Scalability of U-Shape Magnetic Nanoparticles-Based Microreactor–Lipase-Catalyzed Preparative Scale Kinetic Resolutions of Drug-like Fragments
Abstract
1. Introduction
2. Results and Discussion
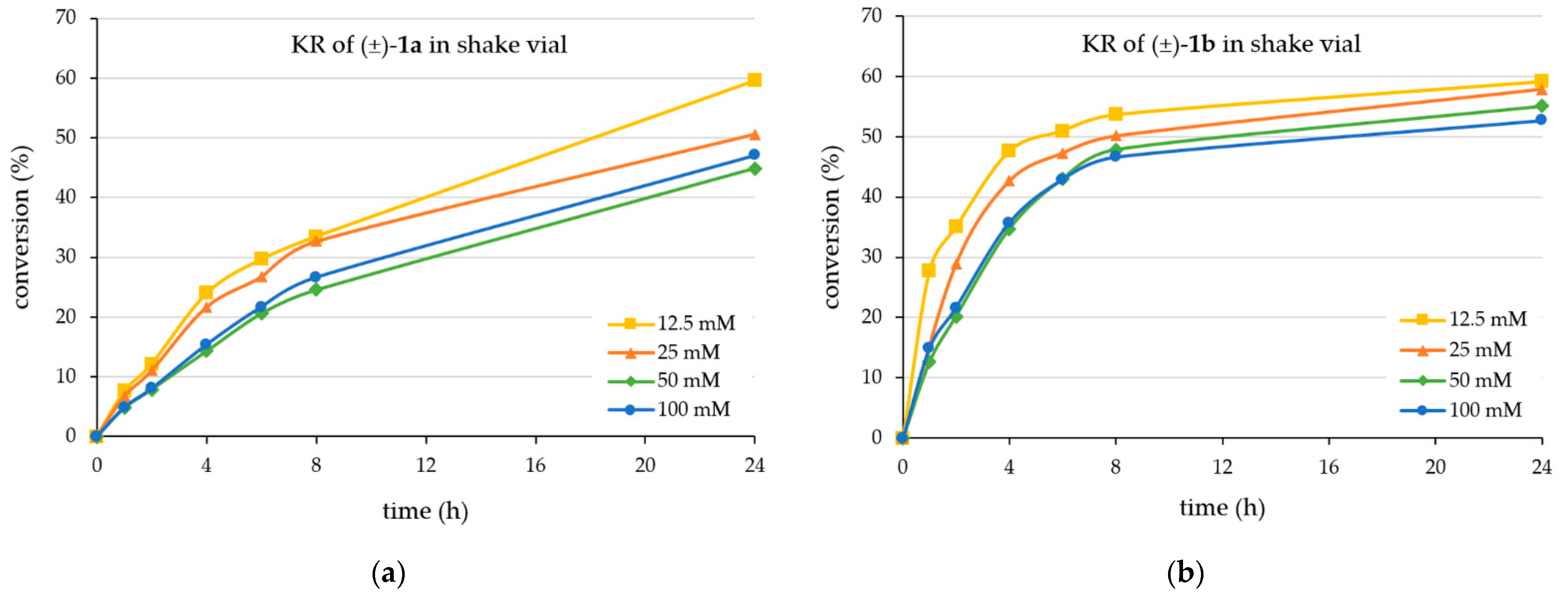
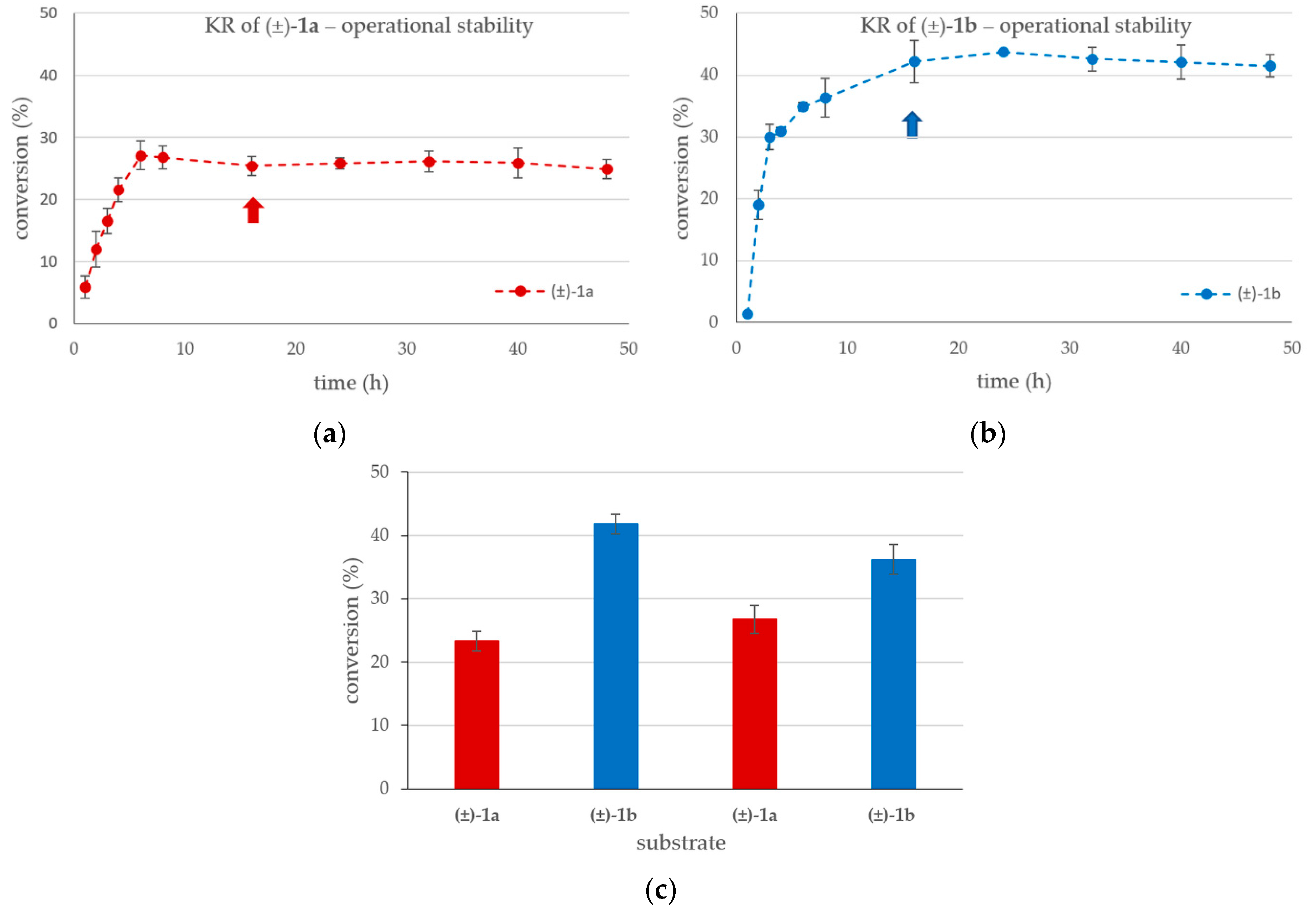
2.1. Kinetic Resolution of Racemic 4-(3,4-Dihydroisoquinolin-2-(1H)-yl)butan-2-ol (±)-1a and 4-(3,4-Dihydroquinolin-1-(2H)-yl)butan-2-ol (±)-1b with CaLB-MNP Biocatalyst in Batch Mode
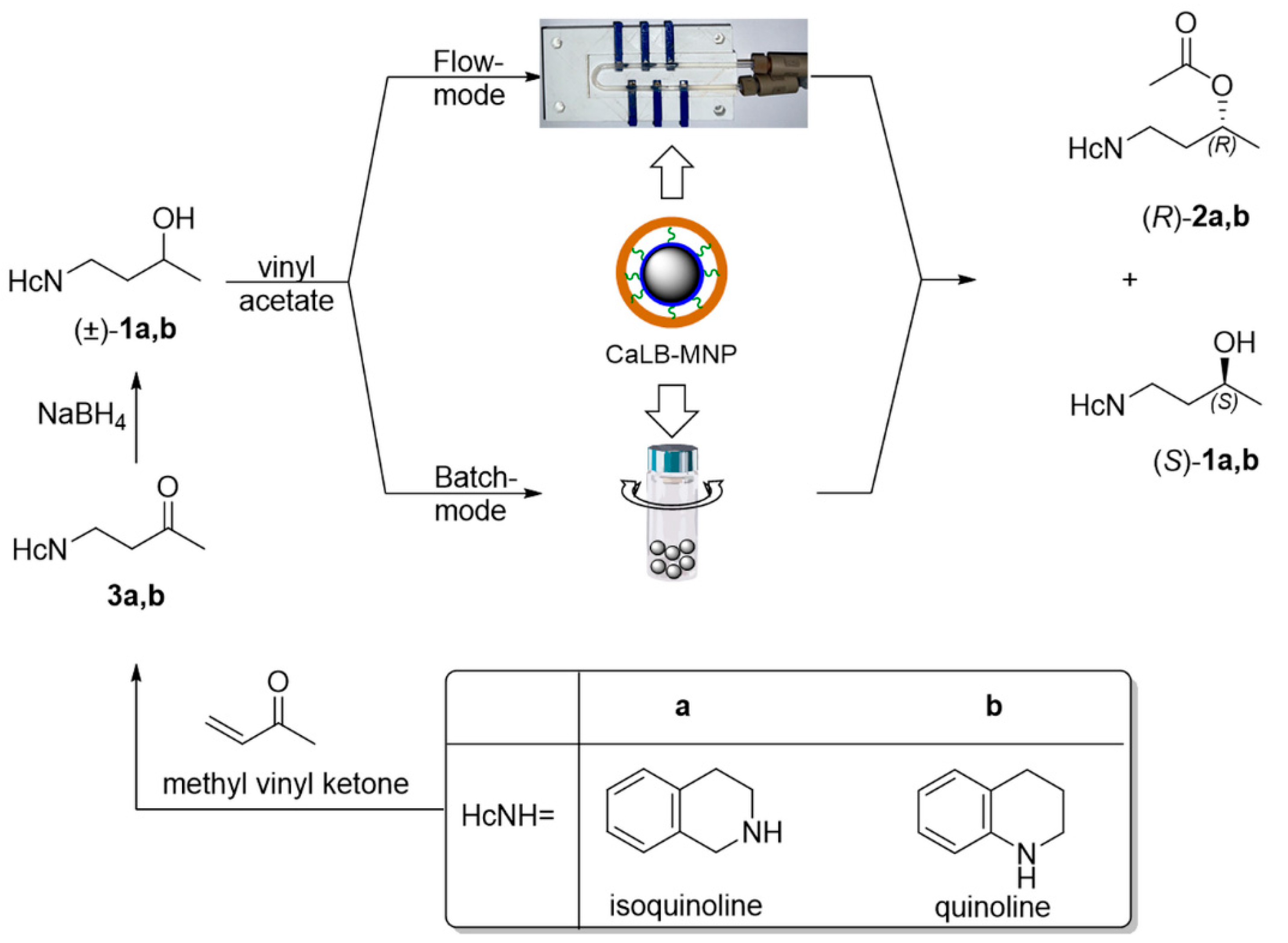
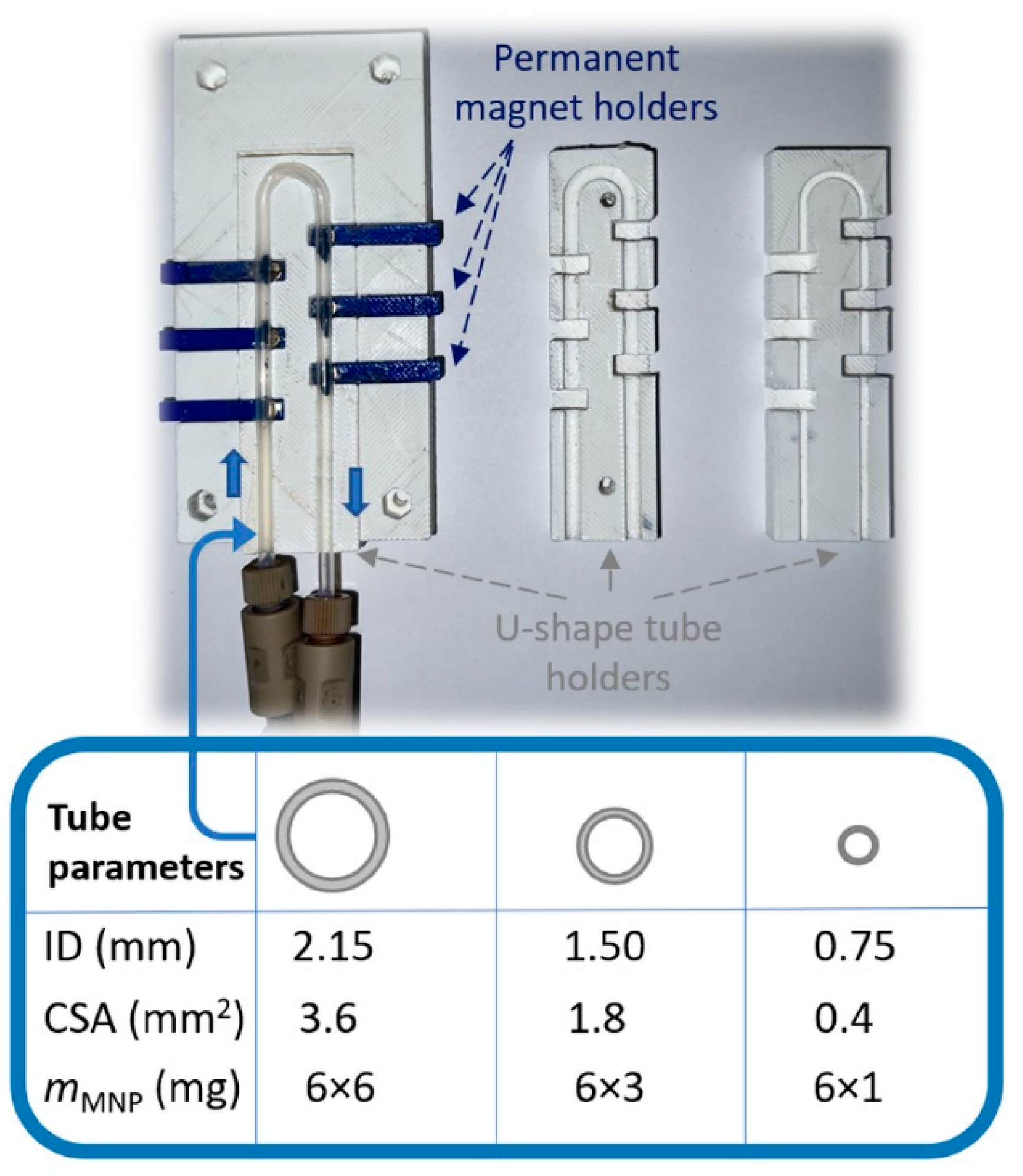
2.2. Kinetic Resolution Experiments with Racemic 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-ol (±)-1a and 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-ol (±)-1b in Continuous Flow Mode U-Shape Reactor Utilizing CaLB-MNPs
3. Materials and Methods
3.1. Materials
3.2. Analytical and Separation Methods
3.3. Assaying the Kinetic Resolution of 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-ol (±)-1a and 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-ol (±)-1b by CaLB-MNP Biocatalyst in Batch Mode
3.4. Kinetic Resolution of 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-ol (±)-1a and 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-ol (±)-1b by CaLB-MNPs Biocatalyst at Preparative Scale in Batch Mode
3.4.1. 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-yl Acetate (R)-2a
3.4.2. 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-ol (S)-1a
3.4.3. 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-yl Acetate (R)-2b
3.4.4. 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-ol (S)-1b
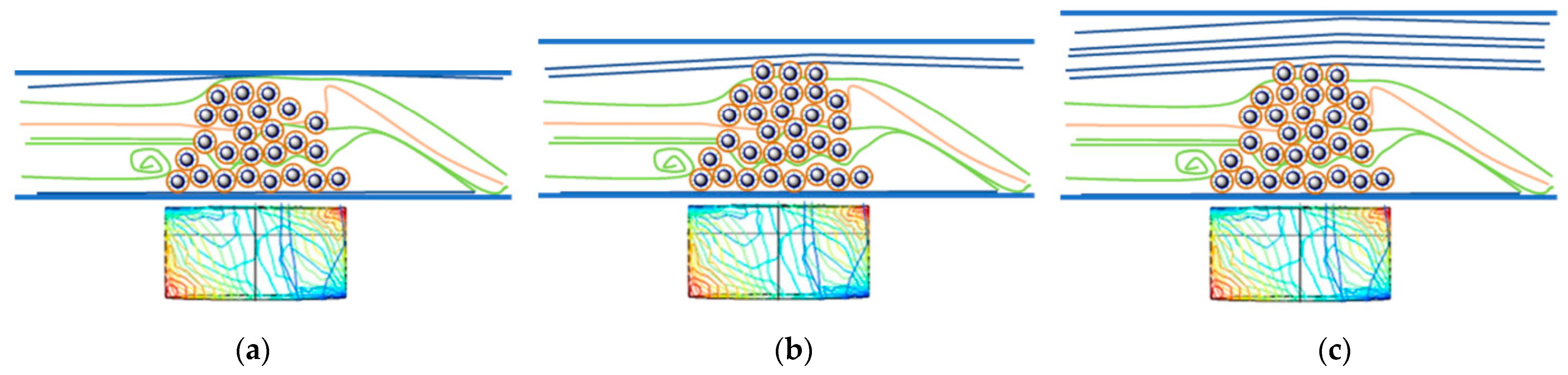
3.5. Design and Assembly of the U-Shape MNP Reactor
3.6. Kinetic Resolution of 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-ol (±)-1a and 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-ol (±)-1b by CaLB-MNPs Biocatalysts in the Continuous Flow U-Shape Reactor
3.6.1. 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-yl Acetate (R)-2a
3.6.2. 4-(3,4-Dihydroisoquinolin-2(1H)-yl)butan-2-ol (S)-1a
3.6.3. 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-yl Acetate (R)-2b
3.6.4. 4-(3,4-Dihydroquinolin-1(2H)-yl)butan-2-ol (S)-1a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, D.; Bayer, T.; Badenhorst, C.P.S.; Wu, S.; Doerr, M.; Höhne, M.; Bornscheuer, U.T. Recent trends in biocatalysis. Chem. Soc. Rev. 2021, 50, 8003–8049. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Prim. 2021, 1, 46. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A compreenhesive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Contesini, F.J.; Davanço, M.G.; Borin, G.P.; Vanegas, K.G.; Cirino, J.P.G.; de Melo, R.R.; Mortensen, U.H.; Hildén, K.; Campos, D.R.; Carvalho, P.d.O. Advances in Recombinant Lipases: Production, Engineering, Immobilization and Application in the Pharmaceutical Industry. Catalysts 2020, 10, 1032. [Google Scholar] [CrossRef]
- Kamble, M.P.; Yadav, G.D. Kinetic Resolution of (R,S)-α-Tetralol by Immobilized Candida antarctica Lipase B: Comparison of Packed-Bed over Stirred-Tank Batch Bioreactor. Ind. Eng. Chem. Res. 2017, 56, 1750–1757. [Google Scholar] [CrossRef]
- Zhong, L.; He, C.; Xiao, C.; Yao, C.; Pyatt, I.H.; Lu, Y. Covalent Immobilization of Candida antarctica Lipase B on Functionalized Hollow Mesoporous Silica Nanoparticles. ChemistrySelect 2021, 6, 3453–3460. [Google Scholar] [CrossRef]
- Palomo, J. Synthetic complexity created by lipases. Nat. Catal. 2020, 3, 335–336. [Google Scholar] [CrossRef]
- Pérez-Venegas, M.; Juaristi, E. Mechanoenzymology: State of the Art and Challenges towards Highly Sustainable Biocatalysis. ChemSusChem 2021, 14, 2682–2688. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Savina, A.A.; Zaitsev, I.S. Biochemical aspects of lipase immobilization at polysaccharides for biotechnology. Adv. Colloid Interface Sci. 2019, 272, 102016. [Google Scholar] [CrossRef]
- Coşkun, G.; Çıplak, Z.; Yıldız, N.; Mehmetoğlu, Ü. Immobilization of Candida antarctica Lipase on Nanomaterials and Investigation of the Enzyme Activity and Enantioselectivity. Appl. Biochem. Biotechnol. 2021, 193, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Cipolatti, E.P.; Valério, A.; Carbonera, N.G.; Soares, N.S.; Theilacker, E.; Ninow, J.L.; de Oliveira, D. Evaluation of different methods for immobilization of Candida antarctica lipase B (CalB lipase) in polyurethane foam and its application in the production of geranyl propionate. Bioprocess Biosyst. Eng. 2015, 38, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.-C.; Della Negra, M.; Kaiser, A.; Pinelo, M. Enzyme Immobilization on Inorganic Surfaces for Membrane Reactor Applications: Mass Transfer Challenges, Enzyme Leakage and Reuse of Materials. Adv. Synth. Catal. 2018, 360, 2578–2607. [Google Scholar] [CrossRef]
- Saikia, K.; Rathankumar, A.K.; Vaithyanathan, V.K.; Cabana, H.; Vaidyanathan, V.K. Preparation of Highly Diffusible Porous Cross-Linked Lipase B from Candida antarctica Conjugates: Advances in Mass Transfer and Application in Transesterification of 5-Hydroxymethylfurfural. Int. J. Biol. Macromol. 2021, 170, 583–592. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Rios, N.S.; Sousa, J.S.; Robert, J.D.M.; da Silva, A.A.T.; Pinto, M.C.; Simas, A.B.C.; Vilarrasa-García, E.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; et al. Synthesis of lipase/silica biocatalysts through the immobilization of CALB on porous SBA-15 and their application on the resolution of pharmaceutical derivatives and on nutraceutical enrichment of natural oil. Mol. Catal. 2021, 505, 111529. [Google Scholar] [CrossRef]
- Wang, H.; Yue, W.; Zhang, S.; Zhang, Y.; Li, C.; Su, W. Modification of Silica Xerogels with Polydopamine for Lipase B from Candida antarctica Immobilization. Catalysts 2021, 11, 1463. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Antunes, A.; Oro, C.E.D.; Dallago, R.M.; Mignoni, M.L. Immobilization of Candida antarctica B (CALB) in Silica Aerogel: Morphological Characteristics and Stability. Biointerface Res. Appl. Chem. 2010, 10, 6744–6756. [Google Scholar] [CrossRef]
- Sulym, I.; Zdarta, J.; Ciesielczyk, F.; Sternik, D.; Derylo-Marczewska, A.; Jesionowski, T. Pristine and Poly(Dimethylsiloxane) Modified Multi-Walled Carbon Nanotubes as Supports for Lipase Immobilization. Materials 2021, 14, 2874. [Google Scholar] [CrossRef]
- Szelwicka, A.; Siewniak, A.; Kolanowska, A.; Boncel, S.; Chrobok, A. PTFE-Carbon Nanotubes and Lipase B from Candida antarctica—Long-Lasting Marriage For Ultra-Fast And Fully Selective Synthesis Of Levulinate Esters. Materials 2021, 14, 1518. [Google Scholar] [CrossRef]
- Xing, X.; Jia, J.; Zhang, J.; Zhou, Z.; Li, J.; Wang, N.; Yu, X. CALB Immobilized onto Magnetic Nanoparticles for Efficient Kinetic Resolution of Racemic Secondary Alcohols: Long-Term Stability and Reusability. Molecules 2019, 24, 490. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; Dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2009, 349, 1289–1307. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. Magnetic Nanoparticle-Containing Supports as Carriers of Immobilized Enzymes: Key Factors Influencing the Biocatalyst Performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol 2022, 2, 374–390. [Google Scholar] [CrossRef]
- Slabu, I.; Galman, J.L.; Lloyd, R.C.; Turner, N.J. Discovery, Engineering, and Synthetic Application of Transaminase Biocatalysts. ACS Catal. 2017, 7, 8263–8284. [Google Scholar] [CrossRef]
- Dong, T.; Zhou, X.; Dai, Y.; Yang, X.; Zhang, W.; Yu, D.; Liu, T. Application of magnetic immobilized enzyme of nano dialdehyde starch in deacidification of rice bran oil. Enzym. Microb. Technol. 2022, 161, 110116. [Google Scholar] [CrossRef] [PubMed]
- Amini, Y.; Shahedi, M.; Habibi, Z.; Yousefi, M.; Ashjari, M.; Mohammadi, M. A multi-component reaction for covalent immobilization of lipases on amine-functionalized magnetic nanoparticles: Production of biodiesel from waste cooking oil. Bioresour. Bioprocess. 2022, 9, 60. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Abdellatiff, M.H.; Saidi, M.; Bozorgian, A.; Nodeh, H.R.; Rezania, S. Biodiesel production from waste cooking oil using a novel biocatalyst of lipase enzyme immobilized magnetic nanocomposite. Fuel 2022, 313, 123057. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- de Souza, M.C.M.; Dos Santos, K.P.; Freire, R.M.; Barreto, A.C.H.; Fechine, P.B.A.; Gonçalves, L.R.B. Production of flavor esters catalyzed by Lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Eng. 2017, 34, 681–690. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jang, W.Y.; Chang, J.H. Reusable and rapid esterolysis of nitrophenyl alkanoates with CalB enzyme-immobilized magnetic nanoparticles. J. Korean Ceram. Soc. 2022, 59, 527–535. [Google Scholar] [CrossRef]
- Song, M.; Chang, J.-H. Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification. Int. J. Mol. Sci. 2022, 23, 2459. [Google Scholar] [CrossRef] [PubMed]
- de Barros, H.R.; Tanaka, L.Y.; da Silva, R.T.P.; Santiago-Arcos, J.; Torresi, S.I.C.; López-Gallego, F. Assembly of Nano-Biocatalyst for the Tandem Hydrolysis and Reduction of p-Nitrophenol Esters. Part. Part. Syst. Charact. 2021, 38, 2100136. [Google Scholar] [CrossRef]
- Netto, C.G.C.M.; Andrade, L.H.; Toma, H.E. Enantioselective transesterification catalysis by Candida antarctica lipase immobilized on superparamagnetic nanoparticles. Tetrahedron Asymmetry 2009, 20, 2299–2304. [Google Scholar] [CrossRef]
- Spelmezan, C.G.; Bencze, L.C.; Katona, G.; Irimie, F.D.; Paizs, C.; Tosa, M.I. Efficient and Stable Magnetic Chitosan-Lipase B from Candida antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols. Molecules 2020, 25, 350. [Google Scholar] [CrossRef]
- Seddigi, Z.S.; Malik, M.S.; Ahmed, S.A.; Babalghith, A.O.; Kamal, A. Lipases in asymmetric transformations: Recent advances in classical kinetic resolution and lipase-metal combinations for dynamic processes. Coord. Chem. Rev. 2017, 348, 54–70. [Google Scholar] [CrossRef]
- Hamidović, M.; Ender, F.; Springer, A.A. A Novel Enzymatic Microreactor: Towards Transforming the Pharmaceutical Industry. In CMBEBIH 2019, IFMBE Proceedings; Badnjevic, A., Škrbić, R., Gurbeta Pokvić, L., Eds.; Springer: Cham, Switzerland, 2020; Volume 73, pp. 303–308. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A Perspective on Continuous Flow Chemistry in the Pharmaceutical Industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- de Santis, P.; Meyer, L.-E.; Kara, S. The Rise of Continuous Flow Biocatalysis—Fundamentals, Very Recent Developments and Future Perspectives. React. Chem. Eng. 2020, 5, 2155–2184. [Google Scholar] [CrossRef]
- Žnidaršič-Plazl, P. Biotransformations in microflow systems: Bridging the gap between academia and industry. J. Flow Chem. 2017, 7, 111–117. [Google Scholar] [CrossRef]
- Nagy, C.; Szabo, R.; Gaspar, A. Microfluidic Immobilized Enzymatic Reactors for Proteomic Analyses-Recent Developments and Trends (2017–2021). Micromachines 2022, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Schejbal, J.; Řemínek, R.; Zeman, L.; Mádr, A.; Glatz, Z. On-line coupling of immobilized cytochrome P450 microreactor and capillary electrophoresis: A promising tool for drug development. J. Chromatogr. A 2016, 1437, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gkantzou, E.; Patila, M.; Stamatis, H. Magnetic Microreactors with Immobilized Enzymes—From Assemblage to Contemporary Applications. Catalysts 2018, 8, 282. [Google Scholar] [CrossRef]
- Mandai, K.; Fukuda, T.; Miyazaki, Y.; Hashimoto, H.; Mandai, H.; Ema, T.; Takada, J.; Suga, S. Magnetic Attachment of Lipase Immobilized on Bacteriogenic Iron Oxide Inside a Microtube Reactor for the Kinetic Resolution of Secondary Alcohols. Synlett 2017, 28, 805–810. [Google Scholar] [CrossRef]
- Sans, V. Emerging trends in flow chemistry enabled by 3D printing: Robust reactors, biocatalysis and electrochemistry. Curr. Opin. Green Sustain. Chem. 2020, 25, 100367. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, W.; Chen, Y.; Guo, L.; Yang, L. A replaceable dual-enzyme capillary microreactor using magnetic beads and its application for simultaneous detection of acetaldehyde and pyruvate. Electrophoresis 2012, 33, 2145–2151. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, L.; Lei, J.; Ju, H. Fabrication of tunable microreactor with enzyme modified magnetic nanoparticles for microfluidic electrochemical detection of glucose. Anal. Chim. Acta 2012, 709, 41–46. [Google Scholar] [CrossRef]
- Weiser, D.; Bencze, L.C.; Bánóczi, G.; Ender, F.; Kiss, R.; Kókai, E.; Szilágyi, A.; Vértessy, B.G.; Farkas, Ö.; Paizs, C.; et al. Phenylalanine Ammonia-Lyase-Catalyzed Deamination of an Acyclic Amino Acid: Enzyme Mechanistic Studies Aided by a Novel Microreactor Filled with Magnetic Nanoparticles. ChemBioChem 2015, 16, 2283–2288. [Google Scholar] [CrossRef]
- Ender, F.; Weiser, D.; Nagy, B.; Bencze, C.L.; Paizs, C.; Pálovics, P.; Poppe, L. Microfluidic multiple cell chip reactor filled with enzyme-coated magnetic nanoparticles—An efficient and flexible novel tool for enzyme catalyzed biotransformations. J. Flow Chem. 2016, 6, 43–52. [Google Scholar] [CrossRef]
- Hübner, J.; Brakowski, R.; Wohlgemuth, J.; Brenner-Weiß, G.; Franzreb, M. Compartmented microfluidic bioreactor system using magnetic enzyme immobilisates for fast small-scale biotransformation studies. Eng. Life Sci. 2015, 15, 721–726. [Google Scholar] [CrossRef]
- Jussen, D.; Soltner, H.; Stute, B.; Wiechert, W.; von Lieres, E.; Pohl, M. μMORE: A microfluidic magnetic oscillation reactor for accelerated parameter optimization in biocatalysis. J. Biotechnol. 2016, 231, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Imarah, A.O.; Silva, F.M.W.G.; Tuba, L.; Malta-Lakó, Á.; Szemes, J.; Sánta-Bell, E.; Poppe, L. A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase-Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol. Catalysts 2022, 12, 1065. [Google Scholar] [CrossRef]
- Aldeghi, M.; Malhotra, S.; Selwood, D.L.; Chan, A.W.E. Two- and Three-dimensional Rings in Drugs. Chem. Biol. Drug Des. 2014, 83, 450–461. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative Analyses of Biochemical Kinetic Resolutions of Enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, Y.K.; Kim, Y.H.; Park, E.S.; Kim, E.J.; Kim, M.-J.; Park, J. Aminocyclopentadienyl Ruthenium Complexes as Racemization Catalysts for Dynamic Kinetic Resolution of Secondary Alcohols at Ambient Temperature. J. Org. Chem. 2004, 69, 1972–1977. [Google Scholar] [CrossRef]
- Toşa, M.; Pilbák, S.; Moldovan, P.; Paizs, C.; Szatzker, G.; Szakács, G.; Novák, L.; Irimie, F.-D.; Poppe, L. Lipase-catalyzed kinetic resolution of racemic 1-heteroarylethanols—Experimental and QM/MM study. Tetrahedron Asymmetry 2008, 19, 1844–1852. [Google Scholar] [CrossRef]
- Hii, K.K.; Hellgardt, K. Catalysis in Flow: Why Leaching Matters. Top. Organomet. Chem. 2015, 57, 249–262. [Google Scholar] [CrossRef]
- Csuka, P.; Molnár, Z.; Tóth, V.; Imarah, A.O.; Balogh-Weiser, D.; Vértessy, B.G.; Poppe, L. Immobilization of the Aspartate Ammonia-Lyase from Pseudomonas fluorescens R124 on Magnetic Nanoparticles: Characterization and Kinetics. ChemBioChem 2022, 23, e202100708. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Pedrali, A.; Urbano, M.; Gaggeri, R.; Serra, M.; Fernandez, L.; Fernandez, M.; Caballero, J.; Ronsisvalle, S.; Prezzavento, O.; et al. Identification of a potent and selective σ1 receptor agonist potentiating NGF-induced neurite outgrowth in PC12 cells. Bioorg. Med. Chem. 2011, 19, 6210–6224. [Google Scholar] [CrossRef]
- Kuriyama, M.; Nakashima, S.; Miyagi, T.; Sato, K.; Yamamoto, K.; Onomura, O. Palladium-catalyzed chemoselective anaerobic oxidation of N-heterocycle-containing alcohols. Org. Chem. Front. 2018, 5, 2364–2369. [Google Scholar] [CrossRef]
- Ouyang, W.; Liu, B.; He, Y.; Wen, Y.; Gao, Y.; Huo, Y.; Chen, Q.; Li, X. Modular construction of functionalized anilines via switchable C-H and N-alkylations of traceless N-nitroso anilines with olefins. Org. Chem. Front. 2022, 9, 2746–2752. [Google Scholar] [CrossRef]
- Deb, M.L.; Dey, S.S.; Bento, I.; Barros, M.T.; Maycock, C.D. Copper-catalyzed regioselective intramolecular oxidative α-functionalization of tertiary amines: An efficient synthesis of dihydro-1,3-oxazines. Angew. Chem. Int. Ed. 2013, 52, 9791–9795. [Google Scholar] [CrossRef] [PubMed]

| Subst. | Time (h) | Temp. (°C) | Conv. (%) | Product | Yield 1 (%) | ee 2 (%) | E 3 | [α]365 4 | [α]578 4 |
|---|---|---|---|---|---|---|---|---|---|
| (±)-1a | 90 | 25 | 44 | (R)-2a | 41.3 | 96.7 | 137 | +9.1 | +6.2 |
| 25 | 44 | (S)-1a | 17.5 | 76.0 5 | 137 | −6.4 | +1.8 | ||
| (±)-1b | 30 | 25 | 41 | (R)-2b | 29.2 | 96.8 | 126 | −29.8 | −0.3 |
| 25 | 41 | (S)-1b | 13.1 | 67.3 5 | 126 | +3.6 | +12.2 | ||
| (±)-1b | 36 | 20 | 38 | (R)-2b | 24.6 | 97.9 | 180 | −27.3 | −0.3 |
| (±)-1b | 75 | 20 | 49 | (R)-2b | 41.2 | 95.4 | 136 | −26.8 | −0.4 |
| (S)-1b | 6 | 6 | 6 | (S)-2b 6 | 60.2 | 87.2 | +24.3 | +0.3 |
| Subst. | Conv. 1 (%) | Product | Yield (%) | ee 2 (%) |
|---|---|---|---|---|
| (±)-1a | 25.4 | (R)-2a | 21 | 93.1 |
| 25.4 | (S)-1a | 36 3 | 4 | |
| (±)-1b | 43.7 | (R)-2b | 39 | 91.8 |
| 43.7 | (S)-1b | 34 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.M.W.G.; Imarah, A.O.; Takács, O.; Tuba, L.; Poppe, L. Scalability of U-Shape Magnetic Nanoparticles-Based Microreactor–Lipase-Catalyzed Preparative Scale Kinetic Resolutions of Drug-like Fragments. Catalysts 2023, 13, 384. https://doi.org/10.3390/catal13020384
Silva FMWG, Imarah AO, Takács O, Tuba L, Poppe L. Scalability of U-Shape Magnetic Nanoparticles-Based Microreactor–Lipase-Catalyzed Preparative Scale Kinetic Resolutions of Drug-like Fragments. Catalysts. 2023; 13(2):384. https://doi.org/10.3390/catal13020384
Chicago/Turabian StyleSilva, Fausto M. W. G., Ali O. Imarah, Orsolya Takács, László Tuba, and László Poppe. 2023. "Scalability of U-Shape Magnetic Nanoparticles-Based Microreactor–Lipase-Catalyzed Preparative Scale Kinetic Resolutions of Drug-like Fragments" Catalysts 13, no. 2: 384. https://doi.org/10.3390/catal13020384
APA StyleSilva, F. M. W. G., Imarah, A. O., Takács, O., Tuba, L., & Poppe, L. (2023). Scalability of U-Shape Magnetic Nanoparticles-Based Microreactor–Lipase-Catalyzed Preparative Scale Kinetic Resolutions of Drug-like Fragments. Catalysts, 13(2), 384. https://doi.org/10.3390/catal13020384










