Mannuronan C-5 Epimerases: Review of Activity Assays, Enzyme Characteristics, Structure, and Mechanism
Abstract
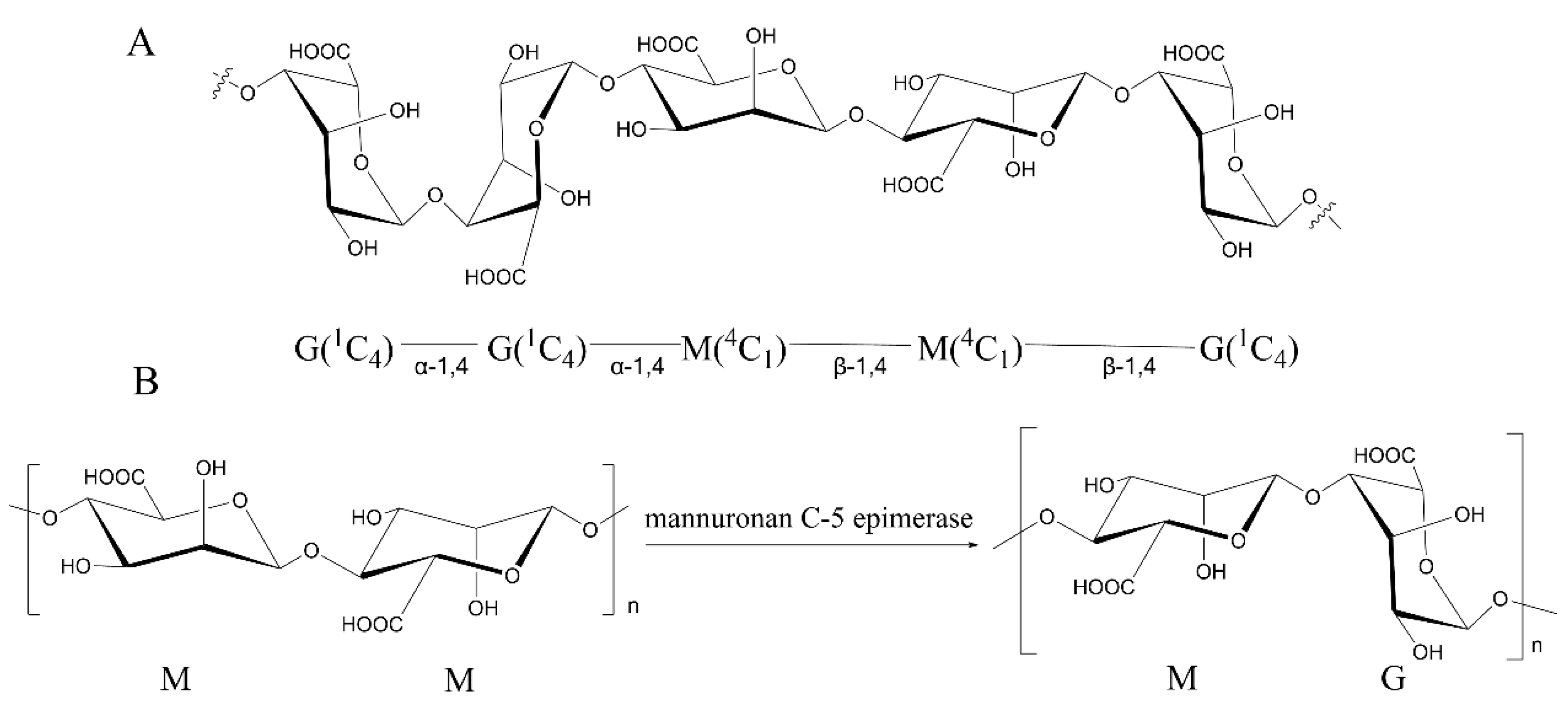
1. Introduction
2. Methods for Analyzing of the Mannuronan C-5 Epimerases Reaction
2.1. Spectrophotometric Assays for the Epimerase Activity of ManC5-Es
2.2. 1H-NMR and 13C-NMR Spectroscopy for C5 Epimerization
2.3. Other Methods for Assaying ManC5-Es Epimerization
3. Characteristics of Mannuronan C-5 Epimerases from Different Sources
3.1. Mannuronan C-5 Epimerases from Brown Algae
3.2. Characterization of Mannuronan C-5 Epimerases from Azotobacter Species
3.3. Characterization of Mannuronan C-5 Epimerases from Pseudomonas Species
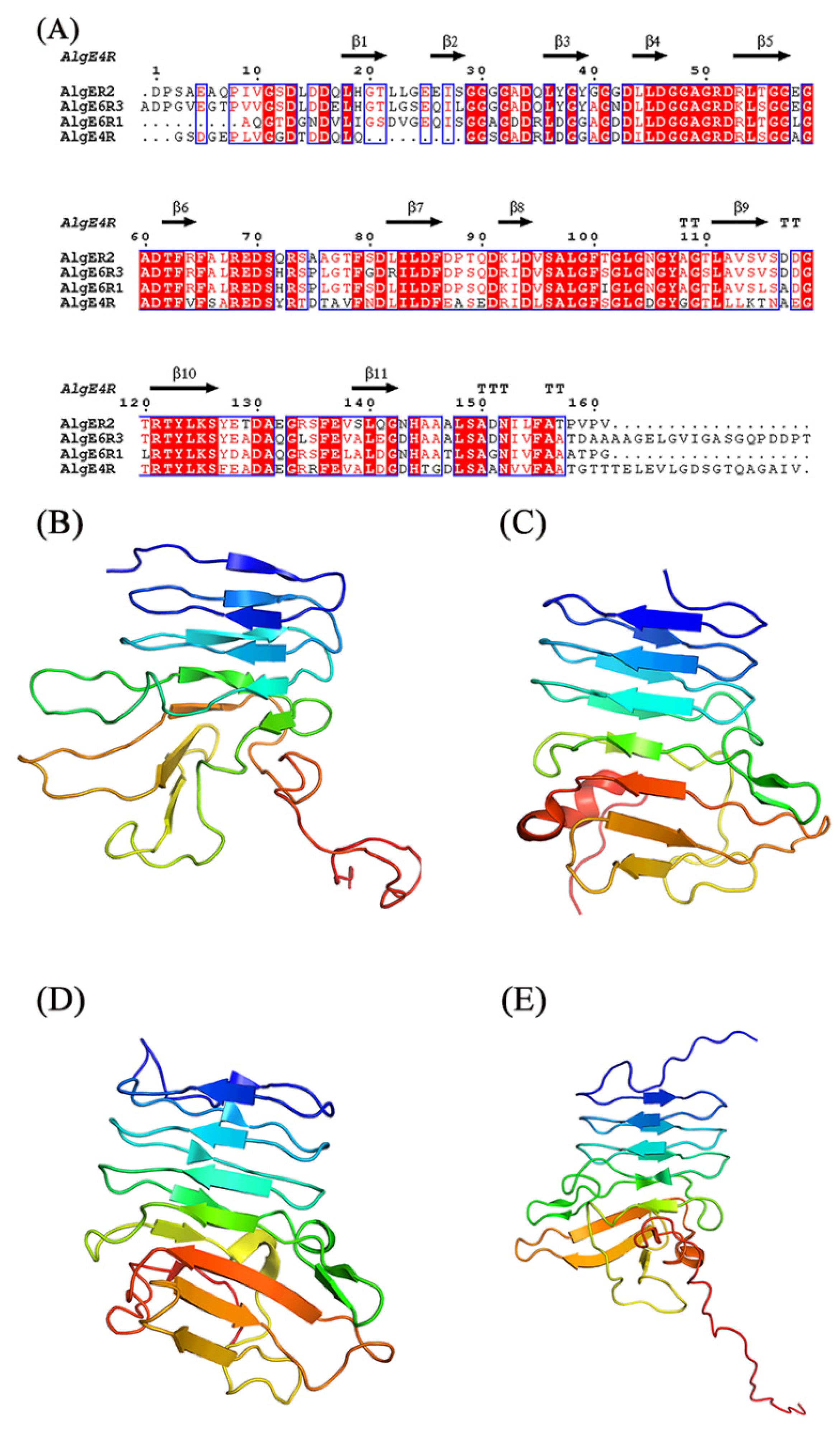
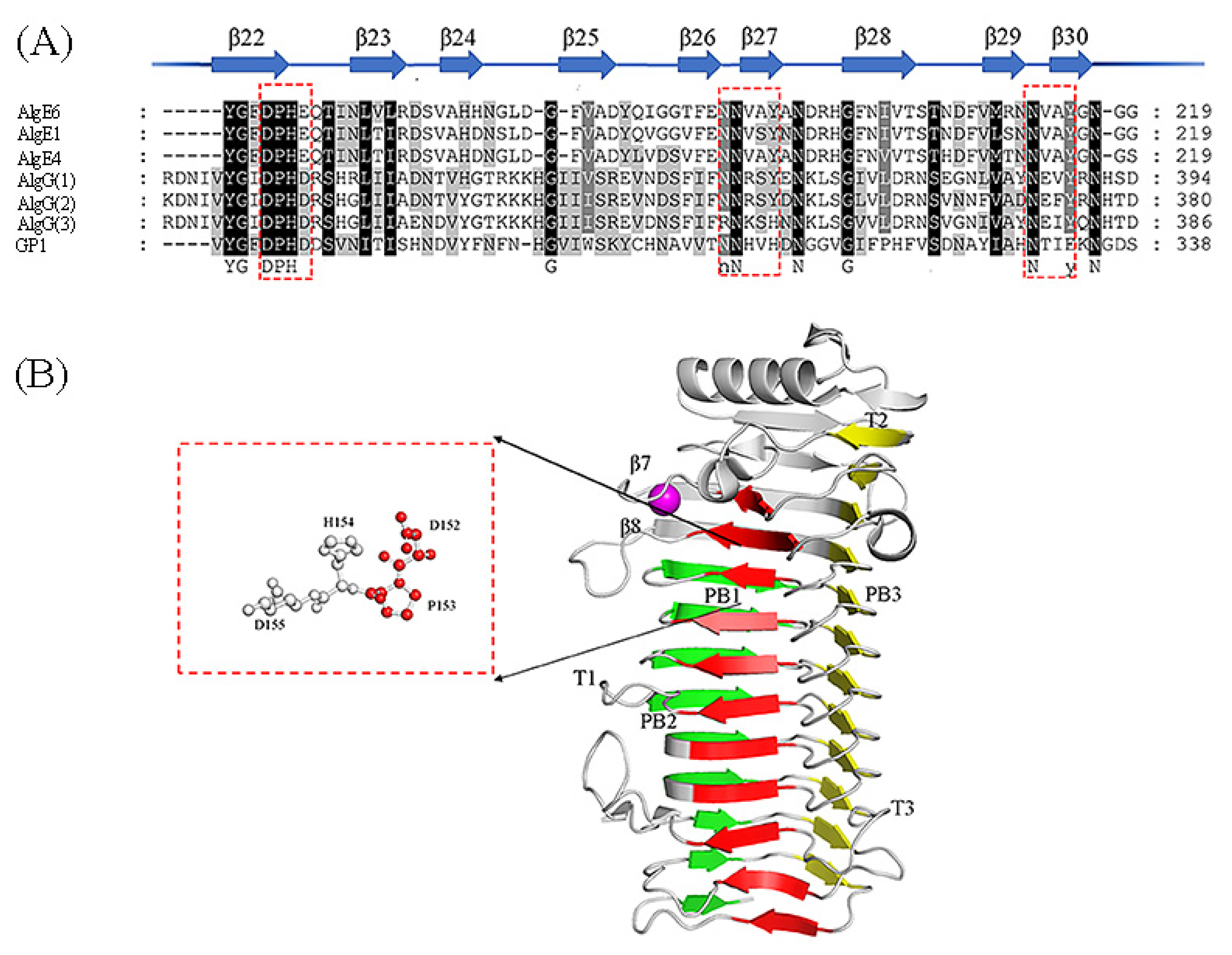
4. Structure and Mechanism of Mannuronan C-5 Epimerases
4.1. The Structure Analysis of Mannuronan C-5 Epimerases
4.2. The Catalytic Mechanism of Mannuronan C-5 Epimerases
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernando, I.P.S.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Black, W.A.P.; Woodward, F.N. Alginates from common British brown marine algae. In Natural Plant Hydrocolloids; ACS Publications: Washington, DC, USA, 1954; Volume 11, pp. 83–91. [Google Scholar]
- Kloareg, B.; Quatrano, R.S. Structure of the cell-walls of marine-algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. 1988, 26, 259–315. [Google Scholar]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010, 188, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.D.T.; Mackie, W.; Smolko, E.E. Crystalline structures of alginic acids. Nature 1970, 225, 626–628. [Google Scholar] [CrossRef]
- Ertesvag, H. Alginate-modifying enzymes: Biological roles and biotechnological uses. Front. Microbiol. 2015, 6, 523. [Google Scholar]
- Donati, I.; Holtan, S.; Morch, Y.A.; Borgogna, M.; Dentini, M.; Skjak-Braek, G. New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules 2005, 6, 1031–1040. [Google Scholar] [CrossRef]
- Smidsrød, O. Molecular basis for some physical properties of alginates in the gel state. Faraday Discuss. Chem. Soc. 1974, 57, 263–274. [Google Scholar] [CrossRef]
- Draget, K.I.; Braek, G.S.; Smidsrod, O. Alginic acid gels—The effect of alginate chemical-composition and molecular-weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Hay, I.D.; Ur Rehman, Z.; Ghafoor, A.; Rehm, B.H.A. Bacterial biosynthesis of alginates. J. Chem. Technol. Biotechnol. 2010, 85, 752–759. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in synthesis of microbial polysaccharides: Properties and development trends. Catalysts 2022, 12, 1377. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—Aa review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Minghou, J.; Yujun, W.; Zuhong, X.; Yucai, G. Studies on the M:G ratios in alginate. In Developments in Hydrobiology; Eleventh International Seaweed Symposium; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 554–556. [Google Scholar]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C.Y. A brief review on the development of alginate extraction process and its sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Ci, F.; Jiang, H.; Zhang, Z.; Mao, X. Properties and potential applications of mannuronan C5-epimerase: A biotechnological tool for modifying alginate. Int. J. Biol. Macromol. 2021, 168, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Clementi, F. Alginate production by Azotobacter vinelandii. Crit. Rev. Biotechnol. 1997, 17, 327–361. [Google Scholar] [CrossRef]
- Gorin, P.; Spencer, J. Exocellular alginic acid from Azotobacter vinelandii. Can. J. Chem. 2011, 44, 993–998. [Google Scholar] [CrossRef]
- Moe, S.; Draget, K.; Skjâk-Bпек, G.; Smidsrod, O. Alginates. In Food Polysaccharides and Their Applications; Stephen, A.M., Ed.; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Linker, A.; Jones, R.S. A new polysaccharide resembling alginic acid isolated from Pseudomonads. J. Biol. Chem. 1966, 241, 3845–3851. [Google Scholar] [CrossRef]
- Ertesvåg, H.; Høidal, H.K.; Schjerven, H.; Svanem, B.I.; Valla, S. Mannuronan C-5-epimerases and their application for in vitro and in vivo design of new alginates useful in biotechnology. Metab Eng 1999, 1, 262–269. [Google Scholar] [CrossRef]
- Wolfram, F.; Kitova, E.N.; Robinson, H.; Walvoort, M.T.; Codée, J.D.; Klassen, J.S.; Howell, P.L. Catalytic mechanism and mode of action of the periplasmic alginate epimerase AlgG. J. Biol. Chem 2014, 289, 6006–6019. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Lu, C.; Shao, Z.R.; Liu, F.L.; Yao, J.T.; Duan, D.L. Genome-wide transcriptome profiling and characterization of mannuronan C5-epimerases in Saccharina japonica. Algal Res. 2021, 60. [Google Scholar] [CrossRef]
- Svanem, B.I.G.; Strand, W.I.; Ertesvag, H.; Skjak-Braek, G.; Hartmann, M.; Barbeyron, T.; Valla, S. The catalytic activities of the bifunctional Azotobacter vinelandii mannuronan C-5-epimerase and alginate lyase AlgE7 probably originate from the same active site in the enzyme. J. Biol. Chem. 2001, 276, 31542–31550. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, S.; Bao, S.; Mo, K.; Sun, D.; Hu, Y. Expression and characterization of a cold-adapted alginate lyase with exo/endo-type activity from a novel marine bacterium Alteromonas portus HB161718. Mar. Drugs 2021, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biotechnol. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Dische, Z. A new specific color reaction of hexuronic acids. J. Biol. Chem. 1947, 167, 189–198. [Google Scholar] [CrossRef]
- Gregory, J.D. The effect of borate on the carbazole reaction. Arch. Biochem. Biophys. 1960, 89, 157–159. [Google Scholar] [CrossRef]
- Larsen, B.; Haug, A. Biosynthesis of alginate: Part III. Tritium incorporation with polymannuronic acid 5-epimerase from Azotobacter vinelandii. Carbohydr. Res. 1971, 20, 225–232. [Google Scholar] [CrossRef]
- Page, W.J.; Sadoff, H.L. Relationship between calcium and uronic acids in encystment of Azotobacter vinelandii. J. Bacteriol. 1975, 122, 145–151. [Google Scholar] [CrossRef]
- Gurin, S.; Hood, D.B. The identification and estimation of hexoses in polysaccharides and glycoproteins by the carbazole method. J. Biol. Chem. 1939, 131, 211–223. [Google Scholar] [CrossRef]
- Taylor, K. Protocols in biotechnology—A colorimetric method for the quantitation of galacturonic acid. Appl. Biochem. Biotechnol. 1993, 43, 51–54. [Google Scholar] [CrossRef]
- Currie, A.J.; Turvey, J.R. An enzymic method for the assay of d-mannuronan c-5 epimerase activity. Carbohydr. Res. 1982, 107, 156–159. [Google Scholar] [CrossRef]
- Gaardlos, M.; Samsonov, S.A.; Sletmoen, M.; Hjornevik, M.; Satrom, G.I.; Tondervik, A.; Aachmann, F.L. Insights into the roles of charged residues in substrate binding and mode of action of mannuronan C-5 epimerase AlgE4. Glycobiology 2021, 31, 1616–1635. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Larsen, B.; Smidsrod, O. A Study of Constitution of Alginic Acid by Partial Acid Hydrolysis. In Proceedings of the Fifth International Seaweed Symposium, Halifax, NS, USA, 25–28 August 1965; Elsevier: Amsterdam, The Netherlands, 1966; Voume 20, pp. 271–277. [Google Scholar]
- Grasdalen, H.; Larsen, B.; Smidsrod, O. PMR study of the composition and sequence of uronate residues in alginates. Carbohydr. Res. 1979, 68, 23–31. [Google Scholar] [CrossRef]
- Sun, M.; Sun, C.; Li, T.; Li, K.; Yan, S.; Yin, H. Characterization of a novel bifunctional mannuronan C-5 epimerase and alginate lyase from Pseudomonas mendocina. sp. DICP-70. Int. J. Biol. Macromol. 2020, 150, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Gaardløs, M.; Heggeset, T.M.B.; Tøndervik, A.; Tezé, D.; Svensson, B.; Ertesvåg, H.; Sletta, H.; Aachmann, F.L. Mechanistic basis for understanding the dual activities of the bifunctional Azotobacter vinelandii Mannuronan C-5-Epimerase and alginate lyase AlgE7. Appl. Environ. Microbiol. 2022, 88. [Google Scholar] [CrossRef] [PubMed]
- Salomonsen, T.; Jensen, H.M.; Larsen, F.H.; Engelsen, S.B. The quantitative impact of water suppression on NMR spectra for compositional analysis of alginates. In Proceedings of the 9th International Conference on Applications of Magnetic Resonance in Food Science, Reykjavik, Iceland, 15–17 September 2008; Nordic House: Reykjavik, Iceland; p. 12. [Google Scholar]
- Salomonsen, T.; Jensen, H.M.; Larsen, F.H.; Engelsen, S.B. The Quantitative Impact of Water Suppression on NMR Spectra for Compositional Analysis of Alginates. In Magnetic Resonance in Food Science: Challenges in a Changing World; The Royal Society of Chemistry: London, UK, 2009; pp. 12–19. [Google Scholar]
- Skjåk-Br˦k, G.; Larsen, B. A new assay for mannuronan C-5-epimerase activity. Carbohydr. Res. 1982, 103, 133–136. [Google Scholar] [CrossRef]
- Larsen, B.; Grasdalen, H. Investigation by nmr-spectroscopy of the site of proton-exchange catalyzed by poly(mannuronic acid) c-5 epimerase. Carbohydr. Res. 1981, 92, 163–167. [Google Scholar] [CrossRef]
- Holtan, S.; Zhang, Q.; Strand, W.; Skjåk-Bræk, G. Characterization of the hydrolysis mechanism of polyalternating alginate in weak acid and assignment of the resulting mg-oligosaccharides by nmr spectroscopy and ESI−Mass Spectrometry. Biomacromolecules 2006, 7, 2108–2121. [Google Scholar] [CrossRef]
- Inoue, A.; Satoh, A.; Morishita, M.; Tokunaga, Y.; Miyakawa, T.; Tanokura, M.; Ojima, T. Functional heterologous expression and characterization of mannuronan C5-epimerase from the brown alga Saccharina japonica. Algal Res. 2016, 16, 282–291. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Thom, D. Characterization of polysaccharide structure and interactions by circular-dichroism—Order-disorder transition in calcium alginate system. J. Chem. Soc. Chem. Commun. 1973, 7, 245–246. [Google Scholar] [CrossRef]
- Donati, I.; Gamini, A.; Skjak-Braek, G.; Vetere, A.; Campa, C.; Coslovi, A.; Paoletti, S. Determination of the diadic composition of alginate by means of circular dichroism: A fast and accurate improved method. Carbohydr. Res. 2003, 338, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Stenta, M.; Calvaresi, M.; Altoe, P.; Spinelli, D.; Garavelli, M.; Galeazzi, R.; Bottoni, A. Catalytic mechanism of diaminopimelate epimerase: A QM/MM Investigation. J. Chem. Theory Comput. 2009, 5, 1915–1930. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Dutta Dubey, K. Combined MD and QM/MM Calculations Reveal Allostery-Driven Promiscuity in Dipeptide Epimerases of Enolase Family. Chem.: Asian J. 2022, 17. [Google Scholar] [CrossRef]
- Penman, A.; Sanderson, G.R. Method for determination of uronic acid sequence in alginates. Carbohydr. Res. 1972, 25, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Gawin, A.; Tietze, L.; Aarstad, O.A.; Aachmann, F.L.; Brautaset, T.; Ertesvag, H. Functional characterization of three Azotobacter chroococcum alginate-modifying enzymes related to the Azotobacter vinelandii AlgE mannuronan C-5-epimerase family. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Rees, D.A.; Thom, D. Characterization of alginate composition and block-structure by circular-dichroism. Carbohydr. Res. 1980, 81, 305–314. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef]
- Nyvall, P.; Corre, E.; Boisset, C.; Barbeyron, T.; Rousvoal, S.; Scornet, D.; Kloareg, B.; Boyen, C. Characterization of mannuronan C-5-epimerase genes from the brown alga Laminaria digitata. Plant Physiol. 2003, 133, 726–735. [Google Scholar] [CrossRef]
- Tonon, T.; Rousvoal, S.; Roeder, V.; Boyen, C. Expression profiling of the mannuronan c5-epimerase multigenic family in the brown alga laminaria digitata (phaeophyceae) under biotic stress conditions. J. Phycol. 2008, 44, 1250–1256. [Google Scholar] [CrossRef]
- Ye, N.H.; Zhang, X.W.; Miao, M.; Fan, X.; Zheng, Y.; Xu, D.; Wang, J.F.; Zhou, L.; Wang, D.S.; Gao, Y.; et al. Saccharina genomes provide novel insight into kelp biology. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Shan, T.F.; Pang, S.J.; Li, J.; Li, X. De novo transcriptome analysis of the gametophyte of Undaria pinnatifida (Phaeophyceae). J. Appl. Phycol. 2015, 27, 1011–1019. [Google Scholar] [CrossRef]
- Fischl, R.; Bertelsen, K.; Gaillard, F.; Coelho, S.; Michel, G.; Klinger, M.; Boyen, C.; Czjzek, M.; Herve, C. The cell-wall active mannuronan C5-epimerases in the model brown alga Ectocarpus: From gene context to recombinant protein. Glycobiology 2016, 26, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Kurland, C.; Gallant, J. Errors of heterologous protein expression. Curr. Opin. Biotechnol. 1996, 7, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, W.J.; Lu, Y.Y.; Li, H.; Xu, S.; Wang, X.; Chen, K.Q. Enhancing soluble expression of phospholipase b for efficient catalytic synthesis of l-alpha-glycerylphosphorylcholine. Catalysts 2022, 12, 650. [Google Scholar] [CrossRef]
- Sadoff, H.L. Encystment and germination in Azotobacter vinelandii. Bacteriol. Rev. 1975, 39, 516–539. [Google Scholar] [CrossRef]
- Hoidal, H.K.; Svanem, B.I.G.; Gimmestad, M.; Valla, S. Mannuronan C-5 epimerases and cellular differentiation of Azotobacter vinelandii. Environ. Microbiol. 2000, 2, 27–38. [Google Scholar] [CrossRef]
- Ertesvåg, H.; Høidal, H.K.; Hals, I.K.; Rian, A.; Doseth, B.; Valla, S. A family of modular type mannuronan C-5-epimerase genes controls alginate structure in Azotobacter vinelandii. Mol. Microbiol. 1995, 16, 719–731. [Google Scholar] [CrossRef]
- Svanem, B.I.G.; Skjåk-Bræk, G.; Ertesvåg, H.; Valla, S. Cloning and expression of three new Azotobacter vinelandii genes closely related to a previously described gene family encoding mannuronan C-5-epimerases. J. Bacteriol. 1999, 181, 68–77. [Google Scholar] [CrossRef]
- Steigedal, M.; Sletta, H.; Moreno, S.; Mærk, M.; Christensen, B.E.; Bjerkan, T.; Ellingsen, T.E.; Espìn, G.; Ertesvåg, H.; Valla, S. The Azotobacter vinelandii AlgE mannuronan C-5-epimerase family is essential for the in vivo control of alginate monomer composition and for functional cyst formation. Environ. Microbiol. 2008, 10, 1760–1770. [Google Scholar] [CrossRef]
- Ertesvag, H.; Hoidal, H.K.; Skjak-Braek, G.; Valla, S. The Azotobacter vinelandii mannuronan C-5-epimerase AlgE1 consists of two separate catalytic domains. J. Biol. Chem. 1998, 273, 30927–30932. [Google Scholar] [CrossRef]
- Ertesvåg, H.; Valla, S. The A modules of the Azotobacter vinelandii mannuronan-C-5-epimerase AlgE1 are sufficient for both epimerization and binding of Ca2+. J. Bacteriol. 1999, 181, 3033–3038. [Google Scholar] [CrossRef] [PubMed]
- Ertesvåg, H.; Doseth, B.; Larsen, B.; Skjåk-Bræk, G.; Valla, S. Cloning and expression of an Azotobacter vinelandii mannuronan C-5-epimerase gene. J. Bacteriol. 1994, 176, 2846–2853. [Google Scholar] [CrossRef] [PubMed]
- Buchinger, E.; Knudsen, D.H.; Behrens, M.A.; Pedersen, J.S.; Aarstad, O.A.; Tøndervik, A.; Valla, S.; Skjåk-Bræk, G.; Wimmer, R.; Aachmann, F.L. Structural and functional characterization of the R-modules in alginate C-5 epimerases AlgE4 and AlgE6 from Azotobacter vinelandii. J. Biol. Chem. 2014, 289, 31382–31396. [Google Scholar] [CrossRef]
- Bjerkan, T.M.; Lillehov, B.E.; Strand, W.I.; Skjå‹ Braek, G.; Valla, S.; Ertesvåg, H. Construction and analyses of hybrid Azotobacter vinelandii mannuronan C-5 epimerases with new epimerization pattern characteristics. Biochem. J. 2004, 381 Pt 3, 813–821. [Google Scholar] [CrossRef]
- Rehm, B.H.; Ertesvag, H.; Valla, S. New Azotobacter vinelandii mannuronan C-5-epimerase gene (algG) is part of an alg gene cluster physically organized in a manner similar to that in Pseudomonas aeruginosa. J. Bacteriol. 1996, 178, 5884–5889. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, C.; Ohman, D. Cloning of Pseudomonas aeruginosa algG, which controls alginate structure. J. Bacteriol. 1990, 172, 2894–2900. [Google Scholar] [CrossRef]
- Franklin, M.J.; Chitnis, C.E.; Gacesa, P.; Sonesson, A.; White, D.C.; Ohman, D.E. Pseudomonas aeruginosa algG is a polymer level alginate c5-mannuronan epimerase. J. Bacteriol. 1994, 176, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Franklin, M.J.; Ertesvag, H.; Valla, S.; Ohman, D.E. The dual roles of AlgG in C-5-epimerization and secretion of alginate polymers in Pseudomonas aeruginosa. Mol. Microbiol. 2003, 47, 1123–1133. [Google Scholar] [CrossRef]
- Skj, G.; Grasdalen, H.; Larsen, B. Monomer sequence and acetylation pattern in some bacterial alginates. Carbohydr. Res. 1986, 154, 239–250. [Google Scholar]
- Bjerkan, T.M.; Bender, C.L.; Ertesvag, H.; Drablos, F.; Fakhr, M.K.; Preston, L.A.; Skjak-Braek, G.; Valla, S. The pseudomonas syringae genome encodes a combined mannuronan C-5-epimerase and O-acetylhydrolase, which strongly enhances the predicted gel-forming properties of alginates. J. Biol. Chem. 2004, 279, 28920–28929. [Google Scholar] [CrossRef]
- Ramstad, M.V.; Ellingsen, T.E.; Josefsen, K.D.; Hoidal, H.K.; Valla, S.; Skjak-Braek, G.; Levine, D.W. Properties and action pattern of the recombinant mannuronan C-5-epimerase AlgE2. Enzyme Microb. Technol. 1999, 24, 636–646. [Google Scholar] [CrossRef]
- Hoidal, H.K.; Ertesvag, H.; Skjak-Braek, G.; Stokke, B.T.; Valla, S. The recombinant Azotobacter vinelandii mannuronan C-5-epimerase AlgE4 epimerizes alginate by a nonrandom attack mechanism. J. Biol. Chem. 1999, 274, 12316–12322. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, P. Alginate-modifying enzymes: A proposed unified mechanism of action for the lyases and epimerases. FEBS Lett. 1987, 212, 199–202. [Google Scholar] [CrossRef]
- Rozeboom, H.J.; Bjerkan, T.M.; Kalk, K.H.; Ertesvag, H.; Holtan, S.; Aachmann, F.L.; Valla, S.; Dijkstra, B.W. Structural and mutational characterization of the catalytic A-module of the mannuronan C-5-epimerase AlgE4 from Azotobacter vinelandii. J. Biol. Chem. 2008, 283, 23819–23828. [Google Scholar] [CrossRef]
- Xu, F.; Wang, P.; Zhang, Y.-Z.; Chen, X.-L. Diversity of three-dimensional structures and catalytic mechanisms of alginate lyases. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Mikami, B.; Ban, M.; Suzuki, S.; Yoon, H.J.; Miyake, O.; Yamasaki, M.; Ogura, K.; Maruyama, Y.; Hashimoto, W.; Murata, K. Induced-fit motion of a lid loop involved in catalysis in alginate lyase A1-III. Prog. Polym. Sci. 2012, 68, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Dypås, L.B. Modification and characterization of the catalytic subunit, the a-module, of the mannuronan C-5 Epimerases AlgE4 and AlgE6. Master’s Thesis, NTNU, Trondheim, Norway, 2015. [Google Scholar]
- Aachmann, F.L.; Svanem, B.I.; Güntert, P.; Petersen, S.B.; Valla, S.; Wimmer, R. NMR structure of the R-module: A parallel β-roll subunit from an Azotobacter vinelandii mannuronan C-5 epimerase. J. Biol. Chem. 2006, 281, 7350–7356. [Google Scholar] [CrossRef]
- Andreassen, T.; Buchinger, E.; Skjåk-Bræk, G.; Valla, S.; Aachmann, F.L. 1H, 13C and 15N resonances of the AlgE62 subunit from Azotobacter vinelandii mannuronan C5-epimerase. Biomol. NMR Assign. 2011, 5, 147–149. [Google Scholar] [CrossRef]
- Buchinger, E.; Skjåk-Bræk, G.; Valla, S.; Wimmer, R.; Aachmann, F.L. NMR assignments of 1H, 13C and 15N resonances of the C-terminal subunit from Azotobacter vinelandii mannuronan C5-epimerase 6 (AlgE6R3). Biomol. NMR Assign. 2011, 5, 27–29. [Google Scholar] [CrossRef]
- Aachmann, F.L.; Skjåk-Bræk, G. 1H, 15N, 13C resonance assignment of the AlgE6R1 subunit from the Azotobacter vinelandii mannuronan C5-epimerase. Biomol. NMR Assign. 2008, 2, 123–125. [Google Scholar] [CrossRef]
- Delepelaire, P. Type I secretion in gram-negative bacteria. Biochim. Biophys. Acta 2004, 1694, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Baumann, U.; Wu, S.; Flaherty, K.M.; McKay, D. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: A two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993, 12, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Baumann, U. Crystal structure of the 50 kDa metallo protease from Serratia marcescens. J. Mol. Biol. 1994, 242, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Pickersgill, R. The architecture of parallel beta-helices and related folds. Prog. Biophys. Mol. 2001, 77, 111–175. [Google Scholar] [CrossRef]
- Tondervik, A.; Klinkenberg, G.; Aachmann, F.L.; Svanem, B.I.G.; Ertesvag, H.; Ellingsen, T.E.; Valla, S.; Skjak-Braek, G.; Sletta, H. Mannuronan C-5 Epimerases Suited for Tailoring of Specific Alginate Structures Obtained by High-Throughput Screening of an Epimerase Mutant Library. Biomacromolecules 2013, 14, 2657–2666. [Google Scholar] [CrossRef]
- Xavier Robert, P.G. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, 320–324. [Google Scholar] [CrossRef]
- Gao, S.K.; Yin, R.; Wang, X.C.; Jiang, H.N.; Liu, X.X.; Lv, W.; Ma, Y.; Zhou, Y.X. Structure characteristics, biochemical properties, and pharmaceutical applications of alginate lyases. Mar. Drugs 2021, 19, 628. [Google Scholar] [CrossRef]
- Yoder, N.T.K. Frances Jurnak, New domain motif: The structure of pectate lyase C, a secreted plant virulence factor. Science 1993, 260, 1503–1507. [Google Scholar] [CrossRef]
- Pickersgill, R.; Smith, D.; Worboys, K.; Jenkins, J. Crystal Structure of Polygalacturonase from Erwinia carotovora ssp. carotovora. Journal of Biological Chemistry 1998, 273, 24660–24664. [Google Scholar] [CrossRef]
- Jerga, A.; Stanley, M.D.; Tipton, P.A. Chemical mechanism and specificity of the C5-mannuronan epimerase reaction. Biochemistry 2006, 45, 9138–9144. [Google Scholar] [CrossRef]
- Stanisci, A.; Tondervik, A.; Gaardlos, M.; Lervik, A.; Skjak-Braek, G.; Sletta, H.; Aachmann, F.L. Identification of a pivotal residue for determining the block structure-forming properties of alginate C-5 Epimerases. Acs Omega 2020, 5, 4352–4361. [Google Scholar] [CrossRef] [PubMed]


| Methods | Strength | Limitations | Reference |
|---|---|---|---|
| Carbazole’s reaction | Simple | Low reproducibility, and response | [28] |
| Enzyme-coupled assay | Suitable for measuring low level epimerase activity | Limited by bifunctional activities; Require specific lyase with high activity and stability | [34] |
| NMR | Accurate; More information about products | Require sample pretreatment | [23] |
| Radiometric assay | Sensitive | Require for substrate preparation | [42] |
| Acid hydrolysis | Simple | Low accuracy | [50] |
| Ca2+-induced gel precipitation | Simple | Low accuracy | [51] |
| Circular dichroism | Simple | Low response | [52] |
| Name | Orgnism | Optimal Temperature (°C) | Optimal pH | Products | Reference |
|---|---|---|---|---|---|
| Ca2+-dependent | |||||
| AlgE1 | A. vineland | 37 | 6.9 | GG and MG blocks | [66] |
| AlgE2 | A. vineland | 55 | 6.5–7.0 | GG blocks | [77] |
| AlgE3 | A. vineland | - | - | GG and MG blocks | [63] |
| AlgE4 | A. vineland | 37 | 6.7–7.0 | MG blocks | [78] |
| AlgE5 | A. vineland | - | - | GG blocks | [63] |
| AlgE6 | A. vineland | - | - | GG blocks | [64] |
| AlgE7 | A. vineland | - | - | G residues and GG blocks | [64] |
| AcAlgE1 | A. chroococcum | - | - | GG blocks | [51] |
| AcAlgE2 | A. chroococcum | - | - | GG blocks, Mred and ΔM | |
| AcAlgE3 | A. chroococcum | - | - | Mred, Gred, ΔM and ΔG | |
| PsmE | P. syringae | 37 | 6.8 | GG blocks | [76] |
| MEP13 | Ectocarpus | - | - | GG blocks | [58] |
| Ca2+-independent | |||||
| PmC5A | P. mendocina | 30 | 9 | Δ | [38] |
| SjC5-VI | S. japonica | 35 | 7.0–8.2 | - | [45] |
| SjMC5E2 | S. japonica | - | - | GG blocks | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Sun, M.; Li, T.; Zhao, M.; Yin, H. Mannuronan C-5 Epimerases: Review of Activity Assays, Enzyme Characteristics, Structure, and Mechanism. Catalysts 2023, 13, 28. https://doi.org/10.3390/catal13010028
Xiao Z, Sun M, Li T, Zhao M, Yin H. Mannuronan C-5 Epimerases: Review of Activity Assays, Enzyme Characteristics, Structure, and Mechanism. Catalysts. 2023; 13(1):28. https://doi.org/10.3390/catal13010028
Chicago/Turabian StyleXiao, Zhongbin, Ming Sun, Tang Li, Miao Zhao, and Heng Yin. 2023. "Mannuronan C-5 Epimerases: Review of Activity Assays, Enzyme Characteristics, Structure, and Mechanism" Catalysts 13, no. 1: 28. https://doi.org/10.3390/catal13010028
APA StyleXiao, Z., Sun, M., Li, T., Zhao, M., & Yin, H. (2023). Mannuronan C-5 Epimerases: Review of Activity Assays, Enzyme Characteristics, Structure, and Mechanism. Catalysts, 13(1), 28. https://doi.org/10.3390/catal13010028







