Abstract
Plastic waste poses a serious threat to the environment and it has been increasing at an alarming rate. In 2022, global plastic waste generation was reported to be around 380 million tonnes as compared to 353 million tonnes in 2019. Production of liquid fuel from plastic waste is regarded as a viable method for disposing of the plastic and utilizing its energy. Currently, a wide range of technologies have been explored for turning plastic waste into fuel, including the conventional pyrolysis, incineration, gasification and advanced oxidation. However, a systematic summary and comparative analysis of various technologies has still not reported. Traditional non-biodegradable plastic waste (NPW) treatment methods include landfilling and incineration, but these methods encounter bottlenecks and are unable to adequately address NPW issues. This review attempts to present a thorough summary of treatment methods for plastic waste (both conventional and novel treatment technologies that have recently been reported), examine their mechanism and their current state of development. Furthermore, the superiority and drawbacks of each technology are analysed and the prospects of technology application are proposed. By tackling the problems of white pollution and energy scarcity, this review intends to inspire the use of solid waste as a source of energy.
1. Introduction
Plastic materials like polyethylene terephthalate (PET), low-density polyethylene (LDPE), high-density polyethylene (HDPE), polyvinyl chloride (PVC), polypropylene (PP) and polystyrene (PS) play a significant role in our daily lives including uses in a wide variety of products, like packaging, electronics, aircrafts, sporting goods and automobiles. Its widespread use results from its unmatchable advantages and characteristics when compared to other available materials [1]. One of the most significant components of solid waste management, and a notable fraction of municipal solid waste (MSW), are plastics with an overall global production of around 390 million metric tonnes in 2021, much higher than in the year 1950 (1.7 million metric tonnes). This is expected to reach 1800 million metric tonnes in 2050 [2,3]. While plastic production has increased at such a rapid rate, environmental issues have recently drawn attention and concern on a global scale.
Today’s greatest challenges are the management of plastic waste and the reduction of environmental plastic pollution, especially keeping in mind the increasing demand for plastic products. Owing to the challenges present in the current scenario, there is a need to urgently switch the strategy of plastic handling from landfilling and incineration towards a more sustainable and environmentally friendly alternative. Plastic waste can be treated via different pathways as illustrated in Figure 1.
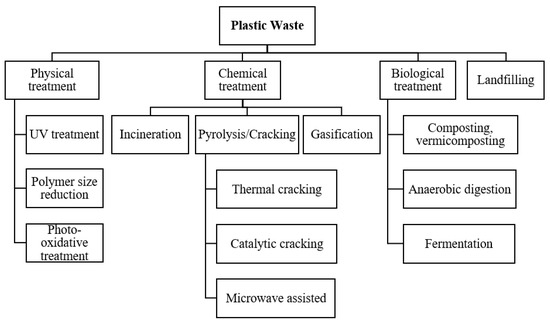
Figure 1.
Pathways for plastic waste management.
One of the most efficient ways of handling plastic waste is to extract and utilize energy from it. Compared to incineration and landfill disposal, fuel conversion from plastics can reduce hazardous emissions and pathogen contamination. In the process of creating fuel from waste plastic, reaction factors (such as temperature, residence time and rate of increase of temperature) can control the overall conversion process.
Various fractions of hydrocarbons can be produced from plastic waste when subjected to chemical treatment (namely, gasification, pyrolysis and catalytic degradation) [4]. By using these thermochemical processes, plastic waste can be transformed into variety of energy products (electricity, process heat for industrial facilities and automobile fuels) [5]. In this regard, chemical treatment of plastic waste emerges as a more sustainable approach as the conversion efficiency is sufficiently high and energy can be recovered from the plastic waste, leading to fuel production, syngas/hydrogen (H2) production and various other chemical productions [6]. Reviews on the pyrolysis and catalytic cracking of plastic waste into fuels with C8-C22 aromatic and aliphatic hydrocarbon have been published [7,8,9]. The composition and characteristics of product fuels were analysed in relation to the effects of pyrolysis and catalytic cracking parameters [10,11,12].
Notably, many studies on innovative technologies, like hydrothermal liquefaction and advanced oxidation processes, have been undertaken recently describing plastic waste conversion [13,14]. Compared to conventional pyrolysis, hydrothermal liquefaction allows for plastic waste conversion into excellent liquid oils at a significantly lower temperature. Additionally, high purity oil can be produced by advanced oxidation of plastic at normal temperature and pressure. In advanced oxidation systems, polymers break down to H2 in the presence of hydroxyl (·OH) species [15]. During the process, formation of carboxylic acids (R-COOH) takes place, making plastics act as carbon source for chemical sector. Additionally, while using photoreduction or electrocatalysis, the CO2 generated during the advanced oxidation process can be transformed into acetic acid [16].
Even though a number of review papers on plastic treatment technologies have been published, they were unable to offer a systematic and in-depth analysis of both conventional and advanced treatment methods. Therefore, without concentrating explicitly on a single polymer or technique, this study summarizes various conventional and advanced oxidation strategies for the treatment of plastic waste and examines their processes and states of development. The review also highlights the merits and demerits of various available techniques while introducing readers to cutting-edge technologies for turning plastic waste into fuel. Based on various kinds of plastic waste, more effective methods can be created, encouraging the production of target fuels by further regulating system parameters. This review focused on advanced oxidation and conventional thermal treatment techniques for efficiently utilizing energy from plastics. The goal of this review is to enhance knowledge about different techniques that could help in achieving efficient plastic waste conversion while promoting the safe disposal of solid waste and its utilization in the form of energy. Researchers with specialization in solid waste may find this review to be useful as a source of key information.
2. Bibliometric Analysis
The goal of bibliometric analysis on plastic waste is to identify the importance and levels of different research that have been completed or remain in progress pertaining to the conversion of plastic waste through different routes. Researchers in this field could use critical analysis to construct extended research methods employing this information. Using Web of Science (WOS), an online scientific citation indexing database managed by Clarivate Analytics, a thorough analysis of the currently available scientific literature in this field of study was conducted. The topic field tag was used to conduct the literature search using the terms “plastic waste treatment” or “pyrolysis and gasification of plastic waste” or “plastic waste to fuel” and “advanced oxidation technique for plastic waste”. 2237 publications that matched this search criteria were returned by the database, with environmental sciences, environmental engineering, energy and fuels, chemical engineering, green sustainable science and technology, environmental studies and polymer science making up the top seven fields of study.
In this study, a network map was created using VOSviewer software for analysing and visualising the keywords. The circles in the network map (Figure 2) show the frequency of keywords in the chosen dataset; the larger the bubble, the more frequently the keyword occurs. The lines that connect the bubbles also demonstrate how the keywords are related to one another; the stronger the association, the closer the circles, and the shorter the lines. By default, only 1000 lines representing the 1000 strongest relationships are shown.
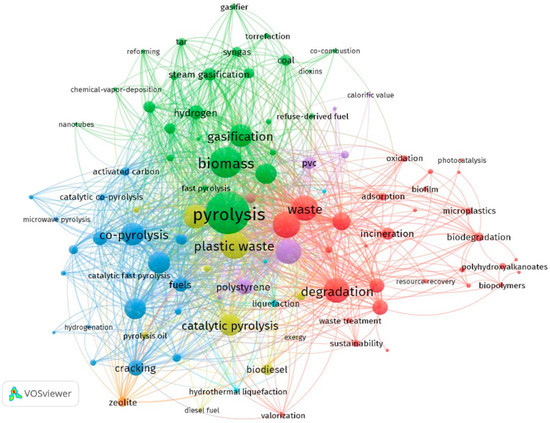
Figure 2.
Network map showing co-occurrence of keywords for plastic waste.
The network map was created by choosing keywords that appeared at least ten times. Out of 7971 total keywords, 418 matched the criteria and were grouped into eight major clusters. The keywords that have found most importance in the past three years include “plastic pyrolysis”, “catalytic pyrolysis”, “photocatalysis” and “gasification”. Although conversion of plastic waste to valuable products has been under investigation for a significant period, it wasn’t until 2015 to 2019 that major publications in the field of plastic waste pyrolysis and gasification showed the technique of pyrolysis being exploited to its full potential. However, gasification is still under research and is in its development stage in terms of parameter optimization and improved product yield. Much scope exists to improve the yield of liquid product obtained through catalytic pyrolysis by changing catalyst or optimizing process parameters in accordance with catalyst. Recently, research on advanced oxidation techniques, such as photodegradation using photocatalyst, has taken a leap and researchers are investigating and analysing its potential for obtaining valuable product.
3. Conventional Techniques for Thermal Treatment of Plastic Waste
3.1. Pyrolysis
Pyrolysis refers to thermal degradation of plastic waste in the absence of oxygen to produce liquid and gaseous fuels [17]. Temperature for the pyrolysis process generally ranges between 300–700 °C. It offers several benefits as compared to other ways of handling plastic waste, such as greater strength and flexibility, elimination of cost associated with segregation, washing and blending before mechanical recycling, the ability to treat both thermoset and thermoplastic and in the handling of polymer composites in engineering applications [18]. Composition of final product and yield is dependent on the composition of feedstock, temperature, reactor configuration, rate of heating and catalyst [19]. The choice of reactor has a significant impact on the efficiency of the reaction leading to the desired end product, residence time, heat transfer, and mixing of the polymers and catalysts. Most of the plastic pyrolysis in laboratories was carried out in batch, semi-batch or continuous-flow reactors like fluidized beds, fixed-bed reactors and conical spouted beds (CSBR).
A batch reactor is essentially a closed system as no reactants or products can enter or leave it while the reaction is taking place. One of the benefits of a batch reactor is that a high conversion can be attained by keeping the reactant in the reactor for a long time. The variability of the product from batch to batch, high labor expenses in each batch and the difficulties of large-scale manufacturing are the drawbacks associated with the batch reactor. A semi-batch reactor, however, enables simultaneous reactant addition and product removal. Regarding reaction selectivity, the semi-batch reactor also has the option of gradually introducing reactants. Semi-batch reactors have similar labor cost issues as batch reactors. As such, they are better suited for small-scale production.
The catalyst is often palletized and packed in a static bed in fixed-bed reactors. Although it is simple to design, there are certain limitations, like the irregular particle size and type of plastics used as feedstock, which present issues during the feeding process. Additionally, the reaction has a finite amount of access to the catalyst’s surface area. In some circumstances, fixed-bed reactors are only employed as the secondary pyrolysis reactor, because the product from primary pyrolysis may be readily fed into the fixed-bed reactor, which typically comprises liquid and gaseous phases.
However, some of the issues that arise within fixed-bed reactors are resolved by fluidized bed reactors. In contrast to a fixed-bed reactor, the catalyst in fluidized bed reactors is carried in a fluid condition by the fluidizing gas as it travels over a distributer plate. Since the catalyst is well-mixed with the fluid, a greater surface area for the reaction to occur is provided and there is improved access to the catalyst [20]. Due to the lower operating cost, a fluidized bed reactor would therefore be the optimal reactor to deploy in the pilot plant on a conventional design size.
The conical spouted bed reactor (CSBR) offers good mixing and can handle larger particles, a wide range of particle densities and broad particle size distribution. Olazar et al. [21] asserted that a CSBR outperformed bubbling fluidized beds in terms of attrition and bed segregation. Additionally, it had a moderate de-fluidization issue while working with sticky solids and a high heat transfer rate between phases. However, several technical difficulties with the reactor’s operation, including catalyst feeding, catalyst entrainment and product (solid and liquid) collection, make it less advantageous.
3.1.1. Thermal Pyrolysis
In this process, depolymerization or cracking of plastics take place at a temperature ranging between 350–900 °C in absence of oxygen, thus producing liquid and gaseous fuel and carbonized char [22]. The condensable component of the volatile product is normally recovered after condensation as liquid fuel, while the remaining portion is a non-condensable gas of high calorific value. Thermal pyrolysis of plastic waste can used to convert the plastic waste to both liquid and gaseous fuel on an economically viable scale. Table 1 summarizes various studies that have been undertaken on thermal pyrolysis.

Table 1.
Summary of research studies carried out by various research groups on thermal pyrolysis of plastic waste.
3.1.2. Microwave-Assisted Pyrolysis
In this process, a dielectric material is heated by microwave radiation and thus referred to as microwave pyrolysis. The interaction of microwave radiation with materials depends on their dielectric qualities. The incoming microwaves may be reflected as with conductors or transmitted as in the case of perfect insulators. They may even get absorbed and decay inside some material. Agitation of molecules in the presence of electromagnetic fields results in the generation of heat. With plastic, the microwave energy is absorbed through the absorbent and, then it transfers thermal heat to the plastic by conduction. The uniformity of heating dispersion is influenced by the absorbent’s physical qualities and volume ratio. Additionally, varying microwave power results in radically different product dispersion. Microwave-induced pyrolysis has been proven to be a viable method for producing value-added chemicals and fuels due to its significant advantages over thermal and catalytic pyrolysis [49]. The method could be used to heat plastics quickly, volumetrically and selectively for energy recovery. Plastics, meanwhile, are unable to absorb microwave energy due to their low dielectric loss factor. Therefore, to favour pyrolysis, plastic must be combined with the absorbent. Plastic materials with a high dielectric loss factor, like shredded tyres and silicon carbide, carbon and iron mesh, are ideal choices [32,33,34]. Various studies have been conducted on pyrolysis of plastic waste using microwave dielectric heating as shown in Table 2.

Table 2.
Summary of studies on microwave pyrolysis of plastic waste.
3.1.3. Catalytic Pyrolysis
A catalyst speeds up the chemical reactions without affecting the process as it progresses. In industry and research, catalysts are frequently employed to enhance product selectivity and optimize product distribution. In order to produce products with significant economic value like automobile fuel (diesel and gasoline) and C2-C4 olefins, which are in high demand in the petrochemical industry, catalytic degradation is therefore particularly intriguing. When a catalyst is utilized, the process activation energy is reduced, accelerating the pace of reaction. As a result, a catalyst lowers the temperature needed, which is significant as the pyrolysis process consumes a lot of energy (it is extremely endothermic). Heat is one of the most expensive factors in any industry; therefore, using a catalyst may assist in saving energy. Additionally, numerous researchers employed catalysts for product improvement and to enhance the hydrocarbon distribution in order to produce pyrolysis liquid with qualities comparable to those of conventional fuels like gasoline and diesel.
Polymer degradation is brought about by catalytic pyrolysis in which the material is heated in the presence of catalyst and in absence of oxygen. Catalyst usage offers several advantages like reduced consumption of energy, modified composition of product on cracking and reduced process time. In literature, zeolites, fluid catalytic cracking (FCC) and silica-alumina based catalysts are mostly reported for pyrolysis of plastic waste [35,36,37]. Zeolite-based catalysts have a greater acid strength compared to non-zeolite catalysts, and they often accomplish higher conversion and produce more gaseous product.
There are two methods for catalytic upgradation of pyrolysis vapors: in-situ and ex-situ. In the in-situ approach, the catalyst is combined with the feedstock and added to the pyrolysis reactor, while in the ex-situ mode, the catalyst is added to a different reactor situated downstream of the pyrolysis reactor. In-situ catalytic pyrolysis does not require a separate catalytic reactor, thereby lowering the overall capital cost of setup manufacturing and operational costs. However, the catalyst deactivates more quickly if the feedstock has a high mineral and ash content [50].
The fact that the catalytic temperature is the same as the pyrolysis temperature, and that the optimal pyrolysis temperature may not be ideal for catalytic cracking, is another drawback of the in-situ catalyst application approach [51]. An ex-situ catalytic upgrading mode is thought to be more appealing for feedstock with a high ash concentration than an in-situ mode. Additionally, char may be obtained as a useful product and catalyst regeneration is also simple. An additional element that makes this mode more adaptable and hence desirable is the provision of separate temperature controls for pyrolysis and catalysis [52].
Either mode of adding the catalyst will result in the conversion of oxygenated molecules into stable hydrocarbons. However, the yield of important hydrocarbons in oil also varies based on the feedstock, the kind of reactor and the operating circumstances. An in-situ contact method increased aromatics production in the catalytic pyrolysis of polyethylene (PE), polypropylene (PP) and polyethylene terephthalate (PET), but an ex-situ mode was shown to be more successful in increasing aromatic yield in the case of polystyrene (PS) [50]. However, there are very little research examining the impact of the catalyst contact method in the simultaneous pyrolysis of plastic and biomass.
Zeolite Catalyst
Zeolites are 3-D crystalline solids of aluminosilicate with small openings/pores that have ion-exchange properties [53]. It is made up of various SiO2/Al2O3 ratios, depending on the kind. The zeolite reactivity is determined by the SiO2/Al2O3 ratio, which has an impact on the pyrolysis end product. According to research by Artetxe et al. [54], the HZSM-5 zeolite’s SiO2/Al2O3 ratio had a significant impact on the yield of the product fraction during HDPE pyrolysis. The strong acidity of zeolite was revealed by its low SiO2/Al2O3 ratio. In comparison to the least acidic catalyst (SiO2/Al2O3 = 280), the highest acidic catalyst (SiO2/Al2O3 = 30) was more active in cracking waxes, yielding higher light olefins and a lower heavy fraction of C12-C20. The yield of light olefins increased from 35.5 to 58.0 wt% with the reduction of the SiO2/Al2O3 ratio from 280 to 30, while the yield of C12-C20 reduced from 28.0 to 5.3 wt%.
Other zeolite catalyst examples include HUSY and HMOR, which are frequently employed in the catalytic pyrolysis of plastic. With a polymer to catalyst ratio of 40%, Garfoth et al. [55] investigated the effectiveness of three distinct zeolite catalysts for the HDPE pyrolysis: HZSM-5, HUSY and HMOR. According to their research, HZSM-5 showed better catalytic activity than HUSY and HMOR since it left significantly less residue behind (4.53 wt%) than HUSY and HMOR (7.07 wt% and 8.93 wt%, respectively). This demonstrates that, in comparison to other zeolites, HZSM-5 can maximize the total product conversion in plastic pyrolysis.
Marcilla et al. [31] investigated the performance of the HZSM-5 and HUSY catalysts on HDPE and LDPE at a constant temperature of 550 °C and a polymer to catalyst ratio of 10%. When utilizing the HUSY catalyst (HDPE = 41.0 wt%, LDPE = 61.6 wt%) as opposed to the HZSM-5 catalyst (HDPE = 17.3 wt%, LDPE = 18.3 wt%), more liquid oil was recovered. HZSM-5 catalyst, on the other hand, produced more of the gaseous product (HDPE = 72.6 wt%, LDPE = 70.7 wt%). This demonstrates that the product selectivity of various catalysts varies. Lin and Yen [56] reported the similar trend of product selectivity on PP pyrolysis employing the HZSM-5 and HUSY zeolites.
Seo et al. [57] looked into the impact of HZSM-5 during the pyrolysis of HDPE at 450 °C with a catalyst to polymer ratio of 20 wt%. They noticed that HZSM-5 produced more gaseous product with 63.5 wt% and a relatively low liquid yield of roughly 35 wt%. When conducting HDPE pyrolysis at 500 °C with the same catalyst to polymer ratio as Seo et al. [57], Hernández et al. [58] got considerably lower liquid yields of around 4.4 wt% but a 86.1 wt% gaseous output. Lin and Yen [56] also used HZSM-5 and HUSY zeolite catalysts and obtained a very small amount of liquid product during the pyrolysis of PP at 360 °C. Only 2.31 wt% and 3.75 wt% of liquid were produced, respectively.
Researchers have examined the efficacy of the zeolite catalyst in two-step reactions comprising sequential thermal and catalytic reactors in addition to the direct cracking of plastics. Aguado et al. [59] investigated the catalytic conversion of LDPE in a two-step reaction process, using batch and fixed-bed reactors. The batch reactor conducted the thermal cracking of the plastic and the vapors produced were transferred to the fixed-bed reactor, which contained the 10 wt% HZSM-5 catalyst. The pyrolysis was carried out between 425 and 475 °C. According to the findings, the gas fraction significantly increased during catalytic reforming over the zeolite catalyst. At the highest temperature, the gaseous product increased to 74.3 wt% while only 21.9 wt% of the liquid hydrocarbons were collected. As a result, the pattern of results that were seen was very similar to direct catalytic cracking, which generated a considerable amount of gaseous product while using the HZSM-5 catalyst.
FCC Catalyst
Zeolite crystals and a non-zeolite acid matrix known as silica-alumina, which are joined by a binder, make up the FCC catalyst [38,39,40,41]. Due to its great product selectivity and thermal stability, zeolite-Y has been the primary component of FCC catalyst for more than 40 years [60]. The FCC catalyst is typically employed in the petroleum refining industry to break heavy oil fractions from crude petroleum into lighter and more marketable gasoline and liquefied petroleum gas (LPG) [60].
At 400 °C and a catalyst/polymer ratio of 10%, Kyong et al. [33] investigated the efficacy of the FCC catalyst to the various types of plastics. They noticed that the liquid yields for HDPE, LDPE, PP and PS ranged from 80 to 90 wt%. This outcome demonstrates that the FCC catalyst can increase the liquid yield during the pyrolysis process for most plastic types. In PP pyrolysis at 450 °C with a 10 wt% catalyst/polymer ratio, Abbas-Abadi et al. [41] likewise got a very high liquid oil yield of around 92.3 wt%. Spent FCC catalyst, which is referred to as used FCC catalyst, is often available from commercial FCC processes in petroleum refineries. Although it contains varying degrees of contamination, it is still valuable and can be utilized again throughout the pyrolysis process.
The impact of using spent FCC catalyst on the pyrolysis of HDPE, LDPE, PP and PS in a stirred semi-batch reactor at 400 °C was examined by Kyong et al. [33]. 200 g of reactants and 20 g of catalyst were combined and they were heated at a rate of 7 °C/min. All polymers produced more liquid oil than 80 wt%, with PS having the highest yield (about 90 wt%). According to the different plastic kinds, the liquid yields were sorted as follows: PS–PP–PE (HDPE, LDPE). The yield of the gaseous product was in the following order, which was reversed from that of the liquid: PS > PP > PE. This demonstrates that PS had a more stable structure since it contained a benzene ring, which caused less cracking of the gaseous product. Overall, it can be said that wasted FCC catalyst still exhibits strong catalytic performance because the liquid yield for all plastic samples was above 80 wt%. Since it is a “used” catalyst, it is also more affordable.
To maximize the pyrolysis product conversion, it is also necessary to consider the restriction of the polymer ratio to the catalyst. Abbas-Abadi et al. [41] used a semi-batch stirred reactor to test various ratios of HDPE to FCC catalyst ranging from 10 to 60 wt% at a constant temperature of 450 °C. According to the study, a catalyst/polymer ratio of 20 wt% provided the highest conversion into liquid. The amount of liquid produced was extremely high (91.2 wt%), while gaseous and coke content were 4.1 wt% and 4.7 wt%, respectively. More coke and gas were created as the catalyst/polymer ratio was raised above 20 wt%, which reduced the amount of liquid produced.
Silica–Alumina Catalyst
The amorphous acid catalyst silica–alumina has Bronsted acid sites with ionizable H2 atoms and Lewis’s acid sites, which accept electrons. The mole ratio of SiO2/Al2O3 determines the acid content of silica alumina catalyst. Unlike zeolite, the acid strength of silica–alumina is determined in the other direction, with a high SiO2/Al2O3 ratio indicating a high acid strength. For example, SA-1 (SiO2/Al2O3 = 4.99) has a higher acidity than SA-2 (SiO2/Al2O3 = 0.27) and both are commercially available silica–alumina [61].
The end product of plastic pyrolysis is significantly influenced by the different acidity strengths of the catalyst. Sakata et al. [61] investigated how the acidity of the catalysts (SA-1, SA-2, ZSM-5) affected the distribution of the HDPE pyrolysis product. In the experiment, 1 g of catalyst and 10 g of HDPE were combined in a semi-batch reactor operating at 430 °C. Using NH3 temperature programmed desorption (TPD), the acidity of the catalysts was evaluated and presented in the following order: SA-1 > ZSM-5 > SA-2. Consequently, it was found that SA-2 catalyst, which had a lower acidity than SA-1 and ZSM-5, produced a higher amount of liquid oil (74.3 wt%). Strong acid sites in ZSM-5 allowed it to create more gaseous products than the other two acid catalysts, but with much lower liquid yields.
At the same temperature and catalyst/polymer ratio, the impact of SA-2 on HDPE and LDPE has also been investigated by Uddin et al. [38]. Their results for liquid oil yield did not differ significantly from those of Sakata et al. When SA-2 catalyst was used, HDPE and LDPE pyrolysis yielded 77.4 wt% and 80.2 wt%, respectively. Low liquid yield was obtained in HDPE pyrolysis because of its stronger structure resulting from the presence of linear chain.
Sakata et al. [36] also studied the silica-alumina catalyst’s efficacy on PP at 380 °C with different catalyst contact modes (liquid phase and vapor phase). For the liquid phase, the PP pellets and the catalyst were combined before being placed into the batch reactor. In contrast, the catalyst for the vapor phase was suspended 10 cm from the reactor’s bottom on a stainless-steel net. They discovered that the liquid mode of contact produced higher liquid yield (68.8 wt%) because the wax residue decomposed into a lighter hydrocarbon on the silica-alumina catalyst. On the other hand, more gaseous product (35 wt%) was produced by the catalyst in the vapor phase as the hydrocarbon continued to decompose over the silica-alumina catalyst. Therefore, since it affected the distribution of the final product in plastic pyrolysis, the catalyst mode was a significant issue that requires attention.
Under specific temperature ranges, the reactivity of a catalyst can also be tuned. HDPE and PP were pyrolyzed using a silica-alumina catalyst at 500 °C in a fluidized bed reactor by Luo et al. [34]. The liquid oil obtained exceeded the levels found in the tests carried out by Sakata et al. [36] and Uddin et al. [38] for HDPE (85.0 wt% on average) and PP (90 wt% on average). This demonstrates that temperature is crucial for maximizing catalyst performance and increasing the liquid oil production during the plastic pyrolysis process.
The different catalysts that have been used to date for various plastic types have been summarized in Table 3.

Table 3.
Overview of catalyst and its interaction with different plastics.
It is important to note that using zeolite as a catalyst for plastic pyrolysis only increased the amount of volatile hydrocarbon produced. HZSM is suggested because of its increased efficiency for regeneration and longer cycle times due to the catalyst’s low deactivation rate. To increase the output of liquid oil, use of the FCC catalyst during plastic pyrolysis is recommended. Additionally, using spent FCC catalyst is also advantageous from an economic standpoint.
3.2. Gasification
Gasification is a thermo-chemical process involving multiple chemical reactions wherein a carbon containing feedstock like plastic waste is converted into synthetic gas in a partial supply of air, oxygen or steam [69]. The process operates at a sufficiently high temperature (>600–1000 °C) in order to thermally degrade the plastic waste for yielding syngas [70].
Several advantages are associated with gasification, like increased heating value of fuel by rejection of non-combustibles like nitrogen and water, reduction in oxygen content of fuel, exposure to H2 at high pressure or exposure to steam at high temperatures and pressures where H2 is added to the product will raise the products relative hydrogen content (H/C ratio).
3.2.1. Gasifying Medium
One of the important parameters to be considered during gasification of plastic waste is the gasifying medium. Gasification can be done in mediums like steam, air, carbon dioxide or oxygen or a combination of these gases [71]. The gasifying medium plays a significant role in converting solid carbon and heavier hydrocarbons into CO and H2, which are low molecular weight gases. Oxygen is primarily used as a gasifying medium in either pure form or via air, and the products include CO and CO2 when subjected to low and high oxygen, respectively. Gasification proceeds towards combustion when oxygen is supplied over a threshold limit, resulting in the formation of flue gas instead of synthesis/producer gas. The formed combustion product or the flue gases possess no residual heating value.
Similarly, when steam is used as a gasifying medium, the formed product contains more hydrogen per unit of carbon, thus increasing the H/C ratio. On the other hand, if air is directly used instead of oxygen, nitrogen present in air would dilute the product and the heating value of the gas would be reduced when compared to the heating value of the gas produced from oxygen/steam gasification. Thus, it can be concluded that the highest heating value is obtained when oxygen is used as a gasifying medium, followed by steam and air. The gases produced after air gasification is generally called producer gas whereas the gases produce through oxygen/steam gasification are known as synthesis gas.
Air gasification is a simple process and is advantageous as no external energy is required. Additionally, the output gas has less tar when compared to when steam gasification is used [72]. The syngas produced by steam gasification has a higher H2/CO ratio than the syngas produced by direct air gasification, making it more suitable for use in chemical synthesis applications [73]. The biggest difficulty with this method is the amount of heat needed to fuel the endothermic steam reforming reactions inside the reactor. Gasification with pure O2 is an alternative to air and steam as it includes the benefits of both gasifying agents. This method is more complex and expensive, especially for medium-scale applications, due to the high fixed assets and running costs for air separation [74]. More recently, it has been suggested that the pyrolysis and in-line reformation of pyrolysis volatiles is a potential method for valorizing waste plastics for production of H2 [31,53,54,55]. Additionally, this method uses very effective reforming catalysts to produce syngas that is entirely tar-free, addressing the primary difficulty in the traditional gasification of plastic.
3.2.2. Classification of Gasifiers
The primary criteria used to classify gasifiers are the gas-solid contacting mechanism and the gasification medium. Gasifiers have been divided into three main categories based on the manner of gas-solid contact: (1) Fixed or moving bed, (2) Fluidized bed, and (3) Entrained-flow bed. As illustrated in Figure 3, the direction in which the gasifying medium passes through the bed further categorizes each type of gasifier [6].
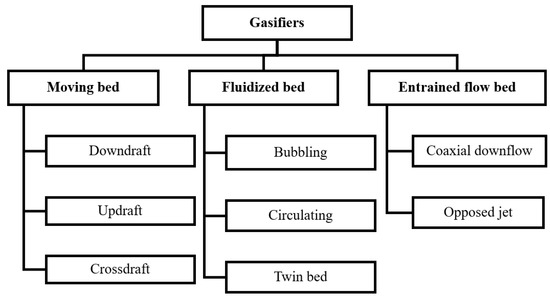
Figure 3.
Classification of gasifiers.
A specific gasifier type might not be appropriate for the entire range of gasifier capacities. For example, moving-bed (updraft and downdraft) types are used for smaller units (under 10 MWth); fluidized-bed types are better suited for intermediate units (between 5 and 100 MWth); and entrained flow reactors are used for high capacity units (above 50 MWth). Major differences between the three gasifier types are summarized in Table 4.

Table 4.
Major differences between the gasifier types.
In a fixed-bed gasifier, sometimes referred to as a moving-bed gasifier, the fuel is supported on a grate. Due to the fuel’s ability to slide down the gasifier like a plug, this type is also known as a moving bed. One of the main benefits of fixed-bed gasifiers is that they may be built in small quantities at low cost. Because of this, there are a lot of small-scale moving-bed biomass gasifiers in operation all over the world. It is challenging to produce equal distribution of fuel, temperature and gas composition over the cross-section of the gasifier due to poor mixing and heat transfer in the moving (fixed) bed. As a result, during gasification, fuels that are prone to agglomeration may form agglomerates. This is why large-capacity fixed-bed gasifiers are not very successful for biomass fuels or coal with a high caking index.
Fluidized-bed gasifiers are well known for temperature uniformity and mixing. A fluidized bed is made up of bed materials, which are granular particles that are retained in a semi-suspended form (fluidized state) by the movement of the gasifying medium across them at proper velocities. This type of gasifier is essentially unaffected by the quality of the fuel due to the superior gas solid mixing and the substantial thermal inertia of the bed. Additionally, the temperature homogeneity significantly lowers the possibility of fuel agglomeration. For gasifying biomass, the fluidized-bed concept has proven to be quite beneficial.
For large-scale gasification of coal, petroleum coke and refinery waste, entrained bed gasifiers are the most effective and popular form of gasifier. Except for low-rank coal, like lignite and biomass, which is unattractive due to its high moisture content, entrained-flow gasifiers are best suited for most forms of coal. Since cold-gas efficiency declines with increasing ash content, high-ash coal is likewise less suited. The economic limit for slurry-fed coal is 20% ash, while the limit for dry feed is 40% ash. For a variety of reasons, the appropriateness of entrained-flow gasification for biomass is debatable. The fuel must be extremely small due to the short residence period (a few seconds) in entrained-flow reactors. It is challenging to crush fibrous biomass into such small particles.
3.2.3. Other Gasifier Types
Spouted Bed
For waste valorization operations, spouted beds are an alternative to fluidized beds due to their specific characteristics like rapid heat and mass transfer rates, effective solid mixing and relatively better gas-solid contact. Additionally, their vigorous cyclic solid circulation prevents de-fluidization issues and makes it easier to handle sticky materials, irregular particles and particles with a wide size range. Their use in gasification processes is primarily constrained by the volatile’s short residence times, which impede tar cracking reactions. This technology has been extensively employed in bench scale setups for the pyrolysis of various solid wastes. The biomass pyrolysis process has been successfully scaled up to 25 kg per hour.
Plasma Gasifier
Waste materials are converted into gaseous products by plasma gasification operations in an oxidizing atmosphere. The primary benefit of plasma reactors for plastic gasification is the high temperature attainment, which encourages practically complete breaking of tar compounds and, consequently, high gas yields by improving the elimination of hazardous compounds. According to the methods used for plasma discharge, three kinds of plasma technologies—radio frequency, microwave, and direct current—have been established.
Pyrolysis-Reforming Process
This procedure, which aims to produce hydrogen, is carried out in two reactors that are connected in order to perform the pyrolysis and reforming processes. Most of the bench and laboratory scale units with various reactor configurations have been used to study this unique method. Czernik and French [75] conducted the ground-breaking research in a continuous system that consumed 0.06 kg of plastic every hour. The experimental unit consisted of a fluidized bed for plastic pyrolysis and a stationary bed for the catalytic reforming of the volatiles produced. Two fixed bed reactors are joined in line to form the continuous process in study done by Yoshikawa et al. [76,77]. The plastic feed was 0.06 kg per hour. The pyrolysis and steam reforming of polymers in a batch unit composed of two fixed bed reactors have been thoroughly explored by Williams et al. [78,79,80]. Erkiaga et al. integrated a fixed bed reactor for in-line reforming with a conical spouted bed reactor for continuous pyrolysis (0.05 kg.h−1 of plastic in the feed). In later research, the fluidized bed was used to reform the system instead of the fixed bed, which increased its performance [81,82]. Ouadi et al. [83] designed a pilot plant for the continuous regime (2 kg.h−1 of feed) of pyrolysis and in-line reforming of MSW. The pyrolysis step took place at 450 °C in an auger reactor, and the volatiles were then reformed in a fixed bed reactor on pyrolysis char.
This method has several benefits over conventional steam gasification and steam reforming of bio-oil or plastic pyrolysis oil, including: (a) operation under optimum conditions because the two reactors are integrated into one unit (the reforming temperature is lower than that used in the gasification process, reducing potential catalyst sintering issues); (b) prevention of tar formation; and (c) direct contact between the feedstock and the catalyst. However, the effectiveness of the reforming catalyst will determine the development of the combined pyrolysis and in-line reforming process. Thus, to increase H2 production, obtain complete conversion of pyrolysis volatiles and prevent tar formation, highly active and selective catalysts are needed [84].
3.2.4. Gasification Reactions
The reactions that occur inside the gasifier are complicated reactions as shown in Table 5. The reactions are generally classified into five types: (i) carbon reactions, (ii) oxidation reactions, (iii) shift reactions, (iv) methanation reactions and (v) steam reforming reactions.

Table 5.
Main gasification reactions [85].
An overview of several studies for the effect of temperature on gas production, as well as composition during gasification of plastic waste, can be found in Table 6.

Table 6.
Summary of plastic waste gasification studies carried out by various research groups around the globe.
Syngas is rich in H2 and CH4, which can be used as combustible gases, as opposed to pyrolysis, which produces gas products that primarily consist of C3–C6. However, because of the dilution effect, the increased gas flow rate during gasification operation leads to lower throughputs, more difficult separation and lower calorific values of products, which has a detrimental influence on the overall economic benefits. Additionally, the gasification process harms the environment by producing noxious gases like NOX. Additionally, the produced syngas contains various pollutants such NH3, H2S, NOx, alkali metals and significant amounts of tars, necessitating an extra step in purification and raising the cost. A high-temperature environment is necessary for gasification, which raises the cost and requires the use of expensive machinery.
4. Advanced Oxidation Techniques for Treatment of Plastic Waste
4.1. Photocatalytic Oxidation
Photocatalysis is a technology that imitates the photosynthesis occurring in nature. Usually, there are three separate steps involved: (1) Charge carriers are excited via light absorption by photocatalysts, (2) the photogenerated charge carriers are separated and transported, and (3) the catalyst surface undergoes redox reaction. Photocatalytic oxidation involves oxidative breakdown of polymers into lower molecular weight materials in the presence of ultraviolet (UV) radiation and a photocatalyst which dominates the conversion process.
For photocatalysis to take place, incident photon energies must be greater than or equal to the band gap of the photocatalyst. While the reduction potential of photoelectrons in the conduction band should be greater than that of the reactant to be reduced, the oxidation potential of holes in the valence band of the photocatalyst should be greater than that of the substrate to be oxidised. For the photocatalytic valorisation of plastic waste, the holes in the photocatalysts’ valence band are frequently used to oxidise the plastic to produce organic products or degrade it to CO2, while the electrons in the photocatalyst’s conduction band can be used to reduce the protons in water to H2, CO2 to carbon derived fuels or capturing by oxygen to involve it in the subsequent plastic oxidation.
The mechanism of photocatalytic conversion of plastic waste can be found in Figure 4. Semiconductor photocatalysts typically feature a band structure and narrow band gap. If the photon energy absorbed by the semiconductor is higher than its band gap, the electrons may be stimulated from the valence band to the conduction band, leaving photoinduced holes on the valence band. Additionally, holes can oxidise water to ·OH, which then oxidises plastic to CO2. Furthermore, the reduction of electrons on the conduction band causes CO2 to be transformed into C2 fuels like acetic acid.
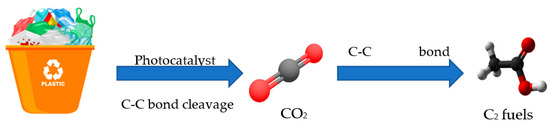
Figure 4.
Photocatalytic oxidation of plastic waste.
Under UV light, polyacrylamide (PAM)/TiO2 and PAM/ZnO, respectively, could convert LDPE into C2 fuel [31,56]. The formation of oxidative species take place via PAM/TiO2 or PAM/ZnO, which further leads to production of low molecular weight fuels. Under solar light, the photocatalytic conversion of polylactic acid (PLA), PET and polyurethane (PUR) to fuels using CdS/CdOx resulted in H2 production of 38.8%, 16.6% and 22.5%, respectively [96]. The Nb2O5 photocatalyst was also used to construct a two-step conversion pathway for the cleavage and coupling of C-C bonds. The degradation of PE, PP and PVC resulted in CO2, which was then reduced to CH3COOH (around 40.0%) via photocatalysis [97]. Some of the studies on plastic waste conversion to fuels via photocatalysis has been summarized in Table 7.

Table 7.
Photocatalytic conversion of plastic waste to fuel.
The existing photocatalysts are not very effective at reducing CO2, resulting in a low yield of C2 fuels. To effectively convert plastic waste into long chain carbon products at room temperature and pressure, rational photocatalyst design and optimization of cleavage and coupling of C-C bonds are therefore essential. Since solar light is the main source of energy for the photocatalytic conversion of plastic waste into fuels, and owing to the fact that photocatalysis uses no thermal energy compared to pyrolysis, plastic conversion by this method might therefore reduce operational costs and would serve as an efficient way of treatment.
4.2. Electrocatalytic Oxidation
Conversion of plastics through electrocatalytic oxidation can be brought about using two different methods: direct oxidation and indirect oxidation. Direct oxidation refers to the electrophilic attack on a polymer by ·OH produced by water discharge on the anode surface. When strong oxidising intermediates dominate in the plastic conversion process, it is referred to as indirect oxidation. With an external voltage (0.55 V) applied in H3PO4 solution at 200 °C, polyvinyl alcohol (PVA) was successfully converted to H2 (9.5 mol/min) [101]. Also, for the production of carboxylic acid (75%), electrocatalytic degradation of PVC was carried out on TiO2/C cathode (−0.7 V) at 100 °C [102].
A plastic polymer is reduced when it receives electrons from the cathode (TiO2/C), which undergoes dechlorination at a high temperature. Additionally, the polymer is oxidised with ·OH to produce carbonyl and hydroxyl groups, which subsequently breaks down into tiny molecules (e.g., alcohols, carboxylic acids and esters). Finally, these chemicals partially mineralize to CO2 and H2O.
Electrocatalytic breakdown of plastic wastes may result in a single product that could be turned directly into fuels. Electrolysis alone cannot yield as many fuel components as pyrolysis and electrolysis combined. Solar thermo-coupled electrolysis was used to convert PP to H2 and C1–C5 fuels. Additionally, increasing temperature (350–390 °C) and electrolysis current (0–400 mA) improved the PP depolymerization [103]. Some of the studies on conversion of plastic waste to value added chemicals and gases via electrocatalysis has been summarized in Table 8.

Table 8.
Summary of studies on electrocatalytic conversion.
4.3. Fenton Oxidation
The Fenton reaction is an advanced technique that is often used to degrade chemical compounds that are resistant to conventional methods. Fenton oxidation is the process of converting polymers into small molecules by generating ·OH from Fe2+ activated H2O2. The Fenton oxidation process for plastic conversion is usually carried out under mild reaction conditions. Due to rapid breakdown of hydrogen peroxide (H2O2), the conversion process is effective, making it advantageous for large-scale applications. However, the procedure uses a lot of H2O2, which increases the cost of operation. Through chemical oxidation reactions, the Fenton reaction can regulate the decomposition of polymer waste to create high-value fine chemicals.
The Fenton oxidation method for converting plastic into fuels is depicted in Figure 5. The C-C bond is broken when the Fenton reagent is added to plastic, producing ·OH. As a result, the polymer gets decomposed into different dicarboxylic acids. H2O2 and Fe2+ react to produce Fe3+ and ·OH. Additionally, H2O2 is catalyzed by Fe3+ to become ·HO2, which can then react with H2O2 to form more OH. In addition, R· is produced through the reaction between ·OH and RH. The C-C bond of the polymer breaks, converting it into CO2 and H2O.
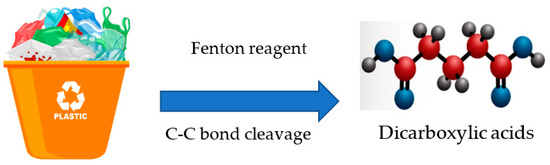
Figure 5.
Plastic waste treatment via Fenton oxidation process.
A new chemical method involving sulfonation and the Fenton reaction for conversion of LDPE and HDPE into organic acids was developed. When LDPE and HDPE were converted to carboxylic acids, the yields were around 87.5% and 88.8%, respectively [104]. The sulfonated PVC also underwent Fenton oxidation at 65 °C and degraded to carboxylic acids with a yield of around 75.5% [105]. Evidently, PE can produce carboxylic acids more efficiently and rapidly than PVC. Sulfonated PE, PP and PVC have been observed to undergo photo-Fenton oxidation as well, leading to generation of CO2 as the primary product, which, following photo-reduction, could be changed into acetic acid. Some of the studies on Fenton oxidation process has been summarized in Table 9.

Table 9.
Fenton oxidation process for plastic waste conversion.
Advanced oxidation of plastics produces liquid products with a low calorific value. Through the right modification of active sites on the catalyst surface, the species and density of oxidants in the system can be controlled. As a result, it is possible to realize the selective breakage of chemical bonds in polymers and the directional conversion of target fuels. Furthermore, by varying the energy band, building heterojunctions and loading co-catalysts, the photocatalysts should be rationally designed. In order to increase the efficiency of photocatalysts, it is desirable to speed the separation of photogenerated carriers. Additionally, by optimizing the process of coupling intermediates and breaking C-C bonds, multi-carbon fuel can be effectively converted from plastic waste under natural light.
5. Conclusions
Plastic waste conversion to value added products has gained extensive importance in alleviating environmental pollution and the energy crisis. Thermal processing of waste plastics via pyrolysis results in the generation of high calorific fuels with better performance when compared to conventional diesel or gasoline. However, thermal processing involves high reaction temperatures, large residence time and the clogging of pipelines due to the production of olefins and paraffins. Therefore, in order to increase fuel yield and maximize economic value, further research must be done to optimize the process parameters while also taking into account product quality.
Thus, an efficient way for the conversion of plastic wastes is via catalytic pyrolysis at low temperatures, thereby overcoming the increasing problem of its management. However, recovering catalysts that are in high demand for the conversion of polymers is challenging, as the formation of coke leads to catalyst deactivation. Moreover, there exist different plastic waste types, but most of the catalysts are effective for depolymerization of selected ones. Therefore, it is crucial to develop catalysts that are highly active, excellently adaptable and recyclable.
Owing to the progress and development in research, advanced oxidation techniques have been formulated for converting plastic waste into the desired fuel at room temperature and pressure, using much less energy than conventional thermal treatment processes with an aim to produce product with high purity and controlled conversion. A significant drawback associated with advanced oxidation techniques is the low calorific value of the liquid products obtained and the requirement of extremely effective catalysts for the reaction. Overall, advanced oxidation technique has the potential for plastic waste conversion in the near future.
Author Contributions
A.R.: Conceptualization, Investigation, Software, Data curation, Formal analysis, Writing—original draft. K.P.: Methodology, Conceptulization, Visualization, Writing original draft, Funding acquisition. A.B.: Investigation, Resources, Writing—review & editing, Funding acquisition. A.M.: Resources, Writing—review & editing. K.K.P.: Conceptualization, Resources, Funding acquisition, Writing—review & editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Science and Engineering Research Board (SERB) New Delhi, National Post Doctoral Fellowship (NPDF) project grant PDF/2020/000634 to Kunwar Pal and Department of Biotechnology, Ministry of Science and Technology, Government of India, Ramalingaswami Re-entry Fellowship grant BT/RLF/Re-entry/61/2020 to Ashish Bohre.
Data Availability Statement
New data are contained within the article. Other data were cited and listed in the bibliography.
Acknowledgments
Abdul Rafey acknowledges the Prime Minister Research Fellowship Scheme for the financial support and Indian Institute of Technology Delhi. Kunwar Pal would like to thank the Science and Engineering Research Board (SERB) New Delhi and acknowledges the SERB National PostDoctoral Fellowship (NPDF) project grant PDF/2020/000634. Ashish Bohre acknowledges Department of Biotechnology, Ministry of Science and Technology, Government of India for the grant BT/RLF/Re-entry/61/2020 under DBT Ramalingaswami Re-entry Fellowship.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Weiland, F.; Lundin, L.; Celebi, M.; van der Vlist, K.; Moradian, F. Aspects of chemical recycling of complex plastic waste via the gasification route. Waste Manag. 2021, 126, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manag. 2019, 89, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018. [Google Scholar] [CrossRef] [PubMed]
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of Plastic Waste as Waste-to-Energy or Waste-to-Syngas Recovery Route. In Solid Waste as a Renewable Resource; Apple Academic Press: Palm Bay, FL, USA, 2015; pp. 241–263. ISBN 9780429154485. [Google Scholar]
- Arena, U.; Di Gregorio, F.; Amorese, C.; Mastellone, M.L. A techno-economic comparison of fluidized bed gasification of two mixed plastic wastes. Waste Manag. 2011, 31, 1494–1504. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; El-Fatah Abomohra, A.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manage. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Mohanraj, C.; Senthilkumar, T.; Chandrasekar, M. A review on conversion techniques of liquid fuel from waste plastic materials. Int. J. Energy Res. 2017, 41, 1534–1552. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Pichler, C.M.; Bhattacharjee, S.; Rahaman, M.; Uekert, T.; Reisner, E. Conversion of Polyethylene Waste into Gaseous Hydrocarbons via Integrated Tandem Chemical-Photo/Electrocatalytic Processes. ACS Catal. 2021, 11, 9159–9167. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S.; Ramasamy, K.K. Electrochemical oxidation of lignin and waste plastic. ACS Omega 2020, 5, 27735–27740. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Eze, W.U.; Madufor, I.C.; Onyeagoro, G.N.; Obasi, H.C. The effect of Kankara zeolite-Y-based catalyst on some physical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polym. Bull. 2020, 77, 1399–1415. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Kaminsky, W.; Kim, J.S. Pyrolysis of mixed plastics into aromatics. J. Anal. Appl. Pyrolysis 1999, 51, 127–134. [Google Scholar] [CrossRef]
- Olazar, M.; Lopez, G.; Amutio, M.; Elordi, G.; Aguado, R.; Bilbao, J. Influence of FCC catalyst steaming on HDPE pyrolysis product distribution. J. Anal. Appl. Pyrolysis 2009, 85, 359–365. [Google Scholar] [CrossRef]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Thermal pyrolysis of high density polyethylene (HDPE) in a novel fixed bed reactor system for the production of high value gasoline range hydrocarbons (HC). Process Saf. Environ. Prot. 2019, 127, 171–179. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: Product yield analysis and their characterization. J. Energy Inst. 2019, 92, 1647–1657. [Google Scholar] [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Ahmad, I.; Ismail Khan, M.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.M.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013, 7–9. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. Evaluation of pyrolysis product of virgin high density polyethylene degradation using different process parameters in a stirred reactor. Fuel Process. Technol. 2013, 109, 90–95. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Mastral, F.J.; Esperanza, E.; Garciía, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Lee, K.H.; Noh, N.S.; Shin, D.H.; Seo, Y. Comparison of plastic types for catalytic degradation of waste plastics into liquid product with spent FCC catalyst. Polym. Degrad. Stab. 2002, 78, 539–544. [Google Scholar] [CrossRef]
- Luo, G.; Suto, T.; Yasu, S.; Kato, K. Catalytic degradation of high density polyethylene and polypropylene into liquid fuel in a powder-particle fluidized bed. Polym. Degrad. Stab. 2000, 70, 97–102. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Sakata, Y.; Uddin, M.A.; Muto, A. Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J. Anal. Appl. Pyrolysis 1999, 51, 135–155. [Google Scholar] [CrossRef]
- Adnan; Shah, J.; Jan, M.R. Thermo-catalytic pyrolysis of polystyrene in the presence of zinc bulk catalysts. J. Taiwan Inst. Chem. Eng. 2014, 45, 2494–2500. [Google Scholar] [CrossRef]
- Uddin, M.A.; Koizumi, K.; Murata, K.; Sakata, Y. Thermal and catalytic degradation of structurally different types of polyethylene into fuel oil. Polym. Degrad. Stab. 1997, 56, 37–44. [Google Scholar] [CrossRef]
- Williams, P.T.; Williams, E.A. Fluidised bed pyrolysis of low density polyethylene to produce petrochemical feedstock. J. Anal. Appl. Pyrolysis 1999, 51, 107–126. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Deák, G.; Jóver, B.; Kalló, D. Thermal and thermo-catalytic degradation of high-density polyethylene waste. J. Anal. Appl. Pyrolysis 2004, 72, 235–242. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G. Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis 2014, 109, 272–277. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Artetxe, M.; Bilbao, J. Product yields and compositions in the continuous pyrolysis of high-density polyethylene in a conical spouted bed reactor. Ind. Eng. Chem. Res. 2011, 50, 6650–6659. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Cho, M.H.; Jung, S.H.; Kim, J.S. Pyrolysis of mixed plastic wastes for the recovery of benzene, toluene, and xylene (BTX) aromatics in a fluidized bed and chlorine removal by applying various additives. Energy Fuels 2010, 24, 1389–1395. [Google Scholar] [CrossRef]
- Grause, G.; Matsumoto, S.; Kameda, T.; Yoshioka, T. Pyrolysis of mixed plastics in a fluidized bed of hard burnt lime. Ind. Eng. Chem. Res. 2011, 50, 5459–5466. [Google Scholar] [CrossRef]
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Ali, M.F.; Ahmed, S.; Qureshi, M.S. Catalytic coprocessing of coal and petroleum residues with waste plastics to produce transportation fuels. Fuel Process. Technol. 2011, 92, 1109–1120. [Google Scholar] [CrossRef]
- Kaminsky, W. Plastics recycling. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 1992; ISBN 3-52730-385-5. [Google Scholar]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Wan, S.; Wang, Y. A review on ex situ catalytic fast pyrolysis of biomass. Front. Chem. Sci. Eng. 2014, 8, 280–294. [Google Scholar] [CrossRef]
- Luo, G.; Resende, F.L.P. In-situ and ex-situ upgrading of pyrolysis vapors from beetle-killed trees. Fuel 2016, 166, 367–375. [Google Scholar] [CrossRef]
- Degnan, T.F. Applications of zeolites in petroleum refining. Top. Catal. 2000, 13, 349–356. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind. Eng. Chem. Res. 2013, 52, 10637–10645. [Google Scholar] [CrossRef]
- Garforth, A.A.; Lin, Y.H.; Sharratt, P.N.; Dwyer, J. Production of hydrocarbons by catalytic degradation of high density polyethylene in a laboratory fluidised-bed reactor. Appl. Catal. A Gen. 1998, 169, 331–342. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yen, H.Y. Fluidised bed pyrolysis of polypropylene over cracking catalysts for producing hydrocarbons. Polym. Degrad. Stab. 2005, 89, 101–108. [Google Scholar] [CrossRef]
- Seo, Y.H.; Lee, K.H.; Shin, D.H. Investigation of catalytic degradation of high-density polyethylene by hydrocarbon group type analysis. J. Anal. Appl. Pyrolysis 2003, 70, 383–398. [Google Scholar] [CrossRef]
- del Remedio Hernández, M.; Gómez, A.; García, Á.N.; Agulló, J.; Marcilla, A. Effect of the temperature in the nature and extension of the primary and secondary reactions in the thermal and HZSM-5 catalytic pyrolysis of HDPE. Appl. Catal. A Gen. 2007, 317, 183–194. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; San Miguel, G.; Castro, M.C.; Madrid, S. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J. Anal. Appl. Pyrolysis 2007, 1–2, 415–423. [Google Scholar] [CrossRef]
- Marcilly, C.R. Where and how shape selectivity of molecular sieves operates in refining and petrochemistry catalytic processes. Top. Catal. 2000, 13, 357–366. [Google Scholar] [CrossRef]
- Sakata, Y.; Azhar Uddin, M.; Muto, A.; Kanada, Y.; Koizumi, K.; Murata, K. Catalytic degradation of polyethylene into fuel oil over mesoporous silica (KFS-16) catalyst. J. Anal. Appl. Pyrolysis 1997, 43, 15–25. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Nahil, M.A.; Williams, P.T. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils. J. Anal. Appl. Pyrolysis 2017, 124, 631–637. [Google Scholar] [CrossRef]
- Khan, M.S.; Inamullah; Sohail, M.; Khattak, N.S. Conversion of Mixed Low-Density Polyethylene Wastes into Liquid Fuel by Novel CaO/SiO2 Catalyst. J. Polym. Environ. 2016, 24, 255–263. [Google Scholar] [CrossRef]
- Huang, W.C.; Huang, M.S.; Huang, C.F.; Chen, C.C.; Ou, K.L. Thermochemical conversion of polymer wastes into hydrocarbon fuels over various fluidizing cracking catalysts. Fuel 2010, 89, 2305–2316. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Borsodi, N. Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Product yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Muneer, B.; Zeeshan, M.; Qaisar, S.; Razzaq, M.; Iftikhar, H. Influence of in-situ and ex-situ HZSM-5 catalyst on co-pyrolysis of corn stalk and polystyrene with a focus on liquid yield and quality. J. Clean. Prod. 2019, 237, 117762. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, D.; Lei, H.; Villota, E.; Ruan, R. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl. Energy 2019, 251, 113337. [Google Scholar] [CrossRef]
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Pt-promoted sulfated zirconia as catalyst for hydrocracking of LDPE plastic waste into liquid fuels. Mater. Chem. Phys. 2018, 213, 548–555. [Google Scholar] [CrossRef]
- Hu, M.; Guo, D.; Ma, C.; Luo, S.; Chen, X.; Cheng, Q.; Laghari, M.; Xiao, B. A novel pilot-scale production of fuel gas by allothermal biomass gasification using biomass micron fuel (BMF) as external heat source. Clean Technol. Environ. Policy 2016, 18, 743–751. [Google Scholar] [CrossRef]
- Nyakuma, B.B.; Ivase, T.J.P. Emerging trends in sustainable treatment and valorisation technologies for plastic wastes in Nigeria: A concise review. Environ. Prog. Sustain. Energy 2021, 40, e13660. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Arku, P.; Dutta, A. Gasification of Plastic Solid Waste and Competitive Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131404. [Google Scholar]
- Gil, J.; Corella, J.; Aznar, M.P.; Caballero, M.A. Biomass gasification in atmospheric and bubbling fluidized bed: Effect of the type of gasifying agent on the product distribution. Biomass Bioenergy 1999, 17, 389–403. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Syngas from steam gasification of polyethylene in a conical spouted bed reactor. Fuel 2013, 109, 461–469. [Google Scholar] [CrossRef]
- Xiao, R.; Jin, B.; Zhou, H.; Zhong, Z.; Zhang, M. Air gasification of polypropylene plastic waste in fluidized bed gasifier. Energy Convers. Manag. 2007, 48, 778–786. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.J. Production of hydrogen from plastics by pyrolysis and catalytic steam reform. Energy Fuels 2006, 20, 754–758. [Google Scholar] [CrossRef]
- Namioka, T.; Saito, A.; Inoue, Y.; Park, Y.; Min, T.J.; Roh, S.A.; Yoshikawa, K. Hydrogen-rich gas production from waste plastics by pyrolysis and low-temperature steam reforming over a ruthenium catalyst. Appl. Energy 2011, 88, 2019–2026. [Google Scholar] [CrossRef]
- Park, Y.; Namioka, T.; Sakamoto, S.; Min, T.J.; Roh, S.A.; Yoshikawa, K. Optimum operating conditions for a two-stage gasification process fueled by polypropylene by means of continuous reactor over ruthenium catalyst. Fuel Process. Technol. 2010, 91, 951–957. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. A novel Ni–Mg–Al–CaO catalyst with the dual functions of catalysis and CO2 sorption for H2 production from the pyrolysis–gasification of polypropylene. Fuel 2010, 89, 1435–1441. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Pyrolysis-gasification of plastics, mixed plastics and real-world plastic waste with and without Ni-Mg-Al catalyst. Fuel 2010, 89, 3022–3032. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Investigation of coke formation on Ni-Mg-Al catalyst for hydrogen production from the catalytic steam pyrolysis-gasification of polypropylene. Appl. Catal. B Environ. 2010, 96, 198–207. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Alvarez, J.; Artetxe, M.; Arregi, A.; Bilbao, J.; Olazar, M. A sequential process for hydrogen production based on continuous HDPE fast pyrolysis and in-line steam reforming. Chem. Eng. J. 2016, 296, 191–198. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Hydrogen production from biomass by continuous fast pyrolysis and in-line steam reforming. RSC Adv. 2016, 6, 25975–25985. [Google Scholar] [CrossRef]
- Ouadi, M.; Jaeger, N.; Greenhalf, C.; Santos, J.; Conti, R.; Hornung, A. Thermo-Catalytic Reforming of municipal solid waste. Waste Manag. 2017, 68, 198–206. [Google Scholar] [CrossRef]
- Olazar, M.; Santamaria, L.; Lopez, G.; Fernandez, E.; Cortazar, M.; Arregi, A.; Bilbao, J. Progress on catalyst development for the steam reforming of biomass and waste plastics pyrolysis volatiles: A review. Energy Fuels 2021, 35, 17051–17084. [Google Scholar] [CrossRef]
- Kannan, P.; Al Shoaibi, A.; Srinivasakannan, C. Energy recovery from co-gasification of waste polyethylene and polyethylene terephthalate blends. Comput. Fluids 2013, 88, 38–42. [Google Scholar] [CrossRef]
- Arena, U.; Zaccariello, L.; Mastellone, M.L. Fluidized bed gasification of waste-derived fuels. Waste Manag. 2010, 30, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lera, S.; Torrico, J.; Pallarés, J.; Gil, A. Design and first experimental results of a bubbling fluidized bed for air gasification of plastic waste. J. Mater. Cycles Waste Manag. 2013, 15, 370–380. [Google Scholar] [CrossRef]
- Sancho, J.A.; Aznar, M.P.; Toledo, J.M. Catalytic Air Gasification of Plastic Waste (Polypropylene) in Fluidized Bed. Part I: Use of in-Gasifier Bed Additives. Ind. Eng. Chem. Res. 2008, 47, 1005–1010. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F. Energy generation by air gasification of two industrial plastic wastes in a pilot scale fluidized bed reactor. Energy 2014, 68, 735–743. [Google Scholar] [CrossRef]
- Lee, J.W.; Yu, T.U.; Lee, J.W.; Moon, J.H.; Jeong, H.J.; Park, S.S.; Yang, W.; Lee, U. Do Gasification of mixed plastic wastes in a moving-grate gasifier and application of the producer gas to a power generation engine. Energy Fuels 2013, 27, 2092–2098. [Google Scholar] [CrossRef]
- Zaccariello, L.; Mastellone, M.L. Fluidized-bed gasification of plastic waste, wood, and their blends with coal. Energies 2015, 8, 8052–8068. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Conversion of mixed plastic wastes in a dual fluidized bed steam gasifier. Fuel 2013, 107, 787–799. [Google Scholar] [CrossRef]
- Friengfung, P.; Jamkrajang, E.; Sunphorka, S.; Kuchonthara, P.; Mekasut, L. NiO/dolomite catalyzed steam/O2 gasification of different plastics and their mixtures. Ind. Eng. Chem. Res. 2014, 53, 1909–1915. [Google Scholar] [CrossRef]
- Rutberg, P.G.; Kuznetsov, V.A.; Serba, E.O.; Popov, S.D.; Surov, A.V.; Nakonechny, G.V.; Nikonov, A.V. Novel three-phase steam-air plasma torch for gasification of high-caloric waste. Appl. Energy 2013, 108, 505–514. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Artetxe, M.; Arregi, A.; Santamaria, L.; Bilbao, J.; Olazar, M. Pyrolysis and in-line catalytic steam reforming of polystyrene through a two-step reaction system. J. Anal. Appl. Pyrolysis 2016, 122, 502–510. [Google Scholar] [CrossRef]
- Uekert, T.; Kuehnel, M.F.; Wakerley, D.W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11, 2853–2857. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Chen, Q.; Li, X.; Li, Y.; Shao, W.; Xu, J.; Zhu, J.; Pan, Y.; Sun, Y.; et al. Photocatalytic Conversion of Waste Plastics into C2 Fuels under Simulated Natural Environment Conditions. Angew. Chemie-Int. Ed. 2020, 59, 15497–15501. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Luo, Y.; Song, S.; Dong, X.; Yu, X. High photocatalytic degradation activity of polyethylene containing polyacrylamide grafted TiO2. Polym. Degrad. Stab. 2013, 98, 1754–1761. [Google Scholar] [CrossRef]
- Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of Nonrecyclable Plastic Waste over a Carbon Nitride/Nickel Phosphide Catalyst. J. Am. Chem. Soc. 2019, 141, 15201–15210. [Google Scholar] [CrossRef]
- Kamalian, P.; Khorasani, S.N.; Abdolmaleki, A.; Karevan, M.; Khalili, S.; Shirani, M.; Neisiany, R.E. Toward the development of polyethylene photocatalytic degradation. J. Polym. Eng. 2020, 40, 181–191. [Google Scholar] [CrossRef]
- Hori, T.; Kobayashi, K.; Teranishi, S.; Nagao, M.; Hibino, T. Fuel cell and electrolyzer using plastic waste directly as fuel. Waste Manag. 2020, 102, 30–39. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, X.; Gu, D.; Yan, C.; Jiang, H.; Wu, H.; Wang, B.; Wang, X. STEP polymer degradation: Solar thermo-coupled electrochemical depolymerization of plastics to generate useful fuel plus abundant hydrogen. Sol. Energy Mater. Sol. Cells 2020, 204, 110208. [Google Scholar] [CrossRef]
- Chow, C.F.; Wong, W.L.; Ho, K.Y.F.; Chan, C.S.; Gong, C.B. Combined Chemical Activation and Fenton Degradation to Convert Waste Polyethylene into High-Value Fine Chemicals. Chem.-A Eur. J. 2016, 22, 9513–9518. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.F.; Wong, W.L.; Chan, C.S.; Li, Y.; Tang, Q.; Gong, C.B. Breakdown of plastic waste into economically valuable carbon resources: Rapid and effective chemical treatment of polyvinylchloride with the Fenton catalyst. Polym. Degrad. Stab. 2017, 146, 34–41. [Google Scholar] [CrossRef]
- Feng, H.M.; Zheng, J.C.; Lei, N.Y.; Yu, L.; Kong, K.H.K.; Yu, H.Q.; Lau, T.C.; Lam, M.H.W. Photoassisted fenton degradation of polystyrene. Environ. Sci. Technol. 2011, 45, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.F.; Wong, W.L.; Chan, C.W.; Chan, C.S. Converting inert plastic waste into energetic materials: A study on the light-accelerated decomposition of plastic waste with the Fenton reaction. Waste Manag. 2018, 75, 174–180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
