Partially Reduced Ni-NiO-TiO2 Photocatalysts for Hydrogen Production from Methanol–Water Solution
Abstract
1. Introduction
2. Results and Discussion
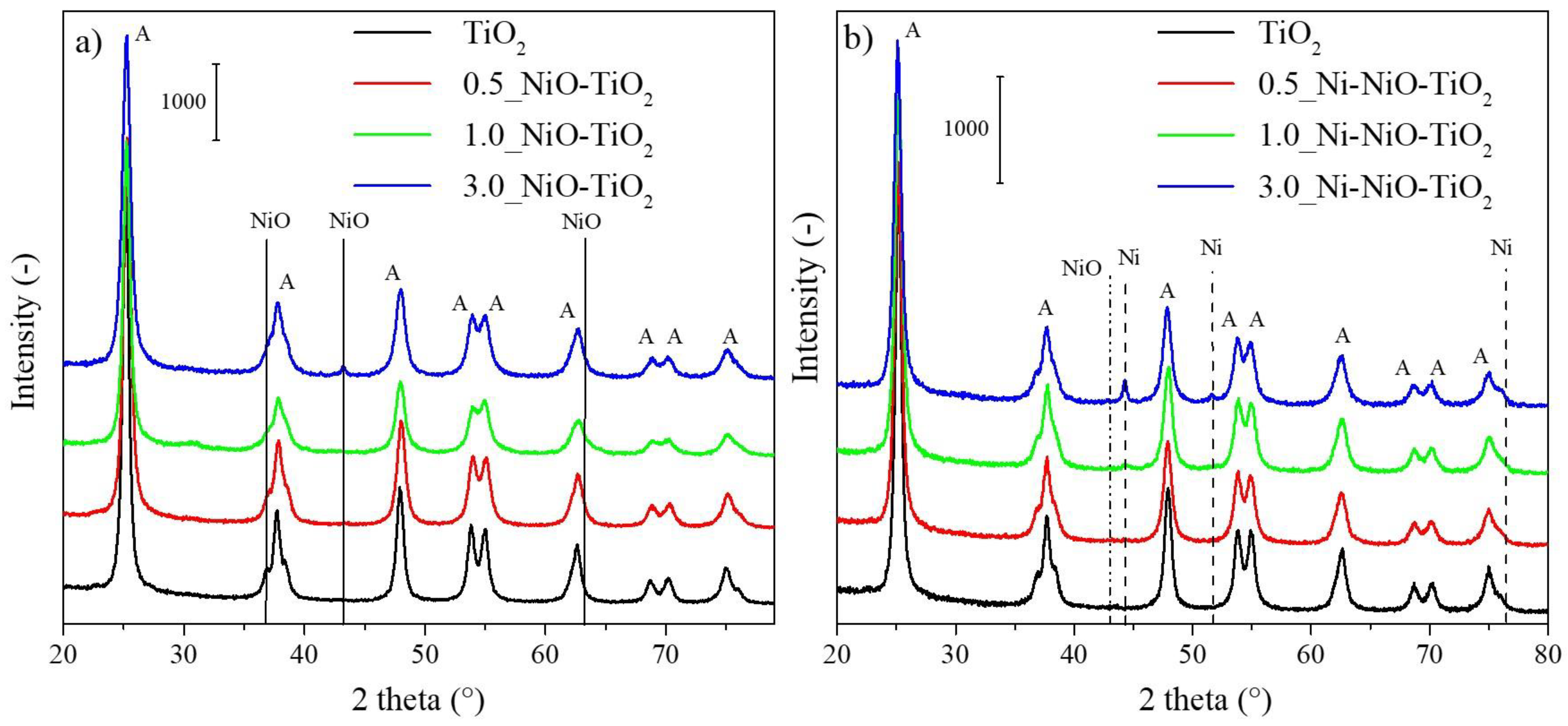
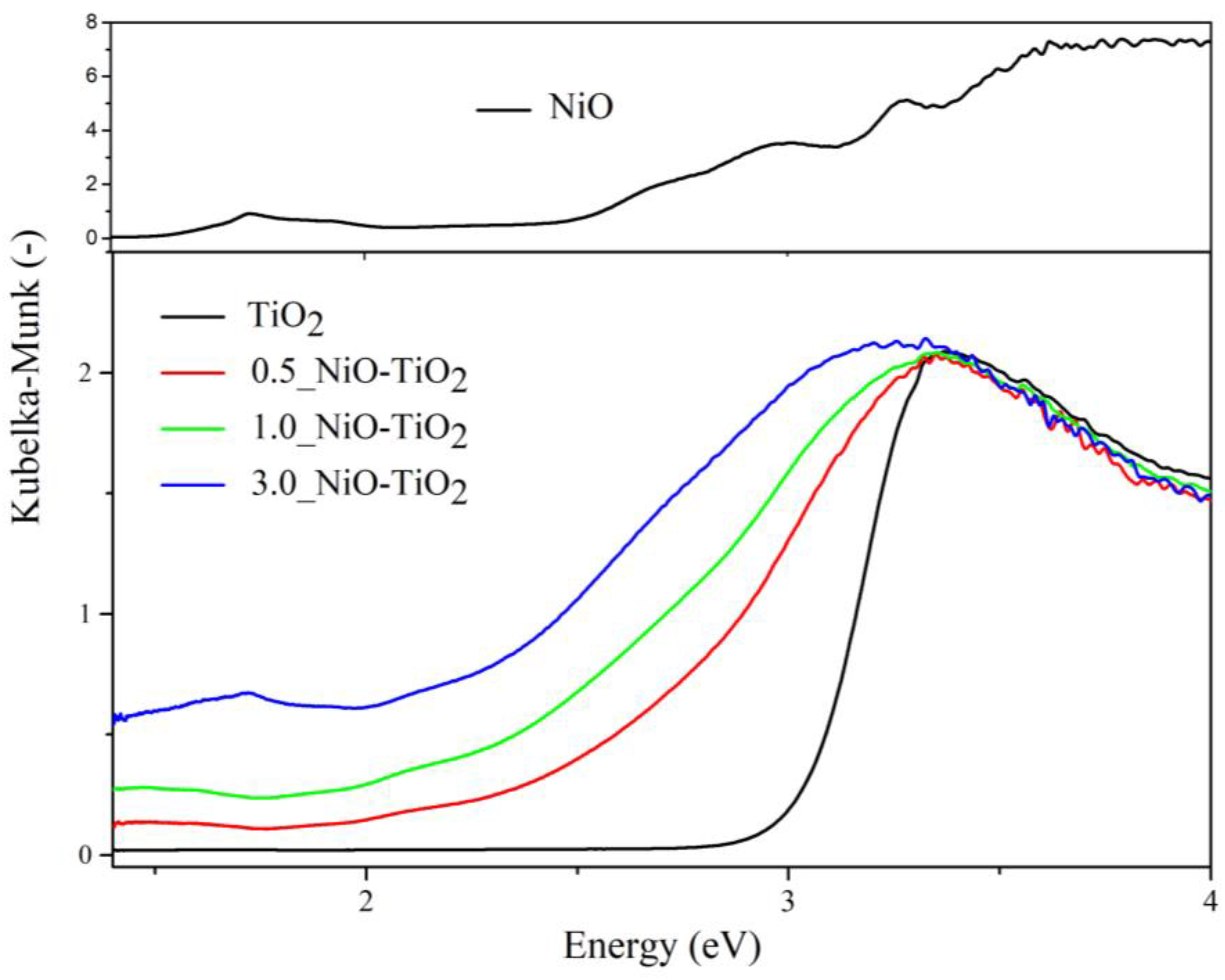
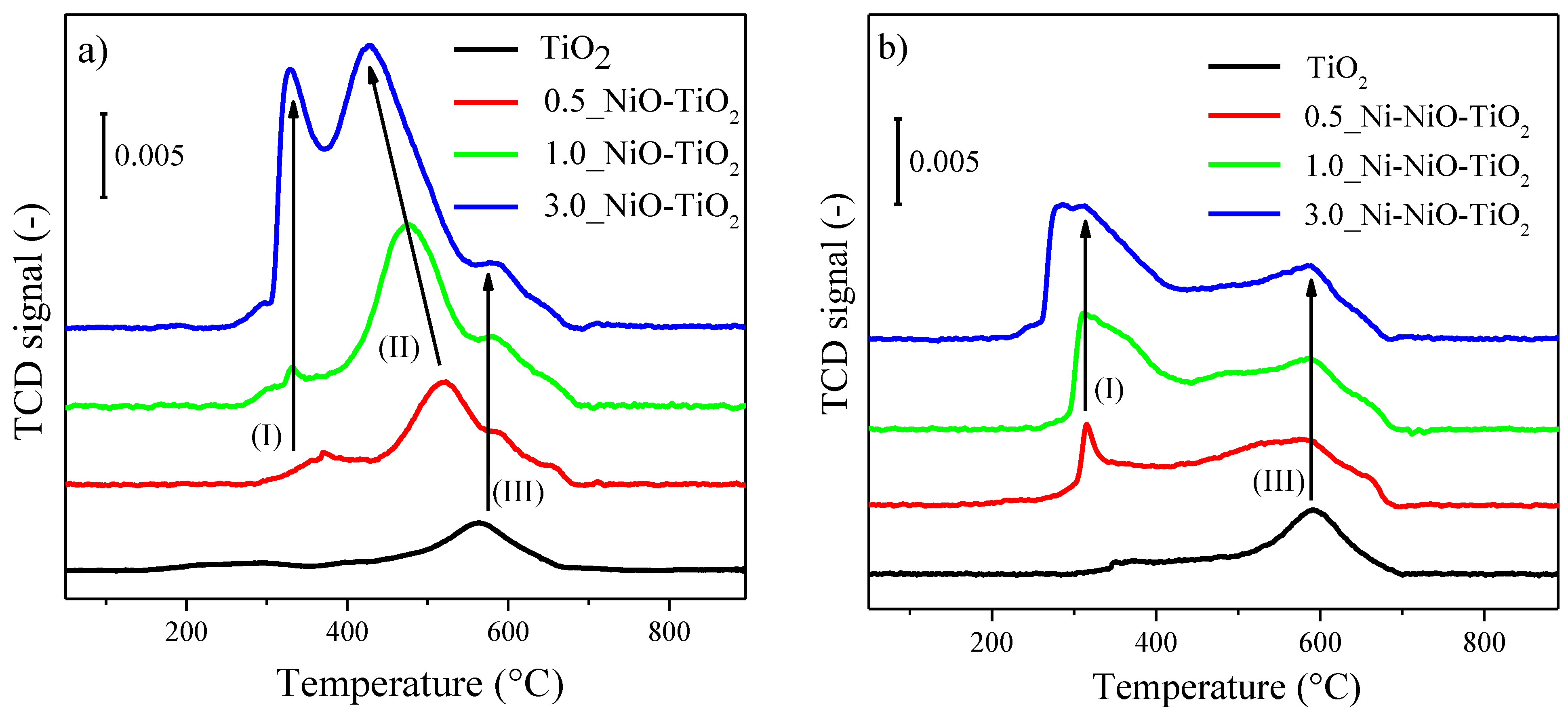
2.1. Photocatalysts’ Characterization
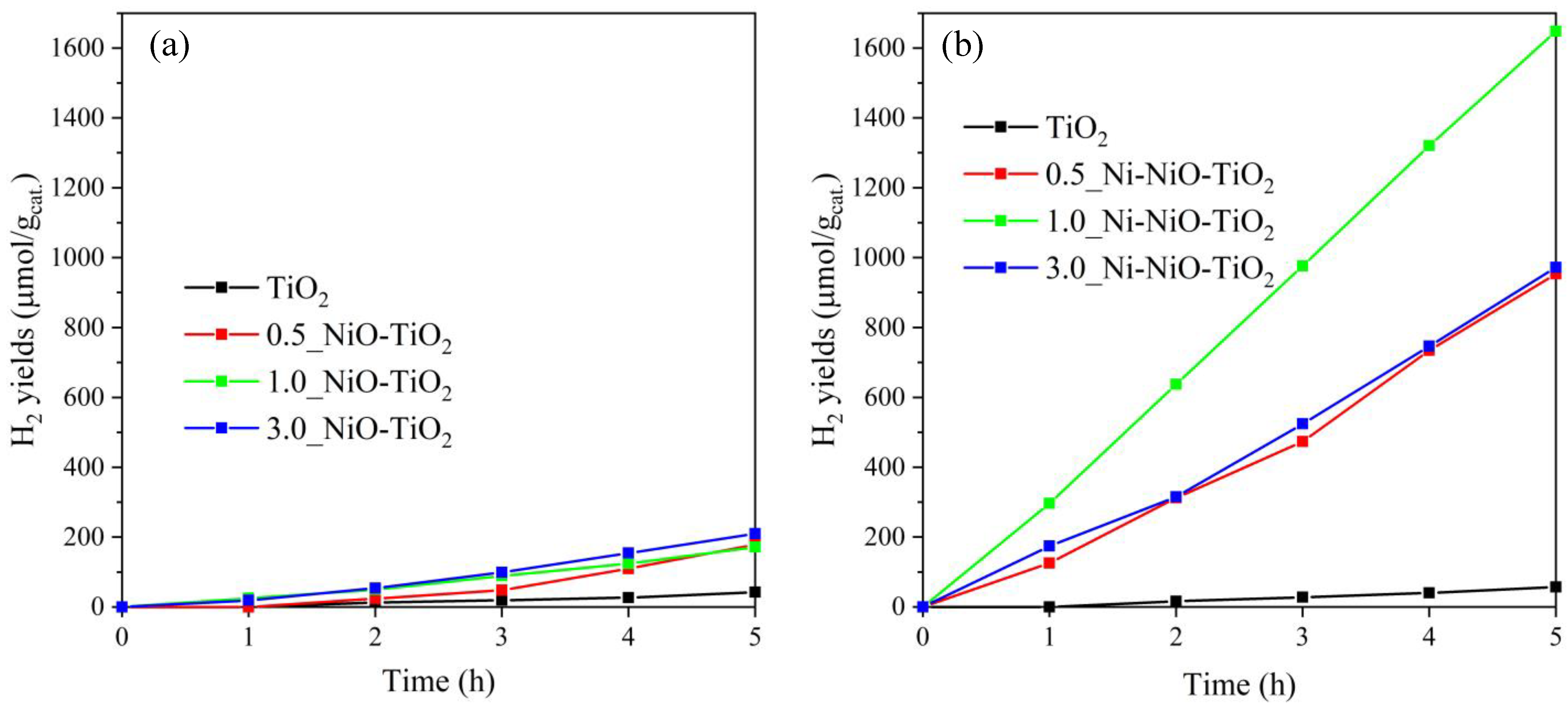
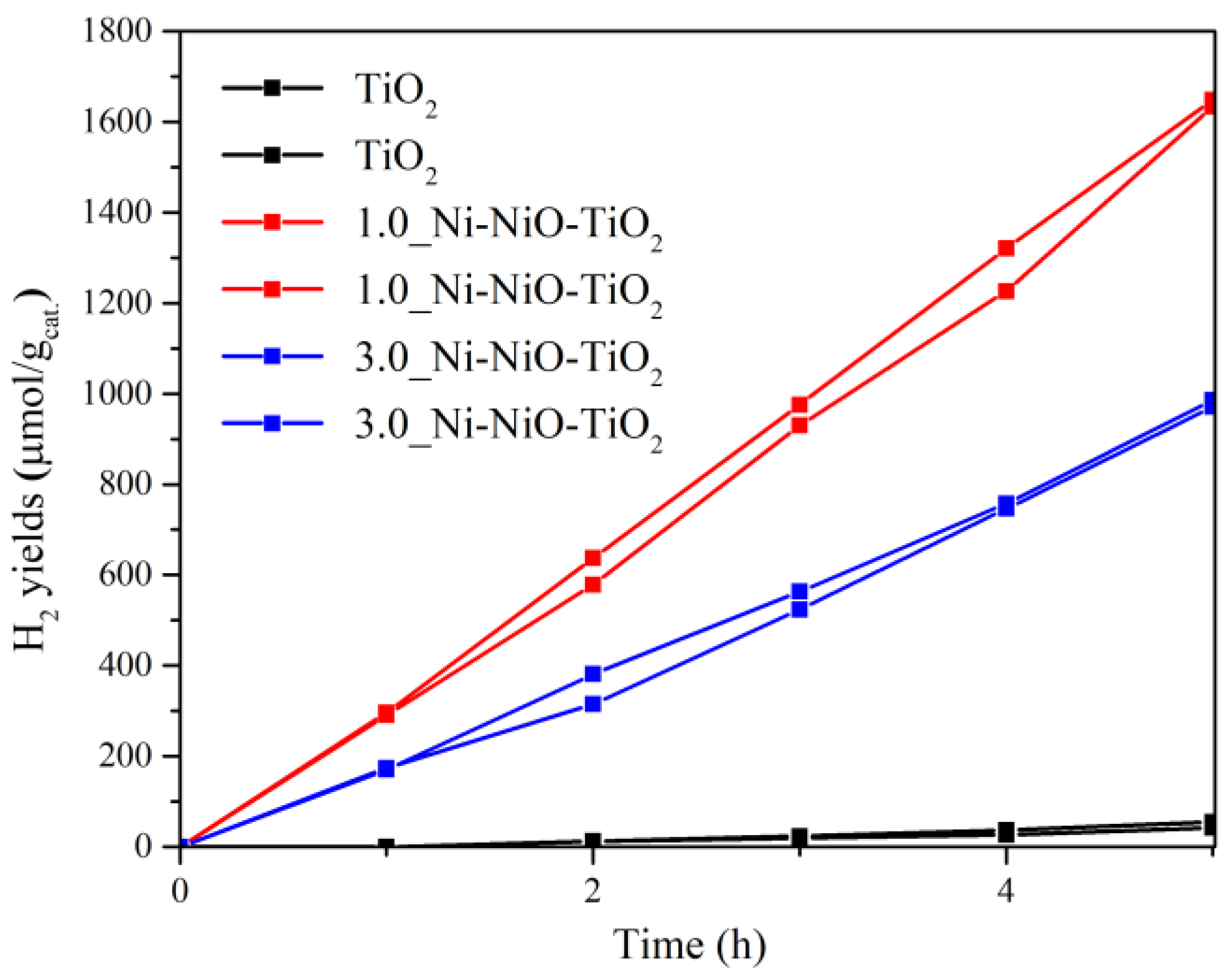
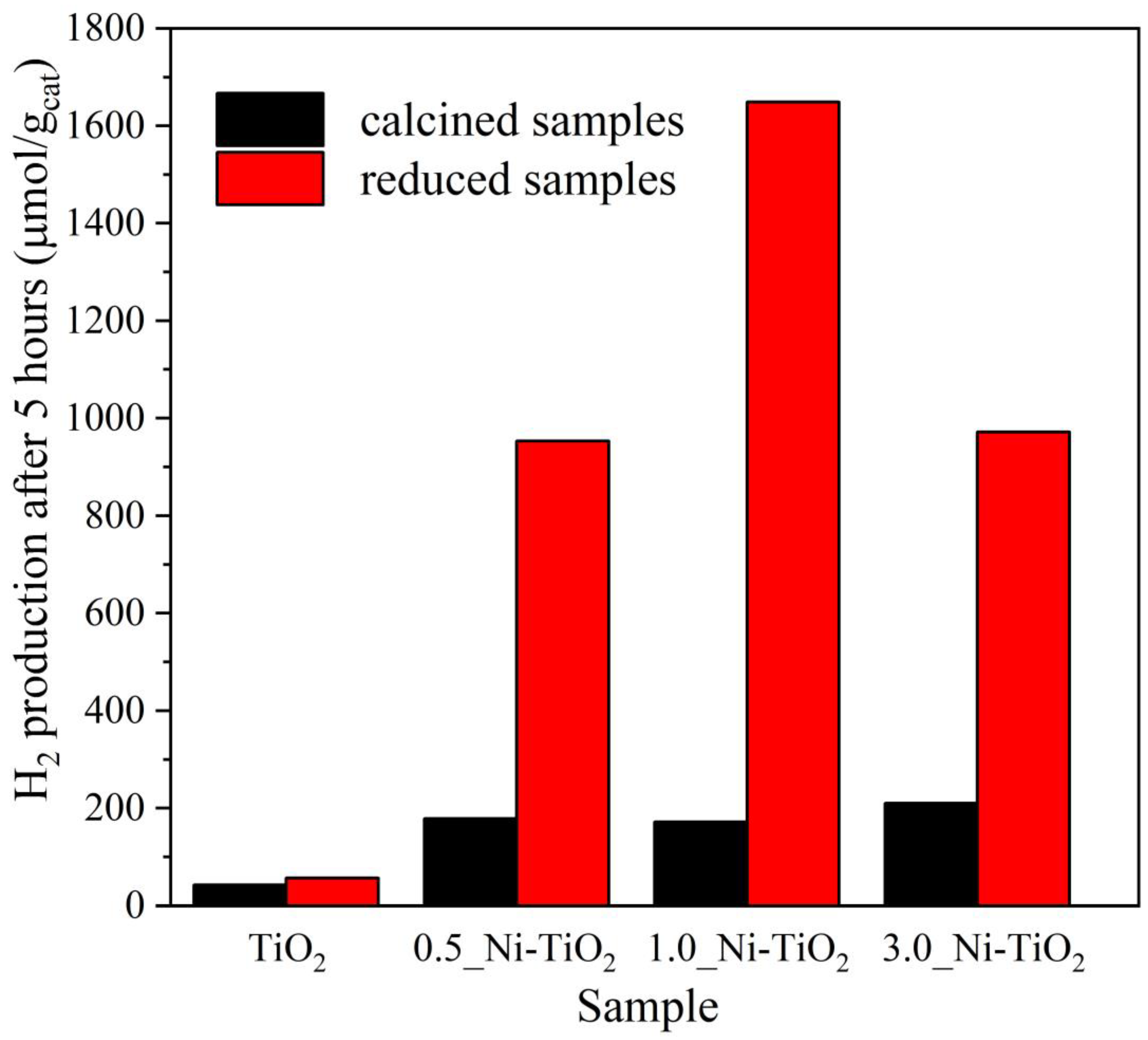
2.2. Photocatalytic Hydrogen Production
2.3. The Contribution of Photocatalysts Properties to Its Photocatalytic Behaviour
3. Conclusions
4. Materials and Methods
4.1. Photocatalysts’ Preparation
4.2. Photocatalysts’ Characterization
4.3. Photocatalytic Test
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lin, L.; Hisatomi, T.; Chen, S.; Takata, T.; Domen, K. Visible-Light-Driven Photocatalytic Water Splitting: Recent Progress and Challenges. Trends Chem. 2020, 2, 813–824. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic water splitting hydrogen production via environmental benign carbon based nanomaterials. Int. J. Hydrog. Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Ng, K.H.; Lai, S.Y.; Cheng, C.K.; Cheng, Y.W.; Chong, C.C. Photocatalytic water splitting for solving energy crisis: Myth, Fact or Busted? Chem. Eng. J. (Lausanne) 2021, 417, 128847. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Clarizia, L.; Di Somma, I.; Onotri, L.; Andreozzi, R.; Marotta, R. Kinetic modeling of hydrogen generation over nano-Cu(s)/TiO2 catalyst through photoreforming of alcohols. Catal. Today 2017, 281, 117–123. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Hu, Y.H. Bimetallic cocatalysts for photocatalytic hydrogen production from water. Chem. Eng. J. (Lausanne) 2021, 409, 128250. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Prahov, L.; Disdier, J.; Herrmann, J.-M.; Pichat, P. Room temperature hydrogen production from aliphatic alcohols over uv-illuminated powder Ni/TiO2 catalysts. Int. J. Hydrog. Energy 1984, 9, 397–403. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, Y.; Guo, L. Study on the synthesis of Ni doped mesoporous TiO2 and its photocatalytic activity for hydrogen evolution in aqueous methanol solution. Chem. Phys. Lett. 2005, 415, 74–78. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Nie, L.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of TiO2 using Ni (NO3)2 as an additive. Phys. Chem. Chem. Phys. 2013, 15, 12033–12039. [Google Scholar] [CrossRef]
- Hu, S.; Li, F.; Fan, Z.; Gui, J. Improved photocatalytic hydrogen production property over Ni/NiO/N–TiO2−x heterojunction nanocomposite prepared by NH3 plasma treatment. J. Power Sources 2014, 250, 30–39. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Aoki, T.; Crozier, P.A. Structural evolution during photocorrosion of Ni/NiO core/shell cocatalyst on TiO2. J. Phys. Chem. C 2015, 119, 7207–7214. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. Band alignment investigations of heterostructure NiO/TiO2 nanomaterials used as efficient heterojunction earth-abundant metal oxide photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 19279–19288. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Moriga, T.; Idriss, H.; Waterhouse, G.I. Ni/TiO2: A promising low-cost photocatalytic system for solar H2 production from ethanol–water mixtures. J. Catal. 2015, 326, 43–53. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H.; Waterhouse, G.I. Performance comparison of Ni/TiO2 and Au/TiO2 photocatalysts for H2 production in different alcohol-water mixtures. J. Catal. 2018, 367, 27–42. [Google Scholar] [CrossRef]
- Wang, P.; Xu, S.; Chen, F.; Yu, H. Ni nanoparticles as electron-transfer mediators and NiSx as interfacial active sites for coordinative enhancement of H2-evolution performance of TiO2. Chin. J. Catal. 2019, 40, 343–351. [Google Scholar] [CrossRef]
- Ren, X.; Gao, P.; Kong, X.; Jiang, R.; Yang, P.; Chen, Y.; Chi, Q.; Li, B. NiO/Ni/TiO2 nanocables with Schottky/p-n heterojunctions and the improved photocatalytic performance in water splitting under visible light. J. Colloid Interface Sci. 2018, 530, 1–8. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.; Kumar, P.; Sekhar, P.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and Characterization of Ni-Doped TiO2 Materials for Photocurrent and Photocatalytic Applications. Sci. World J. 2012, 2012, 127326. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiong, J.; Shi, J.; Xia, S.; Gui, S.; Shangguan, W. Roles of various Ni species on TiO2 in enhancing photocatalytic H2 evolution. Front. Energy 2019, 13, 684–690. [Google Scholar] [CrossRef]
- Bhavani, P.; Kumar, D.P.; Hussain, M.; Chen, W.-H.; Lam, S.S.; Park, Y.-K. Surface ligand functionalized Few-layered MoSe2 nanosheets decorated CdS nanorods for spectacular rate of H2 production. Fuel 2023, 334, 126551. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Wang, F.; Zhang, L.; Li, L.; Chen, H.; Wei, Z. Rational design of porous Ni-Co-Fe ternary metal phosphides nanobricks as bifunctional electrocatalysts for efficient overall water splitting. Appl. Catal. B Environ. 2022, 310, 121353. [Google Scholar] [CrossRef]
- Bhavani, P.; Hussain, M.; Park, Y.-K. Recent advancements on the sustainable biochar based semiconducting materials for photocatalytic applications: A state of the art review. J. Clean. Prod. 2022, 330, 129899. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, J.; Liu, Y.; Hou, L.; Deng, J.; Yuan, C. Construction of hetero-phase Mo2C-CoO@N-CNFs film as a self-supported Bi-functional catalyst towards overall water splitting. Chem. Eng. J. 2023, 451, 139025. [Google Scholar] [CrossRef]
- Jia, L.; Du, G.; Han, D.; Wang, Y.; Zhao, W.; Su, Q.; Ding, S.; Xu, B. Magnetic electrode configuration with polypyrrole-wrapped Ni/NiFe2O4 core–shell nanospheres to boost electrocatalytic water splitting. Chem. Eng. J. 2023, 454, 140278. [Google Scholar] [CrossRef]
- Zabelina, A.; Miliutina, E.; Zabelin, D.; Burtsev, V.; Buravets, V.; Elashnikov, R.; Neubertova, V.; Šťastný, M.; Popelková, D.; Lancok, J. Plasmon coupling inside 2D-like TiB2 flakes for water splitting half reactions enhancement in acidic and alkaline conditions. Chem. Eng. J. 2023, 454, 140441. [Google Scholar] [CrossRef]
- Alijani, M.; Ilkhechi, N.N. Effect of Ni Doping on the Structural and Optical Properties of TiO2 Nanoparticles at Various Concentration and Temperature. Silicon 2018, 10, 2569–2575. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, H.; Ma, Q.; Cui, Y.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q. Fabrication of p-NiO/n-TiO2 nano-tube arrays photoelectrode and its enhanced photocatalytic performance for degradation of 4-chlorphenol. Sep. Purif. Technol. 2017, 186, 1–9. [Google Scholar] [CrossRef]
- Dai, L.; Sun, F.; Fan, Q.; Li, H.; Yang, K.; Guo, T.; Zheng, L.; Fu, P. Carbon-based titanium dioxide materials for hydrogen production in water-methanol reforming: A review. J. Environ. Chem. Eng. 2022, 107326. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Han, J.; Guo, R. TiO2 nanosheet/NiO nanorod hierarchical nanostructures: P–n heterojunctions towards efficient photocatalysis. J. Colloid Interface Sci. 2020, 562, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; El-Toni, A.M.; Al-Sayari, S.; Al-Hajry, A.; Al-Assiri, M. Novel mesoporous NiO/TiO2 nanocomposites with enhanced photocatalytic activity under visible light illumination. Ceram. Int. 2018, 44, 7047–7056. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, H.; Wang, S.; Hu, J.; Chen, Z. NiO-TiO2 p-n Heterojunction for Solar Hydrogen Generation. Catalysts 2021, 11, 1427. [Google Scholar] [CrossRef]
- Yu, C.; Li, M.; Yang, D.; Pan, K.; Yang, F.; Xu, Y.; Yuan, L.; Qu, Y.; Zhou, W. NiO nanoparticles dotted TiO2 nanosheets assembled nanotubes P-N heterojunctions for efficient interface charge separation and photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 568, 150981. [Google Scholar] [CrossRef]
- Jasim, M.M.; Dakhil, O.A.A.; Abdullah, H.I. Synthesis of NiO/TNTs p-n junction for highly photocatalysis activity under sunlight irradiation. Solid State Sci. 2020, 107, 106342. [Google Scholar] [CrossRef]
- Sreethawong, T.; Ngamsinlapasathian, S.; Yoshikawa, S. Surfactant-aided sol–gel synthesis of mesoporous-assembled TiO2–NiO mixed oxide nanocrystals and their photocatalytic azo dye degradation activity. Chem. Eng. J. (Lausanne) 2012, 192, 292–300. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Chen, S.; Qian, T.T.; Ling, L.L.; Zhang, W.; Gong, B.B.; Jiang, H. Hydrogenation of Furfural to Cyclopentanone under Mild Conditions by a Structure-Optimized Ni− NiO/TiO2 Heterojunction Catalyst. ChemSusChem 2020, 13, 5507–5515. [Google Scholar] [CrossRef]
- Kočí, K.; Troppová, I.; Edelmannová, M.; Starostka, J.; Matějová, L.; Lang, J.; Reli, M.; Drobná, H.; Rokicińska, A.; Kuśtrowski, P. Photocatalytic decomposition of methanol over La/TiO2 materials. Environ. Sci. Pollut. Res. 2018, 25, 34818–34825. [Google Scholar] [CrossRef] [PubMed]
- Jitianu, M.; Jitianu, A.; Zaharescu, M.; Crisan, D.; Marchidan, R. IR structural evidence of hydrotalcites derived oxidic forms. Vib. Spectrosc. 2000, 22, 75–86. [Google Scholar] [CrossRef]
- Kim, P.; Kim, Y.; Kim, H.; Song, I.K.; Yi, J. Synthesis and characterization of mesoporous alumina with nickel incorporated for use in the partial oxidation of methane into synthesis gas. Appl. Catal. A: Gen. 2004, 272, 157–166. [Google Scholar] [CrossRef]
- Shibiao, R.; Jinheng, Q.; Chunyan, W.; Bolian, X.; Yining, F.; Yi, C. Influence of nickel salt precursors on the hydrogenation activity of Ni/γ-Al2O3 catalyst. Chin. J. Catal. 2007, 28, 651–656. [Google Scholar]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Alagarasi, A.; Rajalakshmi, P.; Shanthi, K.; Selvam, P. Ordered mesoporous nanocrystalline titania: A promising new class of photocatalytic materials. Catal. Today 2018, 309, 202–211. [Google Scholar] [CrossRef]
- Sahoo, S.; Arora, A.; Sridharan, V. Raman line shapes of optical phonons of different symmetries in anatase TiO2 nanocrystals. J. Phys. Chem. C 2009, 113, 16927–16933. [Google Scholar] [CrossRef]
- Pal, M.; Pal, U.; Jiménez, J.M.G.Y.; Pérez-Rodríguez, F. Effects of crystallization and dopant concentration on the emission behavior of TiO2: Eu nanophosphors. Nanoscale Res. Lett. 2012, 7, 1–12. [Google Scholar] [CrossRef]
- Sanchis, R.; Delgado, D.; Agouram, S.; Soriano, M.; Vázquez, M.; Rodríguez-Castellón, E.; Solsona, B.; Nieto, J.L. NiO diluted in high surface area TiO2 as an efficient catalyst for the oxidative dehydrogenation of ethane. Appl. Catal. A Gen. 2017, 536, 18–26. [Google Scholar] [CrossRef]
- Varkolu, M.; Velpula, V.; Pochamoni, R.; Muppala, A.R.; Burri, D.R.; Kamaraju, S.R.R. Nitrobenzene hydrogenation over Ni/TiO2 catalyst in vapour phase at atmospheric pressure: Influence of preparation method. Appl. Petrochem. Res. 2016, 6, 15–23. [Google Scholar] [CrossRef]


| Calcined Samples | Reduced Samples | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Crystallite Size (nm) | Phase Content (%) | Sample | Crystallite Size (nm) | Phase Content (%) | ||
| Anatase | NiO | Anatase | Ni0 | ||||
| TiO2 | 12.9 | 100 | n.d. 1 | TiO2 | 10.0 | 100 | n.d. 1 |
| 0.5_NiO-TiO2 | 7.0 | 100 | n.d. 1 | 0.5_Ni-NiO-TiO2 | 9.9 | 100 | n.d. 1 |
| 1.0_NiO-TiO2 | 10.3 | 100 | n.d. 1 | 1.0_Ni-NiO-TiO2 | 7.3 | 100 | n.d. 1 |
| 3.0_NiO-TiO2 | 6.6 | 99 | 1.0 | 3.0_Ni-NiO-TiO2 | 6.9 | 97.9 | 2.1 |
| Calcined Samples | Atomic Concentration (mol. %) | NiO | |||||||
| Carbon | Ti-O | C=O | -OH | O Total | Titanium | Ni2+ | Ni0 | (wt. %) | |
| TiO2 | 13.98 | 53.88 | 4.94 | 2.19 | 60.91 | 25.02 | - | - | - |
| 0.5_NiO-TiO2 | 13.00 | 54.24 | 5.02 | 1.97 | 60.96 | 25.77 | 0.10 | n.d. 1 | 0.32 |
| 1.0_NiO-TiO2 | 14.90 | 51.95 | 5.54 | 1.90 | 59.17 | 24.91 | 0.74 | l.d. 2 | 2.35 |
| 3.0_NiO-TiO2 | 15.70 | 45.82 | 9.68 | 2.89 | 58.33 | 24.19 | 1.63 | l.d. 2 | 5.16 |
| Reduced Samples | Atomic Concentration [%] | NiO | |||||||
| Carbon | Ti-O | C=O | -OH | O Total | Titanium | Ni2+ | Ni0 | (wt. %) | |
| TiO2 | 16.02 | 52.21 | 4.97 | 2.16 | 59.29 | 24.64 | - | - | - |
| 0.5_Ni-NiO-TiO2 | 17.43 | 49.05 | 3.84 | 2.44 | 55.40 | 23.59 | 0.07 | n.d. 1 | 0.24 |
| 1.0_Ni-NiO-TiO2 | 12.77 | 52.45 | 6.26 | 2.19 | 60.77 | 25.65 | 0.65 | l.d. 2 | 2.03 |
| 3.0_Ni-NiO-TiO2 | 14.02 | 52.93 | 5.07 | 1.72 | 59.49 | 25.66 | 0.54 | l.d. 2 | 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobná, H.; Meinhardová, V.; Dubnová, L.; Kozumplíková, K.; Reli, M.; Kočí, K.; Čapek, L. Partially Reduced Ni-NiO-TiO2 Photocatalysts for Hydrogen Production from Methanol–Water Solution. Catalysts 2023, 13, 293. https://doi.org/10.3390/catal13020293
Drobná H, Meinhardová V, Dubnová L, Kozumplíková K, Reli M, Kočí K, Čapek L. Partially Reduced Ni-NiO-TiO2 Photocatalysts for Hydrogen Production from Methanol–Water Solution. Catalysts. 2023; 13(2):293. https://doi.org/10.3390/catal13020293
Chicago/Turabian StyleDrobná, Helena, Vendula Meinhardová, Lada Dubnová, Kateřina Kozumplíková, Martin Reli, Kamila Kočí, and Libor Čapek. 2023. "Partially Reduced Ni-NiO-TiO2 Photocatalysts for Hydrogen Production from Methanol–Water Solution" Catalysts 13, no. 2: 293. https://doi.org/10.3390/catal13020293
APA StyleDrobná, H., Meinhardová, V., Dubnová, L., Kozumplíková, K., Reli, M., Kočí, K., & Čapek, L. (2023). Partially Reduced Ni-NiO-TiO2 Photocatalysts for Hydrogen Production from Methanol–Water Solution. Catalysts, 13(2), 293. https://doi.org/10.3390/catal13020293








