Electrochemical Synthesis of Versatile Pyrimidine and Oxadiazoles Tethered Triazoles as Inhibitors of VEGFR-2 in Human Breast Cancer Cells
Abstract
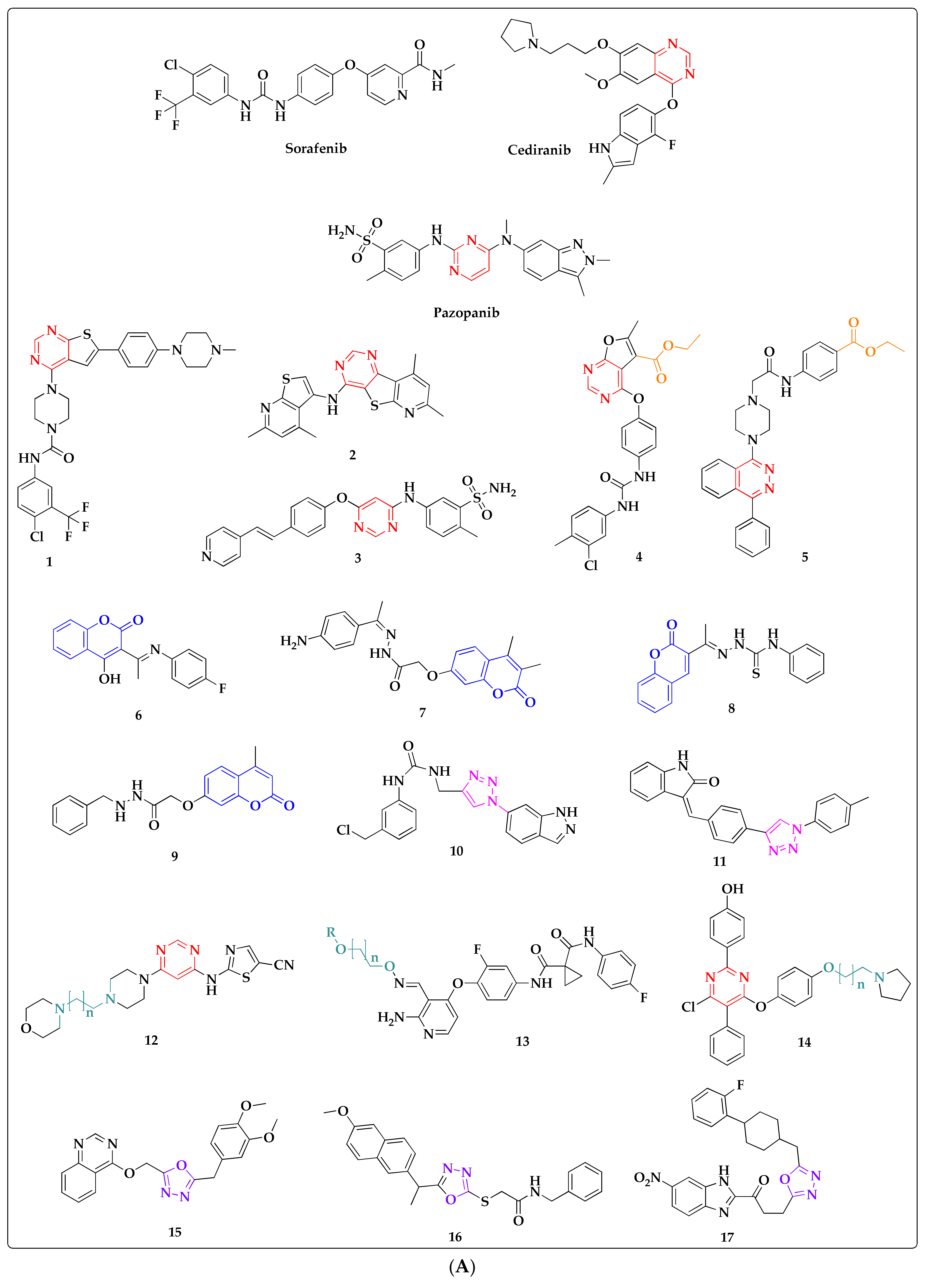
:1. Introduction
2. Results and Discussion
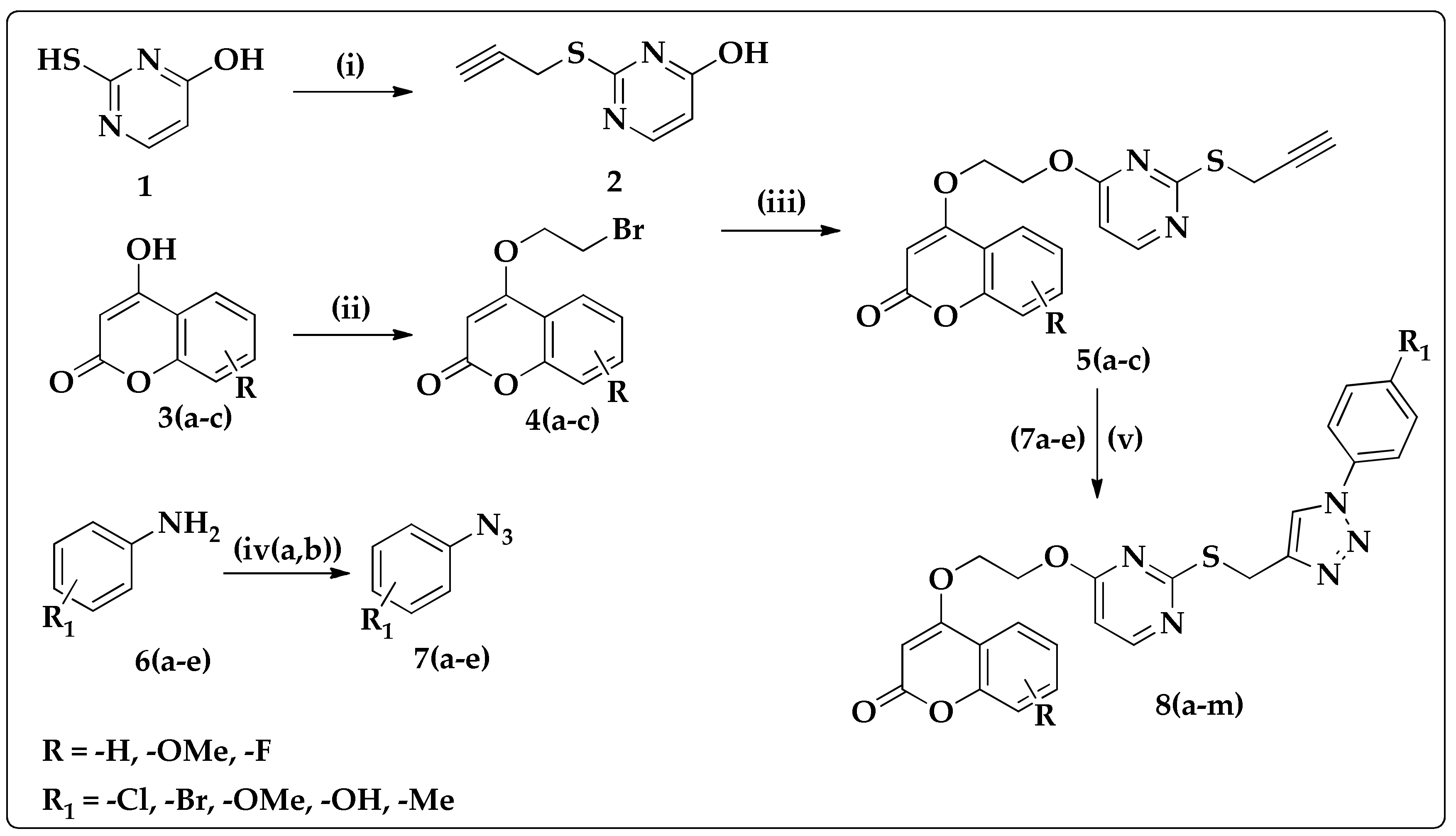
2.1. Synthesis of Coumarin–Pyrimidine–Alkynes
2.2. Synthesis of Oxadiazole–Alkynes
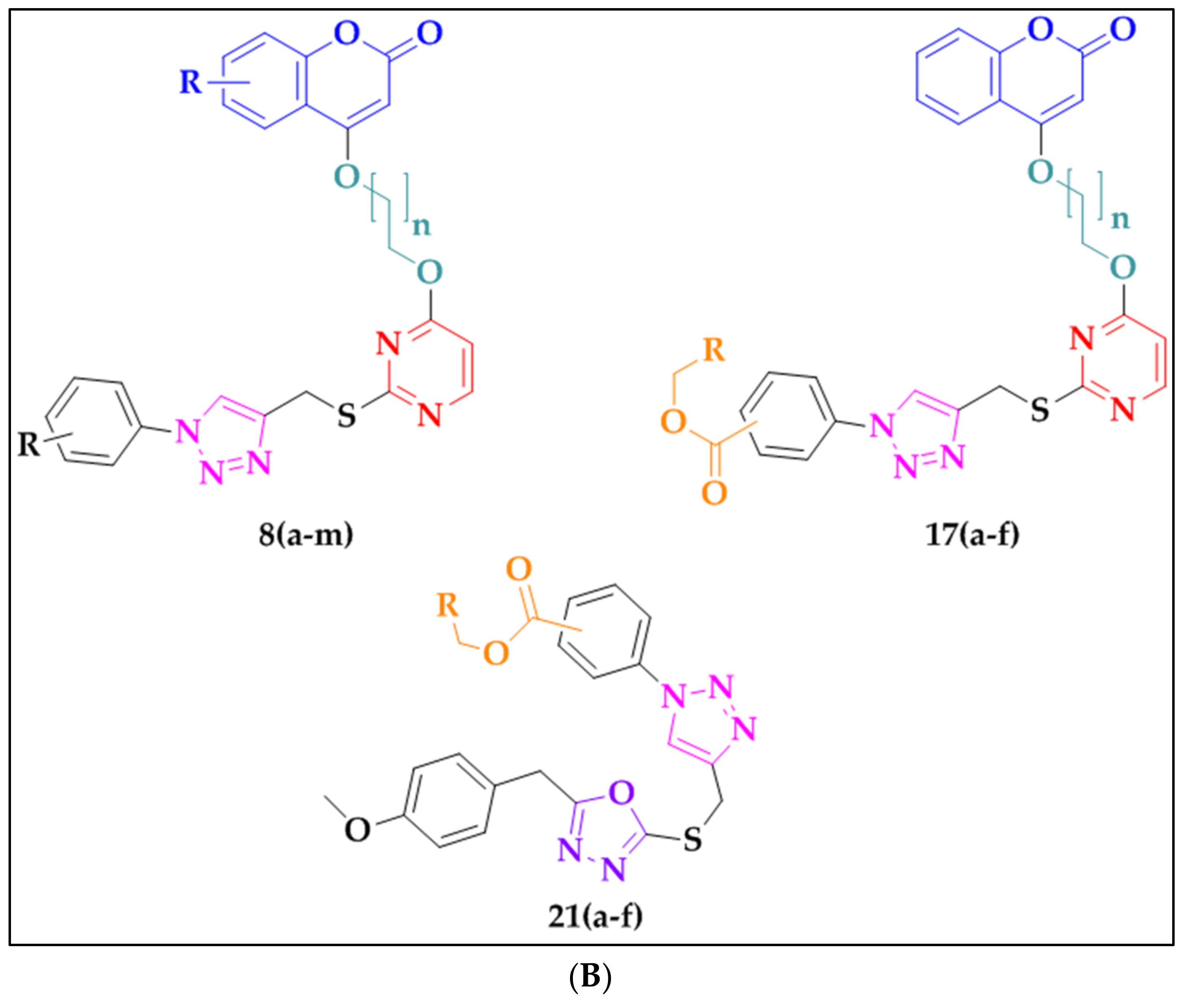
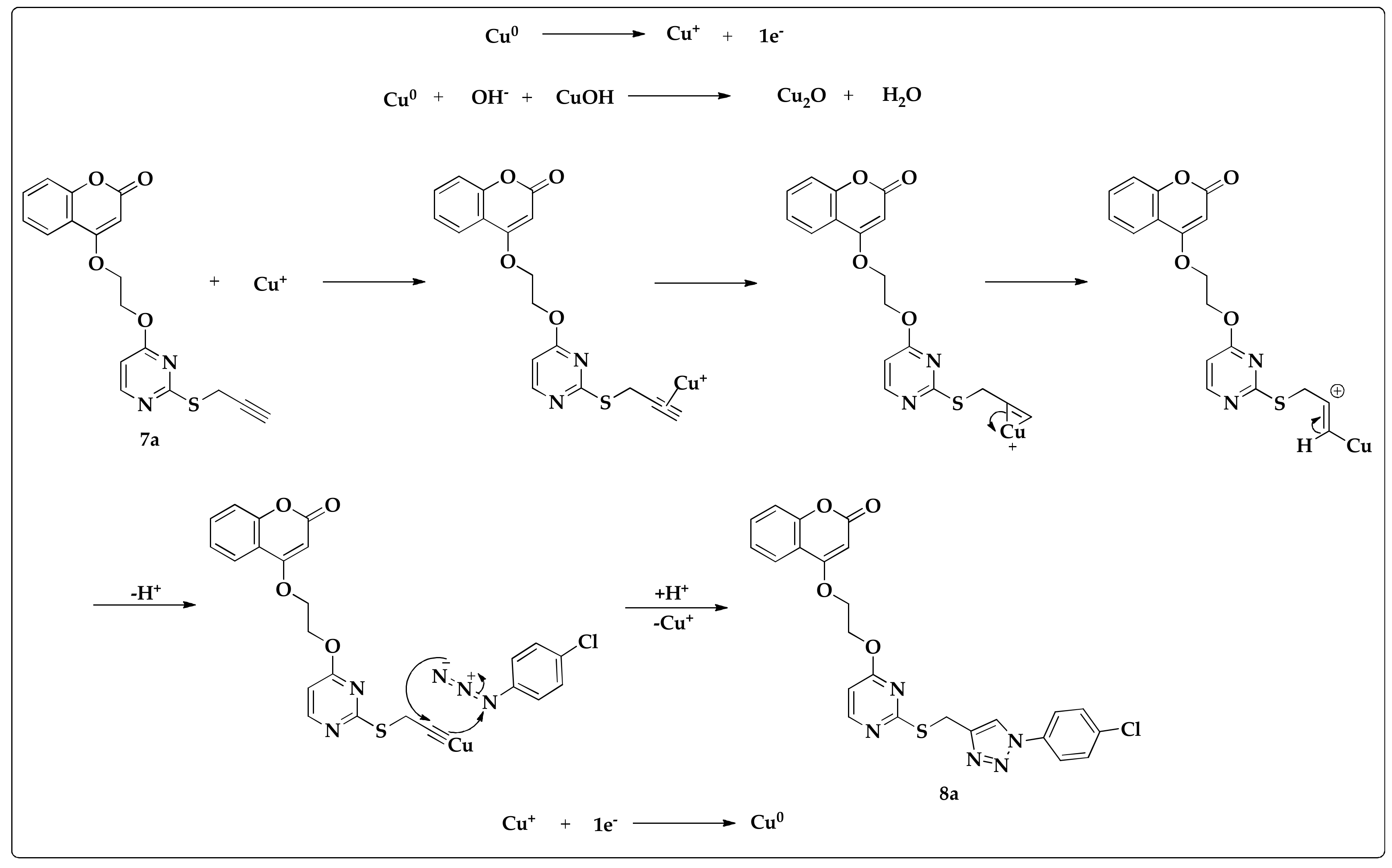
2.3. Synthesis of Triazoles
2.4. Efficacy of Triazoles in Breast Cancer Cells
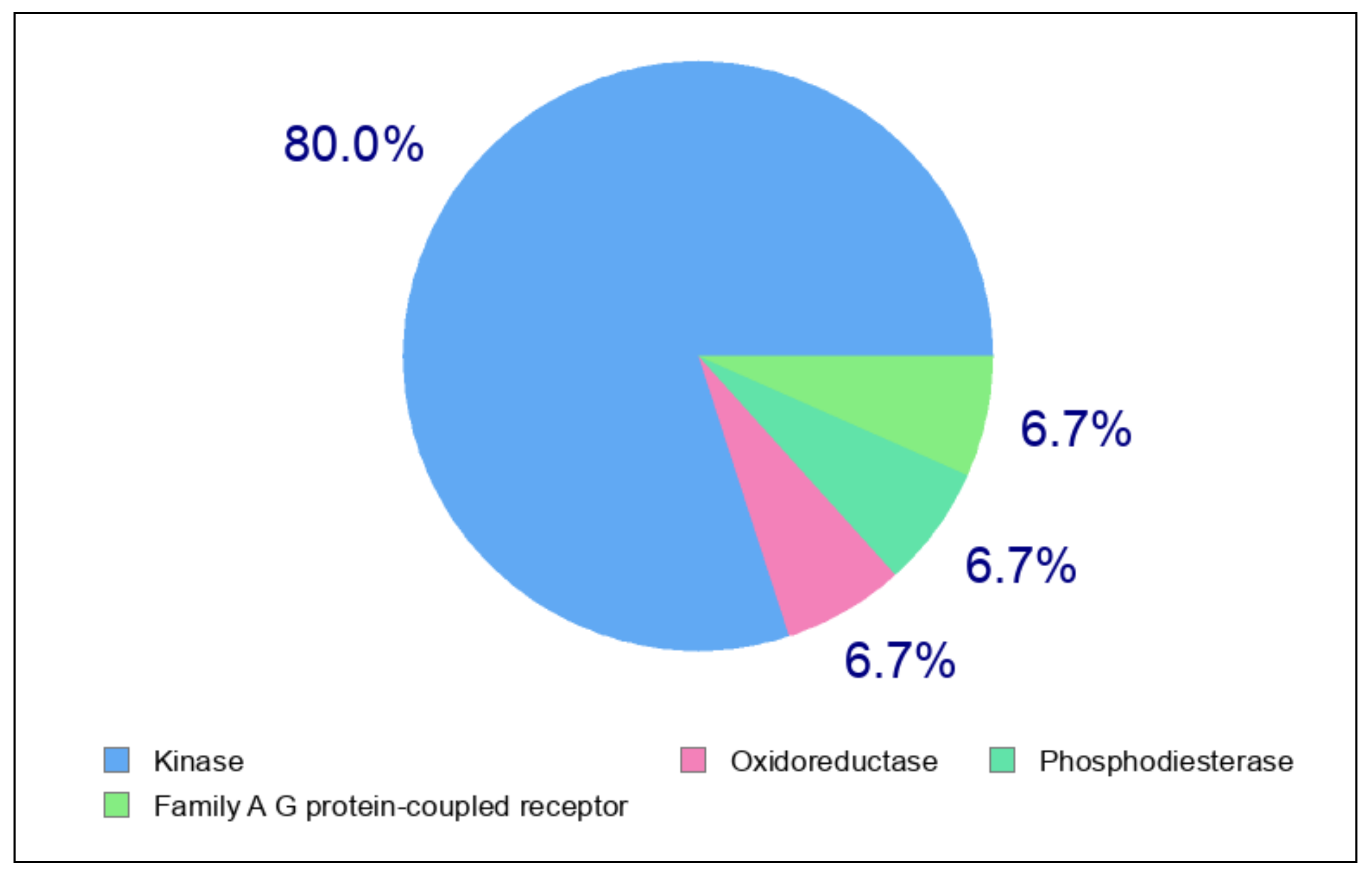
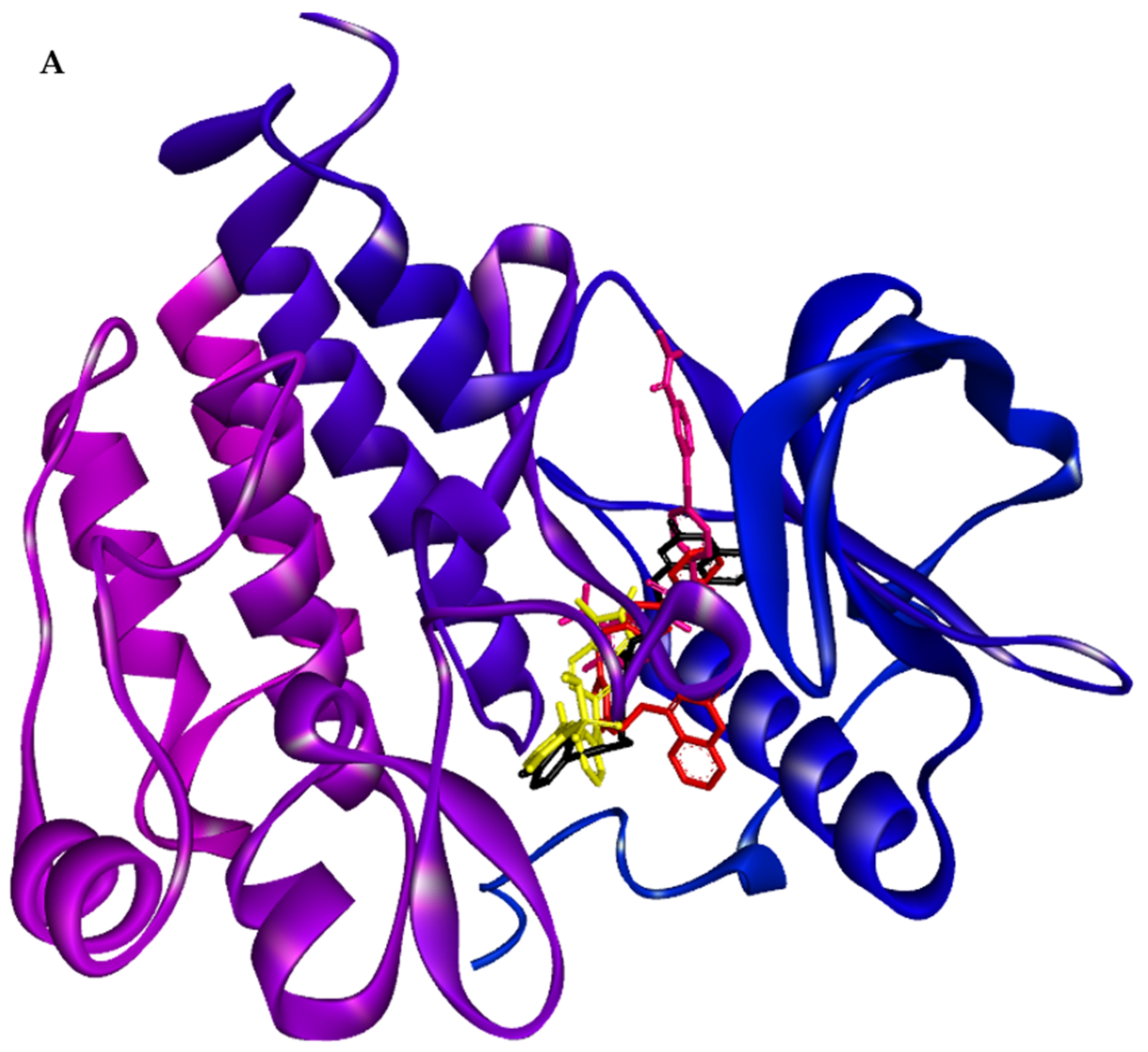
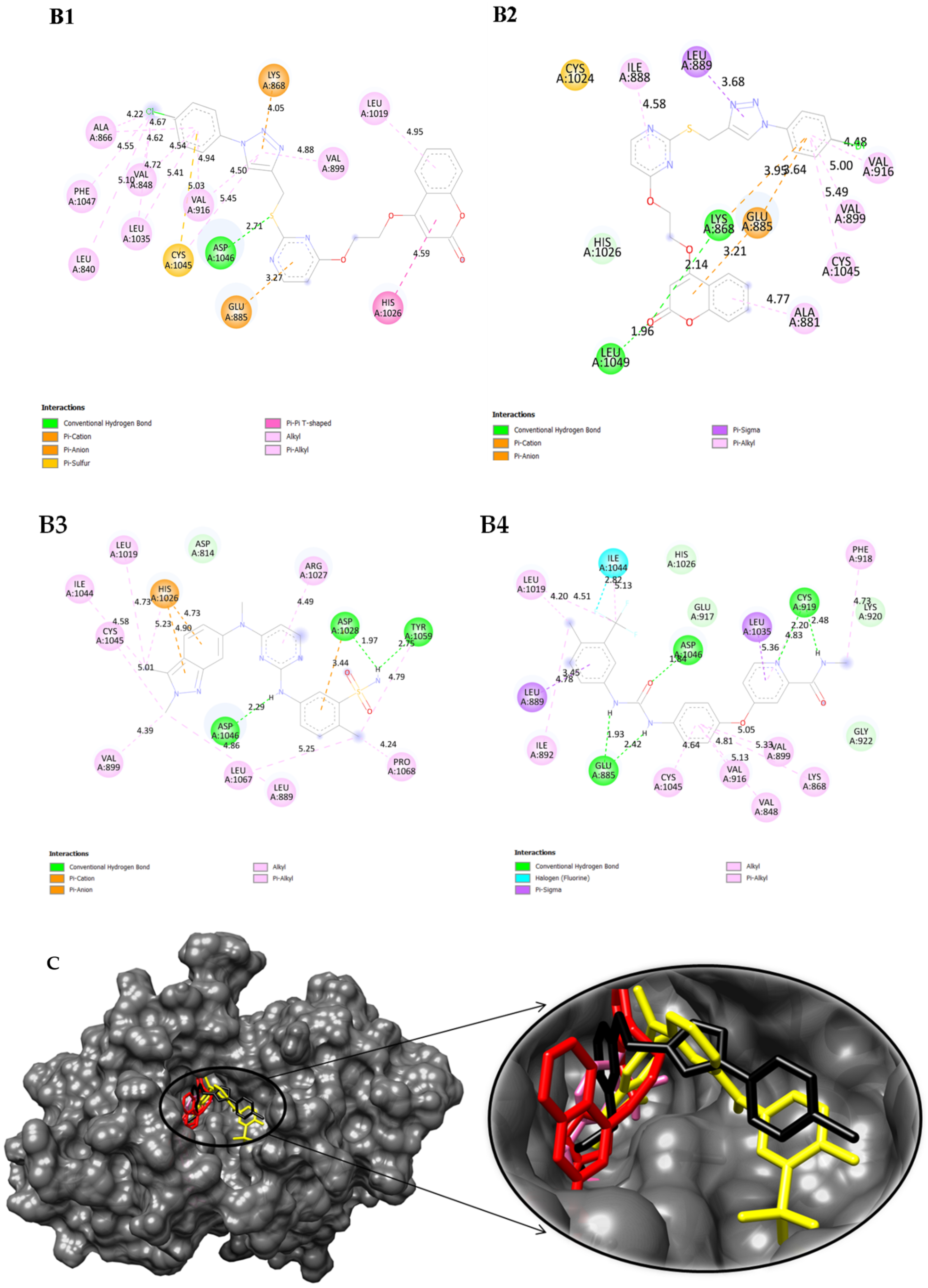
2.5. Swiss TargetPrediction and In Silico Molecular Interactions of Compound 8a or 8b with VEGFR-2
2.6. ADMET Predictions of Compound 8a and 8b with Doxorubicin
3. Materials and Methods
3.1. Synthesis of Coumarin–Pyrimidine–Alkynes Derivatives 5(a–c) and 13
3.2. Synthesis of Oxadiazole–Alkyne Derivatives 20
3.3. Synthesis of Triazoles 8(a–m), 17(a–f), and 21(a–f)
3.4. 4-(2-((2-(((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-2H-chromen-2-one (8a)
3.5. 4-(2-((2-(((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)2H-chromen-2-one (8b)
3.6. 4-(2-((2-(((1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-2H-chromen-2-one (8c)
3.7. 4-(2-((2-(((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-7-methoxy-2H-chromen-2-one (8e)
3.8. 4-(2-((2-(((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-7-methoxy-2H-chromen-2-one (8f)
3.9. 7-Methoxy-4-(2-((2-(((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrim-idin-4-yl)oxy)ethoxy)-2H-chromen-2-one (8g)
3.10. 4-(2-((2-(((1-(4-Hydroxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-7-methoxy-2H-chromen-2-one (8h)
3.11. 7-Methoxy-4-(2-((2-(((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)eth-oxy)-2H-chromen-2-one (8i)
3.12. 4-(2-((2-(((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-6-fluoro-2H-chromen-2-one (8j)
3.13. 4-(2-((2-(((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-6-fluoro-2H-chromen-2-one (8k)
3.14. 6-Fluoro-4-(2-((2-(((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)-oxy)ethoxy)-2H-chromen-2-one (8l)
3.15. 6-Fluoro-4-(2-((2-(((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)thio)pyrimidin-4-yl)oxy)ethoxy)-2H-chromen-2-one (8m)
3.16. Ethyl-4-(4-(((4-methyl-6-(3-((2-oxo-2H-chromen-4-yl)oxy)propoxy)pyrimidin-2-yl)thio)methyl)-1H-1,2,3-triazol-1-yl)benzoate (17a)
3.17. Ethyl-3-(4-(((4-methyl-6-(3-((2-oxo-2H-chromen-4-yl)oxy)propoxy)pyrimidin-2-yl)thio)-methyl)-1H-1,2,3-triazol-1-yl)benzoate (17b)
3.18. Methyl 3-(4-(((4-methyl-6-(3-((2-oxo-2H-chromen-4-yl)oxy)propoxy)pyrimidin-2-yl)thio)-methyl)-1H-1,2,3-triazol-1-yl)benzoate (17c)
3.19. Ethyl-3-(4-(((4-methyl-6-(3-((2-oxo-2H-chromen-4-yl)oxy)propoxy)pyrimidin-2-yl)thio)-methyl)-1H-1,2,3-triazol-1-yl)benzoate (17d)
3.20. Methyl-3-(4-(((4-methyl-6-(3-((2-oxo-2H-chromen-4-yl)oxy)propoxy)pyrimidin-2-yl)thio)-methyl)-1H-1,2,3-triazol-1-yl)benzoate (17e)
3.21. Butyl-3-(4-(((4-methyl-6-(3-((2-oxo-2H-chromen-4-yl)oxy)propoxy)pyrimidin-2-yl)thio)-methyl)-1H-1,2,3-triazol-1-yl)benzoate (17f)
3.22. Butyl-4-(4-(((5-(4-methoxybenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-1H-1,2,3-triazol-1-yl)-benzoate (21a)
3.23. Ethyl-3-(4-(((5-(4-methoxybenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-1H-1,2,3-triazol-1-yl)-benzoate (21b)
3.24. Methyl-4-(4-(((5-(4-methoxybenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-1H-1,2,3-triazol-1-yl)benzoate (21c)
3.25. Methyl-3-(4-(((5-(4-methoxybenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-1H-1,2,3-triazol-1-yl)benzoate (21e)
3.26. Butyl-3-(4-(((5-(4-methoxybenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-1H-1,2,3-triazol-1-yl)benzoate (21f)
3.27. Cell Lines and Culture Conditions
3.28. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O.; Ilbawi, A.M.; Fidarova, E.; Weiderpass, E.; Stevens, L.; Abdel-Wahab, M.; Mikkelsen, B. The Global Breast Cancer Initiative: A Strategic Collaboration to Strengthen Health Care for Non-Communicable Diseases. Lancet Oncol. 2021, 22, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Peerzada, M.N.; Hamel, E.; Bai, R.; Supuran, C.T.; Azam, A. Deciphering the Key Heterocyclic Scaffolds in Targeting Microtubules, Kinases and Carbonic Anhydrases for Cancer Drug Development. Pharmacol. Ther. 2021, 225, 107860. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Vishwanath, D.; Shete-Aich, A.; Honnegowda, M.B.; Anand, M.P.; Chidambaram, S.B.; Sapkal, G.; Basappa, B.; Yadav, P.D. Discovery of Hybrid Thiouracil-Coumarin Conjugates as Potential Novel Anti-SARS-CoV-2 Agents Targeting the Virus’s Polymerase “RdRp” as a Confirmed Interacting Biomolecule. ACS Omega 2023, 8, 27056–27066. [Google Scholar] [CrossRef]
- Albratty, M.; Alhazmi, H.A. Novel Pyridine and Pyrimidine Derivatives as Promising Anticancer Agents: A Review. Arab. J. Chem. 2022, 15, 103846. [Google Scholar] [CrossRef]
- Kim, N.Y.; Vishwanath, D.; Xi, Z.; Nagaraja, O.; Swamynayaka, A.; Kumar Harish, K.; Basappa, S.; Madegowda, M.; Pandey, V.; Sethi, G.; et al. Discovery of Pyrimidine- and Coumarin-Linked Hybrid Molecules as Inducers of JNK Phosphorylation through ROS Generation in Breast Cancer Cells. Molecules 2023, 28, 3450. [Google Scholar] [CrossRef]
- Keerthy, H.K.; Mohan, C.D.; Siveen, K.S.; Fuchs, J.E.; Rangappa, S.; Sundaram, M.S.; Li, F.; Girish, K.S.; Sethi, G.; Basappa; et al. Novel Synthetic Biscoumarins Target Tumor Necrosis Factor-α in Hepatocellular Carcinoma in Vitro and in Vivo. J. Biol. Chem. 2014, 289, 31879–31890. [Google Scholar] [CrossRef]
- Pandey, V.; Zhang, X.; Poh, H.-M.; Wang, B.; Dukanya, D.; Ma, L.; Yin, Z.; Bender, A.; Periyasamy, G.; Zhu, T.; et al. Monomerization of Homodimeric Trefoil Factor 3 (TFF3) by an Aminonitrile Compound Inhibits TFF3-Dependent Cancer Cell Survival. ACS Pharmacol. Transl. Sci. 2022, 5, 761–773. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of Recent Developments of Pyrazole Derivatives as an Anticancer Agent in Different Cell Line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef]
- Basappa, B.; Poonacha, L.K.; Xi, Z.; Vishwanath, D.; Yang, J.-R.; Nagaraja, O.; Swamynayaka, A.; Madegowda, M.; Chinnathambi, A.; Alharbi, S.A.; et al. Nano-Zirconium Dioxide Catalyzed Multicomponent Synthesis of Bioactive Pyranopyrazoles That Target Cyclin Dependent Kinase 1 in Human Breast Cancer Cells. Biomedicines 2023, 11, 172. [Google Scholar] [CrossRef]
- Ravish, A.; Shivakumar, R.; Xi, Z.; Yang, M.H.; Yang, J.-R.; Swamynayaka, A.; Nagaraja, O.; Madegowda, M.; Chinnathambi, A.; Alharbi, S.A.; et al. De Novo Design of Imidazopyridine-Tethered Pyrazolines That Target Phosphorylation of STAT3 in Human Breast Cancer Cells. Bioengineering 2023, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef] [PubMed]
- Basappa, B.; Jung, Y.Y.; Ravish, A.; Xi, Z.; Swamynayaka, A.; Madegowda, M.; Pandey, V.; Lobie, P.E.; Sethi, G.; Ahn, K.S. Methyl-Thiol-Bridged Oxadiazole and Triazole Heterocycles as Inhibitors of NF-κB in Chronic Myelogenous Leukemia Cells. Biomedicines 2023, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Al Sheikh Ali, A.; Khan, D.; Naqvi, A.; Al-blewi, F.F.; Rezki, N.; Aouad, M.R.; Hagar, M. Design, Synthesis, Molecular Modeling, Anticancer Studies, and Density Functional Theory Calculations of 4-(1,2,4-Triazol-3-Ylsulfanylmethyl)-1,2,3-Triazole Derivatives. ACS Omega 2020, 6, 301–316. [Google Scholar] [CrossRef]
- Benassi, A.; Doria, F.; Pirota, V. Groundbreaking Anticancer Activity of Highly Diversified Oxadiazole Scaffolds. Int. J. Mol. Sci. 2020, 21, 8692. [Google Scholar] [CrossRef]
- Malojirao, V.H.; Girimanchanaika, S.S.; Shanmugam, M.K.; Sherapura, A.; Dukanya; Metri, P.K.; Vigneshwaran, V.; Chinnathambi, A.; Alharbi, S.A.; Rangappa, S.; et al. Novel 1,3,4-Oxadiazole Targets STAT3 Signaling to Induce Antitumor Effect in Lung Cancer. Biomedicines 2020, 8, 368. [Google Scholar] [CrossRef]
- Vishwanath, D.; Girimanchanaika, S.S.; Dukanya, D.; Rangappa, S.; Yang, J.-R.; Pandey, V.; Lobie, P.E.; Basappa, B. Design and Activity of Novel Oxadiazole Based Compounds That Target Poly(ADP-Ribose) Polymerase. Molecules 2022, 27, 703. [Google Scholar] [CrossRef]
- Samie, N.; Muniandy, S.; Kanthimathi, M.S.; Haerian, B.S.; Raja Azudin, R.E. Novel Piperazine Core Compound Induces Death in Human Liver Cancer Cells: Possible Pharmacological Properties. Sci. Rep. 2016, 6, 24172. [Google Scholar] [CrossRef]
- Deveshegowda, S.N.; Metri, P.K.; Shivakumar, R.; Yang, J.-R.; Rangappa, S.; Swamynayaka, A.; Shanmugam, M.K.; Nagaraja, O.; Madegowda, M.; Babu Shubha, P.; et al. Development of 1-(4-(Substituted)Piperazin-1-Yl)-2-((2-((4-Methoxybenzyl)Thio)Pyrimidin-4-Yl)Oxy)Ethanones That Target Poly (ADP-Ribose) Polymerase in Human Breast Cancer Cells. Molecules 2022, 27, 2848. [Google Scholar] [CrossRef]
- Brogowska, K.K.; Zajkowska, M.; Mroczko, B. Vascular Endothelial Growth Factor Ligands and Receptors in Breast Cancer. J. Clin. Med. 2023, 12, 2412. [Google Scholar] [CrossRef] [PubMed]
- Arias-Pulido, H.; Chaher, N.; Gong, Y.; Qualls, C.; Vargas, J.; Royce, M. Tumor Stromal Vascular Endothelial Growth Factor A Is Predictive of Poor Outcome in Inflammatory Breast Cancer. BMC Cancer 2012, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Elebiyo, T.C.; Rotimi, D.; Evbuomwan, I.O.; Maimako, R.F.; Iyobhebhe, M.; Ojo, O.A.; Oluba, O.M.; Adeyemi, O.S. Reassessing Vascular Endothelial Growth Factor (VEGF) in Anti-Angiogenic Cancer Therapy. Cancer Treat. Res. Commun. 2022, 32, 100620. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal Blood Vessel Development and Lethality in Embryos Lacking a Single VEGF Allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Dore-Savard, L.; Lee, E.; Kakkad, S.; Popel, A.S.; Bhujwalla, Z.M. The Angiogenic Secretome in VEGF Overexpressing Breast Cancer Xenografts. Sci. Rep. 2016, 6, 39460. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and Its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef]
- Al-Sanea, M.M.; Chilingaryan, G.; Abelyan, N.; Sargsyan, A.; Hovhannisyan, S.; Gasparyan, H.; Gevorgyan, S.; Albogami, S.; Ghoneim, M.M.; Farag, A.K.; et al. Identification of Novel Potential VEGFR-2 Inhibitors Using a Combination of Computational Methods for Drug Discovery. Life 2021, 11, 1070. [Google Scholar] [CrossRef]
- Al Nazawi, A.M.; Aqili, J.; Alzahrani, M.; McCall, P.J.; Weetman, D. Combined Target Site (Kdr) Mutations Play a Primary Role in Highly Pyrethroid Resistant Phenotypes of Aedes Aegypti from Saudi Arabia. Parasites Vectors 2017, 10, 161. [Google Scholar] [CrossRef]
- Benzinger, P.; Martiny-Baron, G.; Reusch, P.; Siemeister, G.; Kley, J.T.; Marmé, D.; Unger, C.; Massing, U. Targeting of Endothelial KDR Receptors with 3G2 Immunoliposomes in Vitro. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2000, 1466, 71–78. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Sivridis, E.; Chlouverakis, G.; Vourvouhaki, E.; Turley, H.; Harris, A.L.; Gatter, K.C. Activated Vegfr2/Kdr Pathway In Tumour Cells And Tumour Associated Vessels of Colorectal Cancer. Eur. J. Clin. Investig. 2007, 37, 878–886. [Google Scholar] [CrossRef]
- Gampel, A.; Moss, L.; Jones, M.C.; Brunton, V.; Norman, J.C.; Mellor, H. VEGF Regulates the Mobilization of VEGFR2/KDR from an Intracellular Endothelial Storage Compartment. Blood 2006, 108, 2624–2631. [Google Scholar] [CrossRef]
- Modi, S.J.; Kulkarni, V.M. Vascular Endothelial Growth Factor Receptor (VEGFR-2)/KDR Inhibitors: Medicinal Chemistry Perspective. Med. Drug Discov. 2019, 2, 100009. [Google Scholar] [CrossRef]
- Harrison, C. A Deeper Understanding of VEGFR Inhibitors. Nat. Rev. Drug Discov. 2012, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Plate, K.H.; Breier, G.; Weich, H.A.; Risau, W. Vascular Endothelial Growth Factor Is a Potential Tumour Angiogenesis Factor in Human Gliomas in Vivo. Nature 1992, 359, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Polena, H.; Creuzet, J.; Dufies, M.; Sidibé, A.; Khalil-Mgharbel, A.; Salomon, A.; Deroux, A.; Quesada, J.-L.; Roelants, C.; Filhol, O.; et al. The Tyrosine-Kinase Inhibitor Sunitinib Targets Vascular Endothelial (VE)-Cadherin: A Marker of Response to Antitumoural Treatment in Metastatic Renal Cell Carcinoma. Br. J. Cancer 2018, 118, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gray, N.S. Rational Design of Inhibitors That Bind to Inactive Kinase Conformations. Nat. Chem. Biol. 2006, 2, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; Soliman, A.M.; Ragab, F.A. VEGFR-2 Inhibitors and Apoptosis Inducers: Synthesis and Molecular Design of New Benzo[g]Quinazolin Bearing Benzenesulfonamide Moiety. J. Enzym. Inhib. Med. Chem. 2017, 32, 893–907. [Google Scholar] [CrossRef]
- Harris, P.A.; Boloor, A.; Cheung, M.; Kumar, R.; Crosby, R.M.; Davis-Ward, R.G.; Epperly, A.H.; Hinkle, K.W.; Hunter, R.N.; Johnson, J.H.; et al. Discovery of 5-[[4-[(2,3-Dimethyl-2H-Indazol-6-Yl)Methylamino]-2-Pyrimidinyl]Amino]-2-Methyl-Benzenesulfonamide (Pazopanib), a Novel and Potent Vascular Endothelial Growth Factor Receptor Inhibitor. J. Med. Chem. 2008, 51, 4632–4640. [Google Scholar] [CrossRef]
- Orbegoso, C.; Marquina, G.; George, A.; Banerjee, S. The Role of Cediranib in Ovarian Cancer. Expert Opin. Pharmacother. 2017, 18, 1637–1648. [Google Scholar] [CrossRef]
- Woo, H.Y.; Heo, J. Sorafenib in Liver Cancer. Expert Opin. Pharmacother. 2012, 13, 1059–1067. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Zhang, J.; Tang, P.; Yang, C.; Wang, G.; Chen, J.; Liu, J.; Zhang, L.; Ouyang, L. Discovery, Synthesis, and Evaluation of Highly Selective Vascular Endothelial Growth Factor Receptor 3 (VEGFR3) Inhibitor for the Potential Treatment of Metastatic Triple-Negative Breast Cancer. J. Med. Chem. 2021, 64, 12022–12048. [Google Scholar] [CrossRef]
- Abdel Aziz, Y.M.; Said, M.M.; El Shihawy, H.A.; Abouzid, K.A.M. Discovery of Novel Tricyclic Pyrido[3′,2′:4,5]Thieno[3,2-d]Pyrimidin-4-Amine Derivatives as VEGFR-2 Inhibitors. Bioorg. Chem. 2015, 60, 1–12. [Google Scholar] [CrossRef]
- Sun, W.; Hu, S.; Fang, S.; Yan, H. Design, Synthesis and Biological Evaluation of Pyrimidine-Based Derivatives as VEGFR-2 Tyrosine Kinase Inhibitors. Bioorg. Chem. 2018, 78, 393–405. [Google Scholar] [CrossRef]
- Aziz, M.A.; Serya, R.A.T.; Lasheen, D.S.; Abdel-Aziz, A.K.; Esmat, A.; Mansour, A.M.; Singab, A.N.B.; Abouzid, K.A.M. Discovery of Potent VEGFR-2 Inhibitors Based on Furopyrimidine and Thienopyrimidne Scaffolds as Cancer Targeting Agents. Sci. Rep. 2016, 6, 24460. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Eldehna, W.M.; Ali, M.M.; Abou El Ella, D.A. 1-Piperazinylphthalazines as Potential VEGFR-2 Inhibitors and Anticancer Agents: Synthesis and in Vitro Biological Evaluation. Eur. J. Med. Chem. 2016, 107, 165–179. [Google Scholar] [CrossRef]
- Batran, R.Z.; Dawood, D.H.; El-Seginy, S.A.; Ali, M.M.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. New Coumarin Derivatives as Anti-Breast and Anti-Cervical Cancer Agents Targeting VEGFR-2 and P38α MAPK. Arch. Der Pharm. 2017, 350, 1700064. [Google Scholar] [CrossRef]
- Abdel Ghany, L.M.A.; El-Dydamony, N.M.; Helwa, A.A.; Abdelraouf, S.M.; Abdelnaby, R.M. Coumarin-Acetohydrazide Derivatives as Novel Antiproliferative Agents via VEGFR-2/AKT Axis Inhibition and Apoptosis Triggering. N. J. Chem. 2022, 46, 17394–17409. [Google Scholar] [CrossRef]
- Emam, S.H.; Hassan, R.A.; Osman, E.O.; Hamed, M.I.A.; Abdou, A.M.; Kandil, M.M.; Elbaz, E.M.; Mikhail, D.S. Coumarin Derivatives with Potential Anticancer and Antibacterial Activity: Design, Synthesis, VEGFR-2 and DNA Gyrase Inhibition, and in silico Studies. Drug Dev. Res. 2023, 84, 475–499. [Google Scholar] [CrossRef]
- Ahmed, E.Y.; Abdel Latif, N.A.; El-Mansy, M.F.; Elserwy, W.S.; Abdelhafez, O.M. VEGFR-2 Inhibiting Effect and Molecular Modeling of Newly Synthesized Coumarin Derivatives as Anti-Breast Cancer Agents. Bioorg. Med. Chem. 2020, 28, 115328. [Google Scholar] [CrossRef]
- Liang, T.; Sun, X.; Li, W.; Hou, G.; Gao, F. 1,2,3-Triazole-Containing Compounds as Anti-Lung Cancer Agents: Current Developments, Mechanisms of Action, and Structure-Activity Relationship. Front. Pharmacol. 2021, 12, 661173. [Google Scholar] [CrossRef]
- Sanphanya, K.; Wattanapitayakul, S.K.; Phowichit, S.; Fokin, V.V.; Vajragupta, O. Novel VEGFR-2 Kinase Inhibitors Identified by the Back-to-Front Approach. Bioorg. Med. Chem. Lett. 2013, 23, 2962–2967. [Google Scholar] [CrossRef]
- Wang, D.; Liu, K.; Li, X.; Lu, G.; Xue, W.; Qian, X.; Mohamed, O.K.; Meng, F. Design, Synthesis, and in Vitro and in Vivo Anti-Angiogenesis Study of a Novel Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) Inhibitor Based on 1,2,3-Triazole Scaffold. Eur. J. Med. Chem. 2021, 211, 113083. [Google Scholar] [CrossRef]
- Sisko, J.T.; Tucker, T.J.; Bilodeau, M.T.; Buser, C.A.; Ciecko, P.A.; Coll, K.E.; Fernandes, C.; Gibbs, J.B.; Koester, T.J.; Kohl, N.; et al. Potent 2-[(Pyrimidin-4-Yl)Amine}-1,3-Thiazole-5-Carbonitrile-Based Inhibitors of VEGFR-2 (KDR) Kinase. Bioorg. Med. Chem. Lett. 2006, 16, 1146–1150. [Google Scholar] [CrossRef]
- Qiang, H.; Gu, W.; Huang, D.; Shi, W.; Qiu, Q.; Dai, Y.; Huang, W.; Qian, H. Design, Synthesis and Biological Evaluation of 4-Aminopyrimidine-5-Cabaldehyde Oximes as Dual Inhibitors of c-Met and VEGFR-2. Bioorg. Med. Chem. 2016, 24, 3353–3358. [Google Scholar] [CrossRef]
- Luo, G.; Tang, Z.; Lao, K.; Li, X.; You, Q.; Xiang, H. Structure-Activity Relationships of 2, 4-Disubstituted Pyrimidines as Dual ERα/VEGFR-2 Ligands with Anti-Breast Cancer Activity. Eur. J. Med. Chem. 2018, 150, 783–795. [Google Scholar] [CrossRef]
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 3361. [Google Scholar] [CrossRef]
- Qiao, F.; Yin, Y.; Shen, Y.-N.; Wang, S.-F.; Sha, S.; Wu, X.; Lu, A.-M.; Xu, C.; Zhang, W.-M.; Zhu, H.-L. Synthesis, Molecular Modeling, and Biological Evaluation of Quinazoline Derivatives Containing the 1,3,4-Oxadiazole Scaffold as Novel Inhibitors of VEGFR2. RSC Adv. 2015, 5, 19914–19923. [Google Scholar] [CrossRef]
- Hagras, M.; Saleh, M.A.; Ezz Eldin, R.R.; Abuelkhir, A.A.; Khidr, E.G.; El-Husseiny, A.A.; El-Mahdy, H.A.; Elkaeed, E.B.; Eissa, I.H. 1,3,4-Oxadiazole-Naphthalene Hybrids as Potential VEGFR-2 Inhibitors: Design, Synthesis, Antiproliferative Activity, Apoptotic Effect, and in silico Studies. J. Enzym. Inhib. Med. Chem. 2021, 37, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Awadallah, F.M.; Refaat, H.M.; Amin, K.M. Molecular Docking Simulation, Synthesis and 3D Pharmacophore Studies of Novel 2-Substituted-5-Nitro-Benzimidazole Derivatives as Anticancer Agents Targeting VEGFR-2 and c-Met. Bioorg. Chem. 2018, 77, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, S.R.; Janza, B. Renaissance of Electrosynthetic Methods for the Construction of Complex Molecules. Angew. Chem. Int. Ed. 2014, 53, 7122–7123. [Google Scholar] [CrossRef] [PubMed]
- Baburajeev, C.P.; Dhananjaya Mohan, C.; Ananda, H.; Rangappa, S.; Fuchs, J.E.; Jagadish, S.; Sivaraman Siveen, K.; Chinnathambi, A.; Ali Alharbi, S.; Zayed, M.E.; et al. Development of Novel Triazolo-Thiadiazoles from Heterogeneous “Green” Catalysis as Protein Tyrosine Phosphatase 1B Inhibitors. Sci. Rep. 2015, 5, 14195. [Google Scholar] [CrossRef]
- Frontana-Uribe, B.A.; Little, R.D.; Ibanez, J.G.; Palma, A.; Vasquez-Medrano, R. Organic Electrosynthesis: A Promising Green Methodology in Organic Chemistry. Green Chem. 2010, 12, 2099. [Google Scholar] [CrossRef]
- van der Vlugt, J.I. Radical-Type Reactivity and Catalysis by Single-Electron Transfer to or from Redox-Active Ligands. Chem.-A Eur. J. 2018, 25, 2651–2662. [Google Scholar] [CrossRef]
- Maljuric, S.; Jud, W.; Kappe, C.O.; Cantillo, D. Translating Batch Electrochemistry to Single-Pass Continuous Flow Conditions: An Organic Chemist’s Guide. J. Flow Chem. 2020, 10, 181–190. [Google Scholar] [CrossRef]
- Lodh, J.; Paul, S.; Sun, H.; Song, L.; Schöfberger, W.; Roy, S. Electrochemical Organic Reactions: A Tutorial Review. Front. Chem. 2023, 10, 956502. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. 76. Concerted Nature of 1,3-Dipolar Cycloadditions and the Question of Diradical Intermediates. J. Org. Chem. 1976, 41, 403–419. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Wirtanen, T.; Rodrigo, E.; Waldvogel, S.R. Selective and Scalable Electrosynthesis of 2H-2-(Aryl)-benzo[d]-1,2,3-triazoles and Their N-Oxides by Using Leaded Bronze Cathodes. Chem.-A Eur. J. 2020, 26, 5592–5597. [Google Scholar] [CrossRef]
- Krishnan, M.; Kathiresan, M.; Praveen, C. Electrochemically Generated Copper(I)-Catalyzed Click Chemistry: Triazole Synthesis and Insights into Their Photophysical Properties. Eur. J. Org. Chem. 2023, 26, e202201405. [Google Scholar] [CrossRef]
- Carre-Rangel, L.; Espinoza, K.; Oropeza-Guzmán, M.; Rivero, I. Synthesis of Triazoles by Electro-Assisted Click Reaction Using a Copper Foil Electrode. J. Braz. Chem. Soc. 2021, 32, 1373–1380. [Google Scholar] [CrossRef]
- Vishwanath, D.; Xi, Z.; Ravish, A.; Mohan, A.; Basappa, S.; Krishnamurthy, N.P.; Gaonkar, S.L.; Pandey, V.; Lobie, P.E.; Basappa, B. Electrochemical Synthesis of New Isoxazoles and Triazoles Tethered with Thiouracil Base as Inhibitors of Histone Deacetylases in Human Breast Cancer Cells. Molecules 2023, 28, 5254. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Feng, P.; Zhang, Y.; Wang, F.; Wang, G.; Fei, H. Scutebarbatine A Induces ROS-Mediated DNA Damage and Apoptosis in Breast Cancer Cells by Modulating MAPK and EGFR/Akt Signaling Pathway. Chem.-Biol. Interact. 2023, 378, 110487. [Google Scholar] [CrossRef]
- Basappa; Kavitha, C.V.; Rangappa, K.S. Simple and an Efficient Method for the Synthesis of 1-[2-Dimethylamino-1-(4-Methoxy-Phenyl)-Ethyl]-Cyclohexanol Hydrochloride: (±) Venlafaxine Racemic Mixtures. Bioorg. Med. Chem. Lett. 2004, 14, 3279–3281. [Google Scholar] [CrossRef]
- Basappa, B.; Chumadathil Pookunoth, B.; Shinduvalli Kempasiddegowda, M.; Knchugarakoppal Subbegowda, R.; Lobie, P.E.; Pandey, V. Novel Biphenyl Amines Inhibit Oestrogen Receptor (ER)-α in ER-Positive Mammary Carcinoma Cells. Molecules 2021, 26, 783. [Google Scholar] [CrossRef]
- Bharathkumar, H.; Mohan, C.D.; Ananda, H.; Fuchs, J.E.; Li, F.; Rangappa, S.; Surender, M.; Bulusu, K.C.; Girish, K.S.; Sethi, G.; et al. Microwave-Assisted Synthesis, Characterization and Cytotoxic Studies of Novel Estrogen Receptor α Ligands towards Human Breast Cancer Cells. Bioorg. Med. Chem. Lett. 2015, 25, 1804–1807. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A Semiempirical Free Energy Force Field with Charge-Based Desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- BIOVIA Dassault Systèmes. Discovery Studio Visualizer, 21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Schrödinger, L.L.C.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 8 July 2022).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]



| Entry | R | R1 | MCF-7 IC50 (μM) |
|---|---|---|---|
| 8a | –H | –Cl | 5.29 |
| 8b | –H | –Br | 15.25 |
| 8c | –H | –OMe | 138.60 |
| 8d | –H | –Me | 126.80 |
| 8e | –OMe | –Cl | 172.30 |
| 8f | –OMe | –Br | >100 |
| 8g | –OMe | –OMe | 214.90 |
| 8h | –OMe | –OH | 41.87 |
| 8i | –OMe | –Me | 71.89 |
| 8j | –F | –Cl | >100 |
| 8k | –F | –Br | >100 |
| 8l | –F | –OMe | >100 |
| 8m | –F | –Me | >100 |
| Entry | R | MCF-7 IC50 (μM) |
|---|---|---|
| 17a | –4COOEt | >100 |
| 17b | –4COOBu | >100 |
| 17c | –4COOMe | >100 |
| 17d | –3COOEt | >100 |
| 17e | –3COOBu | >100 |
| 17f | –3COOMe | >100 |
| Entry | R | MCF-7 IC50 (μM) |
|---|---|---|
| 21a | –4COOBu | >100 |
| 21b | –4COOEt | >100 |
| 21c | –4COOMe | >100 |
| 21d | –3COOEt | >100 |
| 21e | –3COOMe | >100 |
| 21f | –3COOBu | >100 |
| Compound | Binding Scores (kcal/mol) | Interactions | Residue | Bond Length Å |
|---|---|---|---|---|
| 8a | −8.44 | Hydrogen π-cation π-anion | ASP-1046 GLU-885 LYS-868 | 2.71 3.27 4.05 |
| Hydrophobic | LEU-840 VAL-848 ALA-866 VAL-899 VAL-916 LEU-1019 HIS-1026 CYS-1045 PHE-1047 | 5.10 4.62, 4.54 4.22 4.88 4.94.4.50 4.95 4.59 5.45 4.55 | ||
| 8b | −8.90 | Hydrogen π-cation π-anion | LYS-868, LEU-1049 LYS-868 GLU-885 | 1.96, 2.14 3.95 3.64 |
| Hydrophobic | ALA-881 ILE-888 VAL-889 VAL-899 VAL-916 CYS-1045 | 4.77 4.58 3.68 5.00 4.48 5.49 | ||
| Pazopanib | −9.25 | Hydrogen π-cation π-anion | ASP-1028, ASP-1046 HIS-1026 HIS-1026 | 1.97, 2.29 4.73 4.90 |
| Hydrophobic | LEU-889 LEU-899 LEU-1019 ARG-1027 ILE-1044 CYS-1045 TYR-1059 LEU-1067 | 4.86 4.39 4.73 4.49 4.58 5.01 4.79 4.86 | ||
| Co-crystal ligand (Sorafenib) | −11.15 | Hydrogen | ASP-1046, GLU-885, CYS-919 | 1.84, 1.93, and 2.42 2.20, and 2.48 |
| Halogen (Fluorine) Hydrophobic | ILE-1044 VAL-848 ALA-866 LYS-868 LEU-889 ILE-892 VAL-899 VAL-916 PHE-918 LEU-1019 ILE-1044 CYS-1045 | 2.82 5.13 3.94 5.33 3.45 4.78 5.05 4.81 4.73 4.20 5.13 4.64 |
| Query | Liver Toxicity | Metabolism | Membrane Transporters | Others | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYP Inhibitors | |||||||||||||||
| DILI | CT | HLM | 1A2 | 3A4 | 2D6 | 2C9 | 2C19 | BBB | Pgp In | Pgp Sub | hERG | MMP | AMES | MRTD (mg/day) | |
| 8a | Y | N | Y | N | N | N | N | N | N | N | N | Y | N | Y | 244 |
| 8b | Y | N | Y | N | N | N | N | N | Y | N | N | N | N | Y | 274 |
| TAM | Y | N | N | N | Y | N | N | N | Y | Y | Y | Y | N | N | 161 |
| DOX | N | Y | Y | N | N | N | N | N | N | N | Y | N | Y | Y | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravish, A.; Siddappa, T.P.; Xi, Z.; Vishwanath, D.; Mohan, A.; Basappa, S.; Krishnamurthy, N.P.; Lobie, P.E.; Pandey, V.; Basappa, B. Electrochemical Synthesis of Versatile Pyrimidine and Oxadiazoles Tethered Triazoles as Inhibitors of VEGFR-2 in Human Breast Cancer Cells. Catalysts 2023, 13, 1353. https://doi.org/10.3390/catal13101353
Ravish A, Siddappa TP, Xi Z, Vishwanath D, Mohan A, Basappa S, Krishnamurthy NP, Lobie PE, Pandey V, Basappa B. Electrochemical Synthesis of Versatile Pyrimidine and Oxadiazoles Tethered Triazoles as Inhibitors of VEGFR-2 in Human Breast Cancer Cells. Catalysts. 2023; 13(10):1353. https://doi.org/10.3390/catal13101353
Chicago/Turabian StyleRavish, Akshay, Tejaswini P. Siddappa, Zhang Xi, Divakar Vishwanath, Arunkumar Mohan, Shreeja Basappa, Niranjan Pattehalli Krishnamurthy, Peter E. Lobie, Vijay Pandey, and Basappa Basappa. 2023. "Electrochemical Synthesis of Versatile Pyrimidine and Oxadiazoles Tethered Triazoles as Inhibitors of VEGFR-2 in Human Breast Cancer Cells" Catalysts 13, no. 10: 1353. https://doi.org/10.3390/catal13101353
APA StyleRavish, A., Siddappa, T. P., Xi, Z., Vishwanath, D., Mohan, A., Basappa, S., Krishnamurthy, N. P., Lobie, P. E., Pandey, V., & Basappa, B. (2023). Electrochemical Synthesis of Versatile Pyrimidine and Oxadiazoles Tethered Triazoles as Inhibitors of VEGFR-2 in Human Breast Cancer Cells. Catalysts, 13(10), 1353. https://doi.org/10.3390/catal13101353








