Development and Optimization of Air-Electrodes for Rechargeable Zn–Air Batteries
Abstract
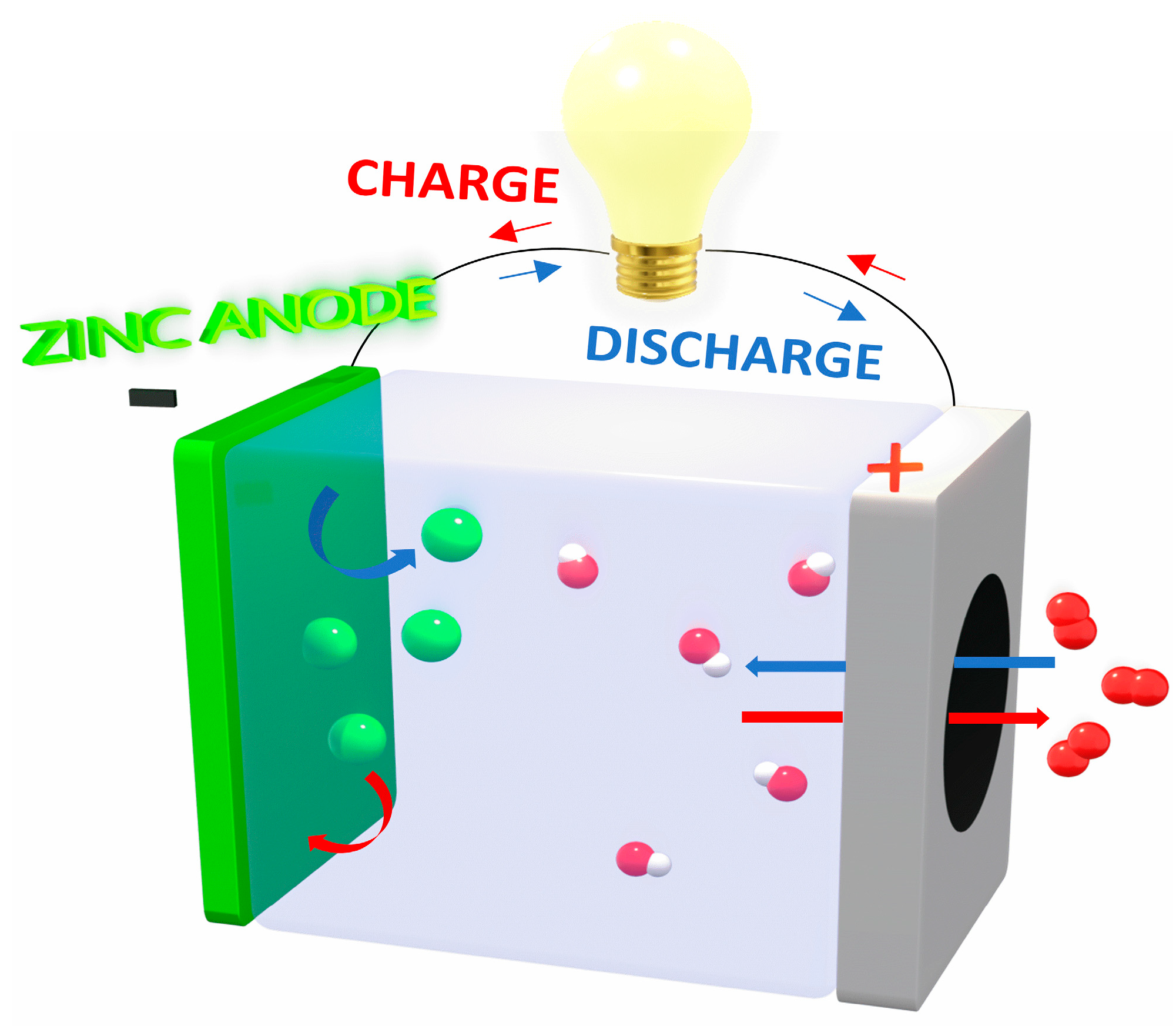
:1. Introduction
1.1. Types of Commercial Gas Diffusion Layers (GDLs) and Their Role
1.2. Current Collectors and Catalyst Layer (CL)
2. Results and Discussion
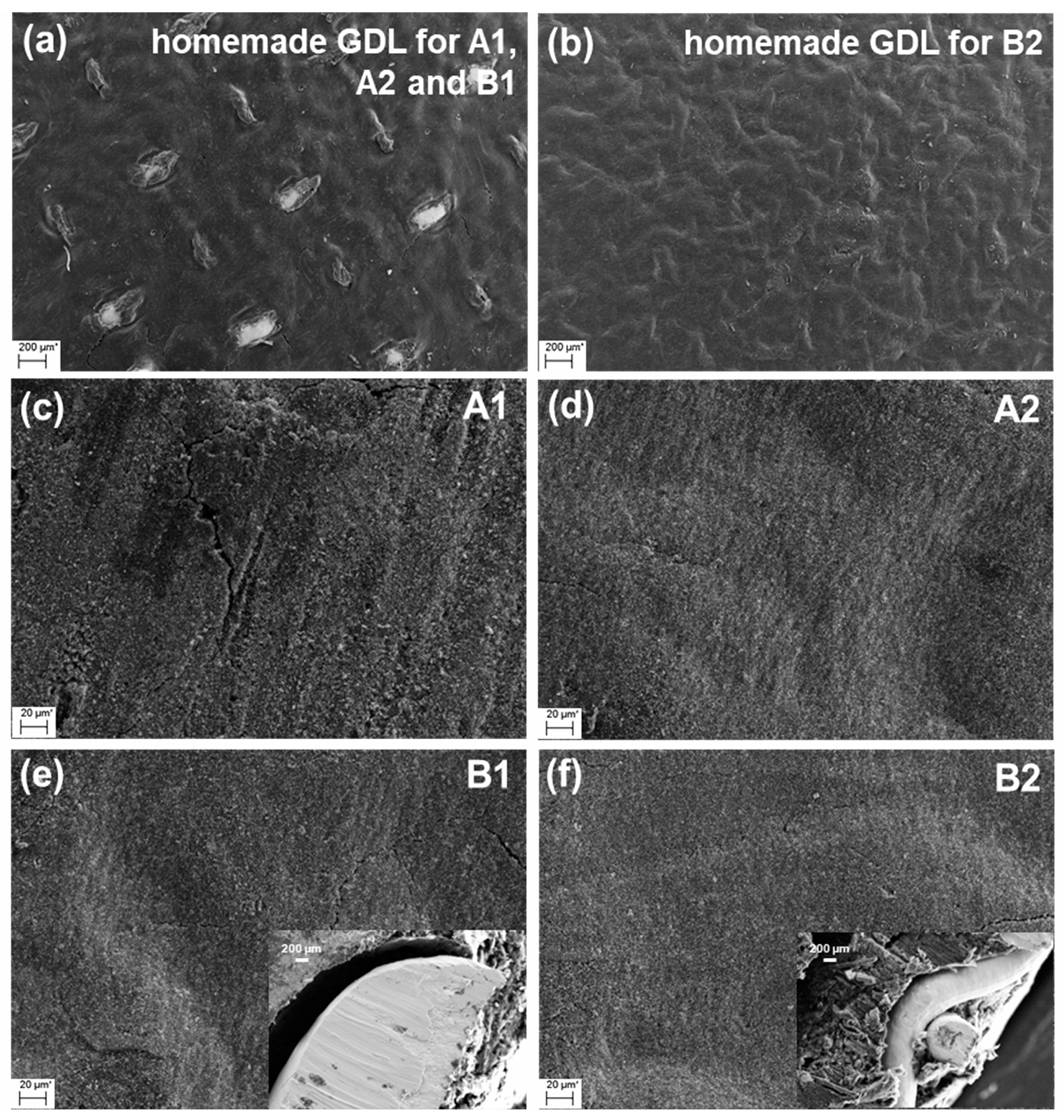
2.1. Morphology of the Prepared Gas Diffusion and Catalyst Layers
2.2. Catalyst Layer Optimization
2.3. Commercial GDL and SS Mesh Pore Size Optimization
3. Materials and Methods
3.1. Materials
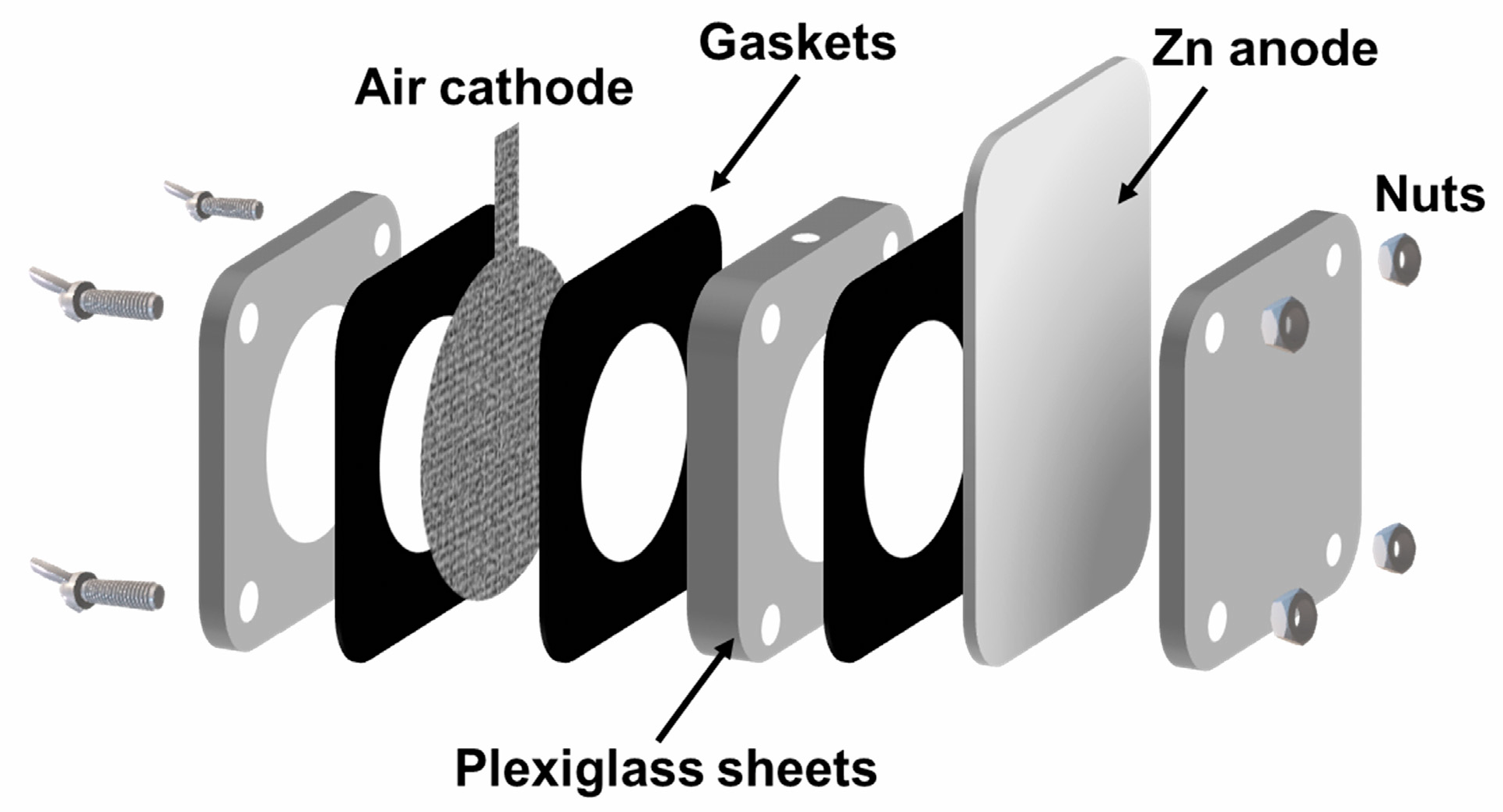
3.2. Preparation of Air Cathodes for Rechargeable ZABs
3.2.1. Preparation of the Homemade Gas Diffusion Layer
3.2.2. Preparation of the Catalyst Layer
3.3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koolen, C.D.; Luo, W.; Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2 Electroreduction. ACS Catal. 2023, 13, 948–973. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y. Recent Development of CO2 Electrochemistry from Li-CO2 Batteries to Zn-CO2 Batteries. Acc. Chem. Res. 2019, 52, 1721–1729. [Google Scholar] [CrossRef]
- Montero, J.; Navalpotro, P.; D’Epifanio, A.; Mecheri, B.; Licoccia, S.; Carretero-González, J. Redox-Active Coordination Polymers as Bifunctional Electrolytes in Slurry-Based Aqueous Batteries at Neutral pH. J. Electroanal. Chem. 2021, 895, 115442. [Google Scholar] [CrossRef]
- Zhang, J.; Pham, T.H.M.; Ko, Y.; Li, M.; Yang, s.; Koolen, C.D.; Zhong, L.; Luo, W.; Züttel, A. Tandem effect of Ag@C@Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep. Phys. Sci. 2022, 3, 100949. [Google Scholar] [CrossRef]
- Montero, J.; da Silva Freitas, W.; Mecheri, B.; Forchetta, M.; Galloni, P.; Licoccia, S.; D’Epifanio, A. A Neutral-pH Aqueous Redox Flow Battery Based on Sustainable Organic Electrolytes. ChemElectroChem 2023, 10, e202201002. [Google Scholar] [CrossRef]
- He, J.; Meng, J.; Huang, Y. Challenges and Recent Progress in Fast-Charging Lithium-Ion Battery Materials. J. Power Sources 2023, 570, 232965. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Meng, J.-K.; Yue, X.-Y.; Qiu, Q.-Q.; Song, Y.; Wu, X.-J.; Fu, Z.-W.; Xia, Y.-Y.; Shadike, Z.; Wu, J.; et al. Tuning P2-Structured Cathode Material by Na-Site Mg Substitution for Na-Ion Batteries. J. Am. Chem. Soc. 2019, 141, 840–848. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Meng, J.-K.; Xiao, N.; Shao, R.-W.; Wu, X.-J.; Gao, P.; Shadike, Z.; Zhou, Y.-N. Sodium Storage Mechanism of a GeP5/C Composite as a High Capacity Anode Material for Sodium-Ion Batteries. Chem. Commun. 2022, 58, 10345–10348. [Google Scholar] [CrossRef]
- Yue, X.-Y.; Wang, W.-W.; Wang, Q.-C.; Meng, J.-K.; Wang, X.-X.; Song, Y.; Fu, Z.-W.; Wu, X.-J.; Zhou, Y.-N. Cuprite-Coated Cu Foam Skeleton Host Enabling Lateral Growth of Lithium Dendrites for Advanced Li Metal Batteries. Energy Stor. Mater. 2019, 21, 180–189. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Wang, L.; Jiao, Z.; Xiao, M.; Huang, Q.; Liu, M.; Shao, Q.; Sun, X.; Zhang, J. Prussian Blue and Its Analogues as Cathode Materials for Na-, K-, Mg-, Ca-, Zn- and Al-Ion Batteries. Nano Energy 2022, 99, 107424. [Google Scholar] [CrossRef]
- Wang, H.-F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, C.; Wang, B.; Li, Y.; Zhang, L.; Zhang, W.; Huang, Y.; Zhang, R. Rational Design of Advanced Oxygen Electrocatalysts for High-Performance Zinc-Air Batteries. Chem Catal. 2022, 2, 3357–3394. [Google Scholar] [CrossRef]
- Radenahmad, N.; Khezri, R.; Mohamad, A.A.; Nguyen, M.T.; Yonezawa, T.; Somwangthanaroj, A.; Kheawhom, S. A Durable Rechargeable Zinc-Air Battery via Self-Supported MnOx-S Air Electrode. J. Alloys Compd. 2021, 883, 160935. [Google Scholar] [CrossRef]
- Abbasi, A.; Xu, Y.; Abouzari-Lotf, E.; Etesami, M.; Khezri, R.; Risse, S.; Kardjilov, N.; Van Tran, K.; Jia, H.; Somwangthanaroj, A.; et al. Phosphonated Graphene Oxide-Modified Polyacrylamide Hydrogel Electrolytes for Solid-State Zinc-Ion Batteries. Electrochim. Acta 2022, 435, 141365. [Google Scholar] [CrossRef]
- Khezri, R.; Motlagh, S.R.; Etesami, M.; Mohamad, A.A.; Pornprasertsuk, R.; Olaru, S.; Kheawhom, S. High Current Density Charging of Zinc-Air Flow Batteries: Investigating the Impact of Flow Rate and Current Density on Zinc Electrodeposition. Appl. Energy 2023, 348, 121564. [Google Scholar] [CrossRef]
- Bósquez-Cáceres, M.F.; De Lima, L.; Morera Córdova, V.; Delgado, A.D.; Béjar, J.; Arjona, N.; Álvarez-Contreras, L.; Tafur, J.P. Chitosan-Carboxymethylcellulose Hydrogels as Electrolytes for Zinc-Air Batteries: An Approach to the Transition towards Renewable Energy Storage Devices. Batteries 2022, 8, 265. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent Advances in Zinc-Air Batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn–air Batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef]
- Yao, Z.C.; Tang, T.; Hu, J.S.; Wan, L.J. Recent Advances on Nonprecious-Metal-Based Bifunctional Oxygen Electrocatalysts for Zinc-Air Batteries. Energy Fuels 2021, 35, 6380–6401. [Google Scholar] [CrossRef]
- Dong, F.; Wu, M.; Chen, Z.; Liu, X.; Zhang, G.; Qiao, J.; Sun, S. Atomically Dispersed Transition Metal-Nitrogen-Carbon Bifunctional Oxygen Electrocatalysts for Zinc-Air Batteries: Recent Advances and Future Perspectives. Nano-Micro Lett. 2022, 14, 36. [Google Scholar] [CrossRef]
- Li, W.; Cheng, L.; Chen, X.; Liu, Y.; Liu, Y.; Liu, Q.; Huang, Y. Key Materials and Structural Design in Flexible and Stretchable Zinc-Air Batteries. Nano Energy 2023, 106, 108039. [Google Scholar] [CrossRef]
- Béjar, J.; Delgado, A.D.; Espinosa-Magaña, F.; Aguilar-Elguezabal, A.; Guerra-Balcázar, M.; Arjona, N.; Álvarez-Contreras, L. Electrodeposition of Smallsized NiM2O4 Spinels (M: Co, Mn) as Bifunctional Nanomaterials for Rechargeable Zinc-Air Batteries. J. Alloys Compd. 2022, 929, 167266. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, D.; Cao, X.; Ma, Y.; Kou, Y.; Liu, Z.; Hassan, A.; Guo, C.; Yue, F.; Wang, J. Phosphorus Modified Hollow, Porous Nickel-Cobalt Oxides Nanocubes with Heterostructure for Oxygen Evolution Reaction in Alkaline. J. Alloys Compd. 2022, 925, 166338. [Google Scholar] [CrossRef]
- Li, S.; Xie, Y.; Feng, C.; Hassan, A.; Wang, J. Nitrogen-Rich Porous Carbon Nanotubes Coated Co/Mo2N Composites Derived from Metal-Organic Framework as Efficient Bifunctional Oxygen Electrocatalysts. Catalysts 2023, 13, 801. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, C.; Guo, Y.; Hassan, A.; Li, S.; Zhang, Y.; Wang, J. Dimethylimidazole and Dicyandiamide Assisted Synthesized Rich-Defect and Highly Dispersed CuCo-Nx Anchored Hollow Graphite Carbon Nanocages as Efficient Trifunctional Electrocatalyst in the Same Electrolyte. J. Power Sources 2022, 517, 230721. [Google Scholar] [CrossRef]
- Qiao, S.; Guo, J.; Wang, D.; Zhang, L.; Hassan, A.; Chen, T.; Feng, C.; Zhang, Y.; Wang, J. Core-Shell Cobalt Particles Co@CoO Loaded on Nitrogen-Doped Graphene for Photocatalytic Water-Splitting. Int. J. Hydrog. Energy 2020, 45, 1629–1639. [Google Scholar] [CrossRef]
- Béjar, J.; Espinosa-Magaña, F.; Avelar, J.; Aguilar-Elguezabal, A.; Guerra-Balcázar, M.; Arjona, N.; Álvarez-Contreras, L. Rational Design of Nitrogen-doped Carbon Nanotubes by Defect Engineering for Zn–air Batteries with High Performance. Carbon 2023, 204, 411–426. [Google Scholar] [CrossRef]
- Davari, E.; Ivey, D. Bifunctional Electrocatalysts for Zn–air Batteries. Sustain. Energy Fuels 2017, 2, 39–67. [Google Scholar] [CrossRef]
- da Silva Freitas, W.; Gemma, D.; Mecheri, B.; D’Epifanio, A. Air-Breathing Cathodes for Microbial Fuel Cells Based on Iron-Nitrogen-Carbon Electrocatalysts. Bioelectrochemistry 2022, 146, 108103. [Google Scholar] [CrossRef]
- Sumboja, A.; Lübke, M.; Wang, Y.; An, T.; Zong, Y.; Liu, Z. All-Solid-State, Foldable, and Rechargeable Zn–air Batteries Based on Manganese Oxide Grown on Graphene-Coated Carbon Cloth Air Cathode. Adv. Energy Mater. 2017, 7, 1700927. [Google Scholar] [CrossRef]
- Wang, P.; Wan, L.; Lin, Y.; Wang, B. Construction of Mass-Transfer Channel in Air Electrode with Bifunctional Catalyst for Rechargeable Zinc-Air Battery. Electrochim. Acta 2019, 320, 134564. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Recent Advances in Air Electrodes for Zn–air Batteries: Electrocatalysis and Structural Design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- da Silva Freitas, W.; D’Epifanio, A.; Lo Vecchio, C.; Gatto, I.; Baglio, V.; Ficca, V.C.A.; Placidi, E.; Mecheri, B. Tailoring MOF Structure via Iron Decoration to Enhance ORR in Alkaline Polymer Electrolyte Membrane Fuel Cells. J. Chem. Eng. 2023, 465, 142987. [Google Scholar] [CrossRef]
- Ricciardi, B.; Mecheri, B.; da Silva Freitas, W.; Ficca, V.C.A.; Placidi, E.; Gatto, I.; Carbone, A.; Capasso, A.; D’Epifanio, A. Porous Iron-Nitrogen-Carbon Electrocatalysts for Anion Exchange Membrane Fuel Cells (AEMFC). ChemElectroChem 2023, 10, e202201115. [Google Scholar] [CrossRef]
- Park, S.; Park, Y. Fabrication of Gas Diffusion Layer (GDL) Containing Microporous Layer Using Flourinated Ethylene Prophylene (FEP) for Proton Exchange Membrane Fuel Cell (PEMFC). Int. J. Precis. Eng. Manuf. 2012, 13, 1145–1151. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Goh, F.W.T.; Li, B.; Geng, D.; Hor, T.S.A.; Zong, Y.; Liu, Z. Manganese Oxide Catalyst Grown on Carbon Paper as an Air Cathode for High-Performance Rechargeable Zinc-Air Batteries. ChemPlusChem 2015, 80, 1341–1346. [Google Scholar] [CrossRef]
- Meng, J.-K.; Wang, W.-W.; Yue, X.-Y.; Xia, H.-Y.; Wang, Q.-C.; Wang, X.-X.; Fu, Z.-W.; Wu, X.-J.; Zhou, Y.-N. Cotton-Derived Carbon Cloth Enabling Dendrite-Free Li Deposition for Lithium Metal Batteries. J. Power Sources 2020, 465, 228291. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, S.; Li, D.; Liao, J.; Ji, F.; Liu, H.; Ci, L. Commercial Carbon Cloth: An Emerging Substrate for Practical Lithium Metal Batteries. Energy Storage Mater. 2022, 48, 172–190. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.-Y.; Chen, K. Elucidating Differences between Carbon Paper and Carbon Cloth in Polymer Electrolyte Fuel Cells. Electrochim. Acta 2007, 52, 3965–3975. [Google Scholar] [CrossRef]
- Li, M.; Xiong, D.; Liu, W.; Liu, J.; Dai, L.; Xiao, X. Boronised Electrodeposited Nickel on Carbon Paper for the Oxygen Evolution Reactions. Mater. Lett. 2023, 336, 133867. [Google Scholar] [CrossRef]
- Shi, Q.; Feng, C.; Tang, F.; Li, B.; Ming, P.; Zhang, C. Bulk Resistance and Internal Contacts of Carbon Fiber Paper Determined via X-Ray Computed Tomography. Mater. Chem. Phys. 2023, 296, 127137. [Google Scholar] [CrossRef]
- Ji, H.; Wang, M.; Liu, S.; Sun, H.; Liu, J.; Qian, T.; Yan, C. Pyridinic and Graphitic Nitrogen-Enriched Carbon Paper as a Highly Active Bifunctional Catalyst for Zn–air Batteries. Electrochim. Acta 2020, 334, 135562. [Google Scholar] [CrossRef]
- Ma, T.Y.; Ran, J.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Phosphorus-Doped Graphitic Carbon Nitrides Grown In Situ on Carbon-Fiber Paper: Flexible and Reversible Oxygen Electrodes. Angew. Chem. Int. Ed. 2015, 54, 4646–4650. [Google Scholar] [CrossRef]
- Levinas, R.; Tsyntsaru, N.; Cesiulis, H. Insights into Electrodeposition and Catalytic Activity of MoS2 for Hydrogen Evolution Reaction Electrocatalysis. Electrochim. Acta 2019, 317, 427–436. [Google Scholar] [CrossRef]
- Kong, S.; Xiang, X.; Jin, B.; Guo, X.; Wang, H.; Zhang, G.; Huang, H.; Cheng, K. B, O and N Codoped Biomass-Derived Hierarchical Porous Carbon for High-Performance Electrochemical Energy Storage. Nanomaterials 2022, 12, 1720. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Kong, S.; Jin, B. Spinel LiMn2O4 as Electrocatalyst toward Solid-State Zinc-Air Batteries. Catalysts 2023, 13, 860. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Yu, P.; Kwon, T.; Lee, K.; Li, J. Ideal Design of Air Electrode-A Step Closer toward Robust Rechargeable Zn–air Battery. APL Mater. 2020, 8, 050905. [Google Scholar] [CrossRef]
- Jaouen, F.; Jones, D.; Coutard, N.; Artero, V.; Strasser, P.; Kucernak, A. Toward Platinum Group Metal-Free Catalysts for Hydrogen/Air Proton-Exchange Membrane Fuel Cells. Johns. Matthey Technol. Rev. 2018, 62, 231–255. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, Y.; Wang, Z.; Xiao, W.; Wang, X.; Li, B.; Li, Z.; Liu, X.; Ma, T.; Wang, L. Surface-Enriched Ultrafine Pt Nanoparticles Coupled with Defective CoP as Efficient Trifunctional Electrocatalyst for Overall Water Splitting and Flexible Zn–air Battery. Chin. J. Catal. 2023, 46, 36–47. [Google Scholar] [CrossRef]
- da Silva Freitas, W.; D’Epifanio, A.; Ficca, V.C.A.; Placidi, E.; Arciprete, F.; Mecheri, B. Tailoring Active Sites of Iron-Nitrogen-Carbon Catalysts for Oxygen Reduction in Alkaline Environment: Effect of Nitrogen-Based Organic Precursor and Pyrolysis Atmosphere. Electrochim. Acta 2021, 391, 138899. [Google Scholar] [CrossRef]
- Ficca, V.C.A.; Santoro, C.; Marsili, E.; da Silva Freitas, W.; Serov, A.; Atanassov, P.; Mecheri, B. Sensing Nitrite by Iron-Nitrogen-Carbon Oxygen Reduction Electrocatalyst. Electrochim. Acta 2022, 402, 139514. [Google Scholar] [CrossRef]
- Hess, F. Corrosion Mechanism and Stabilization Strategies for RuO2 and IrO2 Catalysts in the Electrochemical Oxygen Evolution Reaction. Curr. Opin. Electrochem. 2023, 41, 101349. [Google Scholar] [CrossRef]
- Briguglio, N.; Siracusano, S.; Bonura, G.; Sebastián, D.; Aricò, A.S. Flammability Reduction in a Pressurised Water Electrolyser Based on a Thin Polymer Electrolyte Membrane through a Pt-Alloy Catalytic Approach. Appl. Catal. B 2019, 246, 254–265. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Ding, Y.; Ma, J.; Das, P.; Zhang, B.; Wu, Z.-S.; Bao, X. Spatially Confined Sub-Nanometer Pt in RuO2 Nanosheet as Robust Bifunctional Oxygen Electrocatalyst for Stabilizing Li-O2 Batteries. Chem Catal. 2023, 100658. [Google Scholar] [CrossRef]
- He, X.; Tian, Y.; Huang, Z.; Xu, L.; Wu, J.; Qian, J.; Zhang, J.; Li, H. Engineering the Electronic States of Ni3FeN via Zinc Ion Regulation for Promoting Oxygen Electrocatalysis in Rechargeable Zn–air Batteries. J. Mater. Chem. A 2021, 9, 2301–2307. [Google Scholar] [CrossRef]
- Vincent, I.; Lee, E.-C.; Kim, H.-M. Highly Cost-Effective Platinum-Free Anion Exchange Membrane Electrolysis for Large Scale Energy Storage and Hydrogen Production. RSC Adv. 2020, 10, 37429–37438. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.; Lee, E.-C.; Kim, H.-M. Highly Active Ni–Fe Based Oxide Oxygen Evolution Reaction Electrocatalysts for Alkaline Anion Exchange Membrane Electrolyser. Catalysts 2022, 12, 476. [Google Scholar] [CrossRef]
- Hu, B.; Yang, Y.; Cao, W.; Wang, X.; Zhou, C.; Mao, Y.; Ge, L.; Ran, R.; Zhou, W. Patchy Fe-N-C Supported Low-Loading Pt Nanoparticles as a Highly Active Cathode for Proton Exchange Membrane Fuel Cells. J. Alloys Compd. 2023, 951, 169867. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Zhou, C.; Jiang, J.; Cheng, X.; Yang, L.; Wu, Q.; Wang, X.; Hu, Z. Constructing Membrane Electrodes of Low Pt Areal Loading with the New Support of N-Doped Carbon Nanocages for PEMFC. FlatChem 2023, 40, 100515. [Google Scholar] [CrossRef]
- da Silva Freitas, W.; Mecheri, B.; Lo Vecchio, C.; Gatto, I.; Baglio, V.; Ficca, V.C.A.; Patra, A.; Placidi, E.; D’Epifanio, A. Metal-Organic-Framework-Derived Electrocatalysts for Alkaline Polymer Electrolyte Fuel Cells. J. Power Sources 2022, 550, 232135. [Google Scholar] [CrossRef]
- da Silva Freitas, W.; Machado Pico, P.P.; D’Epifanio, A.; Mecheri, B. Nanostructured Fe-N-C as Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution. Catalysts 2021, 11, 1525. [Google Scholar] [CrossRef]
- Costa de Oliveira, M.A.; Mecheri, B.; D’Epifanio, A.; Zurlo, F.; Licoccia, S. Optimization of PGM-Free Cathodes for Oxygen Reduction in Microbial Fuel Cells. Electrochim. Acta 2020, 334, 135650. [Google Scholar] [CrossRef]
- Andersen, S.M.; Dhiman, R.; Larsen, M.J.; Skou, E. Importance of Electrode Hot-Pressing Conditions for the Catalyst Performance of Proton Exchange Membrane Fuel Cells. Appl. Catal. B 2015, 172–173, 82–90. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem 2020, 1, 124–159. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A Review of Current Collectors for Lithium-Ion Batteries. J. Power Sources 2020, 485, 229321. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, N.; Espiñeira, P.P.; Manian, A.P.; Bechtold, T. Three-Dimensional Embroidered Current Collectors for Ultra-Thick Electrodes in Batteries. RSC Adv. 2016, 6, 69685–69690. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.Y.W. Stainless Steel as Low-Cost High-Voltage Cathode via Stripping/Deposition in Metal-Lithium Battery. Electrochim. Acta 2019, 298, 186–193. [Google Scholar] [CrossRef]
- Zhang, X.; Chu, Y.; Xiong, X.; Fan, Q.; Han, D.; Wu, C. CoP-Loaded BiVO4 for Highly Active and Robust Photocathodic Protection of 304 Stainless Steel. Mater. Today Commun. 2023, 35, 105848. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Kadier, A.; Hao, B.; Li, H.; Ma, P.-C. Performance Optimization of a Batch Scale Electrocoagulation Process Using Stainless Steel Mesh (304) Cathode for the Separation of Oil-in-Water Emulsion. Chem. Eng. Process.-Process Intensif. 2022, 174, 108901. [Google Scholar] [CrossRef]
- Zhang, Y.; Merrill, M.D.; Logan, B.E. The Use and Optimization of Stainless Steel Mesh Cathodes in Microbial Electrolysis Cells. Int. J. Hydrog. Energy 2010, 35, 12020–12028. [Google Scholar] [CrossRef]
- Daio, T.; Staykov, A.; Guo, L.; Liu, J.; Tanaka, M.; Matthew Lyth, S.; Sasaki, K. Lattice Strain Mapping of Platinum Nanoparticles on Carbon and SnO2 Supports. Sci. Rep. 2015, 5, 13126. [Google Scholar] [CrossRef]
- Mauger, S.A.; Pfeilsticker, J.R.; Wang, M.; Medina, S.; Yang-Neyerlin, A.C.; Neyerlin, K.C.; Stetson, C.; Pylypenko, S.; Ulsh, M. Fabrication of High-Performance Gas-Diffusion-Electrode Based Membrane-Electrode Assemblies. J. Power Sources 2020, 450, 227581. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Zheng, G.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Durable Rechargeable Zinc-Air Batteries with Neutral Electrolyte and Manganese Oxide Catalyst. J. Power Sources 2016, 332, 330–336. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, L.; Yuan, Y.; Zhong, L.; Chen, Z.; Chi, X.; Lu, H.; Chen, Z.; Zou, R.; Li, T.; et al. An Iron-Decorated Carbon Aerogel for Rechargeable Flow and Flexible Zn–air Batteries. Adv. Mater. 2020, 32, 2002292. [Google Scholar] [CrossRef]
- Shao, Q.; Liu, J.; Wu, Q.; Li, Q.; Wang, H.; Duan, Q. In Situ Coupling Strategy for Anchoring Monodisperse Co9S8 Nanoparticles on S and N Dual-Doped Graphene as a Bifunctional Electrocatalyst for Rechargeable Zn–air Battery. Nano-Micro Lett. 2019, 11, 4. [Google Scholar] [CrossRef]
- Shahbazi Farahani, F.; Rahmanifar, M.S.; Noori, A.; El-Kady, M.F.; Hassani, N.; Neek-Amal, M.; Kaner, R.B.; Mousavi, M.F. Trilayer Metal-Organic Frameworks as Multifunctional Electrocatalysts for Energy Conversion and Storage Applications. J. Am. Chem. Soc. 2022, 144, 3411–3428. [Google Scholar] [CrossRef]
- Han, J.; Meng, X.; Lu, L.; Bian, J.; Li, Z.; Sun, C. Single-Atom Fe-Nx-C as an Efficient Electrocatalyst for Zinc-Air Batteries. Adv. Funct. Mater. 2019, 29, 1808872. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zeng, Y.; Chen, J.; Qiu, L.; Zhou, H.; Sun, C.; Yu, Y.; Zhu, C.; Zhu, Z. Single Fe Atom on Hierarchically Porous S, N-Codoped Nanocarbon Derived from Porphyra Enable Boosted Oxygen Catalysis for Rechargeable Zn–air Batteries. Small 2019, 15, 1900307. [Google Scholar] [CrossRef]
- Niu, H.-J.; Wang, A.-J.; Zhang, L.; Feng, J.-J. Bioinspired One-Step Pyrolysis Fabrication of 3D Porous Co, N, P-Doped Carbon Nanosheets with Enriched CoNx Active Sites as High-Performance Bifunctional Oxygen Electrocatalyst for Rechargeable Zn–air Battery. ACS Appl. Energy Mater. 2020, 3, 2781–2790. [Google Scholar] [CrossRef]

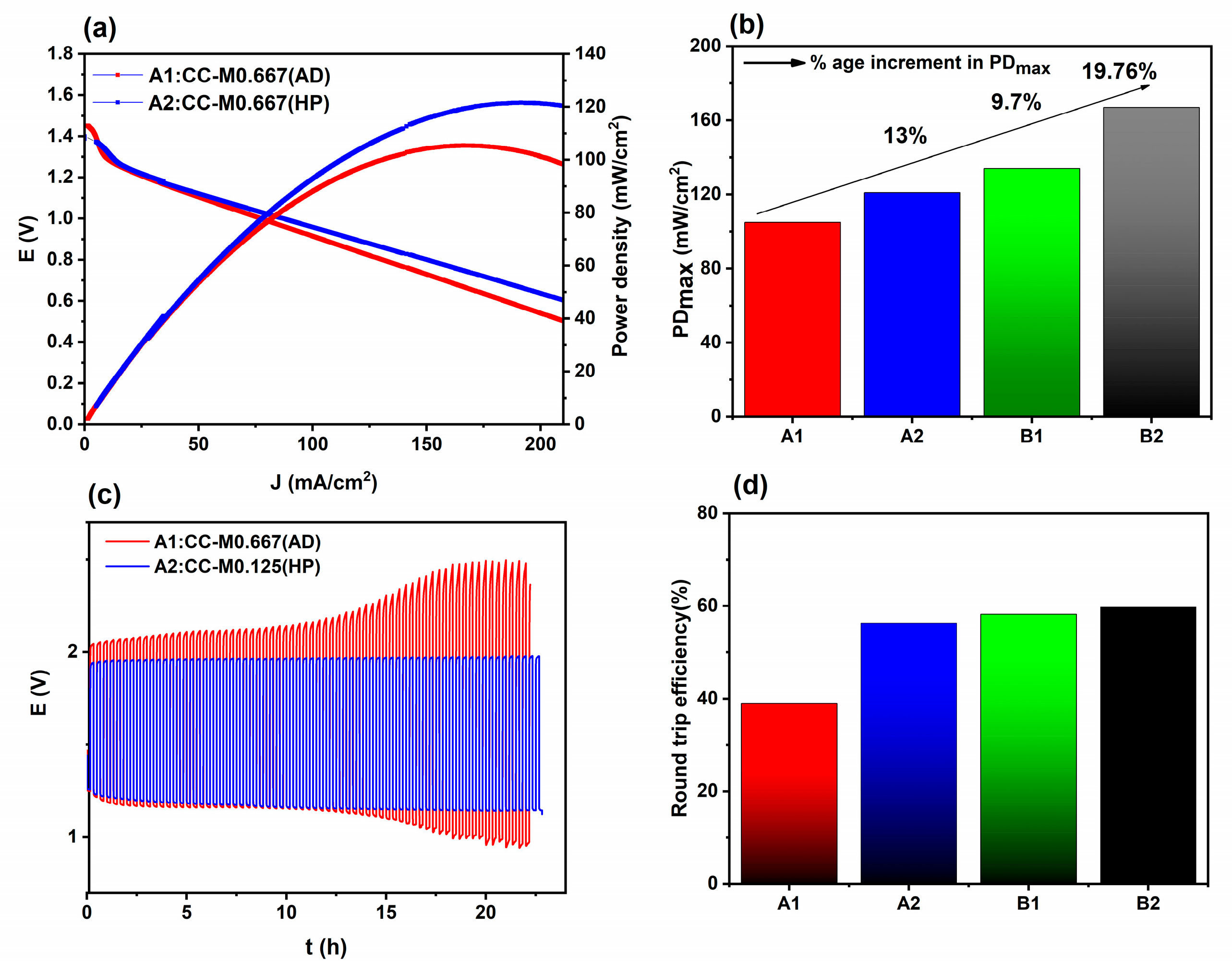

| Pt/C-RuO2-Based Air Cathodes | PDmax (mW/cm2) | Charge Potential(V) | Discharge Potential (V) | Egap (V) | Round Trip Efficiency (%) | |
|---|---|---|---|---|---|---|
| A1 | CC-M0.667(AD) | 105 ± 0.2 | 2.43 | 0.95 | 1.47 ± 0.2 | 39.1 |
| A2 | CC-M0.125(HP) | 121 ± 1.0 | 1.97 | 1.14 | 0.83 ± 0.3 | 57.9 |
| B1 | CP-M0.667 | 134 ± 1.2 | 1.94 | 1.13 | 0.81 ± 0.1 | 58.2 |
| B2 | CP-M0.125 | 167 ± 0.3 | 1.94 | 1.16 | 0.78 ± 0.05 | 59.8 |
| Pt/C-RuO2-Based Cathodes(V) | Egap | Durability Hours/No. of Cycles (n) @J(mA/cm2) | PDmax (mW/cm2) | References |
|---|---|---|---|---|
| 1 | 1.45 | 28h@10 | 110 | [56] |
| 2 | 1.30 | 110h@5 | 112 | [75] |
| 3 | 1.70 | 35h@10 | 75 | [76] |
| 4 | 1.10 | 20h@5 | 129 | [77] |
| 5 | 1.69 | 300cycles@10 | 82 | [78] |
| 6 | 1.16 | 72cycles@4 | - | [79] |
| 7 | 1.60 | 24h@10 | 40 | [80] |
| 8 | 0.78 | 24h/75cycles@5 | 167 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisa, K.U.; da Silva Freitas, W.; Montero, J.; D’Epifanio, A.; Mecheri, B. Development and Optimization of Air-Electrodes for Rechargeable Zn–Air Batteries. Catalysts 2023, 13, 1319. https://doi.org/10.3390/catal13101319
Nisa KU, da Silva Freitas W, Montero J, D’Epifanio A, Mecheri B. Development and Optimization of Air-Electrodes for Rechargeable Zn–Air Batteries. Catalysts. 2023; 13(10):1319. https://doi.org/10.3390/catal13101319
Chicago/Turabian StyleNisa, Khair Un, Williane da Silva Freitas, Jorge Montero, Alessandra D’Epifanio, and Barbara Mecheri. 2023. "Development and Optimization of Air-Electrodes for Rechargeable Zn–Air Batteries" Catalysts 13, no. 10: 1319. https://doi.org/10.3390/catal13101319
APA StyleNisa, K. U., da Silva Freitas, W., Montero, J., D’Epifanio, A., & Mecheri, B. (2023). Development and Optimization of Air-Electrodes for Rechargeable Zn–Air Batteries. Catalysts, 13(10), 1319. https://doi.org/10.3390/catal13101319








