Abstract
Exclusive chemical conversions of polyesters [poly(ethylene adipate) (PEA), poly(butylene adipate) (PBA), poly(ethylene terephthalate) (PET), poly(butylene terephthalate) (PBT)] to the corresponding monomers (diethyl adipate, diethyl terephthalate, ethylene glycol, 1,4-butane diol) by transesterification with ethanol using Cp’TiCl3 (Cp’ = Cp, Cp*) catalyst have been demonstrated. The present acid-base-free depolymerizations by Cp’TiCl3 exhibited completed conversions (>99%) of PET, PBT to afford diethyl terephthalate and ethylene glycol or 1,4-butane diol exclusively (selectivity >99%) without formation of any other by-products in the NMR spectra (150–170 °C, Ti 1.0, or 2.0 mol%). The resultant reaction mixture after the depolymerization of PBA with ethanol via the CpTiCl3 catalyst (1.0 mol%, 150 °C, 3 h), consisting of diethyl adipate and 1,4-butane diol, was heated at 150 °C in vacuo for 24 h to afford high molecular weight recycled PBA with unimodal molecular weight distribution (Mn = 11,800, Mw/Mn = 1.6), strongly demonstrating a possibility of one-pot (acid-base-free) closed-loop chemical recycling.
1. Introduction
The chemical conversion of plastic waste to monomers (chemical recycling) or value-added fine chemicals (upcycling) is considered an important subject concerning establishing the circular economy [,,,]. Recently, there have thus been many reports related to the topic of the chemical recycling of (aliphatic) polyesters [,,,,]. Poly(ethylene terephthalate) (PET), one of the major commodity plastics, has been reused by mechanical recycling through the collection, sorting, cleaning, melting, and re-processing of transparent bottles. However, due to the limitation of supplying high-quality recycled resin as well as the strong demand to use more recycled resin in the long term, the importance of chemical recycling, where PET waste is depolymerized to monomers and purified into resin equivalent to the original one initially derived (produced) from petroleum, has been emphasized more recently [,,,,,,,,,]. There have been many studies concerning the depolymerization of (aliphatic) polyesters [,,,,,,,], including PET [,,,,,,,,,,,,,,,,,,,,]. Most methods required harsh conditions (high temperature, pressure) and/or excess base, acids, and/or inorganic salts [,,]. For example, PET was treated with ethylene glycol (called glycolysis) in the presence of a catalyst system of Zn(OAc)2—Na2CO3 (at 196 °C) [] or Zn(OAc)2—1,3-dimethylurea (at 190 °C) [], or a certain combination of acids and base (5 mol%, 180 °C) [] to afford bis(hydroxyethyl) terephthalate (BHET). In another approach, PET was treated with n-butanol in the presence of [HO3S-(CH2)3-NEt3]Cl–ZnCl2 at 205 °C [] or treated with methanol (called methanolysis) under high temperature (e.g., 280–310 °C) and high-pressure conditions (ca. 4 MPa, the addition of K2CO3 reduced the harsh conditions) [,,,]. Therefore, studies on the development of the “acid-base-free efficient catalytic chemical recycling to monomers (depolymerization of polyesters) under mild conditions” has been considered as one of the key subjects for the purpose [,,,,,,,,,,,]. The achievement should contribute to developing better environmentally benign chemical recycling and the circular economy.
Our laboratory focused on using the efficient transesterification catalysts of aliphatic fatty acid esters (FAEs, Scheme 1) for the depolymerization (transesterification) of polyesters by treating them with alcohol. We recently communicated that Cp’TiCl3 (Cp’ = Cp, Cp*), which enabled the efficient transesterification of FAEs (methyl-10-undecenoate as a model compound) with various alcohols, depolymerized poly(ethylene adipate) (PEA) and poly(butylene adipate) (PBA) via transesterification with alcohols (ethanol, cyclohexane methanol) to afford the corresponding monomers exclusively (Scheme 1, >99% conversion, >99% selectivity) []. Moreover, CaO, a known catalyst for the transesterification of plant oils (triglycerides) with ethanol [], was also an effective catalyst for the depolymerization of PEA and PBA with alcohols [] and La(acac)3 (acac = acetylacetonato) also depolymerized PET by treating it with methanol at 150 °C [].
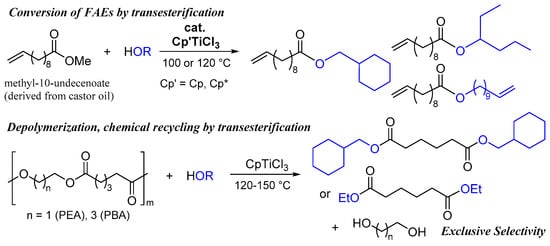
Scheme 1.
Acid-base-free catalytic transesterification of methyl-1-undecenoate and poly(ethylene adipate) (PEA), poly(butylene adipate) (PBA) with alcohol [].
In this paper, we present more results regarding the Cp’TiCl3 catalyzed transesterification (depolymerization) of PEA, PBA with ethanol and the results of that method applied to depolymerization of PET (one of the commodity polyesters, pellet and sheet by cutting the drink bottle) and PBT with ethanol (Scheme 2). The efforts should introduce the utilization of this simple (acid-base-free) catalytic chemical recycling of polyesters through simply mixing polymers and alcohols upon heating to convert monomers with exclusive selectivity []. Moreover, a possibility of one-pot re-preparation of polyester (PBA), closed-loop one-pot chemical recycling, has been demonstrated by subsequent condensation polymerization by removing ethanol in vacuo [].
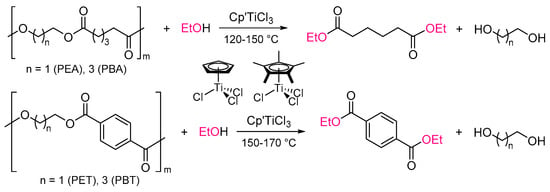
Scheme 2.
Depolymerization of polyesters (PEA, PBA, PET, PBT) by transesterification with ethanol.
2. Results and Discussion
2.1. Transesterification (Depolymerization) of Poly(Ethylene Adipate) (PEA) and Poly(Butylene Adipate) (PBA) with Ethanol
Depolymerizations (transesterifications) of PEA (500 mg scale, commercially available sample from Sigma-Aldrich, Mw = 10,000) with ethanol (5.0 mL) were conducted in the presence of CpTiCl3 [Cp’ = Cp, Cp* (C5Me5)], in a sealed reaction tube [], according to the previous report []. As shown in Figure 1a, the 13C NMR spectrum (in CDCl3 at 25 °C) for the mixture after the reaction (obtained by removing ethanol under reduced pressure) at 120 °C for 24 h (CpTiCl3 1.0 mol% based on monomer repeat unit) showed that resonances ascribed to PEA disappeared and the corresponding resonances ascribed to diethyl adipate (DEA) and ethylene glycol (EG) were observed without any other remarkable by-products []. Moreover, only DEA and EG were observed on the GC chart []. The results conducted at 150 °C are summarized in Table 1. As also communicated previously [], a similar NMR spectrum (Figure 1b) was observed from the reaction mixture after 3 h (run 2), and the GC yield of DEA was >99%; no other peaks ascribed to the by-products were observed on the GC chart []. The conversion of PEA to DEA may not be completed after 3 h (run 1), although no significant differences were observed in the 13C NMR spectra of the reaction mixture between (runs 1 and 2, with complete conversion of PEA). The depolymerization with ethanol was also completed with low catalyst loading (CpTiCl3 0.5 mol%) after 6 h (run 3). The reaction with Cp*TiCl3 showed similar catalyst performances (runs 4, 5), whereas the GC yield after the reaction with low catalyst loading (Cp*TiCl3 0.5 mol%, run 6) seemed rather low compared to that conducted with CpTiCl3 (run 3). The results could thus suggest that CpTiCl3 is more suited as the catalyst rather than Cp*TiCl3.
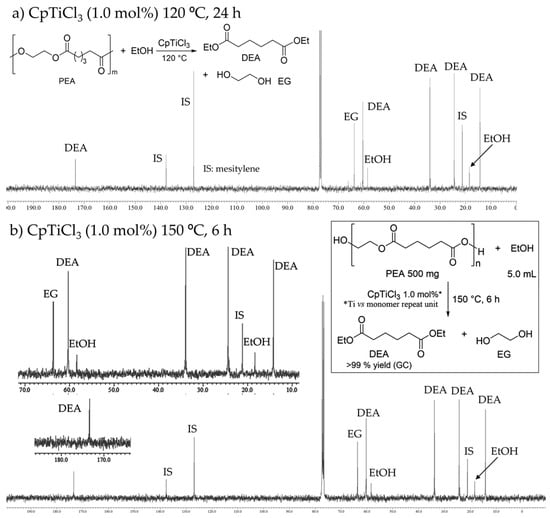
Figure 1.
13C NMR spectra (in CDCl3 at 25 °C) for transesterification of poly(ethylene adipate) (PEA) with ethanol by CpTiCl3 (1.0 mol%). Conditions: (a) CpTiCl3, 120 °C 24 h, (b) Cp*TiCl3, 150 °C 6 h (run 2).

Table 1.
Depolymerization (transesterification) of poly(ethylene adipate) (PEA), poly(butylene adipate) (PBA) with ethanol by Cp’TiCl3 (Cp’ = Cp, Cp*) at 150 °C 1.
The reactions with PBA (500 mg scale, commercially available sample from Sigma-Aldrich, Mw = 12,000) instead of PEA were also conducted, and the results at 150 °C are summarized in Table 1. As observed in the reaction of PEA, the 13C NMR spectrum for the mixture after the reaction (obtained by removing ethanol under reduced pressure) at 120 °C for 24 h (CpTiCl3 1.0 mol%) showed that resonances ascribed to PBA clearly disappeared, and the corresponding resonances ascribed to DEA and 1,4-butane diol (BD) were solely observed without any other by-products (Figure 2a); only DEA and BD were observed on the GC chart []. Moreover, as shown in Figure 2b, a similar NMR spectrum was observed from the reaction mixture when the reaction was conducted at 150 °C for 3 h (run 7), and GC yield was higher than 99% []. It seems that the conversion of PBA to DEA may not be reached completion when the reaction was conducted with low catalyst loading (CpTiCl3 0.5 mol%, run 8), although no significant differences were observed in the 13C NMR spectrum of the reaction mixture (with complete conversion of PBA). As observed in the reactions with PEA, Cp*TiCl3 showed rather low catalyst performances under the same conditions (runs 9–11, Table 1).
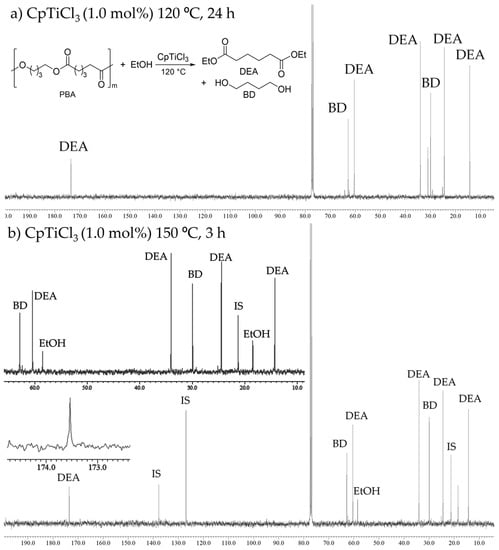
Figure 2.
13C NMR spectra (in CDCl3 at 25 °C) for transesterification of poly(butylene adipate) (PBA) with ethanol by CpTiCl3 (1.0 mol%). Conditions: (a) 120 °C 24 h, (b) 150 °C 3 h (run 7).
2.2. Transesterification (Depolymerization) of Poly(Ethylene Terephthalate) (PET) and Poly(Butylene Terephthalate) (PBT) with Ethanol
PET pellet (commercially available resin, IV = 0.80 ± 0.02 dL/g) and sheets prepared by cutting the used drink bottle (Figure 3) were used for the depolymerization via transesterification with ethanol. The resultant reaction mixture after 170 °C for 24 h (CpTiCl3 2.0 mol%, PET pellet 500 mg, EtOH 5.0 mL, run 15) showed a clear solution, and, as shown in Figure 4, both 1H and 13C NMR spectra (in CDCl3 at 25 °C) showed resonances ascribed to diethyl terephthalate (DET) and EG and no additional resonances ascribed to the by-products were seen. Only DEP and EG were also observed in the GC chart, as expected from the results of PEA and PBA. The result thus suggests that the present catalyst is also effective for acid-base-free depolymerization of PET. The results (with pellet) conducted under various conditions are summarized in Table 2.
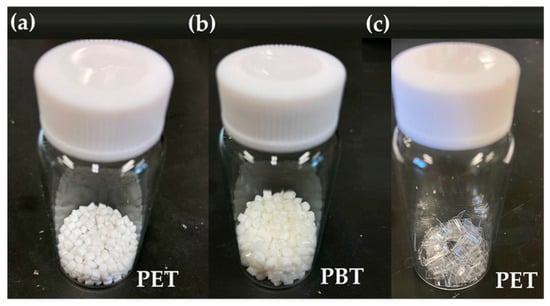
Figure 3.
Samples for commercially available (a) poly(ethylene terephthalate) (PET), (b) poly(butylene phthalate) (PBT) pellets, and (c) PET sheets by cutting the drink PET bottle.
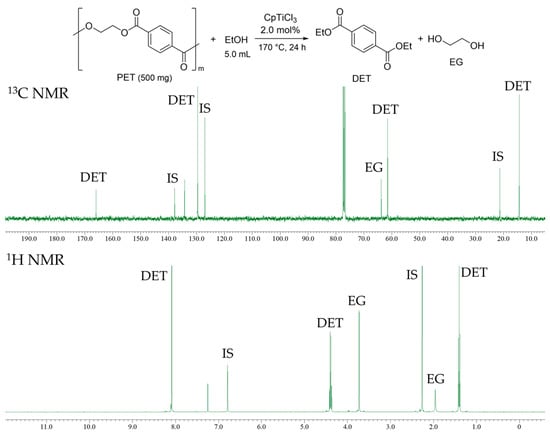
Figure 4.
13C (up) and 1H (bottom) NMR spectra (in CDCl3 at 25 °C) for the reaction mixture of transesterification of PET with ethanol by CpTiCl3 (2.0 mol%). Conditions: PET 500 mg, ethanol 5.0 mL, 170 °C, 24 h (run 15).

Table 2.
Depolymerization of PET with ethanol by Cp’TiCl3 (Cp’ = Cp, Cp*) 1.
Figure 5a shows the 13C NMR spectrum (in CDCl3) for the reaction mixture (obtained by removing ethanol under reduced pressure) in the transesterification of PET at 150 °C for 24 h (CpTiCl3 0.5 mol%, run 12). As observed in Figure 4, the spectrum consisted of resonances ascribed to DET and EG, and other resonances ascribed to the by-products were not seen, although the GC yield (vs. internal standard) was somewhat low (84%). The GC yield, however, seemed slightly increasing when the reaction was conducted with 1.0 mol% of CpTiCl3. The exclusive conversions of PET and yields of DET were achieved when these reactions were conducted at 160 and 170 °C (runs 14 and 15). Moreover, the reaction with PET sheets (Figure 3c) prepared by cutting the used drink bottle was also conducted at 170 °C for 24 h (run 16), affording DET and EG exclusively (93% isolated yield). The reaction mixture showed a clear, viscous liquid (photo in Figure 5b), and the 13C NMR spectrum only showed the resonances ascribed to DET and EG. The result thus clearly indicates no significant differences in the catalyst performances between the PET pellet and the sheets from the used drink bottle (run 15 vs. run 16), strongly suggesting that the present catalyst system can be applied to conventional PET resin.
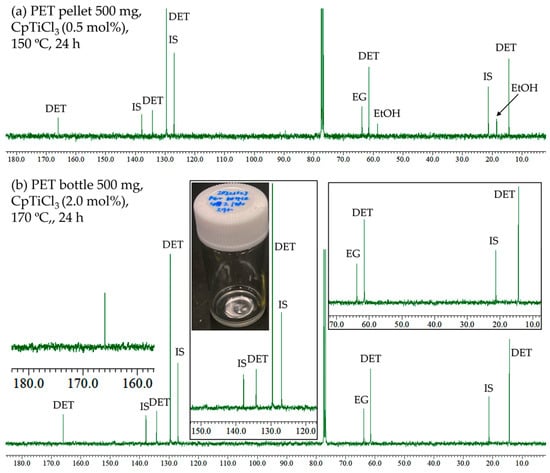
Figure 5.
13C NMR spectra (in CDCl3 at 25 °C) for transesterification of PET with ethanol by CpTiCl3. Conditions: (a) PET pellet 500 mg, ethanol 5.0 mL, CpTiCl3 (0.5 mol%), 150 °C, 24 h (run 12). (b) PET sheets (from the drinking bottle) 500 mg, ethanol 5.0 mL, CpTiCl3 (2.0 mol%), 170 °C, 24 h (run 16).
The results in the presence of Cp*TiCl3 conducted under similar conditions are also summarized in Table 2 (runs 17–21). As observed in the reaction by CpTiCl3, the complete conversions of PET were achieved even at 150 °C (run 17), although the GC yield was somewhat low compared to those conducted at 160 °C (run 19) and 170 °C (run 20) with higher Ti loading (2.0 mol%). The reaction with the PET sheets (prepared from the used bottle) also proceeded at 170 °C to afford both DET and EG exclusively (97% isolated yield, run 21). The resultant reaction mixture showed the pale yellow viscous liquid (photo in Figure 6, probably due to contamination of residual species formed from Cp*TiCl3), and both the 1H and the 13C NMR spectra showed that resonances ascribed to DET and EG were observed without resonances ascribed to the by-products.
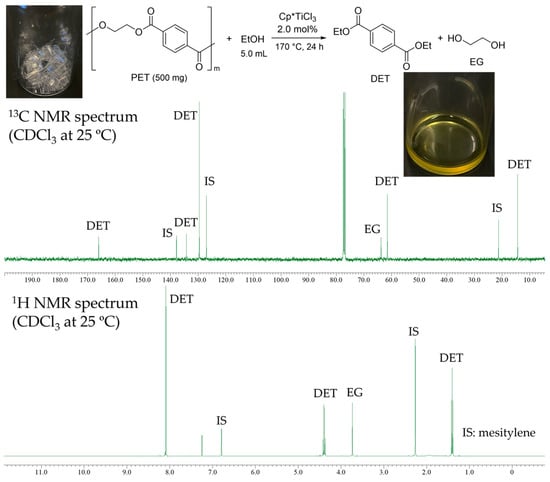
Figure 6.
NMR spectra (in CDCl3 at 25 °C) for transesterification of PET with ethanol by Cp*TiCl3 (2.0 mol%). Conditions: PET sheets (from the bottle) 500 mg, ethanol 5.0 mL, 170 °C, 24 h (run 21).
The transesterifications (depolymerizations) of the PBT pellet (Figure 3b, commercially available resin, Mn = 32,000) with ethanol were also conducted under similar conditions. As shown in Figure 7a,b, the NMR spectra after the reaction (170 °C, 24 h) showed resonances ascribed to DET and BD exclusively, and the other resonances were not observed. Moreover, the 13C NMR spectrum of the reaction mixture conducted at 150 °C showed similar to those conducted at 170 °C (Figure 7c). In both cases, the GC charts showed the two products (DET and BD) solely. Although the GC yields of DET were ca. 80% (79, 82%, at 150 °C and 170 °C, respectively), this could be refined after isolation of the DET and/or another analysis method. These results also indicate that Cp’TiCl3 enabled the depolymerization of PBT by transesterification with ethanol.
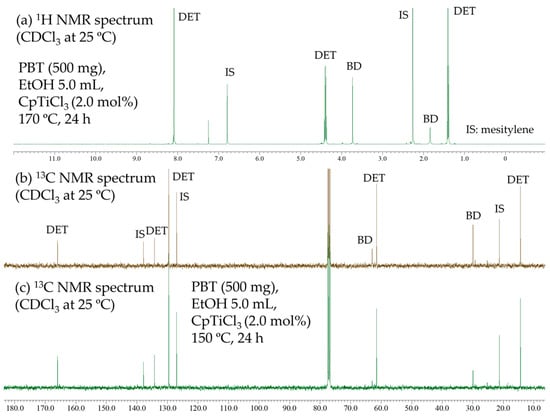
Figure 7.
NMR spectra (in CDCl3 at 25 °C) for transesterification of PBT with ethanol by CpTiCl3 (2.0 mol%). Conditions: PBT 500 mg, ethanol 5.0 mL, (a,b) 170 °C, 24 h; (c) 150 °C, 24 h.
2.3. One-Pot Synthesis of Poly(Butylene Adipate) by Condensation Polymerization from the Depolymerization Reaction Mixture
The resultant reaction mixture after the transesterification of PBA with ethanol should remain titanium alkoxide species derived from CpTiCl3 []; the mixture consisting of DEA and BD was heated in vacuo to proceed with the condensation polymerization with the removal of ethanol accompanied at 150 °C (Scheme 3). Certain titanium alkoxides, like Ti(OiPr)4, were used for condensation polymerization of the synthesis of polyesters []. After the reaction, the resultant highly viscous liquid (solid) was washed with methanol to collect as the white precipitates. The resultant PBA possessed high molecular weight with unimodal molecular weight distribution (Mn = 11,800, Mw/Mn = 1.6). The result demonstrates a possibility of the one-pot closed-loop chemical recycling (one-pot depolymerization and re-condensation polymerization) in the presence of titanium catalyst and ethanol.
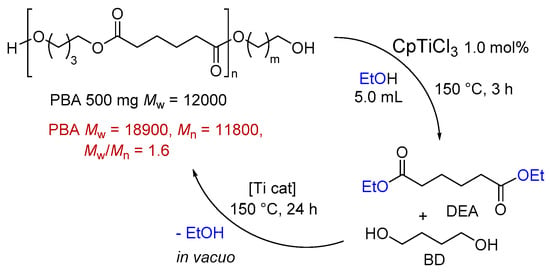
Scheme 3.
Closed-loop chemical recycling (one-pot depolymerization and re-condensation polymerization) of PBA by one-pot depolymerization/re-polycondensation.
3. Concluding Remarks
We have shown that Cp’TiCl3 (Cp’ = Cp, Cp*) enabled acid-base-free depolymerizations of a series of polyesters (PEA, PBA, PET, PBT) with ethanol by transesterification under rather mild conditions. These acid-base-free catalytic reactions proceeded to afford monomers (DEA, DET, EG, BD) with exclusive selectivity without any other by-products observed in both NMR spectra and GC charts. At this moment, it is not clear the reason for the rather low yields conducted at low temperatures (or terminated at the initial stage); one simple assumption is that these polymers were depolymerized to oligomer mixtures and converted to monomers eventually. The present simple, clean depolymerization methods should provide a new possibility for efficient chemical recycling of polyesters. Moreover, the one-pot closed-loop chemical recycling (one-pot depolymerization and re-condensation polymerization) should also provide another new possibility of chemical recycling and upcycling, a new methodology of depolymerization of plastic waste and subsequent condensation to afford new polyesters in the same catalysis.
4. Materials and Methods
All experiments were conducted under a nitrogen atmosphere using a glove box. Poly(ethylene adipate) (PEA, Mw ~ 10,000, Sigma-Aldrich Japan K.K., Tokyo, Japan), poly(1,4-butylene adipate) (PBA, Mw ~ 12,000, Sigma-Aldrich Japan K.K., Tokyo, Japan), ethanol (>99.5%, Kanto Chemical Co., Inc., Tokyo, Japan), and CpTiCl3 and Cp*TiCl3 (Tokyo Chemical Industry, Co., Ltd., Tokyo, Japan) were used as received. Poly(ethylene terephthalate (PET) resin (as pellet shown in Figure 3a, IV = 0.80 ± 0.02 dL/g) and poly(butylene terephthalate (PBT) resin (as pellet shown in Figure 3b, Mn = 32,000) were received from companies (by donation for research purpose). PET sheets (Figure 3c) were prepared by cutting the PET drink bottle. All NMR spectra (in CDCl3 at 25 °C) were acquired on a Bruker AV500 spectrometer (500.13 MHz for 1H, 125.77 MHz for 13C, Bruker Japan Com., Tokyo, Japan) using SiMe4 as the reference at 0.00 ppm (chemical shifts were reported in parts per million). The GC analysis used a Shimadzu gas chromatograph (GC-2014, Shimadzu Corp., Tokyo, Japan) equipped with a flame ionization detector (FID) through nitrogen as a carrier gas. Conditions are as follows (DB-1MS column, 30 m × 0.250 mm × 0.25 μm): column temp, 80 °C (4 min) followed by increasing up to 320 °C (20 °C/min) [injection 300 °C, flow (column) 1.71 mL/min]. Typical conditions and charts (exemplified in the depolymerization of PEA, PBA) in the depolymerization experiments are shown in the previous reports, supporting information [,].
General procedure for the transesterification of poly(ethylene adipate) (PEA), poly(butylene adipate) (PBA). An oven-dried reaction apparatus (15.0 mL scale screw cap) was charged with the prescribed amount of CpTiCl3, 200 mg (or 500 mg) of PEA or PBA, and 2.0 mL (or 5.0 mL) of ethanol under a nitrogen atmosphere. The reaction mixture was stirred at 120 °C or 150 °C using an alumina bath (sealed tube). After completion of the reaction, the mixture was cooled to room temperature and washed with CHCl3 (ca. 3 mL). The solution was then analyzed by GC to estimate the yield (quantitative analysis using a calibration curve vs. internal standard). Samples for 13C NMR spectra were prepared by removing volatiles (CHCl3, ethanol, etc.) in vacuo, and the remained solution was dissolved in CDCl3. Transesterification of poly(ethylene terephthalate) (PET), poly(butylene terephthalate) (PBT) was conducted under similar conditions to those in the transesterification (depolymerization) of PEA, PBA with ethanol, except that PET, PBT were used.
Author Contributions
Conceptualization, methodology, supervision, data curation, project administration, funding acquisition, K.N.; investigation, formal analysis, data curation, Y.O. (Yuriko Ohki); investigation, data curation, Y.O. (Yohei Ogiwara). All authors have read and agreed to the published version of the manuscript.
Funding
This project was partly supported by JST-CREST (Grant Number JPMJCR21L5).
Acknowledgments
Authors thank S. M. A. H. Siddiki and S. Sudhakaran (Tokyo Metropolitan University) for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mat. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Collias, D.I.; James, M.I.; Layman, J.M. (Eds.) Circular Economy of Polymers: Topics in Recycling Technologies; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Worch, J.C.; Dove, A.P. 100th Anniversary of macromolecular science viewpoint: Toward catalytic chemical recycling of waste (and future) plastics. ACS Macro Lett. 2020, 9, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Liu, Y.; Lou, X.; Zhang, Q.; Chen, J. Rational design of chemical catalysis for plastic recycling. ACS Catal. 2022, 12, 4659–4679. [Google Scholar] [CrossRef]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef] [PubMed]
- Westhues, S.; Idel, J.; Klankermayer, J. Molecular catalyst systems as key enablers for tailoredpolyesters and polycarbonate recycling concepts. Sci. Adv. 2018, 4, eaat966. [Google Scholar] [CrossRef]
- Basterretxea, A.; Jehanno, C.; Mecerreyes, D.; Sardon, H. Dual organocatalysts based on ionic mixtures of acids and bases: A step toward high temperature polymerizations. ACS Macro Lett. 2019, 8, 1055–1062. [Google Scholar] [CrossRef]
- Payne, J.; Jones, M.D. The chemical recycling of polyesters for a circular plastics economy: Challenges and emerging opportunities. ChemSusChem 2021, 14, 4041–4070. [Google Scholar] [CrossRef]
- Häußler, M.; Eck, M.; Rothauer, D.; Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 2021, 590, 423–427. [Google Scholar] [CrossRef]
- Allen, R.D.; James, M.I. Chemical Recycling of PET. In Circular Economy of Polymers: Topics in Recycling Technologies; Collias, D.I., James, M.I., Layman, J.M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; pp. 61–80. [Google Scholar]
- Paszun, D.; Spychaj, T. Chemical recycling of poly(ethylene terephthalate). Ind. Eng. Chem. Res. 1997, 36, 1373–1383. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Dupont, L.A.; Gupta, V.P. Degradative transesterification of terephthalate polyesters to obtain DOTP plasticizer for flexible PVC. J. Vinyl. Technol. 1993, 15, 100–104. [Google Scholar] [CrossRef]
- Chen, J.-W.; Chen, L.-W. The glycolysis of poly(ethylene terephthalate). J. Appl. Polym. Sci. 1999, 73, 35–40. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, C.-Y.; Lo, Y.-W.; Mao, C.-F.; Liao, W.-T. Studies of glycolysis of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. I. Influences of glycolysis conditions. J. Appl. Polym. Sci. 2001, 80, 943–948. [Google Scholar] [CrossRef]
- Mansour, S.H.; Ikladious, N.E. Depolymerization of poly(ethylene terephthalate) wastes using 1,4-butanediol and triethylene glycol. Polym. Test. 2002, 21, 497–505. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ohshima, M.; Sugiyama, K.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Satbil. 2003, 79, 529–533. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R.; Gitsov, I. A novel catalyst for the glycolysis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2003, 90, 1148–1152. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Arnaiz, S.; Gutiérrez-Ortiz, J.I. Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts. Polym. Degrad. Stab. 2010, 95, 1022–1028. [Google Scholar] [CrossRef]
- Fukushima, K.; Coulembier, O.; Lecuyer, J.M.; Almegren, H.A.; Alabdulrahman, A.M.; Alsewailem, F.D.; Mcneil, M.A.; Dubois, P.; Waymouth, R.M.; Horn, H.W.; et al. Organocatalytic depolymerization of poly(ethylene terephthalate). J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1273–1281. [Google Scholar] [CrossRef]
- Fukushima, K.; Coady, D.J.; Jones, G.O.; Almegren, H.A.; Alabdulrahman, A.M.; Alsewailem, F.D.; Horn, H.W.; Rice, J.E.; Hedrick, J.L. Unexpected efficiency of cyclic amidine catalysts in depolymerizing poly(ethylene terephthalate). J. Polym. Sci. Part A Polym.Chem. 2013, 51, 1606–1611. [Google Scholar] [CrossRef]
- Imran, M.; Kim, D.H.; Al-Masry, W.A.; Mahmood, A.; Hassan, A.; Haider, S.; Ramay, S.M. Manganese-, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly(ethylene terephthalate) via glycolysis. Polym. Degrad. Satbil. 2013, 98, 904–915. [Google Scholar] [CrossRef]
- Liu, S.W.; Wang, Z.P.; Li, L.; Yu, S.T.; Xie, C.X.; Liu, F.S. Butanol alcoholysis reaction of polyethylene terephthalate using acidic ionic liquid as catalyst. J. Appl. Polym. Sci. 2013, 130, 1840–1844. [Google Scholar] [CrossRef]
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis acid–base synergistic catalysis for polyethylene terephthalate degradation by 1,3-Dimethylurea/Zn(OAc)2 deep eutectic solvent. ACS Sustain. Chem. Eng. 2018, 7, 3292–3300. [Google Scholar] [CrossRef]
- Kaiho, S.; Hmayed, A.A.R.; Chiaie, K.R.D.; Worch, J.C.; Dove, A.P. Designing thermally stable organocatalysts for poly(ethylene terephthalate) synthesis: Toward a one-pot, closed-loop chemical recycling system for PET. Macromolecules 2022, 55, 10628–10639. [Google Scholar] [CrossRef]
- Chiaie, K.R.D.; McMahon, F.R.; Williams, E.J.; Price, M.J.; Dove, A.P. Dual-catalytic depolymerization of polyethylene terephthalate (PET). Polym. Chem. 2020, 11, 1450–1453. [Google Scholar] [CrossRef]
- Du, J.-T.; Sun, Q.; Zeng, X.-F.; Wang, D.; Wang, J.-X.; Chen, J.-F. ZnO Nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate. Chem. Eng. Sci. 2020, 220, 115642–115651. [Google Scholar] [CrossRef]
- Pham, D.D.; Cho, J. Low-energy catalytic methanolysis of poly(ethylene terephthalate). Green Chem. 2021, 23, 511–525. [Google Scholar] [CrossRef]
- Tanaka, S.; Sato, J.; Nakajima, Y. Capturing ethylene glycol with dimethyl carbonate towards depolymerisation of polyethylene terephthalate at ambient temperature. Green Chem. 2021, 23, 9412–9416. [Google Scholar] [CrossRef]
- Abe, R.; Komine, N.; Nomura, K.; Hirano, M. La(iii)-Catalysed degradation of polyesters to monomers via transesterifications. Chem. Commun. 2022, 58, 8141–8144. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Aoki, T.; Ohki, Y.; Kikkawa, S.; Yamazoe, S. Transesterification of methyl-10-undecenoate and poly(ethylene adipate) catalyzed by (cyclopentadienyl)titanium trichlorides as model chemical conversions of plant oils and acid-, base-free chemical recycling of aliphatic polyesters. ACS Sustain. Chem. Eng. 2022, 10, 12504–12509. [Google Scholar] [CrossRef]
- Unruean, P.; Nomura, K.; Kitiyanan, B. High conversion of CaO-catalyzed transesterification of vegetable oils with ethanol using closed reactor system. J. Oleo Sci. 2022, 71, 1051–1062. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Siddiki, S.M.A.H.; Kitiyanan, B.; Nomura, K. CaO catalyzed transesterification of ethyl 10-undecenoate as a model reaction for efficient conversion of plant oils and their application to depolymerization of aliphatic polyesters. ACS Sustain. Chem. Eng. 2022, 10, 12864–12872. [Google Scholar] [CrossRef]
- The Results Were Partly Introduced in 11th The International Symposium on Feedstock Recycling of Polymeric Materials (ISFR2022), Keynote X, Pattaya, Thailand, December 2022. Available online: https://www.chula.ac.th/en/news/85547/ (accessed on 3 October 2022).
- A High-Performance Catalyst That Dissolves Polyester and Realizes Chemical Recycling. Available online: https://www.eurekalert.org/multimedia/960202 (accessed on 3 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).