Bacterial Cellulose and Biodegradable Superbase for Heterogeneous Transesterification to Alkyl Esters
Abstract
:1. Introduction
2. Results and Discussion
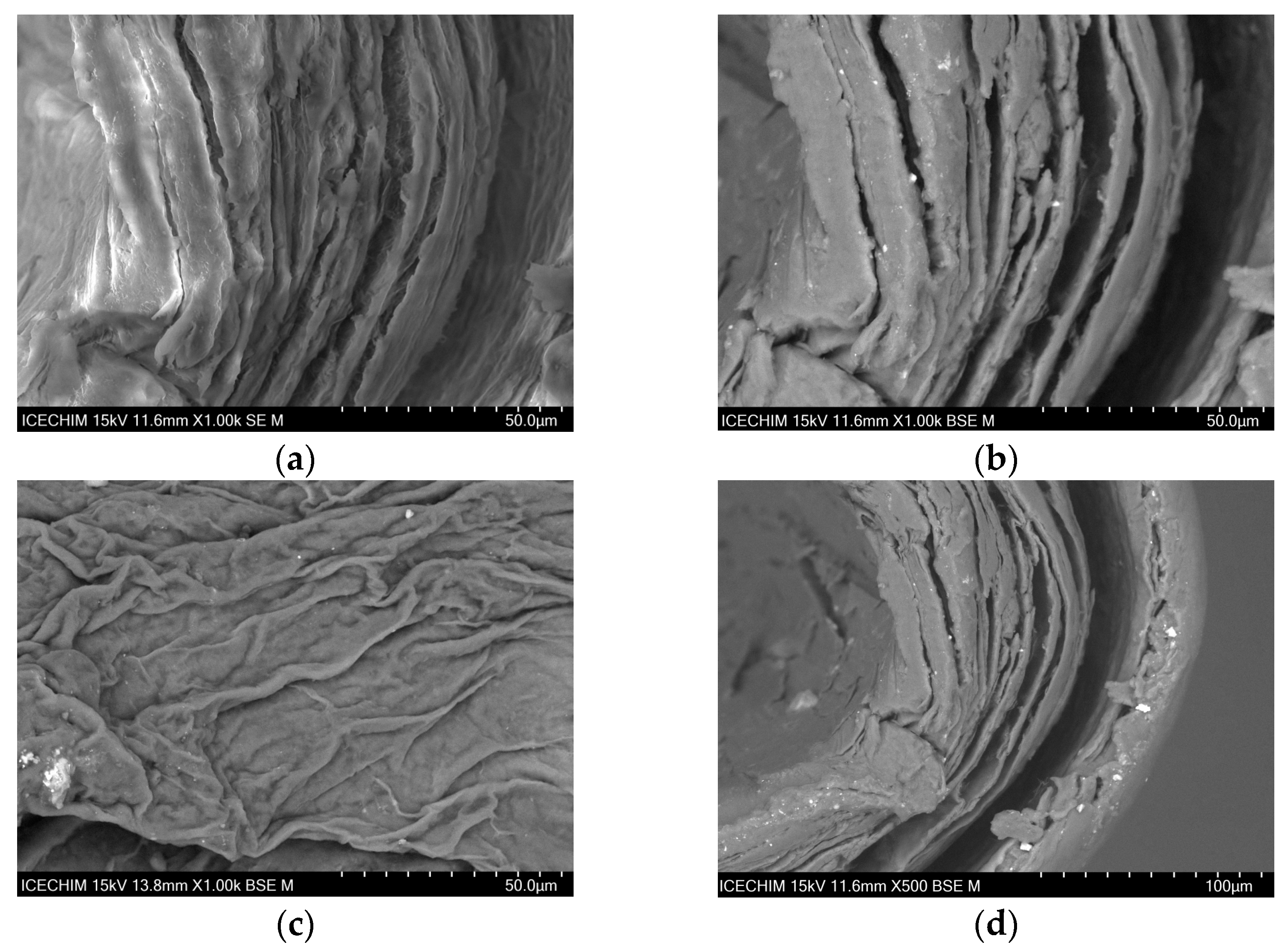
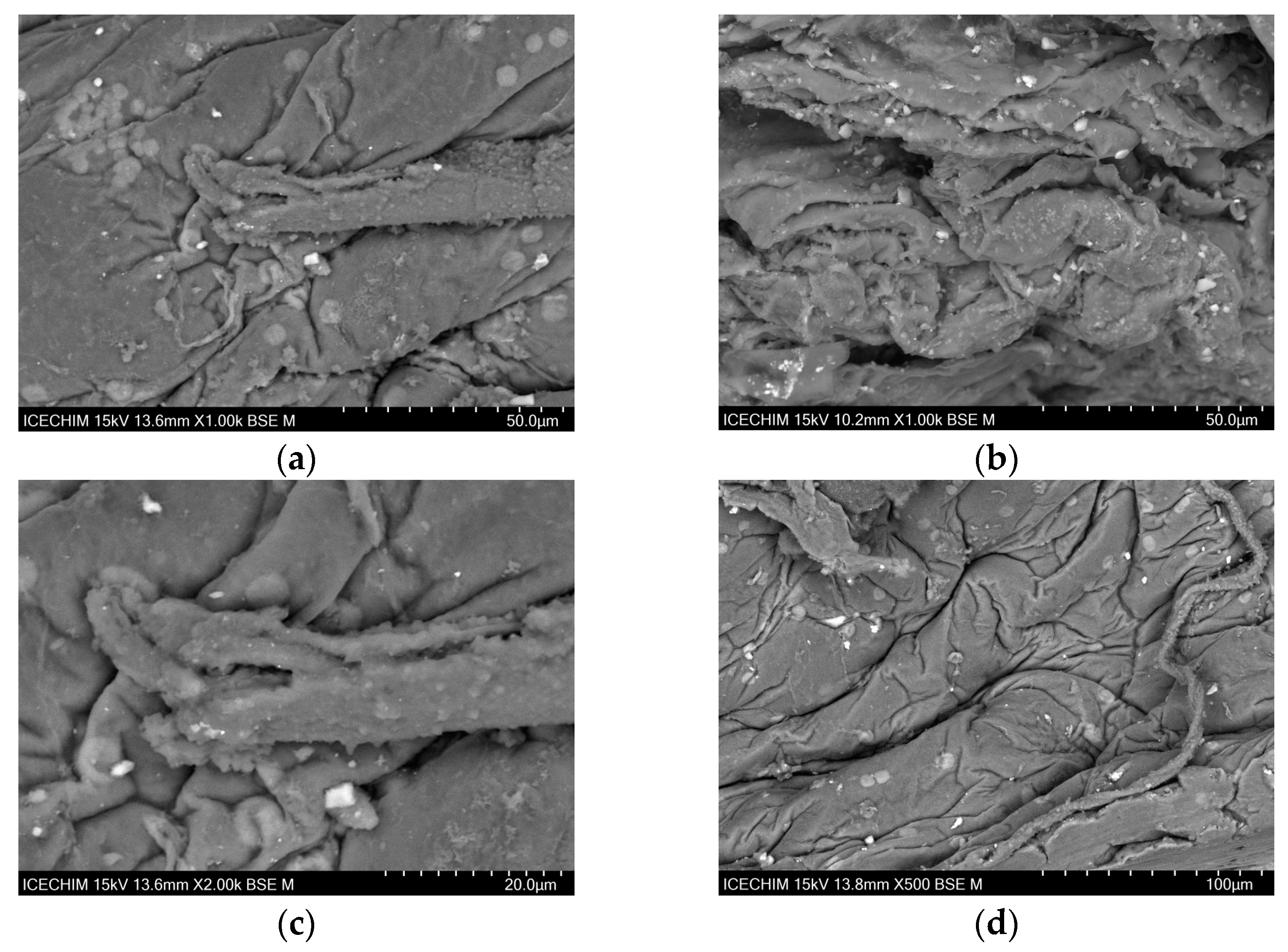
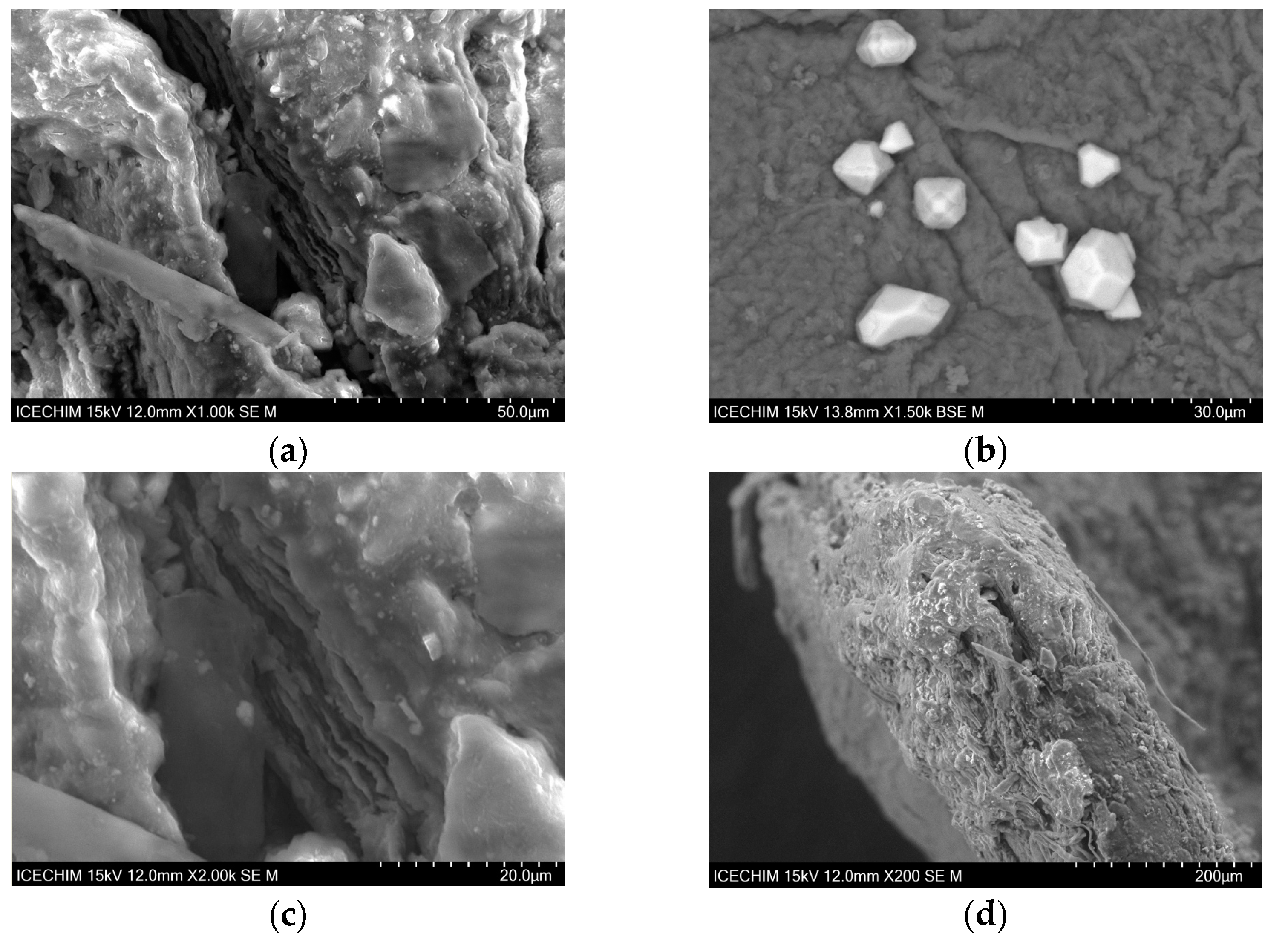
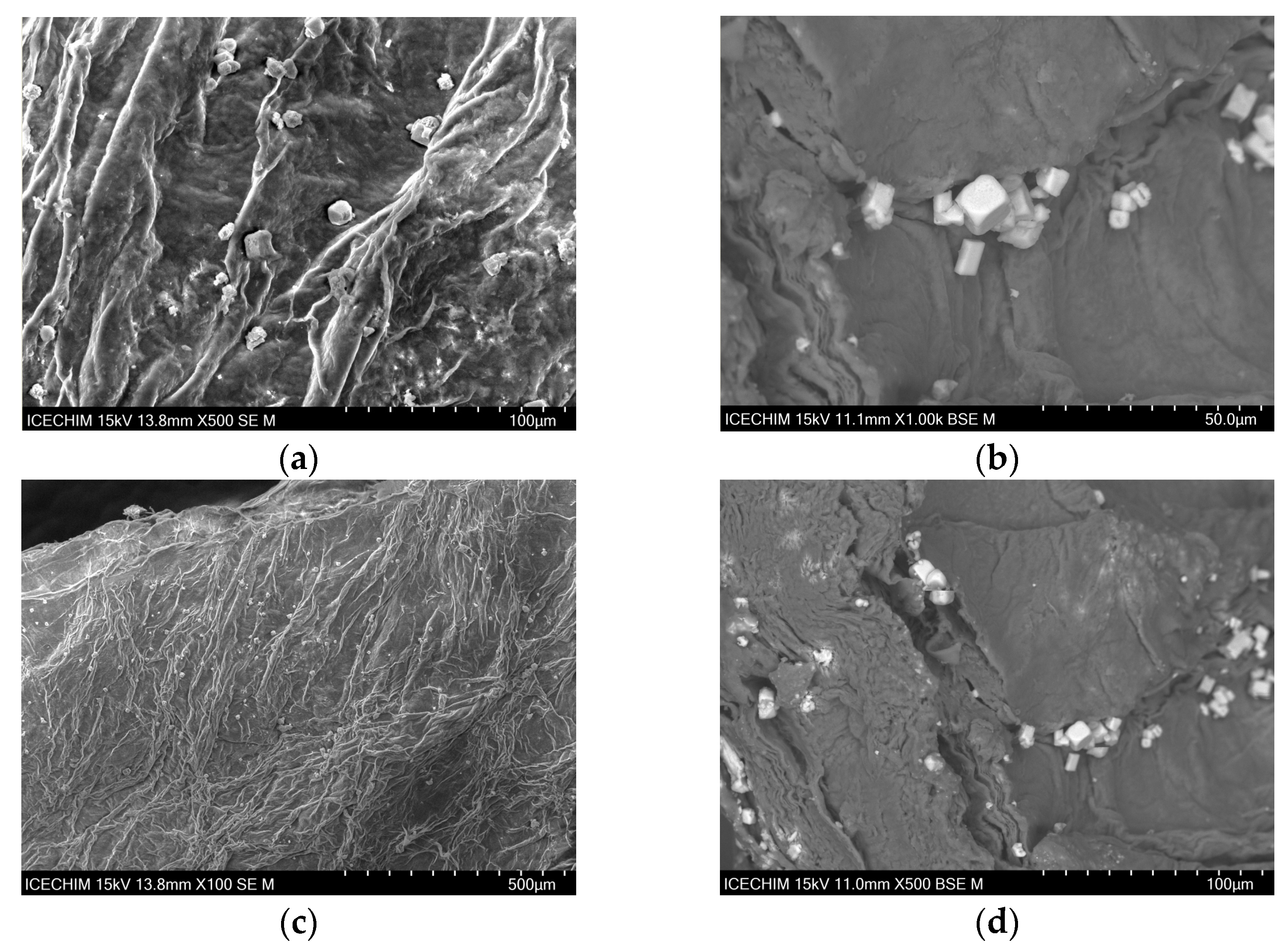
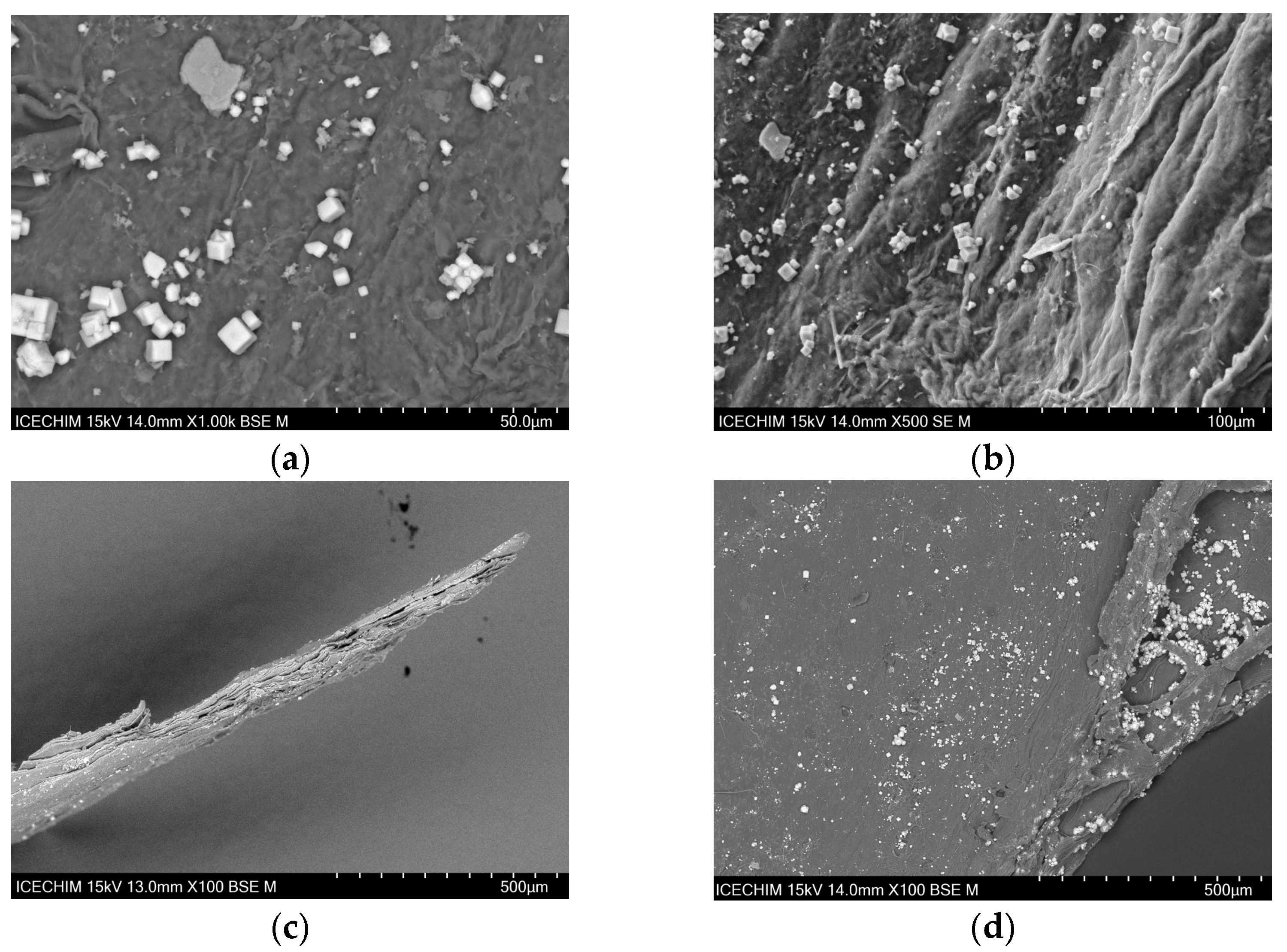
2.1. Scanning Electron Microscope (SEM) Analysis of Heterogeneous Catalyst and Catalytic Support
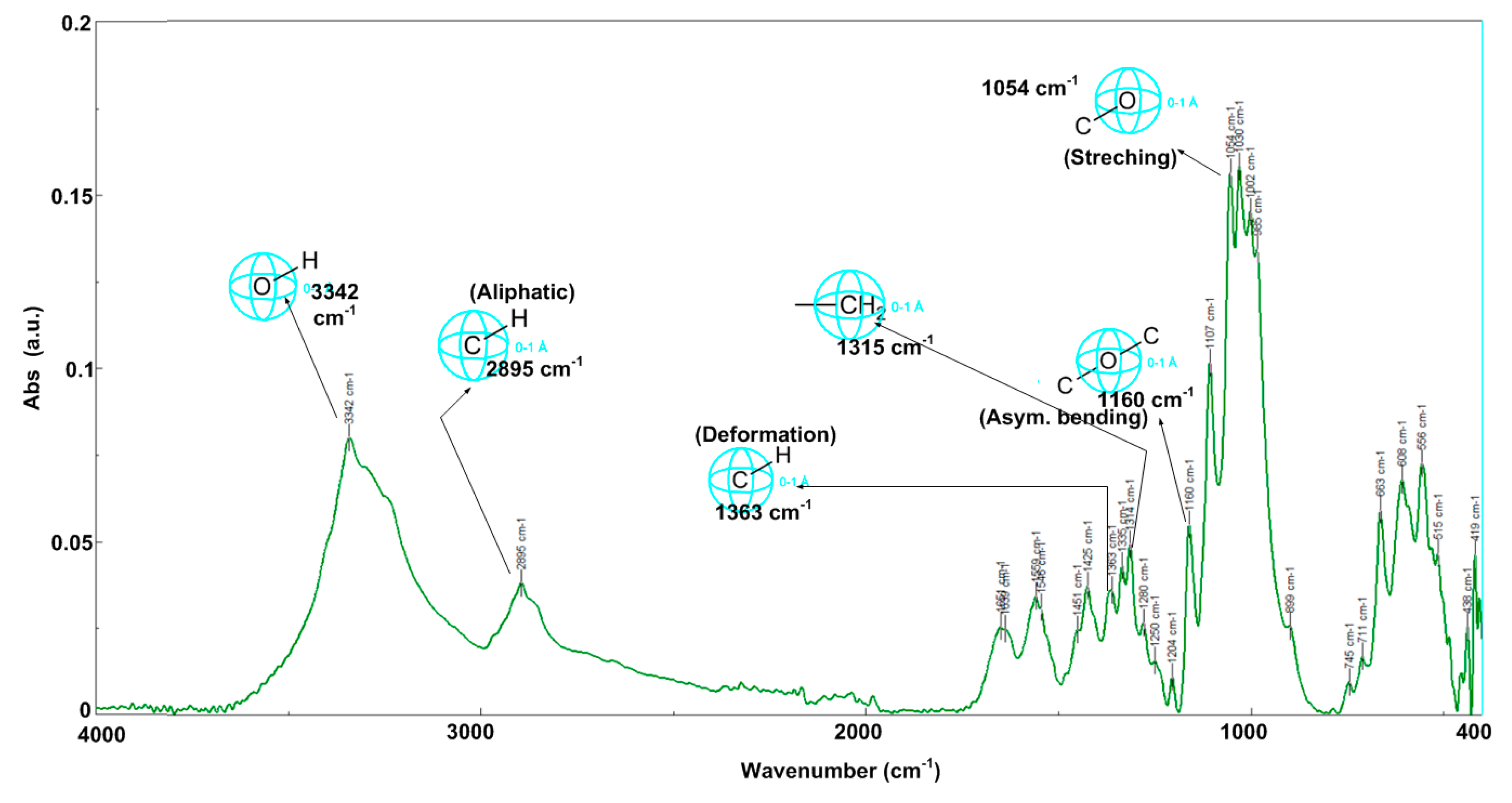
2.2. Fourier Transform Infrared Spectroscopy for Heterogeneous Catalyst and Catalytic Support
2.3. Predicted Responses and Process Factor Optimization
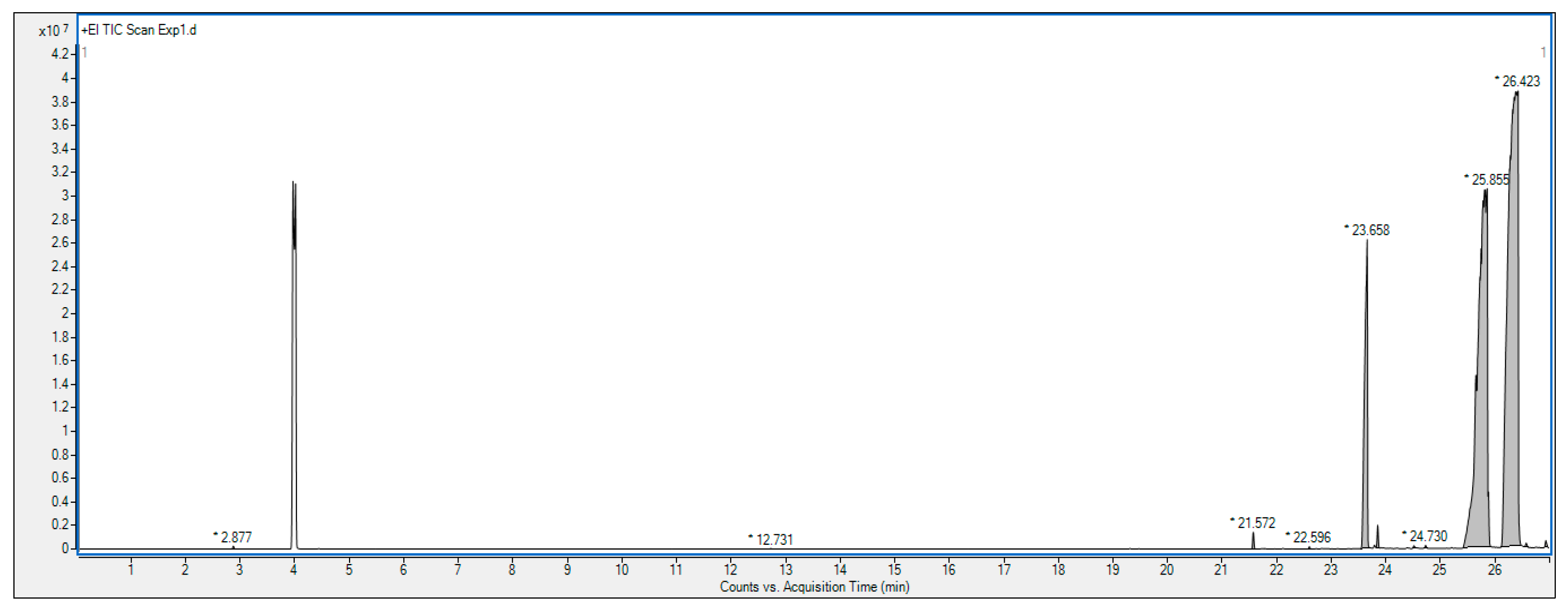
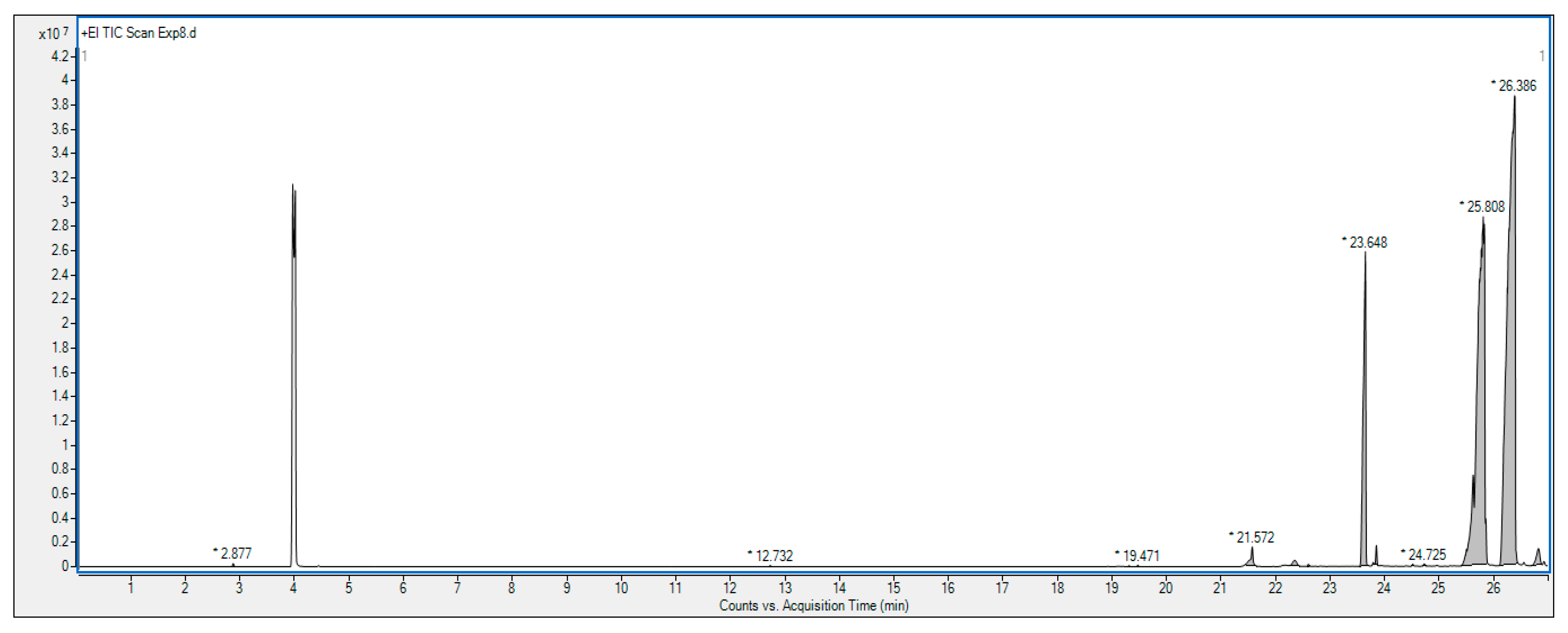
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3. Materials and Methods
3.1. Materials
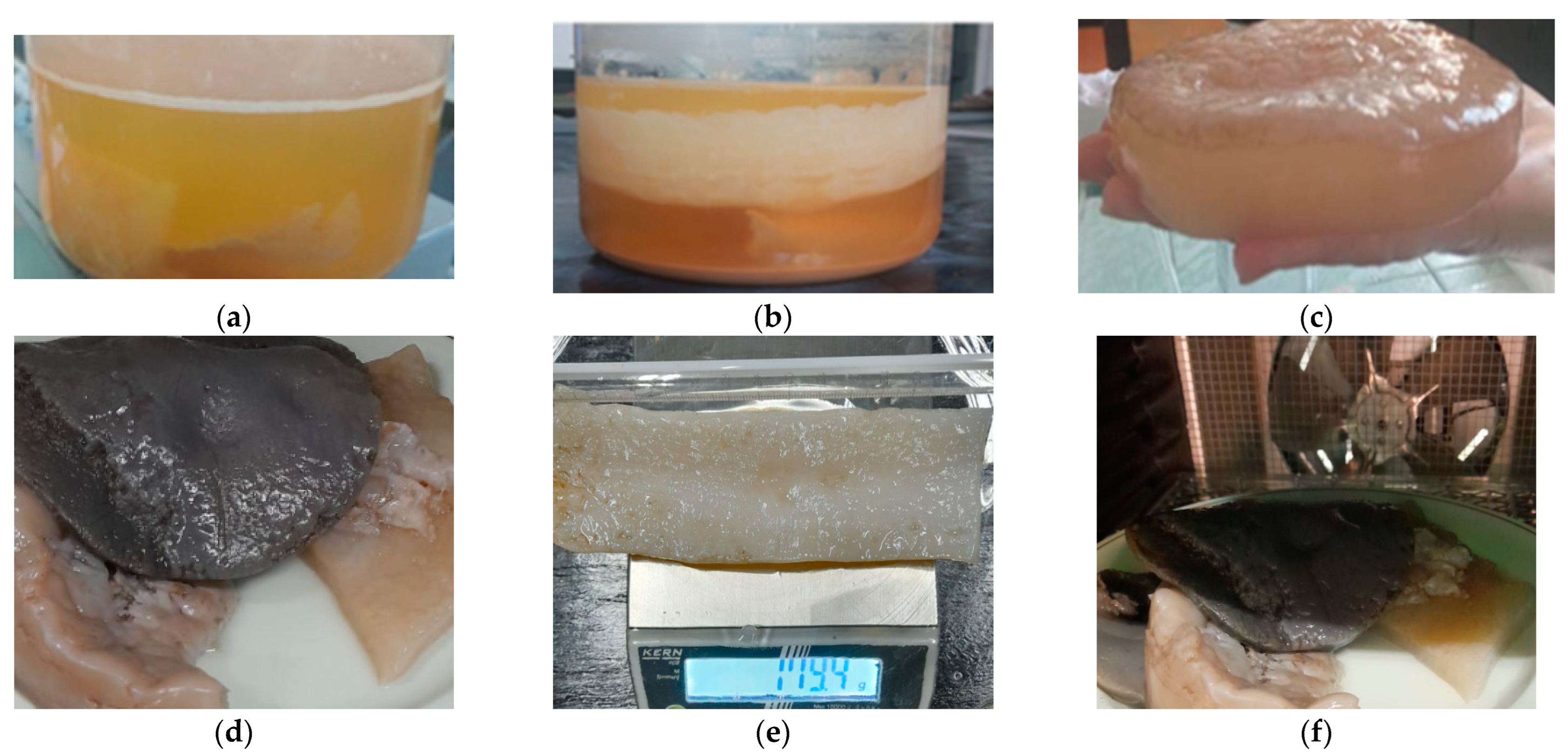
3.2. Bacterial Cellulose Production
3.3. Catalyst Preparation
3.4. Catalyst Characterization
3.4.1. Scanning Electron Microscope (SEM) Analysis
3.4.2. Fourier Transform Infrared Spectroscopy
3.5. Catalyst Testing and Analysis
3.5.1. Catalyst Testing in Transesterification Reactions to Alkyl Esters
3.5.2. Analysis of Main Product in Transesterification Reactions to Alkyl Esters
3.5.3. Soap Content in FAME Test Reaction Product
3.5.4. Independent and Dependent Process Variables
3.5.5. Experimental Design, Statistical Analysis, and Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knothe, G.; Van Gerpen, J. Chapter 2—The History of Vegetable Oil-Based Diesel Fuells. In The Biodiesel Handbook, 1st ed.; Knothe, G., Van Gerpen, J., Krahl, J., Eds.; AOCS Press Publishing: Champaigne, IL, USA, 2005; pp. 12–24. [Google Scholar] [CrossRef]
- Liu, Y.; Boyás, R.S.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of vegetable oil to produce bio-hydrogenated diesel and liquefied petroleum gas fuel over catalysts containing sulfided Ni–Mo and solid acids. Energy Fuel 2011, 25, 4675–4685. [Google Scholar] [CrossRef]
- Murata, K.; Liu, Y.; Inaba, M.; Takahara, I. Production of synthetic diesel by hydrotreatment of Jatropha oils using Pt–Re/H-ZSM-5 catalyst. Energy Fuel 2010, 24, 2404–2409. [Google Scholar] [CrossRef]
- Veriansyah, B.; Han, J.Y.; Kim, S.K.; Hong, S.A.; Kim, Y.J.; Lim, J.S.; Shu, Y.W.; Oh, S.G.; Kim, J. Production of renewable diesel by hydroprocessing of soybean oil: Effect of catalysts. Fuel 2011, 94, 578–585. [Google Scholar] [CrossRef]
- Hydrotreating Catalysts and Technologies for All Crude Oil Fractions. Available online: http://www.topsoe.com/processes/hydrotreating (accessed on 30 September 2023).
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef]
- Castro, E.; Strætkvern, K.O.; Romero-García, J.M.; Martín, C. Pretreatment and Bioconversion for Valorization of Residues of Non-Edible Oilseeds. Agronomy 2023, 13, 2196. [Google Scholar] [CrossRef]
- Neto, J.M.; Komesu, A.; Da Silva Martins, L.H.; Gonçalves, V.O.O.; De Oliveira, J.A.R.; Rai, M.; Ingle, A.P. Chapter 10—Third generation biofuels: An overview. In Sustainable Bioenerg; Rai, M., Ingle, A.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–298. [Google Scholar] [CrossRef]
- Bhatia, K.S.; Gurav, R.; Choi, T.R.; Kim, H.J.; Yang, S.Y.; Hong, H.S.; Park, J.Y.; Park, Y.L.; Han, Y.H.; Choi, Y.K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Singh, H.; Ali, A. Esterification as well as transesterification of waste oil using potassium imbued tungstophosphoric acid supported graphene oxide as heterogeneous catalyst: Optimization and kinetic modeling. Renew. Energy 2023, 207, 422–435. [Google Scholar] [CrossRef]
- Chinglenthoiba, C.; Das, A.; Vandana, S. Enhanced biodiesel production from waste cooking palm oil, with NaOH-loaded Calcined fish bones as the catalyst. Environ. Sci. Pollut. Res. 2020, 27, 15925–15930. [Google Scholar] [CrossRef]
- Poddar, M.K.; Pritam Kumar Dikshit, P.K. Recent development in bacterial cellulose production and synthesis of cellulose based conductive polymer nanocomposites. Nano Select 2021, 2, 1605–1628. [Google Scholar] [CrossRef]
- Sharma, P.; Mittal, M.; Yadav, A.; Aggarwal, N.K. Bacterial cellulose: Nano-biomaterial for biodegradable face masks—A greener approach towards environment. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100759. [Google Scholar] [CrossRef] [PubMed]
- Bueno, F.; Fultz, L.; Husseneder, C.; Keenan, M.; Sathivel, S. Biodegradability of bacterial cellulose polymer below the soil and its effects on soil bacteria diversity. Polym. Degrad. Stab. 2023, 217, 110535. [Google Scholar] [CrossRef]
- Fernando, G.; Torres, F.G.; Solene Commeaux, S.; Omar, P.; Troncoso, O.P. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef]
- Păvăloiu, R.D.; Stroescu, M.; Pârvulescu, O.C.; Dobre, T. Composite hydrogels of bacterial cellulose-carboxymethyl cellulose for drug release. Rev. Chim. 2014, 65, 948–951. [Google Scholar]
- Parvulescu, O.P.; Isopencu, G.; Busuioc, C.; Raducanu, C.; Mocanu, A.; Deleanu, I.; Stoica-Guzun, A. Chapter 17—Antimicrobial bacterial cellulose composites as textile materials. In Antimicrobial Textiles from Natural Resources; Mondal, I.H., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 513–556. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, J.; Zheng, J.; Fu, B. Immobilization of UiO-66-NH2 into Bacterial Cellulose Aerogels for Efficient Particulate Matter Filtration. Sustainability 2023, 15, 13382. [Google Scholar] [CrossRef]
- Tahir, D.; Karim, M.R.A.; Hu, H.; Naseem, S.; Rehan, M.; Ahmad, M.; Zhang, M. Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose Based Composites: A Review. Polymers 2022, 14, 4468. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.M.A.E.; Ghanem, A.F.; Sheir, D.H.; Selim, M.M.; Zaher, F.A.; Badawy, A.A. Assessment of a green zeolite/bacterial cellulose nanocomposite membrane as a catalyst to produce biodiesel from waste cooking oil. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 10350–10365. [Google Scholar] [CrossRef]
- Raducanu, C.E. New Solutions Regarding Catalytic Transesterification Integration with Separation in Biodiesel Technology. Ph.D. Thesis, Chemical and Biochemical Engineering Department, Faculty of Chemical Engineering and Biotechnologies, National University of Sciences and Technologies POLITEHNICA Bucharest, Bucharest, Romania, 2020. [Google Scholar]
- Drozd, M.; Dudzic, D.; Pietraszko, A. Crystal structure, differential scanning calorimetric, infrared spectroscopy and theoretical studies of C(NH2)2(NH)CH2@CHCOOH noncentrosymmetric crystal. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 135–148. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. Chapter 8—Analytical Chemistry. In Handbook of chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 1, pp. 43–48. [Google Scholar]
- Nelli Atykyan, N.; Victor Revin, V.; Shutova, V. Raman and FT-IR Spectroscopy investigation the cellulose structural differences from bacteria Gluconacetobacter sucrofermentans during the different regimes of cultivation on a molasses media. AMB Express 2020, 10, 84. [Google Scholar] [CrossRef]
- Dima, A.D.; Pârvulescu, O.C.; Mateescu, C.; Dobre, T. Optimization of substrate composition in anaerobic co-digestion of agricultural waste using central composite design. Biomass Bioenerg. 2020, 138, 105602. [Google Scholar] [CrossRef]
- Drăghici-Popa, A.-M.; Boscornea, A.C.; Brezoiu, A.-M.; Tomas, S.T.; Pârvulescu, O.C.; Stan, R. Effects of extraction process factors on the composition and antioxidant activity of blackthorn (Prunus spinosa L.) fruit extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef]
- Dobre, T.; Stoica, A.; Pârvulescu, O.C.; Stroescu, M.; Iavorschi, G. Factors influence on bacterial cellulose growth in static reactors. Rev. Chim. 2008, 59, 591–594. [Google Scholar] [CrossRef]
- Chanakaewsomboon, I.; Tongurai, C.; Photaworn, S.; Kungsanant, S.; Nikhom, R. Investigation of saponification mechanisms in biodiesel production: Microscopic visualization of the effects of FFA, water and the amount of alkaline catalyst. J. Environ. Chem. Eng. 2020, 8, 103538. [Google Scholar] [CrossRef]






| Exp. | A (g/mL) | B (cm2) | C (rpm) | D (°C) | E (mol/mol) | F (°C) | G (h) | Y (%) | S (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.053 | 6.250 | 300 | 70 | 12 | 70 | 96 | 89.08 | 1303 |
| 2 | 0.053 | 6.250 | 0 | 25 | 6 | 25 | 96 | 86.32 | 1583 |
| 3 | 0.053 | 0.640 | 300 | 70 | 6 | 25 | 24 | 93.12 | 3386 |
| 4 | 0.053 | 0.640 | 0 | 25 | 12 | 70 | 24 | 99.81 | 1364 |
| 5 | 0.027 | 6.250 | 300 | 25 | 12 | 25 | 24 | 93.32 | 1394 |
| 6 | 0.027 | 6.250 | 0 | 70 | 6 | 70 | 24 | 98.81 | 2061 |
| 7 | 0.027 | 0.640 | 300 | 25 | 6 | 70 | 96 | 99.31 | 1819 |
| 8 | 0.027 | 0.640 | 0 | 70 | 12 | 25 | 96 | 93.92 | 1603 |
| 9 | 0.027 | 0.640 | 0 | 25 | 12 | 70 | 96 | 99.91 | 721.9 |
| 10 | 0.027 | 0.640 | 0 | 25 | 12 | 70 | 96 | 99.88 | 704.8 |
| 11 | 0.027 | 0.640 | 0 | 25 | 12 | 70 | 96 | 99.79 | 691.4 |
| Exp.No. | Catalytic Supp./Catalyst | Comments | Observations |
|---|---|---|---|
| 1 | BC | BC, plain, simple | Evaluation |
| 2 | BCGu | BC in alcoholic guanidine, without KOH | Evaluation |
| 3 | BCGu-4K24h | BC/superbase (Gu-KOH, 4K, impregnation time 24 h) * | Heterogeneous catalyst |
| 4 | BCGu-4K96h | BC/superbase (Gu-KOH, 4K, impregnation time 96 h) | Heterogeneous catalyst |
| 5 | BCGu-8K24h | BC/superbase (Gu-KOH, 8K, impregnation time 24 h) ** | Heterogeneous catalyst |
| 6 | BCGu-4K96h | BC/superbase (Gu-KOH, 8K, impregnation time 96 h) | Heterogeneous catalyst |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogoaşă, C.I.; Răducanu, C.E.; Petraş, L.E.; Cioroiu Tîrpan, D.R.; Vasilievici, G.; Mîrţ, A.L.; Dobre, T.; Pârvulescu, O.C. Bacterial Cellulose and Biodegradable Superbase for Heterogeneous Transesterification to Alkyl Esters. Catalysts 2023, 13, 1431. https://doi.org/10.3390/catal13111431
Gogoaşă CI, Răducanu CE, Petraş LE, Cioroiu Tîrpan DR, Vasilievici G, Mîrţ AL, Dobre T, Pârvulescu OC. Bacterial Cellulose and Biodegradable Superbase for Heterogeneous Transesterification to Alkyl Esters. Catalysts. 2023; 13(11):1431. https://doi.org/10.3390/catal13111431
Chicago/Turabian StyleGogoaşă, Cristina Ionela, Cristian Eugen Răducanu, Laura Elisabeta Petraş, Doinița Roxana Cioroiu Tîrpan, Gabriel Vasilievici, Andreea Luiza Mîrţ, Tănase Dobre, and Oana Cristina Pârvulescu. 2023. "Bacterial Cellulose and Biodegradable Superbase for Heterogeneous Transesterification to Alkyl Esters" Catalysts 13, no. 11: 1431. https://doi.org/10.3390/catal13111431
APA StyleGogoaşă, C. I., Răducanu, C. E., Petraş, L. E., Cioroiu Tîrpan, D. R., Vasilievici, G., Mîrţ, A. L., Dobre, T., & Pârvulescu, O. C. (2023). Bacterial Cellulose and Biodegradable Superbase for Heterogeneous Transesterification to Alkyl Esters. Catalysts, 13(11), 1431. https://doi.org/10.3390/catal13111431










