Abstract
This article discusses the potential applications of the Fuel Shot liquid catalyst in compression ignition (CI) engines for reducing toxic substances in exhaust gases. Incorporating catalysts into fuel can optimize the combustion process, consequently reducing the emission of toxic substances into the atmosphere. Toxic compounds, such as nitrogen oxides, particulate matter, and hydrocarbons, adversely affect flora and fauna. Various methods are known for reducing their concentration in engine exhaust gases, one of which is the Fuel Shot liquid catalyst. The authors conducted experiments on a Fiat 1.3 JTD engine with a Common Rail system. The results indicate that the application of the liquid catalyst reduces the content of nitrogen oxides and hydrocarbons in the exhaust gases and slightly decreases fuel consumption. Additionally, investigations were carried out on the engine’s injection apparatus, which was fueled with modified fuel. The findings demonstrate that the fuel additive does not affect the wear of precision parts of fuel injectors and high-pressure pumps.
1. Introduction
The aim of the conducted research is to analyze the operation of a modern Common Rail engine running on conventional fuel, to which a liquid Eco Fuel Shot catalyst has been added. The purpose of the catalyst is to reduce toxic substances in the exhaust gases. According to the manufacturer, the Eco Fuel Shot additive is a selective catalyst that does not deteriorate the physical and chemical properties of the fuel, and its task is to reduce toxic compounds in the exhaust gases, especially nitrogen oxides, and to decrease fuel consumption.
Catalysts in automotive vehicles are primarily located in the exhaust systems. Their role is to neutralize toxic substances such as nitrogen oxides, hydrocarbons, carbon monoxide, and particulate matter. These types of catalytic reactors do not influence the combustion process but assist in purifying the exhaust gases [1]. The liquid catalyst’s function is to support combustion processes and to prevent the formation of and neutralize toxic substances during combustion. Additionally, the catalyst prevents the formation of hydrocarbon bacteria in the fuel, which is responsible for aging processes. The Eco Fuel Shot catalyst was used in the fuel. Analyzing the literature, one way to utilize catalytic converters is their installation in the combustion chamber of CI engines. In study [2], the authors used catalysts based on zinc and vanadium. The research was conducted on a single-cylinder four-stroke CI engine. The results showed a reduction in nitrogen oxides and hydrocarbons by 70%, carbon monoxide by 60%, and carbon dioxide by 50%. In study [3], the results of engine characteristic measurements operating on biofuel from lemon oil were presented. Additionally, engine components such as the piston crown, cylinder head, and valves were coated with a stabilized zirconia catalyst. After conducting the research, it was observed that the engine operating on biofuel with a catalyst installed on its components showed lower emissions of carbon monoxide, hydrocarbons, nitrogen oxides, and smoke compared to a standard engine fueled with conventional fuel. In study [4], gaseous fuels such as hydrogen and methane and natural gas were used. The fuels were mixed with liquid fuel in a 50:50 ratio. A platinum–aluminum catalyst was installed in the engine. The research showed that the catalytic converter reduces nitrogen oxides. Additionally, the fuel mixture with hydrogen caused an increase in cylinder pressure and emissions of nitrogen oxides and hydrocarbons and a reduction in smoke. However, using natural gas and methane reduced the emission of nitrogen oxides. Additionally, the research showed that using an additional catalyst reduces smoke for these fuels. In CI engines, a substance containing ammonia AdBlue is used for the catalytic removal of nitrogen oxides from exhaust gases [5]. However, this substance is expensive and requires frequent replenishment. In paper [6], considerations on emulsion (W/O) as fuel for CI engines were presented. The research results showed that water added to the fuel reduced nitrogen oxide emissions by 19.6% and smoke by 66.3%. According to the authors, this is due to water lowering the combustion temperature caused by heat absorption of evaporation and micro-explosions caused by rapid vaporization of water particles. This allows for a simultaneous reduction in nitrogen oxides and exhaust smoke. It is possible to produce emulsion fuel from non-biodegradable shopping bags (PGB) [7]. The research showed that using the water–oil emulsion derived from PGB reduces smoke and nitrogen oxide emissions. The emulsion with a 10% addition of diesel oil showed the highest exergetic efficiency. An exergetic analysis of a CI engine operating on biofuel obtained from cottonseed oil with the addition of titanium dioxide and silver oxide nanoparticles was conducted in study [8]. Analyzing the research results, it can be stated that fuels with nanoparticle additives showed lower exergy losses. It should be noted that nanoparticles have a high price. Cerium oxide nanoparticles were used as an additive to diesel oil, and the research results were presented in study [9]. The results showed that the use of this type of catalyst reduces soot and hydrocarbon emissions by 30% at partial engine load. However, at maximum engine load, a slight reduction of about 5% in nitrogen oxide emissions was observed. No difference in fuel consumption was noticed for both medium and maximum engine loads. In article [10], the influence of diesel oil mixtures with methanol, ethanol, n-butanol, methanol/n-butanol, and ethanol/n-butanol on combustion was analyzed. The research results showed that compared to pure diesel oil, the use of additives caused a shortening of the autoignition delay period and an improvement of the combustion process. The most favorable results were obtained with a mixture of 80% diesel oil, 10% methanol, and 10% n-butanol. The use of a catalyst in the nozzle of fuel injectors in an engine with direct mechanical fuel injection was described in studies [11,12]. The research showed that the use of a catalytic reactor in the form of platinum on the non-working part of the needle reduced the emission of toxic substances to the atmosphere. In study [13], a method of partial dehydrogenation of diesel oil to produce high-quality hydrogen was presented. The aim of the research was to assess the possibility of applying such a system in automotive vehicles. The catalyst used in the experiment was platinum on a zinc and aluminum base. Studies [14,15] showed that an appropriate element to be used as a catalyst to activate hydrocarbon dehydrogenation reactions is platinum on a tin and aluminum substrate. The developmental trends of combustion engines are aimed at reducing toxic substances in exhaust gases. In studies [16,17], research results on an engine operating on ethanol as a fuel additive were presented. The additive content caused a reduction in nitrogen oxide emissions in the exhaust gases. In study [18], the author proposed powdered biofuel as an alternative for liquid fuel. The research results showed that the use of powdered biofuel caused a shortening of the autoignition delay period. In turn, studies [19,20] focused on the research of ceramic catalysts based on ceramics for engines working in extreme conditions. Ceramic catalysts are used to increase the exhaust gas temperature, reduce smoke, and reduce nitrogen oxide emissions. In study [21], the possibility of using nano additives in fuel for a compression ignition engine was discussed. The research utilized conventional fuel and biofuel, including substances like cerium oxide, copper oxide, aluminum oxide, carbon nanotube, and titanium oxide. The analysis conducted by the authors demonstrated that the use of nano additives improves the combustion process and reduces the emission of toxic substances into the atmosphere. In the research presented in paper [22], the authors explored the possibility of using a nano additive in the form of cerium oxide in plant-derived fuel. The studies showed that the modified fuels were characterized by improved heat release during combustion and reduced emissions of carbon monoxide and hydrocarbons, with a slight increase in nitrogen oxide emissions. The authors in study [23] investigated the performance of a CI engine using diesel fuel with an additive made from used tire oil and cerium oxide nanoparticles. The additive ratios used in the study were as follows: Diesel fuel was blended with tire-derived oil in various concentrations, specifically 5%, 10%, 15%, and 20%. Additionally, for the blend containing 5% tire-derived oil, cerium oxide nanoparticles were also added to the mixture. The studies showed that a 5% addition of tire oil reduces hydrocarbon emissions and exhaust smokiness. Additionally, cerium oxide particles increase engine efficiency. This mixture represents an interesting alternative to standard fuel. In study [24], the authors examined the performance of a CI engine using fuel derived from plastic with the addition of aluminum oxide nanoparticles in proportions of 100, 150, and 200 mg/L. The analysis showed that the addition of aluminum oxide shortens the autoignition delay period. The emission of carbon monoxide and hydrocarbons was lower for fuels with the additive compared to pure plastic fuel without the additive and conventional fuel at full load. Another additive that may reduce the emission of carbon monoxide and soot in exhaust gases could be titanium nanoparticles and butanol [25]. A laboratory analysis showed that the physical and chemical properties of the fuel were not altered by the alcohol and additive. The studies were conducted at partial and maximum engine load. The addition of alcohol alone led to reduced fuel consumption and emissions of carbon monoxide and soot, while the emission of nitrogen oxides increased.
Analyzing the literature regarding the scope of conducted research, it can be concluded that studies on the application of catalysts in internal combustion engines are being conducted in various directions. One of the approaches to reduce emissions and fuel consumption is the utilization of a liquid catalyst, such as Eco Fuel Shot. This catalyst, when introduced into the fuel, aims to optimize the combustion process, thereby aiming to reduce harmful emissions and enhance fuel efficiency in internal combustion engines. The catalyst is a hygroscopic substance and mixes very well with fuel in the proportion of 1 L of catalyst to 50,000 L of diesel oil. According to the manufacturer, the cost of adding the Eco Fuel Shot product to 1 L of fuel is just a few cents. The environmental benefits of its use can be immense as its purpose is to reduce the emission of toxic nitrogen oxides in exhaust gases. What makes it innovative is its high hygroscopicity and the small amount of catalyst needed per 1 L of fuel. The adaptation of such catalysts appears to be a promising strategy for improving engine performance and sustainability, aligning with global emissions reduction objectives.
2. Results
During the tests, the concentrations of toxic substances in the exhaust were measured, including nitrogen oxides, hydrocarbons, and soot, and the specific fuel consumption was also calculated. The results of the direct measurements of the engine’s ecological parameters are presented in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7.
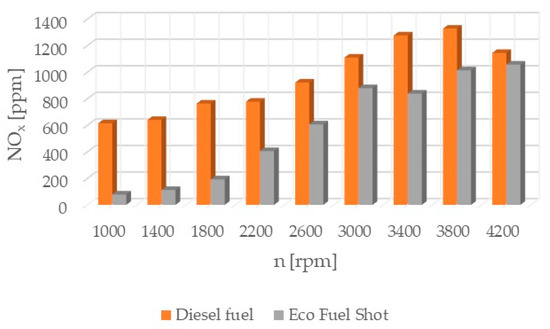
Figure 1.
Concentration of nitrogen oxides in exhaust gases.
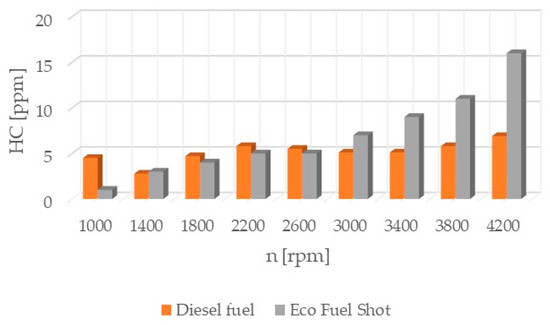
Figure 2.
Concentration of hydrocarbons in exhaust gases.
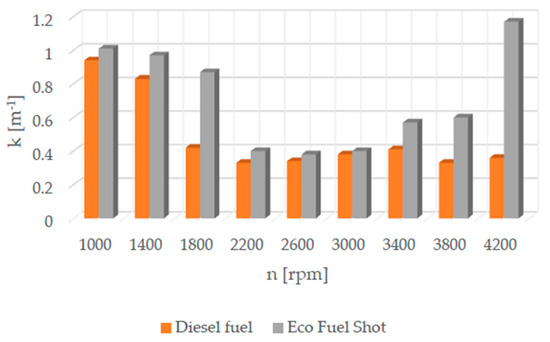
Figure 3.
Soot content in exhaust gases.
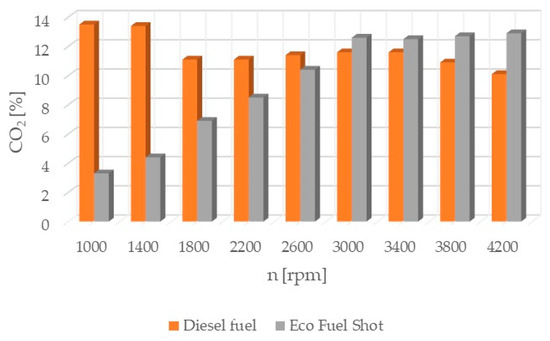
Figure 4.
Carbon dioxide content in exhaust gases.
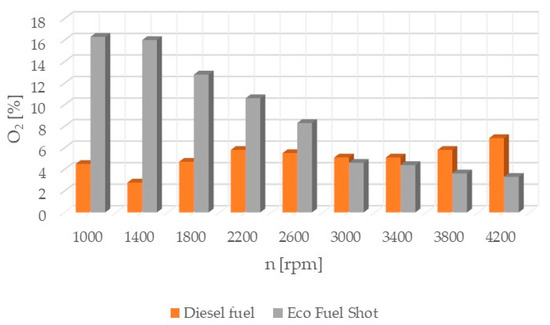
Figure 5.
Oxygen content in exhaust gases.
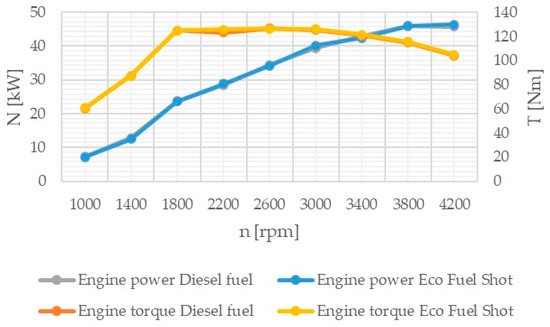
Figure 6.
Engine power and torque.
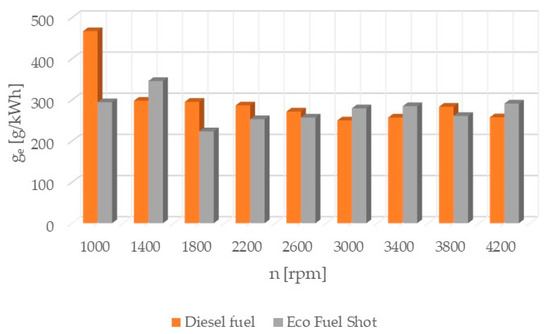
Figure 7.
Specific Fuel Consumption.
Nitrogen oxides (NOx) (Figure 1) are inorganic chemical compounds composed of oxygen and nitrogen. They are formed during combustion at high temperatures and in the presence of excess air. Nitrogen oxides are a toxic substance that can impair the functioning of the immune system in the lungs, leading to increased susceptibility to respiratory infections. Prolonged inhalation of these substances increases the risk of respiratory diseases. Reducing nitrogen oxide emissions can be achieved by using a smaller excess air ratio, limiting heat release, cooling the combustion chamber of the engine, and using fuel additives. Additionally, exhaust systems use catalysts to reduce NOx in the exhaust gases.
Hydrocarbons (HC) (Figure 2) are chemical compounds consisting of carbon and hydrogen. They are produced as a result of incomplete or partial combustion of fuel, as well as from the partial breakdown of hydrocarbon fractions in the fuel during chain reactions in the combustion process. Hydrocarbons are formed near the walls of the combustion chamber because low temperatures limit soot formation. Prolonged exposure to hydrocarbons can cause cancers, immune system failure, damage to the kidneys and liver, and cataracts.
Soot (particulate matter PM) (Figure 3) consists of toxic airborne particles formed as a result of incomplete and partial combustion of fuel. The smokiness of exhaust gases is mainly measured in compression ignition (CI) engines. The formation of particulate matter is primarily due to poor fuel atomization, which subsequently affects evaporation. Soot is generated in areas of the combustion chamber where there is a low oxygen content. It penetrates into the pulmonary alveoli and the bloodstream, causing lung and heart diseases. Soot is associated with aromatic hydrocarbons and increases the risk of cancerous diseases.
Carbon dioxide CO2 (Figure 4) is a gas that is non-toxic to living organisms. However, its excess in the atmosphere leads to the retention of thermal radiation, which causes the greenhouse effect. Therefore, it is crucial to limit the emission of this gas into the atmosphere.
Oxygen O2 (Figure 5) from the atmosphere is an essential element in the oxidation process. It reacts with hydrocarbons to form combustion products, which are emitted in the exhaust gases. The presence of either an excess or a deficiency of oxygen in the engine’s combustion chamber leads to the formation of pollutants.
The power and torque (Figure 6) of the tested Fiat 1.3 JTD engine were measured using an eddy current brake, Automex AMX 100. The measurement error of the test stand is 2%.
The specific fuel consumption (Figure 7) was calculated based on the following relationship:
where Ge is the instantaneous fuel consumption [g/s] and N is the engine power [kW].
The results of the calculations are presented in Figure 7.
Before the dynamometer tests, fuel injectors were removed from the engine (Figure 6) and examined in the laboratory for leak tightness and dosing on the STPiW (AUTOELEKTRONIKA, Poznań Poland) test bench. The aim of these tests was to verify whether the injection equipment operates correctly to eliminate measurement errors during the experiment (Table 1, Table 2, Table 3 and Table 4).

Table 1.
Measurements of the injection doses Bosch 0445110083 fuel injector 1 (AUTOELEKTRONIKA, Poznań, Poland).

Table 2.
Measurements of the injection doses Bosch 0445110083 fuel injector 2.

Table 3.
Measurements of the injection doses Bosch 0445110083 fuel injector 3.

Table 4.
Measurements of the injection doses Bosch 0445110083 fuel injector 4.
The tests of injection quantities conducted on the STPiW 3 test bench aimed to examine whether the fuel injectors remained efficient after dyno testing and whether the Eco Fuel Shot additive affected their operating parameters. The analysis showed that the injection and overflow quantities are within the acceptable ranges.
Additionally, during the tests, the actual parameters of the engine were monitored using a diagnostic interface. The maximum measurement uncertainty of the research station is 3%. Eco Fuel Shot is a liquid catalyst added to fuel with the purpose of improving the combustion of the fuel mixture and preventing the deposition of soot in the engine. The fuel additive is mixed at a ratio of 1 L of Eco Fuel Shot to 50,000 L of diesel fuel. Thanks to the use of a carrier, it emulsifies with the fuel very quickly. Studies conducted by the manufacturer regarding the impact of the additive on engine components have shown that it is neutral.
3. Discussion
Eco Fuel Shot is a liquid catalyst aimed at the reduction and oxidation of toxic substances (mainly NOx and HC) in exhaust gases during the combustion process and preventing the accumulation of deposits. Reviewing the literature, one way to reduce the content of nitrogen oxides and hydrocarbons in exhaust gases is to lower the temperature in the engine combustion chamber using various engine design solutions or fuel additives [26]. These solutions lead to a reduction in the combustion temperature in the engine chamber, resulting in lower emissions of nitrogen oxides. The liquid catalyst reduces the content of nitrogen oxides and hydrocarbons by oxidizing them during combustion (Figure 1 and Figure 2), without changing the parameters of this process. The remaining parameters remain at a similar level as when using standard fuel. The Eco Fuel Shot catalyst contains an active substance that reduces the surface tension of carbon, resulting in the complete combustion of soot and naphthalene. Its operation affects the rate of chain combustion reactions by changing the activation energy of chemically inert particles that react with each other. Eco Fuel Shot is a selective catalyst with an approximate empirical formula of C5H5FeC5H4COCmHn. Table 5 presents the chemical composition of the catalyst.

Table 5.
Chemical composition of catalyst.
During combustion, the catalyst induces redox reactions involving the reduction and oxidation of chemical compounds. In the case of combustion processes, these compounds include nitrogen oxides and hydrocarbons, as well as carbon monoxide and carbon dioxide. The combustion process in the engine chamber is a rapidly occurring reaction of the substance in combination with oxygen, accompanied by the release of a significant amount of heat. This reaction is initiated by the collision of molecules, whose energy exceeds a certain threshold value for each reaction, sufficient to break existing intramolecular bonds and form new bonds within the molecule. The energy possessed by active chemical molecules necessary to engage in a reaction is called activation energy. The physical meaning of activation energy in the context of internal combustion engines can be explained as follows. To initiate a reaction, it is necessary for the reacting molecules to collide. However, not all collisions lead to reactions between the colliding particles. If every collision led to a reaction, all reactions would occur almost instantly. Nevertheless, all reactions have a finite speed, meaning that only a fraction of all collisions lead to a reaction. Effective collisions are defined as those collisions whose energy at the moment of collision is slightly higher than the average energy specified for a given temperature. Activation energy is precisely the excess energy that molecules must have at the moment of collision to undergo a chemical reaction, and it is a fundamental factor determining the course of a chemical reaction. The lower the activation energy, the higher the rate constant of the reaction, and the reaction occurs faster. For polyatomic systems (including hydrocarbon fuels), activation energy is defined as the minimum kinetic energy that the potential energy of the system must exceed to allow a chemical reaction to occur within it [27]. Since activation energy depends on the molecule’s structure and bonding strength, the behavior of paraffinic hydrocarbons CnH2n+2 has been presented in a very clear manner. In these hydrocarbons, the energy of breaking C-H bonds is greater than the energy of breaking C-C bonds. Therefore, as the number of carbon atoms increases, less activation energy is needed to dissociate the molecule. Eco Fuel Shot is an active substance with a low activation energy which reacts with substrates, and the resulting compound easily transitions into the final product. This leads to reduced emissions of toxins in exhaust gases. Substances that reduce the interfacial tension between two phases are called surfactants. These are active surface substances that affect the activation energy size between reacting substrates. In study [28], the authors proposed two types of surfactants as additives to fuels emulsified in water to improve the combustion process and reduce toxin emissions in exhaust gases. Research shows that surfactants reduce the hydrocarbon content in the environment [6].
Analyzing the results of the conducted engine tests, the use of the catalyst resulted in a significant reduction in both nitrogen oxides and hydrocarbon content in the exhaust gases. The fuel combustion process in the engine combustion chamber consists of several stages: ignition delay period, kinetic combustion, diffusive combustion, and secondary combustion. Among these stages, the ignition delay period has the most significant impact on the others. There is an aim to minimize the duration of this stage. The ignition delay period is the time from the beginning of fuel injection into the combustion chamber to the appearance of the first auto-ignition foci. During this period, the fuel jet breaks up, heats up, and mixes with the air. If the ignition delay period is too long, the fuel delivered to the cylinder before ignition does not have enough time for evaporation and mixing with the air. This leads to the engine’s dynamic loading, noise, and increased emission of nitrogen oxides (a rapid pressure increase occurs during the second stage). Factors affecting the ignition delay include:
- Cetane number of the fuel, which determines the fuel’s ability to auto-ignite. The higher its value, the better the combustibility properties of the fuel. The cetane number of fuels can be increased by using various additives.
- The type of combustion chamber affects the size of the auto-ignition delay because there are differences in the fuel distribution in the space and boundary layer and in the temperature distribution of the combustion chamber walls.
- The pressure and temperature of the fuel at the beginning of the fuel injection process influence the first stage of the combustion process. Increasing the pressure and consequently the temperature at lower injection advance angles shortens the auto-ignition delay. Due to the operational wear of the engine, the pressure and temperature at the end of the compression stroke in the cylinder decrease due to leakages, extending the auto-ignition delay.
- Quantitative and qualitative characteristics of fuel injection. The size of the dose and the shape of the jet affect the auto-ignition delay period. Proper injection quantities (pilot, main) generated by the fuel injectors and the appropriate atomization of fuel jets shorten the auto-ignition delay.
- Increasing the engine’s rotational speed improves atomization and increases the pressure and temperature in the combustion chamber, which shortens the auto-ignition delay.
- Directed intensity of charge movement in the combustion chamber shortens the auto-ignition delay period.
- The dependence on the auto-ignition delay period has been presented in work [29]:
Based on relation (3), the auto-ignition delay was calculated (Figure 8).
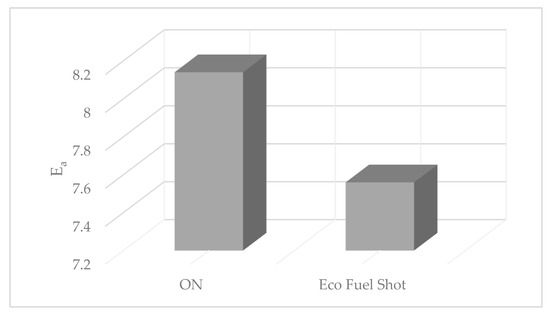
Figure 8.
Activation energy value for ON fuels and ON with Eco Fuel Shot additive.
Then, the auto-ignition delay period across the entire range of rotational speeds of the external characteristics was evaluated for the examined fuels (Figure 9).
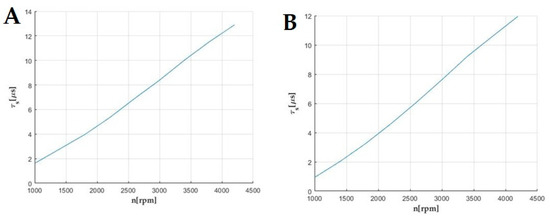
Figure 9.
Ignition delay (A) ON fuel across the entire range of rotational speeds of the engine’s external characteristics. (B) ON fuel with Eco Fuel Shot additive across the entire range of rotational speeds of the engine’s external characteristics.
The Eco Fuel Shot catalyst increases the cetane number of the fuel, thereby affecting the magnitude of the activation energy by reducing its value. By analyzing relations (2) and (3), the use of the catalyst shortens the ignition delay period, which results in improving combustion processes.
The combustion process consists of a set of chemical chain reactions of oxidizing fuel and air components, resulting in mechanical energy. Analyzing the results of conducted studies, it can be concluded that the tested catalyst achieves the greatest reduction in nitrogen oxides in exhaust gases. Shortening the autoignition delay period improves the combustion process by damping the abrupt pressure surge in the engine chamber, thereby achieving a lower temperature. Nitrogen oxides are formed in high-temperature areas of the engine combustion chamber. Lowering the temperature reduces their content in the exhaust gases. However, lowering the temperature increases the content of particulate matter in the exhaust gases due to their incomplete combustion. The research results show that the soot content increased only slightly compared to standard fuel. Theoretically, the content of hydrocarbons should also increase. However, the studies showed that the concentration of hydrocarbons in the exhaust gases was reduced, especially at higher rotational speeds. The formation of particulate matter and hydrocarbons is influenced by the physical parameters of fuel properties. Regardless of the engine load, it is possible to improve the atomization of the injected fuel by using fuel additives. Atomization is the breakup of the fuel jet flowing out at high speed, creating a large number of droplets near the outlet of the nozzle holes. The formation of the combustible mixture consists of atomization and spreading of the fuel jet, heating and evaporation of the fuel, and mixing of the fuel with air. The preparation of the combustible mixture lasts from the start of fuel injection to the end of its combustion. The course of the injection process depends on the quantitative and qualitative characteristics of the fuel jet, the design of the combustion chamber, fuel properties, the speed and direction of the charge spread, the location of fuel injectors, and the mutual orientation of fuel jets. The ignition of atomized fuel is a chain, multi-stage process. The first ignition foci are formed according to the volumetric combustion process. The flame spreads and the combustion of the fuel-air mixture prepared during the autoignition delay period begins. The combustible mixture enters the stage of diffusive combustion and then completes its combustion. The use of the Eco Fuel Shot catalyst affects not only the chemical properties of the fuel but also its physical properties. The additive improves atomization and shortens the fuel evaporation time. This leads to a reduction in hydrocarbons and soot in the exhaust gases, despite the lower temperature in the engine combustion chamber. Analyzing the percentage concentration of carbon dioxide in the exhaust gases, it is evident that the engine running on fuel with the Eco Fuel Shot additive had lower emissions at low and medium rotational speeds, while above 2600 [rpm] carbon dioxide emissions were lower for the engine powered by standard diesel fuel. The oxygen content in the exhaust gases, on the other hand, is the opposite. A similar relationship occurs in the emission of nitrogen oxides: the higher the rotational speed, the smaller the differences in their content in the exhaust gases between the tested fuels. This may be related to the temperature in the combustion chamber. Higher rotational speeds result in higher temperatures, which affect the emission of individual toxic substances. The Eco Fuel Shot additive does not affect the maximum pressure in the engine combustion chamber. The power and torque of the engine depend on the size of the gas forces acting on the piston crown. For both fuels, these parameters were at a similar level. Analyzing the course of specific fuel consumption, it can be concluded that the use of the catalyst lowers engine efficiency. Specific fuel consumption is higher for fuel with the additive throughout the entire range of rotational speeds.
4. Materials and Methods
The engine tests were conducted at the measurement station equipped with a Fiat 1.3 JTD engine featuring a Common Rail system, Automex AMX 100 brake (ODIUT Automex Sp z o. o., Gdańsk, Poland) (Figure 10), Automex fuel meter ((ODIUT Automex Sp z o. o., Gdańsk, Poland), MDO Maha smoke meter (MAHA, Haldenwang, Germany), and Capelec exhaust gas analyzer (Capelec Group, Montpellier, France), which meets the latest European requirements for MID (Measuring Instruments Directive) with the highest accuracy class of 0.

Figure 10.
Fiat 1.3 JTD test bench with control system.
The measurement error of the engine test bench with the fuel meter is 2%. Standard diesel fuel (ON) and fuel with the Eco Fuel Shot liquid catalyst were used for the tests. The exhaust emission measurement was carried out in accordance with the Polish Standard PN—EN ISO 8178—1: 2020 [30]. Emission measurements were taken at the engine outlet about 2 m from the exhaust manifold (Figure 11). For the research, standard diesel fuel with a 7% bio-component additive was used. Data regarding the cetane number content of the standard fuel with the catalyst were provided by the manufacturer.

Figure 11.
Emission measuring point A.
External characteristics were conducted at full engine load for each rotational speed. During the studies, the parameters of the engine running on standard and modified fuels were compared. The results of measurements with the catalyst fuel were referenced to the measurements on standard fuel under the same conditions.
The liquid catalyst Eco Fuel Shot is a hygroscopic substance that is dosed directly into the fuel tank. The additive ensures that the fuel is homogeneous and undivided, leading to its complete combustion in the engine chamber. The task of the active substance is to reduce the magnitude of the activation energy due to the formation of a transitional compound with reactants. The analysis of conducted research showed that the application of the liquid catalyst as a fuel additive significantly limited the emission of certain substances. The chemical properties of the active substance induce secondary combustion and reduce the emissions of nitrogen oxides, hydrocarbons, and carbon dioxide. The smoke emission slightly increased. The catalyst removes deposits from engine components. The increased smokiness could have been caused by the engine operating on modified fuel for too short a period. The soot present on the engine components did not have time to burn off, which could have affected the emission of particulates. The specific fuel consumption was at a comparable level for both tested fuels.
The catalyst initiates a redox reaction during combustion, involving the transfer of electrons between chemical particles. Particles losing electrons undergo oxidation, while molecules gaining electrons undergo reduction. The action of the liquid catalyst occurs inside the combustion chamber, which, thanks to the low activation energy, increases the reaction rate constant, optimizing and improving the combustion process of the fuel mixture in the engine chamber. The cetane number of the tested diesel fuel was 51, and with the addition of Eco Fuel Shot, it was 57. Mathematical analysis showed that the liquid catalyst shortens the ignition delay period, affecting the combustion process of the fuel mixture in the cylinder’s working space.
Further research on the application of the liquid catalyst as an Eco Fuel Shot additive to fuel will include long-term engine tests, analyses of engine operation on plant-derived fuels with the addition of Eco Fuel Shot, and analyses of engine operation with a liquid catalyst on modified fuel supply equipment.
5. Conclusions
The Eco Fuel Shot fuel additive is a liquid catalyst that reduces toxic substances in exhaust gases. Manufacturer data showed that it increases the cetane number of the fuel, which affects the activation energy value and shortens the delay period of fuel autoignition in the engine’s combustion chamber. The studies showed that an engine running on fuel with the catalyst had a lower concentration of nitrogen oxides (NOx) across the entire range of rotational speeds. To reduce nitrogen oxides in exhaust gases, the AdBlue liquid is used, which is dosed from a separate tank in the vehicle and added to the SCR catalyst in the exhaust system. By reducing the content of nitrogen oxides in the exhaust gases, the consumption of AdBlue by the vehicle during operation can be reduced. The emission of hydrocarbons (HC) is lower for the fuel with the additive up to 2600 [rpm] at lower rotational speeds, which may be attributable to a lower amount of oxygen in the combustion chamber. A similar relationship occurs in the emission of carbon dioxide (CO2), where above 2600 [rpm] its emission is higher compared to standard fuel. The velocity characteristic of oxygen (O2) concentration in the exhaust gases shows that the oxygen content above 2600 [rpm] is lower for the modified fuel compared to the standard. The worst performance was noted in the content of particulate matter in the exhaust gases for the fuel with the additive. Across the entire range of engine rotational speeds, its content was lower for standard fuel. The power and torque of the tested engine were at a similar level for both fuels, so the engine efficiency is influenced by the instantaneous fuel consumption, which was lower for conventional fuel. In conclusion, the authors of the study believe that the Eco Fuel Shot catalyst is a developmental product that can be improved or an engine can be adapted for its utilization.
Further research related to the use of the catalyst as a fuel additive should be conducted in the following directions:
- Laboratory tests of the spray patterns of standard and modified fuels with the additive.
- Measurement of pressure and temperature progression in the combustion chamber of the engine for standard and modified fuels.
- Analysis of the operation of CI (compression ignition) engines with modified fuel injectors as presented in study [19], using fuel with the catalyst.
- Analysis of the operation of engines on plant-derived fuels with the Eco Fuel Shot catalyst.
Author Contributions
Supervision, writing—review, A.K., V.T. and K.F.A.; Conceptualization, data acquisition and interpretation, investigation and writing—original draft preparation and editing, T.O.; software, investigation, writing—review and editing, Ł.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vembathu Rajesh, A.; Mathalai Sundaram, C.; Sivaganesan, V.; Nagarajan, B.; Harikishore, S. Emission reduction techniques in CI engine with catalytic converter. Mater. Today Proc. 2020, 21, 98–103. [Google Scholar] [CrossRef]
- Udhayakumar, N.; Mani, M.; Ramesh Babu, S.; Karthikkeyan, G.; Peace John Samuel, K.; Karna, V. An experimental investigation on emission characteristics in CI engine with zinc and vanadium coated catalytic converter. Mater. Today Proc. 2022, 62, 2250–2255. [Google Scholar] [CrossRef]
- Karthickeyan, V.; Thiyagarajan, S.; Edwin Geo, V.; Ashok, B.; Nanthagopal, K.; Ong Hwai Chyuan Vignesh, R. Simultaneous reduction of NOx and and smoke emissions with low viscous biofuel in low heat rejection engine using selective catalytic reduction technique. Fuel 2019, 255, 115854. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Al-Muhtaseb, A.H.; Hasan, A.O. Combustion, performance, and selective catalytic reduction of NOx for a diesel engine operated with combined tri fuel (H2, CH4, and conventional diesel). Energy 2017, 115, 901–910. [Google Scholar] [CrossRef]
- Guziałkowska Tic, J.; Tic, W.J. Modyfikatory stosowane w procesie spalania olejów opałowych i paliw stałych. CHEMIK 2012, 66, 1203–1210. [Google Scholar]
- Park, J.; Oh, J. Study on the characteristics of performance, combustion, and emissions for a diesel water emulsion fuel on a combustion visualization engine and a commercial diesel engine. Fuel 2022, 311, 122520. [Google Scholar] [CrossRef]
- Saha, D.; Roy, B. Effects of plastic-grocery-bag derived oil-water-diesel emulsions on combustion, performance and emission characteristics, and exergoeconomic aspects of compression ignition engine. Sustain. Energy Technol. Assess. 2022, 54, 102877. [Google Scholar] [CrossRef]
- Dogan, B.; Celik, M.; Bayindirli, C.; Erol, D. Exergy, exergyeconomic, and sustainability analyses of a diesel engine using biodiesel fuel blends containing nanoparticles. Energy 2023, 274, 127278. [Google Scholar] [CrossRef]
- Leach, F.C.P.; Davy, M.; Terry, B. Combustion and emissions from cerium oxide nanoparticle dosed diesel fuel in a high speed diesel research engine under low temperature combustion (LTC) conditions. Fuel 2021, 288, 119636. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Li, J.; Lv, J.; Wang, S.; Zhong, Y.; Dong, R.; Gao, S.; Cao, C.; Tan, D. Investigation on combustion, performance and emission characteristics of a diesel engine fueled with diesel/alcohol/n-butanol blended fuels. Fuel 2022, 320, 123975. [Google Scholar] [CrossRef]
- Klyus, O. The use of turbulization in preliminary fuel treatment in self-ignition engines. Combust. Engines 2009, 138, 49–53. [Google Scholar] [CrossRef]
- Sa, B.; Klyus, O.; Markov, V.; Kamaltdinov, V. A numerical study of the effect of spiral counter grooves on a needle on flow turbulence in a diesel injector. Fuel 2021, 290, 120013. [Google Scholar] [CrossRef]
- Gianotti, E.; Taillades-Jacquin, M.; Carmona, A.R.; Taillades, G.; Rozière, J.; Jones, D.J. Hydrogen generation via catalytic partial dehydrogenation of gasoline and diesel fuels. Appl. Catal. B Environ. 2016, 185, 233–241. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, J.; Wu, J.; Yuan, X.; You, K.; Luo, H. Microwave assisted synthesis of Sn–modified MgAlO as support for platinum catalyst in cyclohexane dehydrogenation to cyclohexene. Appl. Catal. A Gen. 2016, 516, 9–16. [Google Scholar] [CrossRef]
- Echeverri, A.; Gomez, T.; Hadad, C. Ammonia borane dehydrogenation tendencies using Pt4, Au4, and Pt2Au2 clusters as catalysts. Mol. Catal. 2019, 471, 9–20. [Google Scholar] [CrossRef]
- Lodi, F.; Zare, A.; Arora, P.; Stevanovic, S.; Ristovski, Z.; Brown, R.J.; Bodisco, T. Combustion characteristics of microalgae–based dioctyl phthalate biofuel during ambient, preheated and hot engine operation. Fuel 2023, 331, 125890. [Google Scholar] [CrossRef]
- Mendiburu, A.Z.; Lauermann, C.H.; Hayashi, T.C.; Marinos, D.J.; Rodrigues da Costa, R.B.; Coronado, C.J.; Roberst, J.J.; De Carvahlo, J.A., Jr. Ethanol as a renewable biofuel: Combustion characteristics and application in engines. Energy 2022, 257, 124288. [Google Scholar] [CrossRef]
- Elfasakhany, A. Investigation of biomass powder as a direct solid biofuel in combustion engines: Modelling assessment and comparisons. Aim Shams Eng. J. 2021, 12, 2991–2998. [Google Scholar] [CrossRef]
- Eliasz, J.; Osipowicz, T.; Abramek, K.F.; Mozga, Ł. Model Issues Regarding Modification of Fuel Injector Components to Improve the Injection Parameters of a Modern Compression Ignition Engine Powered by Biofuel. Appl. Sci. 2019, 9, 5479. [Google Scholar] [CrossRef]
- Krakowski, R. The effect of adding effective microorganism and silver compounds to flash point of engine oil. Combust. Engines 2023, 192, 97–104. [Google Scholar] [CrossRef]
- Lv, J.; Wang, S.; Meng, B. The Effects of Nano Additives Added to Diesel-Biodiesel Fuel Blends on Combustion and Emission Characteristics of Diesel Engine: A Review. Energies 2022, 15, 1032. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Dandakouta, H.; Yahuza, I.; Yusuf, D.A.; Mujtaba, M.A.; El-Shafay, A.S.; Soudagar, M.E.M. Effect of low CeO2 nanoparticles dosage in biodiesel-blends on combustion parameters and toxic pollutants from common rail Diesel engine. Atmos. Pollut. Res. 2022, 13, 101305. [Google Scholar] [CrossRef]
- Thangavelu, K.; Arthanarisamy, M. Experimental investigation on engine performance, emission, and combustion characteristics of a DI CI engine using tyre pyrolysis oil and diesel blends doped with nanoparticles. Environ. Prog. Sustain. Energy 2020, 39, 13321. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P.; Balachandar, M.; Sundar, S.P.; Sathyamurthy, R. Effect of using plastic nanofuel as a fuel in a light-duty diesel engine. Heat Transf. 2020, 49, 726–742. [Google Scholar] [CrossRef]
- Yasar, A.; Keskin, A.; Yildizhan, S.; Uludamar, E.; Ocakoglu, K. Effects of titanium-based additive with blends of butanol and diesel fuel on engine characteristics. Int. J. Glob. Warm. 2018, 15, 38–53. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Liao, C.; Liu, S.; Wick, L.Y.; Peng, D.; Yi, X.; Lu, G.; Yin, H.; Lin, Z.; et al. Drivers and applications of integrated clean-up technologies forsurfactant-enhanced remediation of environments contaminated with polycyclic aromatic hydrocarbons (PAHs). Environ. Pollut. 2017, 225, 129–140. [Google Scholar] [CrossRef]
- Ambrozik, A. Wybrane Zagadnienia Procesów Cieplnych w Tłokowych Silnikach Spalinowych; Politechnika Świętokrzyska: Kielce, Poland, 2003. [Google Scholar]
- Kashinath, S.A.A.; Hashim, H.; Yunus, N.A.; Mustaffa, A.A. Design of Surfactant for Water in Diesel Emulsion Fuel for Designing Eco-Friendly Fuel. Chem. Eng. Trans. 2018, 63, 433–438. [Google Scholar]
- Heywood, J.B. Correlations for ignition delay in engines. In Internal Combustion Engines Fundamentals; McCraw–Hill Book Co.: New York, NY, USA, 1988; Volume 1, pp. 553–554. [Google Scholar]
- ISO 8178-1:2020; Reciprocating Internal Combustion Engines: Exhaust Emission Measurement. International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/79330.html (accessed on 30 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).