Pd+Al2O3-Supported Ni-Co Bimetallic Catalyst for H2 Production through Dry Reforming of Methane: Effect of Carbon Deposition over Active Sites
Abstract
:1. Introduction
2. Results
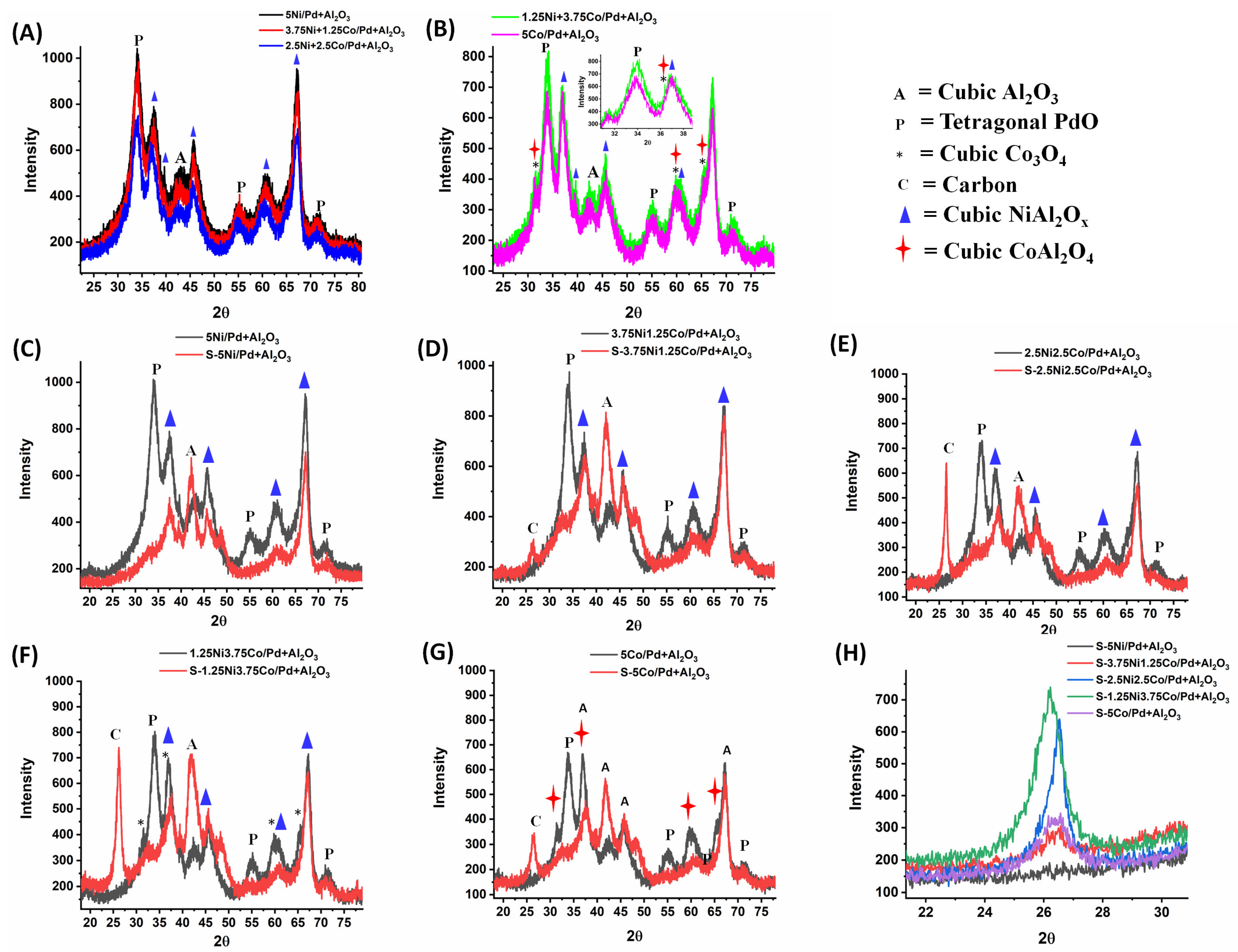
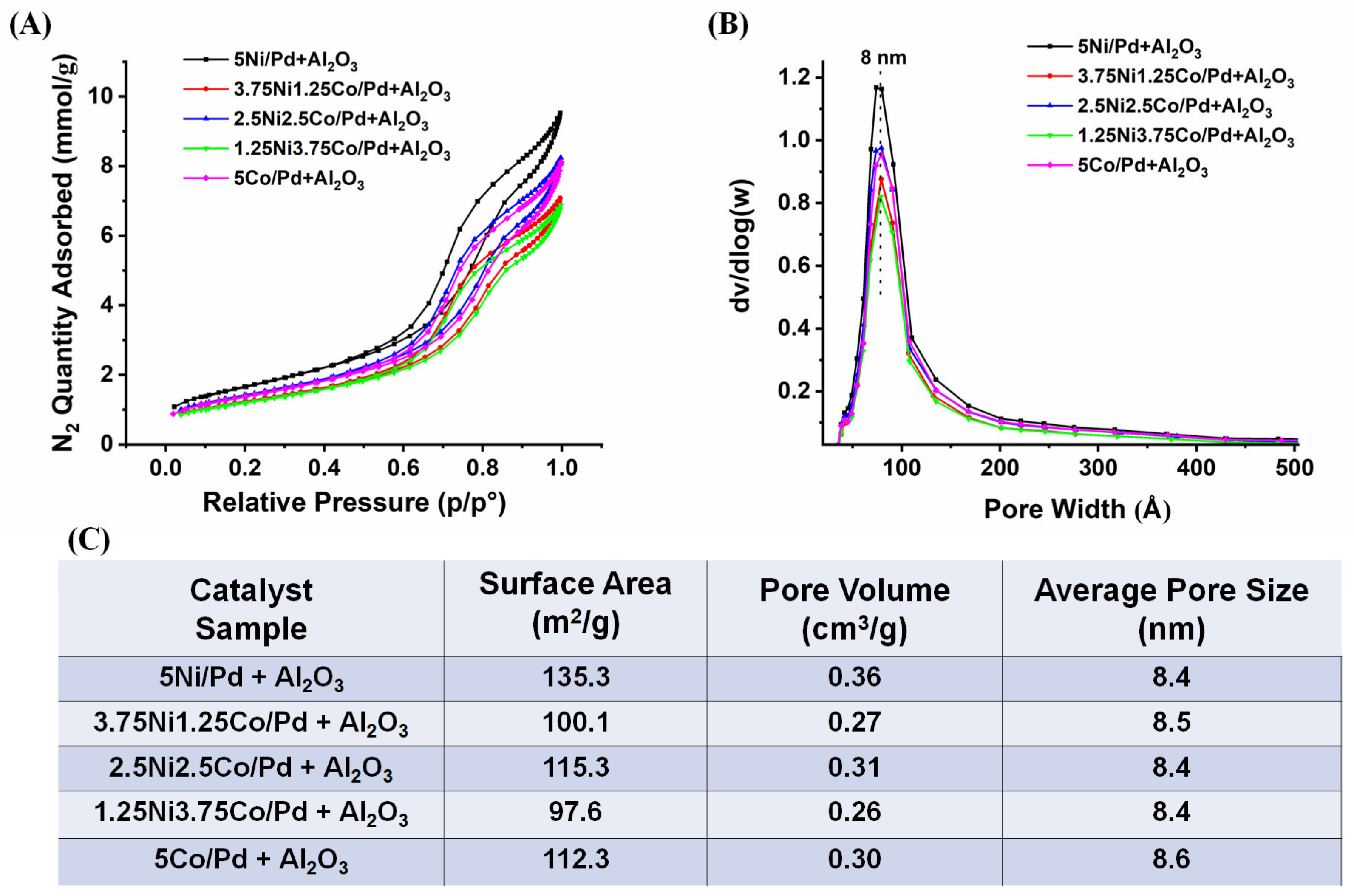
2.1. Characterization Results and Discussion
2.2. Catalytic Activity Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Performance Evaluation
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quiroga, M.M.B.; Luna, A.E.C. Kinetic Analysis of Rate Data for Dry Reforming of Methane. Ind. Eng. Chem. Res. 2007, 46, 5265–5270. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Fakeeha, A.H.; Alotibi, M.F.; Alreshaidan, S.B.; Kumar, R. Reforming of Methane: Effects of Active Metals, Supports, and Promoters. Catal. Rev. Sci. Eng. 2023, 1–99. [Google Scholar] [CrossRef]
- Liao, M.-S.; Au, C.-T.; Ng, C.-F. Methane Dissociation on Ni, Pd, Pt and Cu Metal (111) a Theoretical Comparative Study. Chem. Phy. Lett. 1997, 272, 445–452. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Z.; Lin, S.; Chen, Y.; Zhang, L.; Chen, S.; Zhang, X.; Lin, J.; Zhang, Z.; Wan, S.; et al. Conversion of Syngas to Methanol and DME on Highly Selective Pd/ZnAl2O4 Catalyst. J. Energy Chem. 2021, 58, 564–572. [Google Scholar] [CrossRef]
- Gallego, G.S.; Batiot-Dupeyrat, C.; Barrault, J.; Florez, E.; Mondragón, F. Dry Reforming of Methane over LaNi1-YByO3±δ (B = Mg, Co) Perovskites Used as Catalyst Precursor. Appl. Catal. A Gen. 2008, 334, 251–258. [Google Scholar] [CrossRef]
- Touahra, F.; Chebout, R.; Lerari, D.; Halliche, D.; Bachari, K. Role of the Nanoparticles of Cu-Co Alloy Derived from Perovskite in Dry Reforming of Methane. Energy 2019, 171, 465–474. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Zeaiter, J.; Kwapinski, W.; Leahy, J.J.; Ahmad, M.N. Trimetallic Ni-Co-Ru Catalyst for the Dry Reforming of Methane: Effect of the Ni/Co Ratio and the Calcination Temperature. Fuel 2021, 300, 120950. [Google Scholar] [CrossRef]
- Erdogan, B.; Arbag, H.; Yasyerli, N. SBA-15 Supported Mesoporous Ni and Co Catalysts with High Coke Resistance for Dry Reforming of Methane. Int. J. Hydrogen Energy 2018, 43, 1396–1405. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Wang, J.; Liu, J.; Lv, Y. Enhanced Activity of Co Catalysts Supported on Tungsten Carbide-Activated Carbon for CO2 Reforming of CH4 to Produce Syngas. Int. J. Hydrogen Energy 2021, 46, 28613–28625. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Gao, J.; Zhang, Q. Recent Advances in Vacancy Engineering of Metal-organic Frameworks and Their Derivatives for Electrocatalysis. SusMat 2021, 1, 66–87. [Google Scholar] [CrossRef]
- Huang, H.; Yu, Y.; Zhang, M. Mechanistic Insight into Methane Dry Reforming over Cobalt: A Density Functional Theory Study. Phys. Chem. Chem. Phys. 2020, 22, 27320–27331. [Google Scholar] [CrossRef]
- Jana, P.; De La Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. H2 Production by CH4 Decomposition over Metallic Cobalt Nanoparticles: Effect of the Catalyst Activation. Appl. Catal. A Gen. 2013, 467, 371–379. [Google Scholar] [CrossRef]
- Zardin, L.; Perez-Lopez, O.W. Hydrogen Production by Methane Decomposition over Co-Al Mixed Oxides Derived from Hydrotalcites: Effect of the Catalyst Activation with H2 or CH4. Int. J. Hydrogen Energy 2017, 42, 7895–7907. [Google Scholar] [CrossRef]
- Chen, S.; Zaffran, J.; Yang, B. Dry Reforming of Methane over the Cobalt Catalyst: Theoretical Insights into the Reaction Kinetics and Mechanism for Catalyst Deactivation. Appl. Catal. B 2020, 270, 118859. [Google Scholar] [CrossRef]
- Gonzalez-Delacruz, V.M.; Pereñiguez, R.; Ternero, F.; Holgado, J.P.; Caballero, A. In Situ XAS Study of Synergic Effects on Ni-Co/ZrO2 Methane Reforming Catalysts. J. Phys. Chem. C 2012, 116, 2919–2926. [Google Scholar] [CrossRef]
- Al-Fatesh, A.; Singh, S.K.; Kanade, G.S.; Atia, H.; Fakeeha, A.H.; Ibrahim, A.A.; El-Toni, A.M.; Labhasetwar, N.K. Rh Promoted and ZrO2/Al2O3 Supported Ni/Co Based Catalysts: High Activity for CO2 Reforming, Steam–CO2 Reforming and Oxy–CO2 Reforming of CH4. Int. J. Hydrogen Energy 2018, 43, 12069–12080. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, B.; Miao, S.; Liu, W.; Xie, J.; Lee, S.; Pellin, M.J.; Xiao, D.; Su, D.; Ma, D. Lattice Strained Ni-Co Alloy as a High-Performance Catalyst for Catalytic Dry Reforming of Methane. ACS Catal. 2019, 9, 2693–2700. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni Supported on CeO2 as Selective Bimetallic Catalyst for Dry Reforming of Methane. Int. J. Hydrogen Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Utilization of Greenhouse Gases through Carbon Dioxide Reforming of Methane over Ni-Co/MgO-ZrO2: Preparation, Characterization and Activity Studies. Appl. Catal. B 2010, 100, 365–377. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Jafari, M.F.; Khorram, S. Non-Thermal Plasma Assisted Synthesis and Physicochemical Characterizations of Co and Cu Doped Ni/Al2O3 Nanocatalysts Used for Dry Reforming of Methane. Int. J. Hydrogen Energy 2013, 38, 16048–16061. [Google Scholar] [CrossRef]
- Sirois, S.; Castro, M.; Salahub, D.R. A Density Functional Study of the Interaction of CO2 with a Pd Atom. Int. J. Quantum Chem. 1994, 52, 645–654. [Google Scholar] [CrossRef]
- Jiménez, J.D.; Betancourt, L.E.; Danielis, M.; Zhang, H.; Zhang, F.; Orozco, I.; Xu, W.; Llorca, J.; Liu, P.; Trovarelli, A.; et al. Identification of Highly Selective Surface Pathways for Methane Dry Reforming Using Mechanochemical Synthesis of Pd-CeO2. ACS Catal. 2022, 12, 12809–12822. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Guo, Z.; Dai, H.; Ren, R.; Chu, W. Anti-Sintering Mesoporous Ni–Pd Bimetallic Catalysts for Hydrogen Production via Dry Reforming of Methane. Int. J. Hydrogen Energy 2020, 45, 16133–16143. [Google Scholar] [CrossRef]
- Steinhauer, B.; Kasireddy, M.R.; Radnik, J.; Martin, A. Development of Ni-Pd Bimetallic Catalysts for the Utilization of Carbon Dioxide and Methane by Dry Reforming. Appl. Catal. A Gen. 2009, 366, 333–341. [Google Scholar] [CrossRef]
- Zambaldi, P.; Haug, L.; Penner, S.; Klötzer, B. Dry Reforming of Methane on NiCu and NiPd Model Systems: Optimization of Carbon Chemistry. Catalysts 2022, 12, 311. [Google Scholar] [CrossRef]
- Ahn, S.H.; Klein, M.J.; Manthiram, A. 1D Co- and N-Doped Hierarchically Porous Carbon Nanotubes Derived from Bimetallic Metal Organic Framework for Efficient Oxygen and Tri-Iodide Reduction Reactions. Adv. Energy Mater. 2017, 7, 1601979. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Chaudhary, M.L.; Fakeeha, A.H.; Ibrahim, A.A.; Al-Mubaddel, F.; Kasim, S.O.; Albaqmaa, Y.A.; Bagabas, A.A.; Patel, R.; Kumar, R. Role of Mixed Oxides in Hydrogen Production through the Dry Reforming of Methane over Nickel Catalysts Supported on Modified γ-Al2O3. Processes 2021, 9, 157. [Google Scholar] [CrossRef]
- Hassan, S.; Kumar, R.; Tiwari, A.; Song, W.; van Haandel, L.; Pandey, J.K.; Hensen, E.; Chowdhury, B. Role of Oxygen Vacancy in Cobalt Doped Ceria Catalyst for Styrene Epoxidation Using Molecular Oxygen. Mol. Catal. 2018, 451, 238–246. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, Q.; Wu, H.; Wang, Y. Epoxidation of Styrene with Molecular Oxygen Catalyzed by Cobalt(II)-Containing Molecular Sieves. J. Catal. 2005, 230, 384–397. [Google Scholar] [CrossRef]
- Luo, M.-F.; Hou, Z.-Y.; Yuan, X.-X.; Zheng, X.-M. Characterization Study of CeO2 Supported Pd Catalyst for Low-Temperature Carbon Monoxide Oxidation. Catal. Lett. 1998, 50, 205–209. [Google Scholar] [CrossRef]
- Patel, R.; Al-Fatesh, A.S.; Fakeeha, A.H.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Al-Zahrani, S.A.; Abasaeed, A.E.; Srivastava, V.K.; Kumar, R. Impact of Ceria over WO3–ZrO2 Supported Ni Catalyst towards Hydrogen Production through Dry Reforming of Methane. Int. J. Hydrogen Energy 2021, 46, 25015–25028. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the Reaction Route of CO2 Methanation: Promotion Effect of Medium Basic Sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Srivastava, V.; Osman, A.I.; Rooney, D.W.; Fakeeha, A.H.; Abasaeed, A.E.; Alotibi, M.F.; Kumar, R. Iron-Promoted Zirconia-Alumina Supported Ni Catalyst for Highly Efficient and Cost-Effective Hydrogen Production via Dry Reforming of Methane. J. Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Abasaeed, A.E.; Lanre, M.S.; Kasim, S.O.; Ibrahim, A.A.; Osman, A.I.; Fakeeha, A.H.; Alkhalifa, A.; Arasheed, R.; Albaqi, F.; Kumar, N.S.; et al. Syngas Production from Methane Dry Reforming via Optimization of Tungsten Trioxide-Promoted Mesoporous γ-Alumina Supported Nickel Catalyst. Int. J. Hydrogen Energy 2022, 48, 26492–26505. [Google Scholar] [CrossRef]
- Li, R.; Jin, B.; Ma, T.; Liang, Y.; Wei, Y.; Yu, X.; Li, J.; Liu, J.; Zhao, Z. Highly Efficient Catalysts of AuPd Alloy Nanoparticles Supported on 3D Ordered Macroporous Al2O3 for Soot Combustion. ChemCatChem 2022, 14, e202201020. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman Spectroscopy of Disordered, Amorphous, and Diamondlike Carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 64. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Investigating the Correlation between Deactivation and the Carbon Deposited on the Surface of Ni/Al2O3 and Ni/La2O3-Al2O3 Catalysts during the Biogas Reforming Reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef]
- Kim, H.; Robertson, A.W.; Kwon, G.H.; Jang-Won, O.; Warner, J.H.; Kim, J.M. Biomass-Derived Nickel Phosphide Nanoparticles as a Robust Catalyst for Hydrogen Production by Catalytic Decomposition of C2H2 or Dry Reforming of CH4. ACS Appl. Energy Mater. 2019, 2, 8649–8658. [Google Scholar] [CrossRef]
- Li, X.; Chang, J.-S.; Tian, M.; Park, S.-E. CO2 Reforming of Methane over Modi®ed Ni/ZrO2 Catalysts. Appl. Organomet. Chem. 2001, 15, 109–112. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in Depth Investigation of Deactivation through Carbon Formation during the Biogas Dry Reforming Reaction for Ni Supported on Modified with CeO2 and La2O3 Zirconia Catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Mourhly, A.; Kacimi, M.; Halim, M.; Arsalane, S. New Low Cost Mesoporous Silica (MSN) as a Promising Support of Ni-Catalysts for High-Hydrogen Generation via Dry Reforming of Methane (DRM). Int. J. Hydrogen Energy 2020, 45, 11449–11459. [Google Scholar] [CrossRef]
- Titus, J.; Goepel, M.; Schunk, S.A.; Wilde, N.; Gläser, R. The Role of Acid/Base Properties in Ni/MgO-ZrO2–Based Catalysts for Dry Reforming of Methane. Catal. Commun. 2017, 100, 76–80. [Google Scholar] [CrossRef]
- Kurdi, A.N.; Ibrahim, A.A.; Al-Fatesh, A.S.; Alquraini, A.A.; Abasaeed, A.E.; Fakeeha, A.H. Hydrogen Production from CO2 Reforming of Methane Using Zirconia Supported Nickel Catalyst. RSC Adv. 2022, 12, 10846–10854. [Google Scholar] [CrossRef]
- Liang, T.Y.; Lin, C.Y.; Chou, F.C.; Wang, M.; Tsai, D.H. Gas-Phase Synthesis of Ni-CeOx Hybrid Nanoparticles and Their Synergistic Catalysis for Simultaneous Reforming of Methane and Carbon Dioxide to Syngas. J. Phys. Chem. C 2018, 122, 11789–11796. [Google Scholar] [CrossRef]




| Sr. No. | Catalyst Name | Active Sites (wt.%) | CP | AC (g) | GHSV (L/h gcat) | RT (°C) | TOS (h) | Y (H2) % | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni/ZrO2-I | 10 (Ni) | I | 0.05 | 60 | 700 | - | 50 | [38] |
| 2 | Ni/ZrO2 | 5 (Ni) | I | 0.1 | 42 | 700 | 7 | 43 | [39] |
| 3 | Ni/CeO2-ZrO275 | 5 (Ni) | Co-I | 0.1 | 30 | 700 | 24 | 28 | [40] |
| 4 | Ni1Ce/ZrO2 | 5 (Ni) | I | 0.1 | 42 | 700 | 7 | 47 | [41] |
| 5 | Ni/18wt%CeO2-82wt%ZrO2 | 8 (Ni) | I | 0.15 | 40 | 750 | 50 | 35 | [40] |
| 6 | Ni/28mol%CeO2-72mol%ZrO2 | 5 (Ni) | I | 0.1 | 30 | 700 | 21 | 35 | [40] |
| 7 | Ni/SiO2 | 5 (Ni) | I | 0.1 | 24 | 700 | 23 | 22 | [23] |
| 8 | Ni-SiO2-OA | 5 (Ni) | I-OA | 0.1 | 24 | 700 | 23 | 25 | [23] |
| 9 | Ni-MSN | 5 (Ni) | I | 0.1 | 36 | 700 | 25 | 49 | [42] |
| 10 | Ni/Al2O3 | 10 (Ni) | I | - | 70 | 600 | 70 | 22.7 | [38] |
| 11 | Ni3TiAl | 5 (Ni) | MM | 0.1 | 42 | 700 | 7 | 30 | [27] |
| 12 | Ni3MoAl | 5 (Ni) | MM | 0.1 | 42 | 700 | 7 | 39 | [27] |
| 13 | Ni/CeO2-Al2O3 | 10 (Ni) | M1 | 0.1 | 30 | 750 | 12 | 12.5 | [43] |
| 14 | 5Ni/5Y-Zr | 5 (Ni) | Sg | 0.1 | 42 | 700 | 7 | 45 | [44] |
| 15 | 5Ni/5Mg-Zr | 5 (Ni) | Sg | 0.1 | 42 | 700 | 7 | 23 | [44] |
| 16 | 2.5Ni2.5Co/Pd+Al2O3 | 5 (Ni & Co) | I | 0.1 | 42 | 800 | 7 | 53 | This Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakeeha, A.H.; Vadodariya, D.M.; Alotibi, M.F.; Abu-Dahrieh, J.K.; Ibrahim, A.A.; Abasaeed, A.E.; Alarifi, N.; Kumar, R.; Al-Fatesh, A.S. Pd+Al2O3-Supported Ni-Co Bimetallic Catalyst for H2 Production through Dry Reforming of Methane: Effect of Carbon Deposition over Active Sites. Catalysts 2023, 13, 1374. https://doi.org/10.3390/catal13101374
Fakeeha AH, Vadodariya DM, Alotibi MF, Abu-Dahrieh JK, Ibrahim AA, Abasaeed AE, Alarifi N, Kumar R, Al-Fatesh AS. Pd+Al2O3-Supported Ni-Co Bimetallic Catalyst for H2 Production through Dry Reforming of Methane: Effect of Carbon Deposition over Active Sites. Catalysts. 2023; 13(10):1374. https://doi.org/10.3390/catal13101374
Chicago/Turabian StyleFakeeha, Anis H., Dharmesh M. Vadodariya, Mohammed F. Alotibi, Jehad K. Abu-Dahrieh, Ahmed A. Ibrahim, Ahmed E. Abasaeed, Naif Alarifi, Rawesh Kumar, and Ahmed S. Al-Fatesh. 2023. "Pd+Al2O3-Supported Ni-Co Bimetallic Catalyst for H2 Production through Dry Reforming of Methane: Effect of Carbon Deposition over Active Sites" Catalysts 13, no. 10: 1374. https://doi.org/10.3390/catal13101374
APA StyleFakeeha, A. H., Vadodariya, D. M., Alotibi, M. F., Abu-Dahrieh, J. K., Ibrahim, A. A., Abasaeed, A. E., Alarifi, N., Kumar, R., & Al-Fatesh, A. S. (2023). Pd+Al2O3-Supported Ni-Co Bimetallic Catalyst for H2 Production through Dry Reforming of Methane: Effect of Carbon Deposition over Active Sites. Catalysts, 13(10), 1374. https://doi.org/10.3390/catal13101374









