Abstract
A recombinant E. coli, expressing nitrilase from Acidovorax facilis 72W with dual-site expression plasmid pRSFduet (E. coli pRSF-AfNit2), was constructed. It showed higher soluble expression of nitrilase than that in the pET21a plasmid. The recombinant nitrilase can efficiently catalyze the hydrolysis of 3-cyanopyridine to nicotinic acid. The whole cells of E. coli pRSF-AfNit2 were immobilized by using sodium alginate/glutaraldehyde/polyethylene imine as the best immobilized reagents. The immobilized cells showed 95% activity recovery and excellent mechanical strength, with improved thermal stability and pH stability. They also retained 82% of initial activity after nearly two months of storage at 4 °C. A semi-continuous packed-bed bioreactor (sPBR) filled with the immobilized cells was studied for efficient production of nicotinic acid. After optimization, the highest space–time yield of 1576 g/(L·d) was obtained on 0.8 M substrate concentration at 2 mL/min of flow rate. The sPBR was repeatedly operated for 41 batches, keeping 100% conversion in the presence of 30 mM CaCl2. Finally, 95 g of nicotinic acid were obtained at 90% yield after separation and purification. The developed technology has potential application value.
1. Introduction
Nicotinic acid (NA) and nicotinamide are two main forms of vitamin B3, and they are collectively referred to as vitamin PP, and they are also known as anti-sculpturial factors. Named 3-pyridinic acid in the chemistry of NA, commonly known as Nicotinic acid or vitamin B3, is an indispensable nutrient component in the human body [1,2]. NA, usually in the form of coenzymes (coenzyme I and coenzyme II) in the human body, participate in the oxidative reactions in the body, thereby promoting the metabolic activity of the human body, maintaining the normal function of the human organs [3].
Traditional chemical synthetic methods of NA typically require harsh conditions, such as strong alkali, high temperatures, etc., which not only increase the process costs, but also produce certain pollution to the environment. Therefore, biocatalytic methods have been increasingly applied to NA synthesis [4,5,6,7,8,9]. There are two main routes (Scheme 1) for the biocatalytic synthesis of NA. One is the direct hydrolysis of 3-cyanopyridine to NA by nitrilase, releasing the ammonia molecule at the same time. So far, there were many reports on the synthesis of NA by wild nitrile hydrolase from different sources [10,11,12,13]. Mathew, C.D. et al. reported the production of NA from 3-cyanopyridine by resting whole cells of Rhodococcus rhodochrous J1, and the highest yield was achieved, being 172 mg of NA per 1.0 mL of reaction mixture containing 2.89 mg (dry weight) of cells in 26 h according to feed batch addition of substrate [8]. Another route is that the 3-cyanopyridine is hydrolyzed by nitrile hydratase to nicotinamide, which is then hydrolyzed by amidase to NA and ammonia. Cantarella, L. et al. reported a cascade bioconversion of 3-cyanopyridine to NA using a nitrile hydratase–amidase containing resting cells of Microbacterium imperiale CBS 498-74 in an ultrafiltration-membrane reactor, operated in either batch or continuous mode [9]. It is worth mentioning that the Swiss company Lonza commecialized the bioconversion of 3-cyanopyridine to nicotinamide by immobilized cells of Rhodococcus rhodochrous J1 at >10,000 t/year scale [14].
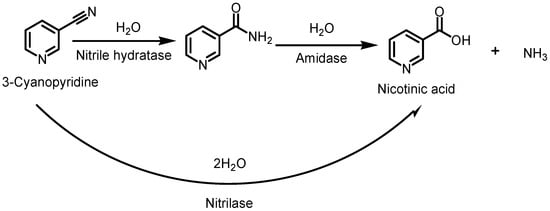
Scheme 1.
Enzymatic synthesis of nicotinic acid via nitrilase or nitrile hydratase–amidase route.
Although the production of NA by recombinant nitrilases were reported, there were several shortcomings, which restricted the practical application of nitrilase, such as low substrate concentration, low catalytic efficiency, and low stability of the enzyme [15,16]. In addition, most of such reaction was carried out in a batch reactor [17,18,19]. There were few reports on using packed-bed bioreactor for the production of NA by recombinant nitrilase. Therefore, we constructed the recombinant nitrile with dual-site expression. In order to achieve the industrial production of niacin, cell immobilization of recombinant bacteria was studied and constructed a semi-continuous packed-bed bioreactor (sPBR).
The vector pRSFDuet-1 contains two multiple cloning sites, and each of them is preceded by a T7 promoter and ribosome binding site. There were several reports on the successful co-expression of two enzymes [20,21,22]. The use of pRSFDuet as recombinant plasmid may improve the expression level of nitrilase in E. coli.
Therefore, the development of a highly efficient bioprocess by high active and stable nitrilase under high substrate concentration in a suitable kind of bioreactor will facilitate the practical application process. The current research was mainly focused on the construction of recombinant E. coli pRSF-AfNit2 capable of two-site expression of nitrilase for the hydrolysis of 3-cyanopyridine to nicotinic acid, cell immobilization, and the development of an efficient process in a semi-continuous packed-bed bioreactor (sPBR). Finally, the preparation of NA by an immobilized whole cell of nitrilase at hectogram scale in sPBR was firstly reported.
2. Results
2.1. Expression of Recombinant Bacteria
After the strains of E. coli pRSF-AfNit2 were constructed, the strains of E. coli pRSF-AfNit2 and E. coli pET21a-AfNit were cultured [23,24]. SDS-PAGE was conducted in order to verify the expression level of nitrile hydrolase from different recombinant strain E. coli. The size of the band was similar to the target band and the theoretical values were consistent. It can be seen, from Figure S1, that the strains of E. coli pRSF-AfNit2 had higher expression of soluble nitrilase. Under the condition of optimal flask level, the cell amount and enzyme activitiy of E. coli pRSF-AfNit2 reached 4.8 gdcw/L and 3218 U/L, respectively (the enzyme activity was determined according to the hydroysis of 3-cyanopyridine at 30 °C and pH 7.0, see Section 4.4 for details).
2.2. Immobilization of Whole Cells of E. coli pRSF-AfNit2 by Different Entrapment Method
This experiment was mainly based on the sodium alginate (SA) gel carrier, selecting other different complexes to further combine with calcium alginate to immobilize the cells [25]. The beads prepared using different immobilization methods were between 3~5 mm in size. Immobilized cells were prepared by different methods, and their enzyme activity and mechanical strength were compared to judge which immobilization method was better (Table 1).

Table 1.
Effect of different cellular immobilization methods on stability of nitrile hydrolase.
It can be seen that, because the polyvinyl alcohol (PVA) solution was too viscous and the solubility was too poor, it was not easy to prepare the polyvinyl alcohol–calcium alginate (PVA-SA) immobilized cells, and other methods were relatively easy to prepare; the mechanical strength test results showed that the calcium alginate, Fe3O4-calcium alginate pellets, and activated carbon–calcium alginate pellets had poor mechanical strength and were easy to swell and break. Calcium alginate–glutaraldehyde (SA–GA) and calcium alginate–glutaraldehyde-polyethyleneimine (SA–GA/PEI) had moderate to high mechnical strength, with both having over 90% activity recovery. Therefore, the best method was the SA–GA/PEI method [25].
2.3. Enzymatic Properties of SA-GA/PEI Immobilized Cells and Free Cells
Temperature has always been an important factor affecting an enzymatic reaction. Too high of a temperature will cause an enzyme to denature and inactivate; if the temperature is too low, the catalytic reaction rate will be slower, and the enzyme activity will not be high. This experiment compared the enzyme activity of free cells and immobilized cells at different temperatures to determine the optimal temperature for free cells and immobilized cells. It can be seen from Figure 1a that the optimum temperature for free cells was 55 °C, and the optimum temperature for immobilized cells was 60 °C. When the temperature was greater than the optimum temperature, as the temperature rised, the enzyme activity of both free cells and immobilized cells all have decreased, but the enzyme activity of immobilized cells was higher than that of free cells at the same temperature. For example, the relative enzyme activity of free cells was only 18% at 70 °C, while the relative enzyme activity of immobilized cells was 50%. This showed that its heat resistance was improved, and the temperature range has been broadened [26].
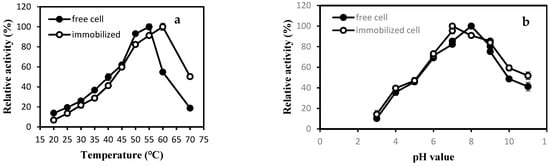
Figure 1.
Effect of reaction temperature (a) and pH (b) on the activity of free and SA–GA/PEI immobilized cells.
In the enzymatic reaction, only when the catalyst exists in an appropriate pH buffer system can the enzyme achieve higher catalytic activity. As shown in Figure 1b, the optimal pH for free cells was 8.0, while it was 7.0 for immobilized cells. The activity of immoilized cells decreased less than that of free cells with the increase in pH value under alkaline conditions, which indicated that immobilized cells had a higher pH tolerance than free cells in the alkaline range [26].
2.4. Storage Stability of Immobilized Cells
In practical industrial applications, an important evaluation criterion for the application value of a catalyst was its storage stability. In this experiment, the storage stability of free cells and immobilized cells was studied, and the results were shown in Figure 2. The residual enzyme activity of free cells was only about 15% after being stored in a refrigerator at 4 °C after 55 days. However, it was still more than 80% for immobilized cells after nearly two months, which showed a much better storage stability of immobilized cells than free cells [27].
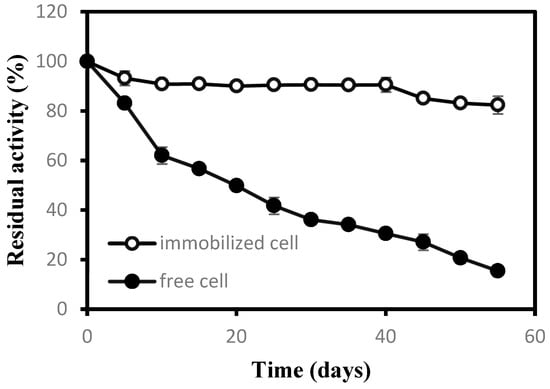
Figure 2.
Storage stability of free and immobilized cells.
2.5. Research on Semi-Continuous Synthesis of NA in a Packed-Bed Reactor with Immobilized Cells
The packed-bed bioreactor can also be called a fixed-bed bioreactor, because the biocatalyst in the reactor was always in a static state, and the reaction happened when substrate solution flowed through the biocatalyst [28]. Since different methods of immobilizing cells produced different cell shapes, the catalyst in the reactor can be filled in different forms. When there existed product inhibition, the use of this type of reactor had certain advantages. Since the catalyst in the reactor is always static, the fluid flow pattern can be regarded as a plug flow. Compared with the continuous stirred tank bioreactor (CSTR), the shear force was smaller, which facilitated keeping the stability of the biocatalyst. Up to now, only scarce research was focused on the biosynthesis of NA in packed-bed bioreactors. In the current research, we studied the effect of flow rate of substrate solution and substrate concentration on the efficiency of catalytic reaction.
It can be seen, from Figure 3a, that the reaction rate increased with the increase in the flow rate when it was increased from 0.5 to 2.0 mL/min. Further increasing the flow rate from 2.0 to 3.0 mL/min, it was maintained in a stable state, which means the influence of external diffusion could be negligible. Therefore, the optimal flow rate was 2.0 mL/min.
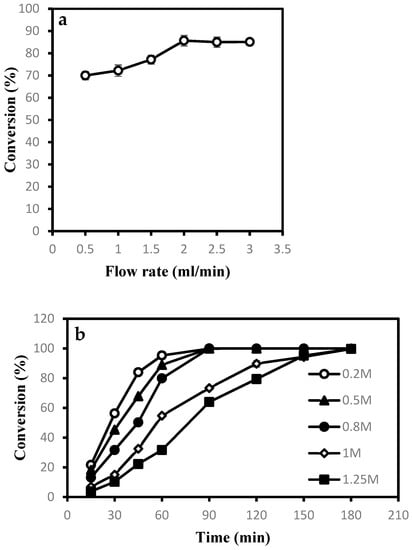
Figure 3.
Study on different parameters on the conversion of NA in semi-continuous packed-bed bioreactor. (a) Flow rate (after operation for 90 min); (b) substrate concentration.
After determining the optimal flow rate, the substrate concentration was investigated. It can be seen from Figure 3b that as the substrate concentration was increased, and the conversion rate was decreased at the same time. When the substrate concentration was greater than 0.8 M, the time for full conversion of the substrate was gradually increased. The space–time yield at different substrate concentrations was shown in Table 2. When the substrate concentration was 0.8 M, the space–time yield reached the highest value of 1576 g/(L·d). Therefore, the optimal substrate concentration was 0.8 M.

Table 2.
The space–time yield at different substrate concentrations.
2.6. Reusability of Immobilized Cells in the Bioreactor
According to the optimal substrate flow rate and substrate concentration, repeated batch experiments were performed in the reactor to verify the operational stability of the immobilized cells. The effect of calcium ions on the stability of immobilized cells was also investigated (Figure 4). When no calcium ions were added to the substrate solution, the immobilized cells began to swell and broke after only five batches, resulting in a sharp decrease in the conversion. However, the immobilized cells with CaCl2 (30 mM) in solution can be reused for at least 41 batches, keeping 100% of conversion. After 41 batches, the immobilized cells started to swell and partially break, leading to a decrease in conversion.
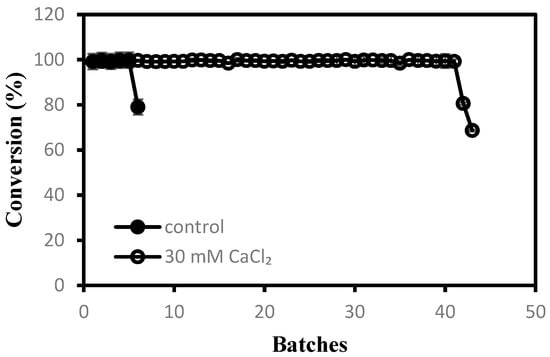
Figure 4.
Repeated use of immobilized cells in sPBR for the production of nicotinic acid.
When the reaction finished, the immobilized cells in the bioreactor were washed. The washing liquid was combined with all the product solutions for each batch, and NA was furthur seperated and purified. Finally, 95 g of pure NA were obtained from 43 batches, corresponding to 90% of isolated yield [28]. Since the immobilized cells packed in sPBR contained around 0.6 g dry cell weight of cells, it can be estimated that preparing 1 ton of NA requires an immobilized biocatalyst containing only 6 kg of dry weight cells.
3. Discussion
In this paper, by molecular cloning, the nitrilase gene derived from Acidovorax facilis was ligated to a vector with two independent multiple cloning sites for two-site expression, and the nitrilase-producing recombinant strain E. coli pRSF-AfNit2 showed higher soluble expression of nitrilase than that of E. coli pET21a-AfNit2.
In order to further improve the stability of nitrilase, the whole cell immobilization of nitrilase was systematically studied, and the immobilized cells were used to catalyze the semi-continuous hydrolysis reaction of 3-cyanopyridine in a packed bed column bioreactor. The results of the study were the following: the best immobilization method was the calcium alginate–glutaraldehyde–polyethyleneimine cross-linking method, and the enzyme activity of immobilized cells was 95% of free cells, and the residual enzyme activity of immobilized cells was still more than 80% after nearly two months storage at 4 °C. In the semi-continuous packed-bed column reactor, it was found that both the substrate solution flow rate and the substrate concentration affected the catalytic efficiency. Regarding this, the catalytic efficiency reached its highest when the substrate flow rate was 2.0 mL/min and the substrate concentration was 0.8 M. The space–time yield of the reactor during stable operation can reach 1576 g/(L·d). When the substrate solution contains 30 mM CaCl2, the immobilized cells can be reused for at least 41 batches, and the conversion was still kept at 100%. Finally, 95 g of NA were obtained in 90% isolated yield through seperation and purification. It can be estimated that producing 1 ton of NA would require immobilized biocatalyst containing only 6 kg of dry weight cells, which fufill the needs of pactical applications.
4. Materials and Methods
4.1. Chemicals, Plasmids and Strains
3-Cyanopridine, nicotinic acid, protein marker, and soluble starch were from the Aladdin reagent company (Shanghai, China); NaCl and yeast extract powder were from sinopharm chemical reagent Co., Ltd. (Shanghai, China); anhydrous ethanol, CaCl2, K2HPO4, KH2PO4 and MgSO4·7H2O were purchased from Shanghai Taitan Technology Co., Ltd. (Shanghai, China); kanamycin was from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China).
Plasmids pET-21a(+) (Novagen, Merck, Darmstadt, Germany) and pRSFDuet-1 (Novagen, Merck, Darmstadt, Germany) were preserved in the Biocatalysis and Biopharmaceutical Laboratory (Shanghai Institute of Technology, Shanghai, China). Restriction endonucleases (NdeI, XhoI, PstI, NcoI) were purchased from Takara Biotechnology (Dalian, China). E. coli BL21 (DE3) and E. coli DH5α were purchased from Friction Biological Engineering (Shanghai) Co., Ltd. (Shanghai, China). The recombinant plasmid pET21a-AfNit was constructed in our previous work [23].
4.2. Construction of Recombinant E. coli Strains Expressing Nitrilase
The nitrilase gene (FJ851547) [29] from Acidovorax facilis 72W (AfNit) was synthesized by Shanghai Generay Biotech Co., Ltd. (Shanghai, China). AfNit was ligated into pET21a via NdeI/XhoI restriction sites to generate expression plasmid pET21a-AfNit. The pET21a-AfNit was transformed into E. coli BL21 (DE3) to construct the recombinant strain E. coli pET21a-AfNit. For the construction of E. coli pRSF-AfNit2, the AfNit gene was ligated into the first multi clone site (MCS1) via PstI/NcoI restriction sites, and the second MCS (MCS2) was ligated via the NdeI/XhoI restriction sites to generate the expression plasmid pRSF-AfNit2. The pRSF-AfNit2 was transformed into E. coli BL21 (DE3) to construct the recombinant strain E. coli pRSF-AfNit2. The construction of recombinant pRSFDuet-1 plasmids and their transformation into E. coli were performed according to standard protocols.
4.3. Cultivation of Recombinant E. coli Expressing Nitrilase
A pure colony (E. coli pRSF-AfNit2 or E. coli pET21a-AfNit) was picked and cultivated overnight in the 50 mL LB medium with 50 μg/mL kanamycin at 37 °C and 200 rpm. Then, the 2 mL culture was incubated in 50 mL of culture medium (Soluble starch: 20 g/L, yeast extract powder: 15 g/L, NaCl: 5 g/L, K2HPO4: 4 g/L, MgSO4·7H2O 1 g/L) with 50 μg/mL kanamycin, and the cells were cultured at 30 °C and 200 rpm. When the OD600 value reached 0.4 to 0.6, IPTG (0.4 mM) was added to the culture to induce enzyme expression at 30 °C for 4 h [23]. Harvested cells were suspended in 50 mM PBS buffer (pH 7.0) and then ultrasonicated on ice. Centrifugation was used to remove cell debris for 10 min at 12,000× g and 4 °C. The protein molecular mass was evaluated by SDS-PAGE [30,31].
4.4. Enzyme Assay
To assay the enzyme activity of AfNit, 0.5 mg of dry weight resting cells were suspended in 475 µL of phosphate buffer (pH 7.0, 100 mM). The cell suspension was preheated for 5 min on thermo-mixer compact at 30 °C and 1000 rpm, then 25 µL of 3-cyanopyridine solution (1 M) in ethanol were added to the cell suspension, corresponding to 50 mM of final substrate concentration. The reaction was performed at 30 °C for 15 min, and the reaction solution was immediately added to 100 µL of HCl (2 M) to terminate the reaction. Then, 1900 µL of anhydrous ethanol was added to the reaction mixture for dilution. After centrifugation at 12,000× g for 5 min, the supernatant was filtered with organic filter membrane (25 mm × 0.45 µm, sinopharm chemical reagent Co., Ltd., Shanghai, China.), and the filtrate was assessed by HPLC. One unit of the enzyme activity was defined as the amount of enzyme releasing 1 µmol of NA per minute under the described conditions [25].
4.5. Analytical Methods
The NA and 3-cyanopridine were quantitatively determined by HPLC (Shimadzu LC-20AT, Kyoto, Japan), equipped with UV detector and a reversed phase column (Diamonsil® Plus C18 5 μm 250 × 4.6 mm, Cat. No.: 99409, Dikma Co., Shanghai, China), and monitored under UV 217 nm at a column temperature of 25 °C. The mobile phase was acetonitrile: phosphate buffer (10 mM sodium dihydrogen phosphate, pH 2.5) = 15:85 (v:v). The sample was eluted at a flow rate of 1.0 mL/min. The retention time of the product and substrate are 3.2 min and 6.8 min, respectively.
The molar response factor of NA to 3-caynopyridine was 0.5345. It was determined by the analysis of equal molar amounts of NA and 3-cyanopyridine standards with HPLC, and the molar response factor was obtained by mapping the peak area of NA and 3-caynopyridine.
4.6. Preparation of Immobilized Cells and Enzyme Assay
General procedue for the immobilization of E. coli pRSF-AfNit2 whole cells (exampled as SA-GA/PEI): the E. coli fermentation broth was washed twice with 0.1 M phosphate buffer (pH 7.0). It was resuspended in certain amount of 0.8% NaCl solutions to 10 g/L. The cell suspension was then mixed with equivalent volume of alginate solution (30 g/L). The mixture was added to 2% (w/v) CaCl2 solution with a syringe or a peristaltic pump, and the curing time was 2 h, then a small ball having an approximately diameter of 3 mm can be obtained, and it is finally washed with deionized water. The prepared alginate immobilized pellets were crosslinked in 2 wt% glutaraldehyde aqueous solution for 1 h. The additional glutaraldehyde was washed away, and 3% of the polyethyleneimine solution was added for 1 h, and the surface residual polyethyleneimine was washed away. After curing for 12 h, it was finally washed three times with deionized water, and the enzyme activity of the immobilized cells was measured [25,26].
Immobilized cells containing 0.5 mg dry weight of free cells were placed in 475 µL of Tris-HCl (0.1 M, pH 7.0) buffer solution, and they were preheated in a constant temperature mixing instrument at 30 °C and 1000 rpm for 5 min, and then 25 µL of 3-cyanopyridine solution (1 M) in ethanol was added to the mixture (final substrate concentration: 50 mM). After reaction for 15 min, the product concentration was determined in the sample by HPLC, as described in Section 4.4, and the enzyme activity was calculated from the degree of conversion. One unit of the immobilized enzyme activity was defined as the amount of enzyme releasing 1 µmol of NA per minute under the described conditions.
4.7. Determination of Mechanical Strength of Immobilized Cells
The immobilized cells studied in this experiment were spherical gel particles. Therefore, when measuring the mechanical strength of immobilized cells, 100 balls of immobilized cells were directly counted and placed in Tris-HCl (0.1 M, pH 7.0) buffer at 30 °C and 180 rpm while shaking, and the gel breakage was observed at different times.
4.8. Effect of Different Reaction Temperature and pH on the Activity of Free and Immobilized Cells
To determine the effect of temperature, the activity of free cells (1 gdcw/L) or immobilized cells (containing 1 gdcw/L free cells) was assayed in Tris-HCl buffers (100 mM, pH 7.0) at various temperatures (20–70 °C). The relative activity referred to the ratio of the enzyme activity at different temperatures to their respective highest activity (free cell: 3076 U/gdcw, immobilized cell: 2462 U/gdcw). Experiments were independently performed in triplicate.
To determine the effect of pH value, the activity of free cells (1 gdcw/L) or immobilized cells (containing 1 gdcw/L free cells) was assayed at 30 °C in various buffers over a pH range of 3.0~11.0 (pH 3.0~6.0, citrate–citric acid buffer; pH 6.0~7.0, PBS buffer; pH 7.0~9.0, Tris-HCl buffer; pH 9.0~11.0, Gly-NaOH buffer). The relative activity was expressed as a percentage of their respective maximum activity of free or immobilized cells. Experiments were independently performed in triplicate.
4.9. Semi-Continuous Packed-Bed Bioreactor
The column bioreactor used in this experiment (Figure 5) was made by Chengxin Glass Technology Co., Ltd. (Shanghai, China). It was a jacketed glass column reactor, and the temperature of the bioreactor was controlled by an external circulating water bath. The height of column was 100 mm, and the inner diameter was 17 mm. The effective volume of the column was approximately 20 mL. There was a round sand core at the bottom of column to prevent the beads from leaking. The wet weight of immobilized cells packed into the bioreactor column was 7.8 g (~10 mL volume, containing 600 mg dry weight of cells). When the reaction begins, the substrate solution entered from the bottom at a constant flow rate, made contact with the immobilized cells, and reacted. The reaction solution flowed out from the top of the column and was pumped back into the substrate storage tank.
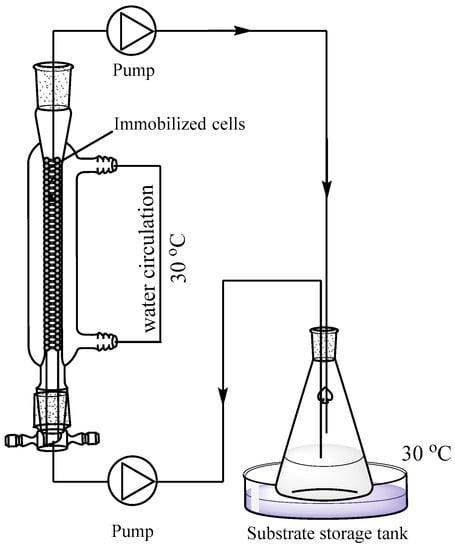
Figure 5.
Enzymatic synthesis of NA by immobilized cells in semi-continuous packed-bed bioreactor.
To investigate the effect of flow rate, 25 mL of 0.8 M substrate solution in (Tris-HCl buffer, 0.1 M, pH 7.0) was pumped into the column with different flow rate (from 0.5 mL/min to 3.0 mL/min). Substrate conversion was determined at different times to determine the best flow rate.
To investigate the effect of substrate concentration, 25 mL of different concentrations of substrate (0.2 M, 0.5 M, 0.8 M, 1.0 M, 1.25 M) solutions were pumped into the column at 2.0 mL/min, and the conversion of the substrate was monitored at different time to determine the optimal substrate concentration.
To study the operational stability of immobilized cells in sPBR, 25 mL of 0.8 M substrate solution (Tris-HCl buffer, 0.1 M, pH 7.0, with or without 30 mM CaCl2) were pumped into the column with a flow rate of 2 mL/min, and the conversion of the substrate was monitored by HPLC. When the reaction for each batch finished, the reaction solution was stored in a collection tank, and 25 mL of fresh substrate solution (0.8 M) was added into substrate storage tank to operate a new batch [28]. The batch reaction was repeated until the conversion at the same time decreased significantly. When the batch reaction finished, the immobilized cells were washed with 0.1 M Tris-HCl buffer (25 mL × 3, pH 7.0). The collected washing liquid was centrifuged (10,000 rpm, 5 min) to obtain the clear supernatant. The supernatant and the accumulated reaction solutions for each batch were combined together for the preparation of NA.
4.10. Preparation of NA
The combined solutions mentioned in Section 4.9 were heated at 70 °C for 30 min, and then they were centrifuged for 30 min at 10,000 rpm. The precipitate (mainly broken immobilized cells) was washed with normal saline (20 mL × 2) to dissolve NA absorbed in the precipitate. Finally, the supernatant and washing solution were collected, and concentrated hydrochloric acid was dropped into the collected solution at pH 3.7 (NA isoelectric points). After all the crystals were precipitated, they were heated to 70 °C, and the obtained solution was filtered while it was hot by a Büchner funnel to remove insoluble impurities. The filtrate was placed at room temperature for natural cooling for 30 min, and then it was placed in a refrigerator at 4 °C for low temperature recrystallization. The crystals were obtained by air pump filtration with a Büchner funnel. The filtrated liquid was concentrated by rotary evaporation, and the above operation of recrystallization was repeated to further obtain more NA and to increase the total yield of NA [32].
The NA product (see Figure S2a for its morphology) was analyzed qualitatively and quantitatively. Its melting point was determined by melting point analyzer (mp. 238.7–238.9 °C, ref. 236–239 °C), and its purity was analyzed by HPLC (Figure S2b). The chemical structure was determined by nuclear magnetic resonance (NMR) 1H NMR (501 MHz, DMSO-d6): δ 13.49 (s, 1H), 9.27–9.06 (m, 1H), 8.83 (dd, J = 4.8, 1.7 Hz, 1H), 8.32 (dt, J = 7.9, 2.0 Hz, 1H), 7.58 (ddd, J = 7.9, 4.8, 0.9 Hz, 1H) (Figure S2c) and Fourier infrared spectroscopy (Figure S2d).
5. Conclusions
A highly efficient biocatalytic process for NA production from 3-cyanopyridine by immobilized whole cells of recombinant strain E. coli pRSF-AfNit2 in sPBR was developed. The immobilized cells prepared by SA-GA/PEI method showed excellent activity recovery, improved stability, and the highest mechanical strength among the tested ones. The immobilized cells were used to catalyze the hydrolysis reaction of 3-cyanopyridine in sPBR. After optimization, the space–time yield of the reaction reached 1576 g/(L·d) during stable operation. The sPBR was repeatedly operated for 41 batches, keeping 100% conversion in the presence of 30 mM CaCl2, resulting in 95 g of NA (90% yield). It can be estimated that producing 1 ton of NA would require an immobilized biocatalyst containing only 6 kg of dry weight cells, which fufill the needs of practical applications. In summary, the developed method exhibits good application potential for the biosynthesis of NA.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13020371/s1, Figure S1: SDS-PAGE of recombinant AfNit from E. coli pET21a-AfNit and E. coli pRSF-AfNit2; Figure S2: Characterization spectrum and picture of prepared nicotinic acid.
Author Contributions
Conceptualization, Y.X.; methodology, B.-D.M. and X.-J.L.; validation, X.-J.L. and Y.X.; formal analysis, X.-J.L.; data curation, X.-J.L. and X.-M.W.; writing—original draft preparation, X.-J.L.; writing—review and editing, X.-M.W., B.-D.M. and Y.X.; funding acquisition, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of Leading Talents in Shandong Taishan Industry (Grant No. LJNY202019).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chuck, R. Technology development in nicotinate production. Appl. Catal. A Gen. 2005, 280, 75–82. [Google Scholar]
- Chuck, R. A catalytic green process for the production of NA. Chim. Int. J. Chem. 2000, 54, 508–513. [Google Scholar]
- Velankar, H.; Clarke, K.G.; Preez, R.; Cowan, D.A.; Burton, S.G. Developments in nitrile and amide biotransformation processes. Trends Biotechnol. 2010, 28, 561–569. [Google Scholar] [PubMed]
- Gong, J.S.; Lu, Z.M.; Li, H.; Shi, J.S.; Zhou, Z.M.; Xu, Z.H. Nitrilases in nitrile biocatalysis: Recent progress and forthcoming research. Microb. Cell Factories 2012, 11, 142–144. [Google Scholar]
- Banerjee, A.; Sharma, R.; Banerjee, U. The nitrile-degrading enzymes: Current status and future prospects. Appl. Microbiol. Biotechnol. 2002, 60, 33–44. [Google Scholar]
- Chen, J.; Zheng, R.C.; Zheng, Y.G.; Shen, Y.C. Microbial transformation of nitriles to high-value acids or amides. Adv. Biochem. Eng.-Biotechnol. 2009, 113, 33–77. [Google Scholar] [PubMed]
- Park, W.J.; Kriechbaumer, V.; Müller, A.; Piotrowski, M.; Meeley, R.B.; Gierl, A.; Glawischnig, E. The nitrilase ZmNIT2 converts indole-3-acetonitrile to indole-3-acetic acid. Plant Physiol. 2003, 133, 794–802. [Google Scholar] [CrossRef]
- Mathew, C.D.; Nagasawa, T.; Kobayashi, M.; Yamada, H. Nitrilase-Catalyzed Production of Nicotinic Acid from 3-Cyanopyridine in Rhodococcus rhodochrous J1. Appl. Environ. Microbiol. 1988, 54, 1030–1032. [Google Scholar]
- Cantarella, L.; Gallifuoco, A.; Malandra, A.; Martínková, L.; Pasquarelli, F.; Spera, A.; Cantarella, M. Application of continuous stirred membrane reactor to 3-cyanopyridine bioconversion using the nitrile hydratase–amidase cascade system of Microbacterium imperiale CBS 498-74. Enzym. Microb. Technol. 2010, 47, 64–70. [Google Scholar]
- Straathof, A.J.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2003, 13, 548–556. [Google Scholar]
- Zhou, Z.M.; Hashimoto, Y.; Kobayashi, M. Nitrile degradation by rhodococcus: Useful microbial metabolism for industrial productiions. Actinomycetologica 2005, 19, 18–26. [Google Scholar] [CrossRef]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Enzymatic mechanism and biochemistry for cyanide degradation: A review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar] [PubMed]
- Fischer-Colbrie, G.; Matama, T.; Heumann, S.; Martinkova, L.; Cavaco Paulo, A.; Guebitz, G. Surface hydrolysis of polyacrylonitrile with nitrile hydrolysing enzymes from Micrococcus luteus BST20. J. Biotechnol. 2007, 129, 62–68. [Google Scholar]
- Robins, K.; Gordon, J. Whole Cell Production of Fine Chemicals and Intermediates. In Biocatalysis for Green Chemistry and Chemical Process Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 299–326. [Google Scholar]
- Pace, H.C.; Brenner, C. The nitrilase superfamily: Classification, structure and function. Genome Biol. 2001, 2, reviews0001.1. [Google Scholar] [CrossRef]
- Desantis, G.; Zhu, Z.; Greenberg, W.A.; Wong, K.; Chaplin, J.; Hanson, S.R.; Farwell, B.; Nicholson, L.W.; Rand, C.L.; Weiner, D.P.; et al. An enzyme library approach to biocatalysis: Development of nitrilases for enantioselective production of carboxylic acid derivatives. J. Am. Chem. Soc. 2002, 124, 9024–9025. [Google Scholar] [CrossRef]
- Obanijesu, E.O.; Bello, O.O.; Osinowo, F.A.O.; Macaulay, S.R.A. Development of a packed-bed reactor for the recovery of metals from industrial wastewaters. Int. J. Environ. Pollut. 2004, 22, 701–702. [Google Scholar]
- Jorge, R.M.F.; Livingston, A.G. Microbial dynamics in a continuous stirred tank bioreactor exposed to alternating organic compounds. Biotechnol. Bioeng. 2000, 69, 409–417. [Google Scholar] [CrossRef]
- England, E.; Fitch, M.W.; Mormile, M.; Roberts, M. Toluene removal in membrane bioreactors under recirculating and non-recirculating liquid conditions. Clean Technol. Environ. Policy 2005, 7, 259–269. [Google Scholar]
- Pham, S.Q.; Gao, P.; Li, Z. Engineering of recombinant E. coli cells coexpressing P450pyrTM monooxygenase and glucose dehydrogenase for highly region and stereoselective hydroxylation of alicycles with cofactor recycling. Biotechnol. Bioeng. 2013, 110, 363–373. [Google Scholar]
- Chen, Y.T.; Ma, B.D.; Cao, S.S.; Wu, X.M.; Xu, Y. Efficient synthesis of Ibrutinib chiral intermediate in high space-time yield by recombinant E. coli coexpressing alcohol dehydrogenase and glucose dehydrogenase. RSC Adv. 2019, 9, 2325–2331. [Google Scholar] [CrossRef]
- Wu, J.; Ma, B.D.; Xu, Y. One–pot synthesis of β–alanine from maleic acid via three–enzyme cascade biotransformation. Catalysts 2023, 13, 267. [Google Scholar]
- Qiu, J.W.; Zheng, X.S.; Ma, B.D.; Xu, Y. Efficient Improvement the Production of Recombinant Nitrilase by Optimizing Culture Conditions Using Response Surface Methodology (RSM). J. Comput. Theor. Nanosci. 2016, 13, 2269–2276. [Google Scholar] [CrossRef]
- Li, H.; Dong, W.L.; Zhang, Y.; Liu, K.; Zhang, W.M.; Zhang, M.; Ma, J.F.; Jiang, M. Enhanced catalytic efficiency of nitrilase from Acidovorax facilis 72W and application in bioconversion of 3-cyanopyridine to nicotinic acid. J. Mol. Catal. B Enzym. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- He, L.; Chen, Y.; Wu, X.M.; Xu, Y. Magnetic Fe3O4/Alginate Cell Beads: Application in Enzymatic Synthesis of Pharmaceutical Intermediate. J. Comput. Theor. Nanosci. 2016, 13, 2264–2268. [Google Scholar]
- Kaul, P.; Banerjee, A.; Banerjee, U.C. Stereoselective Nitrile Hydrolysis by Immobilized Whole-Cell Biocatalyst. Biomacromolecules 2006, 7, 1536–1541. [Google Scholar]
- Zhong, X.; Yang, S.M.; Su, X.Y.; Shen, X.X.; Zhao, W.; Chan, Z. Production of Cyanocarboxylic Acid by Acidovorax facilis 72W Nitrilase Displayed on the Spore Surface of Bacillus subtilis. J. Microbiol. Biotechnol. 2019, 29, 749–757. [Google Scholar]
- Liu, Q.G.; Zhao, N.; Zou, Y.N.; Ying, H.J.; Liu, D.; Chen, Y. Feasibility study on long-term continuous ethanol production from Cassava supernatant by immobilized yeast cells in packed bed reactor. J. Microbiol. Biotechnol. 2020, 30, 1227–1234. [Google Scholar] [CrossRef]
- Chauhan, S.; Wu, S.; Blumerman, S.; Fallon, R.D.; Gavagan, J.E.; Dicosimo, R.; Payne, M.S. Purification, cloning, sequencing and over-expression in Escherichia coli of a regioselective aliphatic nitrilase from Acidovorax facilis 72W. Appl. Microbiol. Biotechnol. 2003, 61, 118–122. [Google Scholar] [CrossRef]
- Gong, J.S.; Dong, T.T.; Gu, B.C.; Li, H.; Dou, W.F.; Lu, Z.M.; Zhou, Z.M.; Shi, J.S.; Xu, Z.H. Semi-rational engineering accelerates the laboratory evolution of nitrilase catalytic efficiency for nicotinic acid biosynthesis. ChemCatChem 2017, 9, 3395–3401. [Google Scholar]
- Gong, J.S.; Li, H.; Lu, Z.M.; Zhang, X.J.; Zhang, Q.; Yu, J.H.; Zhou, Z.M.; Shi, J.S.; Xu, Z.H. Engineering of a fungal nitrilase for improving catalytic activity and reducing by-product formation in the absence of structural information. Catal. Sci. Technol. 2016, 6, 4134–4141. [Google Scholar] [CrossRef]
- Roy, R.B.; Merten, J.J. Evaluation of urea-acid system as medium of extraction for the B-group vitamins. Part II. Simplified semi-automated chemical analysis for niacin and niacinamide in cereal products. J. Assoc. Off. Anal. Chem. 1983, 66, 291–296. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).