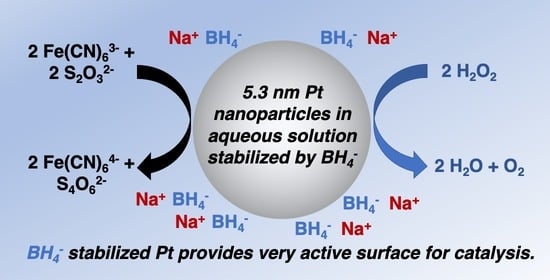
Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications
Abstract
1. Introduction
2. Results
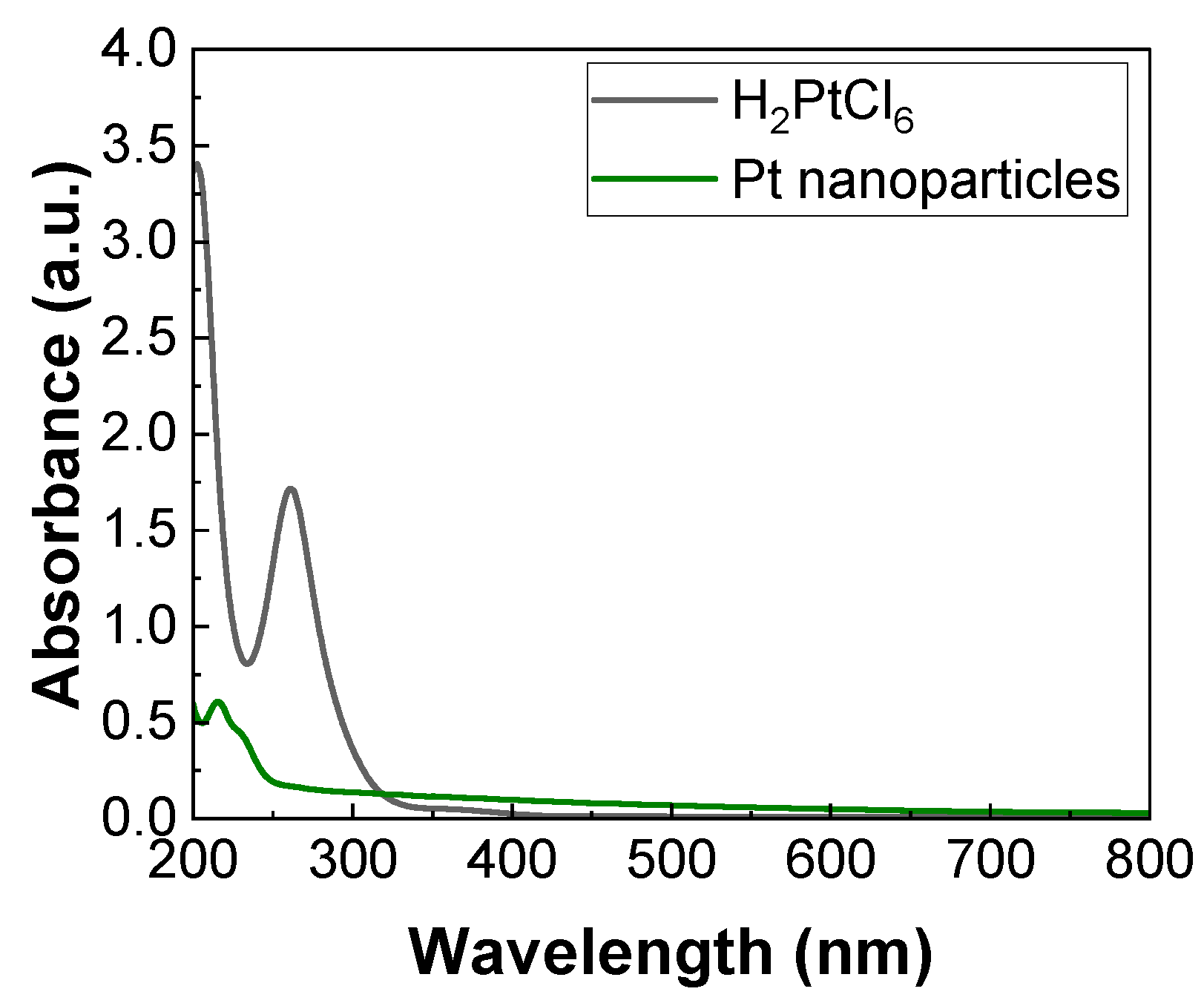
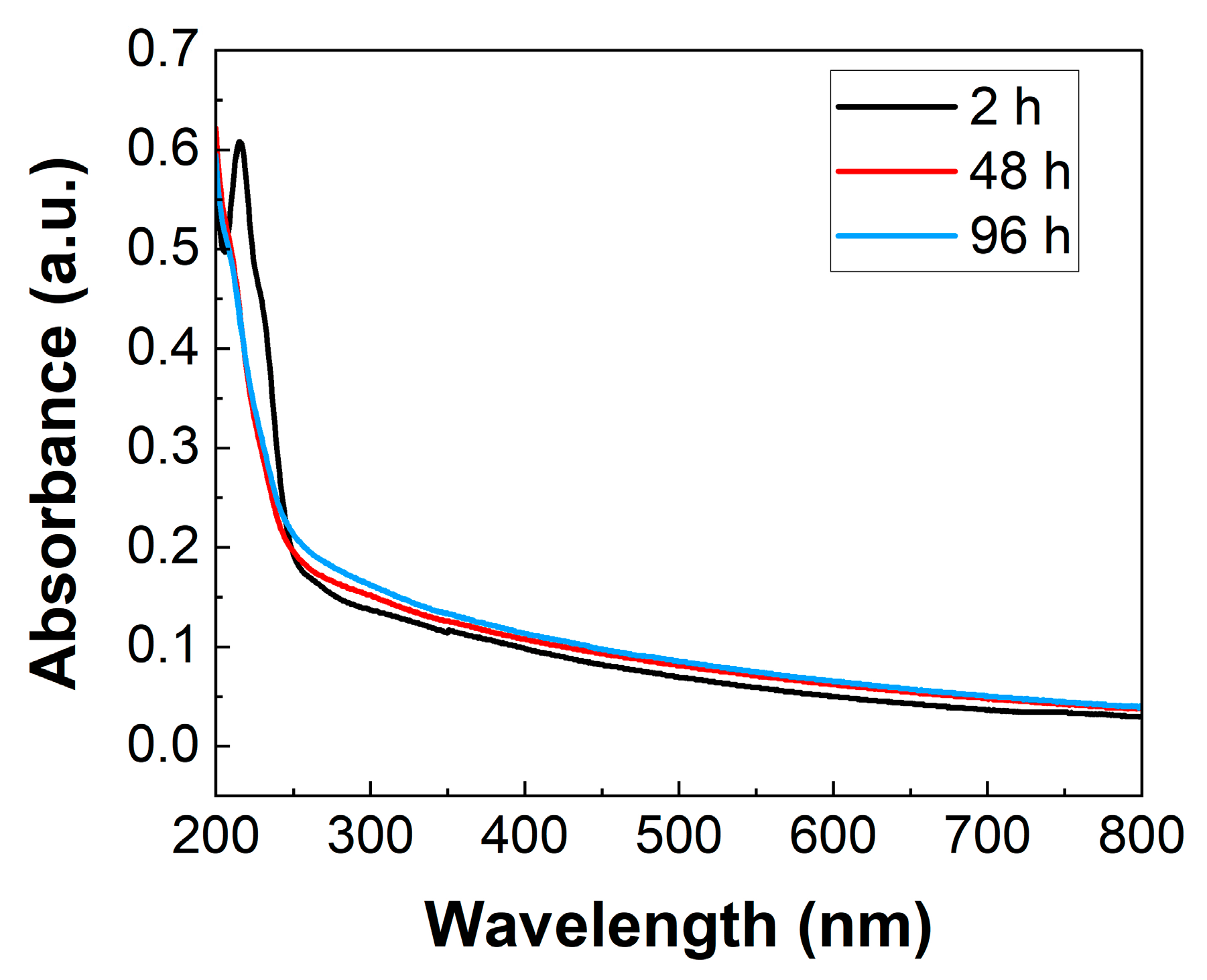
2.1. Synthesis and Characterization
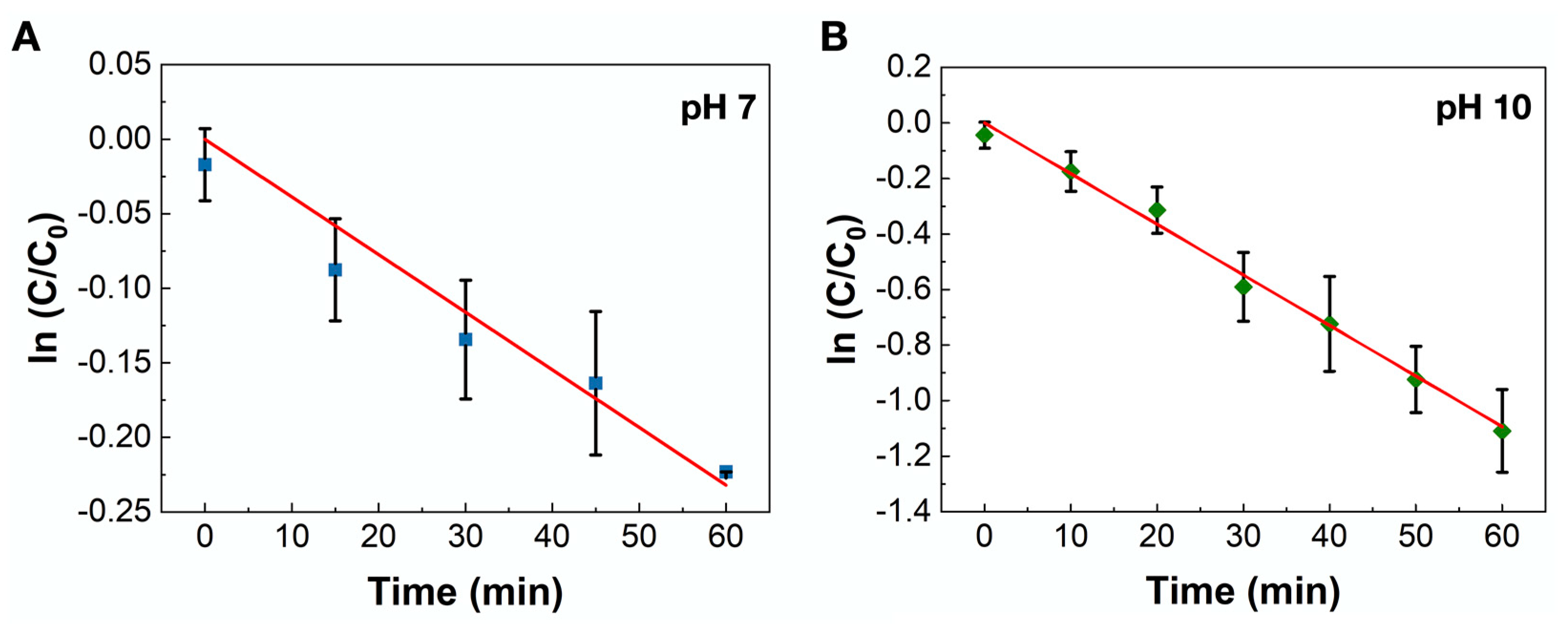
2.2. Catalytic Reaction Testing
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Platinum Nanoparticle Synthesis
3.3. UV-Vis Spectroscopic Measurements
3.4. Transmission Electron Microscopy Measurements
3.5. Pt-Catalyzed Hydrogen Peroxide Decomposition
3.6. Pt-Catalyzed Electron-Transfer Reaction between Hexacyanoferrate(III) and Thiosulfate Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Wang, Z.; Ye, B.; Huang, X.; Deng, H. Ligand effect over gold nanocatalysts towards enhanced gas-phase oxidation of alcohols. J. Catal. 2021, 400, 274–282. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Lei, Z.; Guan, Z.-J.; Wang, Q.-M. Atomically Precise Preorganization of Open Metal Sites on Gold Nanoclusters with High Catalytic Performance. Angew. Chem. Int. Ed. 2021, 60, 5225–5229. [Google Scholar] [CrossRef] [PubMed]
- Brindle, J.; Sufyan, S.A.; Nigra, M.M. Support, composition, and ligand effects in partial oxidation of benzyl alcohol using gold–copper clusters. Catal. Sci. Technol. 2022, 12, 3846–3855. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-Specific Catalytic Activity of Polymer-Stabilized Gold Nanoclusters for Aerobic Alcohol Oxidation in Water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef]
- Sufyan, S.A.; van Devener, B.; Perez, P.; Nigra, M.M. Electronic Tuning of Gold Nanoparticle Active Sites for Reduction Catalysis. ACS Appl. Mater. Interfaces 2023, 15, 1210–1218. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, G.; Jiang, D.-e.; Mullins, D.R.; Zhang, Q.-F.; Allard, L.F.; Wang, L.-S.; Overbury, S.H. Diphosphine-Protected Au22 Nanoclusters on Oxide Supports Are Active for Gas-Phase Catalysis without Ligand Removal. Nano Lett. 2016, 16, 6560–6567. [Google Scholar] [CrossRef]
- Kapil, N.; Weissenberger, T.; Cardinale, F.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.-O. Precisely Engineered Supported Gold Clusters as a Stable Catalyst for Propylene Epoxidation. Angew. Chem. Int. Ed. 2021, 60, 18185–18193. [Google Scholar] [CrossRef]
- Kapil, N.; Cardinale, F.; Weissenberger, T.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.-O. Gold nanoparticles with tailored size through ligand modification for catalytic applications. Chem. Commun. 2021, 57, 10775–10778. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic Oxidation of Cyclohexane Catalyzed by Size-Controlled Au Clusters on Hydroxyapatite: Size Effect in the Sub-2 nm Regime. ACS Catal. 2011, 1, 2–6. [Google Scholar] [CrossRef]
- Cargnello, M.; Chen, C.; Diroll, B.T.; Doan-Nguyen, V.V.T.; Gorte, R.J.; Murray, C.B. Efficient Removal of Organic Ligands from Supported Nanocrystals by Fast Thermal Annealing Enables Catalytic Studies on Well-Defined Active Phases. J. Am. Chem. Soc. 2015, 137, 6906–6911. [Google Scholar] [CrossRef]
- Lu, L.; Zou, S.; Fang, B. The Critical Impacts of Ligands on Heterogeneous Nanocatalysis: A Review. ACS Catal. 2021, 11, 6020–6058. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, L.; Kirkensgaard, J.J.K.; Jensen, K.M.Ø.; Oezaslan, M.; et al. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Kacenauskaite, L.; Quinson, J.; Schultz, H.; Kirkensgaard, J.J.K.; Kunz, S.; Vosch, T.; Arenz, M. UV-Induced Synthesis and Stabilization of Surfactant-Free Colloidal Pt Nanoparticles with Controlled Particle Size in Ethylene Glycol. ChemNanoMat 2017, 3, 89–93. [Google Scholar] [CrossRef]
- Quinson, J.; Dworzak, A.; Simonsen, S.B.; Theil Kuhn, L.; Jensen, K.M.Ø.; Zana, A.; Oezaslan, M.; Kirkensgaard, J.J.K.; Arenz, M. Surfactant-free synthesis of size controlled platinum nanoparticles: Insights from in situ studies. Appl. Surf. Sci. 2021, 549, 149263. [Google Scholar] [CrossRef]
- Quinson, J.; Neumann, S.; Wannmacher, T.; Kacenauskaite, L.; Inaba, M.; Bucher, J.; Bizzotto, F.; Simonsen, S.B.; Theil Kuhn, L.; Bujak, D.; et al. Colloids for Catalysts: A Concept for the Preparation of Superior Catalysts of Industrial Relevance. Angew. Chem. Int. Ed. 2018, 57, 12338–12341. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Kacenauskaite, L.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, L.; Oezaslan, M.; Kunz, S.; Arenz, M. Controlled Synthesis of Surfactant-Free Water-Dispersible Colloidal Platinum Nanoparticles by the Co4Cat Process. ChemSusChem 2019, 12, 1229–1239. [Google Scholar] [CrossRef]
- Quinson, J.; Mathiesen, J.K.; Schröder, J.; Dworzak, A.; Bizzotto, F.; Zana, A.; Simonsen, S.B.; Theil Kuhn, L.; Oezaslan, M.; Jensen, K.M.Ø.; et al. Teaching old precursors new tricks: Fast room temperature synthesis of surfactant-free colloidal platinum nanoparticles. J. Colloid Interface Sci. 2020, 577, 319–328. [Google Scholar] [CrossRef]
- Quinson, J.; Neumann, S.; Kacenauskaite, L.; Bucher, J.; Kirkensgaard, J.J.K.; Simonsen, S.B.; Theil Kuhn, L.; Zana, A.; Vosch, T.; Oezaslan, M.; et al. Solvent-Dependent Growth and Stabilization Mechanisms of Surfactant-Free Colloidal Pt Nanoparticles. Chem. A Eur. J. 2020, 26, 9012–9023. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, H.; Li, Y.; Zhou, Y.; Fang, B. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2023, 451, 138668. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of Surfactant-Free Colloidal Nanoparticles in Heterogeneous Catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Quinson, J. Colloidal surfactant-free syntheses of precious metal nanoparticles for electrocatalysis. Curr. Opin. Electrochem. 2022, 34, 100977. [Google Scholar] [CrossRef]
- Arce-Sarria, A.; Aldana-Villegas, K.M.; Betancourt-Buitrago, L.A.; Colina-Márquez, J.Á.; Machuca-Martínez, F.; Mueses, M.A. Degradation of Hexacyanoferrate (III) from Gold Mining Wastewaters via UV-A/LED Photocatalysis Using Modified TiO2 P25. Water 2020, 12, 2531. [Google Scholar] [CrossRef]
- Brindle, J.S.; Nelson, P.S.; Charde, R.P.; Sufyan, S.A.; Nigra, M.M. Catalytic cooperativity between glucose oxidase and gold nanoparticles in the sequential oxidation of glucose to saccharic acid. Green Chem. 2022, 24, 5162–5170. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Q.; Li, M.; Chao, D.; Huang, L.; Wu, W.; Fang, Y.; Dong, S. Glucose-oxidase like catalytic mechanism of noble metal nanozymes. Nat. Commun. 2021, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Hermans, S.; Deffernez, A.; Devillers, M. Au–Pd/C catalysts for glyoxal and glucose selective oxidations. Appl. Catal. A Gen. 2011, 395, 19–27. [Google Scholar] [CrossRef]
- Lewis, R.J.; Bara-Estaun, A.; Agarwal, N.; Freakley, S.J.; Morgan, D.J.; Hutchings, G.J. The Direct Synthesis of H2O2 and Selective Oxidation of Methane to Methanol Using HZSM-5 Supported AuPd Catalysts. Catal. Lett. 2019, 149, 3066–3075. [Google Scholar] [CrossRef]
- Williams, C.; Carter, J.H.; Dummer, N.F.; Chow, Y.K.; Morgan, D.J.; Yacob, S.; Serna, P.; Willock, D.J.; Meyer, R.J.; Taylor, S.H.; et al. Selective Oxidation of Methane to Methanol Using Supported AuPd Catalysts Prepared by Stabilizer-Free Sol-Immobilization. ACS Catal. 2018, 8, 2567–2576. [Google Scholar] [CrossRef]
- McVicker, R.; Agarwal, N.; Freakley, S.J.; He, Q.; Althahban, S.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Low temperature selective oxidation of methane using gold-palladium colloids. Catal. Today 2020, 342, 32–38. [Google Scholar] [CrossRef]
- Agarwal, N.; Freakley, S.J.; McVicker, R.U.; Althahban, S.M.; Dimitratos, N.; He, Q.; Morgan, D.J.; Jenkins, R.L.; Willock, D.J.; Taylor, S.H.; et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 2017, 358, 223–227. [Google Scholar] [CrossRef]
- Huang, J.; Takei, T.; Ohashi, H.; Haruta, M. Propene epoxidation with oxygen over gold clusters: Role of basic salts and hydroxides of alkalis. Appl. Catal. A Gen. 2012, 435-436, 115–122. [Google Scholar] [CrossRef]
- Huang, J.; Akita, T.; Faye, J.; Fujitani, T.; Takei, T.; Haruta, M. Propene epoxidation with dioxygen catalyzed by gold clusters. Angew. Chem. Int. Ed. Engl. 2009, 48, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Neurock, M.; Manzer, L.E. Theoretical insights on the mechanism of alkene epoxidation by H2O2 with titanium silicalite. Chem. Commun. 1996, 1133–1134. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, W.-J.; Wu, H.; Liebens, A.; Wu, P. Selective synthesis of ethylene oxide through liquid-phase epoxidation of ethylene with titanosilicate/H2O2 catalytic systems. Appl. Catal. A Gen. 2016, 515, 51–59. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, X.; Feng, Z.; Lu, Z.; Zhang, Z.; Huang, W.; Li, Y.; Vuckovic, D.; Li, Y.; Dai, S.; et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 2021, 4, 233–241. [Google Scholar] [CrossRef]
- Agrawal, S.; Mysko, R.A.; Nigra, M.M.; Mohanty, S.K.; Hoepfner, M.P. Plasmonic Photocatalytic Enhancement of L-Cysteine Self-Assembled Gold Nanoparticle Clusters for Fenton Reaction Catalysis. Langmuir 2021, 37, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Y.; Yao, Q.; Wang, Y.; Fang, X.; Shen, C.; Li, F.; Huang, M.; Wang, Z.; Sand, W.; et al. Supported Atomically-Precise Gold Nanoclusters for Enhanced Flow-through Electro-Fenton. Environ. Sci. Technol. 2020, 54, 5913–5921. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, S.; You, M.; Hong, S.; Wu, T.-S.; Soo, Y.-L.; Zhao, Z.; Jiang, G.; Jieshan, Q.; et al. Photocatalytic Fixation of Nitrogen to Ammonia by Single Ru Atom Decorated TiO2 Nanosheets. ACS Sustain. Chem. Eng. 2019, 7, 6813–6820. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Vetter, T.A.; Colombo, D.P. Kinetics of Platinum-Catalyzed Decomposition of Hydrogen Peroxide. J. Chem. Educ. 2003, 80, 788. [Google Scholar] [CrossRef]
- van Wyk, P.-H.; Gerber, W.J.; Koch, K.R. A robust method for speciation, separation and photometric characterization of all [PtCl6−nBrn]2− (n=0–6) and [PtCl4−nBrn]2− (n=0–4) complex anions by means of ion-pairing RP-HPLC coupled to ICP-MS/OES, validated by high resolution 195Pt NMR spectroscopy. Anal. Chim. Acta 2011, 704, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Menumerov, E.; Hughes, R.A.; Golze, S.D.; Neal, R.D.; Demille, T.B.; Campanaro, J.C.; Kotesky, K.C.; Rouvimov, S.; Neretina, S. Identifying the True Catalyst in the Reduction of 4-Nitrophenol: A Case Study Showing the Effect of Leaching and Oxidative Etching Using Ag Catalysts. ACS Catal. 2018, 8, 8879–8888. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Shape-Dependent Catalytic Activity of Platinum Nanoparticles in Colloidal Solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- McKee, D.W. Catalytic decomposition of hydrogen peroxide by metals and alloys of the platinum group. J. Catal. 1969, 14, 355–364. [Google Scholar] [CrossRef]
- Richards, T.; Lewis, R.J.; Morgan, D.J.; Hutchings, G.J. The Direct Synthesis of Hydrogen Peroxide Over Supported Pd-Based Catalysts: An Investigation into the Role of the Support and Secondary Metal Modifiers. Catal. Lett. 2022, 153, 32–40. [Google Scholar] [CrossRef]
- Brehm, J.; Lewis, R.J.; Morgan, D.J.; Davies, T.E.; Hutchings, G.J. The Direct Synthesis of Hydrogen Peroxide over AuPd Nanoparticles: An Investigation into Metal Loading. Catal. Lett. 2022, 152, 254–262. [Google Scholar] [CrossRef]
- Freakley, S.J.; Agarwal, N.; McVicker, R.U.; Althahban, S.; Lewis, R.J.; Morgan, D.J.; Dimitratos, N.; Kiely, C.J.; Hutchings, G.J. Gold–palladium colloids as catalysts for hydrogen peroxide synthesis, degradation and methane oxidation: Effect of the PVP stabiliser. Catal. Sci. Technol. 2020, 10, 5935–5944. [Google Scholar] [CrossRef]
- Bianchi, G.; Mazza, F.; Mussini, T. Catalytic decomposition of acid hydrogen peroxide solutions on platinum, iridium, palladium and gold surfaces. Electrochim. Acta 1962, 7, 457–473. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Michel, F.M.; Douglas, T.A.; Kang, Y.; Stach, E.A. Mechanism and Kinetics of Methane Oxidation to Methanol Catalyzed by AuPd Nanocatalysts at Low Temperature. ACS Catal. 2021, 11, 2837–2845. [Google Scholar] [CrossRef]
- MacInnes, D.A. The Mechanism of the Catalysis of the Decomposition of Hydrogen Peroxide by Colloidal Platinum. J. Am. Chem. Soc. 1914, 36, 878–881. [Google Scholar] [CrossRef]
- Eley, D.D.; Macmahon, D.M. The decomposition of hydrogen peroxide catalysed by palladium-gold alloy wires. J. Colloid Interface Sci. 1972, 38, 502–510. [Google Scholar] [CrossRef]
- Naya, S.-i.; Teranishi, M.; Kimura, K.; Tada, H. A strong support-effect on the catalytic activity of gold nanoparticles for hydrogen peroxide decomposition. Chem. Commun. 2011, 47, 3230–3232. [Google Scholar] [CrossRef] [PubMed]
- Serra-Maia, R.; Winkler, C.; Murayama, M.; Tranhuu, K.; Michel, F.M. Abundance and Speciation of Surface Oxygen on Nanosized Platinum Catalysts and Effect on Catalytic Activity. ACS Appl. Energy Mater. 2018, 1, 3255–3266. [Google Scholar] [CrossRef]
- Weiss, J. The catalytic decomposition of hydrogen peroxide on different metals. Trans. Faraday Soc. 1935, 31, 1547–1557. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Aoki, T.; Kaibara, D.; Seino, S.; Mori, O.; Sasaki, R.; Endo, K.; Yamamura, K. Strong Biomimetic Immobilization of Pt-Particle Catalyst on ABS Substrate Using Polydopamine and Its Application for Contact-Lens Cleaning with H2O2. Nanomaterials 2020, 10, 114. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Radiolytic Synthesis of Pt-Particle/ABS Catalysts for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2017, 7, 235. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Improved Catalytic Durability of Pt-Particle/ABS for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2019, 9, 342. [Google Scholar] [CrossRef]
- Singh, K.S.W.; Rouquerol, J.; Bergeret, G.; Gallezot, P.; Vaarkamp, M.; Koningsberger, D.C.; Datye, A.K.; Niemantsverdriet, J.W.; Butz, T.; Engelhardt, G.; et al. Handbook of Heterogeneous Catalysis; Wiley: Hoboken, NJ, USA, 1997; pp. 427–582. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Shape-Dependent Catalytic Activity of Platinum Nanoparticles in Colloidal Solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Winkler, C.; Murayama, M.; Tranhuu, K.; Michel, F.M. Abundance and Speciation of Surface Oxygen on Nanosized Platinum Catalysts and Effect on Catalytic Activity. ACS Appl. Energy Mater. 2018, 1, 3255–3266. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charde, R.P.; Devener, B.v.; Nigra, M.M. Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications. Catalysts 2023, 13, 246. https://doi.org/10.3390/catal13020246
Charde RP, Devener Bv, Nigra MM. Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications. Catalysts. 2023; 13(2):246. https://doi.org/10.3390/catal13020246
Chicago/Turabian StyleCharde, Rashmi P., Brian van Devener, and Michael M. Nigra. 2023. "Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications" Catalysts 13, no. 2: 246. https://doi.org/10.3390/catal13020246
APA StyleCharde, R. P., Devener, B. v., & Nigra, M. M. (2023). Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications. Catalysts, 13(2), 246. https://doi.org/10.3390/catal13020246