Abstract
Ni-based catalysts supported on Mg-Al mixed oxides (Mg(Al)O) have been intensively investigated as catalysts for CH4 reforming processes (i.e., steam reforming (SMR) and dry reforming (DRM)), which are pivotal actors in the expanding H2 economy. In this review, we provide for the first time an in-depth analysis of homo- and bimetallic Ni-based catalysts supported on Mg(Al)O supports reported to date in the literature and used for SMR and DRM processes. Particular attention is devoted to the role of the synthesis protocols on the structural and morphological properties of the final catalytic materials, which are directly related to their catalytic performance. It turns out that the addition of a small amount of a second metal to Ni (bimetallic catalysts), in some cases, is the most practicable way to improve the catalyst durability. In addition, besides more conventional approaches (i.e., impregnation and co-precipitation), other innovative synthesis methods (e.g., sol-gel, atomic layer deposition, redox reactions) and pretreatments (e.g., plasma-based treatments) have shown relevant improvements in identifying and controlling the interaction among the constituents most useful to improve the overall H2 productivity.
1. Introduction
The rapid expansion of the H2 economy, which has recently received new momentum from institutions such as the European Union with dedicated initiatives [1], calls for mid-term strategies to cope with the surge in demand. In this scenario, the conversion of CH4 and/or biogas via thermo-catalytic reforming processes, that is, steam (SMR) and dry reforming (DRM), represents a solid strategy. The former is one of the best-established H2 production technologies, which today accounts for most of the world’s production, while the latter allows the simultaneous conversion of two potent greenhouse gases into a valuable building block—the syngas, a mixture of CO and H2 [2,3,4,5,6].
Since they are among the most used catalysts, several works have been dedicated to reviewing Ni-based catalysts for both reactions but are either focused on one reaction at a time and/or include the most different catalysts’ compositions [2,3,4,5,6,7]. Here we focused our attention on Ni catalysts supported on Mg-Al mixed oxides and modified with other metals. The reason is that, more than other catalysts, these Ni(M)/Mg(Al)O catalysts offer a unique opportunity to develop new strategies to address the many challenges posed by both SMR and DRM processes since both the synthetic protocol and/or the specific composition have a dramatic influence on the final catalytic activity. After a brief introduction recalling the main aspects of both reactions with a special emphasis on the role of the metal support interaction, the impact of several preparation methods on the structure and catalytic activity of monometallic Ni catalysts will be discussed in detail, followed by a section describing the influence of other metals, i.e., bimetallic catalysts. Considering the sheer mole of data here reviewed and listed in tables that might render reading difficult, the main findings are summarized also in a schematic way in the final paragraph. In the hope of providing a useful guide for the development of future Ni-based catalysts for SMR and DRM reactions, we then singled out the most promising strategies along with some underestimated and/or unexplored pathways for improving the catalytic activity.
1.1. Steam and Dry Reforming Reactions of CH4: General Aspects
In the CH4-H2O system, under the conditions of the steam reforming process, several chemical reactions are realized, of which the main ones are the steam reforming reaction (Equation (1)), which is strongly endothermic, and the water gas shift (WGS) reaction (Equation (2)), which is exothermic:
SMR: CH4 + H2O ⇆ CO + 3H2 (ΔH298 = +205.9 kJ·mol−1)
WGS: CO + H2O ⇆ CO2 + H2 (ΔH298 = −41 kJ·mol−1)
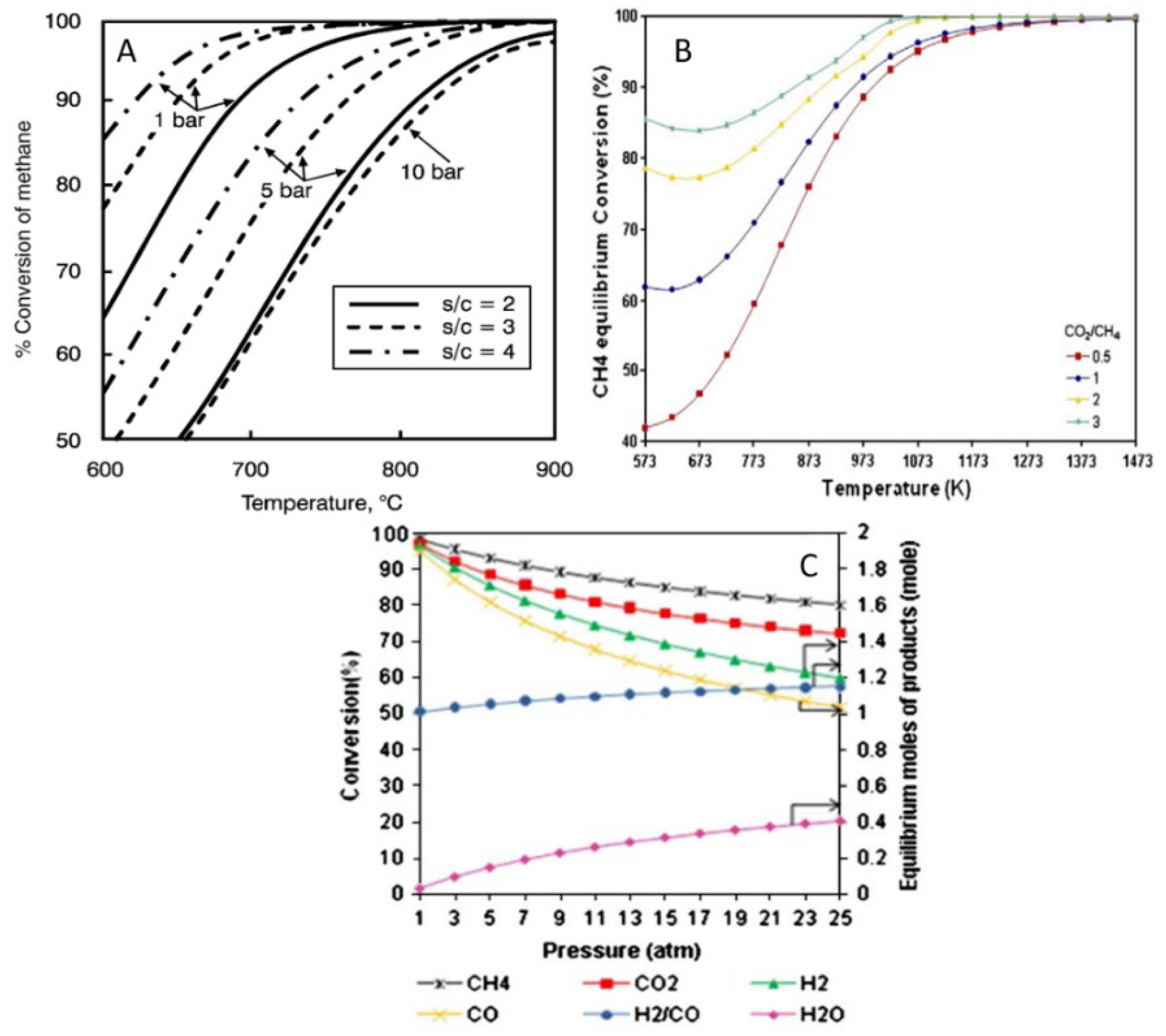
In this process (Equations (1) and (2)), four moles of H2 are produced from each mole of methane, so that a large amount of H2 can be obtained on an industrial scale [7,8]. Figure 1A shows the equilibrium conversion curve of SMR calculated by Joensen and Rostrup-Nielsen [9]. To achieve a high methane conversion, the reforming process should be carried out at a high temperature, low pressure, and a relatively high steam-to-methane molar ratio (3:1). Though SMR is a mature technology to produce H2, the high energy required, the water consumption, and the use of methane with low-sulfur content are the main disadvantages of this process. Other critical issues are the simultaneous production of CO2, which should be captured to avoid its emission into the environment, and the high cost of the overall process, which is still expensive compared to the US Department of Energy (DOE) cost of producing H2 for future cars and other applications [10,11,12].
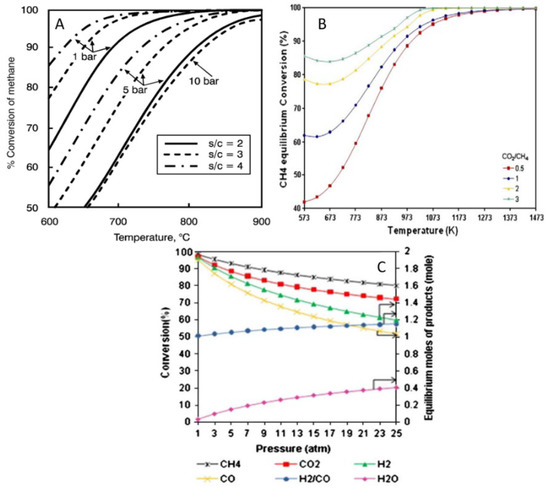
Figure 1.
Equilibrium conversion of (A) SMR against temperature, pressure, and steam/methane ratio. Reprinted with permission from [9], copyright 2022, Elsevier. (B) DRM against temperature and CO2/CH4 ratio (C) DMR against reactants and products distribution for CO2/CH4 = 1, 900 °C and n (CH4 + CO2) = 2 mol. Reprinted with permission from [13], copyright 2011, Elsevier.
Like SMR, the DRM process is highly endothermic and as such is favored at high temperatures (700–900 °C). As CO2 is one of the reactants, this type of methane reforming theoretically does not require any downstream CO2 capture, which is a major perk. Moreover, the H2/CO molar ratio of the produced syngas is typically around 1, which is lower than that of SMR and is particularly suited for the Fischer–Tropsch process [14,15]. The thermodynamics and equilibrium characteristics of DRM are the following:
DRM: CH4 + CO2 ⇆ 2CO + 2H2 (ΔH298 = +247.3 kJ·mol−1)
RWGS: CO2 + H2 ⇆ CO + H2O (ΔH298 = −41 kJ·mol−1)
The reverse water gas shift reaction (RWGS) occurs concurrently with DRM and, according to the studies of Bradford and Vannice, has a lower activation energy compared to H2 formation [16]. In accordance with the equilibrium conversion curve of DRM (Figure 1B), CH4 conversion increases rapidly below 727 °C and is generally favored at high CO2/CH4 molar ratios while it is hampered by high operating pressures [13]. Although the DRM process reduces CH4 and CO2 emissions into the atmosphere and converts them into a valuable product (syngas), it requires a quite high energy input because of two reasons. First, the elevated content of CO in the produced syngas results in an elevated total heating value compared to SMR syngas with a higher H2 content [17]. Second, although DRM on paper is a simpler process than SMR as no steam is needed, the energy requirement is higher due to the exceptional thermodynamic stability of CO2 [13,14,15,17,18,19,20].
CH4 reforming processes are conventionally carried out in catalytic conditions [21]. Noble metals and group VIII metals (Ni, Fe, and Co) are active in both SMR and DRM [21,22,23,24]. However, Fe and Co are susceptible to oxidation under the harsh condition required for reasonably high conversions (high temperature and high steam pressure) while the application of noble metals is limited due to their high market price [6]. Consequently, Ni-based catalysts are extensively studied because of their cost-effectiveness and high reforming activity [25,26]. The main pitfall of using Ni-based catalysts is the very high coke formation rate, which generally causes a rapid failure of the catalysts [27,28,29]. The carbon deposits on the catalyst surface can have different forms (e.g., whisker, gum, pyrolytic coke) and they can seriously affect the reusability of the catalyst by disrupting the catalyst structure (Table 1) [30]. Gum-like (multilayer graphite) and pyrolytic coke can cover the active Ni sites and block the reaction whereas whisker carbon, having high mechanical strength, can break the catalyst pellet, hence causing a pressure build-up and tube failure [22]. Carbon can be formed via two main routes: methane decomposition (Equation (5)) and CO disproportionation (Equation (6)), the latter being dominant at low temperatures due to its exothermicity. When the reforming process is carried out at a high temperature, methane dissociation is the main source of coke buildup. The feed composition also influences the tendency to carbon accumulation. In general, low molar H/C and O/C ratios in the feed stream can promote coke generation [31]. The calculated H/C and O/C molar ratios of stochiometric SMR are six and one, respectively, whereas in DRM feed they are lower (two and one, respectively), thus coke deposition is particularly more severe in DRM.
CH4 dissociation: CH4 ⇆ C + 2H2 (∆H298 = +75 kJ·mol−1)
CO disproportionation: 2CO ⇆ CO2 + C (∆H298 = −172 kJ·mol−1)

Table 1.
Types of carbon formed during reforming reactions, their effects and critical conditions favoring the formation.
1.2. The Ni(M)/Mg(Al)O Catalysts
To overcome the identified challenges in catalytic reforming systems, the selection of the catalyst support plays a crucial role during the catalyst development [32,33,34]. In addition to coke limiting ability, reforming catalysts must also meet stringent requirements due to the harsh conditions required of the processes (high temperature, high pressure, and presence of steam) [22,35]. Common oxide supports are Al2O3, MgO, and their corresponding mixed oxides [22]. Because of its high surface area, thermal and chemical stability, and strong interaction with Ni, Al2O3 is one of the most studied supports [36,37]. Indeed, commercially available catalysts for traditional SMR are mainly constituted by Ni supported on Al2O3 [38]. MgO support is also widely studied, particularly in DRM, because its high basicity is beneficial for adsorbing CO2, which in turn enhances the reforming activity and the coke removal rate [39,40]. Moreover, the ability of MgO to form a strongly interacting solid solution with NiO improves the catalyst stability and resistance to sintering [41,42]. Since these are all highly desirable characteristics for a support, the combination of Al and Mg oxides, that is, Mg-Al mixed oxides (herein denoted as Mg(Al)O), is often exploited to prepare highly performing catalysts and therefore will be discussed in the following sections [22,43].
As for the active metallic constituents, bimetallic systems are attracting considerable research interest thanks to the possible promotional effect of the second (or third) metal on the processes and/or the presence of synergetic effects between the metals [26,44,45]. Particularly for Ni-based catalysts, noble, alkaline, and rare earth metal promoters are the subjects of many studies and demonstrate promising improvements. Among those, the addition of small amounts of noble metals to Ni catalysts is beneficial while remaining cost competitive [31]. The addition of Pt, Ru, and Pd improves reducibility, may provoke an auto-activation of the catalyst, and reduces the coke formation [31]. On the other hand, the addition of Group 11 metals (Au, Ag) reduces the Ni activity but extends the catalyst’s lifetime by inhibiting the coke formation [46].
In addition to the selection of the active metal, support, and promoters, the way the catalyst is prepared also plays an important role in determining its morphological and structural features and, as a result, its catalytic performance [46,47,48]. This is particularly true when preparing Mg-Al mixed oxides, which can form several crystal phases and can have different morphological characteristics [49,50]. Hence, a thorough understanding of each parameter in traditional synthesis procedures as well as the exploration of innovative preparation approaches are both highly desirable to improve homo- and bimetallic Ni catalysts supported on Mg(Al)O for SMR and DRM.
Metal–support interaction is arguably one of the most critical aspects impacting both the activity and the stability of heterogeneous catalysts, especially when used in high-temperature processes. The interaction of Ni with the support is a somewhat textbook example of the tradeoffs to be considered when preparing a heterogeneous catalyst. If, on the one hand, a weak interaction is good for the catalytic activity because it does not impair the Ni reducibility, it may be insufficient to stabilize the nanoparticles against sintering [51,52]. It is also true, however, that it is hard to obtain small Ni nanoparticles, and thus a high number of catalytically active sites with Ni interacting too weakly with the support can be found. In other words, a strong interaction with the support helps suppress the overgrowth of the particles, thereby enhancing their stability at high reaction temperatures, but could also lead to Ni nanoparticles that are hard to reduce, which may limit the availability of metallic Ni [48,53].
The following section delves into the factors influencing the interaction of Ni with Mg and Al oxides separately and then with the Mg(Al)O mixed oxide.
2. Ni-Support Interaction in Ni(M)/Mg(Al)O Catalysts
Ni-based catalysts are generally prepared as NiO followed by reduction, but the final form of the catalyst is heavily influenced by the composition of the Mg(Al)O support because Ni interacts very differently with Al and Mg oxides. The metal–support interaction will be first discussed for Ni catalysts supported on Al and Mg oxides separately; then we will showcase a few examples of how their combination is essential to obtain high-performing reforming Ni-based catalysts.
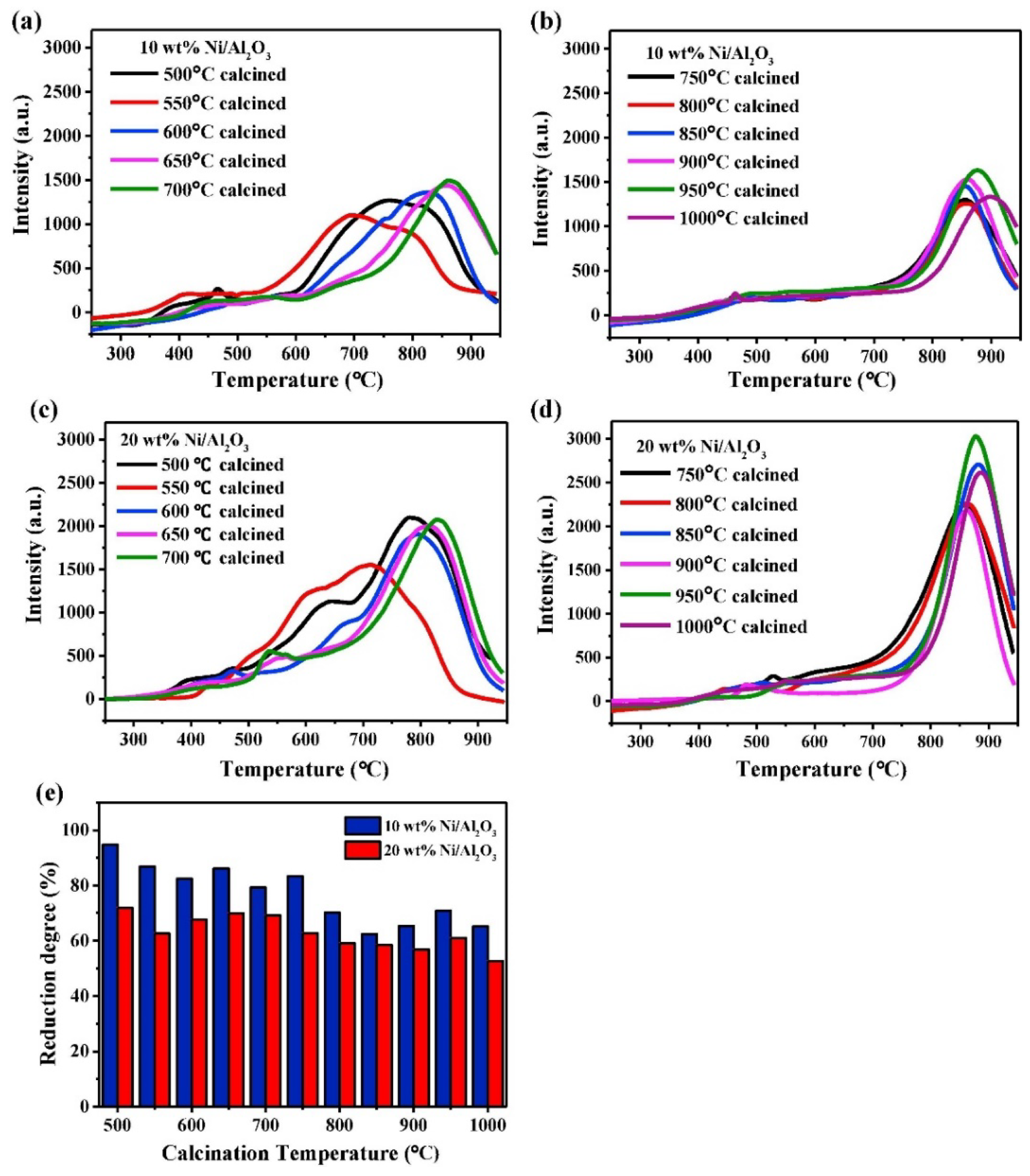
NiO is known to form a stable spinel nickel aluminate with Al2O3 after heat treatment, denoted as NiAl2O4 [54]. In Ni-Al-O systems, three phases of Ni-Al2O3 can be observed: “free” NiO particles, NiO weakly interacting with Al2O3, and NiAl2O4 spinel. The order is based on the strength of interaction between Ni and Al. Temperature-programmed reduction (TPR) can be used to identify the phases of Ni in Al2O3 (Figure 2). Due to the difference in bond strength, each phase displays reduction features in a specific temperature range. Reduction peaks occurring between 300 and 350 °C are originated by the reduction of “free” NiO to metallic Ni, whilst weakly interacting NiO-Al2O3 is reduced around 500–600 °C. The reduction of Ni2+ present in the spinel phase (NiAl2O4) can only be observed at temperatures higher than 800 °C. The formation of such three phases depends largely on calcination temperature, Ni loading, and the preparation method. Generally, high calcination temperatures favor the formation of spinel phases with stronger interaction between Ni and Al2O3 [55,56]. For instance, in catalysts containing 20 wt.% of Ni and calcined at temperature <750 °C, a low amount of the spinel phase could be detected, whereas Ni was present almost quantitatively as spinel when the catalyst was calcined at T > 750 °C. Salhi et al. found that the distribution of the spinel phase is also sensitive to the Ni/Al molar ratio [57]. In Ni-deficient samples, the solid exists as a mixture of Al2O3 and NiAl2O4, whereas NiO aggregates are formed in materials with higher Ni content. The distribution of NiAl2O4 is also affected by the preparation method. For example, while comparing the phase composition between impregnated and co-precipitated catalysts, Li and Chen found that the co-precipitated sample showed less reducibility than the impregnated one, implying the formation of spinel NiAl2O4 by the former method [54].
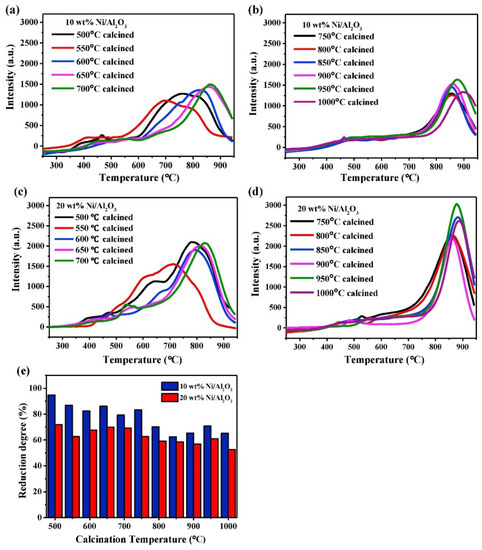
Figure 2.
(a–e) TPR and reduction degree of Ni/Al2O3 with different loading and different calcination temperature. Reprinted with permission from [56], copyright 2018, Elsevier.
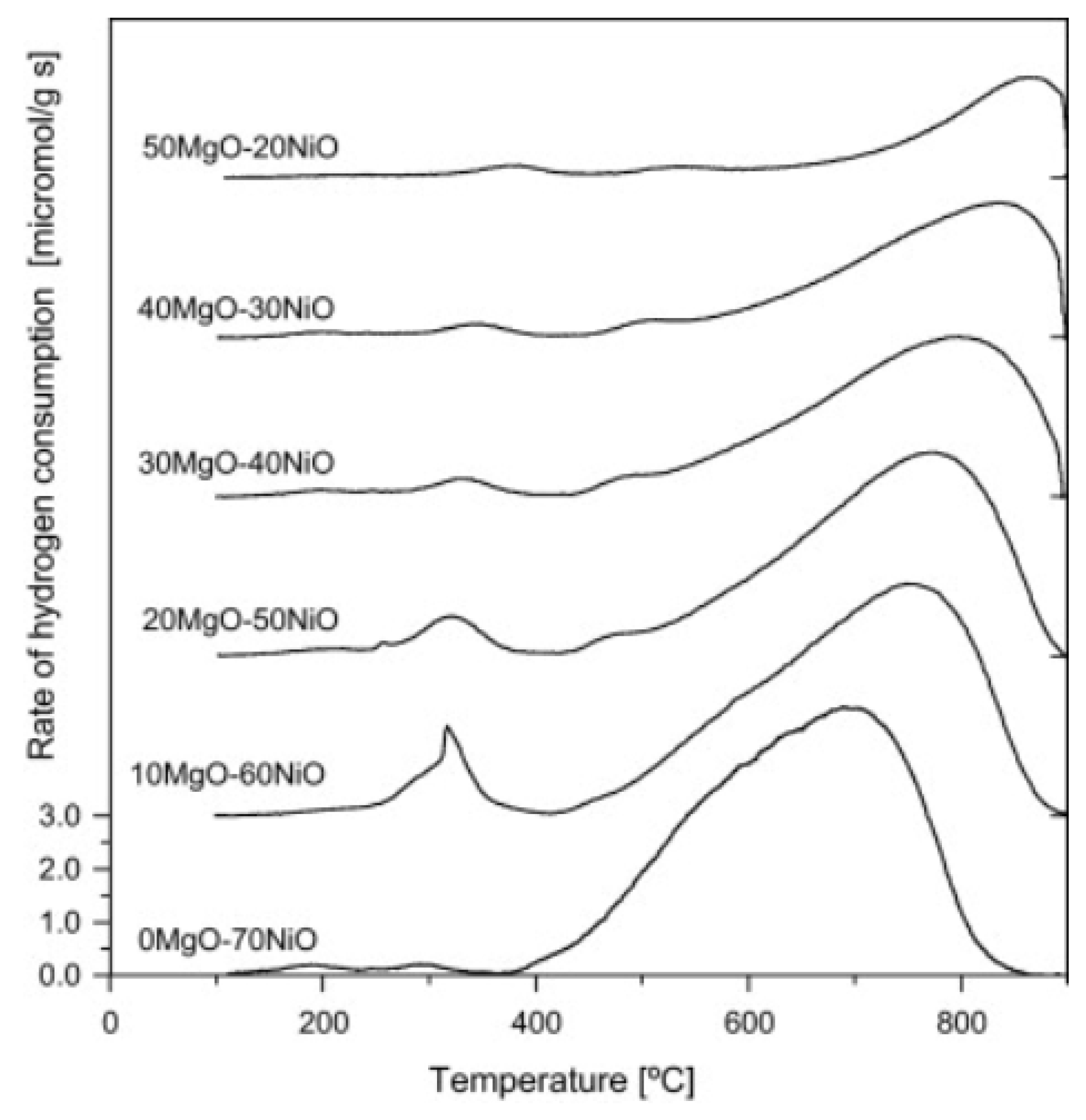
With MgO, NiO can form an ideal NiO-MgO solid solution in the whole range of concentrations due to the similar ionic radii of Ni2+ (69 pm) and Mg2+ (72 pm) [58]. In the solid solution, the interaction of Ni with MgO is particularly strong, resulting in a Ni phase much harder to be reduced. Conventionally, the reduction of NiO-MgO occurs above 800 °C with long reduction times (Figure 3) [59]. Interestingly, the strong interaction between NiO and MgO facilitates the formation of small Ni nanoparticles (NPs) upon reduction, thus preventing particles sintering and coke formation [60,61]. Additionally, the high basicity of MgO could be beneficial for the stability of the catalyst by enhancing the absorption of oxidizing reagents (CO2, H2O), resulting in better gasification of coke species formed on the catalyst surface [62,63].
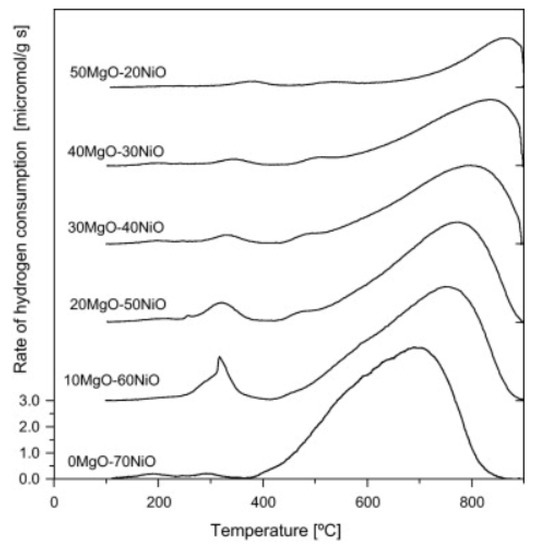
Figure 3.
TPR of Ni-MgO samples with different loading of MgO. Reprinted with permission from [64], copyright 2011, Elsevier.
In addition to the interactions between Ni and each oxide component, the mixed oxide itself can also form the MgAl2O4 spinel phase during the catalyst synthesis [64,65], which provides high surface area, good thermal stability, and strong interaction with Ni [48]. Damyanova et al. prepared Ni catalysts for DRM with different ceramic-based supports (δ,θ-Al2O3, SiO2-Al2O3, ZrO2-Al2O3, MgO-Al2O3) and the results indicated Ni/MgO-Al2O3 as the most active and stable catalyst [66]. Zhang et al. tested various supports in DRM and they found that Al2O3 showed high stability while MgO exhibited great coke resistance [67]. Hence, the authors combined the two benefits by using MgO-Al2O3 mixed oxide as a support for Ni catalysts. The resulting material demonstrated good activity and was stable for over 100 h on stream. Mehr et al. showed that MgO addition reduces carbon deposition, likely due to the Lewis basicity of MgO [68]. It should be noted that Das et al. argued that basicity is not monotonically beneficial, but instead, an optimal acidic/basic balance is required for DRM catalysts [69]. The authors reported that basic sites favor the adsorption of CO2, while acidic sites are necessary for CH4 activation.
Another important aspect of Mg(Al)O systems is the ability to form hydrotalcite. Hydrotalcite is a double-layered lamellar hydroxide (LDH) of Mg and Al with the formula Mg6Al2(OH)16CO3·4H2O [70]. It is demonstrated that materials derived from hydrotalcites are promising for various catalytic processes including CH4 reforming [71,72]. By decomposing the precursors via thermal treatments, homogenized mixed oxides can be obtained. For Ni supported on Mg(Al)O catalysts, hydrotalcite-derived routes are a great way to obtain high surface area, tailored basicity, and uniform distribution of the active metal on the surface [73,74,75,76,77,78].
The first procedure to report is that described by Shishido et al., who named their method “solid phase crystallization” (spc) [77]. The procedure consisted of the replacement of Mg2+ with Ni2+ during the co-precipitation of the LDH precursor, followed by heat treatment to obtain the final catalyst. The authors found that with the spc approach, the catalysts had higher surface area and better dispersion than conventional impregnated samples. They also demonstrated that such catalysts performed well in SMR. Takehira et al. obtained similar results with the same spc approach and the Ni0.5/Mg2.5-Al had high activity and stability under severe conditions of SMR. Moreover, they observed improved performance with the impregnated Ni/Mg3Al due to the reconstruction of the support towards the LDH-like structure during the impregnation process [79]. Further details on hydrotalcite-derived catalysts for SMR and DRM can be found in dedicated review papers [72,73,77,80].
3. Monometallic Ni/Mg(Al)O Catalysts for SMR and DRM
As discussed above, between the components of Ni-Mg-Al systems, various interactions can be established that can drive the efficiency and stability of the catalysts during the reaction. The preparation method therefore becomes crucial in obtaining the desired features. This section will discuss the conventional methods, that is, impregnation and co-precipitation, as well as more advanced techniques such as sol-gel, aerogel, atomic layer deposition (ALD), etc., highlighting the most critical synthesis parameters and their impact on catalytic performance [81].
3.1. Synthesis of Ni/Mg(Al)O Catalysts by Conventional Approaches
Ni catalysts supported on Al-Mg oxides obtained by impregnation and co-precipitation methods with different synthetic parameters, as well as their physicochemical properties and reforming performances for SMR and DMR processes, are summarized in Table 2 and discussed below. Particular attention is devoted to the role of the constituents and the thermal treatments of the catalysts (i.e., calcination and reduction steps).

Table 2.
Monometallic Ni catalysts supported on Mg(Al)O obtained by co-precipitation and impregnation methods for SMR and DRM.
3.1.1. Effect of Ni Loading and Composition Ratio
Ni loading and composition ratio play an important role in the characteristics of the catalysts: High Ni loadings may imply higher activity, but they may result in larger particles, which promote coke formation [82,83,100,101,102].
The study by Guo et al. showed that a catalyst with a low Ni content is not stable in DRM, while the conversion is positively correlated with Ni content only up to 15 wt.% [43]. As expected, above that loading, the coke formation becomes severe. A similar trend of coke deposition was found by Alipour et al. while studying impregnated Ni/Mg(Al)O catalysts obtained by wet impregnation [84]. They showed that after calcination, NiO segregated when above the 15 wt.% of Ni loading. The amount of carbon deposited scaled with the Ni content, but the reforming activity did not. Catalysts prepared via co-precipitation showed a similar behavior [85]. Akbari et al. synthesized mesoporous nanocrystalline Ni-Mg-Al catalysts using that method and studied the effect of Ni loading in DRM. They found again that Ni loading up to 15 wt.% did not dramatically change the textural properties of the catalysts (pore volume, pore size distribution, and surface area) and that above that loading the catalysts experienced severe coke deposition.
Some other studies focused on investigating which pathway of coke formation is preferred with the change in Ni content. Dębek et al. tested co-precipitated Ni/Mg(Al)O catalysts with up to 60 wt.% Ni loading in DRM and they found that the higher the Ni content, the higher the CH4 conversion [86]. Surprisingly, no improvement in CO2 conversion was observed when the Ni content changed. The authors indicated that the marginal increment of CH4 conversion is due to CH4 decomposition, resulting in an increase in coke deposited. Characterization of the Ni-rich catalysts showed large and highly reducible particles, along with a low basicity, which could cause coke formation. The Ni content is also found to affect the CO disproportionation pathway contribution [87]. Lin et al. investigated similar Ni/Mg(Al)O catalysts and found a positive correlation between reforming conversion as well as CO disproportionation activity and Ni loadings at a lower range (3–18 wt.%). In their study, at a low reaction temperature (600 °C), Ni-rich samples significantly promoted the coke deposition via the CO pathway, hindering the stability of the catalysts. At high reaction temperature (750 °C), where the exothermic Boudouard reaction is suppressed, the reverse relationship was observed: Catalysts containing more active metal showed better stability and less coke deposition.
Qi et al. used Ni/Mg(Al)O prepared by the co-precipitation technique and tested them in SMR in comparison with wet impregnated catalysts with varied divalent/trivalent cation molar ratios (Ni+Mg)/Al in the precursors [88]. They reported that the optimal (Ni+Mg)/Al molar ratio in the LDH precursor for SMR is three. The resulting catalyst exhibited the best activity because of its high surface area and high Ni dispersion upon reduction. A similar optimal (Ni+Mg)/Al molar ratio was found by Takehira et al. in SMR by co-precipitated Ni-Mg-Al [79]. The authors found that the high Al content can result in segregated Al(OH)3 and amorphous NiO-MgO, which are detrimental to the catalyst activity and stability. The optimal molar ratio between Ni and Mg (Ni/Mg = 0.5/2.5) was tested and was stable for 600 h. Shishido et al. also found that Ni/Mg = 0.5/2.5 molar ratio gave the highest dispersion for the co-precipitated Ni-Mg-Al catalyst [77].
3.1.2. Effect of Heat Treatment
Both calcination and reduction conditions have a major impact on the physicochemical properties of the resulting catalyst. As stated earlier, higher calcination temperatures favor the formation of stable phases, such as spinel NiAl2O4 or NiO-MgO solid solution. A high calcination temperature can enhance the stability and interaction between components but can also decrease the surface area, thus causing a collapse of the pores, and particle sintering [103,104,105,106]. On the contrary, low reduction temperatures may not guarantee the generation of sufficient active species (Table 3). Hence, various studies have been devoted to the influence of thermal pretreatments on the final structure of the catalysts.

Table 3.
General effects of synthesis conditions on Ni/Mg(Al)O catalysts.
Bian et al. studied the effect of the calcination temperature on the activity of Ni catalysts supported on Al2O3 [89]. They found that high calcination temperatures promote the interaction between Ni and Al and the formation of the spinel phase. The activity and stability of Ni/Al2O3 were optimal when calcined at 700 °C. Higher calcination temperatures added no improvements to the catalysts’ activity. However, in another study, Al-Fatesh et al. argued that the reduction is the step most crucial to activate the catalysts in DRM [90]. The best catalyst that the authors reported was calcined at 900 °C and reduced at 700 °C. Juan-Juan et al. found also that pretreatment influences the coke formation on Ni/Al2O3 in DRM [91]. The uncalcined but reduced catalyst gave the least amount of carbon during the reaction, due to its smaller Ni NPs compared to the calcined and reduced one. Coke deposition dependence on thermal treatment was also studied by Zhou et al. [92]. They observed that the calcination temperature affects the phase distribution of Ni/Al2O3 as higher temperature favors spinel formation. They proposed that spinel phases limit the formation of encapsulated carbon, thus resulting in a stable activity.
The calcination temperature was studied on NiO-MgO systems for DRM [93,107]. At low calcination temperatures, the material contained weakly interacting NiO species, while at 800 °C NiO bonds strongly with MgO in the form of a solid solution. The strong interaction increases the strength of CO2 absorption at low reaction temperatures, thus enhancing the activity and stability of the catalyst. The effect of Mg precursors and heat treatment was also studied by Ruckenstein and Hu [94]. It was observed that the morphology of MgO before Ni impregnation is important to determine the activity and induction time in DRM. Among the investigated systems, the catalyst prepared from [MgCO3]4 Mg(OH)2 precursor through a high calcination temperature protocol carried out in a short time was the optimal catalyst due to high Ni dispersion on the surface of the support.
Katheria et al. investigated the effect of the calcination temperature on Ni/MgAl2O4 in SMR [96]. The authors argued that when a higher calcination temperature is used, smaller Ni crystallites form upon reduction. They attributed such behavior to the strong metal–support interaction generated by the high calcination temperature. The catalyst was most active when calcined at 850 °C, whereas at 1000 °C the activity decreased because of a partial loss of Ni surface due to the incorporation of Ni into an inactive bulk spinel phase. Surprisingly, a different conclusion was drawn by Li et al. [98]. In their study on a complex Ni/Mo-Ce-Zr/MgO-Al2O3 catalyst for DRM, they proposed that the low activity of the catalyst calcined at elevated temperature is to be ascribed to particle sintering. They suggested that high calcination temperatures (900 °C) promote the migration of Ni from stable NiO-MgO solid solution to spinel MgAl2O4. This mobility enables Ni enrichment on the surface upon reduction, thus stimulating particle enlargement. The authors emphasized that an optimal calcination temperature prevents the formation of large particles.
For co-precipitated catalysts, the reduction condition turns out to be more influential than the calcination condition [95,98]. Perez-Lopez et al. found that with co-precipitated Ni-Mg-Al catalysts, the calcination temperature had a limited effect on the activity, mostly by altering the specific surface area of the material [98]. A similar relationship was observed by Djaidja et al. [99]. In addition, the authors reported that a high reduction temperature (up to 700 °C) was beneficial for the catalysts’ activity as it increased the number of active sites. However, sintering can occur at very high reduction temperatures [95]. Mette et al. found that a reduction temperature up to 900 °C did not affect the particle size of Ni but sintering of Ni NPs was observed when the reduction temperature reached 1000 °C.
In summary, different studies show that the effects of Ni loading and pretreatment conditions are complex and possess both advantages and disadvantages. Table 3 sums up the general trends based on the works discussed above. Careful consideration must be given to the calcination and reduction steps. In general, thermal treatments at high temperatures are preferred since they promote the formation of stable phases while an elevated reduction temperature provides activation of the catalysts. Nevertheless, excessive heat treatments can be detrimental causing surface area reduction, sintering of particles, and formation of inactive species. It should be noted that high-temperature treatments need high energy inputs, which could limit the economic viability of the final catalyst.
3.2. Synthesis of Ni/Mg(Al)O Catalysts by Advanced Methods
Besides the more common methods mentioned above, other approaches aimed at obtaining strictly controlled morphological, structural, and textural properties of both the metal active phase and the support have been explored and are here summarized (Table 4).

Table 4.
Monometallic Ni catalysts supported on Mg(Al)O obtained by advanced methods for SMR and DRM.
Among them, the sol-gel method has been extensively investigated [108,109,110,111]. The catalysts obtained via this approach are typically characterized by high surface area, narrow size distribution, high thermal resistance, and homogeneous composition of the mixed oxides when compared to conventional synthesis methods [112,113]. González et al. utilized LDHs prepared in this way as precursors for DRM [114]. Upon mild thermal treatment, the hydrotalcite collapsed and formed nanosphere structures of mixed NiO and Mg(Ni)AlO periclase without the formation of NiAl2O4. The catalyst with 15 wt.% Ni and calcined at 650 °C showed the best activity and low carbon formation in DRM. Sahli et al. used a sol-gel-like method with propionic acid to synthesize Ni-Al oxides for DRM [115]. By varying the ratios between Ni and Al, sub-stoichiometric Ni/Al resulted in a high-surface area solid solution of Al2O3 and NiAl2O4, which was stable for the reaction. Stoichiometric Ni/Al and Ni-excess samples resulted in a segregated NiO phase, which was completely deactivated due to coke formation. Min et al. compared Ni/Mg(Al)O prepared by sol-gel synthesis to the co-precipitated counterpart in DRM. The authors found that the sol-gel catalysts formed a lower amount of coke [116]. They also studied the effect of various Mg/Al molar ratios on catalyst performance. The coke resistance ability of the catalysts was improved by increasing the MgO loading, whereas high activity was expressed at Mg/(Mg+Al) molar ratio from 0.44 to 0.86. The authors attributed the excellent performance to the high surface area of the catalyst and the high dispersion of Ni.
The Pechini method, which is based on the incorporation of metal precursors into a polymeric resin, allows for the preparation of highly controlled and uniform multicomponent phases [117,118]. Rogers et al. used the Pechini method to synthesize Ni-Al catalysts with different molar Ni/Al ratios for both DRM and SMR [119]. By this method, they obtained highly uniform, structurally crystalline, and small grain-sized aluminate. They found that the Ni-rich catalyst (denoted as Ni2Al2O5) featured both NiO and NiAl2O4 phases. In SMR, the active phase was likely reduced Ni NPs but, surprisingly, unreduced stochiometric NiAl2O4 was also active for DRM without the presence of metallic Ni. By X-ray absorption spectroscopy (XAS) analyses, the authors concluded that four-fold-coordinated Ni2+ is responsible for the activity and low carbon deposition of the examined catalyst.
Another attractive synthesis approach is the aerogel method. This approach enables the synthesis of exceptionally high surface area and thermally stable materials [120]. Suh et al. used aerogel Al2O3 as supports for DRM [121]. The high surface area Al2O3 was prepared by supercritical CO2 drying of alcogels. The aerogel-based Ni/Al2O3 catalysts showed high activity and enhanced stability against coking compared to the impregnated ones. The authors assigned such improvements to the high surface area and uniform distribution of the active sites. The follow-up paper investigated the metal loading on this aerogel catalyst, again highlighting that too a high metal content resulted in large particle sizes, which in turn promote coke formation [101].
Sebai et al. sought to improve the traditional incipient wetness by complexing a Ni precursor with aliphatic amine before impregnation [122]. The authors hypothesized that the high steric hindrance of aliphatic amine limits the growth of NiAl2O4, resulting in smaller Ni particle sizes. Indeed, they reported that the average Ni particle size decreased (13 nm to 9 nm) when a bulkier ligand (trialkylamine) was used. High reactivity in SMR was recorded for catalysts using N-triethylamine as a complexing agent.
Atomic layer deposition (ALD) allows for high control of the catalyst surface and might open new pathways for synthesizing stable catalysts [123]. Zhang et al. used this method to prepare Ni/porous γ-Al2O3 for DRM [124]. The catalyst showed higher activity at 850 °C than the one made by wet impregnation but took 10 h to reach its stable conversion. The authors claimed that the ALD synthesis results in spinel NiAl2O4, whose time-consuming reduction was behind the long induction period of the catalyst. Interestingly, they reported that the pretreatment with pure H2 at 700 °C gave no DRM activity, whereas the product stream (H2 and CO) at 850 °C could reduce the spinel phase leading to catalytic activity. Wang et al. reported ALD-made catalysts with remarkably superior activity and stability compared to the impregnated counterparts but came up with a different explanation [125]. They attributed the poor performance of the impregnated samples to the presence of a NiAl2O4 spinel phase, whereas ALD samples contained more NiO, which is easier to reduce, thus resulting in higher activity. The ALD approach was also used for the preparation of NiO-MgO and specifically to improve the basicity of the catalysts [126]. Jeong et al. also utilized the ALD approach to overcoat Ni NPs with MgO. With 200 ALD cycles, the basicity of the catalyst was greatly enhanced. In CO2-TPD, the control sample contains weak and moderate basic surface sites (0.7 µmol CO2/gcat desorbed around 170–400 °C) while 200-ALD-cycled catalysts comprise mostly strong basic sites (1.04 µmol CO2/gcat desorbed around 450–700 °C). The activity and stability of this catalyst were higher than the bare NiO and overcoated 50-ALD-cycled MgO/NiO. Other studies demonstrated that ALD-prepared catalysts present a significant improvement in sintering resistance and stability. Gould et al. synthesized Ni/Al2O3 with an overlayer of porous Al2O3 by molecular layer deposition (MLD) to stabilize the catalyst during DRM [127]. The resulting catalysts were less affected by sintering thanks to the presence of the overcoat. The activity and stability of the catalysts depended significantly on the calcination and reduction temperature. The catalyst with 10 MLD cycles showed high stability with repeated calcination and reduction cycles, and no deactivation over a long running time (up to 108 h) was observed. In another work on ALD-synthesized Ni/Al2O3 catalysts, Littlewood et al. reported that the overcoated catalysts were more resistant to sintering and coke formation compared to uncoated samples [128]. The authors found that the pretreatment of overcoated samples affects the induction time to reach maximal activity since the overlayers restructure themselves through the transformation of inactive spinel NiAl2O4 to active metallic Ni under the reaction stream.
The importance of thermal treatments was stressed also by Kim et al. while preparing Ni catalysts via one-pot evaporation-induced self-assembly (EISA) [129]. They found that the right annealing conditions are vital to obtaining improved morphological and structural characteristics (such as smaller and more dispersed Ni NPs) and, in turn, a superior catalytic activity in SMR.
Recently, Guo et al. synthesized Ni supported on Mg-Al-O by so-called cation-anion double hydrolysis (CADH) for DRM [130]. The preparation is done based on the simultaneous hydrolysis of Ni2+ and Mg2+ cations with AlO2− cation in an aqueous solution. The resulting catalyst possesses high surface area, uniform pore size, high basicity, and ultrasmall Ni nanoparticles upon reduction (2.1 nm). When compared with co-impregnated Ni/MgO-Al2O3 (Ni and Mg were co-impregnated on γ-Al2O3) in DRM, the CADH catalyst showed both higher initial activity and long-term stability. TOF of the CADH catalyst was calculated as 23.9 s−1, twice as much as conventional Ni/MgO-Al2O3. Moreover, virtually no coke was observed during 24 h TOS while the co-impregnated catalyst showed high coke formation accompanied by steady deactivation. The authors attributed the high activity and exceptional coke resistant property to small-sized Ni nanoparticles and enhanced CO2 activation thanks to the high basicity created by the preparation method.
The utilization of the freeze-drying method was investigated by Huang et al. [131]. In their study, Ni was co-precipitated with Mg and Al precursors to form an LDH structure, followed by freeze drying instead of conventional oven drying. The authors claimed that freeze drying helps preserve the platelet-like structure of parent LDH, thus improving the surface area and pore size distribution. The resulting catalysts, containing small Ni nanoparticles with high dispersion, performed exceptionally in DRM with high WHSV, up to 8.3 mmol CH4 s−1 g-cat−1. In the stability test, the freeze-dried catalysts showed higher coke resistance compared to oven-dried samples. The strong interaction between precursor species, accompanied by preserved highly porous morphology and uniform distribution of Ni species, was claimed to be a crucial factor for observed improvement.
In general, these less conventional synthesis methods have proved very useful in amplifying specific attributes of the final catalysts, such as the surface area (sol-gel, aerogel), phase distribution (sol-gel, Pechini method, ALD), Ni dispersion (sol-gel, Pechini method, precursor complexation), basicity (ALD), and sintering resistance (ALD), allowing researchers to understand how they impact on the reforming process. For example, in DRM, it turned out that high stability should be sought by enhancing the Ni-MgO interaction and/or by using the Al-containing phase to increase the surface area.
Scaling up the preparation of a catalyst, also taking into consideration the final shape of the material in a way that can be used in industrial applications, is often a difficult endeavor. Worth mentioning are two patents where two different approaches are disclosed with interesting results. In the first, spherical beads of the catalysts of 2.5 mm in size showed steady activity for about 350 h on stream thanks to particularly stable Ni NPs of 10 nm in size [132]. In the second, it is disclosed that 3D-molding approaches using different kinds of binder are a good way to produce active catalysts in shapes suitable for large reactors [133].
4. Bimetallic Ni-M/Mg(Al)O Catalysts for Reforming Reactions
4.1. Bimetallic Ni-M/Mg(Al)O Catalysts for SMR
Bimetallic catalysts are a promising area of research because they have improved properties compared to their parent metals, resulting in some cases in catalysts with higher selectivity, activity, and stability. Precious metals with high reforming activity and low coking tendency have been studied as promoters for Ni-based catalysts [134,135,136,137,138,139]. Among different synthesis methods reported for CH4 reforming catalysts (i.e., self-combustion, ion exchange, sol-gel, microemulsion, precipitation, wet impregnation, and colloidal), precipitation and impregnation methods are the most common ones [140,141,142,143,144].
The role of Pt as a promoter in Ni/Mg(Al)O catalysts has been investigated in different synthetic ways both for metal deposition and support preparation. Foletto et al. reported a catalyst synthesized by the sequential impregnation method while using the sol-gel method via alkoxide hydrolysis to synthesize the Mg(Al)O support [145]. XRD analyses revealed that as the calcination temperature of the support increased, the size of the crystallites rose exponentially, and the formation of the spinel phase occurred at temperatures higher than 600 °C. The formation of the pure spinel phase and a higher resistance to sintering were observed for calcination temperatures exceeding 700 °C. The catalytic activity of the catalyst as a function of different Pt content showed that 0.1 wt.% Pt is optimal compared to catalysts at higher Pt loadings. According to TPR results, Ni reduction peaks shifted to lower temperatures for small amounts of Pt (0.05–0.1 wt.%), while no significant changes were observed when increasing the Pt content.
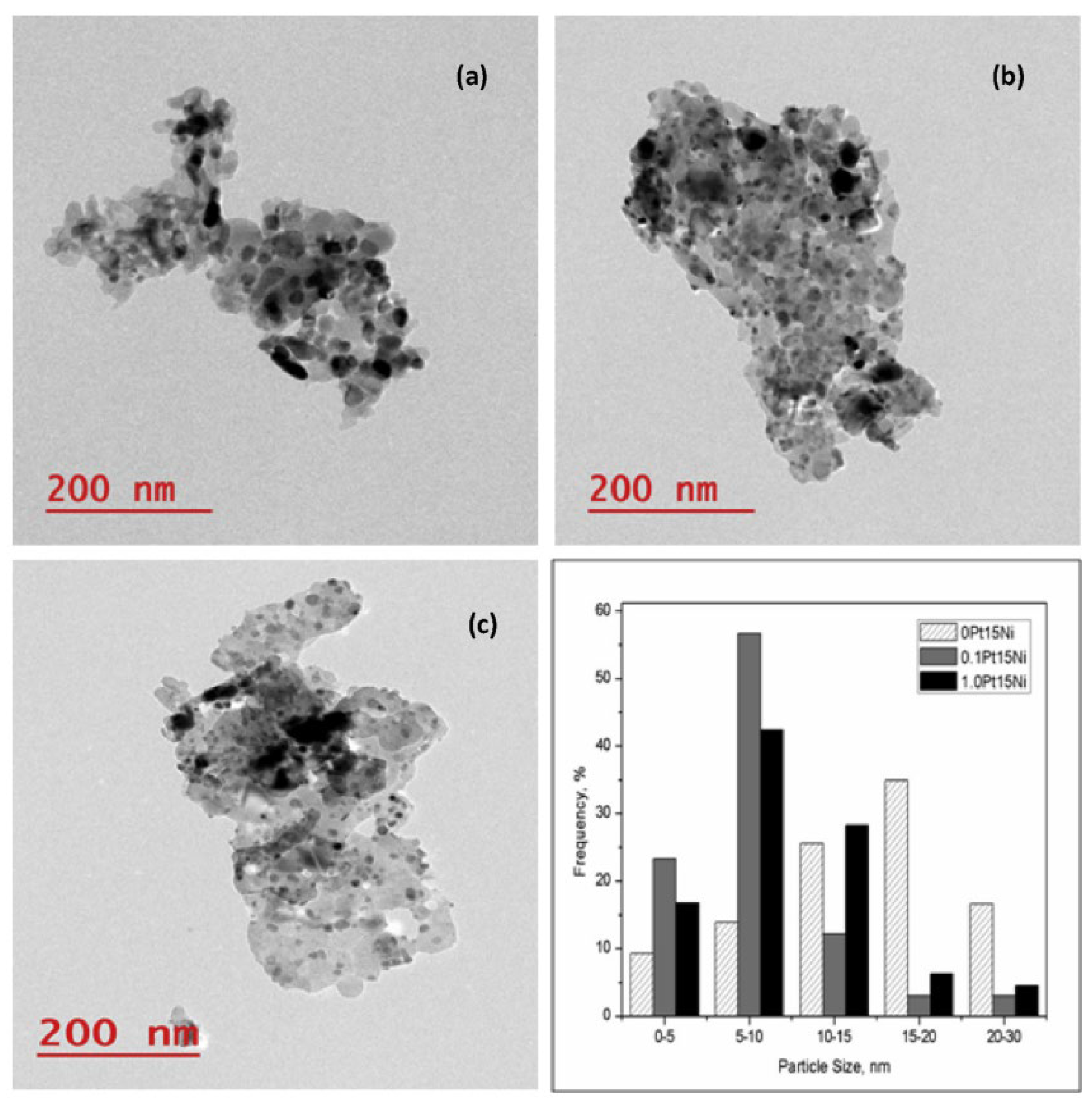
The activity and stability of catalysts obtained by sequential Ni and Pt impregnation were studied by Jaiswar et al. [146]. MG30 (Sasol, 30 wt.% MgO: 70 wt.% Al2O3) was used as support, which experienced a weight loss of about 40 wt.% after the heat treatment due to the conversion of aluminum magnesium hydroxyl carbonate to Mg(Al)O [96]. The effect of Pt doping on Ni/Mg(Al)O catalysts was investigated with different Pt concentrations (0.01, 0.05, 0.1, 0.3, and 1.0 wt.% Pt) and 15 wt.% of Ni. According to the TPR results performed on the calcined samples, the reduction peak appeared at about 800 °C, which was higher than the reduction peak observed for Ni/Al2O3 catalysts doped with Pt and prepared with co-impregnation [147,148]. One reason for this could be the presence of MgO on the catalyst support, which might lead to a strong interaction between the metal oxide and the support itself [149,150]. The different Pt loadings showed that the crystallite size of the active metal increased up to 0.1 wt.%, accompanied by some agglomeration of the active metals on the support. The H2 uptake study also revealed a positive correlation between metal dispersion and Pt loading up to 0.1 wt.%, which was confirmed by TEM analysis. From the TEM images of 0Pt15Ni, 0.1Pt15Ni and 1.0Pt15Ni catalysts (the names referring to 0, 0.1, and 1.0 wt.% Pt, respectively), smaller NPs were observed for the 0.1Pt15Ni catalyst, while larger agglomerated ones were present in the 0Pt15Ni catalyst (Figure 4). In agreement with the dispersion of the active metals after the addition of Pt, the activity was the highest with the 0.1 wt.% Pt-doped Ni/Mg(Al)O catalyst. Higher Pt concentrations led to agglomeration of the active metal, resulting in lower catalytic activity.
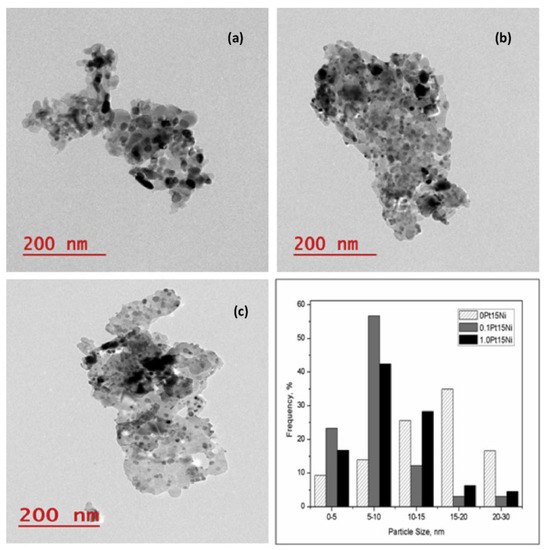
Figure 4.
TEM images and active metal particle size distribution for (a) 0Pt15Ni, (b) 0.1Pt15Ni, and (c) 1.0Pt15Ni catalysts. Reprinted with permission from [146]. Copyright 2017, Elsevier.
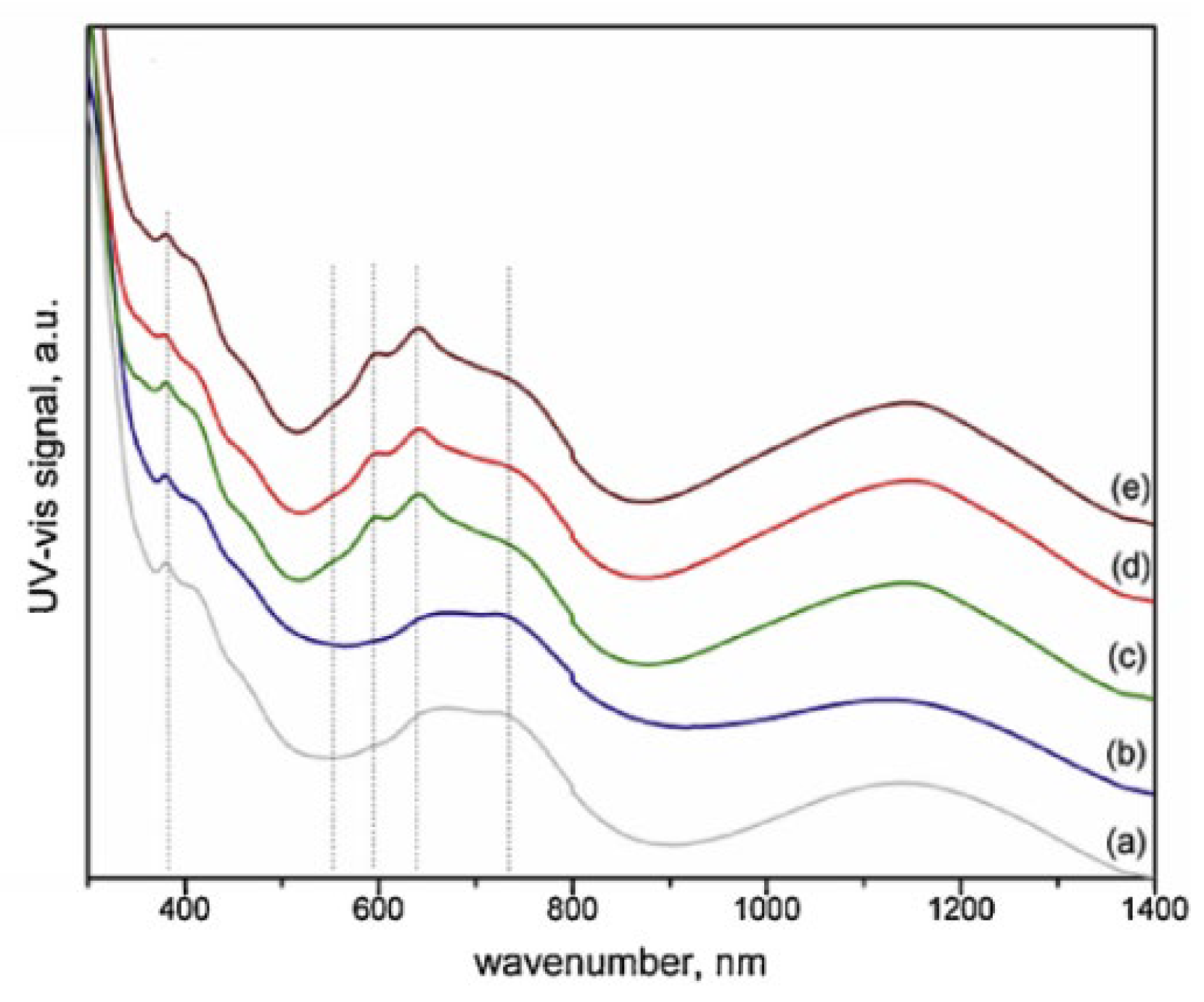
Katheria et al. investigated Rh-Ni/Mg(Al)O catalysts prepared by sequential impregnation, designated as cat600600, cat600850, cat850600, cat850850 (the names referring to the first and second calcination temperatures) and co-impregnation referred to as RhNicoimp calcined at 600 °C [151]. The catalysts prepared by both methods showed the highest activity at lower calcination temperatures (calcined at 600 °C in each step). Basically, above that temperature, and regardless of the presence of Rh, hard-to-reduce Ni aluminate (NiAl2O4) formed, as can be seen in the UV-Vis spectra in Figure 5, which limited the catalytic activity. An improvement in the activity and stability of the catalysts was nonetheless observed after Rh loading mainly due to an increment of the reduction degree, dispersion of the active metals, and the formation of Rh-Ni alloy. It is noteworthy that Rh exerted a similar promoting behavior to Pt. Specifically, Rh loadings up to 0.1 wt.% led to an enhancement in the initial CH4 conversion and H2 yield, while further amounts had no significant effect.
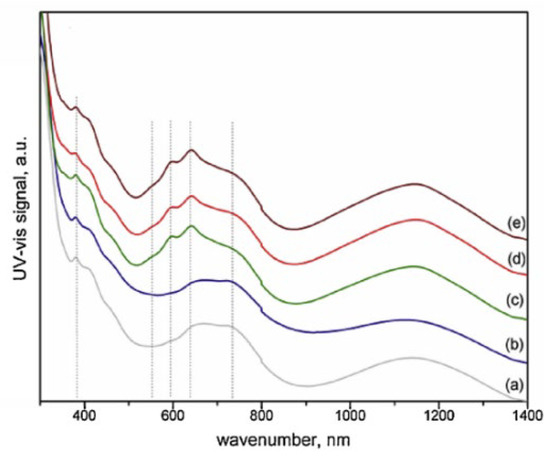
Figure 5.
UV–vis spectroscopic patterns of (a) RhNicoimp, (b) Cat600600, (c) Cat600850, (d) Cat850600, and (e) Cat850850. Reprinted with permission from [151], copyright 2019, Elsevier.
With due care [152], another factor to be considered in catalysts doped with Rh and Pt is the hydrogen spillover effect [153,154,155]. Kinetic studies indeed showed that noble metals can promote the adsorption of the H2 formed during the reaction, which then spill over the non-noble metal thus contrasting its oxidation [155,156]. The presence of Pt-group metals in bimetallic formulations has the positive effect of facilitating the reduction of Ni as well as preventing its undesired oxidation during reforming operations.
Ru-doped Ni catalysts, effective for SMR without the prereduction treatment, were reported by Jeong et al. [157]. They investigated the effect of the addiction of Ru to Ni/Al2O3 and Ni/Mg(Al)O prepared by sequential impregnation under the same conditions. For both catalysts, Ru suppressed the carbon deposition and had a self-activation effect on the oxidized catalysts during reforming; Ni catalysts without Ru, on the contrary, were active only after reduction of the catalysts. As in the case of monometallic Ni catalysts (see Section 2), this study proved also that Ru-Ni/Mg(Al)O is more active than Ru-Ni/Al2O3, likely due to the presence in the latter of Ni species that are hard to reduce because they are dispersed as alluminates. In another study on a similar system, the self-reducibility was ascribed to the H2 spillover that occurred after the addition onto reduced NiO species under the reaction conditions, so that a very small amount of Ru (0.05 wt.%) exerted a positive effect on the conversion of CH4 [158]. The best-performing catalyst showed remarkable stability without deactivation in a long test over a period of 250 h.
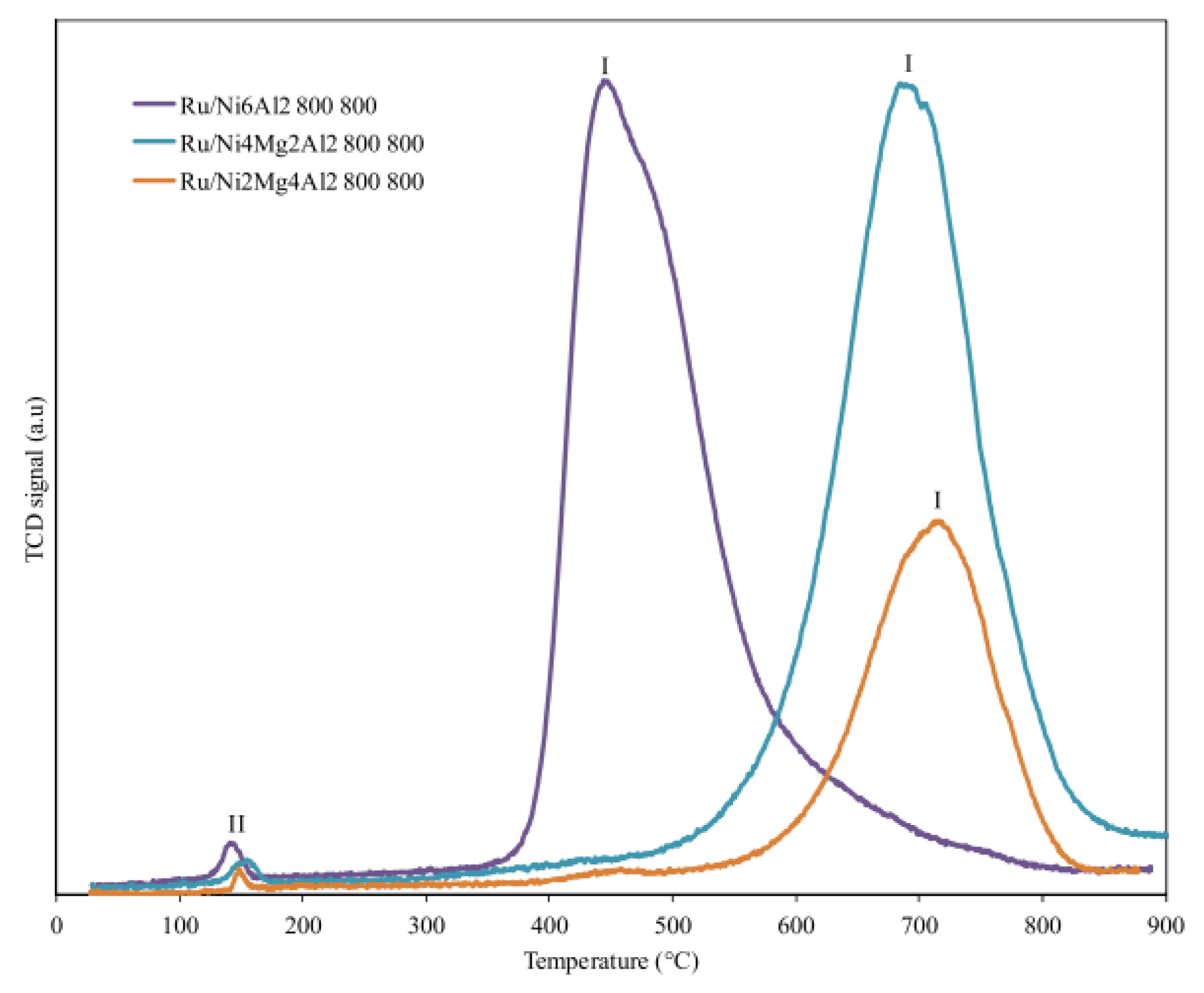
Nawfal et al. studied in a more systematic way the effect of Ru on NixMg6−xAl2, with x = 2, 4, and 6 molar ratios [159]. Mixed oxides based on Ni, Mg, and Al were synthesized by the hydrotalcite route and then Ru was added via an impregnation method. Among the different catalysts tested, Ru/Ni6Al2 showed the best performance, attributed essentially to the stronger interaction between Ru and Ni as the former promotes the reducibility of the NiO species (Figure 6).
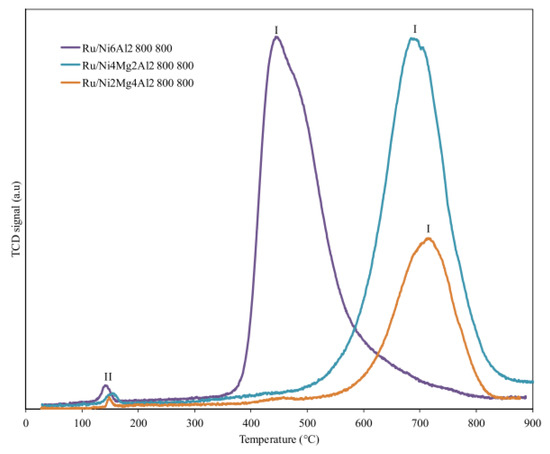
Figure 6.
TPR profiles of Ru/NixMg6-xAl2 samples (x = 2, 4 and 6). Reprinted with permission from [159], copyright 2015, Elsevier.
Kim et al. investigated the use of non-noble promoters for SMR with physically mixed Ni/Mg(Al)O + CrFe3O4 catalysts [160]. They noted that when Cr or Fe was added to the Ni/Mg(Al)O catalyst via classical sequential impregnation, Ni segregated, thus compromising the catalytic activity. On the contrary, the authors reported increased activity with the physically mixed catalysts that could be due to a more facile water activation by CrFe3O4. The recent case of a NiCo catalyst prepared via the one-pot evaporation-induced self-assembly method (EISA) whose activity is markedly higher than the parent monometallic counterpart [161] is notable. Although these catalysts were evaluated at relatively low space velocities (see Table 5 for a comparison with other catalysts), this work shows that co-precipitation methods or similar are a promising way to control the formation and alloying of Ni-M nanoparticles, thus unlocking some perks typical of bimetallic formulations such as a higher presence of catalytically active metal(s) [45,162]. Attempts at preparing active Ni-M catalysts through other procedures have resulted only in a very slightly better coke resistance [163].

Table 5.
Bimetallic Ni catalysts supported on Mg(Al)O for SMR.
Small amounts (<0.5 wt.%) of alkaline metals, such as Ca and Sr, have been reported as beneficial in suppressing the coke formation during the reaction of SMR but only in the presence of CO2, that is, simulated natural gas [164,165].
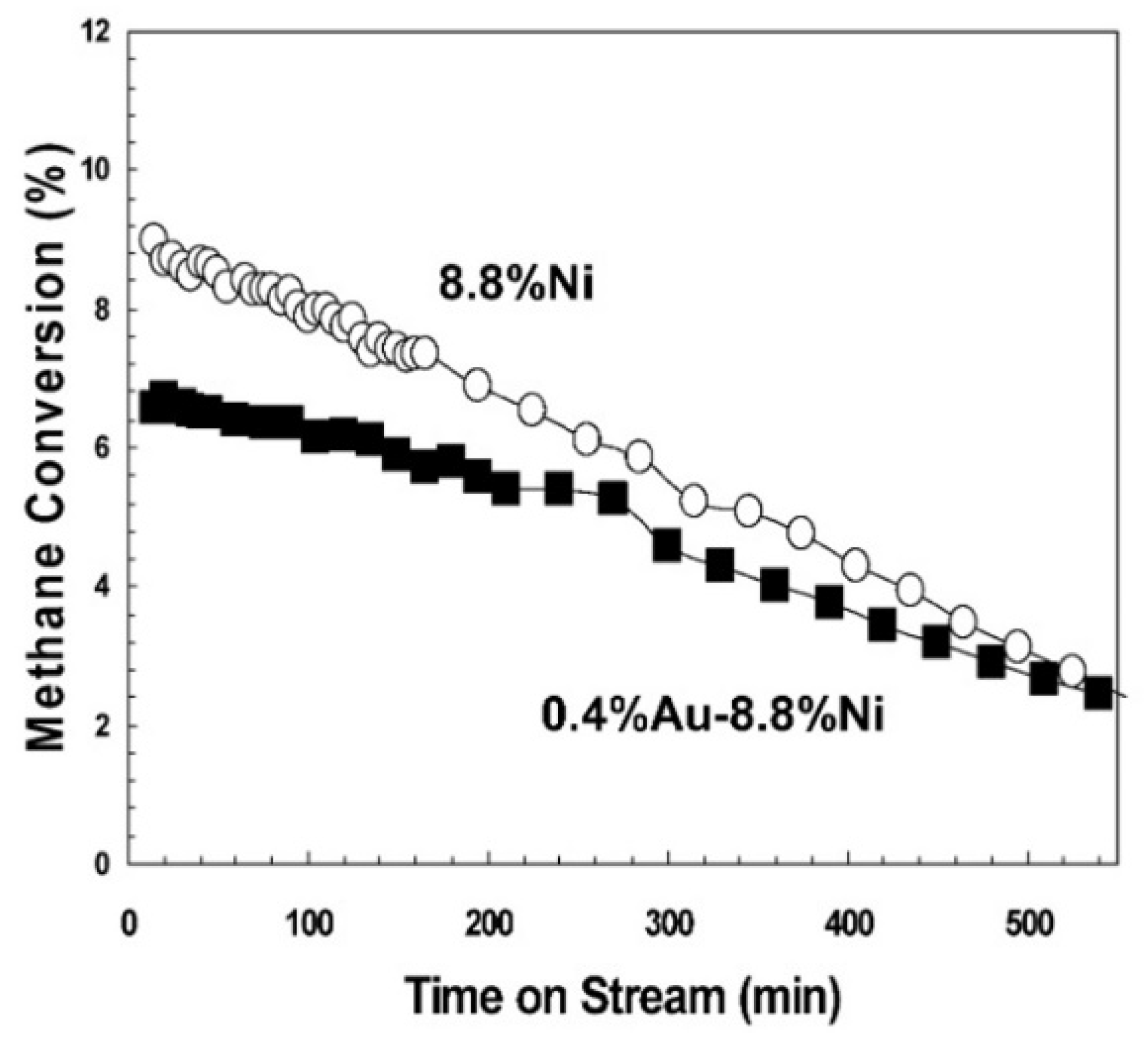
Not all noble metals promote Ni activity though. Indeed, Au-Ni/Mg(Al)O catalysts for SMR prepared by sequential impregnation revealed that the addition of a small amount of Au on Ni-based catalysts caused a decrease in H2 chemisorption and dissociative N2O adsorption, which can be mainly attributed to the effect of Au on the adsorption properties of several adjacent Ni sites [166]. Calculations showed that Au is usually located at step and edge sites, which are active sites for catalytic activity but can also serve as sites for carbon nucleation [167,168]. This study showed that the addition of 0.4 wt.% Au to 8.8 wt.% Ni/Mg(Al)O resulted in a small decrease in catalyst activity (Figure 7).
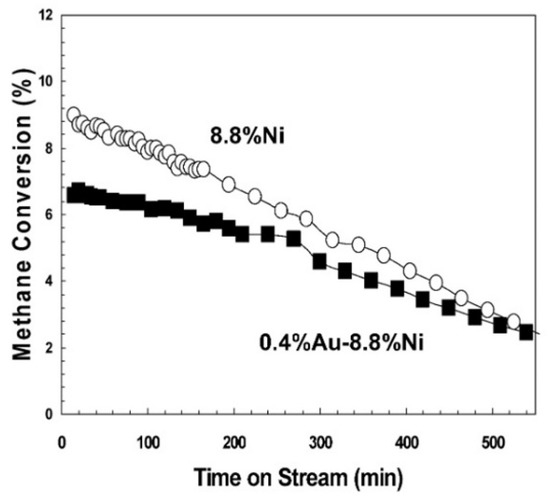
Figure 7.
SMR activity over 8.8 wt.% Ni/Mg(Al)O (O) and 0.4 wt.% Au–8.8 wt.% Ni/Mg(Al)O (■). Reaction conditions: 550 °C, H2O/CH4/H2 = 4.5/4.5/1, 3.3 × 106 cc/(h g). Reprinted with permission from [166], copyright 2006, Elsevier.
4.2. Bimetallic Ni-M/Mg(Al)O Catalysts for DRM
The presence of a metallic phase is necessary for the dissociative adsorption of CH4 and for the generation of H2 as well as chemisorbed carbon species during DRM. Some bimetallic systems are also capable of lowering the activation energy of the rate-limiting step [170,171]. A kinetic study by Niu et al. revealed that the addition of Pt to Ni reduces the activation energy for CH4 dissociation and lowers moderately the CO2 dissociation barrier energy, resulting in higher catalytic activity [172]. Re and Ru have been also described as active sites for CO2 activation, which, due to their electron-rich properties, can donate electrons to the CO2 antibonding orbitals and facilitate the reaction by weakening the C-O bond [173]. In addition, the dilution effect of the noble metal leads to higher metal dispersion, smaller particle size, and improves reducibility and oxidation resistance [174].
Ru, Pt, and Rh generally exhibit higher catalytic activity and stability compared to Ni due to their resistance to coke deposition [175,176,177]. Wu et al. investigated the effect of Pt and Au over Ni/Al2O3 and Ni/Al2O3-MOx (M = Ce or Mg) oxides synthesized by the sequential wet impregnation method [178]. Bimetallic Ni-Pt, Ni-Au, and trimetallic Ni-Au-Pt catalysts were prepared over pure Al2O3 and Al2O3 loaded with 10 wt.% MgO and CeO. The addition of Au and Pt promoted the Ni reduction, while only 0.2 wt.% Pt and Au was sufficient to suppress the coke formation, which was more evident in the case of the trimetallic formulation. In detail, in the monometallic Ni/Al2O3 catalyst, at least two types of carbon, namely amorphous carbon and carbon nanotubes, were found after the catalytic tests. The presence of MgO in the support was reported to suppress the formation of the amorphous type while in the case of CeO, carbonaceous species in the form of nanotubes were also observed. Bamboo-like carbon structures were found only in the most active catalyst, that is, Ni-Au-Pt/Al2O3. Moreover, particle size distributions for Ni/Al2O3 and Ni-Au-Pt/Al2O3 after a long run were measured and Ni-Au-Pt/Al2O3 was characterized by a narrower particle size distribution and by the presence of smaller metallic NPs.
In another impregnation synthesis process, Pt was loaded on a Ni/Mg(Al)O catalyst using glow discharge plasma pretreatment after impregnation and before calcination [179]. Such treatment was useful to improve the Ni dispersion but inhibited its reduction. The addition of Pt helped in this sense and in fact the bimetallic, plasma-treated Pt-Ni/Mg(Al)O catalyst demonstrated a higher conversion of both the reactant and a higher H2 yield. An improved resistance toward coke formation was also reported by the authors. The presence of Pt resulted in smaller Ni NPs in the fresh materials, while the plasma treatment was critical to prevent sintering. It is worth noting the filamentous carbon formations on the spent Ni/Mg(Al)O catalyst, which on the contrary cannot be seen on the spent P-Ni-Pt/Mg(Al)O catalyst. The authors suggest that this was due to the presence of Pt more than the plasma treatment, and specifically to an altered dissociative adsorption capacity of CH4 [180,181].
In a different way, the effect of Pt on Ni/Mg(Al)O catalysts was investigated by the combination of DFT and kinetic studies [172]. In that work, Pt was added to the supported Ni catalyst by a redox reaction. The remarkable feature of this synthesis method is that a very fine size of the Ni NPs can be obtained, likely because the reduced Ni is dissolved in the solution and then redispersed on the support during the redox reaction. For four catalyst models, the adsorption of key intermediates during the reaction was investigated by DFT calculations. The study showed that the energy barriers for both CH and C oxidation were lower after Pt loading, and the energy barrier for CH decomposition leading to surface carbon was higher after Pt loading, demonstrating the effect of Pt in the improvement of the catalytic performance and coke resistance.
The combination of Ni and Co as bimetallic catalysts and their synergistic effect on coke resistance was studied by Li et al. [182]. They attempted to develop a catalyst with high performance by controlling multiple factors including the size of the NPs, the interaction between the support and metal, the structure of the catalyst, and the alloying effect. The catalysts were prepared by co-precipitation in three different metal loadings. Increasing the Ni content of the catalyst resulted in an improved activity, in agreement with the study of Hu et al. [183]. They reported that at low Ni loadings, MgO surrounded the reduced metallic Ni atoms and limited the formation of Ni-Ni bonds and thus prevented the formation of Ni NPs. Metallic NPs of Ni and Co formed a bimetallic alloy, resulting in a more stable catalyst during DRM operations compared to the monometallic Ni catalyst. Since Co can hardly be reduced from its oxides or solid solutions, it can be employed to control the size of Ni NPs. Moreover, the elemental assignment of NiCo2(4,6)/Mg0.9(Al)O showed that the Ni and Co atoms were uniformly distributed into the metal NPs. The size of the metal NPs was close to that of the support in this catalyst, so this nanocomposite structure led to a stronger interaction between the metal NPs and the support. Recently, Duan et al. found that calcination and reduction of Ni-Co-Mg-Al hydrotalcite synthesized by the coprecipitation method yields fine Ni-Co alloy particles with uniform composition [184]. Ni-Co-Mg-Al hydrotalcite decomposes into a solid Mg(Ni,Co,Al)O solution during calcination, resulting in the incorporation of Ni and Co cations into the MgO lattice, and the subsequent reduction step produces an alloy with a similar Ni:Co ratio in the bulk composition. They showed that the prepared Ni-Co alloy effectively inhibited the decomposition of methane and improved the catalytic stability. Moreover, the increment of Co content in the Ni-Co alloy has a direct effect on the removal of the formed carbon species by enhancing the adsorption of CO2 on the metal particle and/or at the metal-support interface.
In general, many alloying effects of Ni-based catalysts with base metals and Ni-based catalysts with noble metals have been reported in DRM and SMR to improve the dispersion and reducibility of the supported metal, modify the catalytic performance such as activity and selectivity, and improve the resistance to carbon deposition, sulfur poisoning, sintering, and so on (see Table 6). However, the effects of alloys often vary with catalysts, compositions, and reaction conditions [185].

Table 6.
Bimetallic Ni catalysts supported on Mg(Al)O for DRM.
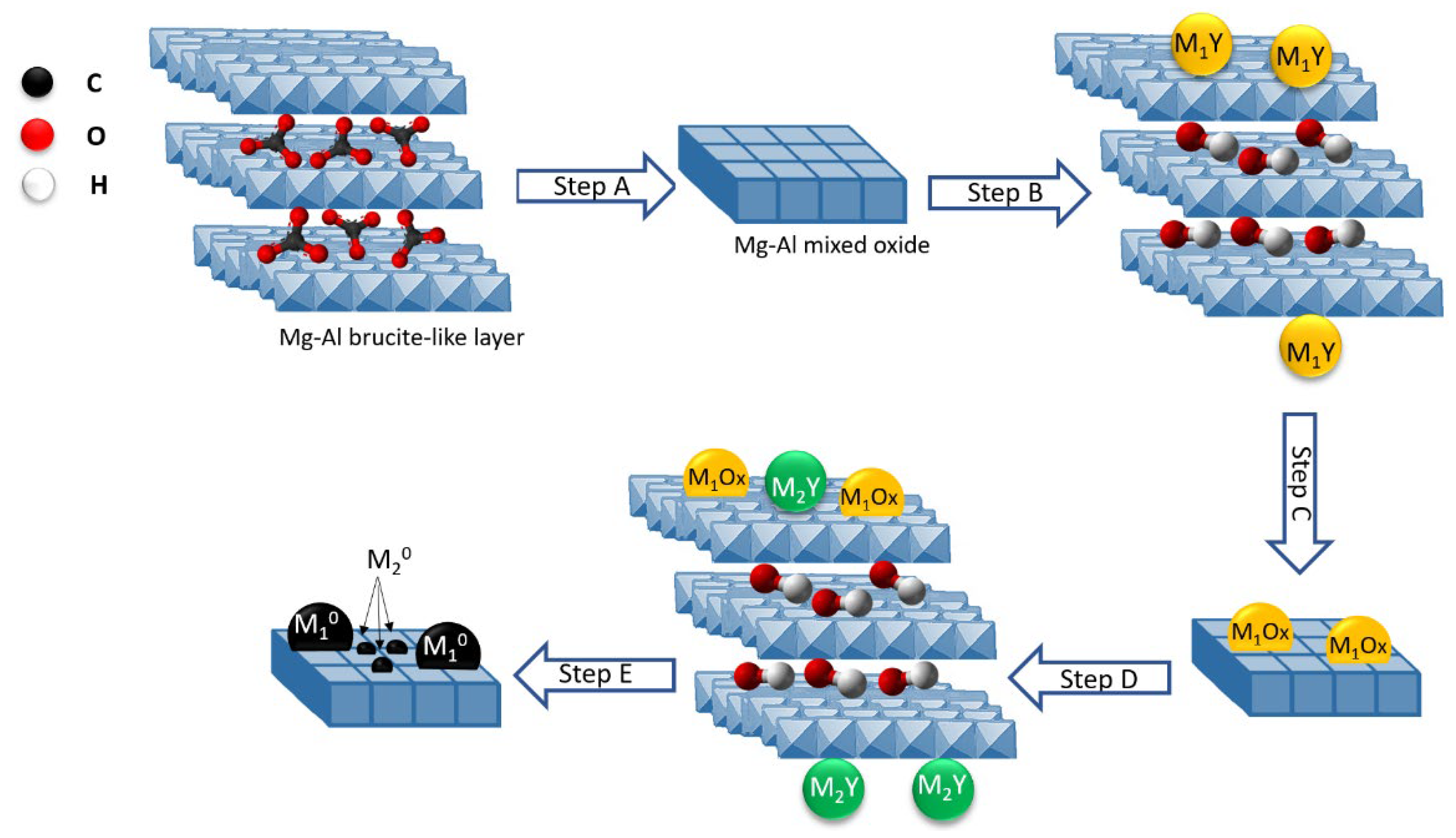
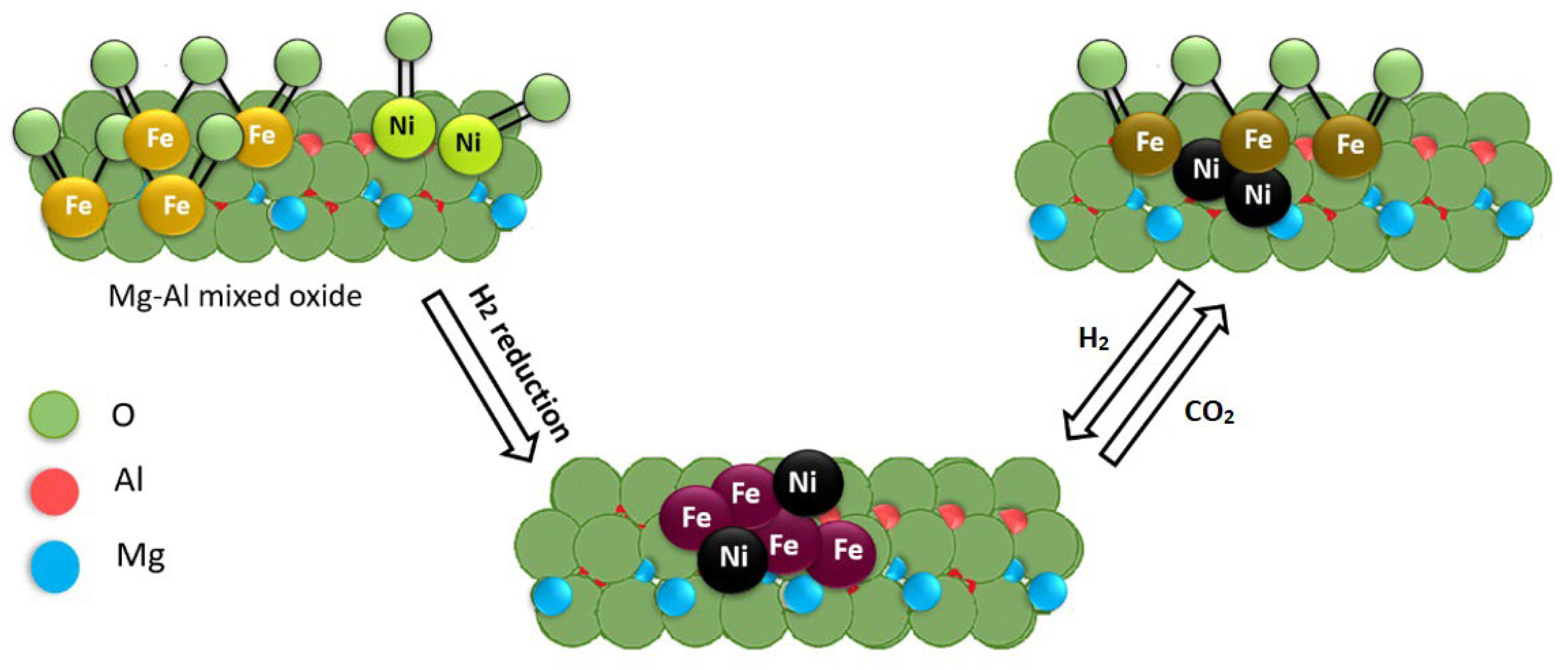
Ru was combined with various metals such as Cr, Fe, Co, Ni, and Cu by Tsyganok et al. using a novel method based on the ability of Mg(Al)O oxide to reconstruct a layered structure following the memory effect of calcined hydrotalcite when rehydrated with aqueous solutions [186,187,188]. Traditionally, LDHs are prepared by co-precipitation of Mg2+ and Al3+ cations at basic pH. Since LDH can incorporate and retain inorganic and organic anions, Ni was used as the anionic species in a complex with ethylenediaminetetra acetic acid [M(EDTA)]n−. After calcination, the first metal was introduced into the support as an anionic chelate by exploiting the memory effect of the calcined hydrotalcite, then the catalyst was calcined again, and the second metal (in this case Ru) was introduced into the catalyst pre-chelated EDTA (Figure 8). In this process, the chelated Ru3+ reduced to atomic species and then sintered to form metallic clusters. When the metallic Ru clusters reached a suitable size, they began to catalyze the dissociation of CH4 to C and H, which in turn can reduce the Ni oxide crystals by binding to the support surface near the Ru clusters. Catalysts obtained from this LDH showed high and sustained catalytic activity for the DRM reaction.
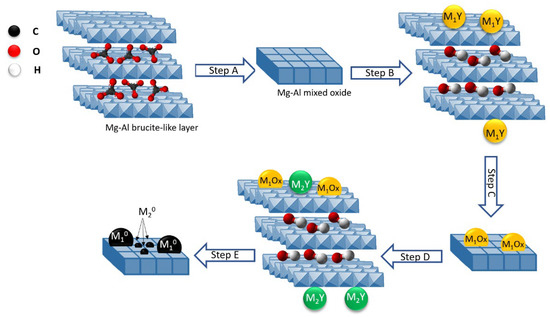
Figure 8.
Synthetic strategy of preparing bimetallic catalysts, supported on Mg–Al mixed oxide: calcination of synthetic hydrotalcite in air at 500 °C to Mg(Al)O mixed oxide (step A), introduction of a transition metal M1 as an anionic chelate (M1Y−) using a ‘‘memory effect’’ of calcined HT (step B), second calcination in air at 500 °C (step C), reconstruction of a layered structure and introduction of a second metal M2, i.e., Ru(III) pre-chelated with EDTA (step D), and an in situ generation of a bimetallic (M2-M1) catalyst from LDH precursor under the DRM reaction conditions (step E). Reprinted with permission from [186], copyright 2005, Elsevier.
The influence of different supports on the catalytic activity of Ni and Ru-Ni catalysts was studied by Wysocka et al. [189]. They prepared bimetallic Ru-Ni catalysts by impregnation and precipitation methods. The highest activity was obtained with those supports that strongly interact with the active metals and have basic properties, which favor the dissociative adsorption of CO2 on the catalyst [5,42]. Thus, the highest activity was observed for Ni/Al2O3 and Ni/Mg(Al)O catalysts, where the addition of Ru particles improved the CH4 conversion and shifted the H2/CO molar ratio to lower values.
The influence of the support on Rh-modified Ni catalysts synthesized by wet impregnation was studied by Lucrédio et al. [190]. The presence of the noble metal, surprisingly, was detrimental to the catalytic activity mostly because of severe coke deposition. On the Al2O3 support the presence of Rh probably caused the segregation of Ni species with time-on-stream; on the Mg(Al)O support, the presence of Rh enhanced the dispersion of Ni by reducing the Ni0 crystallite size, but the Rh-Ni/Mg(Al)O sample still suffered from higher rates of carbon deposition, suggesting that the carbon deposition in this case was due to CH4 decomposition by Rh.
The evaluation of Fe-Ni catalysts synthesized via incipient wet impregnation found a sweet spot at a Fe/Ni molar ratio equal to 0.7 as the most active and least deactivated catalyst [191]. Crystallographic structural studies of bimetallic Fe-Ni/Mg(Al)O catalysts revealed that Fe2O3 and NiO were reduced to a Fe-Ni alloy in the presence of H2 above 634 °C, and this alloy decomposed to metallic Ni and Fe3O4 during CO2 oxidation above 627 °C. A Mars–van Krevelen mechanism was proposed for DRM based on in situ XRD and pulse experiments over Fe/Mg(Al)O, Ni/Mg(Al)O, and Fe-Ni/Mg(Al)O catalysts. Accordingly, CO2 oxidizes Fe to FeOx, and CH4 is activated at Ni sites to form H2 and surface carbon. Carbon can be re-oxidized from FeOx by lattice O, leading to CO (Figure 9).
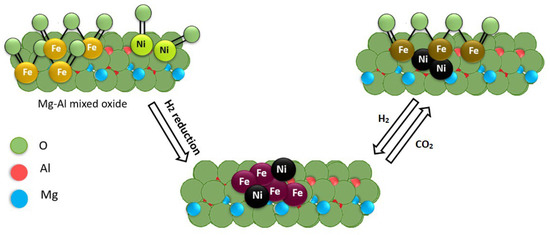
Figure 9.
Schematic diagram of Fe-Ni alloy formation, during H2 reduction, and decomposition, during CO2 oxidation Adapted with permission from [191], copyright 2015, American Chemical Society.
Removal of carbon species from a Fe-Ni catalyst supported on Mg(Al)O after DRM at 750 °C was studied in another work by Theofanidis et al. [192]. The support material and the final catalyst were prepared by co-precipitation and incipient wetness impregnation methods, respectively. The elemental distribution of Fe-Ni proved that after CO2 oxidation, segregation of Ni and Fe particles occurred as Fe was oxidized to Fe3O4, and the Fe-Ni alloy was decomposed. A subsequent H2 reduction step could then lead back to Fe-Ni alloy and restore the original activity. It appears that in the process of carbon removal detected by XRD analyses and temporal analyses of the products, there were two parallel contributions: First the dissociation of CO2 over Ni followed by oxidation of the carbon species by surface oxygen; second, the Fe oxidation by CO2 followed by oxidation of carbon species by Fe oxide lattice oxygen (Fe oxide reduction step).
To study in more detail the effect of Fe addition, Wan et al. reduced a Ni-Fe/Mg(Al)O alloy catalyst at different temperatures [193]. The alloy catalyst was prepared by co-precipitation in one step and compared with Fe/Mg(Al)O and Ni/Mg(Al)O at the same metal content. Since CH4 decomposition is an important reason for coking on Ni/Mg(Al)O, the activation energy of CH4 was measured, indicating that the alloy Ni-Fe inhibited the CH4 dissociation [86]. Compared with Ni-Fe/Al2O3 and Ni-Fe/Mg(Al)O catalysts prepared by the impregnation method, Ni-Fe alloy particles generated in situ from Mg(Ni, Fe, Al)O solid solutions yielded a catalyst with higher activity, stability, and good resistance to coking [194,195]. This can be attributed to the greater homogeneity of the alloy composition, which led to the formation of smaller particle sizes and stronger interaction of the metals with the support. It was proposed that CH4 is mainly dissociated on the Ni sites forming adsorbed carbon, while CO2 can provide adsorbed O through the activation at the metal-support sites to restore the active surface.
5. Conclusions and Perspectives
Ni/Mg(Al)O catalysts have been extensively studied in the last years due to their activity in both SMR and DRM reactions for H2 production. What makes them so interesting is the possibility to finely tune their characteristics such as surface area, metal–support interaction, basicity, etc. by selecting the proper compositions, preparation methods, and thermal treatments. Here we summarized the most recent advances found in the literature about these catalysts, focusing on the strategies and their impact to enhance their catalytic activity and stability (Figure 10).
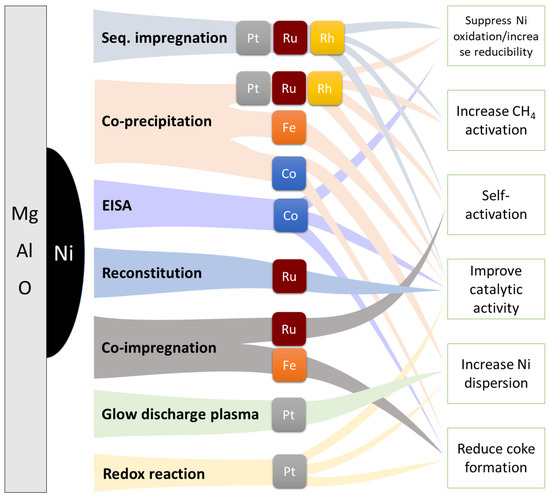
Figure 10.
Schematic depiction of the main strategies adopted to enhance the catalytic activity of Ni-M/Mg(Al)O catalysts for SMR and DRM applications.
Ni/Mg(Al)O catalysts typically feature well-dispersed Ni metal particles (only up to about 15 wt.% Ni loadings) whose reducibility depends strongly on the composition and overall synthesis procedure [84,114]. NiO with little interaction with the support, for instance, may be exploited for low-temperature applications [196], while the formation of the Ni spinel phases with Al or Mg can suppress the Ni NPs sintering during high-temperature operations [55,56,60,61]. It is noteworthy that a certain amount of MgO was found to be beneficial to limit the coke deposition due to its Lewis acidity [68]; an excessive loading, however, was reported to cause the failure of the catalyst [69].
The monometallic Ni/Mg(Al)O catalysts, although very active in both reactions, suffer from Ni oxidation during daily start-up and shut-down operations [197]. Trace amounts of noble metal dopants such as Pt, Rh, and Ru easily suppress such oxidation and hence result in more stable catalysts. For SMR applications, only 0.1 wt.% of Pt or Rh added by the sequential impregnation method is enough to exert such a positive effect [145,146,151,153,198].
A remarkable feature of Ru-doped catalysts prepared by wet impregnation is that they are active without the need for reductive treatments prior to catalytic testing, which is an important economic point [157,158,159]. Not all noble metals are effective promoters though. A good example is Au, which showed a positive effect in retarding the coke formation in butane steam reforming [166]. In SMR, despite some modeling works predicting that alloying Au with Ni would hamper the coke formation [199,200], not only was it not completely suppressed, but the bimetallic Ni-Au catalysts were less active than the monometallic counterpart [166].
Apart from a recent work featuring Ni-Co catalysts prepared via a one-pot evaporation-induced self-assembly method with enhanced coke resistance [161], no promoting effects have been reported by adding non-noble metals to Ni/Mg(Al)O catalysts for SMR.
Pt, Ru, and R are among the most common promoters for Ni/Mg(Al)O catalysts for DRM. Once again, the main purpose of adding such metals is to increase the reducibility of Ni, but there are cases of Pt-modified materials, even in trimetallic formulations with Au [180], that show also a remarkably higher coke resistance [172,175,176,178,179]. Care must be paid as unwanted Ni segregation might occur while adding a noble metal promoter [190].
Contrary to SMR, there are few, but very interesting, examples of Ni catalysts promoted by non-noble metals. For example, Co, added by co-precipitation, can be exploited to control the metal–support interaction and in turn the size of Ni NPs. The resulting bimetallic catalysts showed enhanced stability in DRM [182]. A relevant coke deposition suppression effect was reported using Fe-Ni catalysts [191,192,193]. During the DRM reaction, the active phase is the Fe-Ni alloy, but some Fe oxide phases form and provide a surface O lattice that promotes coke gasification. Careful dosing of the two metals is critical to achieving this result [191,193,201].
To conclude, looking at Figure 10, two main considerations can be drawn about the attempts made at manufacturing highly performing and resistant Ni/Mg(Al)O catalysts for both CH4 reforming processes. Firstly, co-precipitation is the most exploited synthetic method, likely because it has delivered the best results in terms of overall catalytic enhancements. The reason might be that having Ni within the main oxide matrix allows for finer control over the final Ni nanoparticles upon reduction treatments by regulating the metal–support interaction [54,86,161]. Secondly, there is still an overdependence on noble metals as too few base metals have shown sufficient promoting effects. Fe is a promising candidate, but only in DRM, whereas it catastrophically oxidizes during SMR leading to inactive Fe oxide phases [202]. So far, no active Ni-Fe catalysts have been reported for SMR. This hints at the fact that despite sharing many similarities, SMR and DRM reactions are too different to have a catalyst active and stable in both, hence the development of new catalysts should be tailored to the specific reaction.
It is evident from this literature review that some challenges remain for Ni/Mg(Al)O catalysts for SMR and DRM applications. Probably the most crucial is the tendency of Ni to oxidize under the harsh reaction conditions typical of both processes. So far, only noble metals have proved effective in suppressing that, which makes the development of non-noble promoters for SMR even more urgent. Moreover, considering the sensitivity of both the processes to the Ni NPs size, what appears from the literature is that there is still a rather poor control over the final NPs sizes, especially for high temperatures and long-run applications. In addition, while both Mg and Al are necessary to achieve high activity and stability, there is also a lack of control over the final location of the Ni NPs upon reduction, meaning that it is hard to control with which crystal phase the Ni NPs preferentially interact.
Another important factor affecting both reactions is the coke resistance. Again, in SMR, essentially only noble metals can suppress the coke deposition.
What is really lacking in the study and development of Ni(M)/MgAlO catalysts for both SMR and DRM reactions is a deep understanding of the catalysts under working conditions. There are some examples of investigations carried out in operando or in-situ conditions, but they are mostly XRD [119,182] and DRIFTs [144] focused on revealing the crystal phases after reduction and the Ni-Ru interaction, respectively. Others studied the basicity of the catalysts after reductive treatments via CO2-TPD analyses [140] and the Ni NPs’ growth after steam treatments through HRTEM [154]. More detailed studies evidencing the transformations occurring during the reductive treatments and during the catalytic reactions, perhaps focusing on the electronic and actual metal–support interaction, are needed.
One strategy worth pursuing to prepare more active Ni(M)/MgAlO catalysts might consist in better exploiting the phase composition of the Mg-Al-O supports. An effective way to prepare catalysts featuring the correct amounts of MgO and NiO-MgO phases—which improve, respectively, the coke resistance and prevent the Ni NPs sintering upon reduction—is needed, possibly through the memory effect of hydrotalcite materials. More advanced preparation methods, such as ALD, have allowed a deeper understanding as well as finer control over many parameters affecting the final catalytic activity but seem hard to scale up. Some methods to prepare catalysts in large quantities (such as kneading) and already in a suitable shape for large-scale reactors (such as 3D printing) have been patented, but are limited to monometallic formulations for SMR while bimetallic catalysts are reported to show improved coke resistance only when using simulated natural gas, which is, in other words, during the DRM reaction.
Author Contributions
Conceptualization, F.B., C.E. and V.D.S.; writing—original draft preparation, S.S. and X.T.N.; writing—review and editing, F.B., C.E. and V.D.S.; supervision, C.E. and V.D.S.; funding acquisition, C.E. and V.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the financial support through the BIKE project; this project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 813748.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
There are no conflicts to declare.
References
- European Commission. Directorate General for Research and Innovation. In Horizon Europe: Strategic Plan 2021–2024; Publications Office: Luxembourg, 2021. [Google Scholar]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on Methane Steam Reforming to Produce Hydrogen through Membrane Reactors Technology: A Review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on Dry Reforming of Methane, a Potentially More Environmentally Friendly Approach to the Increasing Natural Gas Exploitation. Front. Chem 2014, 2, 81. [Google Scholar] [CrossRef]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A Review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Matus, E.V.; Ismagilov, I.Z.; Sukhova, O.B.; Yashnik, S.A.; Ushakov, V.A.; Kerzhentsev, M.A. Hydrogen Production through Hydrocarbon Fuel Reforming Processes over Ni Based Catalysts. Catal. Today 2019, 323, 166–182. [Google Scholar] [CrossRef]
- Armor, J.N. The Multiple Roles for Catalysis in the Production of H2. Appl. Catal. A Gen. 1999, 176, 159–176. [Google Scholar] [CrossRef]
- Joensen, F.; Rostrup-Nielsen, J.R. Conversion of Hydrocarbons and Alcohols for Fuel Cells. J. Power Sources 2002, 105, 195–201. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Kulkarni, S. A Review on Reforming Reactions with Emphasis on Methane Reforming. Int. J. Res. Rev. 2016, 3, 20–23. [Google Scholar]
- Chai, S.; Zhang, G.; Li, G.; Zhang, Y. Industrial Hydrogen Production Technology and Development Status in China: A Review. Clean Technol. Environ. Policy 2021, 23, 1931–1946. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic Analysis of Carbon Dioxide Reforming of Methane in View of Solid Carbon Formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Özkara-Aydınoğlu, Ş.; Aksoylu, A.E. CO2 Reforming of Methane over Pt–Ni/Al2O3 Catalysts: Effects of Catalyst Composition, and Water and Oxygen Addition to the Feed. Int. J. Hydrogen Energy 2011, 36, 2950–2959. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.-N.; Henkelman, G.; Kim, J.M. Design of a Highly Nanodispersed Pd–MgO/SiO2 Composite Catalyst with Multifunctional Activity for CH4 Reforming. ChemSusChem 2012, 5, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Mondal, K.; Sasmal, S.; Badgandi, S.; Chowdhury, D.R.; Nair, V. Dry Reforming of Methane to Syngas: A Potential Alternative Process for Value Added Chemicals—A Techno-Economic Perspective. Environ. Sci. Pollut. Res. 2016, 23, 22267–22273. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Chatterjee, S.; Huang, K.-W. The Insignificant Role of Dry Reforming of Methane in CO2 Emission Relief. ACS Energy Lett. 2020, 5, 2881–2885. [Google Scholar] [CrossRef]
- Noureldin, M.M.B.; Elbashir, N.O.; Gabriel, K.J.; El-Halwagi, M.M. A Process Integration Approach to the Assessment of CO2 Fixation through Dry Reforming. ACS Sustain. Chem. Eng. 2015, 3, 625–636. [Google Scholar] [CrossRef]
- Cai, X.; Hu, Y.H. Advances in Catalytic Conversion of Methane and Carbon Dioxide to Highly Valuable Products. Energy Sci. Eng. 2019, 7, 4–29. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Sehested, J.; Nørskov, J.K. Hydrogen and Synthesis Gas by Steam-and CO2 Reforming. Adv. Catal. 2002, 47, 65–139. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Christiansen, L.J. Concepts in Syngas Manufacture. Catal. Sci. Ser. 2011, 10, 392. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Hansen, J.H.B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Jones, G.; Jakobsen, J.G.; Shim, S.S.; Kleis, J.; Andersson, M.P.; Rossmeisl, J.; Abild-Pedersen, F.; Bligaard, T.; Helveg, S.; Hinnemann, B.; et al. First Principles Calculations and Experimental Insight into Methane Steam Reforming over Transition Metal Catalysts. J. Catal. 2008, 259, 147–160. [Google Scholar] [CrossRef]
- Wu, H.; La Parola, V.; Pantaleo, G.; Puleo, F.; Venezia, A.; Liotta, L. Ni-Based Catalysts for Low Temperature Methane Steam Reforming: Recent Results on Ni-Au and Comparison with Other Bi-Metallic Systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-Based Bimetallic Heterogeneous Catalysts for Energy and Environmental Applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Trimm, D.L. Coke Formation and Minimisation during Steam Reforming Reactions. Catal. Today 1997, 37, 233–238. [Google Scholar] [CrossRef]
- Trimm, D.L. Catalysts for the Control of Coking during Steam Reforming. Catal. Today 1999, 49, 3–10. [Google Scholar] [CrossRef]
- Sehested, J. Four Challenges for Nickel Steam-Reforming Catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Batchu, R.; Buelens, L.C.; Detavernier, C.; Marin, G.B. Mechanism of Carbon Deposits Removal from Supported Ni Catalysts. Appl. Catal. B Environ. 2018, 239, 502–512. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Methane Reforming to Synthesis Gas over Ni Catalysts Modified with Noble Metals. Appl. Catal. A Gen. 2011, 408, 1–24. [Google Scholar] [CrossRef]
- Roussière, T.; Schulz, L.; Schelkle, K.M.; Wasserschaff, G.; Milanov, A.; Schwab, E.; Deutschmann, O.; Jentys, A.; Lercher, J.; Schunk, S.A. Structure–Activity Relationships of Nickel–Hexaaluminates in Reforming Reactions Part II: Activity and Stability of Nanostructured Nickel–Hexaaluminate-Based Catalysts in the Dry Reforming of Methane. ChemCatChem 2014, 6, 1447–1452. [Google Scholar] [CrossRef]
- Ginsburg, J.M.; Piña, J.; El Solh, T.; de Lasa, H.I. Coke Formation over a Nickel Catalyst under Methane Dry Reforming Conditions: Thermodynamic and Kinetic Models. Ind. Eng. Chem. Res. 2005, 44, 4846–4854. [Google Scholar] [CrossRef]
- Titus, J.; Roussière, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry Reforming of Methane with Carbon Dioxide over NiO–MgO–ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Van Hook, J.P. Methane-Steam Reforming. Catal. Rev. 1980, 21, 1–51. [Google Scholar] [CrossRef]
- Hu, Y.H. Advances in Catalysts for CO2 Reforming of Methane. In Advances in CO2 Conversion and Utilization; American Chemical Society: Washington, DC, USA, 2010; pp. 155–174. [Google Scholar]
- Lee, S. Methane and Its Derivatives; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hu, Y.H.; Ruckenstein, E. Binary MgO-Based Solid Solution Catalysts for Methane Conversion to Syngas. Catal. Rev. 2002, 44, 423–453. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Catalytic Technology for Carbon Dioxide Reforming of Methane to Synthesis Gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. A Review on Coke Management during Dry Reforming of Methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry Reforming of Methane over Nickel Catalysts Supported on Magnesium Aluminate Spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic Catalysts for Upgrading of Biomass to Fuels and Chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Dal Santo, V.; Gallo, A.; Naldoni, A.; Guidotti, M.; Psaro, R. Bimetallic Heterogeneous Catalysts for Hydrogen Production. Catal. Today 2012, 197, 190–205. [Google Scholar] [CrossRef]
- Usman, M.; Daud, W.W.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters—A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Puxley, D.C.; Kitchener, I.J.; Komodromos, C.; Parkyns, N.D. The Effect Of Preparation Method Upon The Structures, Stability And Metal/Support Interactions In Nickel/Alumina Catalysts. In Studies in Surface Science and Catalysis; Poncelet, G., Grange, P., Jacobs, P.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 16, pp. 237–271. [Google Scholar]
- Azancot, L.; Bobadilla, L.F.; Santos, J.L.; Córdoba, J.M.; Centeno, M.A.; Odriozola, J.A. Influence of the Preparation Method in the Metal-Support Interaction and Reducibility of Ni-Mg-Al Based Catalysts for Methane Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 19827–19840. [Google Scholar] [CrossRef]
- Kwon, D.; Kang, J.Y.; An, S.; Yang, I.; Jung, J.C. Tuning the Base Properties of Mg–Al Hydrotalcite Catalysts Using Their Memory Effect. J. Energy Chem. 2020, 46, 229–236. [Google Scholar] [CrossRef]
- Bossola, F.; Evangelisti, C.; Allieta, M.; Psaro, R.; Recchia, S.; Dal Santo, V. Well-Formed, Size-Controlled Ruthenium Nanoparticles Active and Stable for Acetic Acid Steam Reforming. Appl. Catal. B Environ. 2016, 181, 599–611. [Google Scholar] [CrossRef]
- Adamu, S.; Bawah, A.-R.; Muraza, O.; Malaibari, Z.; Hossain, M.M. Effects of Metal Support Interaction on Dry Reforming of Methane over Ni/Ce-Al2O3 Catalysts. Can. J. Chem. Eng. 2020, 98, 2425–2434. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, K.; Pant, K.K.; Choudary, N.V. Tuning the Metal-Support Interaction of Methane Tri-Reforming Catalysts for Industrial Flue Gas Utilization. Int. J. Hydrogen Energy 2020, 45, 1911–1929. [Google Scholar] [CrossRef]
- Özdemir, H.; Öksüzömer, M.A.F.; Gürkaynak, M.A. Effect of the Calcination Temperature on Ni/MgAl2O4 Catalyst Structure and Catalytic Properties for Partial Oxidation of Methane. Fuel 2014, 116, 63–70. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-Programmed-Reduction Studies of Nickel Oxide/Alumina Catalysts: Effects of the Preparation Method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Rynkowski, J.M.; Paryjczak, T.; Lenik, M. On the Nature of Oxidic Nickel Phases in NiO/γ-Al2O3 Catalysts. Appl. Catal. A Gen. 1993, 106, 73–82. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Zhang, Z.; Zhang, L.; Dong, D.; Gao, G.; Westerhof, R.; Syed-Hassan, S.S.A. Steam Reforming of Acetic Acid over Ni/Al2O3 Catalyst: Correlation of Calcination Temperature with the Interaction of Nickel and Alumina. Fuel 2018, 227, 307–324. [Google Scholar] [CrossRef]
- Sahli, N.; Petit, C.; Roger, A.-C.; Kiennemann, A.; Libs, S.; Bettahar, M.M. Ni Catalysts from NiAl2O4 Spinel for CO2 Reforming of Methane. Catal. Today 2006, 113, 187–193. [Google Scholar] [CrossRef]
- Kuzmin, A.; Mironova, N. Composition Dependence of the Lattice Parameter in Solid Solutions. J. Phys. Matter 1998, 10, 7937–7944. [Google Scholar] [CrossRef]
- Parmaliana, A.; Arena, F.; Frusteri, F.; Giordano, N. Temperature-Programmed Reduction Study of NiO–MgO Interactions in Magnesia-Supported Ni Catalysts and NiO–MgO Physical Mixture. J. Chem. Soc. Faraday Trans. 1990, 86, 2663–2669. [Google Scholar] [CrossRef]
- Zanganeh, R.; Rezaei, M.; Zamaniyan, A. Dry Reforming of Methane to Synthesis Gas on NiO–MgO Nanocrystalline Solid Solution Catalysts. Int. J. Hydrogen Energy 2013, 38, 3012–3018. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Carbon Dioxide Reforming of Methane over Nickel/Alkaline Earth Metal Oxide Catalysts. Appl. Catal. A Gen. 1995, 133, 149–161. [Google Scholar] [CrossRef]
- Ou, Z.; Zhang, Z.; Qin, C.; Xia, H.; Deng, T.; Niu, J.; Ran, J.; Wu, C. Highly Active and Stable Ni/Perovskite Catalysts in Steam Methane Reforming for Hydrogen Production. Sustain. Energy Fuels 2021, 5, 1845–1856. [Google Scholar] [CrossRef]
- Hong Phuong, P.; Cam Anh, H.; Tri, N.; Phung Anh, N.; Cam Loc, L. Effect of Support on Stability and Coke Resistance of Ni-Based Catalyst in Combined Steam and CO2 Reforming of CH4. ACS Omega 2022, 7, 20092–20103. [Google Scholar] [CrossRef]
- Gac, W. Acid–Base Properties of Ni–MgO–Al2O3 Materials. Appl. Surf. Sci. 2011, 257, 2875–2880. [Google Scholar] [CrossRef]
- Coleman, L.J.I.; Epling, W.; Hudgins, R.R.; Croiset, E. Ni/Mg–Al Mixed Oxide Catalyst for the Steam Reforming of Ethanol. Appl. Catal. A Gen. 2009, 363, 52–63. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-Based Catalysts for Reforming of Methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, G.; Li, M.; Wu, Y.; Nie, H.; Li, D. Effect of Support on the Performance of Ni-Based Catalyst in Methane Dry Reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Mehr, J.Y.; Jozani, K.J.; Pour, A.N.; Zamani, Y. Influence of MgO in the CO2–Steam Reforming of Methane to Syngas by NiO/MgO/α-Al2O3 Catalyst. React. Kinet. Catal. Lett. 2002, 75, 267–273. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A Study of the Synergy between Support Surface Properties and Catalyst Deactivation for CO2 Reforming over Supported Ni Nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- Williams, G.R.; O’Hare, D. Towards Understanding, Control and Application of Layered Double Hydroxide Chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Sikander, U.; Sufian, S.; Salam, M.A. A Review of Hydrotalcite Based Catalysts for Hydrogen Production Systems. Int. J. Hydrogen Energy 2017, 42, 19851–19868. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.E.; Da Costa, P. A Short Review on the Catalytic Activity of Hydrotalcite-Derived Materials for Dry Reforming of Methane. Catalysts 2017, 7, 32. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chang, V.W.; Schumacher, D.J. CO2 Reforming of Methane to Syngas: I: Evaluation of Hydrotalcite Clay-Derived Catalysts. Appl. Clay Sci. 1998, 13, 317–328. [Google Scholar] [CrossRef]
- Fornasari, G.; Gazzano, M.; Matteuzzi, D.; Trifirò, F.; Vaccari, A. Structure and Reactivity of High-Surface-Area Ni/Mg/Al Mixed Oxides. Appl. Clay Sci. 1995, 10, 69–82. [Google Scholar] [CrossRef]
- Shishido, T.; Sukenobu, M.; Morioka, H.; Kondo, M.; Wang, Y.; Takaki, K.; Takehira, K. Partial Oxidation of Methane over Ni/Mg-Al Oxide Catalysts Prepared by Solid Phase Crystallization Method from Mg-Al Hydrotalcite-like Precursors. Appl. Catal. A Gen. 2002, 223, 35–42. [Google Scholar] [CrossRef]
- Shishido, T.; Wang, P.; Kosaka, T.; Takehira, K. Steam Reforming of CH4 over Ni/Mg-Al Catalyst Prepared by Spc-Method from Hydrotalcite. Chem. Lett. 2002, 31, 752–753. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Wang, P.; Kosaka, T.; Takaki, K. Steam Reforming of CH4 over Supported Ni Catalysts Prepared from a Mg–Al Hydrotalcite-like Anionic Clay. Phys. Chem. Chem. Phys. 2003, 5, 3801–3810. [Google Scholar] [CrossRef]
- Shishido, T.; Sukenobu, M.; Morioka, H.; Furukawa, R.; Shirahase, H.; Takehira, K. CO2 Reforming of CH4 over Ni/Mg–Al Oxide Catalysts Prepared by Solid Phase Crystallization Method from Mg–Al Hydrotalcite-like Precursors. Catal. Lett. 2001, 73, 21–26. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. Chapter One—A Review of Preparation Methods for Supported Metal Catalysts. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 61, pp. 1–35. [Google Scholar]
- Touahra, F.; Sehailia, M.; Ketir, W.; Bachari, K.; Chebout, R.; Trari, M.; Cherifi, O.; Halliche, D. Effect of the Ni/Al Ratio of Hydrotalcite-Type Catalysts on Their Performance in the Methane Dry Reforming Process. Appl. Petrochem. Res. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Zhang, Y.; Li, J. Effect of Ni Loadings on the Catalytic Properties of Ni/MgO(111) Catalyst for the Reforming of Methane with Carbon Dioxide. J. Fuel Chem. Technol. 2015, 43, 315–322. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Ni Loadings on the Activity and Coke Formation of MgO-Modified Ni/Al2O3 Nanocatalyst in Dry Reforming of Methane. J. Energy Chem. 2014, 23, 633–638. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. Synthesis Gas Production over Highly Active and Stable Nanostructured Ni-MgO-Al2O3 Catalysts in Dry Reforming of Methane: Effects of Ni Contents. Fuel 2017, 194, 171–179. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Duraczyska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane Dry Reforming over Hydrotalcite-Derived Ni–Mg–Al Mixed Oxides: The Influence of Ni Content on Catalytic Activity, Selectivity and Stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Lu, M.; Chen, C.; Li, D.; Zhan, Y.; Jiang, L. Carbon Dioxide Reforming of Methane over Ni Catalysts Prepared from Ni–Mg–Al Layered Double Hydroxides: Influence of Ni Loadings. Fuel 2015, 162, 271–280. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, Z.; Zhou, Z. Steam Reforming of Methane over Ni Catalysts Prepared from Hydrotalcite-Type Precursors: Catalytic Activity and Reaction Kinetics. Chin. J. Chem. Eng. 2015, 23, 76–85. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Wang, Z.; Jiang, B.; Kawi, S. Dry Reforming of Methane on Ni/Mesoporous-Al2O3 Catalysts: Effect of Calcination Temperature. Int. J. Hydrogen Energy 2021, 46, 31041–31053. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.A.; Fakeeha, A.H. Effects of Calcination and Activation Temperature on Dry Reforming Catalysts. J. Saudi Chem. Soc. 2012, 16, 55–61. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Nickel Catalyst Activation in the Carbon Dioxide Reforming of Methane: Effect of Pretreatments. Appl. Catal. A Gen. 2009, 355, 27–32. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.-M. Effect of NiAl2O4 Formation on Ni/Al2O3 Stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef]
- Feng, J.; Ding, Y.; Guo, Y.; Li, X.; Li, W. Calcination Temperature Effect on the Adsorption and Hydrogenated Dissociation of CO2 over the NiO/MgO Catalyst. Fuel 2013, 109, 110–115. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hang Hu, Y. The Effect of Precursor and Preparation Conditions of MgO on the CO2 Reforming of CH4 over NiO/MgO Catalysts. Appl. Catal. A Gen. 1997, 154, 185–205. [Google Scholar] [CrossRef]
- Mette, K.; Kühl, S.; Düdder, H.; Kähler, K.; Tarasov, A.; Muhler, M.; Behrens, M. Stable Performance of Ni Catalysts in the Dry Reforming of Methane at High Temperatures for the Efficient Conversion of CO2 into Syngas. ChemCatChem 2014, 6, 100–104. [Google Scholar] [CrossRef]
- Katheria, S.; Gupta, A.; Deo, G.; Kunzru, D. Effect of Calcination Temperature on Stability and Activity of Ni/MgAl2O4 Catalyst for Steam Reforming of Methane at High Pressure Condition. Int. J. Hydrogen Energy 2016, 41, 14123–14132. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Zhang, Q.; Luan, C.; Vinokurov, V.A.; Huang, W. Highly Stable and Anti-Coking Ni/MoCeZr/MgAl2O4-MgO Complex Support Catalysts for CO2 Reforming of CH4: Effect of the Calcination Temperature. Energy Convers. Manag. 2019, 179, 166–177. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Senger, A.; Marcilio, N.R.; Lansarin, M.A. Effect of Composition and Thermal Pretreatment on Properties of Ni–Mg–Al Catalysts for CO2 Reforming of Methane. Appl. Catal. A Gen. 2006, 303, 234–244. [Google Scholar] [CrossRef]
- Djaidja, A.; Libs, S.; Kiennemann, A.; Barama, A. Characterization and Activity in Dry Reforming of Methane on NiMg/Al and Ni/MgO Catalysts. Catal. Today 2006, 113, 194–200. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Dam, A.H.; Xiao, L.; Qi, Y.; Niu, J.; Yang, J.; Zhu, Y.-A.; Holmen, A.; Chen, D. Understanding Effects of Ni Particle Size on Steam Methane Reforming Activity by Combined Experimental and Theoretical Analysis. Catal. Today 2019, 355, 139–147. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of Metal Particle Size on Coking during CO2 Reforming of CH4 over Ni–Alumina Aerogel Catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure Sensitivity in Steam and Dry Methane Reforming over Nickel: Activity and Carbon Formation. ACS Catal. 2020, 10, 1428–1438. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Roh, H.-S.; Eum, I.-H.; Jeong, D.-W.; Yi, B.E.; Na, J.-G.; Ko, C.H. The Effect of Calcination Temperature on the Performance of Ni/MgO–Al2O3 Catalysts for Decarboxylation of Oleic Acid. Catal. Today 2011, 164, 457–460. [Google Scholar] [CrossRef]
- Smoláková, L.; Kout, M.; Koudelková, E.; Čapek, L. Effect of Calcination Temperature on the Structure and Catalytic Performance of the Ni/Al2O3 and Ni–Ce/Al2O3 Catalysts in Oxidative Dehydrogenation of Ethane. Ind. Eng. Chem. Res. 2015, 54, 12730–12740. [Google Scholar] [CrossRef]
- Khan, A.I.; O’Hare, D. Intercalation Chemistry of Layered Double Hydroxides: Recent Developments and Applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Millet, M.-M.; Tarasov, A.V.; Girgsdies, F.; Algara-Siller, G.; Schlögl, R.; Frei, E. Highly Dispersed Ni0/NixMg1–XO Catalysts Derived from Solid Solutions: How Metal and Support Control the CO2 Hydrogenation. ACS Catal. 2019, 9, 8534–8546. [Google Scholar] [CrossRef]
- Ward, D.A.; Ko, E.I. Preparing Catalytic Materials by the Sol-Gel Method. Ind. Eng. Chem. Res. 1995, 34, 421–433. [Google Scholar] [CrossRef]
- Frenzer, G.; Maier, W.F. Amorphous Porous Mixed Oxides: Sol-Gel Ways to a Highly Versatile Class of Materials and Catalysts. Annu. Rev. Mater. Res. 2006, 36, 281–331. [Google Scholar] [CrossRef]
- Othman, M.R.; Helwani, Z.; Martunus; Fernando, W.J.N. Synthetic Hydrotalcites from Different Routes and Their Application as Catalysts and Gas Adsorbents: A Review. Appl. Organomet. Chem. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Miller, J.B.; Ko, E.I. Control of Mixed Oxide Textural and Acidic Properties by the Sol-Gel Method. Catal. Today 1997, 35, 269–292. [Google Scholar] [CrossRef]
- Otero Areán, C.; Peñarroya Mentruit, M.; López López, A.J.; Parra, J.B. High Surface Area Nickel Aluminate Spinels Prepared by a Sol–Gel Method. Colloids Surf. A Physicochem. Eng. Asp. 2001, 180, 253–258. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Wang, J. Methane Reforming with CO2 to Syngas over CeO2-Promoted Ni/Al2O3-ZrO2 Catalysts Prepared via a Direct Sol-Gel Process. J. Nat. Gas Chem. 2011, 20, 1–8. [Google Scholar] [CrossRef]
- González, A.R.; Asencios, Y.J.O.; Assaf, E.M.; Assaf, J.M. Dry Reforming of Methane on Ni–Mg–Al Nano-Spheroid Oxide Catalysts Prepared by the Sol–Gel Method from Hydrotalcite-like Precursors. Appl. Surf. Sci. 2013, 280, 876–887. [Google Scholar] [CrossRef]
- Salhi, N.; Boulahouache, A.; Petit, C.; Kiennemann, A.; Rabia, C. Steam Reforming of Methane to Syngas over NiAl2O4 Spinel Catalysts. Int. J. Hydrogen Energy 2011, 36, 11433–11439. [Google Scholar] [CrossRef]
- Min, J.-E.; Lee, Y.-J.; Park, H.-G.; Zhang, C.; Jun, K.-W. Carbon Dioxide Reforming of Methane on Ni–MgO–Al2O3 Catalysts Prepared by Sol–Gel Method: Effects of Mg/Al Ratios. J. Ind. Eng. Chem. 2015, 26, 375–383. [Google Scholar] [CrossRef]
- Sunde, T.O.L.; Grande, T.; Einarsrud, M.-A. Modified Pechini Synthesis of Oxide Powders and Thin Films. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–30. [Google Scholar]
- Rivas, M.E.; Fierro, J.L.G.; Guil-López, R.; Peña, M.A.; La Parola, V.; Goldwasser, M.R. Preparation and Characterization of Nickel-Based Mixed-Oxides and Their Performance for Catalytic Methane Decomposition. Catal. Today 2008, 133–135, 367–373. [Google Scholar] [CrossRef]
- Rogers, J.L.; Mangarella, M.C.; D’Amico, A.D.; Gallagher, J.R.; Dutzer, M.R.; Stavitski, E.; Miller, J.T.; Sievers, C. Differences in the Nature of Active Sites for Methane Dry Reforming and Methane Steam Reforming over Nickel Aluminate Catalysts. ACS Catal. 2016, 6, 5873–5886. [Google Scholar] [CrossRef]
- Schimmoeller, B.; Pratsinis, S.E.; Baiker, A. Flame Aerosol Synthesis of Metal Oxide Catalysts with Unprecedented Structural and Catalytic Properties. ChemCatChem 2011, 3, 1234–1256. [Google Scholar] [CrossRef]
- Suh, D.J.; Park, T.-J.; Kim, J.-H.; Kim, K.-L. Nickel–Alumina Aerogel Catalysts Prepared by Fast Sol–Gel Synthesis. J. Non-Cryst. Solids 1998, 225, 168–172. [Google Scholar] [CrossRef]
- Sebai, I.; Boulahaouache, A.; Trari, M.; Salhi, N. Preparation and Characterization of 5%Ni/γ-Al2O3 Catalysts by Complexation with NH3 Derivatives Active in Methane Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 9949–9958. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly Active and Stable Alumina Supported Nickel Nanoparticle Catalysts for Dry Reforming of Methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Wang, G.; Luo, F.; Cao, K.; Zhang, Y.; Li, J.; Zhao, F.; Chen, R.; Hong, J. Effect of Ni Content of Ni/γ-Al2O3 Catalysts Prepared by the Atomic Layer Deposition Method on CO2 Reforming of Methane. Energy Technol. 2019, 7, 1800359. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Kim, S.Y.; Kim, D.H.; Han, S.W.; Kim, I.H.; Lee, M.; Hwang, Y.K.; Kim, Y.D. High-Performing and Durable MgO/Ni Catalysts via Atomic Layer Deposition for CO2 Reforming of Methane (CRM). Appl. Catal. A Gen. 2016, 515, 45–50. [Google Scholar] [CrossRef]
- Gould, T.D.; Izar, A.; Weimer, A.W.; Falconer, J.L.; Medlin, J.W. Stabilizing Ni Catalysts by Molecular Layer Deposition for Harsh, Dry Reforming Conditions. ACS Catal. 2014, 4, 2714–2717. [Google Scholar] [CrossRef]
- Littlewood, P.; Liu, S.; Weitz, E.; Marks, T.J.; Stair, P.C. Ni-Alumina Dry Reforming Catalysts: Atomic Layer Deposition and the Issue of Ni Aluminate. Catal. Today 2020, 343, 18–25. [Google Scholar] [CrossRef]
- Kim, H.; Eissa, A.A.-S.; Kim, S.B.; Lee, H.; Kim, W.; Seo, D.J.; Lee, K.; Yoon, W.L. One-Pot Synthesis of a Highly Mesoporous Ni/MgAl2O4 Spinel Catalyst for Efficient Steam Methane Reforming: Influence of Inert Annealing. Catal. Sci. Technol. 2021, 11, 4447–4458. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Ning, Y.; Liu, Q.; Tian, L.; Zhang, R.; Fu, Q.; Wang, Z. CO2 Reforming of Methane over a Highly Dispersed Ni/Mg–Al–O Catalyst Prepared by a Facile and Green Method. Ind. Eng. Chem. Res. 2020, 59, 15506–15514. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Y.; Saqline, S.; Liu, W.; Liu, B. High Performance Ni Catalysts Prepared by Freeze Drying for Efficient Dry Reforming of Methane. Appl. Catal. B Environ. 2020, 275, 119109. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takahashi, S. Catalyst for Decomposition of Hydrocarbons, Process for Producing the Catalyst, and Process for Producing Hydrogen Using the Catalyst. U.S. Patent 7196036B2, 27 March 2007. [Google Scholar]
- Kwak, G.; Park, H.; Wi, S.; Park, S. High Efficiency Ni-based Catalyst for Steam Methane Reforming and Steam Methane Reforming Reaction using the Same. KR Patent 20220052099A, 27 April 2022. [Google Scholar]
- Hou, Z.; Yashima, T. Small Amounts of Rh-Promoted Ni Catalysts for Methane Reforming with CO2. Catal. Lett. 2003, 89, 193–197. [Google Scholar] [CrossRef]
- Fei, J.; Hou, Z.; Zheng, X.; Yashima, T. Doped Ni Catalysts for Methane Reforming with CO2. Catal. Lett. 2004, 98, 241–246. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Minicò, S.; Solarino, L. Ni–Ru Bimetallic Catalysts for the CO2 Reforming of Methane. Appl. Catal. A Gen. 2002, 225, 1–9. [Google Scholar] [CrossRef]
- Tomishige, K.; Kanazawa, S.; Sato, M.; Ikushima, K.; Kunimori, K. Catalyst Design of Pt-Modified Ni/Al2O3 Catalyst with Flat Temperature Profile in Methane Reforming with CO2 and O2. Catal. Lett. 2002, 84, 69–74. [Google Scholar] [CrossRef]
- Dias, J.A.C.; Assaf, J.M. Autothermal Reforming of Methane over Ni/γ-Al2O3 Catalysts: The Enhancement Effect of Small Quantities of Noble Metals. J. Power Sources 2004, 130, 106–110. [Google Scholar] [CrossRef]
- Dias, J.A.C.; Assaf, J.M. Autoreduction of Promoted Ni/γ-Al2O3 during Autothermal Reforming of Methane. J. Power Sources 2005, 139, 176–181. [Google Scholar] [CrossRef]
- Daza, C.E.; Gallego, J.; Mondragón, F.; Moreno, S.; Molina, R. High Stability of Ce-Promoted Ni/Mg–Al Catalysts Derived from Hydrotalcites in Dry Reforming of Methane. Fuel 2010, 89, 592–603. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Sutthisripok, W.; Assabumrungrat, S. Synthesis Gas Production from Dry Reforming of Methane over CeO2 Doped Ni/Al2O3: Influence of the Doping Ceria on the Resistance toward Carbon Formation. Chem. Eng. J. 2005, 112, 13–22. [Google Scholar] [CrossRef]
- Abd Ghani, N.A.; Azapour, A.; Syed Muhammad, A.F.; Abdullah, B. Dry Reforming of Methane for Hydrogen Production over NiCo Catalysts: Effect of NbZr Promoters. Int. J. Hydrogen Energy 2019, 44, 20881–20888. [Google Scholar] [CrossRef]
- Navarro, M.V.; Plou, J.; López, J.M.; Grasa, G.; Murillo, R. Effect of Oxidation-Reduction Cycles on Steam-Methane Reforming Kinetics over a Nickel-Based Catalyst. Int. J. Hydrogen Energy 2019, 44, 12617–12627. [Google Scholar] [CrossRef]
- Álvarez, M.A.; Bobadilla, L.F.; Garcilaso, V.; Centeno, M.A.; Odriozola, J.A. CO2 Reforming of Methane over Ni-Ru Supported Catalysts: On the Nature of Active Sites by Operando DRIFTS Study. J. CO2 Util. 2018, 24, 509–515. [Google Scholar] [CrossRef]
- Foletto, E.L.; Alves, R.W.; Jahn, S.L. Preparation of Ni/Pt Catalysts Supported on Spinel (MgAl2O4) for Methane Reforming. J. Power Sources 2006, 161, 531–534. [Google Scholar] [CrossRef]
- Jaiswar, V.K.; Katheria, S.; Deo, G.; Kunzru, D. Effect of Pt Doping on Activity and Stability of Ni/MgAl2O4 Catalyst for Steam Reforming of Methane at Ambient and High Pressure Condition. Int. J. Hydrogen Energy 2017, 42, 18968–18976. [Google Scholar] [CrossRef]
- Yoshida, K.; Begum, N.; Ito, S.; Tomishige, K. Oxidative Steam Reforming of Methane over Ni/α-Al2O3 Modified with Trace Noble Metals. Appl. Catal. A Gen. 2009, 358, 186–192. [Google Scholar] [CrossRef]
- Li, B.; Kado, S.; Mukainakano, Y.; Miyazawa, T.; Miyao, T.; Naito, S.; Okumura, K.; Kunimori, K.; Tomishige, K. Surface Modification of Ni Catalysts with Trace Pt for Oxidative Steam Reforming of Methane. J. Catal. 2007, 245, 144–155. [Google Scholar] [CrossRef]
- Arena, F.; Horrell, B.A.; Cocke, D.L.; Parmaliana, A.; Giordano, N. Magnesia-Supported Nickel Catalysts I. Factors Affecting the Structure and Morphological Properties. J. Catal. 1991, 132, 58–67. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Mukainakano, Y.; Kado, S.; Miyazawa, T.; Okumura, K.; Miyao, T.; Naito, S.; Suzuki, K.; Fujimoto, K.-I.; Kunimori, K.; et al. Oxidative Steam Reforming of Methane under Atmospheric and Pressurized Conditions over Pd/NiO–MgO Solid Solution Catalysts. Appl. Catal. A Gen. 2006, 308, 1–12. [Google Scholar] [CrossRef]
- Katheria, S.; Deo, G.; Kunzru, D. Rh-Ni/MgAl2O4 Catalyst for Steam Reforming of Methane: Effect of Rh Doping, Calcination Temperature and Its Application on Metal Monoliths. Appl. Catal. A Gen. 2019, 570, 308–318. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef] [PubMed]
- Takehira, K. “Intelligent” Reforming Catalysts: Trace Noble Metal-Doped Ni/Mg(Al)O Derived from Hydrotalcites. J. Nat. Gas Chem. 2009, 18, 237–259. [Google Scholar] [CrossRef]
- Li, D.; Zhan, Y.; Nishida, K.; Oumi, Y.; Sano, T.; Shishido, T.; Takehira, K. “Green” Preparation of “Intelligent” Pt-Doped Ni/Mg(Al)O Catalysts for Daily Start-up and Shut-down CH4 Steam Reforming. Appl. Catal. A Gen. 2009, 363, 169–179. [Google Scholar] [CrossRef]
- Miyata, T.; Li, D.; Shiraga, M.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Promoting Effect of Rh, Pd and Pt Noble Metals to the Ni/Mg(Al)O Catalysts for the DSS-like Operation in CH4 Steam Reforming. Appl. Catal. A Gen. 2006, 310, 97–104. [Google Scholar] [CrossRef]
- Katheria, S.; Kunzru, D.; Deo, G. Kinetics of Steam Reforming of Methane on Rh–Ni/MgAl2O4 Catalyst. React. Kinet. Mech. Catal. 2020, 130, 91–101. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, J.W.; Seo, D.J.; Seo, Y.; Yoon, W.L.; Lee, D.K.; Kim, D.H. Ru-Doped Ni Catalysts Effective for the Steam Reforming of Methane without the Pre-Reduction Treatment with H2. Appl. Catal. A Gen. 2006, 302, 151–156. [Google Scholar] [CrossRef]
- Baek, S.-C.; Jun, K.-W.; Lee, Y.-J.; Kim, J.D.; Park, D.Y.; Lee, K.-Y. Ru/Ni/MgAl2O4 Catalysts for Steam Reforming of Methane: Effects of Ru Content on Self-Activation Property. Res. Chem. Intermed. 2012, 38, 1225–1236. [Google Scholar] [CrossRef]
- Nawfal, M.; Gennequin, C.; Labaki, M.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Hydrogen Production by Methane Steam Reforming over Ru Supported on Ni–Mg–Al Mixed Oxides Prepared via Hydrotalcite Route. Int. J. Hydrogen Energy 2015, 40, 1269–1277. [Google Scholar] [CrossRef]
- Kim, N.Y.; Yang, E.-H.; Lim, S.-S.; Jung, J.S.; Lee, J.-S.; Hong, G.H.; Noh, Y.-S.; Lee, K.Y.; Moon, D.J. Hydrogen Production by Steam Reforming of Methane over Mixed Ni/MgAl + CrFe3O4 Catalysts. Int. J. Hydrogen Energy 2015, 40, 11848–11854. [Google Scholar] [CrossRef]
- Kim, D.H.; Youn, J.-R.; Seo, J.-C.; Kim, S.B.; Kim, M.-J.; Lee, K. One-Pot Synthesis of NiCo/MgAl2O4 Catalyst for High Coke-Resistance in Steam Methane Reforming: Optimization of Ni/Co Ratio. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Bossola, F.; Roongcharoen, T.; Coduri, M.; Evangelisti, C.; Somodi, F.; Sementa, L.; Fortunelli, A.; Dal Santo, V. Discovering Indium as Hydrogen Production Booster for a Cu/SiO2 Catalyst in Steam Reforming of Methanol. Appl. Catal. B Environ. 2021, 297, 120398. [Google Scholar] [CrossRef]
- Etminan, A.; Sadrnezhaad, S.K. A Two Step Microwave-Assisted Coke Resistant Mesoporous Ni-Co Catalyst for Methane Steam Reforming. Fuel 2022, 317, 122411. [Google Scholar] [CrossRef]
- Moon, D.J.; Lee, Y.J.; Jung, J.S.; Lee, J.H.; Lee, S.H.; Kim, B.H.; Kim, H.J.; Yang, E.H. Alkaline Earth Metal Co-Precipitated Nickel-Based Catalyst for Steam Carbon Dioxide Reforming of Natural Gas. US Patent 20140339475A, 3 March 2015. [Google Scholar]
- Du, J.; Liu, P.; Gao, Z.; Zhang, W.; Zhang, L.; Li, X. Magnesium Aluminate Spinel Type Composite Oxide Carrier, Preparation Method Thereof and Steam Reforming Catalyst. CN Patent 112844388A, 29 November 2022. [Google Scholar]
- Chin, Y.-H.; King, D.L.; Roh, H.-S.; Wang, Y.; Heald, S.M. Structure and Reactivity Investigations on Supported Bimetallic AuNi Catalysts Used for Hydrocarbon Steam Reforming. J. Catal. 2006, 244, 153–162. [Google Scholar] [CrossRef]
- Binary Alloy Phase Diagrams, 2nd ed; Massalski, T.B., Okamoto, H., Subramanian, P.R., Kacprzak, L., Eds.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Bengaard, H.S.; Nørskov, J.K.; Sehested, J.; Clausen, B.S.; Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R. Steam Reforming and Graphite Formation on Ni Catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, D.; Nishida, K.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Preparation of “Intelligent” Pt/Ni/Mg(Al)O Catalysts Starting from Commercial Mg–Al LDHs for Daily Start-up and Shut-down Steam Reforming of Methane. Appl. Clay Sci. 2009, 45, 147–154. [Google Scholar] [CrossRef]
- Ko, J.; Kim, B.-K.; Han, J.W. Density Functional Theory Study for Catalytic Activation and Dissociation of CO2 on Bimetallic Alloy Surfaces. J. Phys. Chem. C 2016, 120, 3438–3447. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of Metal Content on Activity and Stability of Ni-Co Bimetallic Catalysts for CO2 Reforming of CH4. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Liland, E.S.; Regli, K.S.; Yang, J.; Rout, K.R.; Luo, J.; Rønning, M.; Ran, J.; Chen, D. Unraveling Enhanced Activity, Selectivity, and Coke Resistance of Pt–Ni Bimetallic Clusters in Dry Reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Álvarez Moreno, A.; Ramirez-Reina, T.; Ivanova, S.; Roger, A.-C.; Centeno, M.Á.; Odriozola, J.A. Bimetallic Ni–Ru and Ni–Re Catalysts for Dry Reforming of Methane: Understanding the Synergies of the Selected Promoters. Front. Chem. 2021, 9, 694976. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A Review on Bimetallic Nickel-Based Catalysts for CO2 Reforming of Methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Aparicio, P.; Fernandez-Garcia, M.; Guerrero-Ruiz, A.; Rodriguez-Ramos, I. Evaluation of the Role of the Metal–Support Interfacial Centers in the Dry Reforming of Methane on Alumina-Supported Rhodium Catalysts. J. Catal. 2000, 190, 296–308. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Khajenoori, M. Combined Dry Reforming and Partial Oxidation of Methane to Synthesis Gas on Noble Metal Catalysts. Int. J. Hydrogen Energy 2011, 36, 2969–2978. [Google Scholar] [CrossRef]
- Wang, H.Y.; Au, C.T. Carbon Dioxide Reforming of Methane to Syngas over SiO2-Supported Rhodium Catalysts. Appl. Catal. A Gen. 1997, 155, 239–252. [Google Scholar] [CrossRef]
- Wu, H.; Pantaleo, G.; La Parola, V.; Venezia, A.M.; Collard, X.; Aprile, C.; Liotta, L.F. Bi- and Trimetallic Ni Catalysts over Al2O3 and Al2O3-MOx (M=Ce or Mg) Oxides for Methane Dry Reforming: Au and Pt Additive Effects. Appl. Catal. B Environ. 2014, 156–157, 350–361. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, F.; Wang, N.; Hao, S.; Chu, W. Plasma-Treated Bimetallic Ni–Pt Catalysts Derived from Hydrotalcites for the Carbon Dioxide Reforming of Methane. Catal. Lett. 2014, 144, 293–300. [Google Scholar] [CrossRef]
- Osaki, T.; Mori, T. Role of Potassium in Carbon-Free CO2 Reforming of Methane on K-Promoted Ni/Al2O3 Catalysts. J. Catal. 2001, 204, 89–97. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Nanostructured Pt- and Ni-Based Catalysts for CO2-Reforming of Methane. J. Catal. 2010, 270, 136–145. [Google Scholar] [CrossRef]
- Li, L.; Anjum, D.H.; Zhu, H.; Saih, Y.; Laveille, P.V.; D’Souza, L.; Basset, J.-M. Synergetic Effects Leading to Coke-Resistant NiCo Bimetallic Catalysts for Dry Reforming of Methane. ChemCatChem 2015, 7, 427–433. [Google Scholar] [CrossRef]
- Hu, Y.H. Solid-Solution Catalysts for CO2 Reforming of Methane. Catal. Today 2009, 148, 206–211. [Google Scholar] [CrossRef]
- Duan, X.; Pan, J.; Yang, X.; Wan, C.; Lin, X.; Li, D.; Jiang, L. Nickel-cobalt Bimetallic Catalysts Prepared from Hydrotalcite-like Compounds for Dry Reforming of Methane. Int. J. Hydrogen Energy 2022, 47, 24358–24373. [Google Scholar] [CrossRef]
- Torimoto, M.; Sekine, Y. Effects of Alloying for Steam or Dry Reforming of Methane: A Review of Recent Studies. Catal. Sci. Technol. 2022, 12, 3387–3411. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Inaba, M.; Tsunoda, T.; Uchida, K.; Suzuki, K.; Takehira, K.; Hayakawa, T. Rational Design of Mg–Al Mixed Oxide-Supported Bimetallic Catalysts for Dry Reforming of Methane. Appl. Catal. A Gen. 2005, 292, 328–343. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Inaba, M.; Tsunoda, T.; Suzuki, K.; Takehira, K.; Hayakawa, T. Combined Partial Oxidation and Dry Reforming of Methane to Synthesis Gas over Noble Metals Supported on Mg–Al Mixed Oxide. Appl. Catal. A Gen. 2004, 275, 149–155. [Google Scholar] [CrossRef]
- Wysocka, I.; Hupka, J.; Rogala, A. Catalytic Activity of Nickel and Ruthenium–Nickel Catalysts Supported on SiO2, ZrO2, Al2O3, and MgAl2O4 in a Dry Reforming Process. Catalysts 2019, 9, 540. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Assaf, J.M.; Assaf, E.M. Methane Conversion Reactions on Ni Catalysts Promoted with Rh: Influence of Support. Appl. Catal. A Gen. 2011, 400, 156–165. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced Carbon-Resistant Dry Reforming Fe-Ni Catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Batchu, R.; Galvita, V.V.; Poelman, H.; Marin, G.B. Carbon Gasification from Fe–Ni Catalysts after Methane Dry Reforming. Appl. Catal. B Environ. 2016, 185, 42–55. [Google Scholar] [CrossRef]
- Wan, C.; Song, K.; Pan, J.; Huang, M.; Luo, R.; Li, D.; Jiang, L. Ni–Fe/Mg(Al)O Alloy Catalyst for Carbon Dioxide Reforming of Methane: Influence of Reduction Temperature and Ni–Fe Alloying on Coking. Int. J. Hydrogen Energy 2020, 45, 33574–33585. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Koso, S.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Catalytic Performance and Characterization of Ni-Fe Catalysts for the Steam Reforming of Tar from Biomass Pyrolysis to Synthesis Gas. Appl. Catal. A Gen. 2011, 392, 248–255. [Google Scholar] [CrossRef]
- Ray, K.; Sengupta, S.; Deo, G. Reforming and Cracking of CH4 over Al2O3 Supported Ni, Ni-Fe and Ni-Co Catalysts. Fuel Process. Technol. 2017, 156, 195–203. [Google Scholar] [CrossRef]
- Boudjeloud, M.; Boulahouache, A.; Rabia, C.; Salhi, N. La-Doped Supported Ni Catalysts for Steam Reforming of Methane. Int. J. Hydrogen Energy 2019, 44, 9906–9913. [Google Scholar] [CrossRef]
- Ohi, T.; Miyata, T.; Li, D.; Shishido, T.; Kawabata, T.; Sano, T.; Takehira, K. Sustainability of Ni Loaded Mg–Al Mixed Oxide Catalyst in Daily Startup and Shutdown Operations of CH4 Steam Reforming. Appl. Catal. A Gen. 2006, 308, 194–203. [Google Scholar] [CrossRef]
- Benito, P.; Dal Santo, V.; De Grandi, V.; Marelli, M.; Fornasari, G.; Psaro, R.; Vaccari, A. Coprecipitation versus Chemical Vapour Deposition to Prepare Rh/Ni Bimetallic Catalysts. Appl. Catal. B Environ. 2015, 179, 150–159. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Ling, C.; Zhou, T.; Wang, S. Methane Dehydrogenation on Au/Ni Surface Alloys—A First-Principles Study. Catal. Sci. Technol. 2013, 3, 1343–1354. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Qi, Y.; Dam, A.H.; Wang, H.; Zhu, Y.-A.; Holmen, A.; Ran, J.; Chen, D. New Mechanism Insights into Methane Steam Reforming on Pt/Ni from DFT and Experimental Kinetic Study. Fuel 2020, 266, 117143. [Google Scholar] [CrossRef]
- De Coster, V.; Srinath, N.V.; Theofanidis, S.A.; Pirro, L.; Van Alboom, A.; Poelman, H.; Sabbe, M.K.; Marin, G.B.; Galvita, V.V. Looking inside a Ni-Fe/MgAl2O4 Catalyst for Methane Dry Reforming via Mössbauer Spectroscopy and in Situ QXAS. Appl. Catal. B Environ. 2022, 300, 120720. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).