Abstract
Catalytic performance of 3 and 5 wt.% of vanadia, supported on zirconia, zirconia-ceria, and zirconia-yttria, tested in the combustion of soot without and in the presence of NO was described. The catalysts were characterized by structural (XRD, RS) and functional (EPR, TPR) methods. The effect of composition on the catalytic performance of the investigated systems in soot combustion was discussed in detail. Zirconia-supported vanadia was found to be the most active catalyst for soot oxidation characterized by the lowest combustion temperature (~375 °C) attributed to the maximal signal of conversion to the detected products. The relationship between the reducibility of surface oxovanadium species and their catalytic activity was established, revealing the involvement of the lattice oxygen in the combustion process. The importance of thermal treatment conditions and the nature of zirconia-based support determining the stability of specific oxovanadium entities on the catalyst surface was emphasized.
1. Introduction
Engines powered by burning fossil fuels are still the most popular in the world [1,2]. The fuel combustion processes in diesel engines are often incomplete, and therefore undesired, and noxious by-products can be formed. One of the most fundamental problems accompanying the exploitation of diesel engines is the associated emission of particulate matter (PM), which causes strong air pollution in urban areas, along roads with heavy traffic and in some industrial zones. On the other hand, emissions of PMs undergo stepwise more and more strict limitations [3,4]. It is a matter of fact that the amount of NOx and soot emitted by diesel engines is much larger than that emitted by Otto engines equipped with catalytic converters [5]. Thus, the practical interest in cleaning diesel exhaust gases significantly increased in the last three decades. Soot emissions from diesel engines can be reduced in several different ways. The most effective option is soot collection on DPF (diesel particulate filter) joined with its simultaneous catalytic oxidation if efficient and cheap catalysts are available [4,6]. Unfortunately, soot oxidation takes place at temperatures of 550–600 °C, while the temperature of diesel exhaust gases is usually about 150–400 °C [7,8]. Such a temperature misfit is the main reason for applying a catalyst to prevent the accumulation of soot on the monolithic filter. The main drawbacks affecting catalytic performance are not only related to the occurrence of the temperature discrepancy but also its variability, depending on engine loading, as well as to the relatively poor soot–catalyst contact [3]. Another vital problem concerns the way of catalyst introduction. It can be applied in the form of a precursor added to the fuel [9]. Another possibility is spraying metal salt solution (as a precursor) on the accumulated soot or deposition of the catalyst directly on the filter walls by impregnation [3,10].
Many different systems were investigated in the field of soot combustion. Reported catalysts can be divided, i.e., following Hernández–Giménez et al. [11] into platinum-based [12,13], non-platinum noble metal-based [14,15,16], perovskites [17,18,19,20], spinels [21,22], ceria-based [19,23,24], molten salts [8,25,26,27], and alkaline metal-based catalysts [28,29,30]. Other classifications and reviews can be found in [1,4,31,32,33,34]. Taking into account all those reports, it can be stated that the supported metal oxides can be considered as very promising candidates.
Easily and reversibly reducible vanadia is a component of many oxidation catalysts [35]. It was thus a natural candidate to be tested in soot oxidation processes, where the presence of stable redox sites remains of vital importance. Additionally, a relatively low melting point of vanadia (ca. 690 °C) can ensure strong contact between soot and the active phase [8]. Therefore, it was not surprising that vanadia-based catalysts showed high activity in soot combustion [8,36,37,38,39,40,41,42,43]. Commercial V-based catalysts for SCR reaction also showed great activity in soot oxidation [44,45]. Another application of vanadium-containing catalysts in the field of soot combustion is plasma-assisted oxidation [46,47].
Catalytic support can substantially influence the activity of vanadia’s active phase in soot combustion as it was shown in [8,37]. It was found that catalytic activity increased in the series from Al2O3 through SiO2 and ZrO2 to TiO2 [8,37]. In addition, ceria as a support for vanadia was investigated [36]. It is worth stressing, however, that even if some authors focused their attention on the effect of support on soot combustion, the elucidation of the effect was far from its maturity. Understanding the intimate mechanism remaining behind the observed catalytic activity is particularly important in the case of vanadia as an active component of deSoot catalysts strongly interacting with such supports as ZrO2, CeO2, Y2O3, and their binary combinations. There is an evident gap in the literature regarding the latter issue, which can be at least partially filled by our studies. The appropriate selection of support can be considered as one of the crucial factors for designing soot combustion catalysts based on vanadia.
Zirconium dioxide is widely used as a catalyst and a catalyst support [48,49]. It has several structural and functional features that favor its application in catalysis. Among the physical properties that make ZrO2 very useful as a support under harsh conditions are: its high melting point (ca. 2370 °C), low thermal conductivity, and mechanical resistance. Chemically, zirconia is a multifunctional support, exhibiting the presence of both acid-base and redox sites. The latter property can be extremely strengthened by an addition of ceria or yttria, which are able to stabilize the high-temperature, high-surface-area zirconia polymorphs, together with making easy oxygen release and uptake, dependently on the conditions accompanying soot oxidation [50,51].
In this work, the results of catalytic tests of soot combustion in both the absence, and the presence of NO are presented. V2O5 catalysts of various carriers (zirconia, zirconia-ceria, and zirconia-yttria) were characterized by structural (X-ray diffraction (XRD)) spectroscopic (Raman spectroscopy (RS), electron paramagnetic resonance (EPR)), and temperature-programmed reduction (TPR) methods. The effects of catalyst composition on the temperature of soot combustion were discussed. Particular attention was devoted to the structural determination of the reducibility of oxovanadium active phase, deposited on various carriers, which remain strongly related to the observed catalytic activity. Our goal was to elucidate the effect of zirconia-based support composition on the activity of vanadia in soot combustion. The described nature of the complex interaction between deposited vanadia and the surfaces of the chosen supports as well as its pronounced influence on soot combustion can be considered as a valuable aspect of novelty.
2. Results and Discussion
2.1. Catalytic Activity
The catalytic performance (parameterized as the temperature of the maximal signal measured for the combustion products, Tmax) of the studied systems in the combustion of soot, in both the absence and the presence of NO, is shown in Figure 1. It can be clearly seen that the 3V/Zr catalyst was the most active system, regardless of NO presence in the reaction mixture. Tmax determined for this catalyst was equal to 392 °C and 375 °C, without or with NO, respectively. In the absence of NO, the activities of 3V/ZrCe and 3V/ZrY systems were distinctly lower, and the corresponding temperatures were shifted up to 493 °C and 504 °C, respectively. In turn, in the presence of NO, respective characteristic temperatures were lowered for 3V/ZrCe and 3V/ZrY catalysts by ca. 40 °C and 30 °C, in comparison to those determined without NO. Simultaneously, the content of nitric oxide in the reaction mixture significantly decreased during soot oxidation, as can be inferred from the corresponding curves, illustrating NO consumption, presented in Figure 2. Comparison of the data from Figure 1 and Figure 2 led to the conclusion that for the series of studied catalysts, there is a correlation between the determined temperatures of soot combustion and the respective temperatures of NO decay in the reaction mixture.
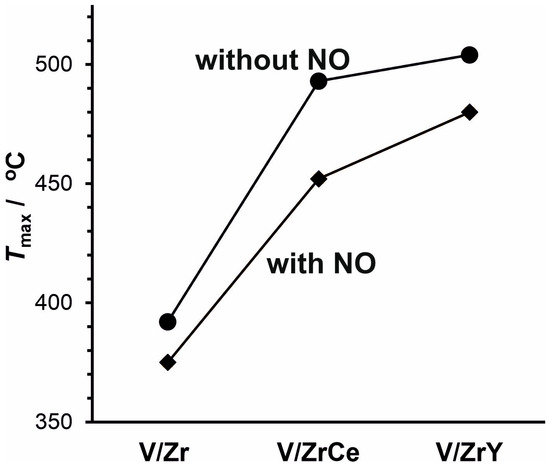
Figure 1.
Catalytic activity (expressed as the temperature of the maximal signal of combustion products) of the systems containing 3 wt.% of V2O5 in soot combustion, in the absence of NO (diamonds) and the presence of 1000 ppm of NO (circles).
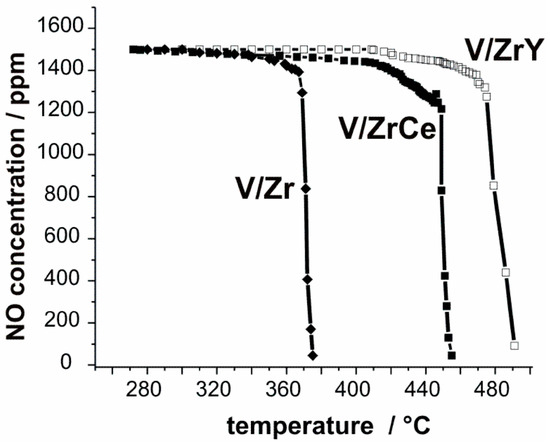
Figure 2.
The progress of NO consumption during soot combustion reaction over catalysts containing 3 wt.% of V2O5.
It is worth noting that for the investigated series of catalysts, the effect of NO on soot oxidation was well-pronounced and suggested its positive influence on soot removal. According to the mechanism of soot oxidation proposed by Weber et al. [52], in the first step of nitric oxide participation, NO is oxidized to NO2, which, in turn, can play the role of an efficient oxidizer for CO, formed in the initial step of soot conversion. Moreover, NO2 can oxidize soot directly to CO2 more efficiently than O2 [53]. The role of NO in soot combustion can be rationalized by the summarized Equation (1):
as well as by the equation of a cooperative reaction (2):
C + 2NO2 → CO2 + 2NO,
C + ½O2 + NO2 → CO2 + NO,
In the observed temperature range (above 300 °C), the contribution of the cooperative reaction becomes significant [44].
2.2. Catalysts Structure and Vanadium Speciation
Zirconia and related materials belong to the supports strongly influencing both the structure and chemical properties of the deposited vanadia phase; therefore, they can strongly affect catalytic activity in redox reactions. To elucidate the catalytic results, structural characterization of both supports and the deposited oxovanadium entities was thus indispensable. As it can be inferred from the careful inspection of the diagnostic XRD region (2Θ = 25–35°) in the patterns collected for pure supports, two zirconia polymorphs can be identified (Figure 3, insert). The mixture of ca. 68.0% of monoclinic (M) and ca. 32.0% of tetragonal (T) phases occurred in the case of bare ZrO2, giving rise to three distinct maxima at 2Θ = 28.2, 31.4, and 30.3°, which can be ascribed to M, M(111), and T(111) reflections, respectively. Only traces of m-ZrO2 with M and M(111) reflections accompanied the predominant t-ZrO2 phase, with the strong T(111) Bragg maximum in the XRD pattern of ZrCe. In turn, ZrY was found to occur in the monophase tetragonal form, giving rise to a single, strong T(111) reflection at 30.2°. The phase composition of the doped ZrO2 supports confirmed that low contents of ceria and yttria additives can efficiently stabilize the high-temperature, high-surface-area metastable t-ZrO2 [54,55].
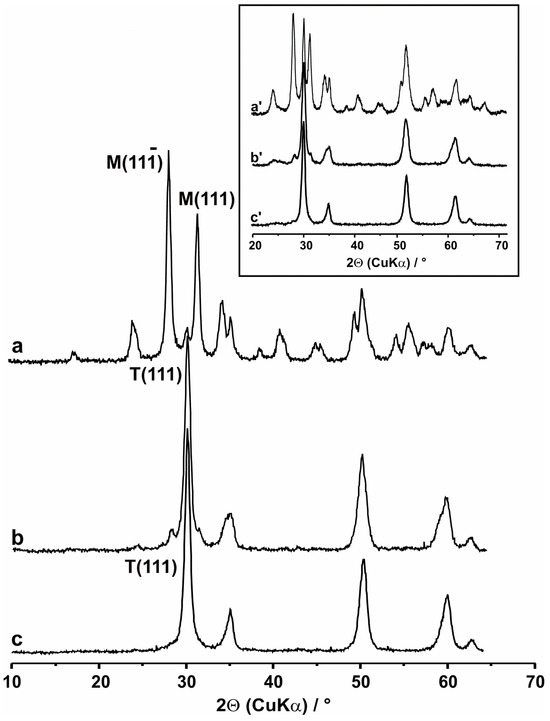
Figure 3.
XRD patterns of (a) V/Zr, (b) V/ZrCe, and (c) V/ZrY samples, containing 3 wt.% of V2O5. In the insert, the corresponding XRD patterns for bare supports are shown: (a’) Zr, (b’) ZrCe, and (c’) ZrY.
Vanadia belongs to the non-stoichiometric oxides; however, the applied preparation method distinctly modified intrinsic V2O5 properties. Its dissolution in oxalic acid favored the reduced vanadium state, according to Equation (3):
V2O5 + 3H2C2O4 = 2VO(C2O4) + 3H2O + 2CO2,
However, further catalyst calcination in the air led to the subsequent vanadium reoxidation to its highest oxidation level. This was clearly observed in the case of 5V/ZrCe and 5V/ZrY systems, which were practically EPR silent. Contrary to this, in the EPR spectra of 5V/Zr samples, the signals from V4+ (3d1) ions in the surrounding oxygen were identified (Figure 4, insert). Most probably the specific interactions between zirconia and vanadia were responsible for stabilizing the partially reduced oxovanadium species, being a source of V4+ ions, which can easily be incorporated within the zirconia matrix, according to Equation (4):
xV2O5 + ZrO2 = 2VxZr1−xO2 + xO2,
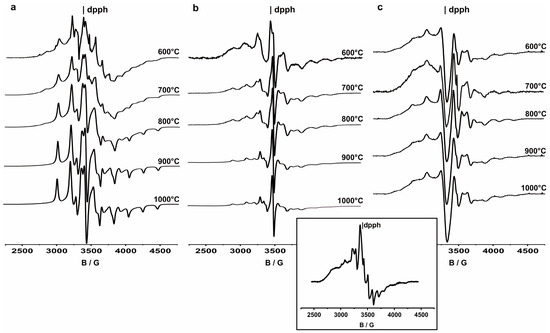
Figure 4.
The sequences of the EPR spectra recorded at LNT as a function of annealing temperatures of (a) V/Zr, (b) V/ZrCe, (c) V/ZrY samples, containing 5 wt.% of V2O5. In the insert, the spectrum of the V/Zr catalyst before annealing is presented.
Only in the case of the V/Zr catalyst did the introduction of 3 or 5 wt.% vanadia over the supported surface result in changes in the phase composition of the studied system (Figure 3a). Stabilized zirconia supports (ZrCe and ZrY) remained unaffected by the presence of such low V2O5 contents (Figure 3b,c). Contrary to this, the contribution of t-ZrO2 in the V/Zr sample dropped to ca. 7.5% (from 32.0% in the case of bare ZrO2). The influence of vanadium on the content of the t-ZrO2 polymorph was also observed previously [56], and can be connected to the shifts in the temperature of the T → M phase transition, which was, in turn, altered by the presence of reduced vanadium ions, migrating within the zirconia matrix. The reducibility of the deposited oxovanadium species remains thus a key parameter controlling vanadium distribution between surface and bulk, which may be essential for catalytic activity. This finding remains of vital importance for practical applications of supported vanadia-based catalysts. Using zirconia as a support and considering the relatively strong interaction between V2O5 and ZrO2 partial reduction of vanadia accompanied by thermally-induced migration of V4+ ions into the bulk must be taken into account. Partial loss of active components (and thus active sites) from the surface can have a negative impact on the catalytic behavior of the studied catalysts. However, on the other hand, the enhanced surface reducibility of vanadium can be beneficial due to the formation of V4+/V5+ mixed valence states. The resultant effect can be dependent on specific kinetic parameters characterizing both processes.
Computer simulation of the EPR spectrum of 5V/Zr, presented in the insert to Figure 4, revealed enhanced vanadium speciation in the zirconia-supported systems. In addition to the oxovandium species isolated on the support surface, giving rise to an axial signal (g|| = 1.924, g⊥ = 1.972) with an 8-line hyperfine structure (|A||| = 185 G, |A⊥| = 64 G), originating from the interaction of an unpaired electron with 51V nucleus (I = 7/2, natural abundance 99.76%), the polymeric VxOy entities were found, which were responsible for the broad structureless axial signal (g|| = 1.941, g⊥ = 1.971). An additional component was an orthorhombic signal (g1 = 1.897, g2 = 1.942, g3 = 1.973) with a clearly resolved hyperfine structure (|A1| = 159 G, |A2| = 67 G, |A3| = 15 G), which was attributed to the V4+ ions isolated inside the zirconia matrix. The approximate relative ratio of isolated surface V4+ sites to the polymeric ones and the V4+ ions stabilized within the bulk of zirconia was found to be 37%:41%:22%, reflecting both vanadium(IV) speciation in the V/Zr samples and its distribution between surface and bulk.
The sequence of EPR spectra recorded in variable-temperature experiments for all three investigated systems (Figure 4a–c), clearly proved that, despite the distinctly easier reducibility exhibited by the V/Zr samples, also in the two other cases, a temperature increase above 600 °C can cause the incorporation of V4+ ions into the bulk. In the case of 5V/Zr the progress of V4+ incorporation was the most pronounced, as it was accompanied by T → M phase transition of zirconia matrix. This effect can be clearly observed if we compare the shape of the EPR spectra recorded after 5V/Zr sample annealing at 700 and 900 °C, typical of isolated V4+ stabilized within tetragonal and monoclinic ZrO2 matrices. Parallel XRD data confirmed the total transformation of the t-ZrO2 into m-ZrO2. Spectral evolution was weaker in the case of samples of stabilized zirconia matrices (ZrCe and ZrY); however, even in these two cases a decay in the hyperfine structure from surface V4+ ions and the appearance of that originating from bulk vanadium(IV) sites can be observed with temperature increases. The local surrounding of V4+ within the three matrices was different, as can be inferred from the differences in the shape of the EPR spectra recorded after heating the samples to 1000 °C. The observed differences reflect structural modifications involved in the ZrO2 matrix by doping with ceria and yttria. The detailed interpretation of the presented spectra can be found in [56].
The limited reducibility of V/ZrCe and V/ZrY systems can be explained by the formation of the new compounds at the interface vanadia/support via thermally-induced solid-state reaction. Up to now, the product of such a reaction was identified by Raman spectroscopy in the case of the 5V/CeZr system. Below calcination temperature (i.e., 600 °C), vanadia is able to react selectively with ceria, forming CeVO4, according to Equation (5):
V2O5 + 2CeO2 = 2CeVO4 + ½O2,
The formation of such a compound in comparable temperature ranges was observed in the case of supported vanadia systems also by the other authors [36,57,58,59]. The product of the solid-state reaction gave rise to the vibration bands in the corresponding Raman spectrum (Figure 5) but its concentration was too small to be detected by XRD. The CeVO4 structure consists of VO4 tetragonal bisphoenohedra, which are edged-linked to CeO8 triangular dodecahedra. The CeO8 chains are interrupted by VO4 units along one axis, while in the plane the CeO8 are edge-sharing [60]. The bands below 750 cm−1 originated from the scattering effects on the ZrCe matrix. The most intense band centered around 472 cm−1, can be attributed to the symmetrical F2g mode of the fluorite-like structure [61] and was additional evidence of the phase composition proposed above based on the diffraction results. The bands above 750 cm−1 (the corresponding fragment was enlarged in the insert) were ascribed to the cerium orthovanadate. The most pronounced band above 850 cm−1, together with the two weaker ones around 800 cm−1, can be attributed to A1g symmetric and the Eg and B2g antisymmetric stretchings of vanadates, respectively [62]. The lack of any bands from the surface oxovanadium species in the Raman spectrum of 5V/ZrCe suggested that the majority of VOx species undergo preferential reaction with CeO2 from the support to form the mixed oxide phase. Due to the much more difficult reducibility of CeVO4 in comparison to that of V2O5 [63], vanadium(V), stabilized in cerium orthovanadate, did not tend to lower its oxidation state. Higher redox stability of vanadium in CeVO4 resulted in lower catalytic activity in soot combustion, which remains in agreement with [36]. The effects described above should be taken into account while designing the vanadium-containing catalysts and also in the practical implementation of the preparative protocols, where the nature of the support and thermal treatment conditions should be well adapted to the specific behavior of the investigated systems described above.
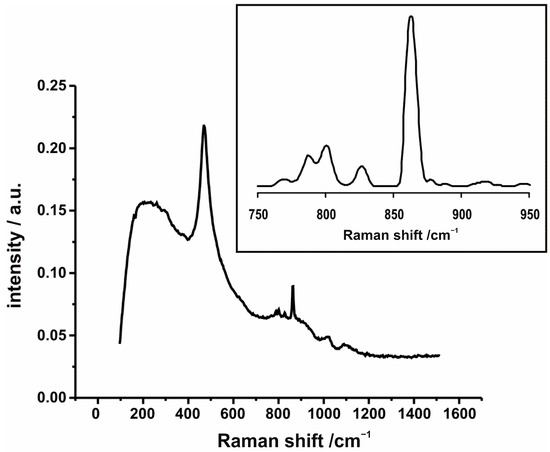
Figure 5.
Raman spectrum of 5V/ZrCe. In the insert, the enlarged region, diagnostic for CeVO4 vibrations, is shown.
TPR profiles for catalysts containing 5 wt.% of V2O5 suggested that in the hydrogen flow, the catalysts underwent one-step reduction below 800 °C (Figure 6). It can be thus assumed that only the reduction of V2O5 → VO2 occurred, and no lower oxidation states of vanadium can be expected. Parallel measurements performed on bare supports confirmed that there was no reduction observed in the studied zirconia-based materials. The highest hydrogen consumption took place at temperatures around 525 °C for all vanadia-containing systems. Only small deviations in reduction temperatures in the studied samples can be distinguished. There are, however, distinct differences in the threshold reduction temperatures (marked with arrows), which indicated that 5V/ZrCe catalysts are more resistant to reduction. Hydrogen consumption started in the case of the 5V/ZrCe catalyst at ca. 400 °C, whereas for two other systems (5V/Zr and 5V/ZrY), the reduction threshold temperatures were 100 °C lower.
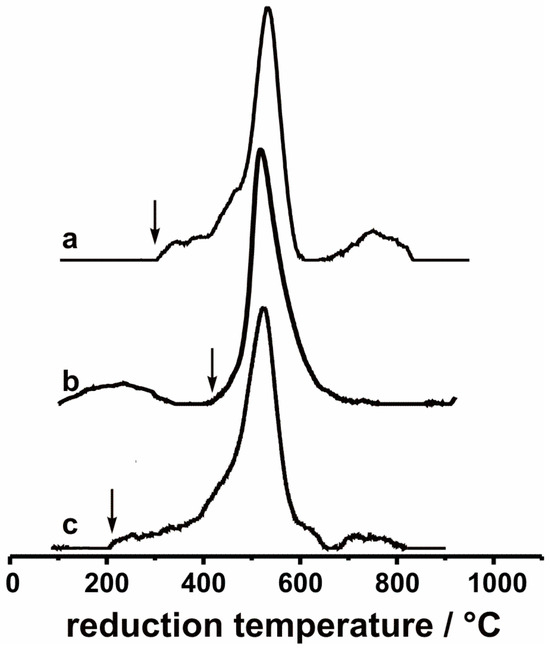
Figure 6.
TPR profiles of (a) V/Zr, (b) V/ZrCe, (c) V/ZrY samples, containing 5 wt.% of V2O5. The thresholds of the reduction temperatures were marked with arrows.
3. Materials and Methods
3.1. Materials
Three types of supports for vanadia were used in the current studies: bare zirconia, zirconia-ceria, and zirconia-yttria, labeled hereafter Zr, ZrCe, and ZrY, respectively. Zirconia supports containing 3 mol.% of Y2O3 (63.9 m2/g) and 10 mol.% of CeO2 (63.8 m2/g), supplied by CEREL (Boguchwała, Poland), were obtained from the corresponding nitrates by coprecipitation with NH3aq at pH = 8. Bare zirconia (96.0 m2/g) was obtained from an aqueous 0.1 M solution of ZrOCl2 by precipitation with a 0.4 M solution of ammonia (NH3aq). The parent mixture was heated to 100 °C for 12 h then dried at 110 °C for 12 h and finally calcined in air at 550 °C for 3 h.
Catalysts containing nominally 3 or 5 wt.% of V2O5 (labeled hereafter 3V/support or 5V/support) were prepared by incipient wetness impregnation. The carriers were contacted with the corresponding amount of an aqueous solution of V2O5 and oxalic acid, mixed in the weight ratio 1:2. Then the samples were dried at 100 °C for 12 h and calcined in air at 600 °C for 3 h.
Commercial carbon black (CB) Printex U was used for the catalytic tests. Its parameters like a specific surface area of 100 m2/g, average pore diameter of ca. 50 nm, and pH of suspension in water equal to 4, made this soot very similar to that produced in diesel engines.
3.2. Characterization Techniques
The N2-BET specific surface areas of the prepared samples were determined based on the flow type BET measurements using the Quantachrome Adsorb-1 analyzer (Boynton Beach, FL, USA).
The crystal structures were identified by X-ray diffraction patterns collected with a 0.5°/min step in the 2Θ range of 20–70° using a DRON-3 X-ray powder diffractometer, operating at Ni-filtered CuKα radiation (λ = 1.5408 Å). The phase composition of the samples was calculated from the following relationships (6):
where Xt and Xm stand for the fraction of the tetragonal and the monoclinic ZrO2 forms, respectively, whereas It and Im are the intensities of their diagnostic peaks [64].
Temperature-programmed reduction (TPR) measurements were performed in a flow system equipped with a fixed-bed reactor and a thermal conductivity detector (TCD) detector with a gas mixture containing 5% of hydrogen in argon. The heating rate was 5 °C/min.
Raman spectra were collected on an FTS 6000 spectrometer (Bio-Rad, Cambridge, MA, USA) equipped with a BIORAD accessory. The samples were excited with a 1064 nm line of a diode-pumped Nd:YAG laser (Spectra Physics Model T108S) and the scattered radiation was collected at 180°, using 4 cm−1 resolution.
The continuous wave electron paramagnetic resonance (CW-EPR) X-band spectra were recorded at room (RT) and liquid nitrogen (LNT, i.e., −196 °C) temperatures with a Bruker ELEXSYS E-500 spectrometer (Billerica, MA, USA) operating at the 100 kHz field modulation. EPR parameters were determined by simulation using the EPRsim32 program [65]. In the variable temperature EPR experiments (VT-EPR), the samples were progressively heated in air in a micro-furnace from RT up to 1000 °C with a rate of 4 °C/min. At every 100 °C the temperature was kept constant for 0.5 h, and then the samples were quenched to the LNT for EPR spectra recording.
3.3. Catalytic Tests
The activity of the prepared catalysts was determined in the reactions of soot combustion and nitrogen oxide reduction with soot. Both tests were performed separately. The catalyst bed was prepared by mixing carefully for ca. 1 min with a spatula the catalyst powder with soot (loose contact) in a ratio of 5:1. Total mixture weight was 0.6 g and the volume of catalyst–soot mixture bed was V = 1.0 cm3.
Soot combustion in the absence or in the presence of NO was tested in a tubular quartz reactor (ϕ = 10 mm). The test gas composition was similar to that of the real diesel engine exhaust (ca. 1400 ppm NO, 10% vol. O2, 1000 ppm water vapor, nitrogen balanced). At the outlet of the reactor, the products were analyzed by Multor 610 gas analyzer (Hamburg, Germany).
4. Conclusions
Catalytic studies of three vanadia-containing systems of various zirconia-based supports, confronted to the spectroscopic and TPR measurements, revealed a strong relationship between catalyst structure and reactivity. The most promising catalyst was V/Zr system, able to oxidize soot even at ~375 °C (Tmax) in the presence of ca. 1400 ppm of NO in loose contact. A beneficial effect of NO in soot combustion was clearly observed in all investigated cases. The specific interactions between the deposited vanadia and the used supports as well as the thermal stability of the zirconia-based matrices were directly responsible for the catalytic performance of the studied systems. The reducibility of surface vanadia, which was the highest in the case of V/Zr samples, was found to be a key parameter, determining the catalytic activity of the investigated systems in soot oxidation. The relatively easy electron exchange within the V4+/V5+ redox couple makes the V/Zr catalyst the most promising system, active in the low-temperature oxidation of soot. However, its thermal stability was the lowest mainly due to the phase transition of the zirconia matrix, occurring between 700 °C and 800 °C, which can additionally be catalyzed by V4+ ions migrating into the bulk. The formation of the CeVO4 of limited reducibility at the interface VOx-ZrCe stabilized V5+, resulting in the lower catalytic activity of this system, in comparison to that exhibited by V/Zr. The obtained results remain of vital importance for the practical design of deSoot catalysts containing vanadia as an active component and can be decisive for an appropriate selection of the support stabilizing vanadium-containing mixed valence entities on the catalyst surface. Mechanistic details, together with more in-depth studies on the evolution of surface architecture (including reducibility), as well as consequent stability tests, will be the subject of our further studies.
Author Contributions
Conceptualization and supervision, J.T.; methodology, preparation of the investigated samples, partial characterization, and catalytic experiments, T.R.; partial characterization, writing—original draft preparation, and discussion of the final version of the manuscript, A.A.; manuscript actualization, methodological improvement, and discussion of the obtained results, P.L.; discussion of the final version of the manuscript A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No data to share.
Acknowledgments
The authors are grateful to Aleksandra Wesełucha-Birczyńska from the Regional Laboratory of Physicochemical Analyses and Structural Research for recording the Raman spectra and to Piotr Kuśtrowski from the Faculty of Chemistry of the Jagiellonian Chemistry for TPR experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neha; Prasad, R.; Singh, S.V. A Review on Catalytic Oxidation of Soot Emitted from Diesel Fuelled Engines. J. Environ. Chem. Eng. 2020, 8, 103945. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Z.; Ye, Y.; Huang, H.; Cao, C. Review of Particle Filters for Internal Combustion Engines. Processes 2022, 10, 993. [Google Scholar] [CrossRef]
- Van Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and Technology of Catalytic Diesel Particulate Filters. Catal. Rev.-Sci. Eng. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A Review on the Catalytic Combustion of Soot in Diesel Particulate Filters for Automotive Applications: From Powder Catalysts to Structured Reactors. Appl. Catal. A Gen. 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Makkee, M.; Moulijn, J.A. Diesel Particulate Emission Control. Fuel Process. Technol. 1996, 47, 1–69. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Di Benedetto, A.; Lisi, L. Synergy Between Ceria and Metals (Ag or Cu) in Catalytic Diesel Particulate Filters: Effect of the Metal Content and of the Preparation Method on the Regeneration Performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Krishna, K.; Makkee, M. Soot Oxidation over NOx Storage Catalysts: Activity and Deactivation. Catal. Today 2006, 114, 48–56. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Duan, A.; Zhu, L.; Wang, X. Diesel Soot Oxidation over Supported Vanadium Oxide and K-Promoted Vanadium Oxide Catalysts. Appl. Catal. B Environ. 2005, 61, 36–46. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Legutko, P.; Kopacz, A.; Jakubek, T.; Indyka, P.; Pietrzyk, P.; Wojtasik, M.; Markowski, J.; Krasodomski, W.; Ziemiański, L.; et al. Role of Chain Length of the Capping Agents of Iron Oxide Based Fuel Borne Catalysts in the Enhancement of Soot Combustion Activity. Appl. Catal. B Environ. 2016, 199, 485–493. [Google Scholar] [CrossRef]
- Fino, D. Diesel Emission Control: Catalytic Filters for Particulate Removal. Sci. Technol. Adv. Mater. 2007, 8, 93–100. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Castelló, D.L.; Bueno-López, A. Diesel Soot Combustion Catalysts: Review of Active Phases. Chem. Pap. 2014, 68, 1154–1168. [Google Scholar] [CrossRef]
- Oi-Uchisawa, J.; Wang, S.; Nanba, T.; Ohi, A.; Obuchi, A. Improvement of Pt Catalyst for Soot Oxidation Using Mixed Oxide as a Support. Appl. Catal. B Environ. 2003, 44, 207–215. [Google Scholar] [CrossRef]
- Tang, M.; Liu, S.; Fu, W.; Wang, J.; Yin, K.; Zhu, M.; Tian, J.; Sun, Y.; Dai, Y. Surface Oxygen Vacancies Promoted Pt Nanoparticles on Celery-like CeO2 Nanofibers for Enhanced Sinter-Resistance and Catalytic Performance. Mater. Today Nano 2022, 20, 100249. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Xiao, J.; Yu, Y.; Xu, L.; Li, X.; Cao, C.; Liu, G. Silver-Modified NiCo2O4 Nanosheets Monolithic Catalysts Used for Catalytic Soot Elimination. Fuel 2022, 326, 125036. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Wei, C.; Wan, X.; Li, W.; Lin, Q. Au Nanoparticles Supported on Iron-Based Oxides for Soot Oxidation: Physicochemical Properties Before and After the Reaction. ACS Omega 2021, 6, 11510–11518. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; Zhang, C.; Song, Q.; Xue, Z.; Zhang, X.; Tong, L. Mechanism of Pd and K Co-Doping to Enhance the Simultaneous Removal of NOxand Soot over LaMnO3. Catal. Sci. Technol. 2020, 10, 6013–6024. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.-S.; Illán-Gómez, M.-J. Analyzing the Role of Copper in the Soot Oxidation Performance of BaMnO3-Perovskite-Based Catalyst Obtained by Modified Sol-Gel Synthesis. Fuel 2022, 328, 125258. [Google Scholar] [CrossRef]
- Neha; Singh, S.V. Facile and Template-Free Synthesis of Nano-Macroporous LaCoO3 Perovskite Oxide for Efficient Diesel Soot Oxidation. React. Kinet. Mech. Catal. 2022, 135, 1607–1620. [Google Scholar] [CrossRef]
- Huo, Z.; Zhao, P.; Miu, P.; Ren, L.; Tan, B.; Feng, N.; Wan, H.; Guan, G. Enhanced Catalytic Oxidation of Soot over 3DOM LaMnO3 by Adding Ag and CeO2: Improving the Generation and Delivery of Active Oxygen Species. Appl. Surf. Sci. 2022, 600, 154204. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Adamski, A.; Ura, B.; Trawczynski, J. Copper Catalysts for Soot Oxidation: Alumina versus Perovskite Supports. Environ. Sci. Technol. 2008, 42, 7670–7675. [Google Scholar] [CrossRef]
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Soot Oxidation over K-Doped Manganese and Iron Spinels—How Potassium Precursor Nature and Doping Level Change the Catalyst Activity. Catal. Commun. 2014, 43, 34–37. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.M.; Portela, R.; Reinosa, J.J.; Rubio-Marcos, F.; Pascual, L.; Fernández, J.F. Performance and Stability of Wet-Milled CoAl2O4, Ni/CoAl2O4, and Pt,Ni/CoAl2O4 for Soot Combustion. Catalysts 2020, 10, 406. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Ao, C.; Zhang, L.; Lei, L. Interactive Effects of NOx Synergistic and Hydrothermal Aging on Soot Catalytic Combustion in Ce-Based Catalysts. Combust. Flame 2022, 245, 112289. [Google Scholar] [CrossRef]
- Sacco, N.A.; Miró, E.E.; Milt, V.G.; Banús, E.D.; Bortolozzi, J.P. Kinetic, Stability and Characterization Studies of Ce, Mn and Mn-Doped Ceria Paper Catalysts Towards Soot Combustion Under Different Reaction Conditions. Top. Catal. 2022, 65, 1262–1272. [Google Scholar] [CrossRef]
- Neri, G.; Rizzo, G.; Galvagno, S.; Donato, A.; Musolino, M.G.; Pietropaolo, R. K- and Cs-FeV Al2O3 Soot Combustion Catalysts for Diesel Exhaust Treatment. Appl. Catal. B Environ. 2003, 42, 381–391. [Google Scholar] [CrossRef]
- Li, Q.; Xin, Y.; Zhang, Z.; Cao, X. Electron Donation Mechanism of Superior Cs-Supported Oxides for Catalytic Soot Combustion. Chem. Eng. J. 2018, 337, 654–660. [Google Scholar] [CrossRef]
- Yongtao, W.; Lina, S.U.I.; Hongquan, K.; Liyan, Y.U. Research on K-V-Rare Earth Metal Catalysts for Diesel Soot Oxidation. J. Wuhan Univ. Technol. Sci. Ed. 2018, 33, 331–337. [Google Scholar]
- Peng, C.; Yu, D.; Zhang, C.; Chen, M.; Wang, L.; Yu, X.; Fan, X.; Zhao, Z.; Cheng, K.; Chen, Y.; et al. Alkali/Alkaline-Earth Metal-Modified MnOx Supported on Three-Dimensionally Ordered Macroporous–Mesoporous TixSi1−XO2 Catalysts: Preparation and Catalytic Performance for Soot Combustion. J. Environ. Sci. 2023, 125, 82–94. [Google Scholar] [CrossRef]
- Legutko, P.; Pęza, J.; Villar Rossi, A.; Marzec, M.; Jakubek, T.; Kozieł, M.; Adamski, A. Elucidation of Unexpectedly Weak Catalytic Effect of Doping with Cobalt of the Cryptomelane and Birnessite Systems Active in Soot Combustion. Top. Catal. 2019, 62, 599–610. [Google Scholar] [CrossRef]
- Legutko, P.; Gryboś, J.; Fedyna, M.; Janas, J.; Wach, A.; Szlachetko, J.; Adamski, A.; Yu, X.; Zhao, Z.; Kotarba, A.; et al. Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance. Catalysts 2020, 10, 1390. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. Ceria-Based Catalysts for Soot Oxidation: A Review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Mishra, A.; Prasad, R. Preparation and Application of Perovskite Catalysts for Diesel Soot Emissions Control: An Overview. Catal. Rev.-Sci. Eng. 2014, 56, 57–81. [Google Scholar] [CrossRef]
- Legutko, P.; Stelmachowski, P.; Yu, X.; Zhao, Z.; Sojka, Z.; Kotarba, A. Catalytic Soot Combustion—General Concepts and Alkali Promotion. ACS Catal. 2023, 13, 3395–3418. [Google Scholar] [CrossRef]
- Lisi, L.; Landi, G.; Di Sarli, V. The Issue of Soot-Catalyst Contact in Regeneration of Catalytic Diesel Particulate Filters: A Critical Review. Catalysts 2020, 10, 1307. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Keller, D.E. Chemistry, Spectroscopy and the Role of Supported Vanadium Oxides in Heterogeneous Catalysis. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Duan, A.; Jiang, G. CeO2-Supported Vanadium Oxide Catalysts for Soot Oxidation: The Roles of Molecular Structure and Nanometer Effect. J. Rare Earths 2010, 28, 198–204. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Duan, A.; Zhu, L.; Wang, X. The Structures of VOx/MOx and Alkali-VOx/MOx Catalysts and Their Catalytic Performances for Soot Combustion. Catal. Today 2006, 118, 315–322. [Google Scholar] [CrossRef]
- Ciambelli, P.; Parrella, P.; Vaccaro, S. Kinetics of Soot Oxidation on Potassium-Copper-Vanadium Catalyst. Stud. Surf. Sci. Catal. 1991, 71, 323–335. [Google Scholar]
- Ahlström, A.F.; Odenbrand, C.U.I. Combustion of Soot Deposits from Diesel Engines on Mixed Oxides of Vanadium Pentoxide and Cupric Oxide. Appl. Catal. 1990, 60, 157–172. [Google Scholar] [CrossRef]
- Trawczynski, J. Catalytic Combustion of Soot. React. Kinet. Catal. Lett. 1998, 63, 41–45. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Duan, A.; Jiang, G.; Gao, J.; Lin, W.; Wachs, I.E. In-Situ UV-Raman Study on Soot Combustion over TiO2 or ZrO2-Supported Vanadium Oxide Catalysts. Sci. China Ser. B Chem. 2008, 51, 551–561. [Google Scholar] [CrossRef]
- Neri, G.; Rizzo, G.; Galvagno, S.; Musolino, M.G.; Donato, A.; Pietropaolo, R. Thermal Analysis Characterization of Promoted Vanadium Oxide-Based Catalysts. Thermochim. Acta 2002, 381, 165–172. [Google Scholar] [CrossRef]
- Cousin, R.; Capelle, S.; Abi-Aad, E.; Courcot, D.; Aboukaïs, A. Copper-Vanadium-Cerium Oxide Catalysts for Carbon Black Oxidation. Appl. Catal. B Environ. 2007, 70, 247–253. [Google Scholar] [CrossRef]
- Schobing, J.; Tschamber, V.; Brilhac, J.-F.; Auclaire, A.; Hohl, Y. Simultaneous Soot Combustion and NOx Reduction over a Vanadia-Based Selective Catalytic Reduction Catalyst. Comptes Rendus Chim. 2018, 21, 221–231. [Google Scholar] [CrossRef]
- Zheng, L.; Casapu, M.; Grunwaldt, J.D. Understanding the Multiple Interactions in Vanadium-Based SCR Catalysts during Simultaneous NOx and Soot Abatement. Catal. Sci. Technol. 2022, 12, 3969–3981. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, X.; Wu, X.; Tu, X.; Chen, G.; Yang, G. Plasma-Catalytic Reactions for Soot Oxidation on VOx/M (M=KIT-6, SBA-15 and SiO2) Catalysts: Influence of Pore Structure. ChemistrySelect 2022, 7, e202103545. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, H.; Luo, J.; Liu, J.; Yan, J.; Zhou, Z.; Yang, Z.; Jiang, Y.; Chen, G.; Yang, G. Soot Oxidation in a Plasma-Catalytic Reactor: A Case Study of Zeolite-Supported Vanadium Catalysts. Catalysts 2022, 12, 677. [Google Scholar] [CrossRef]
- Yamaguchi, T. Application of ZrO2 as a Catalyst and a Catalyst Support. Catal. Today 1994, 20, 199–217. [Google Scholar] [CrossRef]
- Mercera, P.D.L.; van Ommen, J.G.; Doesburg, E.B.M.; Burggraaf, A.J.; Ross, J.R.H. Zirconia as a Support for Catalysts Influence of Additives on the Thermal Stability of the Porous Texture of Monoclinic Zirconia. Appl. Catal. 1991, 71, 363–391. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Promotional Effect of Rare Earths and Transition Metals in the Combustion of Diesel Soot over CeO2 and CeO2-ZrO2. Catal. Today 2006, 114, 40–47. [Google Scholar] [CrossRef]
- Azambre, B.; Collura, S.; Trichard, J.M.; Weber, J.V. Nature and Thermal Stability of Adsorbed Intermediates Formed during the Reaction of Diesel Soot with Nitrogen Dioxide. Appl. Surf. Sci. 2006, 253, 2296–2303. [Google Scholar] [CrossRef]
- Peralta, M.A.; Zanuttini, M.S.; Ulla, M.A.; Querini, C.A. Diesel Soot and NOx Abatement on K/La2O3 Catalyst: Influence of K Precursor on Soot Combustion. Appl. Catal. A Gen. 2011, 399, 161–171. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. The Role of Suprafacial Oxygen in Some Perovskites for the Catalytic Combustion of Soot. J. Catal. 2003, 217, 367–375. [Google Scholar] [CrossRef]
- Howard, C.J.; Hill, R.J.; Reichert, B.E. Structures of ZrO2 Polymorphs at Room Temperature by High-Resolution Neutron Powder Diffraction. Acta Crystallogr. Sect. B Struct. Sci. 1988, 44, 116–120. [Google Scholar] [CrossRef]
- Adamski, A.; Sojka, Z.; Dyrek, K.; Che, M. An XRD and ESR Study of V2O5/ZrO2 Catalysts: Influence of the Phase Transitions of ZrO2 on the Migration of V4+ Ions into Zirconia. Solid State Ionics 1999, 117, 113–122. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Lakshmanan, P.; Aouine, M.; Loridant, S.; Volta, J.C. Structural Characterization of Nanosized CeO2-SiO2, CeO2-TiO2, and CeO2-ZrO2 Catalysts by XRD, Raman, and HREM Techniques. J. Phys. Chem. B 2005, 109, 3355–3363. [Google Scholar] [CrossRef]
- Daniell, W.; Ponchel, A.; Kuba, S.; Anderle, F.; Weingand, T.; Gregory, D.H.; Knözinger, H. Characterization and Catalytic Behavior of VOx-CeO2 Catalysts for the Oxidative Dehydrogenation of Propane. Top. Catal. 2002, 20, 65–74. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Martínez-Huerta, M.V.; Rojas-García, E.; Jehng, J.-M.; Bañares, M.A. On the Nature of the Unusual Redox Cycle at the Vanadia Ceria Interface. J. Phys. Chem. C 2018, 122, 1197–1205. [Google Scholar] [CrossRef]
- Opara Krasovec, U. Structural and Spectroelectrochemical Investigations of Tetragonal CeVO4 and Ce/V-Oxide Sol-Gel Derived Ion-Storage Films. Solid State Ionics 1999, 118, 195–214. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Dulgheru, P.; Atribak, I.; Bueno-López, A.; García-García, A. Attempts at an in Situ Raman Study of Ceria/Zirconia Catalysts in PM Combustion. Appl. Catal. B Environ. 2011, 108–109, 134–139. [Google Scholar] [CrossRef]
- Martinez-Huerta, M.; Coronado, J.; Fernández-García, M.; Iglesias-Juez, A.; Deo, G.; Fierro, J.L.G.; Bañares, M.A. Nature of the Vanadia-Ceria Interface in V5+/CeO2 Catalysts and Its Relevance for the Solid-State Reaction toward CeVO4 and Catalytic Properties. J. Catal. 2004, 225, 240–248. [Google Scholar] [CrossRef]
- Abi-Aad, E.; Matta, J.; Courcot, D.; Aboukaïs, A. EPR and TPR Investigation of the Redox Properties of Vanadia Based Ceria Catalysts. J. Mater. Sci. 2006, 41, 1827–1833. [Google Scholar] [CrossRef]
- Mercera, P.D.L.; Van Ommen, J.G.; Doesburg, E.B.M.; Burggraaf, A.J.; Ross, J.R.H. Zirconia as a Support for Catalysts. Appl. Catal. 1990, 57, 127–148. [Google Scholar] [CrossRef]
- Spałek, T.; Pietrzyk, P.; Sojka, Z. Application of the Genetic Algorithm Joint with the Powell Method to Nonlinear Least-Squares Fitting of Powder EPR Spectra. J. Chem. Inf. Model. 2005, 45, 18–29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).