catalytic Fixed Bed Reactors; Twente University Press: Enschede, The Netherlands, 2002. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; J. Wiley: New York, NY, USA, 1998; ISBN 978-0-471-25424-9. [Google Scholar]
- Zhu, L.T.; Ma, W.Y.; Luo, Z.H. Influence of Distributed Pore Size and Porosity on MTO Catalyst Particle Performance: Modeling and Simulation. Chem. Eng. Res. Des. 2018, 137, 141–153. [Google Scholar] [CrossRef]
- Sun, Y.P.; Liu, S.B.; Keith, S. Approximate Solution for the Nonlinear Model of Diffusion and Reaction in Porous Catalysts by the Decomposition Method. Chem. Eng. J. 2004, 102, 1–10. [Google Scholar] [CrossRef]
- Cutlip, M.B.; Shacham, M. Problem Solving in Chemical and Biochemical Engineering with POLYMATH, Excel and MATLAB, 2nd ed.; Prentice Hall: Boston, MA, USA, 2008. [Google Scholar]

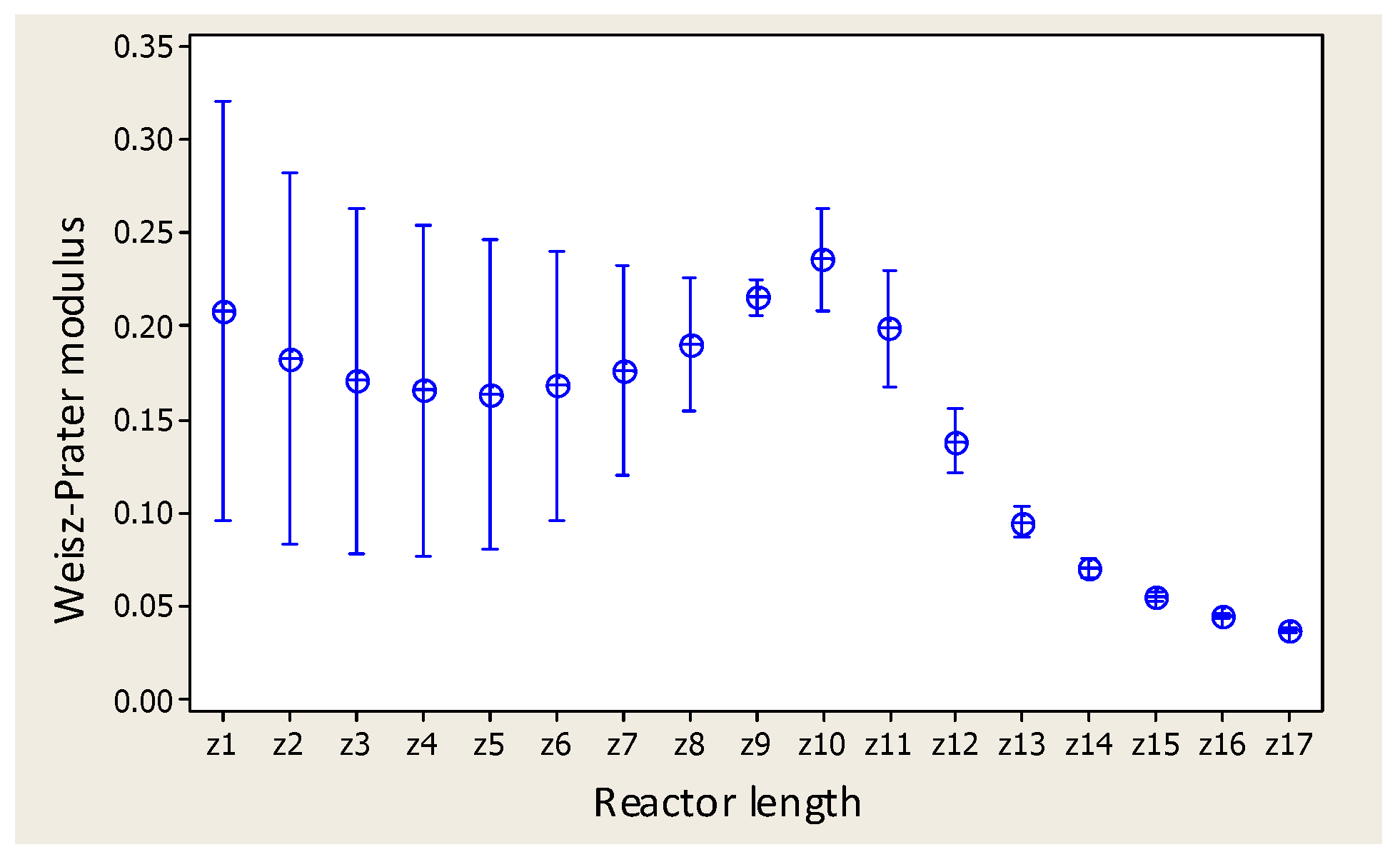
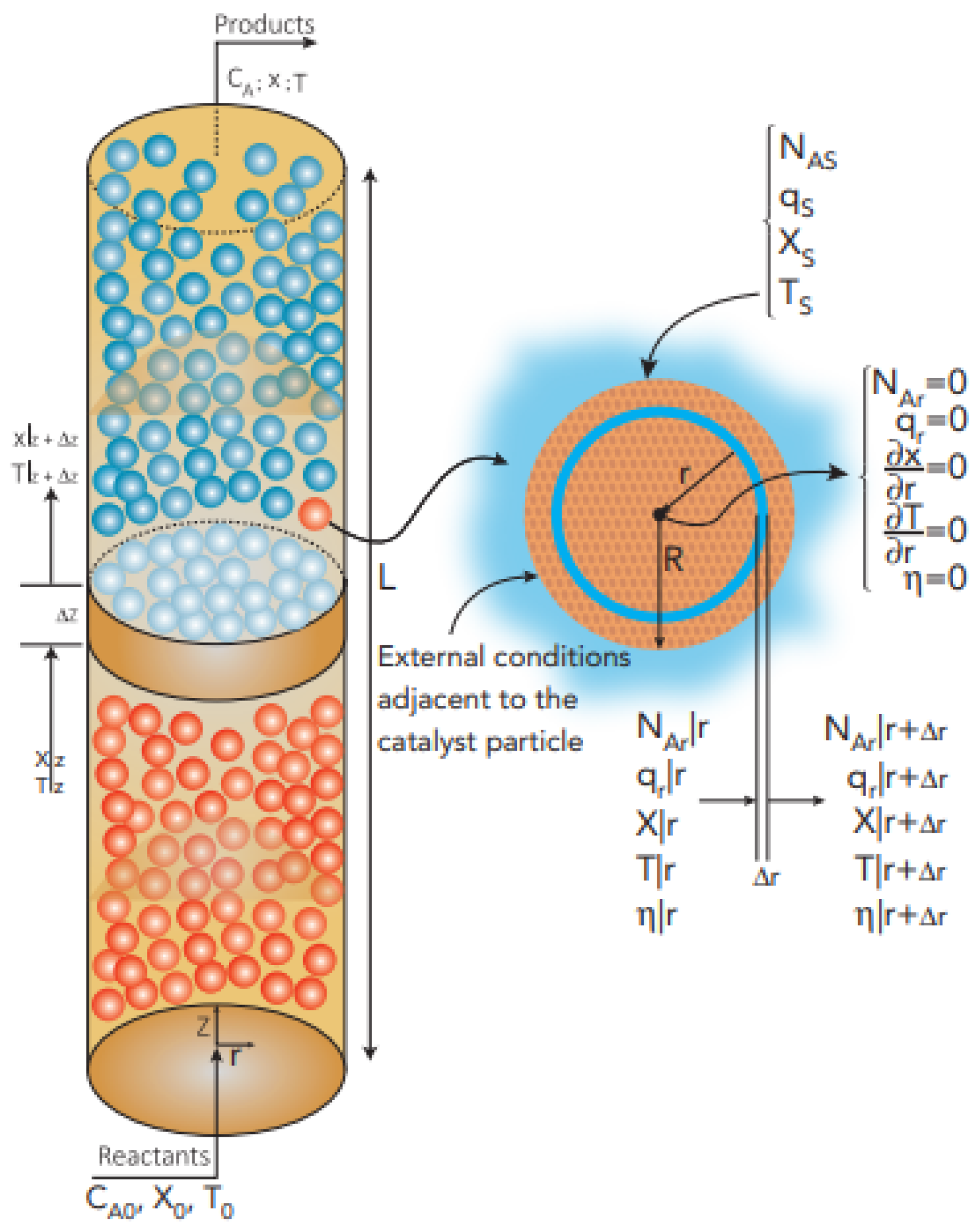
| Food composition | Catalyst and catalytic bed (ZnO spheres) | ||
| Flow properties and fluid phase | Mass flow velocity | ||
| Reaction enthalpy | Feed temperature | ||
| Wall temperature | Tube diameter | ||
| Tube length | Biot number in the wall | ||
| Radial heat transfer Peclet number | Radial mass transfer Peclet number | ||
| Effective diffusivity | * | Radial effective conductivity | * |
| Source | DF | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| Factor | 10 | 0.011734 | 0.001173 | 1.51 | 0.138 |
| Error | 176 | 0.136401 | 0.000775 | ||
| Total | 186 | 0.148135 |
| Source | DF | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| Factor | 16 | 0.074023 | 0.004626 | 10.61 | 0.000 |
| Error | 170 | 0.074112 | 0.000436 | ||
| Total | 186 | 0.148135 |
| Source | DF | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| Factor | 10 | 0.4007 | 0.0401 | 3.13 | 0.001 |
| Error | 176 | 2.2526 | 0.0128 | ||
| Total | 186 | 2.6533 |
| Source | DF | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| Factor | 16 | 1.29019 | 0.08064 | 10.06 | 0.000 |
| Error | 170 | 1.36314 | 0.00802 | ||
| Total | 186 | 2.65333 |
| Source | DF | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| Factor | 10 | 0.40216 | 0.04022 | 4.51 | 0.000 |
| Error | 176 | 1.56792 | 0.00891 | ||
| Total | 186 | 1.97008 |
| Source | DF | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| Factor | 16 | 0.70245 | 0.04390 | 5.89 | 0.000 |
| Error | 170 | 1.26762 | 0.00746 | ||
| Total | 186 | 1.97007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Venegas, L.A.; González-Fernández, J.V.; Castañeda-Pérez, L.G.; Medina-Collana, J.T.; Palomino-Hernández, G.; Martínez-Hilario, D.G.; Trujillo-Pérez, S.A. Analysis of the Effectiveness Factor in a Fixed-Bed Tubular Reactor System: Catalytic Dehydrogenation of Cyclohexanol. Catalysts 2023, 13, 585. https://doi.org/10.3390/catal13030585
Carrasco-Venegas LA, González-Fernández JV, Castañeda-Pérez LG, Medina-Collana JT, Palomino-Hernández G, Martínez-Hilario DG, Trujillo-Pérez SA. Analysis of the Effectiveness Factor in a Fixed-Bed Tubular Reactor System: Catalytic Dehydrogenation of Cyclohexanol. Catalysts. 2023; 13(3):585. https://doi.org/10.3390/catal13030585
Chicago/Turabian StyleCarrasco-Venegas, Luis Américo, José Vulfrano González-Fernández, Luz Genara Castañeda-Pérez, Juan Taumaturgo Medina-Collana, Guido Palomino-Hernández, Daril Giovanni Martínez-Hilario, and Salvador Apolinar Trujillo-Pérez. 2023. "Analysis of the Effectiveness Factor in a Fixed-Bed Tubular Reactor System: Catalytic Dehydrogenation of Cyclohexanol" Catalysts 13, no. 3: 585. https://doi.org/10.3390/catal13030585
APA StyleCarrasco-Venegas, L. A., González-Fernández, J. V., Castañeda-Pérez, L. G., Medina-Collana, J. T., Palomino-Hernández, G., Martínez-Hilario, D. G., & Trujillo-Pérez, S. A. (2023). Analysis of the Effectiveness Factor in a Fixed-Bed Tubular Reactor System: Catalytic Dehydrogenation of Cyclohexanol. Catalysts, 13(3), 585. https://doi.org/10.3390/catal13030585








