Abstract
Endocrine-disrupting chemicals (EDCs) in the aquatic environment have garnered a lot of attention during the past few years. Due to their toxic behavior, which interferes with endocrine functions in both humans and aquatic species, these types of compounds have been recognized as major polluting agents in wastewater effluents. Therefore, the development of efficient and sustainable removal methods for these emerging contaminants is essential. Photocatalytic removal of emerging contaminants using silver carbonate (Ag2CO3)-based photocatalyst is a promising process due to the unique characteristics of this catalyst, such as absorption of a larger fraction of the solar spectrum, wide band gap, non-toxicity, and low cost. The photocatalytic performance of Ag2CO3 has recently been improved through the doping of elements and optimization variation of operational parameters resulting in decreasing the rate of electron–hole pair recombination and an increase in the semiconductor’s excitation state efficiency, which enables the degradation of contaminants under UV or visible light exposure. This review summarized some of the relevant investigations related to Ag2CO3-based photocatalytic materials for EDC removal from water. The inclusion of Ag2CO3-based photocatalytic materials in the water recovery procedure suggests that the creation of a cutting-edge protocol is essential for successfully eliminating EDCs from the ecosystem.
1. Introduction
Water pollution is a global challenge that has increased in both developed and developing countries; it occurs when a body of water becomes contaminated by chemicals, toxic and microorganisms. Typically, this was caused by inappropriate sewage disposal, chemical waste dumping, fertilizer run-off, and rapid urban growth. In addition, a wide range of contaminants from the agricultural, industrial, pharmaceutical, and plastic industries, as well as endocrine disruptors, have been developed and are continuously being released, whether knowingly or unconsciously, into water sources. Many of these compounds are carcinogenic and difficult to biodegrade, posing a short- and long-term threat to our society and the environment.
Endocrine-disrupting compounds (EDCs) are emerging environmental micropollutants that cause serious environmental pollution and consequently affect wildlife and public health concerns due to their hormone-like behaviors [1,2,3]. EDCs are described as substances that prevent the body’s natural hormones from producing, secreting, transporting, binding, acting, or eliminating the hormones necessary for homeostasis, reproduction, development, and/or behavior [4,5,6]. Moreover, EDCs are xenobiotic substances that are typically found in manufactured goods, including toothpaste, cosmetics, plastic bottles, toys for children, and polyvinyl chloride pipes [1]. Disruption of the endocrine system in wildlife and humans has recently garnered considerable interest across the globe as a result of the realization that the environment is contaminated with a range of endocrine-disrupting chemicals (EDCs) that induce hormonal imbalance activities [7].
Various chemical, physical, and biological treatment processes are currently being proposed for the removal of EDCs. However, conventional water and wastewater treatment plants—for instance, sedimentation/filtration, flocculation/coagulation, adsorption by powdered activated carbon (PAC), granular activated carbon (GAC), chemical oxidation, and chlorination—are ineffective and non-destructive for many EDCs, including Bisphenol A (BPA) and chlorophenols [8,9]. The complete elimination of the EDCs remains a difficulty despite the enormous efforts of researchers to advance the currently used conventional treatment technologies, as highlighted above. As a result, various research from the past few years that was reviewed by a number of authors investigated and mostly concentrated on the alternative treatment approach for the removal of EDC micropollutants from diverse water sources.
In recent times, advanced oxidation processes (AOPs) have emerged as a novel and proficient approach for the degradation of non-biodegradable organic waste as well as inorganic pollutants in wastewater [10]. Four different methods are employed with AOPs to generate hydroxyl radicals and treat wastewater, which includes (i) ozone treatment, (ii) electrochemical processes, (iii) direct decomposition of water, and (iv) photocatalysis [11,12]. Heterogeneous photocatalytic processes are one of the many AOPs that are efficient at removing a variety of pollutants at room temperature and pressure without creating potentially harmful intermediates. Thousands of photocatalytic processes have been studied annually by different synthesis approaches, and silver carbonate (Ag2CO3)-based photocatalysts have also been extensively studied for the destruction of EDCs. To date, scientists have published many papers that discuss Ag2CO3-based photocatalysts for degrading EDCs. The entire number of publications about photocatalysts during the past ten years is shown in Figure 1.
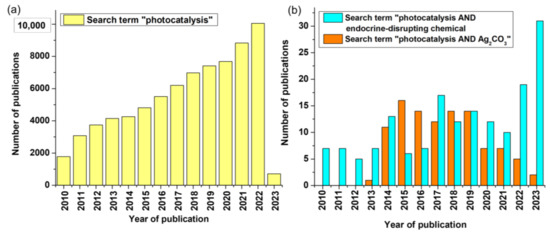
Figure 1.
(a,b) Annual publications of photocatalysis (Scopus search system accessed in December 2022; search terms “photocatalysis”, “photocatalysis AND endocrine disrupting chemical”, and “photocatalysis AND Ag2CO3” from 2010 to 2023).
Recent studies found that most Ag-based semiconductors could exhibit strong visible light absorption and obvious visible light catalytic activity due to their narrow band gap energy [13,14]. This paper mainly demonstrated the recent progress in synthesizing and developing typical Ag-based photocatalysts (including Ag-based heterojunctions, Ag-based solid solutions, loaded Ag-based semiconductors, carbon materials coupled with Ag-based semiconductors, etc.) and their applications in decomposing EDCs, particularly phenol and bisphenol A (BPA). Moreover, the effect of key operational parameters on the Ag2CO3-based photocatalytic degradation of EDCs is thus presented. Finally, the future prospects together with the challenges for the Ag2CO3-based photocatalysis on EDC degradation are summarized and discussed.
2. Photocatalysis for Water Purification
Heterogeneous photocatalysis was first discovered by Fujishima et al. in 1972 during the splitting of H2O in a photoelectrochemical cell comprising a monocrystal-line TiO2 electrode [15]. Since then, a lot of studies have been conducted to comprehend and investigate the basic mechanism and parameters so that this technique can be applied to wastewater treatment. In the realm of decomposing organic pollutants in water, photocatalysis is a “green chemistry” approach that has garnered significant interest [16]. Photocatalysis possesses distinctive advantages in the degradation of these pollutants, such as rapid degradation rate, high mineralization efficiency, and low toxicity, and the mechanism of photocatalysis is primarily described by the semiconductors’ capability to generate charge carriers under light irradiation followed by the generation of free radicals such as OH−, which leads to further reactions, eventually forming CO2 and H2O [12].
The two most extensively researched photocatalysts out of the many available ones are TiO2 and ZnO. Both catalysts have certain evident benefits, such as their significant power oxidizing capacity for the breakdown of organic contaminants, photo-stability, cheap operating costs, and environmental friendliness [17,18,19]. Although these two attractive photocatalysts have high photocatalytic activity compared to other semiconductors, the electron–hole recombination, as well as low photoactivity under visible light irradiation, were the factors that limited their large-scale use in wastewater treatment. The band gap of TiO2 and ZnO (3.2 eV and 3.37 eV) only enables it to absorb the ultraviolet region which makes up 3–5% of sunlight at a wavelength of 380 nm. To prolong the absorption of TiO2 and ZnO from UV light to visible light range, various alternative methods have been developed, such as coupling with small band gap semiconductors, transition metal addition, and non-metal element doping [20,21,22,23].
Very recently, Ag-based semiconductors such as Ag3PO4 [24,25,26], Ag/AgCl [27,28], Ag/AgBr [29,30,31], Ag/Ag2O [32], Ag2S [33], AgVO3 [34,35], Ag/CNTs-TiO2 [36], and silver halides AgX [37,38] have received a lot of interest because of their distinctive ability to absorb visible light and a few other intriguing photocatalytic qualities. However, in real-world applications, Ag-based semiconductor photocatalysts frequently have low stability because, in the absence of a sacrificial reagent, they are easily destroyed by photocorrosion. Additionally, their poor stability causes difficulties in photocatalyst recycling, which increases the operating cost due to the high price of Ag noble metal. Therefore, one of the most challenging issues in the field of photocatalysis research today is to figure out ways to improve the activity and stability of Ag-based semiconductors.
2.1. Endocrine-Disrupting Chemicals (EDCs) Classification
EDCs are a kind of environmental pollutant that relate to the mixture of chemical agents that interfere with the human body’s endocrine system; for instance, metabolic, immune systems, hormones, etc. As the EDC disruptors come from a variety of sources, people and aquatic life are regularly exposed through the air, food, and water. Additionally, EDCs can also enter the human body through the skin. Based on the characteristic functional groups in the target EDC substrates and their application fields, EDCs can be classified into a few types: (1) pesticides and herbicides (aromatic rings and heterocyclic rings containing chlorine substituents) [39,40], (2) hormones and steroids containing phenolic hydroxyl/carbonyl groups (i.e., estron, 17β-estradiol, estriol, and 17α-ethyl estradiol [41]), (3) pharmaceuticals and personal care product [42,43], (4) plasticizers (bisphenols, polystyrene (PS) and phenol-formaldehyde resin (PF) [44,45]), and (5) other organic pollutants (Figure 2).
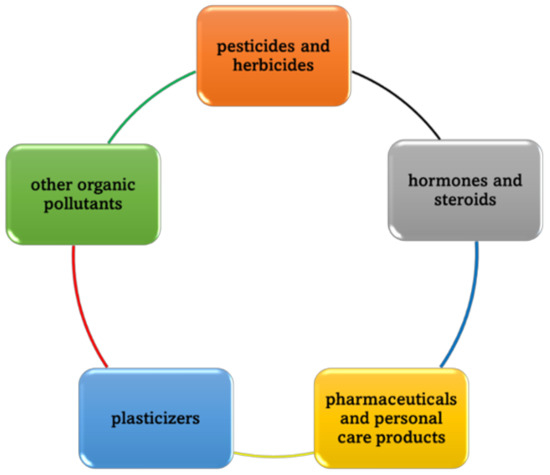
Figure 2.
Classification of EDCs.
The existence of EDCs in aquatic systems such as drinking water, wastewater, and underground water is a pressing environmental concern. It is suspected that a range of natural chemicals and man-made substances may interfere with the endocrine systems of both humans and wildlife, with varying sources of exposure and chemical properties. The proliferation of synthetic EDCs in the environment has added to the already vast array of chemicals that have been discharged worldwide, resulting in a more complex situation [46]. These compounds, which can be either natural or synthetic, are discharged into aquatic environments by multiple sources, including industrial discharge or drain water, domestic sewage effluents, livestock waste, and landfill leachates [47]. While the active components of some pharmaceuticals, cosmetics, and pesticides are crucial to identify the chemical constituents present in the environment, particularly those that are highly water-soluble. Generally, wastewater treatment is the largest contributor to EDC contamination, particularly in the form of untreated and treated sewage effluents and direct discharge to rivers.
In recent times, an increasing number of studies have drawn attention to the diverse health and environmental implications associated with endocrine-disrupting compounds. These compounds are known to interfere with the endocrine systems of humans and animals and can impact hormone synthesis, release, transport, metabolism, and excretion within the body [1]. For instance, the extensive use of pharmaceuticals and personal care products (PPCPs) in daily life has led to the presence of these compounds in rivers, groundwater, and even drinking water [42]. The occurrence of such pollutants in water resources can also be attributed to agricultural practices, accidental spills, leaching from building materials, disposal of prescription drugs, and chemical runoff from wastewater treatment plants, among other factors, aside from natural events. Moreover, phenolic compounds can be detected in water bodies due to various sources such as agricultural and industrial activities, synthetic resin and plastic waste, food packaging, paper coating, surfactants, adhesives, flame retardants, and dyestuffs [48]. These compounds can undergo a gradual biochemical transformation through microbial processes in the water stream, potentially leading to the formation of additional toxic byproducts. Table 1 presents a concise summary of the reported harmful effects of EDC contaminants from various sources.

Table 1.
Sources and harmful effects of endocrine disrupting chemicals (EDCs).
2.2. EDC Removal from Water by Semiconductor-Based Photocatalytic Materials
The performance and stability of photocatalytic materials based on semiconductor-based materials have emerged as a critical issue in oxidative environments, particularly when exposed to light energy (photons), as they are utilized for combating endocrine-disrupting chemicals (EDCs). Recently, researchers have been focusing on enhancing the performance of semiconductor-based photocatalysts in degrading endocrine-disrupting chemicals (EDCs) by designing hybrid or ternary structures or manipulating the surface compositions and structures of photocatalysts.
TiO2 and ZnO are considered the primary contenders for an outstanding semiconductor photocatalyst that can effectively eliminate low concentrations of toxic organic substances. This is primarily due to its non-toxic nature, cost-effectiveness, high activity, photostability, and commercial viability. However, TiO2 and ZnO require UV light to initiate the photocatalytic process due to its bandgap energy, which only allows it to utilize around 3–5% of the solar radiation that reaches the earth’s surface [53,54]. To overcome this limitation, researchers have been focusing on modifying these photocatalysts to reduce their bandgap energy, which would allow them to harvest visible light. Consequently, there have been several studies investigating modified TiO2 and ZnO materials for EDC removal in aqueous media. For instance, Hayati et al. prepared a ZnO@AC@Fe3O4 composite mediated with peroxymonosulfate (PMS) for boosting the degradation efficiency of BP-A under UVC irradiation. The photocatalytic activity of ZnO@AC@Fe3O4 mediated with PMS produced a higher degradation rate of 95.6% BPA, and about 58.9% of TOCs were eliminated within 60 min of the reaction. It was proposed that the synergistic interaction between PMS-based oxidation photocatalysis contributed toward BPA removal in the composite process [55]. The optimization of several morphological and structural properties for improved photocatalytic performance requires the surface modification of TiO2 nanoparticles (NPs). Batista et al. investigated the degradation of acetaminophen (APP) in the aqueous medium using magnetic photocatalysts based on carbon xerogel/TiO2 composites. This composition showed a maximum degradation efficiency of 99.2 of APP, respectively [56]. The ability of carbonaceous materials to act as photosensitizers, coupled with their large surface area and favorable mechanical and electronic properties, has piqued the interest of researchers, leading to a surge in their functionalization [56]. In another study, Silvestri et al. synthesized a combination of TiO2 and Polypyrrole (PPy) for the degradation of pesticide 4-CP and pharmaceutical compound DCF. The performance of combination photocatalysts showed that the PPy-TiO2 was able to convert more than 90% of DCF and 40% of 4-CP in just 60 min compared to TiO2 alone. They postulated that the introduction of polymers constituent to TiO2 facilitates the transfer of charges and prevents electron–hole recombination, hence improving efficiency [57]. While Monfared et al. reported that the combination of nano TiO2 and the ability of polyaniline to excite the electrons under visible light (Eg = 2.8 eV) caused the formation of a nanocomposite with significantly better photocatalytic behavior especially under visible light [58]. Anjum et al. also reported an enhancement of photocatalytic degradation when combining ZnO-ZnS with polyaniline (PANI) [59]. This combination of semiconductor materials and conducting polymer provides a synergistic behavior between the polymer and catalyst and reduces the bandgap energy in the range of visible light, which was also agreed and reported by Thakare et al. [60]. Meanwhile, Baishnisha et al. prepared graphitic carbon nitride (g-CN)/CuO nanocomposite for the photocatalytic degradation of phenol under visible light irradiation [61]. The combination of g-CN/CuO resulted in high degradation efficiency (87.8%) within 120 min. They reported that the formation of heterojunction by combining other semiconductor or carbon materials is a recognized technique to promote the separation of photogenerated electron–hole pairs which exhibit enhanced photocatalytic performance and a high efficiency of utilizing solar energy. This also has been supported by Kumar et al., combining ZnO/SnO2 with reduced graphene oxide (RGO) toward the elimination of different organic pollutants viz. p-bromophenol, bisphenol A, and ofloxacin from water [62]. The nanocomposite containing 5 wt% reduced graphene oxide exhibited a maximum photocatalytic efficiency of approximately 98.64% and 98.50% for removing p-bromophenol and bisphenol A, respectively, when exposed to UV light for 180 min. Additionally, this same level of efficiency was observed for eliminating ofloxacin after 120 min of UV light exposure.
2.2.1. Silver Carbonate (Ag2CO3) Photocatalyst
In recent times, the excellent photocatalytic performance of silver-based semiconductors has led to their increased research attention. Notably, Ag2CO3 has emerged as a highly efficient material for degrading EDCs in wastewater due to its exceptional photocatalytic capabilities. Moreover, as Ag-based compounds, they are well known for antibacterial activity. Ag2CO3, as a highly active visible-light-driven photocatalyst, has been recognized as a good photosensitizer since its narrow band gap (~2.17 V) is advantageous for sunlight absorption [63].
Table 2 presents recent research on the application of composite catalysts, including ternary and quaternary composites, under visible/solar light irradiation for the degradation of various pollutants. The degradation efficiency is dependent on factors such as the type and concentration of the pollutant, the amount of catalyst used, and the light source employed. Notably, the use of Ag2CO3-based catalysts for the degradation of EDCs under light irradiation has been a recent area of focus in these studies. Mergenbayeva et al. prepared Ag2CO3 microparticle photocatalysts for 4-t-BP degradation under solar light irradiation [64]. After 60 min, the complete degradation of 4-t-BP (5 ppm) was accomplished using Ag2CO3 (200 mg/L). They postulated that increasing the amount of the catalyst from 100 mg/L to 300 mg/L resulted in an increase in the final degradation efficiency from 41.6% to 100%. Similarly, Petala et al. observed that increasing Ag2CO3 photocatalyst concentration from 250 mg/L to 1000 mg/L led to an increase in ethyl paraben (EP) removal, while increasing EP concentration from 0.25 mg/L to 1.00 mg/L slightly lowered the photodegradation rate constant, kapp from 0.115 min−1 to 0.085 min−1 [65]. Rosman et al. investigated Ag2CO3/Ag2O heterostructure over a ZnO photocatalyst, and results exhibited a 99.3% photodegradation of ibuprofen (IBF) solution under visible-light irradiation [66]. Accordingly, the effective interfacial charge transfers observed in the ZnO-Ag2CO3-Ag2O sample were facilitated by the compact interfacial heterojunction. This was attributed to the synergistic effect resulting from the Ag2CO3/Ag2O heterostructure. Another study conducted by Rosman and her colleagues worked on Ag2CO3-based photocatalytic membrane combined with poly (vinylidene fluoride) (PVDF) acting as ultrafiltration for degradation similar to the source of EDC, which is ibuforen classified under pharmaceutically active compounds (PhACs) [67]. This hybrid photocatalytic membrane resulted in a removal of 35.27% IBF under visible light irradiation. The use of Ag2CO3 as a photocatalyst is not restricted to foreign materials; it also involves incorporating polymer materials into the design of the photocatalyst to enhance its photocatalytic performance toward EDC removal [68]. Pirzada et al. conducted a study where they synthesized a heterostructure of Ag2CO3 with a perovskite metal ferrite to improve the stability and photocatalytic efficiency through the strong surface plasmon resonance effect of Ag nanoparticles on the surface [69]. They successfully achieved a degradation efficiency of approximately 59% for p-chlorophenol using a novel LaFeO3/Ag2CO3 heterostructure photocatalyst under natural sunlight within 45 min. Notably, the nanocomposite significantly improved the stability of Ag2CO3, and no catalyst decomposition was observed even after multiple photocatalytic cycles.

Table 2.
Photocatalytic degradation of EDCs of over-selected semiconductor-based materials from aqueous solution.
2.2.2. Synthesis Techniques of Ag2CO3-Based Photocatalyst
Today, there are several types of reported Ag-based semiconductors that have been reported, drawing attention toward the elimination of EDCs in wastewater. Basically, all Ag-based semiconductors are responsive in the visible light irradiation due to their small band gap energy (<3.0 eV). Moreover, it has been found as a talented photocatalyst for the elimination of organic contamination due to nontoxicity and unique structural properties. More recently, Ag2CO3 has been considered one of the Ag-based photocatalysts with the distinct advantage of being driven by visible light absorption and relatively narrow band gap (~2.1–2.7 eV) [63,70]. Nevertheless, Ag2CO3 alone suffers from the problem of photocorrosion during the photocatalytic reaction [71,72]. Therefore, various strategies have been researched and implemented to improve its photocatalytic activity and stability. Ag2CO3 has been designed and constructed by a heterojunction photocatalyst via coupling with another semiconductor or carbon element to enhance the separation of photogenerated electron–hole pairs to prevent the occurrence of photochemical corrosion [73]. Moreover, it should be noted that the photocatalytic performance of semiconductors is affected not only by their band gap, but also by their physicochemical properties such as crystal structure, morphology, and crystallinity. Due to their strong visible light absorption, great efforts have been devoted to the design, preparation, and characterization of Ag-based photocatalysts.
There are various approaches that have been conducted in synthesizing and designing Ag2CO3-based photocatalysts. This synthesis method can be categorized into solution-based and vapor-based approaches. Physicochemical properties such as crystal structure, size, and morphology of photocatalysts are significantly influenced by the preparation method, and this also results in the changes of charge career separation and band gap energy of the material. This implies that the good control of preparation conditions determines the efficiency of photocatalysis. A solution-based approach is the simplest and least energy consuming. Therefore, an extensive approach has been developed by researchers; for instance, co-precipitation, sol–gel, and the solid-state reaction method enhance the physicochemical properties of the photocatalyst, hence improving the photocatalytic reaction of EDC removal in aqueous media.
The co-precipitation method is one of the significant and familiar solution-based approaches for developing Ag2CO3-based photocatalyst. Yu et al. developed a series of Ag2S/Ag2CO3 composite photocatalysts at different compositions (Ag2S, Ag2CO3, 4%Ag2S-Ag2CO3, 8%Ag2S-Ag2CO3, 16%Ag2S-Ag2CO3, 32%Ag2S-Ag2CO3 and 40%Ag2S-Ag2CO3 using the co-precipitation method (Figure 3) [16]. This approach includes three steps: (1) mixing of Na2CO3 and Na2S·9H2O in deionized water followed by adding AgNO3 dropwise, (2) stirring for 1 h, and (3) the drying and calcination of the precipitation. Moreover, a successive precipitation route has been introduced to prepare the core-shell-like composite of Ag2CO3@Ag2S and Ag2S@Ag2CO3 composite photocatalysts with 32 wt% Ag2S. They reported that the coupling of Ag2S into Ag2CO3 could produce a well-contacted Ag2S/Ag2CO3 interface as represented in TEM results (Figure 3B), which largely enhanced the photocatalytic activity and stability in decomposing methyl orange and phenol.
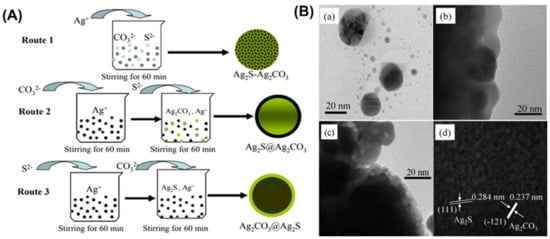
Figure 3.
(A) Fabrication of Ag2S-Ag2CO3, Ag2CO3@Ag2S, and Ag2S@Ag2CO3 composite photocatalysts by coprecipitation/successive precipitation route. (B) Photograph showing TEM images of (a) Ag2S; (b) Ag2CO3; (c) 32%Ag2S@ Ag2CO3. (d) High-resolution image of 32%Ag2S@Ag2CO3. Reproduced with permission from [16].
Another study conducted by Rui Zhang and co-workers also involved the synthesizing of Ag2CO3-based semiconductor photocatalysts via the co-precipitation method [74]. They have designed a Z-scheme heterostructure of ZnFe2O4/Ag2CO3 with an introduction of polyaniline (PANI) as a surface stabilizer to enhance the photocatalytic ability of the composite photocatalyst. For the first step, the ZnFe2O4 was prepared by mixing the Fe(NO3)3·9H2O and Zn(NO3)2·6H2O as a starting material in deionized water followed by hydrothermal and, finally, the drying process. The second step of the preparation of ZnFe2O4/PANI/Ag2CO3 was performed by mixing the ZnFe2O4, PANI, and AgNO3 and undergoing an ultrasonic treatment under dark conditions; finally, the product was dried (Figure 4A). They observed that ZnFe2O4/PANI/Ag2CO3, Ag2CO3 presents a regular rod-like structure, indicating that the addition of ZnFe2O4 and PANI has a great influence on the crystal growth of Ag2CO3 (Figure 4B). For photocatalytic activity, they found that the introduction of PANI and ZnFe2O4 improved the separation efficiency of photogenerated carriers of Ag2CO3.
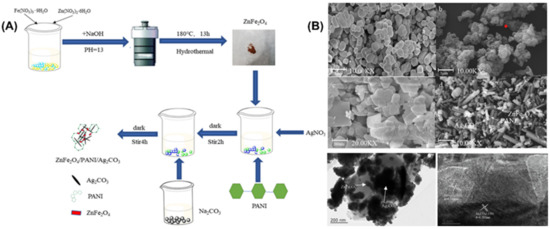
Figure 4.
(A) Synthesis of ZnFe2O4/PANI/Ag2CO3 composite using hydrothermal/co-precipitation method and (B) Photograph showing SEM images of (a) Ag2CO3, (b) PANI, (c) ZnFe2O4, (d) ZnFe2O4/PANI/Ag2CO3, TEM image of (e) ZnFe2O4/PANI/Ag2CO3, and HRTEM images of (f) ZnFe2O4/PANI/Ag2CO3. Reproduced with permission from [74].
Jindou Hu and colleagues constructed the Ag2O/Ag2CO3 heterojunction by adjusting the alkalinity of a room temperature solid-state chemical reaction system [74]. A simple room temperature solid-state (RTSS) chemical reaction through changing the molar ratios of the starting materials, which are AgNO3 and Na2CO3, was introduced. It is a simple process that starts with the grinding of raw material and ends with a drying process. They observed that the molar weight of Na2CO3 is important in the formation of Ag2O/Ag2CO3 composites, and the respective contents of Ag2O and Ag2CO3 in the composites can be adjusted by the addition of an amount of Na2CO3 resulting in the change of physicochemical and photocatalytic properties of the prepared samples. Table 3 represents a summary of previous research on the photocatalytic degradation of EDCs by Ag2CO3-based photocatalysts that were prepared using different approaches.

Table 3.
Photocatalytic degradation of EDCs by Ag2CO3-based photocatalysts prepared by various experimental conditions.
3. Endocrine-Disrupting Chemical Removal from Water by Ag2CO3-Based Photocatalytic Materials
The photostability and behavior of Ag2CO3 and Ag2CO3-based photocatalysts in an aqueous environment has become a significant issue, especially when considering this material for the photocatalytic removal of endocrine-disrupting chemicals (EDCs) in water. The fabrication and selection of the suitable photocatalysts toward certain organic pollutants such as EDCs is crucial when examining the photocatalytic activity, degradation mechanism, kinetics, operational parameters, and reusability of the photocatalysts and the preservation of their physicochemical characteristics during the process. The judicious design of water-stable photocatalytic materials implies that the complex behavior of the photocatalyst during contact with an aqueous medium is a major factor to be explored for practical applications in real-world problems. Moreover, the growing distress about the quality of the freshwater that is being used by the public from several sources has demanded the development of advanced materials to combat the existence of EDCs in water as pollutants of concern.
In this scenario, Perumal et al. incorporated Ag2CO3 with BiOBr/CdS via a hydrothermal and precipitation method to form heterojunctions while simultaneously improving their charge carriers’ efficiency [77]. The ternary composite demonstrated an outstanding photocatalytic performance against antibiotic drugs such as tetracycline (TC), in which almost a complete removal (~99%) was achieved within 70 min of a normal reaction under visible irradiation (Figure 5a). The performance showed 1.5 times higher than that of pure Ag2CO3, 1.28 times higher than pure BiOBr, and 1.1 times for BiOBr/CdS. These findings may be due to the reduced band gap of 2.31 eV, and subsequently increased the absorption toward a higher wavelength (red shift). The lower photoluminescence (PL) emission intensity also infers that there is better electron–hole separation efficiency in the flat square sheet catalyst structure. The plausible mechanism postulated by the study indicates that the excellent photoactivity depends on the higher amount of photon absorption ability and less recombination of the charge carriers. Scavenger analysis confirmed the presence of •OH and •O2− as the major reactive species that are responsible for degradation with good reusability up to five cycles (Figure 5b). Liu et al. intended to investigate the performance of ternary photocatalysts with flower-like sphere structures, namely Ag/Ag2CO3/BiVO4 plasmonic heterojunctions, for the removal of TC [78]. The results demonstrated the highest degradation efficiency up to 94.9% within 150 min (Figure 5c). It was postulated that the degradation mechanism was influenced by •OH and h+, higher photocurrent density, and reduced band gap energy for higher wavelength absorption. The intermediate products were produced through a ring-opening and the cleavage of central carbon [79]. With the presence of active radicals, the ring was further broken, and later, CO2, H2O, and NH4+ were finally generated by the oxidation reactions. The reusability of the photocatalyst was tested up to five runs, and no significant reduction can be seen, suggesting the higher photostability against visible light (Figure 5d).
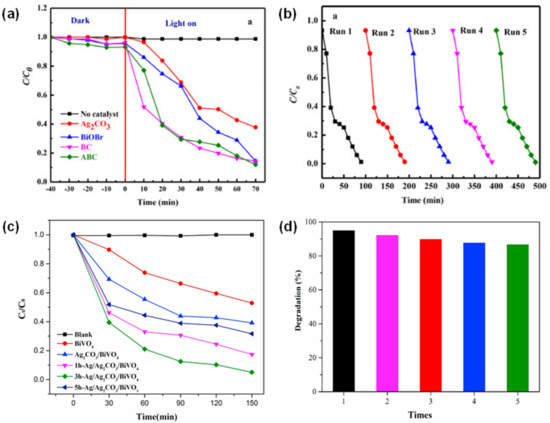
Figure 5.
(a) Photocatalytic TC degradation and (b) recycling runs using Ag2CO3/BiOBr/CdS photocatalysts. Reproduced with permission from [77]. (c) Photocatalytic TC degradation using as-prepared sample and (d) recycling runs of 3h-Ag/Ag2CO3/BiVO4 photocatalysts. Reproduced with permission from [78].
In another approach, Chen et al. constructed step-scheme (S-scheme) heterojunctions by combining Ag2CO3 with Bi4O5I2/g-C3N4 microstructures via heat treatment and in situ wet chemistry [80]. It was hypothesized that an S-scheme heterojunction system have higher potential in strong oxidation ability (lower Fermi level) by accelerating the separation and conversion of photoexcited charge carriers. In contrast, the reduction ability has a smaller work function and higher Fermi level (Figure 6A) [81]. The photoactivity was tested against TC, and the results demonstrated a higher degradation rate of 0.03892 min−1 (~82%), which is about 2.09, 5.80, and 13.33 times higher than that of pure Ag2CO3 (0.01865 min−1), Bi4O5I2 (0.00671 min−1), and g-C3N4 (0.00292 min−1), respectively. The photoactivity is closely related to the visible light absorption performance of the catalyst in which the surface state energy levels formed between the semiconductors in the S-scheme heterojunction, which can effectually exploit more visible irradiation and enhance the degradation rate. Further investigation has been made by Shen et al. regarding the S-scheme heterojunctions through the incorporation of ln2O3 into Ag2CO3 via in situ hydrothermal precipitation methods [82]. The study examines the transfer of photoexcited electrons in the heterojunctions through density functional theory (DFT) calculations and internal electrical field (IEF). The photocatalytic activity was evaluated by degrading levofloxacin (LEV) in an aqueous solution under simulated visible irradiation, and the performance reached 86.1%, the highest for ln2O3/Ag2CO3 (35%) compared with In2O3 (3.1%) and Ag2CO3 (66.0%). The IEF alleviates the potential energy of electron of In2O3 and lessens the potential energy of the electrons of Ag2CO3, resulting in an upward energy band edge in In2O3 and a downward band edge in Ag2CO3. In this case, the S-scheme heterojunction promotes the migration of charge carriers directly from the conduction band (CB) of Ag2CO3 to valence band (VB) of ln2O3, resulting in an outstanding separation of photoexcited electron–hole pairs in the catalyst structure forming more active free radicals in the reaction (Figure 6B). Scavenger analysis showed that holes (h+) act as major role in the degradation of LEV, followed by •OH and •O2−, respectively.
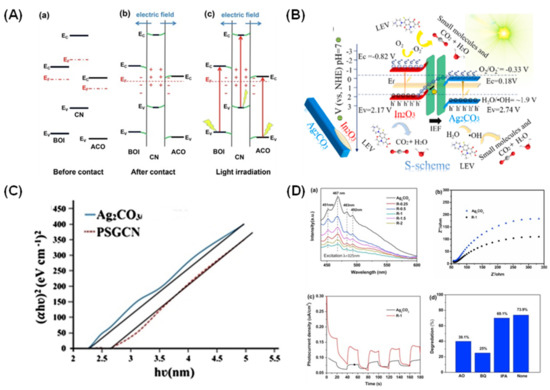
Figure 6.
(A) Schematic diagram of charge transfer mechanism based on dual S-scheme heterojunction in Ag2CO3/Bi4O5I2/g-C3N4 photocatalysts. Reproduced with permission from [80]. (B) S-scheme mechanism in ln2O3/Ag2CO3 photocatalysts. Reproduced with permission from [82]. (C) Tauc plot of pure Ag2CO3 and Ag2CO3/PSGCN. Reproduced with permission from [83]. (D) (a) Photoluminescence (PL) spectra of Ag2CO3 and (R-0.25, R-0.5, R-1, R-1.5, R-2) rGO/Ag2CO3 composites; (b,c) EIS spectra and transient photocurrent response of Ag2CO3 and R-1; (d) photocatalytic degradation efficiency of TC on R-1 by addition of various scavengers (no pH adjustment). Reproduced with permission from [85].
Due to better electron separation and migration in the photocatalytic system, the combination of semiconductor photocatalysts with carbonaceous materials like graphitic carbon nitride (g-C3N4) could be seen as a promising approach. From this aspect, Raizada et al. explored the hybrid combination of a phosphorous and sulfur co-doped g-C3N4 nanosheet (PSGCN) integrated with Ag2CO3 through co-precipitation and a simple ion exchange method [83]. The co-doping contributes to the narrowing of the band gap for the enhanced absorption in the visible region (Figure 6C). The photodegradation of 2,4-dinitrophenol (DNP) showed almost a complete degradation within 180 min under normal conditions, with •OH and h+ as the main active species identified in the process. The reduction in photoexcited charge carriers’ recombination was confirmed by PL and electrochemical impedance (EIS) analyses. The doping with P and S could greatly influence the recombination behavior of the nanocomposite in which they act as electron acceptors and effectively facilitate the separation of electron–hole pairs [84]. The intermediates formed were successfully recorded to be hydroquinone/resorcinol/catechol, benzoquinone, succinic acid, maleic acid, oxalic acid, and formic acid. The recyclability study showed that the degradation efficiency was reduced to 87% after completing the 10th cycle, suggesting the high photostability displayed by the nanocomposite. In another perspective, Reheman et al. integrated Ag2CO3 with reduced graphene oxide (rGO) prepared through a simple photo-ultrasonic assisted reduction method for the photodegradation of TC under visible light [85]. The results revealed a remarkable degradation activity, with 90% of TC being successfully removed by using 1% rGO/Ag2CO3 within 60 min at pH = 4, which was about 1.3 times higher than that of pure Ag2CO3. This is due to the photocurrent response value of the optimized composite being higher than that of pure Ag2CO3, and a lower PL intensity suggesting a higher separation efficiency and charge transfer. The •O2− was found to be the major active species, followed by h+ and •OH (Figure 6D). Moreover, the high specific surface area provides more surface-active sites, which in turn enhances TC degradation. However, increasing the content of rGO up to 2% decreased the degradation efficiency because excess loading would reduce the light penetration and adsorption of pollutants on the surface of the composite. The cycling experiments revealed that the optimized composite exhibited higher photostability and could be reused up to five cycles without significant deactivation.
The development of photocatalysts with better interaction is further illustrated by Li et al. [86] in which they constructed Z-scheme heterojunctions by using an in situ coupling of Ag2CO3 with carbon defect and nitrogen-rich material, and a C3N5 nanosheet through a simple thermal oxidative exfoliation and deposition methods. Due to the synergistic effect of nanosheet structures, carbon defects, and Z-scheme heterojunctions, the Ag2CO3/C3N5 (0.3 g, CNAC-10) displayed the highest photoactivity against tetracycline hydrochloride (TC-HCl), with percentages that reached ~98% with 50 mg/L within 100 min of reaction. The scavenger analysis showed that •O2− and h+ are the dominant radical species in the solution. The results indicate that the nanosheet-like and mesoporous structures are beneficial for the increased number of surface-active sites and specific surface area (177.9 m2/g), and subsequently enhanced the visible light absorption at a reduced band gap (2.34 eV) (Figure 7A) and outstanding migration of the charge carrier [68,87], thereby boosting the photoactivity. In the meantime, Wen et al. prepared ternary photocatalysts of Ag2CO3/CeO2/AgBr via an in situ loading of Ag2CO3 onto CeO2 spindles and subsequent acid corrosion process for degradation of LEV under visible irradiation [88]. The ternary photocatalyst was expected to exhibit enhanced photoactivity by destroying the LEV compound with up to ~87.63% degradation within 40 min of the reaction via a Z-scheme mechanism due to a higher specific surface area (46.87 m2/g) and outstanding photoexcited charge separation (Figure 7B). Scavenger analysis combined with an electron spin resonance technique confirmed that h+, •O2−, and •OH all participated in the reaction and contributed to the photocatalytic stability up to four cycles. Similar observation could also be seen from the research carried out by Li et al., whereby they constructed Ag2CO3 with ZnFe2O4/nitrogen-doped carbon quantum dots (NCDs) and managed to degrade LEV up to 88.75% under visible irradiation with pseudo first-order degradation kinetics (Figure 4C) [89]. In this work, the Z-scheme proved to be the mechanism of action due to the well-matched band structure for wider optical absorbance and superb electron transfer by the NCDs, which act as solid-state electron transfer mediators [90]. The enhanced photoactivity was attributed to the superior charge separation efficiency and increased lifetime of the charge carriers, evidenced by the low intensity of photoluminescence spectra and transient photocurrent response [91]. Superoxide radical anions (•O2−) play a major role among other active species being studied in the work. Accordingly, the degradation pathway of LEV may involve the piperazine ring oxidation process, quinolone moieties transformation, and decarboxylation. Additionally, this ternary photocatalyst also displayed higher photostability up to four cycles.
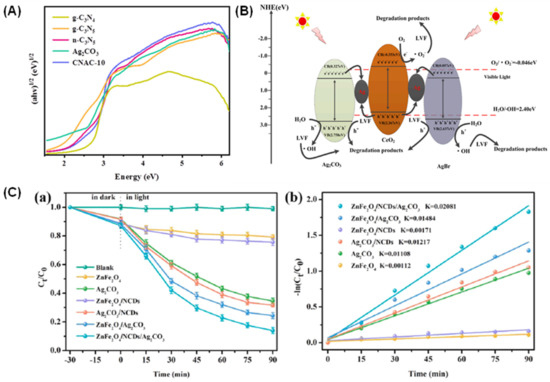
Figure 7.
(A) Band gap energies of g-C3N4, g-C3N5, n-C3N5, and Ag2CO3/C3N5 composite. Reproduced with permission from [86]. (B) The proposed mechanism for the enhancement of photocatalytic activity through Z-scheme route. Reproduced with permission from [88]. (C) (a) Photoactivity for LEV removal under visible light and (b) first-order kinetics of as-synthesized photocatalysts. Reproduced with permission from [89].
Some researchers have also investigated Ag-based coupled photocatalysts and provided some insight for further mechanism understanding. For instance, Jo et al. prepared Ag2O/Ag2CO3 heterojunction photocatalysts with Janus morphology via simple ion exchange followed by an in situ phase transformation method by carefully controlling their nucleation growth [92]. The nanocomposite not only exbibits remarkable photoactivity toward 4-chlorophenol, 4-CP (~90%) as compared to pure Ag2CO3 (43%) and Ag2O (35%) within 180 min according to the pseudo first-order kinetics (Figure 8A), but also photostability during reusability experiment (up to four runs) without significant change in degradation efficiency under visible light. The actives species responsible for the degradation was attributed by the presence of h+, followed by •O2− and •OH. Moreover, the obvious enhancement was ascribed to the intimate Janus interface between Ag2O and Ag2CO3, which endows for rapid charge migration processes. The higher specific surface area (38.5 m2/g) of the nanocomposite benefits the better adsorption of 4-CP on the surface and also provides more available surface-active sites for excellent photocatalytic performance [93]. Similarly, Yu et al. examined the performance of core-shell Ag2S@Ag2CO3 heterojunction photocatalysts for the removal of phenol and bisphenol A (BPA) [16]. Results revealed that outstanding performance was obtained over the composite photocatalyst with the incorporation of 32% Ag2S against phenol (54%) and BPA (~90%), and lower degradation activity was also observed with respect to pure Ag2S and Ag2CO3 (Figure 8B). The core-like structure of the composite photocatalyst could play a role in the degradation activity (Figure 8C). This is because the well-contacted interface contributes to better electron transfer as evidenced by a higher photocurrent response with higher specific surface area (2.65 m2/g). The scavenger experiment indicated that all radical species (•OH, •O2−, and h+) actively participated in the reaction with better photostability up to five cycles.
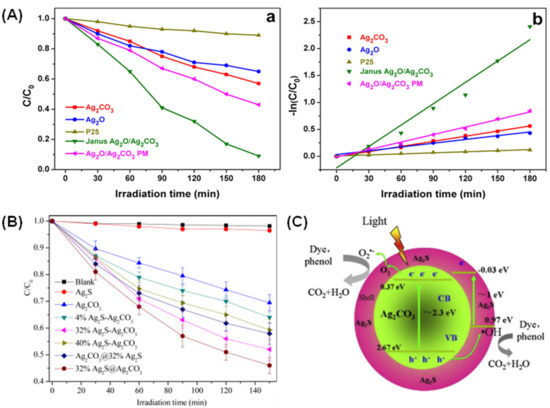
Figure 8.
(A) (a) Comparison of the photocatalytic activities in the decomposition of 4-CP over the prepared samples under visible-light illumination and (b) pseudo first-order kinetics. Reproduced with permission from [92]. (B) Photocatalytic activity comparison of the prepared photocatalysts in the degradation of phenol. (C) The mechanism of enhanced photocatalytic performance in Ag2S@Ag2CO3. Reproduced with permission from [16].
Previous studies also showed that constructing p–n heterojunctions at the photocatalyst interface can be regarded as another alternative to solve the problems of the low separation and migration efficiency of photoexcited electron–hole pairs. Theoretically, when a p-type and an n-type semiconductor are combined to form the p–n heterojunction, strong charge-driven capacity can be formed from the built-in electrical field. This is due to the difference in terms of the large flat band potential between the two semiconductors, which are beneficial for the rapid and effective charge carriers’ separation [94,95]. In this perspective, Sun et al. investigated the performance of Ag3VO4/Ag2CO3 p–n heterojunction photocatalysts prepared via a simple co-precipitation method [96]. The photoactivity toward LEV and TC degradation under visible irradiation reached 82% and ~75%, respectively, using an optimized ratio (1:2). Moreover, the LEV removal alone showed 12.8 and 21.51 times higher when compared with pure Ag3VO4 and Ag2CO3, respectively. The outstanding photoactivity is attributed by the accelerated charge carriers’ separation induced by the internal electrical field, evidenced by the transient photocurrent response and lower PL emission intensity, reduced band gap, and higher specific surface area (3.28 m2/g). Through scavenger experiments, the reactive radical species were dominated by h+ and •O2− while •OH was the species that was least responsible for the degradation. The degradation of LEV was majorly attributed by demethylation, decarboxylation, defluorination, piperazine ring, and quinoline ring destruction. The photostability tests were performed at four cycles without any significant loss of degradation efficiency. Fascinated by the heterojunction applicability, Rosman et al. prepared ternary photocatalysts of ZnO/Ag2CO3/Ag2O via a precipitation and phase transformation approach for the removal of phenol [71]. The results demonstrated that the heterojunction mixed phase could degrade the pollutant much better than their counterparts, reaching up to 41.40% under visible irradiation within 420 min (Figure 9). The higher photocatalytic performance exhibited by the ternary photocatalyst can be ascribed based on a crystalline phase transition along with enhanced specific surface area (11.15 m2/g) and a narrow band gap (2.77 eV). Moreover, the crystal’s growth of Ag2O over the ZnO/Ag2CO3 heterojunction lattice could promote the electron affinity and simultaneously facilitate charge transfer, while subsequently inhibiting the recombination of photoexcited electron–hole pairs. The intermediates formed were identified as hydroquinone, p-benzoquinone, and catechol. The same research team also carried out the immobilization of the ternary photocatalysts onto poly (vinylidene fluoride) (PVDF) membrane via a phase inversion method for the photocatalytic filtration of ibuprofen [67]. The results revealed some enhancement for the ibuprofen removal with the percentage obtained reaching 35.27% under visible irradiation. The incorporation of such ternary metal oxides into the membrane matrix greatly improved the hydrophilicity of the membrane for a better photodegradation interaction in an aqueous solution based on the lower water contact angle value. It was previously reported that the presence of a polar group (-OH) on the surface of the photocatalysts could promote better interaction with water molecules via hydrogen bonds and van der Waal’s forces [97]. Such improvement provides synergistic contact between the mixed phase membrane, water, and pollutant molecules for effective degradation activity.

Figure 9.
UV-vis spectra of phenol photo-oxidation process in the presence of (a) ZnO, (b) ZnO/Ag2CO3, (c) ZnO/Ag2CO3/Ag2O and (d) ZnO/Ag2O nanocomposites. Reproduced with permission from [71].
4. Challenges and Perspectives
Preliminary studies highlighted that Ag2CO3 is a very promising candidate for a high-efficient photocatalyst that can work well under visible light due to its intrinsic electronic property of low-energy bandgaps. Working in the visible spectrum region is crucially important to ensure that natural sunlight can be fully utilized, which will significantly reduce the source of energy and the cost of the photocatalytic process, especially in a large area water treatment reservoir. On top of that, Ag2CO3 has a wider selection of decomposing organic pollutants, which can reduce the type of photocatalyst use and the purifying process of the water treatment. Another important benefit is that the Ag2CO3 has poor solubility in water, which easily can be removed using a proper filtration system after the treatment was performed. This can ensure that the water supply to the users is clean and safe from impurities that may be harmful to the body system in the long term.
Despite this strong point, Ag2CO3 also has some challenges that need to be overcome. The stability of the compound is relatively low and tends to degrade over time. The photocatalytic activity slowly reduces to become weaker and less performative when the same compound was used for several cycles. Although operating in the visible region is an advantage for the low-energy photocatalytic process, it is still not fully utilized in the spectrum range of sunlight as a natural and environmentally friendly resource. The sunlight spectrum covers three main regions, which are ultraviolet (5%), visible (40%), and infrared (55%) [98], as illustrated in Figure 10. It is beneficial if the compound can also operate well in the infrared region, which is wider than the visible region and covers almost all spectrum of the sunlight.
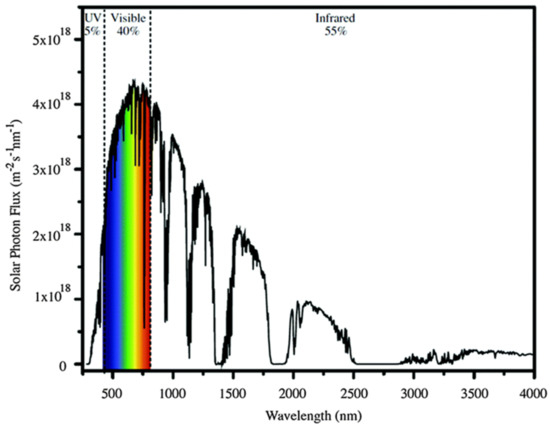
Figure 10.
Sunlight emission spectra. Reproduced with permission from [98].
Owing to metal elements in the compounds make the Ag2CO3 categorized as toxic materials that may be harmful to the human body. Proper segregation and filtration of the compound from the water supply after the pollution treatment are compulsory to avoid or at least reduce the risk of toxicity effects on the human being and the environment. Another issue that should be considered is that the price of this compound is relatively high as compared to the other common photocatalyst agents, such as zinc oxide (ZnO). An amount of 100 g of Ag2CO3 is sold at USD 351, while ZnO only costs about USD 139 for the same amount. High materials costs will contribute to the increase of operating costs for water treatment, for example. A simple but efficient synthesis route with a high yield of end compoundcould be the best solution to lower the product cost in the future.
Notwithstanding all the challenges, Ag2CO3 has a bright prospect to be looked at. Many research works have started to explore the potential of this material. The stability and efficiency of Ag2CO3 for photocatalytics can be enhanced by forming heterojunction structures with other semiconducting materials and modifying the energy band structure. The dimension of the compound could also be compressed as small as possible to form quantum dots for a larger surface area and tunable optical and electronic properties to enhance the photocatalytic activity [99,100]. Beyond that, the compound can be utilized in medical diagnosis and treatment, especially for cancer. For example, in a cancer diagnosis, with a proper surface modification, the compound could self-search and attach to the cancer cell and then be detected by using a bio-imaging method [101]. The cancer cell then could be destroyed by exciting the Ag2CO3 that attached to the cell to produce oxidation agents. This could be a solution for drug-free sustainable cancer therapy that can reduce toxic side effects to the patient [102]. A recent study shows that by manipulating temperature and humidity, the photocatalytic processes can be utilized to inactivate airborne viruses from indoor air by means of altering the photocatalytic rate. The primary mechanisms for the inactivation of airborne viruses are thought to involve chemical oxidation by reactive oxidative species, toxicity of metal ions released from metal-containing photocatalysts, and morphological damage to airborne viruses caused by sharp edges of two-dimensional nano-structural photocatalysts [103]. Prior to that, Ag particles have been shown to be very promising as an antibacterial agent via photocatalysis mechanism [104]. The latest technology shows that the photocatalyst compound was embedded into the paint to improve the hygiene and air quality of the building [105]. With suitable formulation, this is a new direction for Ag2CO3 for the development of smart buildings. Ag2CO3 could also be implemented as a photocatalyst for hydrogen production through the water splinting process, which is recognized as an ideal solution for renewable and green energy [106]. Moreover, hydrogen also can be extracted from biomass to produce valuable carbon-free energy and, at the same time, provide an avenue of production for industrially relevant biomass products [107]. All the prospects of Ag2CO3 that are illustrated in Figure 11 and Figure 12 summarized several strengths, weaknesses, threats, and opportunities of Ag2CO3 as a photocatalysis compound.
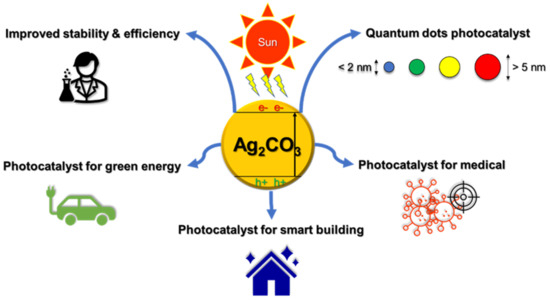
Figure 11.
Prospect of Ag2CO3 photocatalyst.
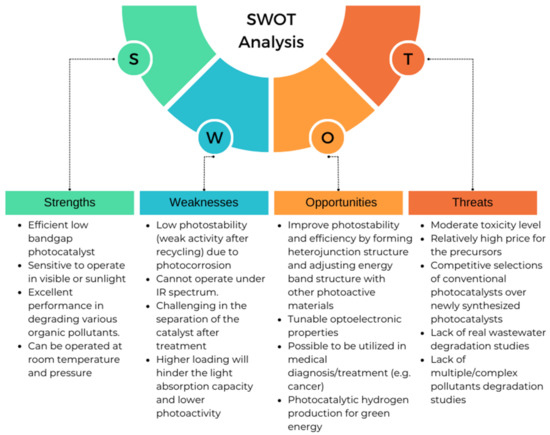
Figure 12.
Strengths, weaknesses, opportunities, and threats (SWOT) analysis for Ag2CO3-based photocatalytic materials.
5. Conclusions and Future Outlooks
Ag2CO3-based photocatalysts via several techniques and reaction conditions have been rapidly established as one of the most highly innovative research in photocatalytic degradation and are especially well suited to degrading organic pollutants in wastewater, specifically EDCs. Much effort has been designed in the development of methods for enhancing and stabilizing Ag2CO3-based photocatalysts. The modification of Ag2CO3-based photocatalysts with other elements were found to increase visible light activity, improve electron migration, and effectively separate electron–hole pairs. Moreover, due to the high aspect ratio and novel physicochemical properties, Ag2CO3-based photocatalysts also significantly enhanced visible-light-driven photoactivity for EDC removal.
However, there are certain crucial considerations in relation to this emerging designed Ag2CO3-based photocatalyst. For instance, improving the stability of the photocatalyst and the viability of the photocatalyst separation along with the reusability factor is a difficult challenge toward eventual commercialization. In fact, creating this photocatalyst on a big scale for water treatment requires considerable costs. Consequently, as a nation that currently emphasizes environmental sustainability, future goals need to identify the practical and sustainable strategies to develop an affordable photocatalyst. Additionally, an increasing amount of research have been published on the processes, methods, and pathways for the degradation of EDC molecules. Generally, this key aspect is crucial and significant since it will help in understanding the relationship between the properties of the photocatalyst and reaction activity, resulting in maximizing the mineralization of the pollutant. With this review article, we seek not only to provide an overview of recent advancements in the Ag2CO3-based photocatalyst for the degradation of EDCs from environmental sources, but also to encourage further research in this area and attract researchers to join this field. With the impressive progress over the last few years and clear goals for future work, we anticipate reading a lot of intriguing study publications in the near future.
Author Contributions
H.A.R.: Conceptualization, Writing—Original Draft and Editing, Funding acquisition, Z.A.M.H.: Conceptualization, Writing—Original Draft and Review, M.A.M.S.: Conceptualization, Writing—Review, N.I.T.R.: Review, Z.K.: Review, and P.D.: Review. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the Ministry of Higher Education (MOHE) and Universiti Teknologi MARA (UiTM) for the YTR grant (600-RMC/YTR/5/3 (005/2020)).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would also like to acknowledge technical and management support from UiTM Pahang Branch, Jengka Campus and the Centre for Functional Materials and Nanotechnology, Institute of Science, UiTM Shah Alam, Selangor.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Surana, D.; Gupta, J.; Sharma, S.; Kumar, S.; Ghosh, P. A review on advances in removal of endocrine disrupting compounds from aquatic matrices: Future perspectives on utilization of agri-waste based adsorbents. Sci. Total Environ. 2022, 826, 154129. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.A.; Gebru, S.B.; Redae, G.H.; Tsige, A.G. Removal of endocrine disrupters from the contaminated environment: Public health concerns, treatment strategies and future perspectives—A review. Heliyon 2022, 8, e09206. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; Nor, M.Z.M.; Azis, R.S.; Umar, A.M. Contemporary Techniques for Remediating Endocrine-Disrupting Compounds in Various Water Sources: Advances in Treatment Methods and Their Limitations. Polymers 2021, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Katibi, K.; Yunos, K.; Man, H.C.; Aris, A.; Nor, M.b.M.; Azis, R.B. Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review. Polymers 2021, 13, 392. [Google Scholar] [CrossRef]
- Sin, J.; Lam, S.; Mohamed, A.; Lee, K. Degrading endocrine disrupting chemicals from wastewater by TiO2 photocatalysis: A review. Int. J. Photoenergy 2012, 2012, 185159. [Google Scholar] [CrossRef]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Ghosh, A.; Orasugh, J.T.; Chattopadhyay, D.; Ghosh, S. Electrospun nanofibres: A new vista for detection and degradation of harmful endocrine-disrupting chemicals. Groundw. Sustain. Dev. 2021, 16, 100716. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic water decontamination using graphene and ZnO coupled photocatalysts: A review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.; He, X.; Duan, X.; Dionysiou, D.D. Degradation and mineralization of organic UV absorber compound 2-phenylbenzimidazole-5-sulfonic acid (PBSA) using UV-254nm/H2O2. J. Hazard. Mater. 2015, 282, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wan, X.; Xu, X.; Chen, X. Facile fabrication of highly efficient AgI/ZnO heterojunction and its application of methylene blue and rhodamine B solutions degradation under natural sunlight. Appl. Surf. Sci. 2014, 321, 10–18. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Shanker, V. In situ growth strategy for highly efficient Ag2CO3/g-C3N4 hetero/nanojunctions with enhanced photocatalytic activity under sunlight irradiation. J. Environ. Chem. Eng. 2015, 3, 852–861. [Google Scholar] [CrossRef]
- Finegold, L.; Cude, J.L. Biological Sciences: One and Two-dimensional Structure of Alpha-Helix and Beta-Sheet Forms of Poly(L-Alanine) shown by Specific Heat Measurements at Low Temperatures (1.5–20 K). Nature 1972, 238, 38–40. [Google Scholar] [CrossRef]
- Yu, C.; Wei, L.; Zhou, W.; Dionysiou, D.D.; Zhu, L.; Shu, Q.; Liu, H. A visible-light-driven core-shell like Ag2S@Ag2CO3 composite photocatalyst with high performance in pollutants degradation. Chemosphere 2016, 157, 250–261. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Y.; Hu, Y.H. Synthesis, structures and applications of single component core-shell structured TiO2: A review. Chem. Eng. J. 2019, 375, 122029. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, S.; Gu, P.; Zhang, T.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Conjugate Polymer-clothed TiO2@V2O5 nanobelts and their enhanced visible light photocatalytic performance in water remediation. J. Colloid Interface Sci. 2020, 578, 402–411. [Google Scholar] [CrossRef]
- Mehta, M.; Krishnamurthy, S.; Basu, S.; Nixon, T.; Singh, A. BiVO4/TiO2 core-shell heterostructure: Wide range optical absorption and enhanced photoelectrochemical and photocatalytic performance. Mater. Today Chem. 2020, 17, 100283. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Raza, W.; Khan, A.; Bahnemann, D.; Muneer, M. Highly efficient Y and V co-doped ZnO photocatalyst with enhanced dye sensitized visible light photocatalytic activity. Catal. Today 2017, 284, 169–178. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R. Photocatalytic decolouration, degradation and disinfection capability of Ag2CO3/ZnO in natural sunlight. J. Indian Chem. Soc. 2022, 99, 100311. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Wang, Z.; Xu, G.; Zheng, H.; Zhou, L.; Chen, Z. Efficient porous carbon nitride/Ag3PO4 photocatalyst for selective oxidation of amines to imines: Z-scheme heterojunction and interfacial adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 652, 129806. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.; Harunsani, M. Ag3PO4 and Ag3PO4—Based visible light active photocatalysts:Recent progress, synthesis, and photocatalytic applications. Catal. Commun. 2022, 172, 106556. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Duan, L.; Shen, H.; Zhang, Q.; Zhao, X. The synergistic effect of graphene oxide and silver vacancy in Ag3PO4-based photocatalysts for rhodamine B degradation under visible light. Appl. Surf. Sci. 2018, 462, 263–269. [Google Scholar] [CrossRef]
- Duan, J.; Fang, X.; Li, C.; Qu, J.; Guo, L.; Zou, Y.; Xiang, M.; Wang, W. Efficient and stable monolithic microreactor with Ag/AgCl photocatalysts coated on polydopamine modified melamine sponge for photocatalytic water purification. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 659, 130759. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Fu, F.; Xu, H.; Da, K.; Cao, S.; Chen, W.; Yang, L.; Fan, X. Construction of Z-scheme Ag/AgCl/Bi2WO6 photocatalysts with enhanced visible-light photocatalytic performance for gaseous toluene degradation. Appl. Surf. Sci. 2023, 610, 155598. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Sun, X.; Zhang, H.; Xian, T. Preparation and photocatalytic application of ternary n-BaTiO3/Ag/p-AgBr heterostructured photocatalysts for dye degradation. Mater. Res. Bull. 2020, 124, 110754. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.; Karimi, H.; Ghaedi, M.; Avargani, V. Construction of efficient and stable ternary ZnFe2O4/Ag/AgBr Z-scheme photocatalyst based on ZnFe2O4 nanofibers under LED visible light. Mater. Res. Bull. 2021, 143, 111449. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Lv, J.; Zhao, J.; Jiang, D.; Zhan, Q. Synthesis and characterization of Bi2SiO5-coated Ag/AgBr photocatalyst with highly efficient decontamination of organic pollutants. Appl. Surf. Sci. 2021, 578, 152074. [Google Scholar] [CrossRef]
- Xu, H.; Xie, J.; Jia, W.; Wu, G.; Cao, Y. The formation of visible light-driven Ag/Ag2O photocatalyst with excellent property of photocatalytic activity and photocorrosion inhibition. J. Colloid Interface Sci. 2018, 516, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhai, J.; Jiang, H.; Liu, D.; Zhang, L. CdS/Ag2S nanocomposites photocatalyst with enhanced visible light photocatalysis activity. Solid State Sci. 2019, 98, 106020. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Wei, Z.; Wang, S.; He, H.; Sun, C.; Yang, S. Fabrication of a ternary plasmonic photocatalyst of Ag/AgVO3/RGO and its excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 179, 9–20. [Google Scholar] [CrossRef]
- Mansha, M.S.; Iqbal, T. Experimental and theoretical study of novel rGO/AgVO3 nano-hetrostructures for their application as efficient photocatalyst. Opt. Mater. 2022, 131, 112591. [Google Scholar] [CrossRef]
- Alheshibri, M.; Elsayed, K.; Haladu, S.A.; Magami, S.M.; Al Baroot, A.; Ercan, I.; Ercan, F.; Manda, A.A.; Çevik, E.; Kayed, T.S.; et al. Synthesis of Ag nanoparticles-decorated on CNTs/TiO2 nanocomposite as efficient photocatalysts via nanosecond pulsed laser ablation. Opt. Laser Technol. 2022, 155, 108443. [Google Scholar] [CrossRef]
- Dai, Y.-D.; Lyu, R.-J.; Wu, T.; Huang, C.-C.; Lin, Y.-W. Influences of silver halides AgX (X = Cl, Br, and I) on magnesium bismuth oxide photocatalyst in methylene blue degradation under visible light irradiation. J. Photochem. Photobiol. A Chem. 2020, 397, 112585. [Google Scholar] [CrossRef]
- Lan, Y.; Qian, X.; Zhao, C.; Zhang, Z.; Chen, X.; Li, Z. High performance visible light driven photocatalysts silver halides and graphitic carbon nitride (X=Cl, Br, I) nanocomposites. J. Colloid Interface Sci. 2013, 395, 75–80. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Saranya, K.; Pandikumar, A.; Lin, K.-C. Palladium and silver nanoparticles embedded on zinc oxide nanostars for photocatalytic degradation of pesticides and herbicides. Chem. Eng. J. 2021, 410, 128434. [Google Scholar] [CrossRef]
- González, V.J.; Vázquez, E.; Villajos, B.; Tolosana-Moranchel, A.; Duran-Valle, C.; Faraldos, M.; Bahamonde, A. Eco-friendly mechanochemical synthesis of titania-graphene nanocomposites for pesticide photodegradation. Sep. Purif. Technol. 2022, 289, 120638. [Google Scholar] [CrossRef]
- Huang, F.; Gao, F.; Li, C.; Campos, L.C. Photodegradation of free estrogens driven by UV light: Effects of operation mode and water matrix. Sci. Total Environ. 2022, 835, 155515. [Google Scholar] [CrossRef]
- Javaid, A.; Imran, M.; Latif, S.; Hussain, N.; Bilal, M. Functionalized magnetic nanostructured composites and hybrids for photocatalytic elimination of pharmaceuticals and personal care products. Sci. Total Environ. 2022, 849, 157683. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals and endocrine disrupting compounds from water by zinc oxide-based photocatalytic degradation: A review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Zhang, X.; Su, H.; Gao, P.; Li, B.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Effects and mechanisms of aged polystyrene microplastics on the photodegradation of sulfamethoxazole in water under simulated sunlight. J. Hazard. Mater. 2022, 433, 128813. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Zhang, S.; Zhang, M.; Hou, J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109275. [Google Scholar] [CrossRef]
- Komesli, O.; Muz, M.; Ak, M.; Bakırdere, S.; Gokcay, C. Occurrence, fate and removal of endocrine disrupting compounds (EDCs) in Turkish wastewater treatment plants. Chem. Eng. J. 2015, 277, 202–208. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.; Brown, R.; Hashib, M. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef]
- Olasupo, A.; Suah, F.B.M. Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: A case of polymer inclusion membranes. J. Hazard. Mater. 2020, 406, 124317. [Google Scholar] [CrossRef]
- Corsini, E.; Ruffo, F.; Racchi, M. Steroid hormones, endocrine disrupting compounds and immunotoxicology. Curr. Opin. Toxicol. 2018, 10, 69–73. [Google Scholar] [CrossRef]
- Vessa, B.; Perlman, B.; McGovern, P.G.; Morelli, S.S. Endocrine disruptors and female fertility: A review of pesticide and plasticizer effects. F&S Rep. 2022, 3, 86–90. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajji, L.; Ismail, A.A.; Bumajdad, A.; Alsaidi, M.; Ahmed, S.; Al-Hazza, A.; Ahmed, N. Photodegradation of powerful five estrogens collected from waste water treatment plant over visible-light-driven Au/TiO2 photocatalyst. Environ. Technol. Innov. 2021, 24, 101958. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.; Rojas, H.; Navío, J.; Hidalgo, M. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Hayati, F.; Moradi, S.; Saei, S.F.; Madani, Z.; Giannakis, S.; Isari, A.A.; Kakavandi, B. A novel, Z-scheme ZnO@AC@FeO photocatalyst, suitable for the intensification of photo-mediated peroxymonosulfate activation: Performance, reactivity and bisphenol A degradation pathways. J. Environ. Manag. 2022, 321, 115851. [Google Scholar] [CrossRef]
- Batista, W.C.; da Cunha, R.; Santos, A.; Reis, P.; Furtado, C.; Silva, M.; de Fátima Gorgulho, H. Synthesis of a reusable magnetic photocatalyst based on carbon xerogel/TiO2 composites and its application on acetaminophen degradation. Ceram. Int. 2022, 48, 34395–34404. [Google Scholar] [CrossRef]
- Silvestri, S.; Burgo, T.; Dias-Ferreira, C.; Labrincha, J.; Tobaldi, D. Polypyrrole-TiO2 composite for removal of 4-chlorophenol and diclofenac. React. Funct. Polym. 2020, 146, 104401. [Google Scholar] [CrossRef]
- Monfared, A.H.; Jamshidi, M. Synthesis of polyaniline/titanium dioxide nanocomposite (PAni/TiO2) and its application as photocatalyst in acrylic pseudo paint for benzene removal under UV/VIS lights. Prog. Org. Coatings 2019, 136, 105257. [Google Scholar] [CrossRef]
- Anjum, M.; Oves, M.; Kumar, R.; Barakat, M. Fabrication of ZnO-ZnS@polyaniline nanohybrid for enhanced photocatalytic degradation of 2-chlorophenol and microbial contaminants in wastewater. Int. Biodeterior. Biodegrad. 2017, 119, 66–77. [Google Scholar] [CrossRef]
- Thakare, S.; Mate, V.; Urkude, K.; Gawande, S. Graphene-TiO2-polyaniline nanocomposite: A new green and efficient catalyst as a alternative for noble metal and NaBH4 induced the reduction of 4-nitro phenol. FlatChem 2020, 22, 100179. [Google Scholar] [CrossRef]
- Baishnisha, A.; Divakaran, K.; Balakumar, N.; Perumal, K.N.; Meenakshi, C.; Kannan, R.S. Synthesis of highly efficient g-CN@CuO nanocomposite for photocatalytic degradation of phenol under visible light. J. Alloys Compd. 2021, 886, 161167. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.; Purohit, L. RGO supported ZnO/SnO2 Z-scheme heterojunctions with enriched ROS production towards enhanced photocatalytic mineralization of phenolic compounds and antibiotics at low temperature. J. Colloid Interface Sci. 2023, 632, 196–215. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mo, L.; Liu, Y.; Zhang, H.; Ge, Y.; Zhou, Y. Ag2CO3 Decorating BiOCOOH Microspheres with Enhanced Full-Spectrum Photocatalytic Activity for the Degradation of Toxic Pollutants. Nanomaterials 2018, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Mergenbayeva, S.; Atabaev, T.; Vakros, J.; Mantzavinos, D.; Poulopoulos, S. Photocatalytic Degradation of 4-tert-butylphenol Using Solar Light Responsive Ag2CO3. Catalysts 2022, 12, 1523. [Google Scholar] [CrossRef]
- Petala, A.; Nasiou, A.; Mantzavinos, D.; Frontistis, Z. Photocatalytic Evaluation of Ag2CO3 for Ethylparaben Degradation in Different Water Matrices. Water 2020, 12, 1180. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.; Mohamed, M.; Harun, Z.; Ismail, A.; Aziz, F. Constructing a compact heterojunction structure of Ag2CO3/Ag2O in-situ intermediate phase transformation decorated on ZnO with superior photocatalytic degradation of ibuprofen. Sep. Purif. Technol. 2020, 251, 117391. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Razali, N.A.M.; Ahmad, S.N.; Ismail, N.H.; Aziz, F.; Harun, Z.; Ismail, A.F.; Yusof, N. Ibuprofen removal through photocatalytic filtration using antifouling PVDF- ZnO/Ag2CO3/Ag2O nanocomposite membrane. Mater. Today Proc. 2019, 42, 69–74. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Abdullah, A.H. Hybrid polymer-based photocatalytic materials for the removal of selected endocrine disrupting chemicals (EDCs) from aqueous media: A review. J. Mol. Liq. 2022, 361, 119632. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Pushpendra; Kunchala, R.K.; Naidu, B. Synthesis of LaFeO3/Ag2CO3 Nanocomposites for Photocatalytic Degradation of Rhodamine B and p-Chlorophenol under Natural Sunlight. ACS Omega 2019, 4, 2618–2629. [Google Scholar] [CrossRef]
- Wu, C. Synthesis of Ag2CO3/ZnO nanocomposite with visible light-driven photocatalytic activity. Mater. Lett. 2014, 136, 262–264. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.; Ismail, A.; Jaafar, J.; Harun, Z.; Aziz, F.; Mohamed, M.; Ohtani, B.; Takashima, M. Photocatalytic degradation of phenol over visible light active ZnO/Ag2CO3/Ag2O nanocomposites heterojunction. J. Photochem. Photobiol. A Chem. 2018, 364, 602–612. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Song, Y.; Zhu, J.; Zhu, T.; Liu, C.; Zhao, D.; Zhang, Q.; Li, H. Synthesis and characterization of g-C3N4/Ag2CO3 with enhanced visible-light photocatalytic activity for the degradation of organic pollutants. RSC Adv. 2014, 4, 34539–34547. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Paez, C.A.J.; Navío, J.; Martín-Gómez, A.; Hidalgo, M.C. Coupling of Ag2CO3 to an optimized ZnO photocatalyst: Advantages vs. disadvantages. J. Photochem. Photobiol. A Chem. 2018, 369, 119–132. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, C.; Yu, J.; Chen, Z.; Jiang, J.; Zeng, K.; Cai, L.; Yang, Z. Synthesis of dual Z-scheme photocatalyst ZnFe2O4/PANI/Ag2CO3with enhanced visible light photocatalytic activity and degradation of pollutants. Adv. Powder Technol. 2021, 33, 103348. [Google Scholar] [CrossRef]
- Hu, J.; Xu, H.; Wang, S.; Jia, W.; Cao, Y. In-situ solid-state synthesis and regulation of Ag2O/Ag2CO3 heterojunctions with promoted visible-light driven photocatalytic decomposition for organic pollutant. Sep. Purif. Technol. 2019, 226, 95–108. [Google Scholar] [CrossRef]
- Ma, L.; Jia, I.; Guo, X.; Xiang, L. High performance of Pd catalysts on bimodal mesopore for the silica catalytic oxidation of toluene. Chin. J. Catal. 2014, 35, 108–119. [Google Scholar] [CrossRef]
- Perumal, K.; Shanavas, S.; Ahamad, T.; Karthigeyan, A.; Murugakoothan, P. Construction of Ag2CO3/BiOBr/CdS ternary composite photocatalyst with improved visible-light photocatalytic activity on tetracycline molecule degradation. J. Environ. Sci. 2023, 125, 47–60. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, J.; Yuan, J.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation Chem. Eng. J. 2018, 331, 242–254. [Google Scholar]
- Zhu, X.-D.; Wang, Y.; Sun, R.-J.; Zhou, D.-M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, H.; Liu, H.; Niu, C.; Huang, D.; Yang, Y.; Liang, C.; Li, L.; Li, J. Construction of dual S-scheme Ag2CO3/Bi4O5I2/g-C3N4 heterostructure photocatalyst with enhanced visible-light photocatalytic degradation for tetracycline. Chem. Eng. J. 2022, 438, 135471. [Google Scholar] [CrossRef]
- Martín, S.S.; Rivero, M.J.; Ortiz, I. Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production. Catalysts 2020, 10, 901. [Google Scholar] [CrossRef]
- Shen, J.; Qian, L.; Huang, J.; Guo, Y.; Zhang, Z. Enhanced degradation toward Levofloxacin under visible light with S-scheme heterojunction In2O3/Ag2CO3: Internal electric field, DFT calculation and degradation mechanism. Sep. Purif. Technol. 2021, 275, 119239. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P.; Shandilya, P.; Thakur, P.; Jung, H. Visible light assisted photodegradation of 2,4-dinitrophenol using Ag2CO3 loaded phosphorus and sulphur co-doped graphitic carbon nitride nanosheets in simulated wastewater. Arab. J. Chem. 2018, 13, 3196–3209. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Chen, X.; Wu, Z.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus- and Sulfur-Codoped g-C3N4: Facile Preparation, Mechanism Insight, and Application as Efficient Photocatalyst for Tetracycline and Methyl Orange Degradation under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- Reheman, A.; Kadeer, K.; Okitsu, K.; Halidan, M.; Tursun, Y.; Dilinuer, T.; Abulikemu, A. Facile photo-ultrasonic assisted reduction for preparation of rGO/Ag2CO3 nanocomposites with enhanced photocatalytic oxidation activity for tetracycline. Ultrason. Sonochemistry 2019, 51, 166–177. [Google Scholar] [CrossRef]
- Li, G.; Zeng, G.; Chen, Z.; Hong, J.; Ji, X.; Lan, Z.; Tan, X.; Li, M.; Hu, X.; Tang, C. In Situ Coupling Carbon Defective C3N5 Nanosheet with Ag2CO3 for Effective Degradation of Methylene Blue and Tetracycline Hydrochloride. Nanomaterials 2022, 12, 2701. [Google Scholar] [CrossRef]
- Bagheri, S.; Hir, Z.A.M.; Yousefi, A.T.; Hamid, S.B.A. Progress on mesoporous titanium dioxide: Synthesis, modification and applications. Microporous Mesoporous Mater. 2015, 218, 206–222. [Google Scholar] [CrossRef]
- Wen, X.; Niu, C.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211–223. [Google Scholar] [CrossRef]
- Li, L.; Niu, C.; Guo, H.; Wang, J.; Ruan, M.; Zhang, L.; Liang, C.; Liu, H.; Yang, Y. Efficient degradation of Levofloxacin with magnetically separable ZnFe2O4/NCDs/Ag2CO3 Z-scheme heterojunction photocatalyst: Vis-NIR light response ability and mechanism insight. Chem. Eng. J. 2020, 383, 123192. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Z.; Zeng, D.; Yu, C.; Liu, X.; Yang, K.; Liu, H. The preparation and characterization of CaMg(CO3)2@Ag2CO3/Ag2S/NCQD nanocomposites and their photocatalytic performance in phenol degradation. J. Nanoparticle Res. 2018, 20, 182. [Google Scholar] [CrossRef]
- Fu, S.; Yuan, W.; Yan, Y.; Liu, H.; Shi, X.; Zhao, F.; Zhou, J. Highly efficient visible-light photoactivity of Z-scheme MoS2/Ag2CO3 photocatalysts for organic pollutants degradation and bacterial inactivation. J. Environ. Manag. 2019, 252, 109654. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Yadav, P.; Tonda, S. In situ phase transformation synthesis of unique Janus Ag2O/Ag2CO3 heterojunction photocatalyst with improved photocatalytic properties. Appl. Surf. Sci. 2018, 445, 555–562. [Google Scholar] [CrossRef]
- Syazwani, O.; Hir, Z.M.; Mukhair, H.; Mastuli, M.; Abdullah, A. Designing visible-light-driven photocatalyst of Ag3PO4/CeO2 for enhanced photocatalytic activity under low light irradiation. J. Mater. Sci. Mater. Electron. 2018, 30, 415–423. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Zeng, G.-M. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: Possible degradation pathways, mineralization activity and an in depth mechanism insight. Appl. Catal. B Environ. 2017, 221, 701–714. [Google Scholar] [CrossRef]
- Sun, H.; Qin, P.; Wu, Z.; Liao, C.; Guo, J.; Luo, S.; Chai, Y. Visible light-driven photocatalytic degradation of organic pollutants by a novel Ag3VO4/Ag2CO3 p–n heterojunction photocatalyst: Mechanistic insight and degradation pathways. J. Alloys Compd. 2020, 834, 155211. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Visible light-active hybrid film photocatalyst of polyethersulfone–reduced TiO2: Photocatalytic response and radical trapping investigation. J. Mater. Sci. 2018, 53, 13264–13279. [Google Scholar] [CrossRef]
- Lazorski, M.S.; Castellano, F.N. Advances in the light conversion properties of Cu(I)-based photosensitizers. Polyhedron 2014, 82, 57–70. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Khadem, S.S.M.; Habibzadeh, S.; Esmaeili, A.; Abida, O.; Vatanpour, V.; Rabiee, N.; Bagherzadeh, M.; Iravani, S.; Saeb, M.R.; et al. Quantum dots for photocatalysis: Synthesis and environmental applications. Green Chem. 2021, 23, 4931–4954. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, N.; Saghy, P.; Dube, L.; Zhu, H.; Chen, O. Quantum Dot Photocatalysts for Organic Transformations. J. Phys. Chem. Lett. 2021, 12, 7180–7193. [Google Scholar] [CrossRef]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2020, 2045, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, Y.; Yao, X.; Chen, D.; Fan, M.; Jin, Z.; He, Q. Photocatalysis-mediated drug-free sustainable cancer therapy using nanocatalyst. Nat. Commun. 2021, 12, 1345. [Google Scholar] [CrossRef] [PubMed]
- Poormohammadi, A.; Bashirian, S.; Rahmani, A.R.; Azarian, G.; Mehri, F. Are photocatalytic processes effective for removal of airborne viruses from indoor air? A narrative review. Environ. Sci. Pollut. Res. 2021, 28, 43007–43020. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Salvadores, F.; Reli, M.; Alfano, O.M.; Kočí, K.; Ballari, M.D.L.M. Efficiencies Evaluation of Photocatalytic Paints Under Indoor and Outdoor Air Conditions. Front. Chem. 2020, 8, 551710. [Google Scholar] [CrossRef] [PubMed]
- Teets, T.S.; Nocera, D.G. Photocatalytic hydrogen production. Chem. Commun. 2021, 47, 9268. [Google Scholar] [CrossRef]
- Davis, K.A.; Yoo, S.; Shuler, E.W.; Sherman, B.D.; Lee, S.; Leem, G. Photocatalytic hydrogen evolution from biomass conversion. Nano Converg. 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).