Au-Deposited Ce0.5Zr0.5O2 Nanostructures for Photocatalytic H2 Production under Visible Light
Abstract
:1. Introduction
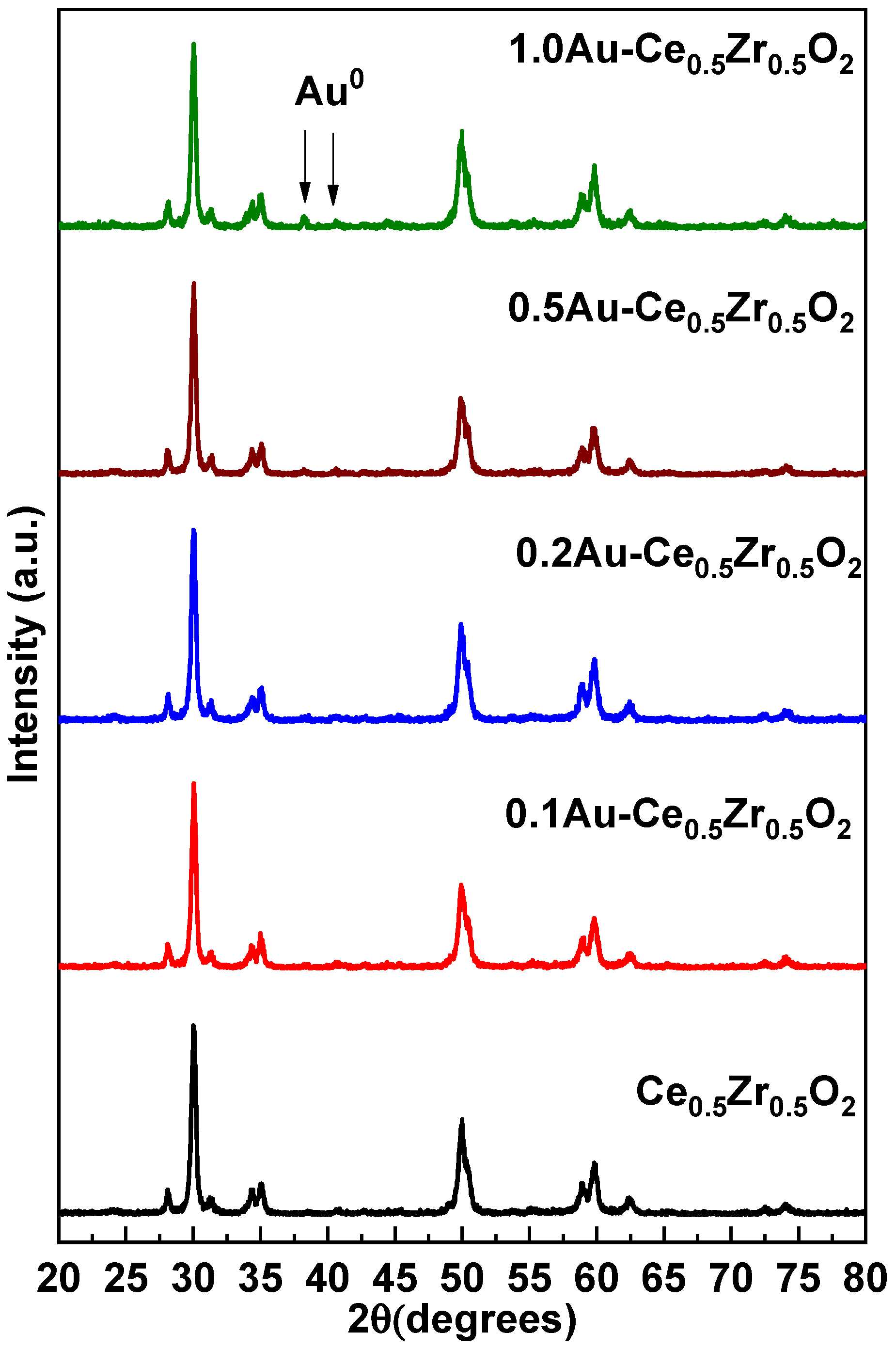
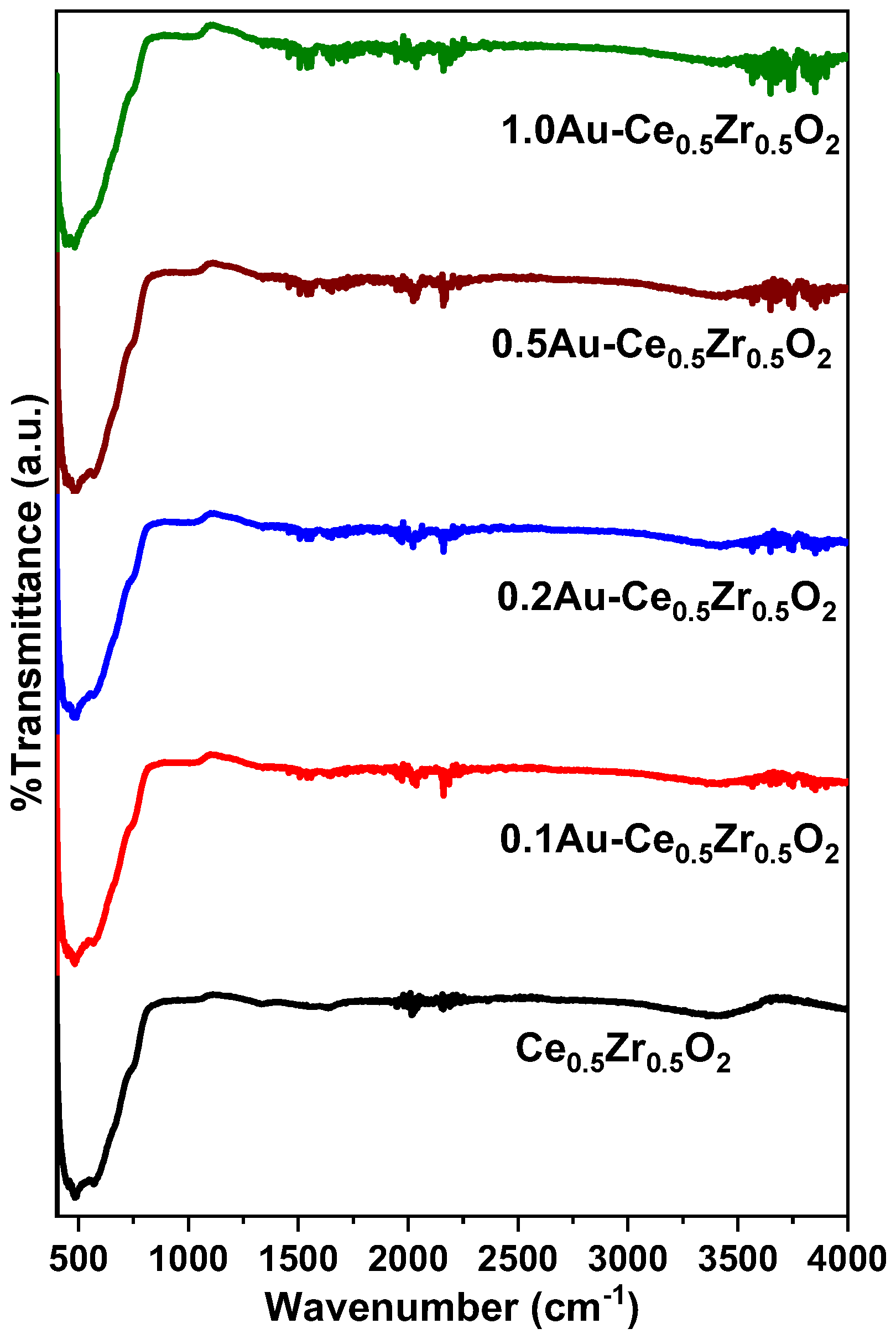
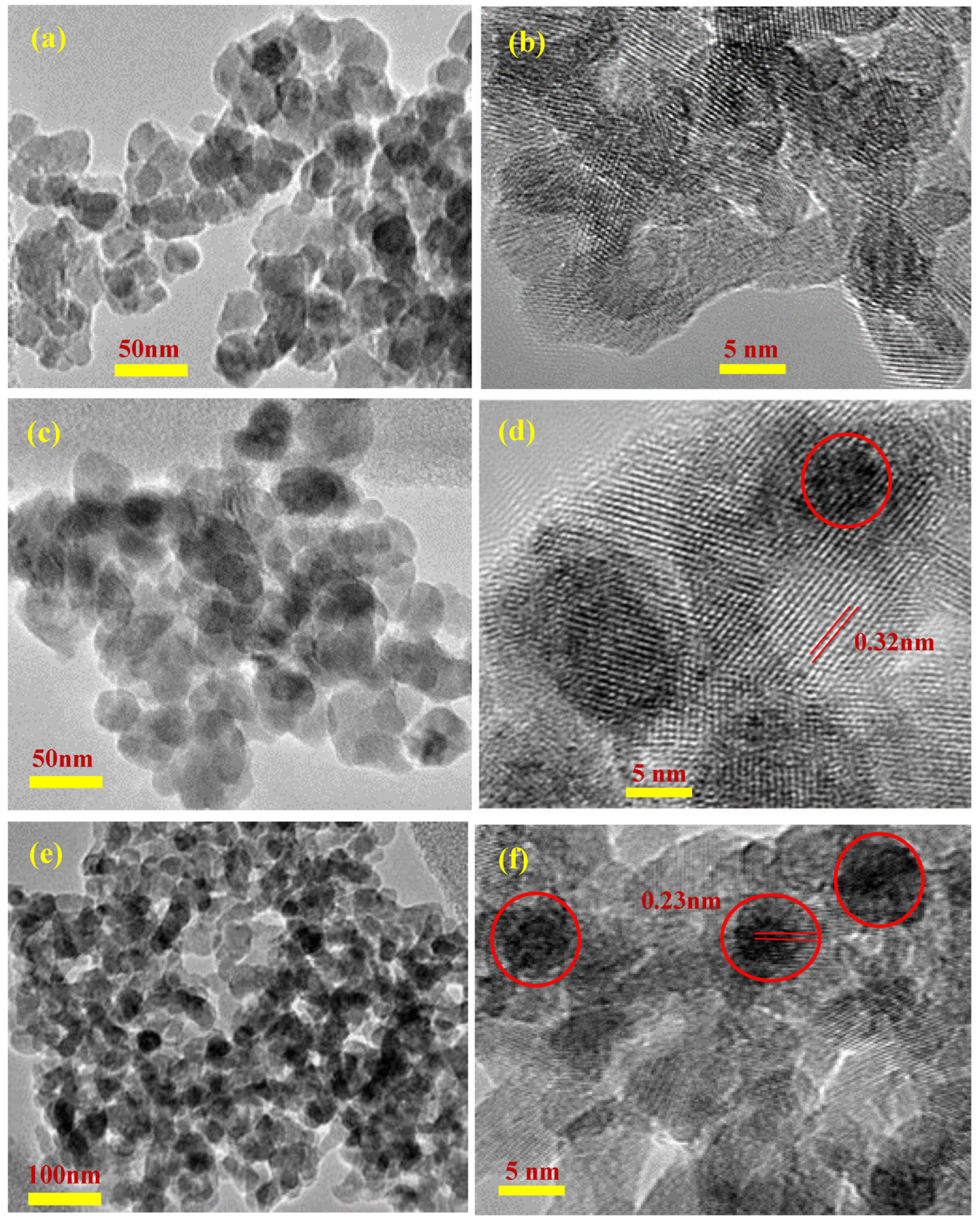
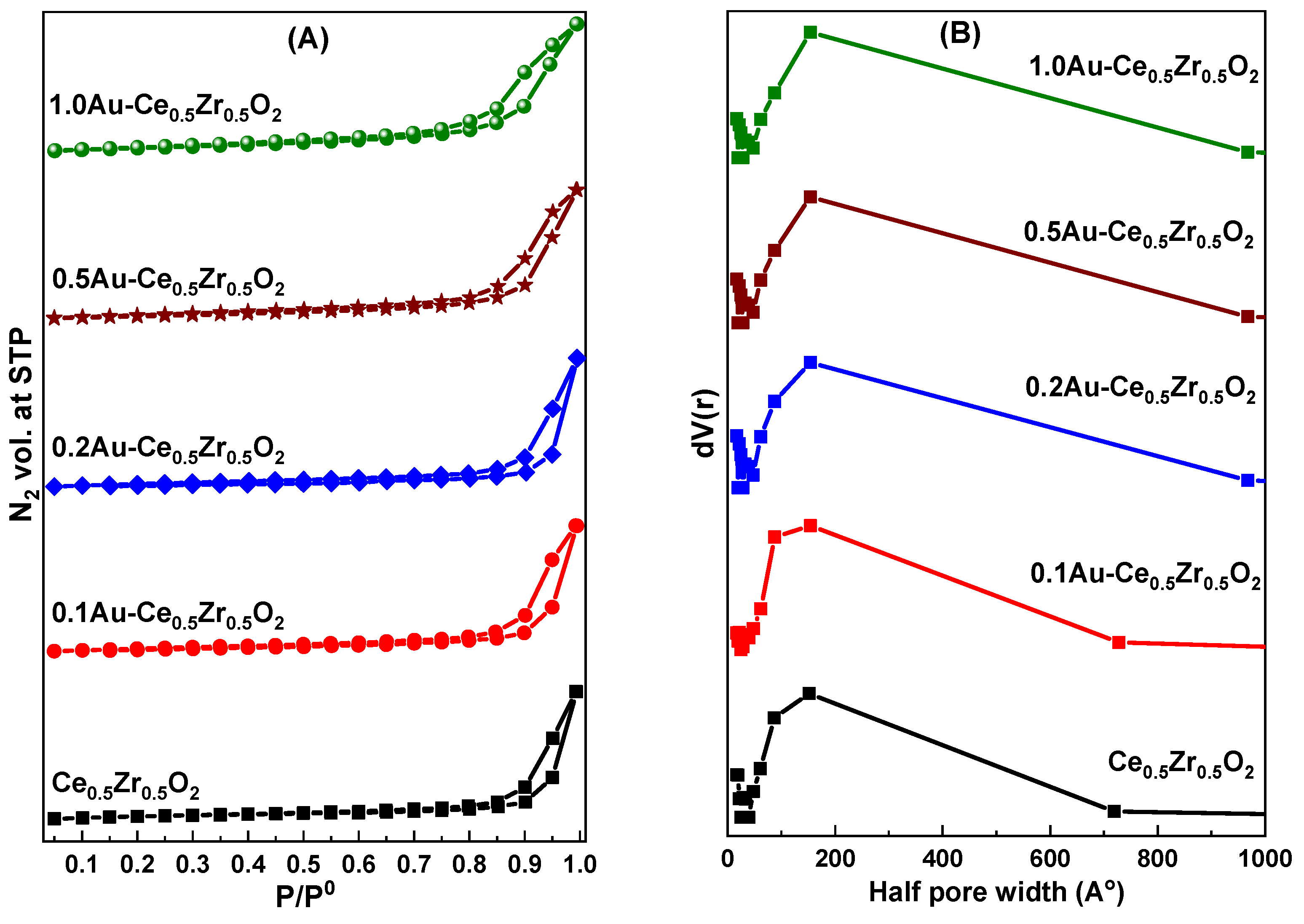
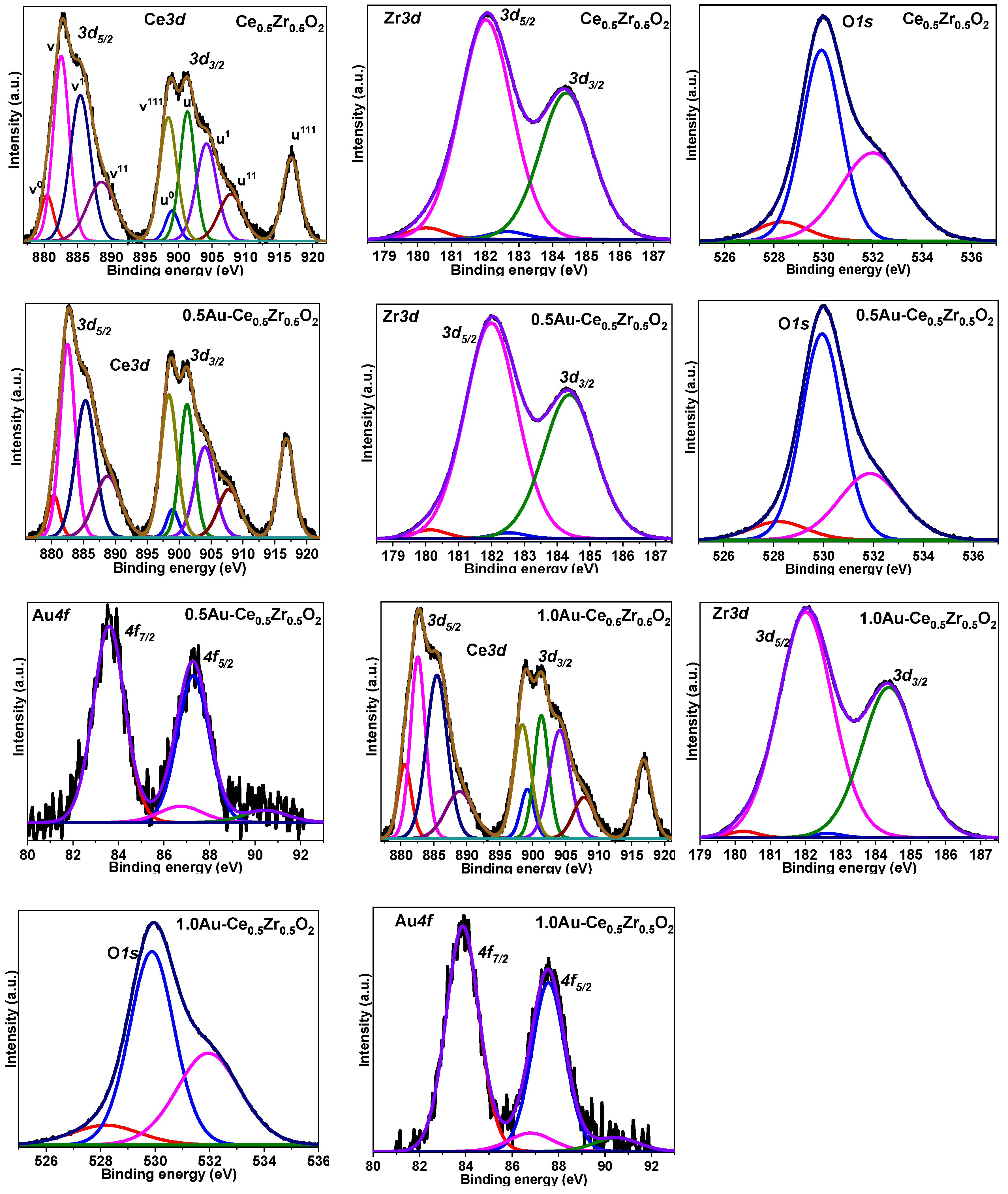
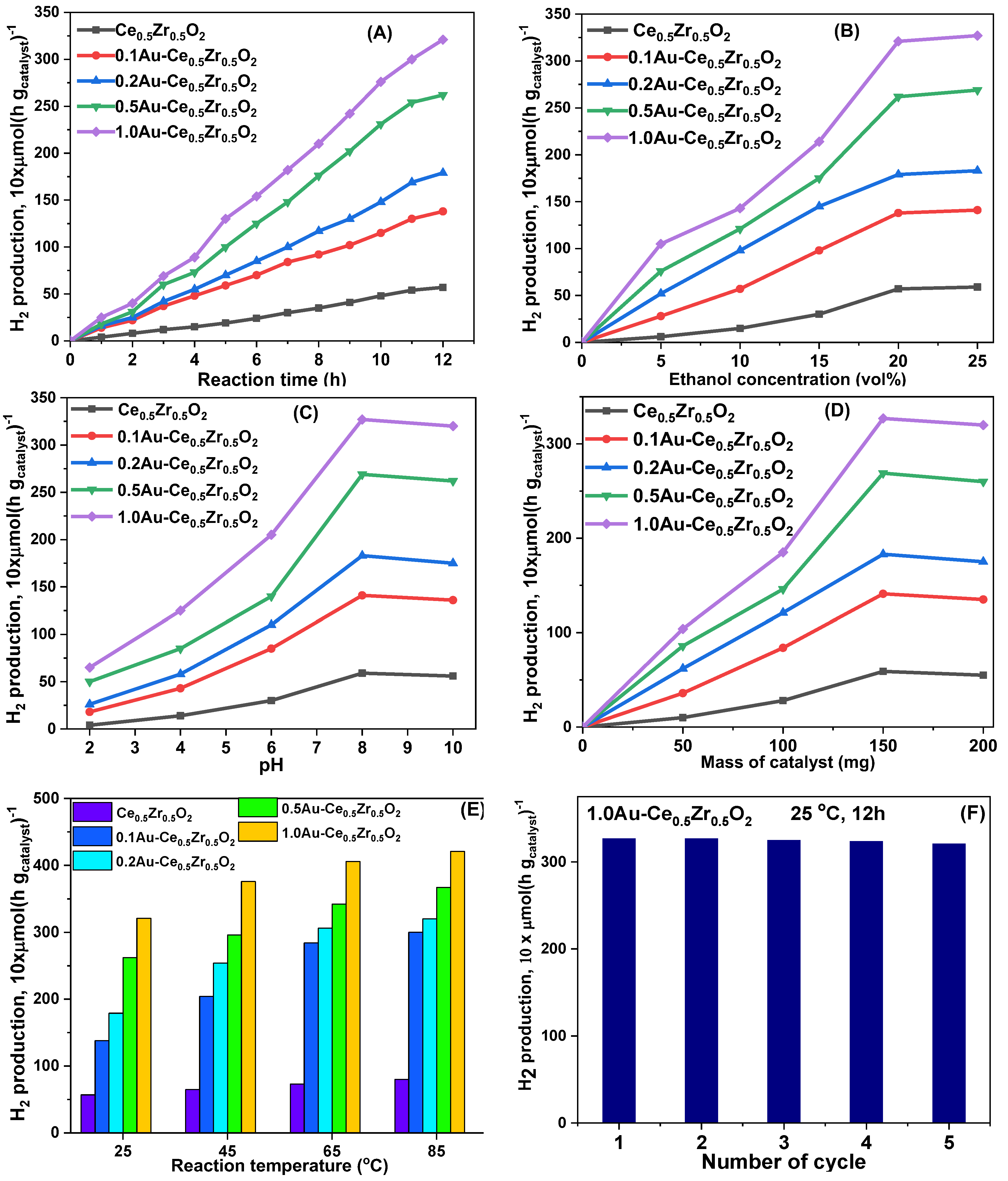
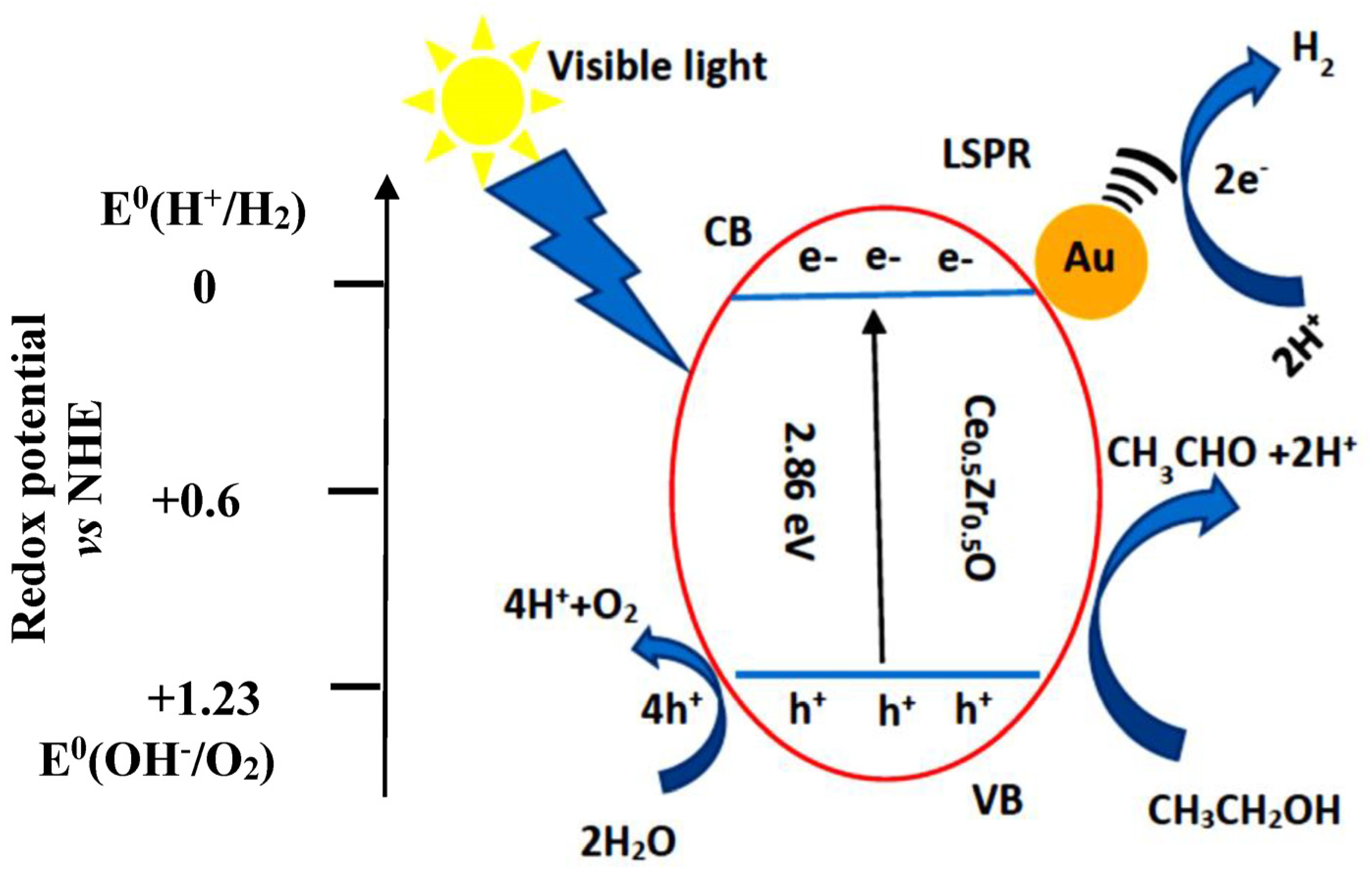
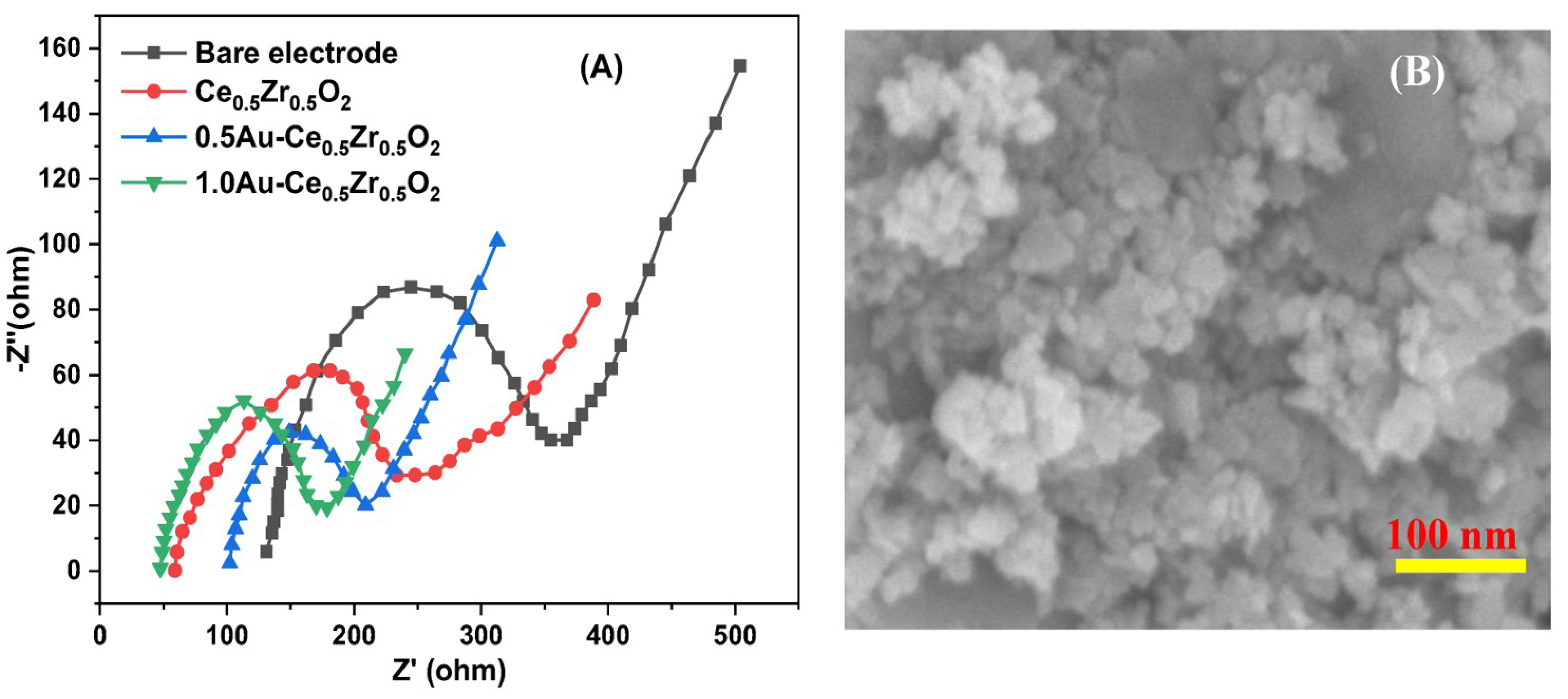
2. Results and Discussion
3. Experimental Section
3.1. Preparation of Catalysts
3.1.1. Ce0.5Zr0.5O2 Nanoparticles
3.1.2. Au-Deposited Ce0.5Zr0.5O2 Samples
3.2. Characterization of Synthesized Nanomaterials
3.3. Photocatalytic Reforming of Bioethanol to Hydrogen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.; Xue, W.; Hu, X.; Fan, J.; Tang, C.; Shi, Y.; Liu, E.; Sun, T. A S-scheme heterojunction of Co9S8 decorated TiO2 for enhanced photocatalytic H2 evolution. J. Alloys Compd. 2023, 930, 167368. [Google Scholar] [CrossRef]
- Li, T.; Tsubaki, N.; Jin, Z. S-scheme heterojunction in photocatalytic hydrogen production. J. Mater. Sci. Technol. 2024, 169, 82–104. [Google Scholar] [CrossRef]
- Idriss, H.; Scott, M.; Llorca, J.; Chan, S.C.; Chiu, W.; Sheng, P.-Y.; Yee, A.; Blackford, M.A.; Pas, S.J.; Hill, A.J.; et al. A Phenomenological Study of the Metal-Oxide Interface: The Role of Catalysis in Hydrogen Production from Renewable Resources. ChemSusChem 2008, 1, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy-A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Zhang, J.; Lin, W.; Wang, J.; Xu, Y.; Xiang, Y.; Hisatomi, T.; Domen, K.; Ma, G. Synthesis of Narrow-Band-Gap GaN:ZnO Solid Solution for Photocatalytic Overall Water Splitting. ACS Catal. 2022, 12, 14637–14646. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Zhang, L.; Li, N.; Lou, Z.; Ma, T.; Zhang, P.; Li, Y.; Li, X. W–N Bonds Precisely Boost Z-Scheme Interfacial Charge Transfer in g-C3N4/WO3 Heterojunctions for Enhanced Photocatalytic H2 Evolution. ACS Catal. 2022, 12, 9994–10003. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, Y.; Liu, F.; Tong, Z.; Sha, J.; Zhao, W.; Liu, M.; Zheng, Y. Seeded Growth of Au@CuxO Core–Shell Mesoporous Nanospheres and Their Photocatalytic Properties. Front. Chem. 2021, 9, 671220. [Google Scholar] [CrossRef] [PubMed]
- Naz, G.; Shamsuddin, M.; Butt, F.K.; Bajwa, S.Z.; Khan, W.S.; Irfan, M.; Irfan, M. Au/Cu2O core/shell nanostructures with efficient photoresponses. Chin. J. Phys. 2019, 59, 307–316. [Google Scholar] [CrossRef]
- Fu, X.; Li, G.G.; Villarreal, E.; Wang, H. Hot carriers in action: Multimodal photocatalysis on Au@SnO2 core–shell nanoparticles. Nanoscale 2019, 11, 7324–7334. [Google Scholar] [CrossRef]
- Yang, L.; Guo, S.; Li, X. Au nanoparticles@MoS2 core-shell structures with moderate MoS2 coverage for efficient photocatalytic water splitting. J. Alloys Compd. 2017, 706, 82–88. [Google Scholar] [CrossRef]
- Lv, Y.; Duan, S.; Zhu, Y.; Guo, H.; Wang, R. Interface control and catalytic performances of Au-NiS heterostructures. Chem. Eng. J. 2019, 382, 122794. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.I.N.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, X.; Cao, J.; Wu, S.; Yan, W.; Liu, G. Plasmonic Au nanoparticles embedding enhances the activity and stability of CdS for photocatalytic hydrogen evolution. Chem. Commun. 2016, 52, 2394–2397. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Calvo, P.; Caps, V.; Keller, V. Plasmonic Au-based junctions onto TiO2, g-C3N4, and TiO2-g-C3N4 systems for photocatalytic hydrogen production: Fundamentals and challenges. Renew. Sustain. Energy Rev. 2021, 149, 111095. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- García-López, E.; Marcì, G.; Pomilla, F.; Paganini, M.; Gionco, C.; Giamello, E.; Palmisano, L. ZrO2 Based materials as photocatalysts for 2-propanol oxidation by using UV and solar light irradiation and tests for CO2 reduction. Catal. Today 2018, 313, 100–105. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, B.; Yang, M.; Han, W.; Shao, X. ZrO2/CeO2 nanocomposite: Two step synthesis, microstructure, and visible-light photocatalytic activity. Mater. Lett. 2013, 112, 90–93. [Google Scholar] [CrossRef]
- Reddy, B.M.; Reddy, G.K.; Katta, L. Structural characterization and dehydration activity of CeO2–SiO2 and CeO2–ZrO2 mixed oxides prepared by a rapid microwave-assisted combustion synthesis method. J. Mol. Catal. A Chem. 2010, 319, 52–57. [Google Scholar] [CrossRef]
- Hao, Y.; Li, L.; Liu, D.; Yu, H.; Zhou, Q. The synergy of SPR effect and Z-scheme of Ag on enhanced photocatalytic performance of 3DOM Ag/CeO2-ZrO2 composite. Mol. Catal. 2018, 447, 37–46. [Google Scholar] [CrossRef]
- Narasimharao, K.; Malkhasian, A.Y. Ag/Ce0.5Zr0.5O2 nanofibers: Visible light photocatalysts for degradation of p-nitrophenol. Mol. Catal. 2022, 524, 112309. [Google Scholar] [CrossRef]
- Bo, Z.J.; Lintao; Maochu, G.; Li, W.J.; Min, L.Z.; Ming, Z.; Chen, Y. Effect of metal doping into Ce0.5Zr0.5O2 on photocatalytic activity of TiO2/Ce0.45Zr0.45M0.1OX (M=Y, La, Mn). J. Hazard. Mater. 2007, 143, 516–521. [Google Scholar] [CrossRef]
- Tesler, A.B.; Chuntonov, L.; Karakouz, T.; Bendikov, T.A.; Haran, G.; Vaskevich, A.; Rubinstein, I. Tunable Localized Plasmon Transducers Prepared by Thermal Dewetting of Percolated Evaporated Gold Films. J. Phys. Chem. C 2011, 115, 24642–24652. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative Methods for the Preparation of Gold Nanoparticles Supported on TiO2. J. Phys. Chem. B 2002, 106, 7634–7642. [Google Scholar] [CrossRef]
- Haruta, M. Catalysis of Gold Nanoparticles Deposited on Metal Oxides. CATTECH 2002, 6, 102–115. [Google Scholar] [CrossRef]
- Vasylkiv, O.; Sakka, Y. Nonisothermal Synthesis of Yttria-Stabilized Zirconia Nanopowder through Oxalate Processing: I, Characteristics of Y-Zr Oxalate Synthesis and Its Decomposition. J. Am. Ceram. Soc. 2004, 83, 2196–2202. [Google Scholar] [CrossRef]
- Katta, L.; Sudarsanam, P.; Thrimurthulu, G.; Reddy, B.M. Doped nanosized ceria solid solutions for low temperature soot oxidation: Zirconium versus lanthanum promoters. Appl. Catal. B Environ. 2010, 101, 101–108. [Google Scholar] [CrossRef]
- Thompson, D. Report on AURICAT Workshop, Gandia, Spain, 11–13 May 2005. Gold Bull. 2005, 38, 135–137. [Google Scholar] [CrossRef]
- Prasad, P.D.H.; Park, S.Y.; Ji, H.I.; Kim, H.R.; Son, J.W.; Kim, B.K.; Lee, H.W.; Lee, J.H. Structural Characterization and Catalytic Activity of Ce0.65Zr0.25RE0.1O2−δ Nanocrystalline Powders Synthesized by the Glycine-Nitrate Process. J. Phys. Chem. C 2012, 116, 3467–3476. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Zhao, S.; Wang, S.; Wang, A.; Hu, Y. CeO2–ZrO2 promoted CuO/ZnO catalyst for methanol steam reforming. Int. J. Hydrogen Energy 2013, 38, 4397–4406. [Google Scholar] [CrossRef]
- Babitha, K.K.; Sreedevi, A.; Priyanka, K.P.; Sabu, B.; Varghese, T. Structural characterization and optical studies of CeO2 nanoparticles synthesized by chemical precipitation. Indian J. Pure Appl. Phys. 2015, 53, 596–603. Available online: http://nopr.niscpr.res.in/handle/123456789/32128 (accessed on 4 September 2023).
- Castillo-López, D.N.; Pal, U. Green synthesis of Au nanoparticles using potato extract: Stability and growth mechanism. J. Nanoparticle Res. 2014, 16, 2571. [Google Scholar] [CrossRef]
- Rao, G.R.; Sahu, H.R. XRD and UV-Vis diffuse reflectance analysis of CeO2-ZrO2 solid solutions synthesized by combustion method. J. Chem. Sci. 2001, 113, 651–658. [Google Scholar] [CrossRef]
- Nandi, M.; Talukdar, A.K. Ceria–zirconia solid solution loaded hierarchical MFI zeolite: An efficient catalyst for solvent free oxidation of ethyl benzene. Arab. J. Chem. 2019, 12, 3753–3763. [Google Scholar] [CrossRef]
- Amoresi, R.A.C.; Oliveira, R.C.; Marana, N.L.; de Almeida, P.B.; Prata, P.S.; Zaghete, M.A.; Longo, E.; Sambrano, J.R.; Simões, A.Z. CeO2 Nanoparticle Morphologies and Their Corresponding Crystalline Planes for the Photocatalytic Degradation of Organic Pollutants. ACS Appl. Nano Mater. 2019, 2, 6513–6526. [Google Scholar] [CrossRef]
- Xuan, Y.; Duan, H.; Li, Q. Enhancement of solar energy absorption using a plasmonic nanofluid based on TiO2/Ag composite nanoparticles. RSC Adv. 2014, 4, 16206–16213. [Google Scholar] [CrossRef]
- Kurochkina, M.; Konshina, E.; Oseev, A.; Hirsch, S. Hybrid structures based on gold nanoparticles and semiconductor quantum dots for biosensor applications. Nanotechnol. Sci. Appl. 2018, 11, 15–21. [Google Scholar] [CrossRef]
- Gaspar, D.; Pimentel, A.C.; Mateus, T.; Leitão, J.P.; Soares, J.; Falcão, B.P.; Araújo, A.; Vicente, A.; Filonovich, S.A.; Águas, H.; et al. Influence of the layer thickness in plasmonic gold nanoparticles produced by thermal evaporation. Sci. Rep. 2013, 3, 1469. [Google Scholar] [CrossRef]
- Antony, J.; Bandyopadhyay, S.; Yang, J.; Rønning, M. Optimizing the shape anisotropy of gold nanoparticles for enhanced light harvesting and photocatalytic applications. Photochem. Photobiol. Sci. 2022, 22, 773–781. [Google Scholar] [CrossRef]
- Abed, J.; Rajput, N.S.; El Moutaouakil, A.; Jouiad, M. Recent Advances in the Design of Plasmonic Au/TiO2 Nanostructures for Enhanced Photocatalytic Water Splitting. Nanomaterials 2020, 10, 2260. [Google Scholar] [CrossRef]
- Rahulan, K.M.; Ganesan, S.; Aruna, P. Synthesis and optical limiting studies of Au-doped TiO2 nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 025012–025017. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Słoczyński, J.; Grabowski, R.; Kozłowska, A.; Olszewski, P.; Lachowska, M.; Skrzypek, J.; Stoch, J. Effect of Mg and Mn oxide additions on structural and adsorptive properties of Cu/ZnO/ZrO2 catalysts for the methanol synthesis from CO2. Appl. Catal. A Gen. 2003, 249, 129–138. [Google Scholar] [CrossRef]
- Wandelt, K. Photoemission studies of adsorbed oxygen and oxide layers. Surf. Sci. Rep. 1982, 2, 1–121. [Google Scholar] [CrossRef]
- Dongare, M.K.; Dongare, A.M.; Tare, V.; Kemnitz, E. Synthesis and characterization of copper-stabilized zirconia as an anode material for SOFC. Solid State Ionics 2002, 152–153, 455–462. [Google Scholar] [CrossRef]
- Navío; Hidalgo; Colón, G.; Botta, S.G.; Litter, M.I. Preparation and Physicochemical Properties of ZrO2 and Fe/ZrO2 Prepared by a Sol−Gel Technique. Langmuir 2000, 17, 202–210. [Google Scholar] [CrossRef]
- Wu, G.-S.; Wang, L.-C.; Liu, Y.-M.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Implication of the role of oxygen anions and oxygen vacancies for methanol decomposition over zirconia supported copper catalysts. Appl. Surf. Sci. 2006, 253, 974–982. [Google Scholar] [CrossRef]
- Reddy, B.M.; Thrimurthulu, G.; Katta, L.; Yamada, Y.; Park, S.-E. Structural Characteristics and Catalytic Activity of Nanocrystalline Ceria−Praseodymia Solid Solutions. J. Phys. Chem. C 2009, 113, 15882–15890. [Google Scholar] [CrossRef]
- Shen, M.; Ding, T.; Rackers, W.H.; Tan, C.; Mahmood, K.; Lew, M.D.; Sadtler, B. Single-Molecule Colocalization of Redox Reactions on Semiconductor Photocatalysts Connects Surface Heterogeneity and Charge-Carrier Separation in Bismuth Oxybromide. J. Am. Chem. Soc. 2021, 143, 11393–11403. [Google Scholar] [CrossRef]
- Topka, P.; Klementová, M. Total oxidation of ethanol over Au/Ce0.5Zr0.5O2 cordierite monolithic catalysts. Appl. Catal. A Gen. 2016, 522, 130–137. [Google Scholar] [CrossRef]
- Sola, A.C.; Homs, N.; de la Piscina, P.R. Photocatalytic H2 production from ethanol (aq) solutions: The effect of intermediate products. Int. J. Hydrog. Energy 2016, 41, 19629–19636. [Google Scholar] [CrossRef]
- Behravesh, E.; Melander, M.M.; Wärnå, J.; Salmi, T.; Honkala, K.; Murzin, D.Y. Oxidative dehydrogenation of ethanol on gold: Combination of kinetic experiments and computation approach to unravel the reaction mechanism. J. Catal. 2020, 394, 193–205. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO 2 nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Alqurashi, G.K.; Alshehri, A.; Narasimharao, K. Noble Metals (Ag, Au, Pd and Pt) Decorated ZnBiVO4 Nanostructures for Enhanced Photocatalytic H2 Production. Top. Catal. 2022, 66, 533–545. [Google Scholar] [CrossRef]
- Althabaiti, S.A.; Khan, Z.; Bawaked, S.M.; Al-Sheheri, S.Z.; Mokhtar, M.; Malik, M.A.; Narasimharao, K. PtOx deposited Fe3O4-ZnO/TiO2 nanocomposites for photocatalytic H2 production under visible light. J. Environ. Chem. Eng. 2023, 11, 110615. [Google Scholar] [CrossRef]
- Bartolomé, J.; Taeño, M.; Martínez-Casado, R.; Maestre, D.; Cremades, A. Ethanol gas sensing mechanisms of p-type NiO at room temperature. Appl. Surf. Sci. 2022, 579, 152134. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, M.; Guan, Z.; Li, Q.; Yang, J. Effect of platinum dispersion on photocatalytic performance of Pt-TiO2. J. Nanoparticle Res. 2018, 20, 60. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Malik, M.A.; Narasimharao, K. Pt-Ag/Ag3PO4-WO3 nanocomposites for photocatalytic H2 production from bioethanol. Fuel 2023, 344, 127998. [Google Scholar] [CrossRef]
- Ramírez-Ortega, D.; Guerrero-Araque, D.; Acevedo-Peña, P.; Reguera, E.; Calderon, H.A.; Zanella, R. Enhancing the photocatalytic hydrogen production of the ZnO-TiO2 heterojunction by supporting nanoscale Au islands. Int. J. Hydrogen Energy 2021, 46, 34333–34343. [Google Scholar] [CrossRef]
- Van Dao, D.; Nguyen, T.T.D.; Le, T.D.; Kim, S.-H.; Yang, J.-K.; Lee, I.-H.; Yu, Y.-T. Plasmonically driven photocatalytic hydrogen evolution activity of a Pt-functionalized Au@CeO2 core–shell catalyst under visible light. J. Mater. Chem. A 2020, 8, 7687–7694. [Google Scholar] [CrossRef]
- Alebachew, N.; Murthy, H.C.A.; Gonfa, B.A.; von Eschwege, K.G.; Langner, E.H.G.; Coetsee, E.; Demissie, T.B. Nanocomposites with ZrO2@S-Doped g-C3N4 as an Enhanced Binder-Free Sensor: Synthesis and Characterization. ACS Omega 2023, 8, 13775–13790. [Google Scholar] [CrossRef] [PubMed]
- Bamwenda, G.R.; Tsubota, S.; Nakamura, T.; Haruta, M. Photoassisted hydrogen production from a water-ethanol solution: A comparison of activities of Au-TiO2 and Pt-TiO2. J. Photochem. Photobiol. A Chem. 1995, 89, 177–189. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, J.; Li, L.; Gong, L.; Zheng, J.; Zhang, L.; Wang, Z.; Zhang, J.; Zhu, Z. Selective oxidation of sacrificial ethanol over TiO2-based photocatalysts during water splitting. Energy Environ. Sci. 2011, 4, 3384–3388. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]

| Sample | Crystal Size (nm) | Cell Parameter (nm) | |
|---|---|---|---|
| Ce0.5Zr0.5O2 | Au | ||
| Ce0.5Zr0.5O2 | 50 | - | a = 0.514 |
| 0.1Au-Ce0.5Zr0.5O2 | 58 | - | a = 0.515 |
| 0.2Au-Ce0.5Zr0.5O2 | 57 | - | a = 0.515 |
| 0.5Au-Ce0.5Zr0.5O2 | 59 | 25 | a = 0.515 |
| 1.0Au-Ce0.5Zr0.5O2 | 59 | 35 | a = 0.516 |
| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Ce0.5Zr0.5O2 | 58 | 0.102 | 1.90 |
| 0.1Au-Ce0.5Zr0.5O2 | 55 | 0.107 | 1.90 |
| 0.2Au-Ce0.5Zr0.5O2 | 51 | 0.110 | 1.95 |
| 0.5Au-Ce0.5Zr0.5O2 | 45 | 0.116 | 2.00 |
| 1.0Au-Ce0.5Zr0.5O2 | 40 | 0.113 | 2.10 |
| Sample | Elemental Composition (Mass %) | ||||||
|---|---|---|---|---|---|---|---|
| Bulk a | Surface b | ||||||
| Ce | Zr | Au | Ce | Zr | Au | O | |
| Ce0.5Zr0.5O2 | 47.6 | 31.1 | - | 45.7 | 30.4 | - | 23.9 |
| 0.1Au-Ce0.5Zr0.5O2 | 47.1 | 31.0 | 0.09 | 45.6 | 30.2 | 0.07 | 23.9 |
| 0.2Au-Ce0.5Zr0.5O2 | 46.6 | 30.7 | 0.18 | 45.5 | 30.6 | 0.17 | 23.8 |
| 0.5Au-Ce0.5Zr0.5O2 | 46.3 | 30.2 | 0.48 | 43.9 | 30.7 | 0.46 | 23.9 |
| 1.0Au-Ce0.5Zr0.5O2 | 45.4 | 30.0 | 0.98 | 43.8 | 30.9 | 0.96 | 23.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Thabaiti, S.A.; Khan, Z.; Al-Thubaiti, K.S.; Bawaked, S.M.; Al-Sheheri, S.Z.; Mokhtar, M.; Malik, M.A.; Narasimharao, K. Au-Deposited Ce0.5Zr0.5O2 Nanostructures for Photocatalytic H2 Production under Visible Light. Catalysts 2023, 13, 1340. https://doi.org/10.3390/catal13101340
Al Thabaiti SA, Khan Z, Al-Thubaiti KS, Bawaked SM, Al-Sheheri SZ, Mokhtar M, Malik MA, Narasimharao K. Au-Deposited Ce0.5Zr0.5O2 Nanostructures for Photocatalytic H2 Production under Visible Light. Catalysts. 2023; 13(10):1340. https://doi.org/10.3390/catal13101340
Chicago/Turabian StyleAl Thabaiti, Shaeel Ahmed, Zaheer Khan, Khloud Saeed Al-Thubaiti, Salem Mohamed Bawaked, Soad Zahir Al-Sheheri, Mohamed Mokhtar, Maqsood Ahmad Malik, and Katabathini Narasimharao. 2023. "Au-Deposited Ce0.5Zr0.5O2 Nanostructures for Photocatalytic H2 Production under Visible Light" Catalysts 13, no. 10: 1340. https://doi.org/10.3390/catal13101340
APA StyleAl Thabaiti, S. A., Khan, Z., Al-Thubaiti, K. S., Bawaked, S. M., Al-Sheheri, S. Z., Mokhtar, M., Malik, M. A., & Narasimharao, K. (2023). Au-Deposited Ce0.5Zr0.5O2 Nanostructures for Photocatalytic H2 Production under Visible Light. Catalysts, 13(10), 1340. https://doi.org/10.3390/catal13101340










