Abstract
The chemical synthesis of heterocycles typically requires elevated temperature and acid or base addition to form the desired product. Moreover, these reactions often involve hazardous reagents, which is why biocatalytic routes for heterocycle formation have gained increasing attention. In recent years, several enzymes belonging to the amidohydrolase superfamily have been identified to generate heterocycles via cyclocondensation reactions. Of particular interest is the amidohydrolase MxcM, which catalyzes the formation of an imidazoline moiety in the biosynthesis of the anti-inflammatory natural product pseudochelin A. In this study, we present a concept for the immobilization of this enzyme using a fused hexahistidine tag for fixation onto a solid, porous carrier. Notably, the immobilization improves the enzyme’s tolerance to organic solvents. The immobilized MxcM exhibits a residual activity of 169% in the polar solvent acetonitrile compared to the free enzyme, and the storage stability in the presence of 20 vol% acetonitrile was ameliorated. In addition, an immobilized enzyme reactor (IMER) was designed that can be operated under flow conditions. The MxcM-IMER retains its biocatalytic activity and mechanic stability over the tested operation time. These results provide important insights for the integration of heterocycle-forming amidohydrolases in chemical processes.
1. Introduction
Heterocycles are defined as molecules that incorporate at least one ring structure with one or more heteroatoms, most commonly nitrogen, oxygen or sulfur [1]. Such compounds are omnipresent in nature, as they can be found in nucleic acids, amino acids, carbohydrates, vitamins, pigments, and natural products [2]. Due to their specific biological effects, heterocycles are important molecular fragments of agrochemicals and active pharmaceutical ingredients [3,4]. Prominent examples include the fungicide cyazofamid [4] or the β-lactam antibiotic penicillin [5]. The heterocyclic core as well as the attached functional groups influence the physicochemical properties, metabolic stability, and bioavailability of a drug [6,7]. A vast number of studies focused on the synthesis of heterocyclic compounds for the discovery of new or optimized pharmaceuticals [8,9,10,11,12]. Over the past decade, the demand for “green” chemistry has triggered the development of flow processes for the synthesis of heterocycles [13,14]. Flow chemistry enables the set-up of fully automated and controlled processes with higher efficiency and safety as well as lower waste generation. Other advantages of flow processes include better heat and mass transfer or simplified scalability [13,14,15,16]. Another aspect of green chemistry is the use of biocatalysts that operate under mild temperatures and in aqueous solutions. In heterocyclic chemistry, biocatalysts are also of particular interest for the development of stereoselective syntheses [17,18,19].
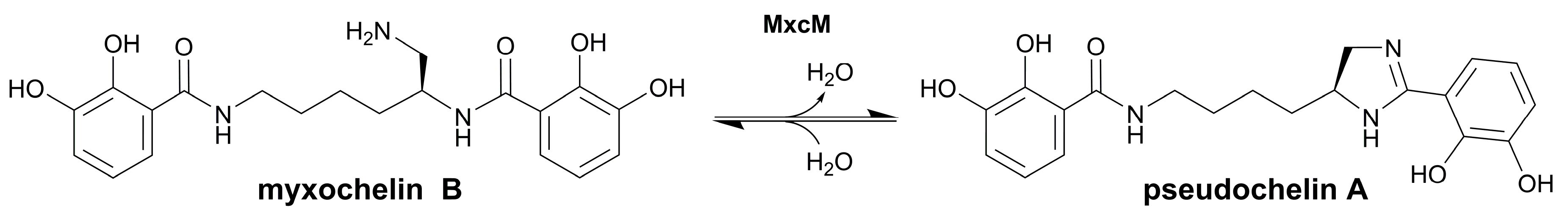
Nature uses different enzyme-catalyzed reactions for the biosynthesis of heterocycles, e.g., alkylation, condensation, decarboxylation, oxidation, or reduction [20,21,22,23]. In recent years, evidence has accumulated that selected enzymes of the amidohydrolase superfamily perform heterocyclizations. While amidohydrolases are usually known to hydrolyze various substrates, the newly identified members catalyze cyclocondensations. In Streptomyces spp., the biosyntheses of different benzoxazole natural products were shown to involve amidohydrolases [24,25,26,27]. For instance, the amidohydrolase NatAM from the nataxazole biosynthetic pathway was demonstrated to convert a substituted 2-aminophenyl benzoate into a benzoxazole via a hemiorthoamide intermediate [27]. A homolog of NatAM was found in the anaerobic bacterium Clostridium cavendishii DSM 21758, which produces meta-substituted benzoxazoles [28]. Another heterocycle-forming amidohydrolase was identified in the myxochelin biosynthesis gene cluster of the marine bacterium Pseudoalteromonas piscicida S2040 [29]. The corresponding enzyme, MxcM, catalyzes an intramolecular condensation of the β-aminoethyl amide moiety in myxochelin B to form an imidazoline ring (Figure 1) [29]. The product of this reaction, pseudochelin A, showed an improved activity against human 5-lipoxygenase compared to other myxochelins, including myxochelin B [30]. Thus, the integration of an imidazoline heterocycle enhanced the anti-inflammatory properties. A preceding study focused on the kinetic and biochemical characterization of the amidohydrolase MxcM [31]. The good stability and the high tolerance towards organic solvents and concentrated salt solutions as well as its cofactor independency imply that this enzyme might be a valuable candidate for integration into chemical process synthesis of heterocyclic compounds. In this study, we further assessed the competence of the amidohydrolase MxcM for utilization in heterocyclic chemistry. For this purpose, we developed a protocol for the immobilization of MxcM on a porous, solid support and characterized the immobilized enzyme with regard to solvent tolerance and storage stability. Subsequently, a packed-bed reactor with immobilized MxcM was designed and used for semicontinuous flow synthesis of imidazoline heterocycles.
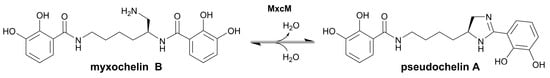
Figure 1.
Reaction catalyzed by the amidohydrolase MxcM. Intramolecular condensation of the β-aminoethyl amide moiety in myxochelin B leads to the formation of the imidazoline moiety in pseudochelin A [29,31].
2. Results and Discussion
2.1. Immobilization of MxcM
2.1.1. Influence of Divalent Metal Ions
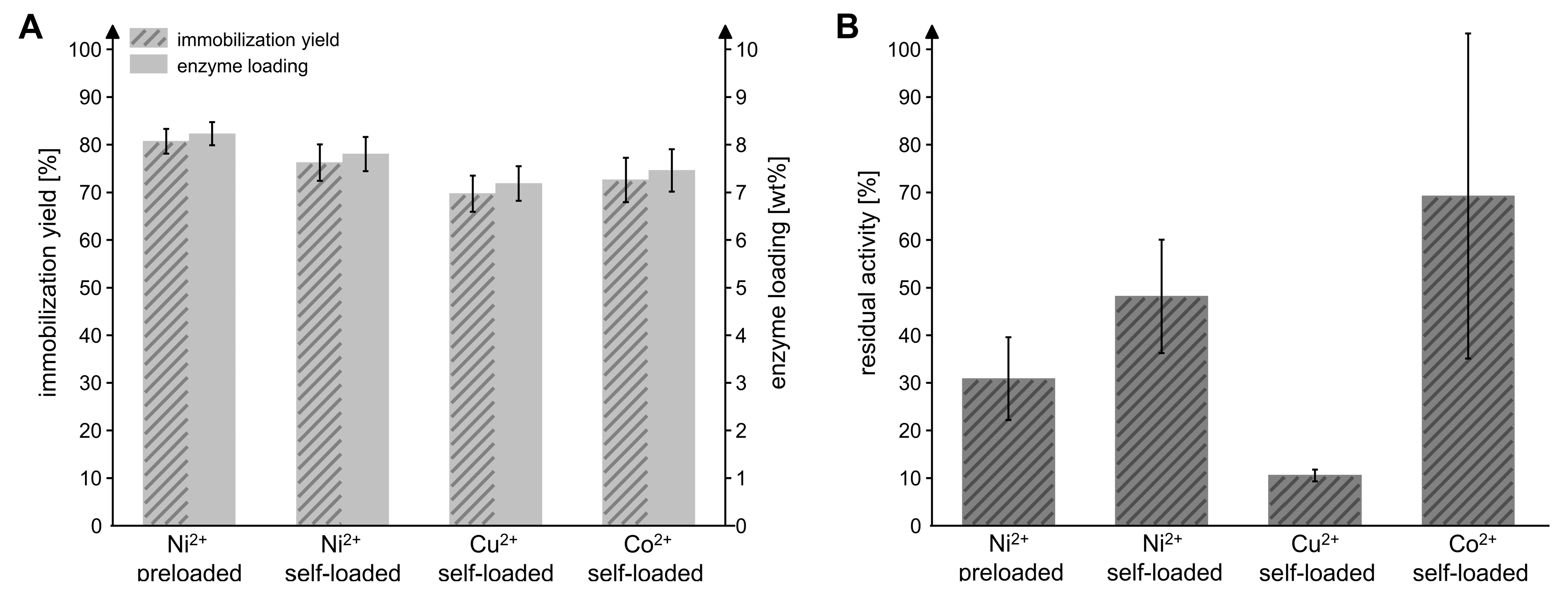
Previous studies demonstrated that the amidohydrolase MxcM is active and stable as a hexahistidine (6xHis)-tagged fusion protein [31]. Because histidine residues promote the binding of a protein to metals on a solid support, a His-tag can be used both for purification and immobilization purposes [32]. Immobilizations based on the metal affinity of His-tags usually result in good enzymatic activity and stability compared to other immobilization methods. Furthermore, they enable the recovery of the support material [33,34]. In this study, recombinantly produced 6xHis-MxcM was immobilized on Chromalite MIDA/M particles. Chromalite MIDA/M is a hydrophilic, polymethacrylic macroporous iminodiacetic acid affinity resin with a mean particle size of 110 µm and a pore diameter of 1000 Å. The resin can be loaded with divalent metal ions that form chelate complexes with the imidazole moieties of the 6xHis-tag. To screen the performance of resins loaded with different metal ions for the immobilization of MxcM, a commercial Chromalite MIDA/M/Ni2+ resin was compared to Ni2+, Cu2+ and Co2+ self-loaded particles. For immobilization, an enzyme-to-particle ratio of 0.1 mgMxcM/mgpdw was applied corresponding to a maximal achievable enzyme loading of 10 wt%. The particle mass refers to the particle dry weight (pdw). With all tested resins, a good immobilization yield of 70–80% and an enzyme loading between 7.2 and 8.2 wt% was achieved (Figure 2A). The most favorable results were obtained with the preloaded Chromalite MIDA/M/Ni2+ particles, which provided an immobilization yield of 80.7 ± 0.3% and an enzyme loading of 8.2 ± 0.2 wt%. For the residual activity of the immobilized enzyme, we observed significant differences (Figure 2B). The MxcM on Ni2+ preloaded particles showed a residual activity of 30.9 ± 8.7% compared to the free enzyme. While the performance of Ni2+ self-loaded particles regarding the enzyme activity was improved to 48.2 ± 11.9%, the Cu2+ self-loaded resin had a residual activity of only 10.6 ± 1.3%. The mean residual activity of MxcM on Co2+ self-loaded Chromalite MIDA/M amounted to 69.2% but exhibited a surprisingly high standard deviation of 34.1%. Generally, many factors can affect the residual activity of an enzyme that is attached to a solid support, e.g., steric hindrance or hydrophobic and ionic interactions [35]. The different metal ions exhibit varying binding affinities to the carrier (Cu2+ > Ni2+ > Co2+) and His-tag selectivity (Cu2+ < Ni2+ < Co2+), which might have influenced the performance of the immobilizate [35,36]. Most amidohydrolases catalyze metal-dependent reactions and, hence, feature one or more metal-binding sites [27,37]. Although experimental data indicate that MxcM does not require metal ions for its biocatalytic activity, a sequence alignment revealed that the crucial metal-coordinating residues are present [31]. For that reason, the metal content as well as the type of divalent metal ion used for immobilization might influence the enzyme’s biocatalytic activity if the metal ions are captured by the putative metal-binding site of MxcM [35,38].
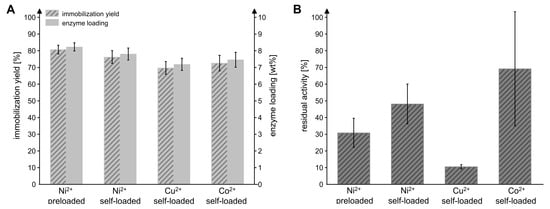
Figure 2.
Influence of the divalent metal ion loaded on Chromalite MIDA/M resin on the immobilization yield, enzyme loading (A) and residual enzymatic activity (B). The Ni2+-preloaded particles were compared to resin that was self-loaded with Ni2+, Cu2+, or Co2+. Activity of free MxcM: 13.5 ± 0.4 U/mgMxcM (Nbiol = 2, Ntech = 3; 0.2 µM MxcM).
2.1.2. Influence of Enzyme Concentration, Immobilization Time, and Enzyme-to-Particle Ratio
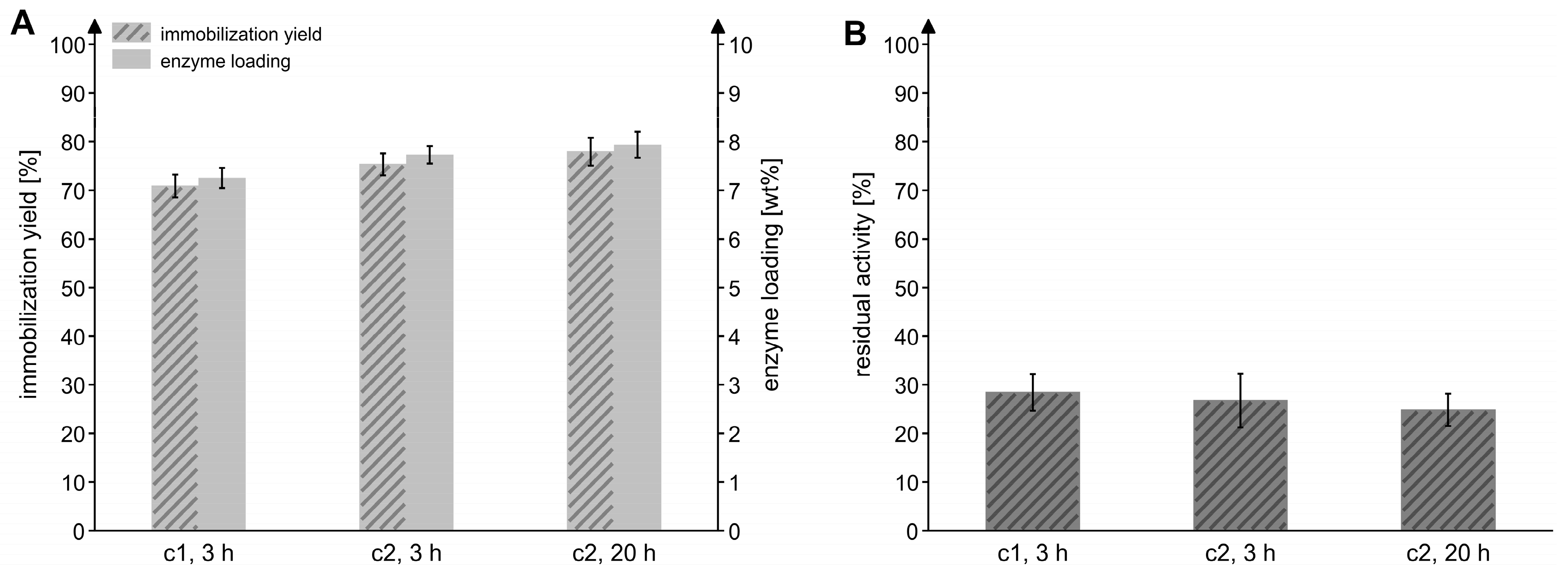
To evaluate the effect of the enzyme concentration and the incubation time during the immobilization process, the Chromalite MIDA/M/Ni2+ resin was incubated with two different concentrations of the enzyme solution for 3 or 20 h at an enzyme-to-particle ratio of 0.1 mgMxcM/mgpdw. As displayed in Figure 3, the higher enzyme concentration and a longer immobilization time marginally improved the yield and loading, but the residual activity was slightly reduced. For this reason, an immobilization time of 3 h and the lower enzyme concentration were applied in the following experiments.
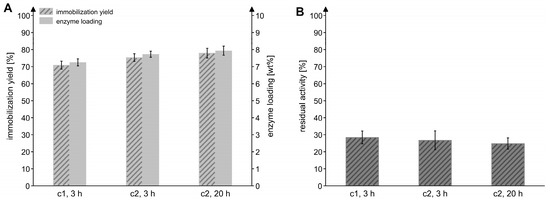
Figure 3.
Effects of enzyme concentration and immobilization time on the immobilization efficiency. (A) Immobilization yield and enzyme loading after varying the concentration of the MxcM solution (c1 = 0.53 mg/mL; c2 = 1.11 mg/mL) and the time used for the immobilization process. Ni2+-preloaded Chromalite MIDA/M particles were used as the immobilization resin (enzyme-to-particle ratio: 0.1 mgMxcM/mgpdw). (B) Residual activity of immobilized MxcM generated in the different immobilization setups. Activity of the free MxcM: 14.5 ± 0.4 U/mgMxcM (Nbiol = 2, Ntech = 3; 0.2 µM MxcM).
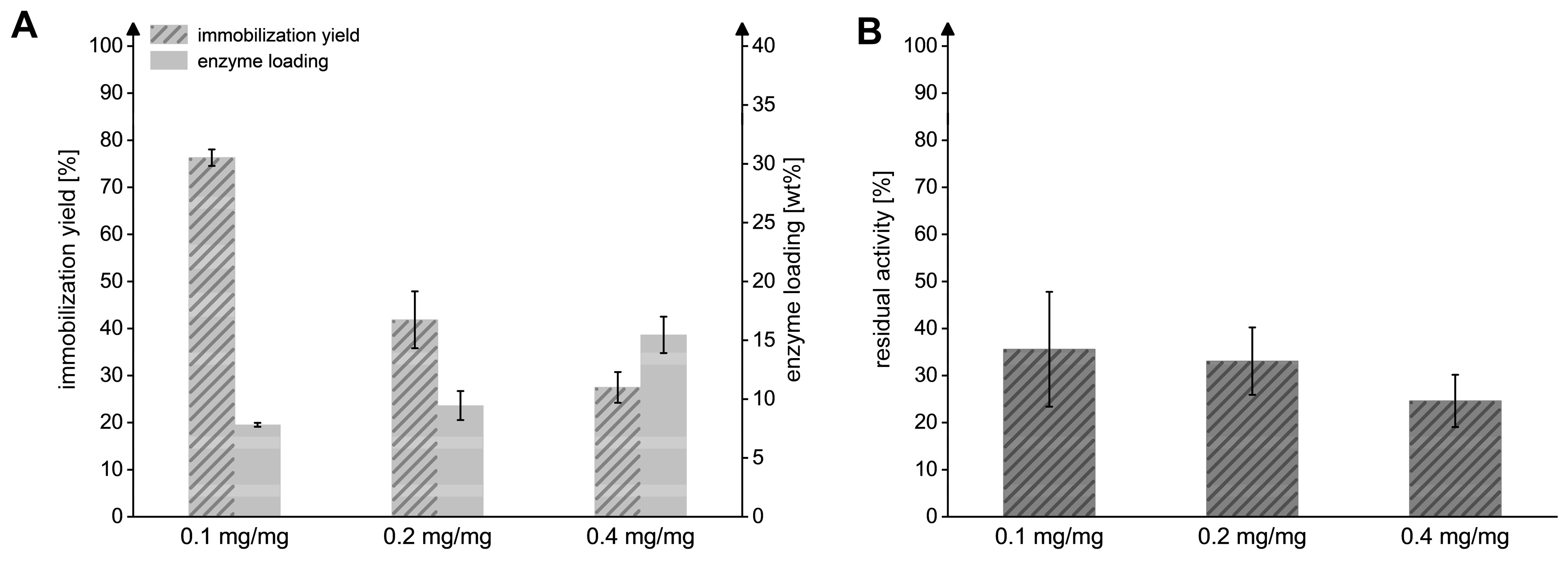
We observed that enhancing the enzyme-to-particle ratios during the immobilization process from 0.1 to 0.2 and 0.4 mgMxcM/mgpdw led to an increased loading of MxcM on the immobilization resin (Figure 4). A ratio of 0.1 mgMxcM/mgpdw effectively resulted in an enzyme loading of 7.8 ± 0.2 wt% and an immobilization yield of 76.3 ± 1.8%. With enzyme-to-particle ratios of 0.2 and 0.4 mgMxcM/mgpdw, the enzyme loading increased by a factor of 1.2 or 2, respectively, while the immobilization yield and residual activity were significantly reduced. The latter can be explained by an increased enzyme concentration on the surface and in the pores of the resin, which might lead to the effects of steric hindrance or altered enzyme–enzyme interactions [39,40]. A maximal loading of 15.5 ± 1.5 wt% was reached with 0.4 mgMxcM/mgpdw. However, because the yield (27.5 ± 3.3%) and residual activity (24.6 ± 5.5%) were notably lowered under these conditions, an enzyme-to-particle ratio of 0.1 mgMxcM/mgpdw was used for the subsequent experiments. Overall, we demonstrate the technical feasibility for the immobilization of a heterocycle-forming amidohydrolase for the first time. This way, the enzyme becomes recyclable, offering new opportunities for process operation.
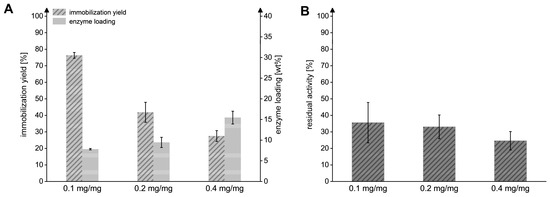
Figure 4.
Influence of the different enzyme-to-particle ratios on the immobilization efficiency. (A) Immobilization yield and enzyme loading reached after the immobilization with ratios of 0.1, 0.2, and 0.4 mgMxcM/mgpdw. (B) Residual activity of immobilized MxcM generated in the three immobilization setups. Activity of the free MxcM: 13.7 ± 0.2 U/mgMxcM (Nbiol = 2, Ntech = 3; 0.2 µM MxcM).
2.1.3. Activity of Immobilized MxcM in Organic Solvents
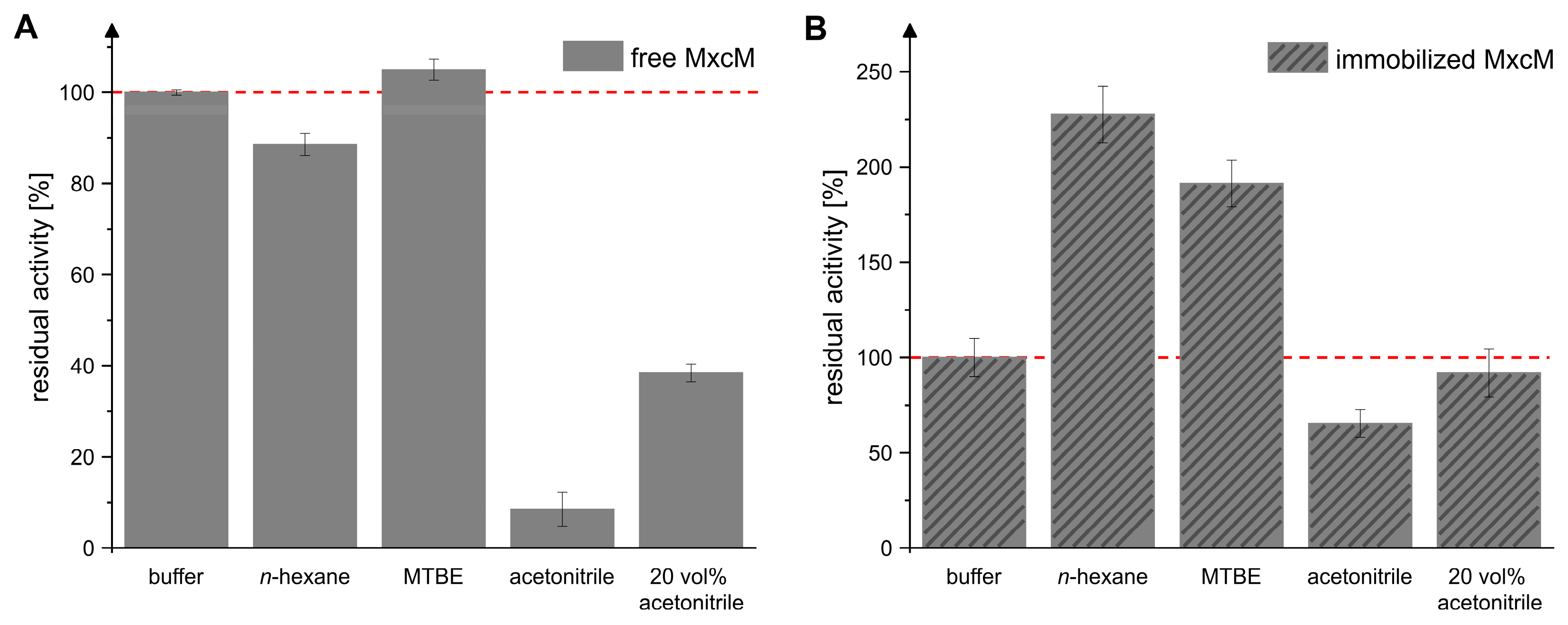
The use of organic solvents can be beneficial for biocatalytic processes, e.g., due to the improved solubility of substrates and products or simplified downstream processing [41,42,43,44]. Previous studies demonstrated that the amidohydrolase MxcM is active in several organic solvents. Herein, the enzymatic activity increased with the log p-value of the organic solvent. High residual activities were observed in hydrophobic solvents. Since MxcM catalyzes a cyclocondensation reaction with water as a byproduct, a reduction in the water activity can be expected to shift the reaction equilibrium towards the product side [31]. Because immobilization is known to ameliorate the solvent tolerance of enzymes [45,46,47], we evaluated this effect for the immobilized MxcM in five different solvent systems (Figure 5). To ensure that the hydration shell of the MxcM was kept intact, 10 vol% water was added to each setup. In accordance with the reported solvent tolerance of the MxcM, we observed high residual activities of the free MxcM in the hydrophobic solvents n-hexane and methyl tertiary-butyl ether (MTBE), while the presence of acetonitrile reduced the enzymatic activity (Figure 5A). Interestingly, the activity of the immobilized MxcM in n-hexane and MTBE was elevated by a factor of 2.3 or 1.9, respectively, compared to the aqueous system (Figure 5B). These hydrophobic solvents formed a liquid two-phase system with water. The immobilized MxcM was found in the aqueous phase. Consequently, the enzymatic reaction occurred in the aqueous phase, where the local concentration of the enzyme was higher than in systems with only one liquid phase. Additionally, transport of the substrate and product between the two liquid phases presumably influenced the measured specific activity. In contrast, the water-miscible solvent acetonitrile had direct contact with the enzyme and disrupted its hydration shell [48,49]. Therefore, the activity in acetonitrile was significantly reduced compared to the aqueous system. Our results indicate that the immobilization positively affected the tolerance of the MxcM towards acetonitrile. The residual activity of the immobilized MxcM in acetonitrile was reduced to 65.4 ± 7.2% compared to the aqueous system (specific activity: 2.2 ± 0.2 U/mgMxcM). Without immobilization, acetonitrile was extremely detrimental for the enzymatic activity (residual activity: 8.5 ± 3.7%; specific activity: 1.3 ± 0.2 U/mgMxcM). If these values are brought into relation, it becomes evident that the immobilization ameliorated the enzyme’s stability in acetonitrile (169.0 ± 18.7% residual activity in acetonitrile after immobilization). Furthermore, the activities of the immobilized MxcM in 20 vol% acetonitrile and in buffer were almost identical (92.0 ± 12.7% residual activity compared to the aqueous system). The hydrophilic surface properties of the immobilization support Chromalite MIDA/M might influence the water distribution in the microenvironment of the enzyme and, thus, protect the enzyme from the detrimental effects of acetonitrile [50,51,52].
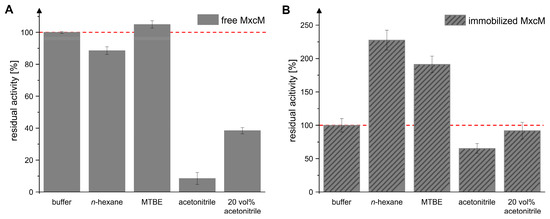
Figure 5.
Residual activity of free (A) and immobilized MxcM (B) in 50 mM phosphate buffer, n-hexane, MTBE, acetonitrile, and 50 mM phosphate buffer with 20 vol% acetonitrile. A total of 10 vol% ddH2O was added to the setups with n-hexane, MTBE, and acetonitrile. The activity of free MxcM in buffer: 15.0 ± 0.1 U/mgMxcM. The red, dashed line indicates the reference activity in buffer, which was set to 100%. The activity of the immobilized MxcM in buffer: 3.3 ± 0.3 U/mgMxcM (Ntech = 3; 0.2 µM MxcM).
2.1.4. Storage Stability of the Immobilized MxcM
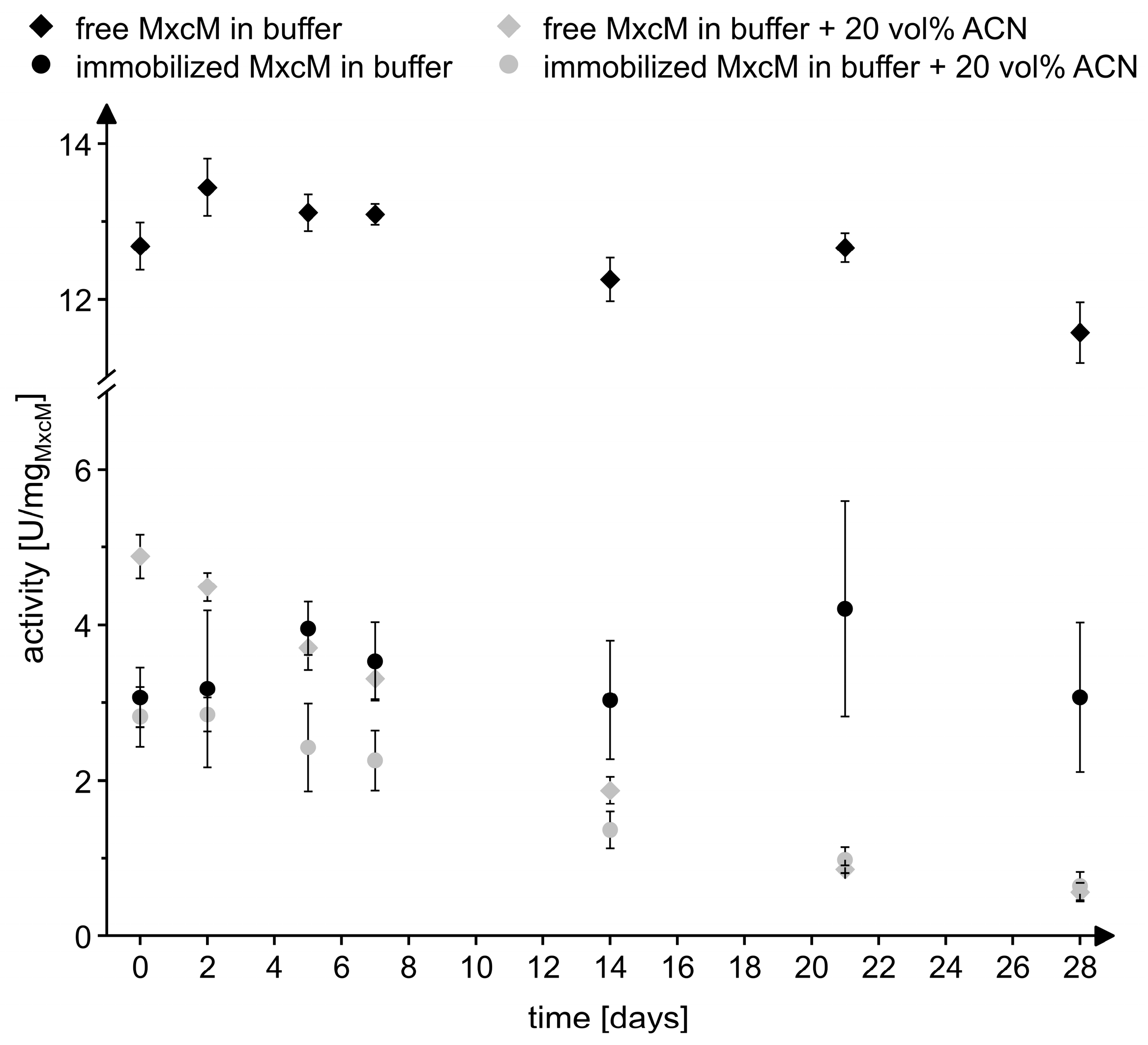
In order to assess the storage stability of the immobilized and free MxcM, the samples were stored for 28 days at 4 °C in phosphate buffer and in phosphate buffer with 20 vol% acetonitrile. At defined time points, the enzyme leaching and residual activity were measured (Figure 6). Only on day 1, a significant amount of enzyme was detected in the supernatant (0.03 ± 0.01 mg/mL), which might have been caused by insufficient washing of the resin on the previous day. On all remaining days of the sampling, no significant enzyme leaching was observed, suggesting a stable binding of the enzyme to the solid support. In buffer, both the immobilized and the free MxcM nearly retained the specific activity. After 28 days of storage, the residual activity of the free MxcM was 91.3 ± 4.2%, while the residual activity of the immobilizate amounted to 89.6 ± 22.9%. As mentioned before, the presence of 20 vol% acetonitrile reduced the specific activity of the free enzyme by approximately 60%, while the activity of the immobilized MxcM was only marginally affected. On day 28, the measured residual activity in 20 vol% acetonitrile compared to day 0 was 11.6 ± 2.7% for the free MxcM and 22.6 ± 5.9% for the immobilized enzyme. For this reason, the immobilization increased the stability in 20 vol% acetonitrile. The reusability, the good storage stability, and the tolerance to organic solvents of the immobilized enzyme prompted us to evaluate the performance of the immobilizate under flow conditions.
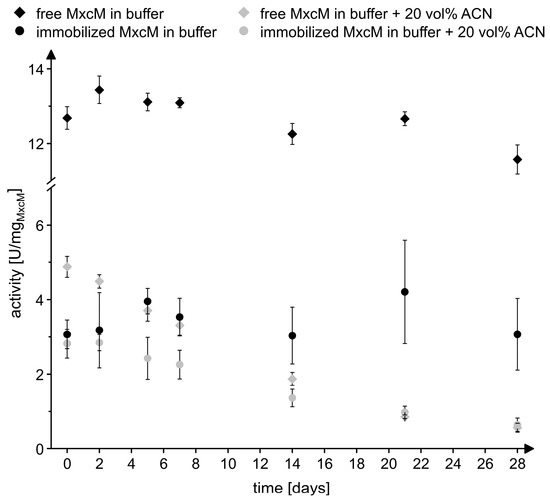
Figure 6.
Storage stability of the free and immobilized MxcM in 50 mM phosphate buffer (pH 8) and 50 mM phosphate buffer with 20 vol% acetonitrile (ACN) stored at 4 °C. The activity of free MxcM: 13.7 ± 0.2 U/mgMxcM (Nbiol = 2, Ntech = 3; 0.2 µM MxcM).
2.2. Design and Characterization of the Immobilized Enzyme Reactor
2.2.1. Reactor Packing
For the preparation of the immobilized enzyme reactor (MxcM-IMER), the immobilization procedure was upscaled, and the immobilizate was packed into empty HPLC columns (50 × 2.1 mm). During the packing process, which was conducted in three iterative runs, the pump pressure was recorded. The maximal packing density was reached with a volumetric flow rate of 1.5 mL/min. The physical parameters of the two separately packed reactors are listed in Table 1. We observed that the immobilization yield after the scale-up was ~10% higher, which can be attributed to a more intensive mixing in the horizontally fixed 5 mL tubes. The pressure drop induced by the packed bed increased in a linear correlation with the volumetric flow rate, indicating that the particles were not deformed (Figure S1). Using the Carman–Kozeny equation (6), the theoretical porosity of the packed bed was calculated to ~20%. The cubic closest packing of a packed bed with ideal spherical particles features a porosity of 26% [53]. This difference can be explained by the particle size distribution of the Chromalite MIDA/M particles.

Table 1.
Parameters of the MxcM-IMERs. The dimensions of the steal columns: 50 × 2.1 mm, empty volume: 173.2 µL.
2.2.2. Residence Time Behavior
To estimate the theoretical residence time, which would be required to reach the reaction equilibrium in an ideal plug flow reactor, we made use of the [𝐸]∙𝜏-concept [54,55]. For this, the reaction time course of the batch reactions with immobilized MxcM was recorded (Figure S2). For further calculations, we anticipated that the reaction equilibrium of 60.5 ± 5.4% pseudochelin A is reached after 30 min with an enzyme concentration of 0.2 µM and 0.5 mM myxochelin B. Using the [𝐸]∙𝜏 approach, a theoretical residence time of 1.3 s was calculated to be required at an enzyme concentration of 0.28 mM to achieve a product yield of 60% for a substrate concentration of 0.5 mM. This theoretical residence time was used to provide a reasonable estimate for the required volumetric flow rate.
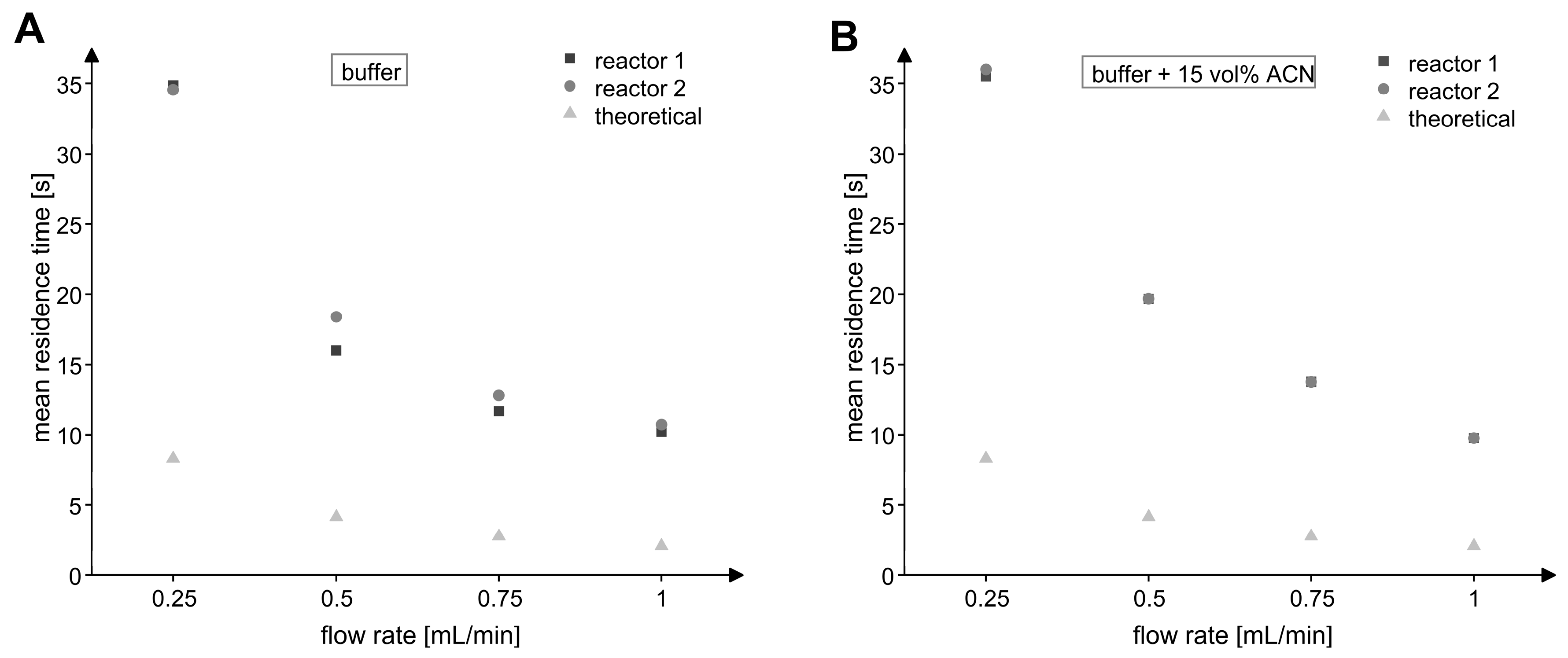
Since it can be assumed that the properties of the MxcM-IMER differed from an ideal plug flow reactor, the real residence times were measured for volumetric flow rates of 0.25 to 1.00 mL/min, which corresponded to theoretical residence times between 8 and 2 s for a theoretical porosity of 20%. For this purpose, we conducted tracer experiments once with phosphate buffer and once with phosphate buffer including 15 vol% acetonitrile as mobile phases. The analysis of the recorded UV profiles revealed that an integration of the flow reactors into the HPLC systems caused a peak broadening of the residence time distribution (Figure S3). For this reason, the mean residence time was calculated and used for the assessment of the residence time behavior (Figure 7). For every tested flow rate, the real residence times were higher than the theoretical ones, which was probably due to backmixing and interactions of the analytes with the particles in the packed bed via pore diffusion and adsorption. In addition, the differences between the theoretical and real porosity would affect the residence times.
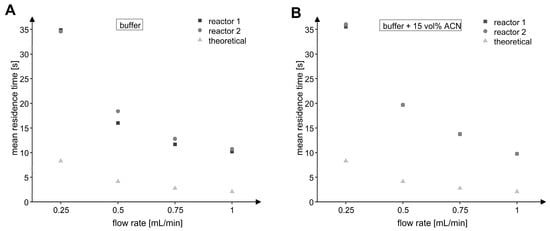
Figure 7.
Mean residence time of analytes in the MxcM-IMERs at different volumetric flow rates. (A) Eluent: 50 mM phosphate buffer (pH 8). (B) Eluent: 50 mM phosphate buffer with 15 vol% acetonitrile. The theoretical residence time was calculated from the packed bed porosity 𝜀 of 20%.
2.2.3. Operation in Semicontinuous Mode
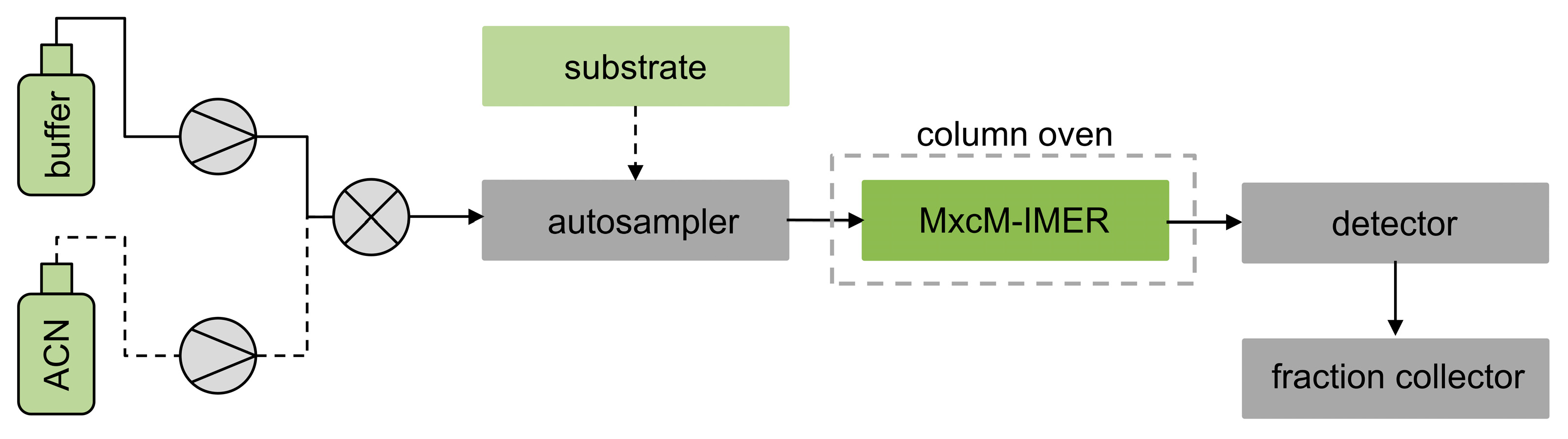
For a semicontinuous mode of operation, the MxcM-IMERs were integrated into the HPLC system and placed into the column oven to achieve a temperature control (Scheme 1). The autosampler was used to inject the substrate myxochelin B into the flow system. Two separate eluent reservoirs, filled with 50 mM phosphate buffer and acetonitrile, allowed the adjustment of the eluent composition.
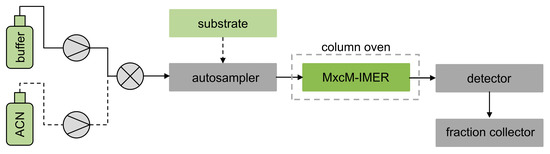
Scheme 1.
Flow chart of an HPLC system with integrated MxcM-IMER for application in the semicontinuous mode; 50 mM phosphate buffer (pH 8) and acetonitrile (ACN) were used as eluents.
First, we evaluated the impact of different volumetric flow rates on myxochelin B conversion (Table 2). The conversion was determined from the mole portion of pseudochelin A at the reactor outlet. The results indicate that the conversion was nearly independent from the residence time. With 100 vol% buffer as mobile phase, the conversion ranged between 25 and 48%. For both reactors, the best conversion was achieved at a flow rate of 0.75 mL/min, which corresponds to a mean residence time of ~12 s. It can be hypothesized that the elevated convection at higher volumetric flow rates reduces the diffusion limitations, which might occur in the packed bed at lower flow rates. Interestingly, the addition of 15 vol% acetonitrile to the mobile phase greatly influenced the performance of the MxcM-IMERs. Under these conditions, the substrate conversion increased to over 90%. The presence of acetonitrile might improve the solubility of the substrate and product in the mobile phase and reduce mass transfer limitations between the bulk phase and the catalytically active particle surface. Acetonitrile might also influence the hydrophobic interactions between the enzymes [56].

Table 2.
Conversion of myxochelin B in a semicontinuous flow system with the MxcM-IMERs under different volumetric flow rates, calculated from the mole portion of pseudochelin A at the reactor outlet; 5 µL of 20 mM myxochelin B (0.1 µmol) were injected into the HPLC system using the autosampler. The experiments were conducted with 50 mM phosphate buffer (pH 8) or 50 mM phosphate buffer with 15 vol% acetonitrile (ACN) (Ntech = 2).
Subsequently, we analyzed the temperature-dependency at flow rates of 0.5 and 0.75 mL/min (Table 3). For both flow rates, a temperature of 30 °C seemed to be advantageous in the flow system. In contrast, previous experiments revealed a temperature optimum of 50 °C for the free MxcM [31]. In batch reactions, the immobilized enzyme also exhibited higher activities at 50 °C (Figure S4). For the MxcM-IMERs, we observed that the temperature had the greatest influence on the conversion in the presence of 15 vol% acetonitrile and a flow rate of 0.5 mL/min. Here, a temperature increase from 30 to 50 °C led to a ~50% reduction in the conversion. All other conditions were only slightly affected by the elevated temperature. The temperature and the presence of polar organic solvents can modify the conformational flexibility and activity of enzymes by influencing hydrogen bonds or hydrophobic interactions [57,58]. In addition, the temperature influences the viscosity of the eluents, which can alter the flow conditions and the mass transport in the flow system.

Table 3.
Temperature-dependent conversion of myxochelin B in a semicontinuous flow system with the MxcM-IMERs at volumetric flow rates of 0.50 and 0.75 mL/min; 5 µL of 20 mM myxochelin B (0.1 µmol) were injected into the HPLC system using the autosampler. The experiments were conducted with 50 mM phosphate buffer (pH 8) or 50 mM phosphate buffer with 15 vol% acetonitrile (ACN) (Ntech = 3).
To assess the process stability of the MxcM-IMERs, we recorded the residual activity after 8 days of operation and 38 semicontinuous runs for the characterization of the reactors (total operation time: ~12 h) at a flow rate of 0.5 mL/min and a temperature of 30 °C. During the downtime periods, the reactors were stored at 4 °C in phosphate buffer. Especially in flow systems, the streaming mobile phases can induce a leaching of the Ni2+-ions and the enzymes, which would lead to a reduction in the enzyme concentration in the reactor [56,59,60]. Compared to day 0, the residual activities were calculated to 102.3 ± 7.6% for reactor 1 and 107.5 ± 5.6% for reactor 2. Consequently, it can be assumed that the enzymes stayed active under the flow operation and that no significant leaching effects occurred in the MxcM-IMERs, providing evidence of the high stability of the enzyme–metal chelate complexes. This result is consistent with several references in the literature, which describe low metal and enzyme leaching and, thus, good stability of His-tag based affinity immobilization resins [34,61,62]. Furthermore, visual inspection of the reactors on day 8 did not reveal a compression of the packed bed, indicating a high mechanical stability of the immobilizate. Since the MxcM-IMERs have not yet reached their maximum performance, the operation time period should be extended in the future to further assess the long-term process stability in flow. Based on these properties, it might also be feasible to operate the MxcM-IMER in the continuous mode.
3. Materials and Methods
3.1. Production and Purification of Myxochelin B and Pseudochelin A
For the production of myxochelin B, M. xanthus FB was cultured in CYE medium (casitone 10 g/L, 5 g/L yeast extract, 2.1 g/L MOPS, 1 g/L MgSO4 × 7 H2O, 0.5 mg/L vitamin B12; pH 7.4) in the presence of 3 wt% of the adsorber resin Amberlite XAD7HP (Sigma Aldrich, Taufkirchen, Germany) and 100 mg/L of the biosynthetic precursor 2,3-dihydroxybenzoic acid. After 7 days of incubation at 30 °C and 130 rpm, the adsorber resin was collected by filtration. Subsequently, the resin was washed with water and adsorbed compounds were eluted with methanol. The eluate was concentrated in a vacuum evaporator (Heidolph Instruments, Schwabach, Germany), and solid particles were removed by centrifugation and filtration. Myxochelin B was isolated from the bacterial raw extract using a semipreparative HPLC system (Shimadzu, Duisburg, Germany) and a Nucleodur 100-5 C18 VarioPrep column (Macherey-Nagel, Düren, Germany) in two consecutive purification steps with the following chromatographic conditions: flow rate—4 mL/min; mobile phases—methanol (MeOH) and water with 0.1 vol% trifluoroacetic acid. Step 1: 0–14 min: 50% MeOH; 14–20 min: 50–100% MeOH; 20–25 min: 100% MeOH; 25–27 min: 100–50% MeOH; 27–20 min: 100% MeOH. Step 2: 50% MeOH isocratic. The identity and purity of the isolated myxochelin B was analyzed via LC-MS and HPLC (Figure S5). Pseudochelin A, which served as an analytical standard, was produced in the recombinant strain M. xanthus DSM16526: pJK5 before [29].
3.2. Enzyme Production and Purification
For the production of 6xHis-tagged MxcM, the recombinant expression strain Escherichia coli BL21(DE3): pET28a(+)-mxcM was cultured according to a previously described protocol [29]. The cells were harvested by centrifugation (4500 rpm, 30 min, 4 °C), the supernatant was discarded, and the cell pellet was resuspended in lysis buffer (50 mM NaH2PO4, 10 mM imidazole, 300 mM NaCl, and 10 vol% glycerol). The cell lysis was conducted via pressure homogenization in a French press at 69 bars. Subsequently, the cell debris was removed by centrifugation (19,000 rpm, 30 min, and 4 °C). For the isolation of the 6xHis-tagged MxcM, an FPLC system (ÄKTA pure, Cytiva Europe, Freiburg, Germany) was used in combination with HisTrap FF crude 1 mL columns (Cytiva Europe). First, the columns were washed with 5 column volumes (CV) water and equilibrated with 5 CV binding buffer (lysis buffer with 40 mM imidazole) at a flow rate of 1 mL/min. Afterwards, the protein sample was applied and washed with 10 CV binding buffer. For protein elution, the amount of elution buffer (lysis buffer with 500 mM imidazole) was successively increased to 100 vol% over 10 CV and kept at 100 vol% for 5 CV. Then, the column was washed with 10 CV binding buffer. Finally, the isolated enzyme was desalted using the PD-10 desalting column (Cytiva Europe) according to the manufacturer’s specification. For the column equilibration and protein elution, 100 mM phosphate buffer (pH 8) was used. The concentration of protein samples was determined by use of the NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, Dreieich, Germany). The purity of the protein solution was analyzed via SDS PAGE (Figure S6).
3.3. Immobilization
The purified 6xHis-tagged MxcM was immobilized on a porous Chromalite MIDA/M (Purolite, an Ecolab Company, Ratingen, Germany) support, which was delivered as wet particles. To determine the actual residual moisture, a defined mass of wet particles was dried for 2 h at 60 °C in a SpeedVac vacuum concentrator (Eppendorf, Hamburg, Germany). Afterwards, the particle dry weight was measured, and the residual moisture was calculated. For the equilibration, 20 µL phosphate buffer (50 mM NaH2PO4 × 2 H2O, pH 8) were added per mg wet particles. For the loading of the Chromalite MIDA/M with Ni2+, Cu2+, or Co2+, the wet resin particles were suspended in a 10 mM metal salt solution (NiSO4 × 6 H2O, CoSO4 × 7 H2O, CuCl2 × 2 H2O; 30 µL per mg resin). This suspension was incubated for 3 h at 21 °C and 200 rpm. Subsequently, the particles were washed four times with 20 µL phosphate buffer per mg wet particles. A defined amount of enzyme solution and the immobilization resin were incubated for 3 h (or 20 h) at 21 °C and 200 rpm. The required volume of the enzyme solution was determined from the enzyme-to-particle ratio and the particle dry weight. After the immobilization, the supernatant was removed and the immobilizate was washed two times with 30 µL phosphate buffer per mg wet particles. The amount of remaining free MxcM in the solution was measured spectrophotometrically. The amount of immobilized enzyme was calculated from a mass balance. The immobilization yield Yimmo (1) and the enzyme loading Wimmo (2) were determined as criteria for the evaluation of the immobilization process.
where mimmo [mg] = mass of immobilized enzyme; cenz [mg/mL] = concentration of the enzyme stock solution; Venz [mL] = volume of enzyme solution; and mPDW [mg] = particle dry weight.
For the determination of the residual activity of the immobilized enzyme, the specific activity of the immobilizate and the free enzyme were measured. The reactions with the free MxcM were performed in 50 mM phosphate buffer (pH 8) with 0.2 µM MxcM and 0.5 mM myxochelin B in a total volume of 250 µL. For the reactions with the immobilized MxcM, the total volume was adjusted to achieve the same enzyme–substrate ratio. Prior to the addition of the substrate, all reactions were preheated to 30 °C. Then, myxochelin B was added and, after 2 min incubation at 30 °C, the reactions were stopped by heat (99 °C, 5 min, 300 rpm). For the removal of the denatured enzyme or the immobilization resin, the reaction mixtures were centrifuged for 2 min at 13,000 rpm. The supernatant was filtered and analyzed by reversed phase HPLC using a Nucleodur Sphinx RP column (150 × 2 mm, 3 μm, Macherey Nagel). The separation of the substrate and product was achieved with an isocratic method (13 vol% acetonitrile, 87 vol% water with 0.1 vol% trifluoracetic acid; flow rate: 0.4 mL/min). The substrate conversion was determined from the peak areas at a wavelength of 254 nm and used for the calculation of the specific activity. Each experiment was performed in two biological (Nbiol) and three technical replicates (Ntech).
3.4. Activity in Organic Solvents and Storage Stability of Immobilized MxcM
To analyze the performance of the immobilized MxcM in organic solvents, the reactions with free and immobilized enzyme were conducted in 90 vol% n-hexane, MTBE, and acetonitrile and 10 vol% ddH2O. Additionally, a setup of 20 vol% acetonitrile and 80 vol% phosphate buffer was evaluated. For each setup, the specific enzymatic activity was determined and used for the calculation of the residual activity regarding immobilization, which describes the effect of the immobilization on the specific activity in each solvent system (Ntech = 3).
The storage stability of the immobilized and free MxcM was evaluated in phosphate buffer and in phosphate buffer with 20 vol% acetonitrile. In these media, the enzyme was stored at 4 °C for 28 days. On defined days of sampling, the specific activity was determined (Nbiol = 2, Ntech = 3). For the investigation of the enzyme leaching, the protein concentration in the supernatant was measured. The supernatant was removed and replaced by fresh storage medium.
3.5. Reactor Packing
First, an empty stainless-steel HPLC column (50 × 2.1 mm, SCP Seitz Chromatographie Produkte, Weiterstadt, Germany) was integrated into the HPLC system (Shimadzu) to record the pressure drop induced by the system at volumetric flow rates of 0.50, 0.75, 1.00, 1.25, and 1.50 mL/min. The immobilization process described in Section 3.3 was upscaled to obtain a sufficient amount of immobilizate. Afterwards, the immobilizate was packed into the empty column. To achieve a homogenous packed bed, the MxcM-IMER was installed into the HPLC system, and the immobilizate was compressed by successively increasing the flow rate from 0.50 to 1.50 mL/min. Each flow rate was maintained for 5 min. Because the height of the packed bed was significantly reduced after the first and second packing iterations, the empty volume was refilled with immobilizate. After the third packing iteration, the height of the packed bed remained constant. The system pressure with integrated MxcM-IMER was measured at different flow rates and used for the calculation of the pressure drop induced by the packed bed (Δpparticles). Assuming that the pressure drop induced by the fittings and the fluid are negligibly small compared to Δpparticles, the equation (3) can be used to describe the correlation between Δpparticles and the friction coefficient (λ), the column length (LS), the mean particle diameter (dp), the fluid density (ρF), and the empty tube velocity () [63,64].
For a laminar flow (Rep < 20), the friction coefficient depends on the porosity of the packed bed (ε) and the Reynolds number (Rep) (4). The Reynolds number depends on , dp, ρF and on the dynamic viscosity (ηF) (5). From Equations (3)–(5), the Carman–Kozeny equation can be deduced (6). The theoretical porosity of the packed bed (ε) is calculated from (6) by the iterative target value search.
3.6. Characterization of the Residence Time Behavior
By use of the integrated UV detector, the residence time distribution of the analytes in the reactor was recorded. The integration of the MxcM-IMER led to a significant peak broadening of the residence time distribution compared to the empty system. For that reason, the mean residence time was calculated from the residence time distribution and used for the evaluation of the residence time behavior (Figure S7).
3.7. Characterization of the Reactor Performance in Semicontinuous Mode
The influence of the different reaction parameters (flow rate, temperature, and mobile phase composition) was tested in a semicontinuous mode of operation. For this, the MxcM-IMER was installed into the column oven of the HPLC system. Prior to the biocatalytic reaction, the reactor was preheated and equilibrated with the required mobile phase for 30 min. The substrate myxochelin B (0.1 µmol) was injected using the autosampler. The reaction mixture from the reactor output was collected and separately analyzed via HPLC-MS (Ntech = 2–3 per reactor).
4. Conclusions
The impressive developments in the fields of molecular biology, bioinformatics, and process engineering have spurred the discovery and engineering of new biocatalysts as well as biocatalytic processes [65,66,67,68]. For this reason, biocatalysis has become a powerful tool for the synthesis of heterocyclic compounds, which are of particular interest for the pharmaceutical industry. Some years ago, genome mining revealed a new enzyme belonging to the superfamily of amidohydrolases that catalyzes the synthesis of imidazoline heterocycles [29]. This enzyme, MxcM, was recently shown to exhibit promising activity and stability properties [31]. In this study, we successfully established an immobilization process for the amidohydrolase MxcM based on immobilized metal affinity chromatography. Our immobilization protocol leads to immobilization yields of ~75% and enzyme loadings between 7 and 8 wt% for an enzyme-to-particle ratio of 0.1 mgMxcM/mgpdw. In buffer, the remaining activity of the immobilized MxcM amounted to 30–40% compared to the free enzyme. We observed that higher enzyme loadings negatively affected the residual activity, probably caused by steric hindrance and modified enzyme–enzyme interactions on the particle surface. The immobilization of MxcM improved the enzyme’s solvent stability. The specific activity of the immobilized enzyme in n-hexane and MTBE was doubled compared to the aqueous system. In the polar solvent acetonitrile, the immobilized enzyme was even more active than the free enzyme (residual activity: 169%). Additionally, the storage stability in the presence of 20 vol% acetonitrile was increased through immobilization. Due to the good solvent and storage stability and the fact that no cofactors are required, we further evaluated the performance of the immobilized MxcM for biocatalysis in flow. For this purpose, packed bed-reactors were designed and installed into an HPLC system. Interestingly, the composition of the mobile phase greatly influenced the conversion, while the residence time and the temperature had only minor impact under the tested conditions. The MxcM-IMERs featured a good operational stability, indicating that no significant leaching events occurred and that the enzyme remained stable under operation in flow. In the future, the presented HPLC-coupled flow system can be used to screen the substrate scope of the amidohydrolase MxcM for the synthesis of imidazoline-containing, heterocyclic compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13020229/s1, Figure S1: Pressure stability of the MxcM-IMERs; Figure S2: Reaction time course of a batch reaction; Figure S3: UV profile at 254 nm after injection of 0.1 µmol myxochelin B into the HPLC system with and without integration of the MxcM-IMERs; Figure S4: Influence of the reaction temperature on the specific activity of the immobilized MxcM in the batch reactions; Figure S5: Quality control of myxochelin B after semi-preparative HPLC purification. Figure S6: SDS PAGE of the purified 6xHis-MxcM; Figure S7: Determination of the mean residence time (τmean) from the residence time distribution.
Author Contributions
Conceptualization, L.W., S.T., S.L., K.R. and M.N.; methodology, L.W. and S.T.; formal analysis, L.W. and S.T.; investigation, L.W. and S.T.; writing—original draft preparation, L.W.; writing—review and editing, L.W., S.L., K.R. and M.N.; visualization, L.W.; supervision, M.N.; project administration, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support from the European Regional Development Fund (grant no. EFRE-0300098 to M.N.) is gratefully acknowledged.
Data Availability Statement
The data presented in this study are available in the supplementary materials.
Acknowledgments
We thank Katharina Kuhr (Laboratory of Technical Biology, TU Dortmund University) for the preparation and purification of myxochelin B.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alvarez-Builla, J.; Vaquero, J.J.; Barluenga, J. Modern Heterocyclic Chemistry; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-63406-4. [Google Scholar]
- Quin, L.D. Fundamentals of Heterocyclic Chemistry: Importance in Nature and in the Synthesis of Pharmaceuticals; Online-Ausg; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2010; ISBN 9780470626535. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Lamberth, C. Heterocyclic chemistry in crop protection. Pest Manag. Sci. 2013, 69, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2018, 71, 153–184. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Comp. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, M.; Tan, W.; Zheng, L.; Tao, K.; Fan, X. Developments towards synthesis of N-heterocycles from amidines via C–N/C–C bond formation. Org. Chem. Front. 2019, 6, 2120–2141. [Google Scholar] [CrossRef]
- Abdella, A.M.; Abdelmoniem, A.M.; Abdelhamid, I.A.; Elwahy, A.H.M. Synthesis of heterocyclic compounds via Michael and Hantzsch reactions. J. Heterocycl. Chem. 2020, 57, 1476–1523. [Google Scholar] [CrossRef]
- Murlykina, M.V.; Morozova, A.D.; Zviagin, I.M.; Sakhno, Y.I.; Desenko, S.M.; Chebanov, V.A. Aminoazole-Based Diversity-Oriented Synthesis of Heterocycles. Front. Chem. 2018, 6, 527. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E.A.; Cravotto, G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020, 10, 14170–14197. [Google Scholar] [CrossRef]
- Ley, S.V. On being green: Can flow chemistry help? Chem. Rec. 2012, 12, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.G.; Jensen, K.F. The role of flow in green chemistry and engineering. Green Chem. 2013, 15, 1456. [Google Scholar] [CrossRef]
- Sharma, U.K.; van der Eycken, E.V. Flow Chemistry for the Synthesis of Heterocycles; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-94327-5. [Google Scholar]
- Pastre, J.C.; Browne, D.L.; Ley, S.V. Flow chemistry syntheses of natural products. Chem. Soc. Rev. 2013, 42, 8849–8869. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Brady, D. Streamlining Design, Engineering, and Applications of Enzymes for Sustainable Biocatalysis. ACS Sustain. Chem. Eng. 2021, 9, 8032–8052. [Google Scholar] [CrossRef]
- Pinho e Melo, T.M.; Pineiro, M. Heterocycles: Synthesis, Catalysis, Sustainability, and Characterization; Wiley-VCH: Weinheim, Germany, 2022; ISBN 978-3-527-83200-2. [Google Scholar]
- Lechner, H.; Pressnitz, D.; Kroutil, W. Biocatalysts for the formation of three- to six-membered carbo- and heterocycles. Biotechnol. Adv. 2015, 33, 457–480. [Google Scholar] [CrossRef][Green Version]
- Diana, P.; Cirrincione, G. Biosynthesis of Heterocycles; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; ISBN 9781118960554. [Google Scholar]
- Hemmerling, F.; Hahn, F. Biosynthesis of oxygen and nitrogen-containing heterocycles in polyketides. Beilstein J. Org. Chem. 2016, 12, 1512–1550. [Google Scholar] [CrossRef]
- Meng, S.; Tang, G.-L.; Pan, H.-X. Enzymatic Formation of Oxygen-Containing Heterocycles in Natural Product Biosynthesis. ChemBioChem 2018, 19, 2002–2022. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yang, B.; Feng, X.; Li, C. Recent advances in the biosynthesis strategies of nitrogen heterocyclic natural products. Nat. Prod. Rep. 2022, 39, 139–162. [Google Scholar] [CrossRef]
- Cano-Prieto, C.; García-Salcedo, R.; Sánchez-Hidalgo, M.; Braña, A.F.; Fiedler, H.-P.; Méndez, C.; Salas, J.A.; Olano, C. Genome Mining of Streptomyces sp. Tü 6176: Characterization of the Nataxazole Biosynthesis Pathway. ChemBioChem 2015, 16, 1461–1473. [Google Scholar] [CrossRef]
- Lv, M.; Zhao, J.; Deng, Z.; Yu, Y. Characterization of the Biosynthetic Gene Cluster for Benzoxazole Antibiotics A33853 Reveals Unusual Assembly Logic. Chem. Biol. 2015, 22, 1313–1324. [Google Scholar] [CrossRef]
- Losada, A.A.; Cano-Prieto, C.; García-Salcedo, R.; Braña, A.F.; Méndez, C.; Salas, J.A.; Olano, C. Caboxamycin biosynthesis pathway and identification of novel benzoxazoles produced by cross-talk in Streptomyces sp. NTK 937. Microb. Biotechnol. 2017, 10, 873–885. [Google Scholar] [CrossRef]
- Song, H.; Rao, C.; Deng, Z.; Yu, Y.; Naismith, J.H. The Biosynthesis of the Benzoxazole in Nataxazole Proceeds via an Unstable Ester and has Synthetic Utility. Angew. Chem. Int. Ed. 2020, 59, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Horch, T.; Molloy, E.M.; Bredy, F.; Haensch, V.G.; Scherlach, K.; Dunbar, K.L.; Franke, J.; Hertweck, C. Alternative Benzoxazole Assembly Discovered in Anaerobic Bacteria Provides Access to Privileged Heterocyclic Scaffold. Angew. Chem. Int. Ed. 2022, 61, e202205409. [Google Scholar] [CrossRef]
- Korp, J.; Winand, L.; Sester, A.; Nett, M. Engineering Pseudochelin Production in Myxococcus xanthus. Appl. Environ. Microbiol. 2018, 84, e01789-18. [Google Scholar] [CrossRef]
- Sester, A.; Winand, L.; Pace, S.; Hiller, W.; Werz, O.; Nett, M. Myxochelin- and Pseudochelin-Derived Lipoxygenase Inhibitors from a Genetically Engineered Myxococcus xanthus Strain. J. Nat. Prod. 2019, 82, 2544–2549. [Google Scholar] [CrossRef]
- Winand, L.; Vollmann, D.J.; Hentschel, J.; Nett, M. Characterization of a Solvent-Tolerant Amidohydrolase Involved in Natural Product Heterocycle Formation. Catalysts 2021, 11, 892. [Google Scholar] [CrossRef]
- Kuo, W.-H.K.; Chase, H.A. Exploiting the interactions between poly-histidine fusion tags and immobilized metal ions. Biotechnol. Lett. 2011, 33, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.; Holtmann, D.; Mangold, K.-M.; Schrader, J. Immobilization of histidine-tagged proteins on electrodes. Colloids Surf. B Biointerfaces 2011, 88, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Brown, M.S.; Cruz-Izquierdo, A.; Martinez, C.A.; Serban, S. Optimization of Metal Affinity Ketoreductase Immobilization for Application in Batch and Flow Processes. Org. Process Res. Dev. 2022, 26, 2075–2084. [Google Scholar] [CrossRef]
- Ueda, E.; Gout, P.; Morganti, L. Current and prospective applications of metal ion–protein binding. J. Chromatogr. A 2003, 988, 1–23. [Google Scholar] [CrossRef]
- Janson, J.-C. Protein Purification: Principles, High Resolution Methods, and Applications; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2011; ISBN 9780470940075. [Google Scholar]
- Sugrue, E.; Fraser, N.J.; Hopkins, D.H.; Carr, P.D.; Khurana, J.L.; Oakeshott, J.G.; Scott, C.; Jackson, C.J. Evolutionary expansion of the amidohydrolase superfamily in bacteria in response to the synthetic compounds molinate and diuron. Appl. Environ. Microbiol. 2015, 81, 2612–2624. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, G.; Zhao, Y.-J.; Porath, J. Carboxypeptidase a: A model for studying the interaction of proteins with immobilized metal ions. J. Inorg. Biochem. 1986, 26, 127–135. [Google Scholar] [CrossRef]
- Zaak, H.; Siar, E.-H.; Kornecki, J.F.; Fernandez-Lopez, L.; Pedrero, S.G.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of immobilization rate and enzyme crowding on enzyme stability under different conditions. The case of lipase from Thermomyces lanuginosus immobilized on octyl agarose beads. Process Biochem. 2017, 56, 117–123. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Effects of Enzyme Loading and Immobilization Conditions on the Catalytic Features of Lipase From Pseudomonas fluorescens Immobilized on Octyl-Agarose Beads. Front. Bioeng. Biotechnol. 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Villela Filho, M.; Stillger, T.; Müller, M.; Liese, A.; Wandrey, C. Is log P a Convenient Criterion to Guide the Choice of Solvents for Biphasic Enzymatic Reactions? Angew. Chem. Int. Ed. 2003, 42, 2993–2996. [Google Scholar] [CrossRef]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Immobilized enzymes in ordered mesoporous silica materials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater. 2001, 44–45, 755–762. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Li, Q.; Fan, F.; Wang, Y.; Feng, W.; Ji, P. Enzyme Immobilization on Carboxyl-Functionalized Graphene Oxide for Catalysis in Organic Solvent. Ind. Eng. Chem. Res. 2013, 52, 6343–6348. [Google Scholar] [CrossRef]
- Lousa, D.; Baptista, A.M.; Soares, C.M. A molecular perspective on nonaqueous biocatalysis: Contributions from simulation studies. Phys. Chem. Chem. Phys. 2013, 15, 13723–13736. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, X.; Zhou, H.; Liu, Y.; Secundo, F.; Liu, Y. Enzyme Stability and Activity in Non-Aqueous Reaction Systems: A Mini Review. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.d.; Ebert, C.; Garau, G.; Gardossi, L.; Linda, P. Penicillin G amidase in low-water media: Immobilisation and control of water activity by means of celite rods. J. Mol. Catal. B Enzym. 1999, 6, 437–445. [Google Scholar] [CrossRef]
- Basso, A.; Martin, L.d.; Ebert, C.; Gardossi, L.; Linda, P. High isolated yields in thermodynamically controlled peptide synthesis in toluene catalysed by thermolysin adsorbed on Celite R-640. Chem. Commun. 2000, 6, 467–468. [Google Scholar] [CrossRef]
- Conway, J.; Sloane, N.J.A. Sphere Packings, Lattices and Groups, 3rd ed.; Softcover Version of Original Hardcover Edition 1999; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-3134-4. [Google Scholar]
- Bommarius, A.S.; Riebel, B. Biocatalysis: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2002; ISBN 3-527-30344-8. [Google Scholar]
- Liese, A.; Seelbach, K.; Wandrey, C. Industrial Biotransformations, 2nd completely rev. and extended ed.; Wiley-VCH: Weinheim, Germany, 2010; ISBN 3-527-31001-0. [Google Scholar]
- Cheung, R.C.F.; Wong, J.H.; Ng, T.B. Immobilized metal ion affinity chromatography: A review on its applications. Appl. Microbiol. Biotechnol. 2012, 96, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Dachuri, V.; Boyineni, J.; Choi, S.; Chung, H.-S.; Jang, S.-H.; Lee, C. Organic solvent-tolerant, cold-adapted lipases PML and LipS exhibit increased conformational flexibility in polar organic solvents. J. Mol. Catal. B Enzym. 2016, 131, 73–78. [Google Scholar] [CrossRef]
- Li, C.; Tan, T.; Zhang, H.; Feng, W. Analysis of the conformational stability and activity of Candida antarctica lipase B in organic solvents: Insight from molecular dynamics and quantum mechanics/simulations. J. Biol. Chem. 2010, 285, 28434–28441. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, G.P. Comparative Studies on the Metal Sorption Characteristics of Chelating Gels for Immobilized Metal Ion Affinity Chromatography. Sep. Sci. Technol. 2002, 37, 3491–3511. [Google Scholar] [CrossRef]
- Tamborini, L.; Previtali, C.; Annunziata, F.; Bavaro, T.; Terreni, M.; Calleri, E.; Rinaldi, F.; Pinto, A.; Speranza, G.; Ubiali, D.; et al. An Enzymatic Flow-Based Preparative Route to Vidarabine. Molecules 2020, 25, 1223. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, W.; Knaus, T.; Volkov, A.; Slot, T.K.; Shiju, N.R.; Engelmark Cassimjee, K.; Mutti, F.G. Highly efficient production of chiral amines in batch and continuous flow by immobilized ω-transaminases on controlled porosity glass metal-ion affinity carrier. J. Biotechnol. 2019, 291, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Liang, M.-R.; Lin, Y.-C.; Chen, C.-T. Specifically and Reversibly Immobilizing Proteins/Enzymes to Nitriolotriacetic-Acid-Modified Mesoporous Silicas through Histidine Tags for Purification or Catalysis. Chem. Eur. J. 2011, 17, 13059–13067. [Google Scholar] [CrossRef]
- Bohnet, M. Mechanische Verfahrenstechnik; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2003; ISBN 9783527663569. [Google Scholar]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 5th ed.; McGraw-Hill: New York, NY, USA, 1993; ISBN 0071127380. [Google Scholar]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef]
- Rosenthal, K.; Lütz, S. Recent developments and challenges of biocatalytic processes in the pharmaceutical industry. Curr. Opin. Green Sustain. Chem. 2018, 11, 58–64. [Google Scholar] [CrossRef]
- Schwarz, J.; Rosenthal, K.; Snajdrova, R.; Kittelmann, M.; Lütz, S. The Development of Biocatalysis as a Tool for Drug Discovery. Chimia (Aarau) 2020, 74, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Kinner, A.; Nerke, P.; Siedentop, R.; Steinmetz, T.; Classen, T.; Rosenthal, K.; Nett, M.; Pietruszka, J.; Lütz, S. Recent Advances in Biocatalysis for Drug Synthesis. Biomedicines 2022, 10, 964. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).