Abstract
The gas-to-liquid (GTL) process is a catalytic technology for achieving carbon neutrality during fuel production. Fischer–Tropsch synthesis (FTS), a core step in this process, converts synthesis gas (CO + H2) to high-value hydrocarbon products. This study synthesized a chabazite-shaped zeolite and a Co/γ-alumina catalyst by using conventional hydrothermal and wet impregnation methods, respectively. Hybrid FTS catalysts were then prepared by mixing the Co/γ-alumina catalyst with supports, including the synthesized and commercial zeolites alone and mixed at various ratios. The effects of these zeolites on the FTS conversion and selectivity were investigated. Additionally, the physicochemical properties of the supports and prepared catalysts were analyzed. The bifunctional hybrid catalyst performance was evaluated in a fixed-bed reactor, and the FTS products were analyzed using online and offline gas chromatography. The hybrid catalysts produced lighter hydrocarbons than the Co/γ-alumina catalyst alone. Meanwhile, heavy hydrocarbons produced over the Co/γ-alumina catalyst were hydrocracked at the acid sites of the silicoaluminophosphate zeolite (SAPO-34) to yield lighter, fuel-range hydrocarbons. Cobalt-based hybrid FTS catalysts were also investigated to determine the optimum support ratio for high carbon conversion and C5+ selectivity. The hybrid catalyst supported on SAPO-34:ZSM-5 (2:8) exhibited the highest CO conversion and favorable C5+ selectivity.
1. Introduction
The effects of global warming have continued to worsen in recent years. Considerable research has been directed toward developing approaches that use chemical technologies to mitigate the greenhouse effect and achieve carbon neutrality. Atmospheric CO2, which is largely emitted by human activity, is a major contributor to the greenhouse effect; therefore, CO2 utilization technologies have become more important and research efforts toward their development have been accelerated [1,2]. The catalytic conversion of CO and CO2 to fuels has attracted considerable attention as a useful approach to achieving carbon neutrality [3,4,5]. The gas-to-liquid (GTL) process is one of several promising methods for reducing CO2 emissions [2,6,7,8,9].
The gas-to-liquid (GTL) process is an appealing and reliable technology that employs Fischer–Tropsch synthesis (FTS) to generate high-value chemicals and vehicle fuels from synthesis gas (syngas) derived from natural gas or flare gas through a reforming process. Similarly, biomass-to-liquid and coal-to-liquid processes involve the gasification of biomass or coal to produce syngas for the synthesis of liquid transportation fuels, waxes, and chemicals using FTS [10,11,12,13,14]. The Fischer–Tropsch (FT) reaction is key in the conversion of natural gas through GTL technology.
The superior grade of liquid hydrocarbons and chemicals reduces the levels of sulfur and nitrogen derived from sources such as natural gas, biomass, coal, and other hydrocarbons serving as alternatives to crude oil. These carbon sources can also be utilized for generating the syngas required for FTS, which can be described as a catalytic step-growth polymerization process [14,15]. The range of active Fe-, Co-, Ni-, or Ru-based catalysts is broad [10,11,13]. The composition of the product distribution, including a range of hydrocarbon mixtures such as alcohol oxygenates, linear and branched olefins, paraffins, C2+ hydrocarbons, and methane, is influenced by the specific catalysts and reaction conditions employed [16]. Through the four catalysts stated above, Fe and Co are most generally applied for industrial FTS. The FT reaction polymerization mechanism is a carbon chain growth reaction involving CH2 groups, which sequentially attach to the hydrocarbon chain. The overall reaction can be detailed as follows [14,15,17]:
Several other reactions can also occur during FTS, but the expressions for these phenomena as described by many other researchers are passionately disputed because the mechanisms have not yet been adequately clarified. At present, none of the suggested mechanisms fully explain all the FTS products produced [10,14,15,18]. Some of these other reported reactions rely on the exothermic nature of FTS as a conventional rule owing to the production of H2O or CO2 in the reaction. The related equations are as follows [14,15]:
In addition, the water–gas shift reaction equation is
The average heat of these exothermic reactions is approximately 10 kJ/g [12]. The most important parameter for researchers to keep in mind, therefore, is the need to cool the reaction to avoid an uncontrolled temperature increase. It is crucial to limit the generation of lower-molecular-weight hydrocarbons, such as methane, while maintaining specific reaction conditions including temperature, pressure, and gas hourly space velocity (GHSV). Additionally, it is important to prevent catalyst sintering and reduce its activity [15]. The stoichiometric reactions necessary for the production of hydrocarbons and oxygenates as the primary products in FTS are outlined as follows [10,14,17]:
Equations (5) and (6) imply reactions that make alkanes and alkenes, respectively, while Equations (7)–(9) produce alcohols/ethers, aldehydes/ketones, and carboxylic acids/esters, respectively. The important products in FTS describe the compounds with functional groups on the terminal carbon [10,14].
GTL floating production, storage, and offloading (FPSO) is a method for utilizing stranded offshore gas fields, which are less efficient than working gas fields. The commercial use of offshore natural gas faces significant challenges, including remote locations, shipment and infrastructure issues, and a shortage of scientific knowledge [19,20,21]. However, GTL-FPSO processes still require an efficient hybrid catalyst for the FTS. Owing to the severe operating environment and inherent space limitations of GTL-FPSO plants, the process should be compact and secure to mitigate risks. The development of high-performance hybrid FTS catalysts can minimize the upgrading process [22,23,24,25,26,27]. High-quality gasoline containing branched paraffins can be produced from FTS diesel using solid catalysts with acidic properties, such as zeolites, which exhibit excellent hydrocracking performance in FTS [28,29].
Despite its potential, the production of clean fuel via FTS presents two important challenges. The first challenge is that the hydrocarbon chain growth kinetics of FTS must comply with the Anderson–Schulz–Flory (ASF) model for the distribution of hydrocarbons, which is determined by a single parameter, the probability of chain growth of hydrocarbon, denoted as α. This parameter can be specified in relation to the rate of completion rt and the degree of chain multiplication or polymerization rp of the increasing hydrocarbon chain, as follows [10,14,18]:
The rates of completion and multiplication can rely on the chain length of the hydrocarbon n and the FT reaction conditions [14,18].
The distribution of FTS products can then be denoted as yn, which is the mole fraction of all FTS components with the chain length or carbon number n, detailed as [14]
Equation (11) assumes that the chain growth probability α is independent of the carbon number or chain length. This is related to the ASF distribution of the carbon number [14,18].
Equation (11) can also be written as
The ASF distribution is a theoretical function that has limited uses because it assumes a constant chain growth probability [18,30,31]. For example, some experimental results show alterations for C1 and C2 in the FTS products. However, the ASF carbon number distribution is preferred for its clarity because, as mentioned above, it has only one parameter, namely, α. The ASF distribution can be experimentally analyzed by plotting the logarithm of the mole fraction yn against the chain length or carbon number from Equations (12) and (13) to yield a linear plot. The resulting slope can be achieved from Equation (13) [14,18]:
The ASF kinetics of FTS define the optimal feasible selectivity for a specified appropriate hydrocarbon fraction.
The second challenge is that the linear structures of the products currently obtained by FTS are considered low quality because of their low octane numbers and inferior cold-flow features; therefore, these products must be further converted before they can be considered usable fuels. Various downstream conversion methods can be used to improve the characteristics of the main FTS hydrocarbons to increase clean fuel production. For instance, alkenes and oxygenated FTS products can be produced over zeolite catalysts, affording gasolines with abundant isoparaffins and aromatics [32], while long-chain hydrocarbons can be hydrocracked to yield high-cetane diesels [33].
Most processes for producing olefins from methanol involve the use of SAPO-34, a silicoaluminophosphate catalyst. These processes require commercialization to satisfy the increasing demand for ethylene, propylene, and other compounds [34]. Molecular sieves with small pore sizes, such as SAPO-34, facilitate the formation of light olefins and are thus suitable raw materials for producing high-value polymers [34,35,36]. The direct production of isoparaffins from syngas has been achieved in one step using zeolite-incorporated bifunctional catalysts, such as physically mixed alumina and acidic zeolite hybrid FTS catalysts [37,38,39]. The Fischer–Tropsch (FT) reaction can be modified by employing hybrid catalysts, which consist of a physical combination of a traditional cobalt-based FT catalyst for the production of long-chain hydrocarbons and heavy waxes and a bifunctional or acidic zeolite containing acid–metal sites for the hydrocracking of these long-chain products, particularly heavy wax. The advantage of adjusting the reaction through the use of hybrid catalysts lies in its ability to enable the selective production of desired products, such as hydrocarbons within the gasoline range [14]. According to most studies, other factors in cobalt hybrid catalysis include coke formation, deactivation properties, selectivity toward diesel-range hydrocarbons, and a decrease in methane selectivity [11,40,41,42,43]. Cobalt hybrid catalysts utilizing zeolite as the acid catalyst can be prepared through physical methods, such as mixing different ratios in a single reactor to form a homogeneous mixed-bed, dual-bed, or hybrid-catalyst pellet bed [40,41]. Martínez et al. [11] conducted an experiment involving a blend of different commercially available zeolites with varying levels of acidity and pore structures, as well as Co/SiO2 catalysts, in a reactor to study coke production and shutdown. The reactivity of the zeolites was found to be influenced by their surface acidity, with ITQ-2 exhibiting the highest initial yield of gasoline-range hydrocarbons due to its larger surface area compared to ZSM-5, MCM-22, and IM-5. This outcome was attributed to the total number of Brønsted acid sites and the limited presence of long-chain hydrocarbons dispersed within the zeolites [11,14]. The physically mixed hybrid catalyst with protonated ZSM-5 produced the highest amount of medium isoparaffins for gasoline-range hydrocarbons [11,14,42].
In this study, hybrid FTS catalysts for the GTL-FPSO process were prepared by physically mixing Co/γ-alumina with hydrocracking catalysts (SAPO-34, ZSM-5, zeolite beta, and γ-alumina). Activity tests were conducted to determine the catalytic effects of physically mixing SAPO-34 with commonly used zeolites like ZSM-5 and zeolite beta. The CO conversion, α values of the C5+ products, and catalytic stability of the catalysts were analyzed for application in the GTL-FPSO process. Cobalt-based hybrid FTS catalysts were further investigated to determine the optimum support ratio in the hybrid catalysts and attain the highest CO conversion and C5+ selectivity.
2. Results and Discussion
2.1. Characterization of Hybrid FTS Catalysts
The XRD patterns of the hybrid FTS catalysts and supports are shown in Figure 1. The patterns of the prepared catalysts showed peaks characteristic of SAPO-34, ZSM-5, and zeolite beta materials [44,45,46,47]. The XRD peaks of SAPO-34 (red line) at 2θ = 9.5°, 12.8°, 20.6°, and 31° are characteristic of a rhombohedral structure (JCPDS: 01-087-1527) [48]. The peaks characteristic of the Co3O4 phase were observed at 2θ = 31.1°, 36.8°, and 59.2°; however, an additional peak was observed at 2θ = 43.8°. We attributed this additional peak to the CoO phase [49,50]. The crystallite sizes of Co3O4 (311) at 2θ = 36.8° were calculated using the Scherrer equation, as listed in Table 1. For the hybrid catalyst, the same Co/γ-alumina was used, so the crystal size of the cobalt was the same.
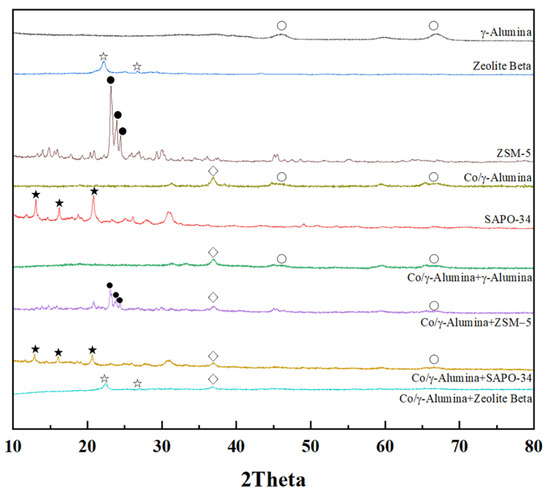
Figure 1.
XRD patterns of supports and prepared hybrid FTS catalysts (○: γ-alumina, ◇: Co/γ-alumina, ●: ZSM-5, ☆: zeolite beta, ★: SAPO-34).

Table 1.
Textural properties of the supports and prepared hybrid FTS catalysts [51].
The specific surface areas of the hybrid FTS catalysts ranged from 163.0 to 382.9 m2/g, with an average pore diameter and volume of 6.05 nm and 0.38 cm3/g, respectively (Table 1). The average pore diameters of the hybrid FTS catalysts with mixed zeolites exceeded those of the supports. However, the average pore diameter decreased marginally when γ-alumina was mixed with Co/γ-alumina. Overall, the physical adsorption qualities decreased substantially after mixing Co/γ-alumina with γ-alumina and zeolite supports [49,50].
The TPR results of the prepared Co/γ-alumina catalysts are shown in Figure 2. Cobalt oxide undergoes two reduction processes, with CoO as an intermediate compound. The reduction process proceeds along the following reactions represented by Equations (14) and (15) [49].
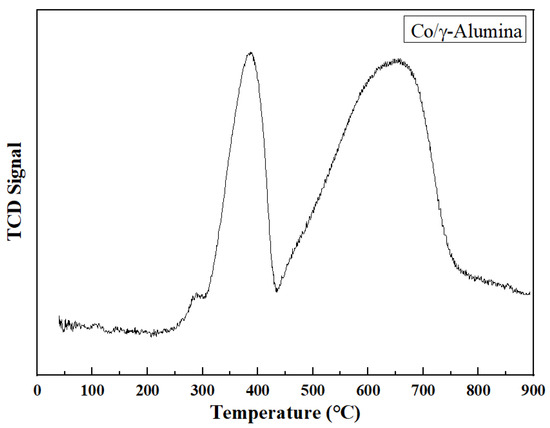
Figure 2.
TPR profile of Co/γ-alumina catalyst. Reprinted with permission from [51].
Co3O4 + H2 → 3CoO + H2O
CoO + H2 → Co0 + H2O
The peak at the lower temperature (∼310 °C) is attributed to the reduction of Co3O4 to CoO, whereas the peak at the higher temperature (∼370 °C) is attributed to the reduction of CoO to Co0. The higher-temperature peak for Co/γ-alumina corresponds to the reduction of CoO combined with alumina supports [50].
The NH3-TPD results for the SAPO-34, ZSM-5, zeolite beta, and γ-alumina supports are shown in Figure 3, and the acidic properties of the samples are listed in Table 2. The desorption temperature in the TCD signal describes the acidity of the site. The NH3-TPD profile for each support revealed two characteristic peaks centered at 150–200 and 400–450 °C, corresponding to weak and strong acid sites, respectively. As shown in Figure 3, SAPO-34 shows the highest peak for the strong acidic site, followed by zeolite beta, ZSM-5, and γ-alumina. The peaks for the weak acidic site were similarly high for SAPO-34 and zeolite beta, followed by γ-alumina and ZSM-5. The weak acid sites contain weak structural OH groups (Si–OH, P–OH, and Al–OH), whereas the strong acid sites contain Si–OH–Al groups (Brønsted acidic sites) [52]. The weak acid sites did not influence the reaction significantly, while the strong acid sites played a crucial role in the FTS hydrocracking reaction, improving coke formation and methane selectivity [53]. SAPO-34 exhibited higher acidity than γ-alumina, zeolite beta, and ZSM-5 (Table 2); therefore, SAPO-34-based catalysts are predicted to exhibit higher hydrocracking activity toward FTS.
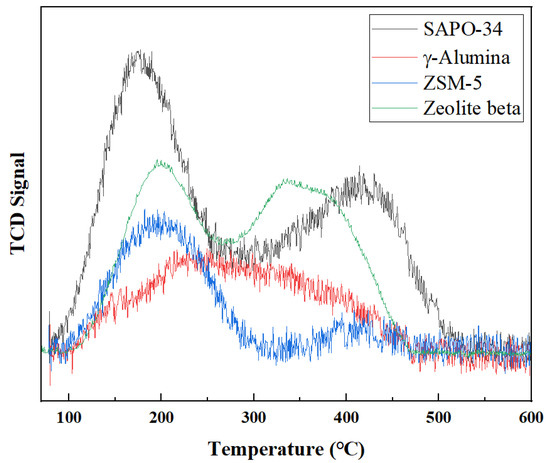
Figure 3.
NH3-TPD profiles of SAPO-34, ZSM-5, zeolite beta, and γ-alumina supports. Reprinted with permission from [51].

Table 2.
Acidities of the SAPO-34, ZSM-5, zeolite beta, and γ-alumina supports analyzed using NH3-TPD [51].
2.2. Performance of Hybrid FTS Catalysts Mixed with Zeolites
In previous experiments, the GHSV was set to 3000 h−1; however, in this experiment, the GHSV was lowered to 1500 h−1, which has been reported to promote hydrocarbon chain growth [51,54]. Therefore, lowering the GHSV increased the production of heavy waxes, which can be used for achieving the hydrocracking effect with zeolites. As shown in Table 3, the CO conversions and methane selectivities of all catalyst systems are approximately 28–73% and 14–23%, respectively.

Table 3.
Catalytic activities and hydrocarbon selectivities of the prepared catalysts.
A high GHSV was previously shown to result in high CO conversion and low C5+ selectivity. However, upon comparing the catalytic activity of the Co/γ-alumina + γ-alumina and Co/γ-alumina + SAPO-34 catalysts in this experiment with the catalytic activity observed in the previous experiment, it was found that the CO conversion rate was lower and the C5+ higher in this experiment than those in the previous experiment, but the present catalysts deactivated more rapidly than those in the previous experiment [51]. Co/γ-alumina with more γ-alumina as a support exhibits the second lowest CO conversion and highest α value. Compared to the α value of the previous catalyst [51], the hybrid catalysts with ZSM-5 had a lower α value in the remaining catalyst, unlike the hybrid catalysts with SAPO-34 alone. One exception to this finding was the hybrid catalyst with SAPO-34 and ZSM-5 in an 8:2 ratio, which had a higher α value than that found in previous experiments [29]. Co/γ-alumina with the SAPO-34 support exhibits the highest methane selectivity owing to the acidity of SAPO-34. In addition, the catalysts mixed with zeolites other than SAPO-34 show a decrease in methane selectivity compared with that mixed with more γ-alumina. Catalysts containing zeolites have been reported to have lower methane selectivity [14]. However, addition of the highly acidic SAPO-34 increases their methane selectivity compared with that of the Co/γ-alumina catalyst mixed with more γ-alumina.
As shown in Table 3, the α values of the hybrid catalysts with zeolite are lower than that of Co/γ-alumina catalyst mixed with more γ-alumina. Additionally, adding a zeolite rather than γ-alumina results in the production of lighter hydrocarbons, as confirmed by comparing the α values of the C5+ products using offline GC analysis. Many researchers have reported that cobalt-containing catalysts are prone to carbon deposition, and hybrid catalysts containing zeolites often suffer from carbon decomposition owing to the acidity of the zeolites [55,56,57]. This carbon decomposition may generate a sufficiently large amount of carbon to deactivate the catalyst [58]. The reports published by these researchers helped us assess the extent of rapid deactivation on zeolite-containing hybrid catalysts. SAPO-34, which is the most acidic, was found to deactivate at the highest rate (Figure 4c).
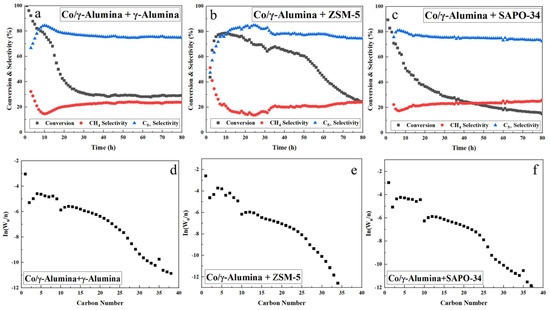
Figure 4.
(a–c) Time-on-stream results and (d–f) ASF product distributions over mixed hybrid FTS catalysts.
As shown in Figure 5a-c, as the proportion of SAPO-34 in the hybrid catalyst composition increases, the deactivation point of the catalyst accelerates, which might be attributable to the acidity of SAPO-34. Meanwhile, Figure 5c demonstrates that a hybrid catalyst prepared by mixing ZSM-5 with a small amount of SAPO-34 exhibits high performance and C5+ selectivity. This suggests that utilizing hybrid catalysts containing a moderate amount of SAPO-34, which is highly acidic, may yield desirable results in terms of catalytic activity and product. The catalyst mixed with both SAPO-34 and zeolite beta supports also exhibits rapid deactivation owing to the high acidity of SAPO-34 (Figure 5 and Figure 6). As shown in Table 3, the hybrid FTS catalyst prepared using Co/γ-alumina and zeolite beta alone is high-performing, but the mixed catalysts incorporating both ZSM-5 and SAPO-34 perform even better. Therefore, mixing small amounts of SAPO-34 with ZSM-5 produces a higher-performance hydrocracking catalyst, which yields lighter hydrocarbons than when using ZSM-5 and γ-alumina separately. The strength of the acidity of SAPO-34 is greater than that of ZSM-5, as determined via NH3-TPD analysis (Table 2). Moreover, the methane selectivity increases and C5+ selectivity decreases with an increasing SAPO-34/ZSM-5 ratio. In comparison to adding both ZSM-5 and SAPO-34, adding only SAPO-34 to the FTS catalyst results in a catalyst with higher hydrocracking activity.
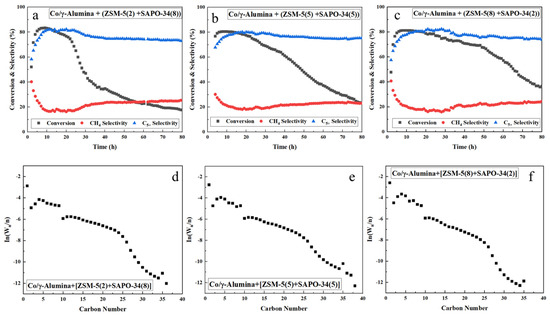
Figure 5.
(a–c) Time-on-stream results and (d–f) ASF product distributions over hybrid FTS catalysts with SAPO-34 and ZSM-5. The support ratio is indicated in parentheses.
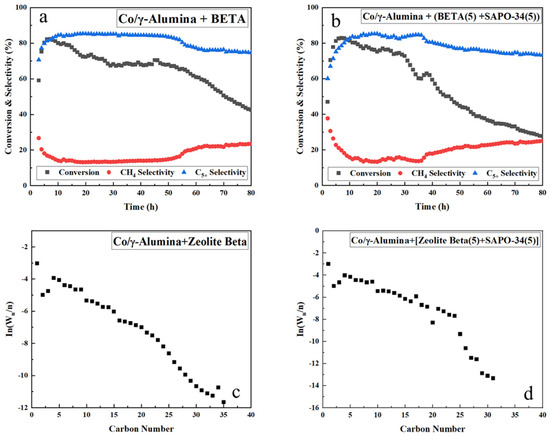
Figure 6.
(a,b) Time-on-stream results and (c,d) ASF product distributions over hybrid FTS catalysts with SAPO-34 and zeolite beta. The support ratio is indicated in parentheses.
As shown in Figure 4b,c and Figure 6a, the hybrid catalyst mixed with zeolite alone exhibited a rapid decline in CO conversion on the conversion rate graph owing to rapid deactivation caused by coke deposition and tended to increase the selectivity for methane. Catalyst deactivation was further confirmed as shown in Figure 4a, likely attributable to the acidity of γ-alumina, as illustrated in Figure 3, which contributed to the deactivation of the catalyst.
Adding SAPO-34 to the hybrid FTS catalyst increases the methane selectivity, as shown previously. Decreasing the SAPO-34/zeolite beta ratio increases the CO conversion because carbon decomposition is reduced as the SAPO-34 content decreases. However, the hybrid FTS catalyst with small amounts of SAPO-34 and ZSM-5 (ratio of 2:8) attains better performance and hydrocracking activity than the other hybrid catalyst systems (Figure 5), corresponding to the highest CO conversion and lowest α value. Compared to the hybrid catalyst with SAPO-34:ZSM-5 (ratio of 2:8), the catalyst reported by Yakovenko et al. [59] showed a higher conversion of CO but a lower selectivity for C5+. Addition of a small amount of the strongly acidic SAPO-34 improves the catalytic activity of the hybrid Co/ZSM-5 catalyst, which is commonly used in commercial FTS reactions [60].
3. Experimental Procedure
3.1. Synthesis of Chabazite-Structured Zeolite SAPO-34 Supports
The procedures for synthesizing starting materials and the detailed hydrothermal method for preparing chabazite-structured zeolite SAPO-34 supports were adopted from previously published reports [34,51].
3.2. Preparation of Hybrid FTS Catalysts via the Physical Mixing Method
Cobalt nitrate hexahydrate (Co(NO3)2, 97 wt.%, Samchun Chemical Co., Ltd., Seoul, Republic of Korea) was dissolved in deionized water. The cobalt solution (15 wt.%) was loaded onto γ-alumina (PURALOX SBa 200, Sasol, Sandton, South Africa) to prepare the γ-alumina-supported cobalt catalyst using a wet impregnation method. After drying at 100 °C overnight, the sample was calcined at 400 °C for 4 h. The fresh Co/γ-alumina catalyst was crushed into particles and sieved to obtain diameters of 300–400 μm, and average particle size of the zeolites was 2 μm. Hybrid FTS catalysts were prepared by physically mixing the Co/γ-alumina catalyst with a single hydrocracking support or a combination of two supports. The weight ratio of the catalyst to the support(s) was 1:1. The supports used for physical mixing were γ-alumina, zeolite beta (Zeolyst, Conshohocken, PA, USA), ZSM-5 (Zeolyst, Conshohocken, PA, USA), and the synthesized SAPO-34 alone, as well as different combinations of these materials at various ratios. The Si/Al ratios in zeolite beta, ZSM-5, and SAPO-34 are 38, 280, and 0.7, respectively.
3.3. Characterization of the Hybrid FTS Catalysts
X-ray diffraction (XRD) analyses of the crystal phases of the supports and catalysts were conducted using an Empyrean XRD instrument (Malvern Panalytical, Malvern, UK, Cu Kα radiation, 1.5406 Å) at diffraction angles (2θ) ranging between 5° and 80°. The Brunauer–Emmett–Teller (BET) method was used to determine the textural properties of the samples. This analysis was performed at −196 °C using a Moonsorp-II (KIST, Seoul, Republic of Korea) instrument to obtain the nitrogen adsorption/desorption isotherms. Temperature-programmed reaction (TPR) and temperature-programmed desorption (TPD) experiments were performed using a Micrometrics Autochem II instrument (Norcross, GA, USA). The detailed procedure for NH3-TPD analysis is described in a previous paper [37].
3.4. Catalytic Activity Test
Catalytic activity was tested using a constant-flow fixed-bed reactor. Cold and hot traps were placed at the outflow end of the reactor to collect water and heavy hydrocarbons, respectively. One gram of the catalyst was placed into a tubular reactor with a diameter of 12.7 mm. The operating conditions for FTS were 220 °C, 2 MPa, a feed gas molar ratio of H2/CO = 2, and a gas hourly space velocity (GHSV) of 1500 h−1. The gas exiting the reactor was analyzed for C1–C9 hydrocarbons using an online gas chromatograph (GC; Agilent, Santa Clara, CA, USA, 7890B) equipped with a flame ionization detector (FID) and a GS Gaspro column (30 m × 0.320 mm). The gas was also analyzed for H2, N2, CO, CO2, water, and methane using a thermal conductivity detector (TCD) and a Carbonsphere column (1.83 m × 1/8″ × 2.0 mm). The collected organic liquid and wax products (C5–C40 hydrocarbons) were analyzed using an offline GC (Agilent, Santa Clara, CA, USA, 7890A) equipped with an FID and an HP-PONA column (50 m × 0.2 mm × 0.5 μm).
4. Conclusions
SAPO-34 and Co/γ-alumina catalysts were synthesized via a conventional hydrothermal method and wet impregnation method, respectively. Hybrid FTS catalysts with varying acidities were prepared by physically mixing the Co/γ-alumina catalyst with γ-alumina and zeolites (ZSM-5, zeolite beta, and SAPO-34). The use of the Co/γ-alumina catalyst mixed with zeolites resulted in the formation of lighter products than those obtained using the Co/γ-alumina catalyst mixed with more γ-alumina. Hybrid catalysts with varying support ratios exhibited differing C5+ and methane selectivities in FTS. Adding a specific quantity of SAPO-34 to the Co/γ-alumina catalyst supported on a 2:8 mixture of SAPO-34 and ZSM-5 improved the CO conversion and C5+ selectivity of FTS. However, there was a significant increase in the methane selectivity and decrease in CO conversion when the SAPO-34 content in the FTS catalyst was increased. Owing to the influence of the FTS reaction conditions and the high acidity of the zeolite, coke deposition occurred and deactivated the catalyst. Therefore, the reaction conditions must be optimized to prevent coke deposition in the next study. In addition, an in-depth examination of the hybrid catalysts by applying catalyst surface analysis for carbon deposition, as well as further screening of the zeolite supports, must be conducted to optimize clean fuel production. These results advance the development of an improved GTL-FPSO process for the offshore production of clean fuel using syngas.
Author Contributions
Formal analysis, J.M.S.; investigation, J.M.S. and Y.-n.C.; data curation, H.-t.S.; writing—original draft, H.D.K.; writing—review and editing, H.D.K. and H.-t.S.; supervision, K.-Y.L. and D.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Science and Technology (Project Nos. 2E32562 and 2B04332).
Data Availability Statement
Restrictions apply to the availability of these data. Some data was obtained from Elsevier and are available at “https://www.sciencedirect.com/science/article/abs/pii/S0920586120304521?via%3Dihub accessed on 1 November 2023” with the permission of Elsevier.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the existing affiliation information. This change does not affect the scientific content of the article.
References
- Mahammadunnisa, S.; Reddy, E.L.; Ray, D.; Subrahmanyam, C.; Whitehead, J.C. CO2 reduction to syngas and carbon nanofibres by plasma-assisted in situ decomposition of water. Int. J. Greenh. Gas Control 2013, 16, 361–363. [Google Scholar] [CrossRef]
- Kang, S.C.; Jun, K.-W.; Lee, Y.-J. Effects of the CO/CO2 ratio in synthesis gas on the catalytic behavior in Fischer–Tropsch synthesis using K/Fe–Cu–Al catalysts. Energy Fuels 2013, 27, 6377–6387. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Advances in CO2 Conversion and Utilization; ACS Publications: Washington, DC, USA, 2010; pp. 125–139. [Google Scholar]
- Ning, W.; Koizumi, N.; Yamada, M. Researching Fe catalyst suitable for CO2-containing syngas for Fischer−Tropsch synthesis. Energy Fuels 2009, 23, 4696–4700. [Google Scholar] [CrossRef]
- Lee, S.-C.; Jang, J.-H.; Lee, B.-Y.; Kim, J.-S.; Kang, M.; Lee, S.-B.; Choi, M.-J.; Choung, S.-J. Promotion of hydrocarbon selectivity in CO2 hydrogenation by Ru component. J. Mol. Catal. A Chem. 2004, 210, 131–141. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, K.B.; Kim, M.Y.; Lee, C.S. Experimental investigation and prediction of density and viscosity of GTL, GTL–biodiesel, and GTL–diesel blends as a function of temperature. Energy Fuels 2013, 27, 56–65. [Google Scholar] [CrossRef]
- Iandoli, C.L.; Kjelstrup, S. Exergy analysis of a GTL process based on low-temperature slurry F− T reactor technology with a cobalt catalyst. Energy Fuels 2007, 21, 2317–2324. [Google Scholar] [CrossRef]
- Saito, M.; Fujitani, T.; Takeuchi, M.; Watanabe, T. Development of copper/zinc oxide-based multicomponent catalysts for methanol synthesis from carbon dioxide and hydrogen. Appl. Catal. A Gen. 1996, 138, 311–318. [Google Scholar] [CrossRef]
- Qi, G.-X.; Fei, J.-H.; Zheng, X.-M.; Hou, Z.-Y. DME synthesis from carbon dioxide and hydrogen over Cu–Mo/HZSM-5. Catal. Lett. 2001, 72, 121–124. [Google Scholar] [CrossRef]
- de Klerk, A.; Furimsky, E. Catalysis in the refining of Fischer–Tropsch syncrude. Platin. Met. Rev. 2011, 55, 263–267. [Google Scholar]
- Martínez, A.; Valencia, S.; Murciano, R.; Cerqueira, H.S.; Costa, A.F.; Aguiar, E.F.S. Catalytic behavior of hybrid Co/SiO2-(medium-pore) zeolite catalysts during the one-stage conversion of syngas to gasoline. Appl. Catal. A Gen. 2008, 346, 117–125. [Google Scholar] [CrossRef]
- Sousa-Aguiar, E.F.; Noronha, F.B.; Faro, A., Jr. The main catalytic challenges in GTL (gas-to-liquids) processes. Catal. Sci. Technol. 2011, 1, 698–713. [Google Scholar] [CrossRef]
- Beaumont, S.K. Recent developments in the application of nanomaterials to understanding molecular level processes in cobalt catalysed Fischer–Tropsch synthesis. Phys. Chem. Chem. Phys. 2014, 16, 5034–5043. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Liu, X.; Lu, X.; Moyo, M.; Hildebrandt, D. Cobalt hybrid catalysts in Fischer-Tropsch synthesis. Rev. Chem. Eng. 2020, 36, 437–457. [Google Scholar] [CrossRef]
- Ralph, C.R. The Fischer–Tropsch Process. In The Biofuels Handbook; Speight, J.G., Ed.; The Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Blekkan, E.A.; Borg, Ø.; Frøseth, V.; Holmen, A. Fischer-Tropsch synthesis on cobalt catalysts: The effect of water. Catalysis 2007, 20, 13–32. [Google Scholar]
- Jacobs, G.; Davis, B.H. Conversion of Biomass to Liquid Fuels and Chemicals via the Fischer–Tropsch Synthesis Route; The Royal Society of Chemistry: London, UK, 2010; Volume 1. [Google Scholar]
- Förtsch, D.; Pabst, K.; Groß-Hardt, E. The product distribution in Fischer–Tropsch synthesis: An extension of the ASF model to describe common deviations. Chem. Eng. Sci. 2015, 138, 333–346. [Google Scholar] [CrossRef]
- Park, D.; Moon, D.J.; Kim, T. Steam-CO2 reforming of methane on Ni/γ-Al2O3-deposited metallic foam catalyst for GTL-FPSO process. Fuel Process. Technol. 2013, 112, 28–34. [Google Scholar] [CrossRef]
- Jung, J.-S.; Choi, G.; Lee, J.-S.; Moon, D.J. Microstructure of FTS studies over spherical Co/γ-Al2O3. Catal. Today 2015, 250, 102–114. [Google Scholar] [CrossRef]
- Hong, G.H.; Noh, Y.S.; Park, J.I.; Shin, S.A.; Moon, D.J. Effect of catalytic reactor bed dilution on product distribution for Fischer-Tropsch synthesis over Ru/Co/Al2O3 catalyst. Catal. Today 2018, 303, 136–142. [Google Scholar] [CrossRef]
- Jung, I.; Kshetrimayum, K.S.; Park, S.; Na, J.; Lee, Y.; An, J.; Park, S.; Lee, C.-J.; Han, C. Computational fluid dynamics based optimal design of guiding channel geometry in U-type coolant layer manifold of large-scale microchannel Fischer–Tropsch reactor. Ind. Eng. Chem. Res. 2016, 55, 505–515. [Google Scholar] [CrossRef]
- Hong, G.H.; Moon, D.J. Development of fixed bed reactor for application in GTL-FPSO: The effect of nitrogen and carbon dioxide contents in feed gas on Fischer-Tropsch synthesis reaction over Ru/Co/Al2O3 catalyst. Catal. Today 2019, 353, 73–81. [Google Scholar] [CrossRef]
- Noh, Y.S.; Lee, K.-Y.; Moon, D.J. Studies on the Fischer-Tropsch synthesis over RuCo/SiC-Al2O3 structured catalyst. Catal. Today 2019, 348, 157–165. [Google Scholar] [CrossRef]
- Koo, H.M.; Park, M.J.; Moon, D.J.; Bae, J.W. Kinetic models of Fischer-Tropsch synthesis reaction over granule-type Pt-promoted Co/Al2O3 catalyst. Korean J. Chem. Eng. 2018, 35, 1263–1273. [Google Scholar] [CrossRef]
- Jung, J.-S.; Lee, J.-S.; Choi, G.; Ramesh, S.; Moon, D.J. The characterization of micro-structure of cobalt on γ-Al2O3 for FTS: Effects of pretreatment on Ru–Co/γ-Al2O3. Fuel 2015, 149, 118–129. [Google Scholar] [CrossRef]
- Jung, J.-S.; Kim, S.W.; Moon, D.J. Fischer–Tropsch Synthesis over cobalt based catalyst supported on different mesoporous silica. Catal. Today 2012, 185, 168–174. [Google Scholar] [CrossRef]
- Feller, A.; Guzman, A.; Zuazo, I.; Lercher, J.A. On the mechanism of catalyzed isobutane/butene alkylation by zeolites. J. Catal. 2004, 224, 80–93. [Google Scholar] [CrossRef]
- Kim, N.Y.; Jung, J.-S.; Lee, J.S.; Yang, E.H.; Hong, G.H.; Lim, S.S.; Noh, Y.S.; Hodala, J.L.; Lee, K.Y.; Moon, D.J. Synthesis and characterization of Al-modified SBA-15 for Fischer–Tropsch synthesis (FTS) reaction. Res. Chem. Intermed. 2016, 42, 319–334. [Google Scholar] [CrossRef]
- Kuipers, E.; Scheper, C.; Wilson, J.; Vinkenburg, I.; Oosterbeek, H. Non-ASF product distributions due to secondary reactions during Fischer–Tropsch synthesis. J. Catal. 1996, 158, 288–300. [Google Scholar] [CrossRef]
- Liu, X.; Hamasaki, A.; Honma, T.; Tokunaga, M. Anti-ASF distribution in Fischer-Tropsch synthesis over unsupported cobalt catalysts in a batch slurry phase reactor. Catal. Today 2011, 175, 494–503. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Corma, A. Applications of zeolites to C1 chemistry: Recent advances, challenges, and opportunities. Adv. Mater. 2020, 32, 2002927. [Google Scholar] [CrossRef]
- Sie, S.; Senden, M.; Van Wechem, H. Conversion of natural gas to transportation fuels via the shell middle distillate synthesis process (SMDS). Catal. Today 1991, 8, 371–394. [Google Scholar] [CrossRef]
- Eslami, A.A.; Haghighi, M.; Sadeghpour, P. Short time microwave/seed-assisted synthesis and physicochemical characterization of nanostructured MnAPSO-34 catalyst used in methanol conversion to light olefins. Powder Technol. 2017, 310, 187–200. [Google Scholar] [CrossRef]
- Dahl, I.M.; Kolboe, S. On the reaction mechanism for propene formation in the MTO reaction over SAPO-34. Catal. Lett. 1993, 20, 329–336. [Google Scholar] [CrossRef]
- Chen, D.; Moljord, K.; Fuglerud, T.; Holmen, A. The effect of crystal size of SAPO-34 on the selectivity and deactivation of the MTO reaction. Microporous Mesoporous Mater. 1999, 29, 191–203. [Google Scholar] [CrossRef]
- Lu, P.; Sun, J.; Zhu, P.; Abe, T.; Yang, R.; Taguchi, A.; Vitidsant, T.; Tsubaki, N. Sputtered nano-cobalt on H-USY zeolite for selectively converting syngas to gasoline. J. Energy Chem. 2015, 24, 637–641. [Google Scholar] [CrossRef]
- Yoneyama, Y.; He, J.; Morii, Y.; Azuma, S.; Tsubaki, N. Direct synthesis of isoparaffin by modified Fischer–Tropsch synthesis using hybrid catalyst of iron catalyst and zeolite. Catal. Today 2005, 104, 37–40. [Google Scholar] [CrossRef]
- Tsubaki, N.; Yoneyama, Y.; Michiki, K.; Fujimoto, K. Three-component hybrid catalyst for direct synthesis of isoparaffin via modified Fischer–Tropsch synthesis. Catal. Commun. 2003, 4, 108–111. [Google Scholar] [CrossRef]
- Sartipi, S.; Parashar, K.; Valero-Romero, M.J.; Santos, V.P.; Van Der Linden, B.; Makkee, M.; Kapteijn, F.; Gascon, J. Hierarchical H-ZSM-5-supported cobalt for the direct synthesis of gasoline-range hydrocarbons from syngas: Advantages, limitations, and mechanistic insight. J. Catal. 2013, 305, 179–190. [Google Scholar] [CrossRef]
- Sartipi, S.; Van Dijk, J.E.; Gascon, J.; Kapteijn, F. Toward bifunctional catalysts for the direct conversion of syngas to gasoline range hydrocarbons: H-ZSM-5 coated Co versus H-ZSM-5 supported Co. Appl. Catal. A Gen. 2013, 456, 11–22. [Google Scholar] [CrossRef]
- Martínez, A.; Rollán, J.; Arribas, M.A.; Cerqueira, H.S.; Costa, A.F.; Aguiar, E.F.S. A detailed study of the activity and deactivation of zeolites in hybrid Co/SiO2-zeolite Fischer–Tropsch catalysts. J. Catal. 2007, 249, 162–173. [Google Scholar] [CrossRef]
- Huang, X.; Hou, B.; Wang, J.; Li, D.; Jia, L.; Chen, J.; Sun, Y. CoZr/H-ZSM-5 hybrid catalysts for synthesis of gasoline-range isoparaffins from syngas. Appl. Catal. A Gen. 2011, 408, 38–46. [Google Scholar] [CrossRef]
- Wu, E.; Lawton, S.; Olson, D.; Rohrman, A.; Kokotailo, G. ZSM-5-type materials. Factors affecting crystal symmetry. J. Phys. Chem. 1979, 83, 2777–2781. [Google Scholar] [CrossRef]
- Aghaei, E.; Haghighi, M. Effect of crystallization time on properties and catalytic performance of nanostructured SAPO-34 molecular sieve synthesized at high temperatures for conversion of methanol to light olefins. Powder Technol. 2015, 269, 358–370. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Q.; He, J.; Wang, Y.; Wei, F. Pore-structure-mediated hierarchical SAPO-34: Facile synthesis, tunable nanostructure, and catalysis applications for the conversion of dimethyl ether into olefins. Particuology 2013, 11, 468–474. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Bao, X.; Liu, X.; Han, X.; He, C.; Zhai, R. Crystallization and Si incorporation mechanisms of SAPO-34. Microporous Mesoporous Mater. 2002, 53, 97–108. [Google Scholar] [CrossRef]
- Sadeghpour, P.; Haghighi, M. DEA/TEAOH templated synthesis and characterization of nanostructured NiAPSO-34 particles: Effect of single and mixed templates on catalyst properties and performance in the methanol to olefin reaction. Particuology 2015, 19, 69–81. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Zhang, Y.; Li, J.; Racoillet, G.; Davis, B.H. Fischer–Tropsch synthesis: Support, loading, and promoter effects on the reducibility of cobalt catalysts. Appl. Catal. A Gen. 2002, 233, 263–281. [Google Scholar] [CrossRef]
- Xu, D.; Li, W.; Duan, H.; Ge, Q.; Xu, H. Reaction performance and characterization of Co/Al2O3 Fischer–Tropsch catalysts promoted with Pt, Pd and Ru. Catal. Lett. 2005, 102, 229–235. [Google Scholar] [CrossRef]
- Kim, H.D.; Song, H.-t.; Fazeli, A.; Eslami, A.A.; Noh, Y.S.; Saeidabad, N.G.; Lee, K.-Y.; Moon, D.J. CO/CO2 hydrogenation for the production of lighter hydrocarbons over SAPO-34 modified hybrid FTS catalysts. Catal. Today 2022, 388, 410–416. [Google Scholar] [CrossRef]
- Kwini, M.N.; Botha, J.M. Influence of feed components on the activity and stability of cobalt molybdenum alumina metathesis catalyst. Appl. Catal. A Gen. 2005, 280, 199–208. [Google Scholar] [CrossRef]
- Aghaei, E.; Haghighi, M.; Pazhohniya, Z.; Aghamohammadi, S. One-pot hydrothermal synthesis of nanostructured ZrAPSO-34 powder: Effect of Zr-loading on physicochemical properties and catalytic performance in conversion of methanol to ethylene and propylene. Microporous Mesoporous Mater. 2016, 226, 331–343. [Google Scholar] [CrossRef]
- De la Osa, A.; De Lucas, A.; Romero, A.; Valverde, J.; Sánchez, P. Fischer–Tropsch diesel production over calcium-promoted Co/alumina catalyst: Effect of reaction conditions. Fuel 2011, 90, 1935–1945. [Google Scholar] [CrossRef]
- Borkó, L.; Horváth, Z.; Schay, Z.; Guczi, L. The role of carbon nanospecies in deactivation of cobalt based catalysts in CH4 and CO transformation. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 167, pp. 231–236. [Google Scholar]
- Martínez, A.; Lopez, C. The influence of ZSM-5 zeolite composition and crystal size on the in situ conversion of Fischer–Tropsch products over hybrid catalysts. Appl. Catal. A Gen. 2005, 294, 251–259. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Huang, J.; Liang, J.; Wang, H.; Li, Z.; Wu, J.; Li, M.; Zhao, Y.; Niu, J. Effect of hierarchical crystal structures on the properties of cobalt catalysts for Fischer–Tropsch synthesis. Fuel 2016, 174, 17–24. [Google Scholar] [CrossRef]
- Goodman, D.; Kelley, R.; Madey, T.; Yates, J., Jr. Kinetics of the hydrogenation of CO over a single crystal nickel catalyst. J. Catal. 1980, 63, 226–234. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Savost’yanov, A.P.; Narochniy, G.B.; Soromotin, V.N.; Zubkov, I.N.; Papeta, O.P.; Svetogorov, R.D.; Mitchenko, S.A. Preliminary evaluation of a commercially viable Co-based hybrid catalyst system in Fischer–Tropsch synthesis combined with hydroprocessing. Catal. Sci. Technol. 2020, 10, 7613–7629. [Google Scholar] [CrossRef]
- Kibby, C.; Jothimurugesan, K.; Das, T.; Lacheen, H.; Rea, T.; Saxton, R. Chevron’s gas conversion catalysis-hybrid catalysts for wax-free Fischer–Tropsch synthesis. Catal. Today 2013, 215, 131–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).