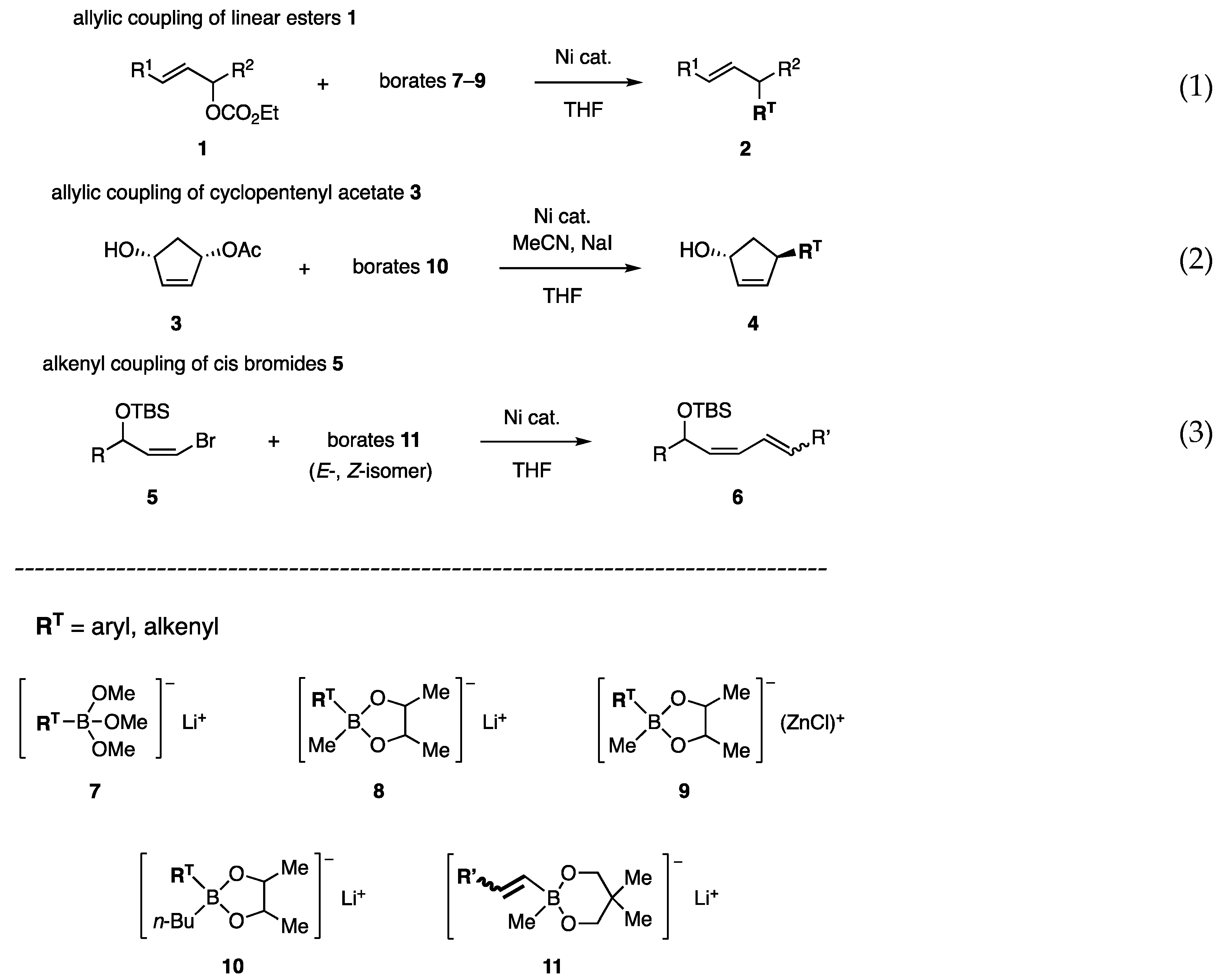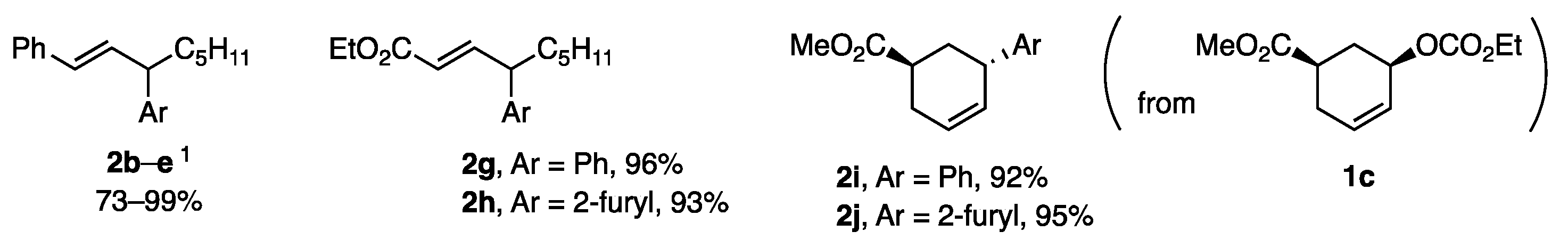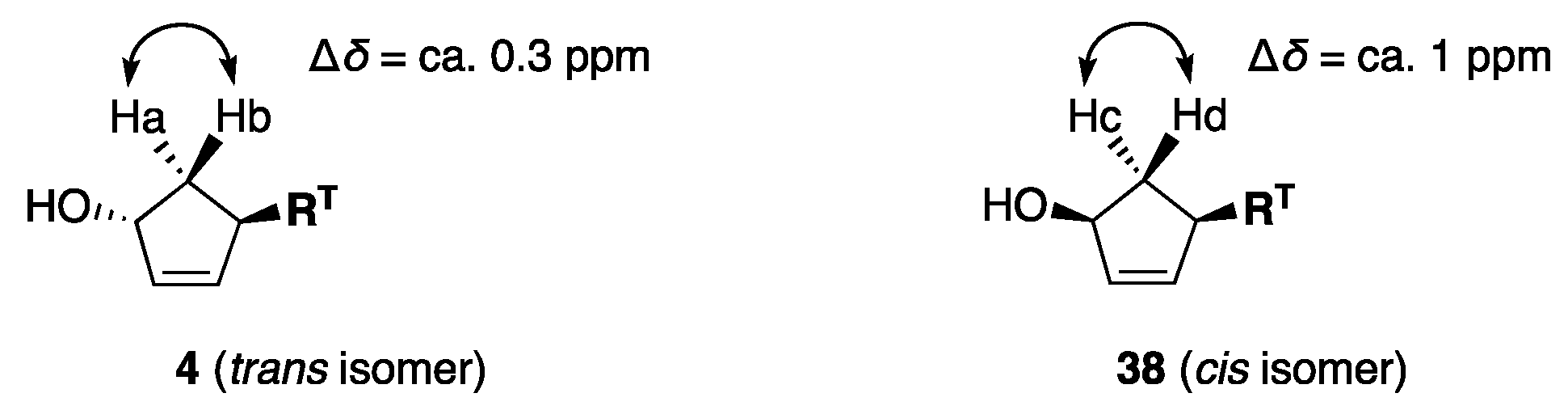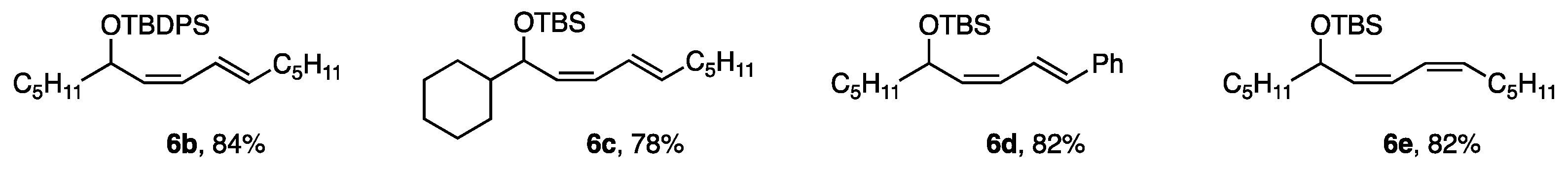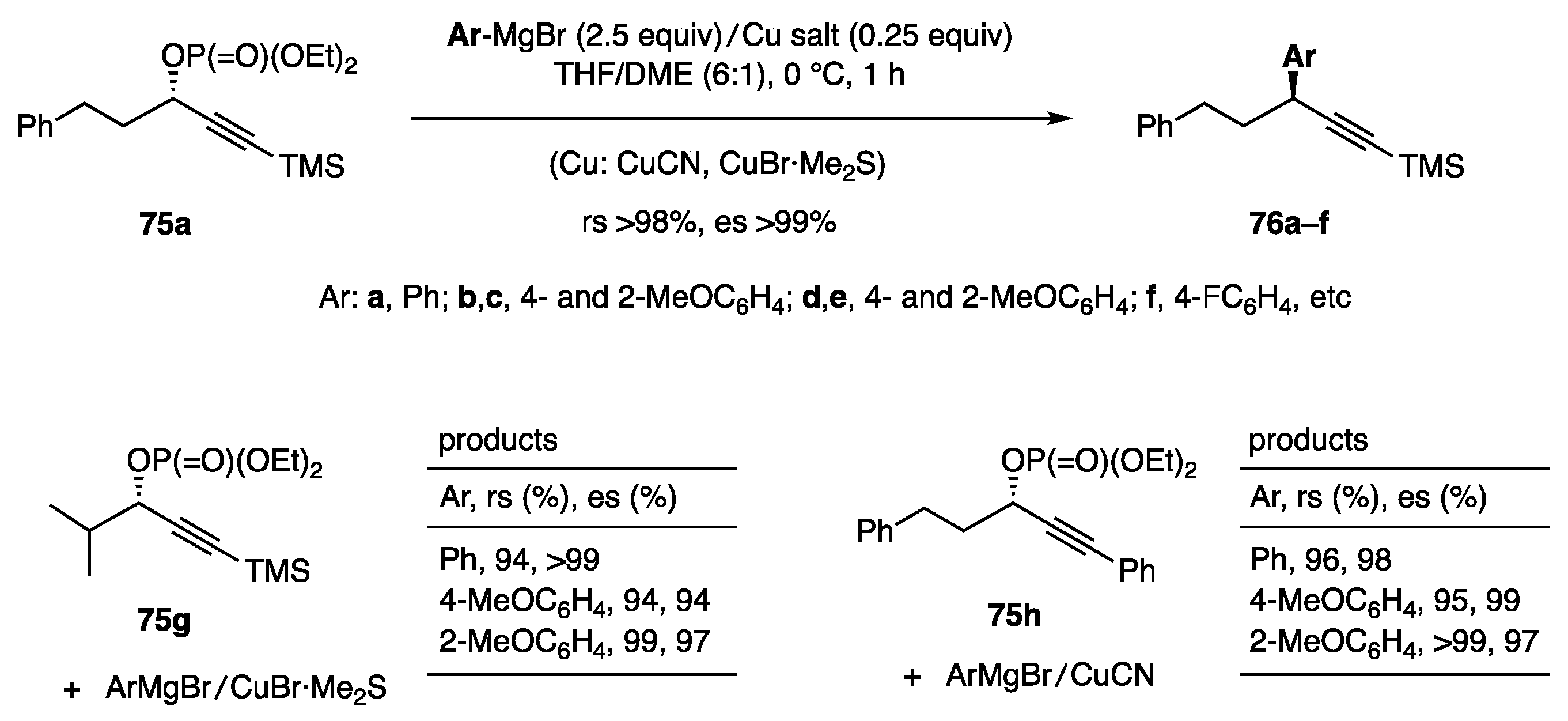Abstract
In the first part of this review, secondary carbon-carbon bond formation by using allylic coupling reactions with aryl and alkenyl borates is presented. Early investigations have revealed the suitability of a nickel catalyst and [RTB(OMe)3]Li (RT: transferable group). Due to their low reactivity, the borates were converted to more reactive congeners possessing an alkanediol ligand, such as 2,3-butanediol and 2,2-dimethyl-1,3-propanediol. Borates with such diol ligands were used to install aryl and alkenyl groups on the monoacetate of 4-cyclopentenyl-1,3-diol. Furthermore, alkenyl borates showed sufficient reactivity toward less reactive allylic alcohol derivatives with bromine atoms at the cis position, producing dieneyl alcohols. In the second part, copper-based and/or copper-catalyzed substitutions of secondary allylic picolinates, propargylic phosphonates, and alkyl (2-pyridine)sulfonates with RMgX are briefly summarized. The application of these reactions to the synthesis of biologically active compounds is also discussed.
Keywords:
borate; boronate ester; nickel; secondary carbon; coupling; substitution; Grignard reagent; copper; organic synthesis 1. Introduction
When I was a doctoral course student of Professor Tsuji (1978–1981), transition metal-catalyzed reactions were little recognized as tools for organic synthesis. The obvious reason for this is the cost of transition metal complexes for stoichiometric use. However, Professor Tsuji emphasized the advantages of using transition metal-catalyzed reactions that could not be attained by the classical reactions [1,2]. Furthermore, Professor Tsuji was interested in carbon-carbon bond-forming reactions and natural product synthesis. According to him, such inclinations were grown in the Stork laboratory at Columbia University in New York, where he pursued his doctoral research [3,4].
Among the studies undertaken in the Tsuji group at that time, the palladium-catalyzed allylic substitution reaction with soft nucleophiles was especially attractive because of the ability to form carbon-carbon bonds. Later, the allylic substitution prompted me to investigate the coupling reaction of secondary allylic substrates with hard nucleophiles to furnish chiral carbon-carbon bonds connecting various types of carbon groups.
Borates were selected as hard nucleophiles because of their expected high nucleophilicity based on the general consideration of ate complexes, although such reactivity was not observed for [BuCH=CHB(Sia)2Me]−Li+ in a mechanistic study of the Suzuki–Miyaura coupling reaction [5]. The recent progress in borates and boronate esters for carbon-carbon bond formation has been well summarized in several reviews [6,7]. In contrast, the reactions of borates in combination with nickel catalysts have been less well documented [8,9]. Herein, we present a review of our studies on nickel-catalyzed allylic coupling reactions on secondary allylic carbons and C(sp2)–C(sp2) coupling reactions with various types of borates, with a hope of spurring future studies on borate chemistry and organic synthesis. In addition, we briefly present our investigation of secondary C–C bond formation using allylic and propargylic substitutions with copper-based Grignard reagents. The substitution of secondary alkyl carbons is also mentioned.
2. Design of Reactive Borates
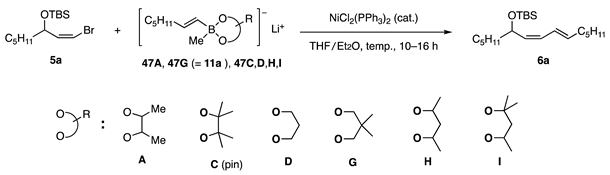
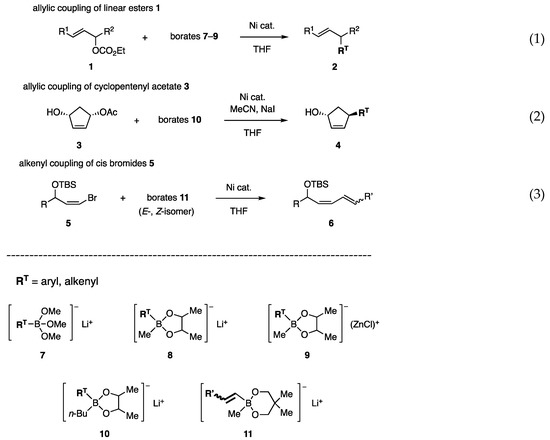
In an early investigation, allylic carbonates 1 and acetates were used as substrates to identify effective borates (RT: transferable group) and nickel catalysts (Scheme 1, Equation (1)). Trimethoxy borate 7 was studied first to find a nickel catalyst [10] and then advanced to borate 8, which possesses the 2,2-butanediol and methyl ligands [11]. Subsequently, carbonyl group-friendly borates 9 were developed [12]. Allylic coupling with cyclopentenyl acetate 3 was established using borate 10, which possesses an n-Bu ligand to produce higher reactivity (Equation (2)) [13]. Products 4 were used to synthesize prostaglandins and aristeromycin. Furthermore, the concept of using a diol ligand led us to develop new alkenyl borates 11 for the coupling reaction of cis-bromo olefins 5, which are readily available but suffer from low reactivity (Equation (3)) [14]. The synthesis of biologically active dienes has been achieved. Due to their high reactivity, borates were successfully used in the coupling reaction with aryl mesylates and tosylates [15], which are less reactive than triflates in catalytic coupling reactions.
Scheme 1.
Allylic and alkenyl coupling reactions with borates.
3. Preparation of Borates
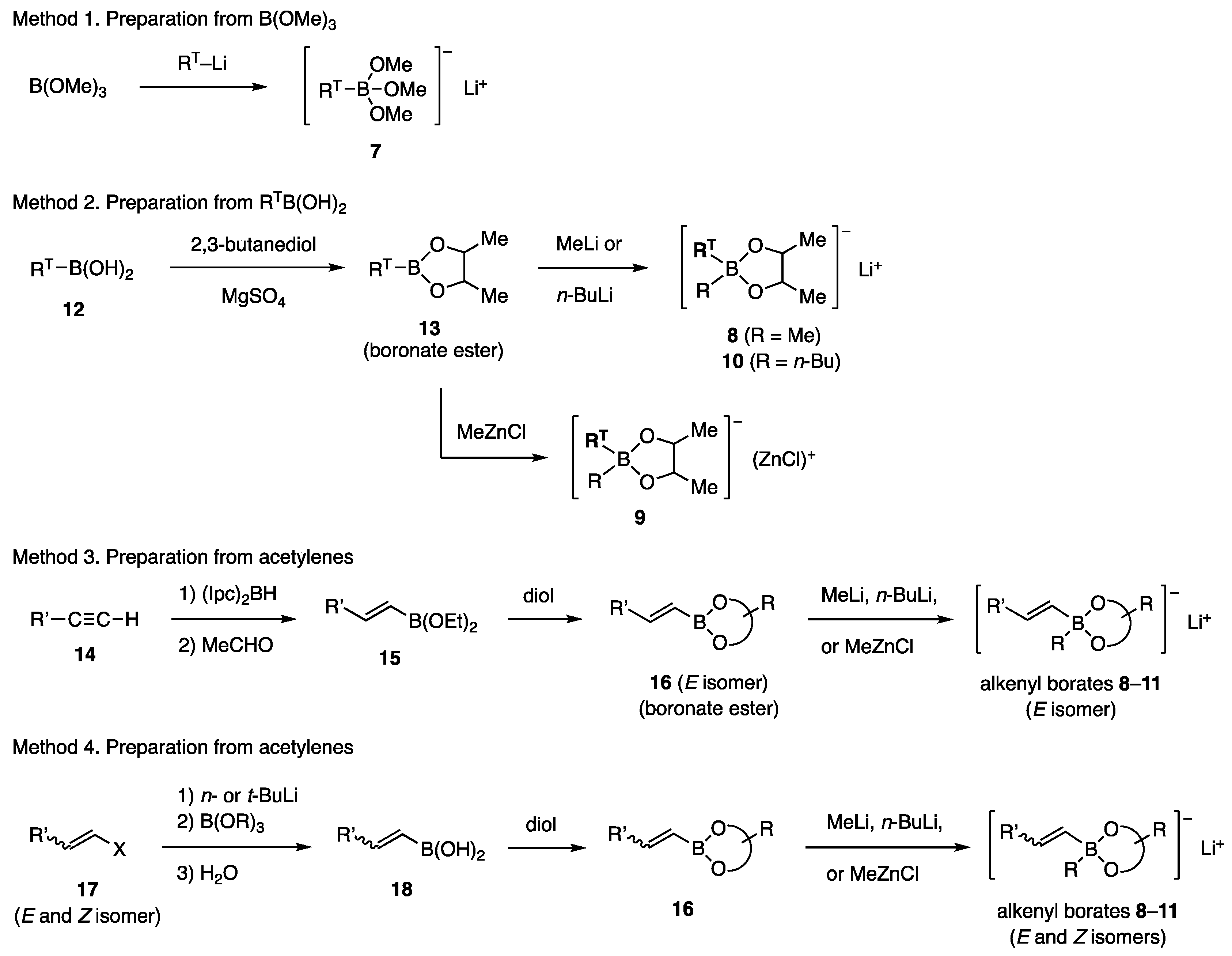
The borates studied in this work were prepared using the methods described in Scheme 2 (RT = aryl or alkenyl). Commercially available B(OMe)3 was converted with RT-Li to borates 7 (Method 1) [10,11], which was used for coupling without isolation. Borates 8–10 were obtained by the esterification of boronic acids RTB(OH)2 (12) with 2,3-butanediol, followed by the addition of MeLi [11], n-BuLi [11], or MeZnCl [12] to the resulting boronate esters 13 (Method 2). Some of the (E)-alkenyl borates 8–10 (RT = (E)-alkenyl) and 11 were obtained via hydroboration of acetylenes 14 with (Ipc)2BH, followed by the conversion of Ipc to EtO with MeCHO (Method 3). Hydroboration with (c-Hex)2BH, followed by oxidation with Me3NO, has also been used to prepare boronic acids. A transformation consisting of lithiation of stereo-defined halo olefins 17 (X = Br, I) followed by reactions similar to Method 1 produced the (E)- and (Z)-isomers of 8–11 (Method 4). Boronate esters 13 and 16 are much less polar than the free diol and are chemically stable, allowing purification by chromatography on silica gel. Distillation of boronate esters is recommended before the reaction with MeLi, n-BuLi, or MeZnCl. Currently, various boronic acids, RTB(OH)2, and boronate esters are commercially available. However, sterically demanding diol ligands decreased their reactivity in our study, as discussed below. Pinacol, known as “pin”, is one of such ligands.
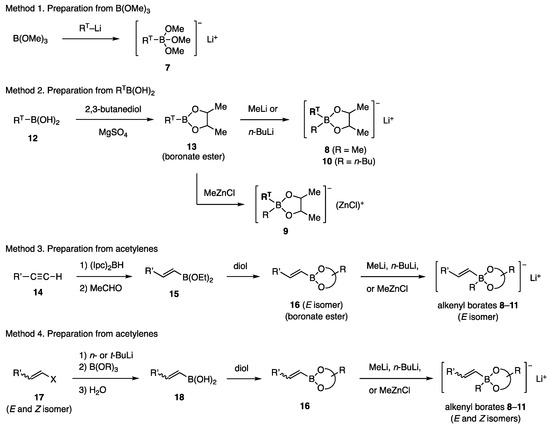
Scheme 2.
Preparation of borates.
4. Allylic Coupling
4.1. Trimethoxyborates
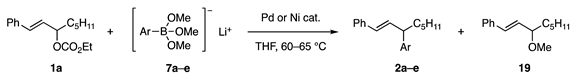
The reaction of carbonate 1a with phenylborate 7a (prepared from B(OMe)3 and PhLi) was first examined [10,11]. Unfortunately, palladium catalysts at 60–65 °C produced methyl ether 19 (Table 1, entries 1 and 2). In contrast, the Ni catalysts unexpectedly afforded coupling product 2a in high yields (entries 3 and 4). Other aryl borates 7b–e afforded 2b–e in good yields (entries 5–8). The borate/Ni catalyst was successfully applied to the reaction of another substrate, 1b (R1 = CO2Et, R2 = C5H11).

Table 1.
Allylic substitution with aryl borates.
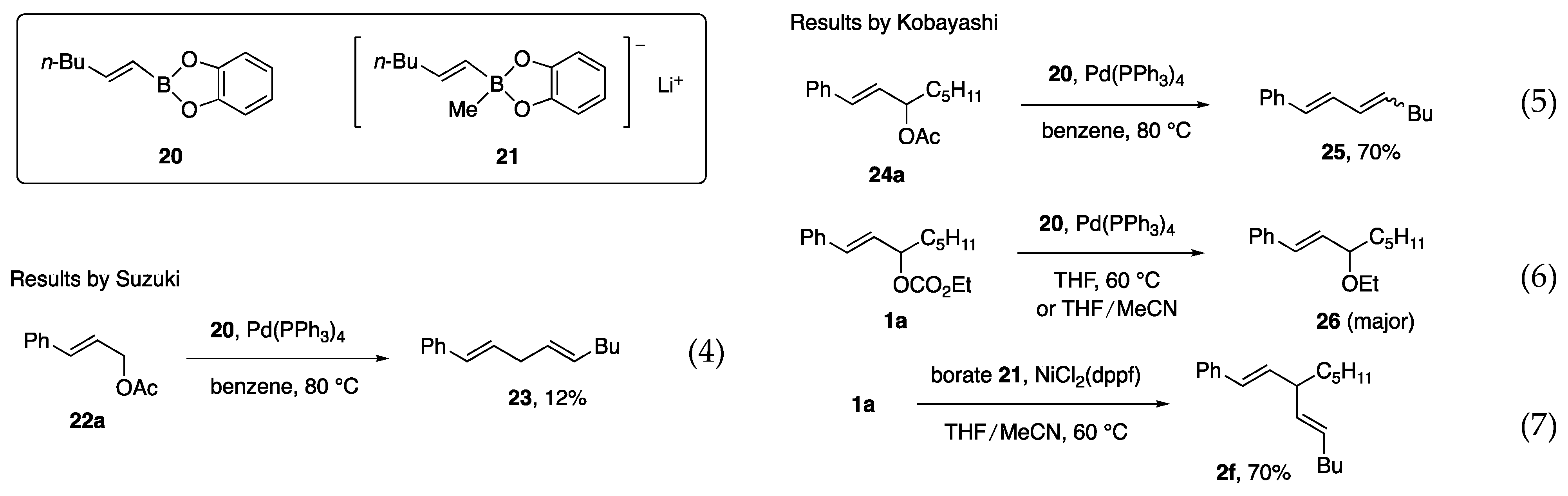
Before our investigation, the Pd-catalyzed reaction of primary allylic acetate 22a with boronate ester 20 was reported by Suzuki and Miyaura in their mechanistic study of the “Suzuki–Miyaura coupling reaction” (Scheme 3, Equation (4)) [5]. Due to the reactivity toward secondary acetates has not been reported, acetate 24a, and carbonate 1a were subjected to Pd-catalyzed reactions in our study. However, undesired diene 25 or Et ether 26 was produced (Equations (5) and (6)) [11]. In contrast, the reaction of 1a with borate 21 and a Ni catalyst proceeded smoothly to afford 2f with a 70% yield (Equation (7)).
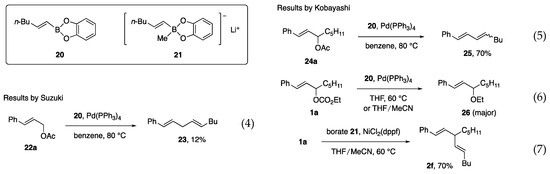
Scheme 3.
Related reactions by Suzuki (Equation (4)) and by us (Equations (5)–(7)).
4.2. Borates with Alkanediol Ligands
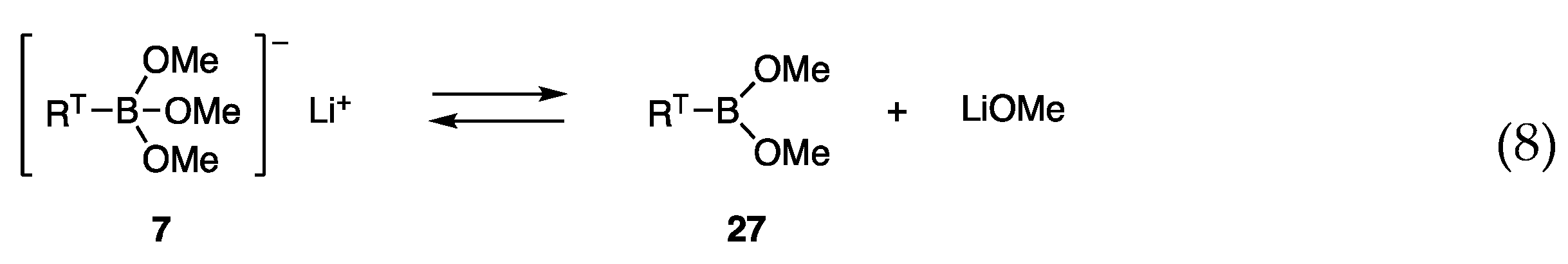
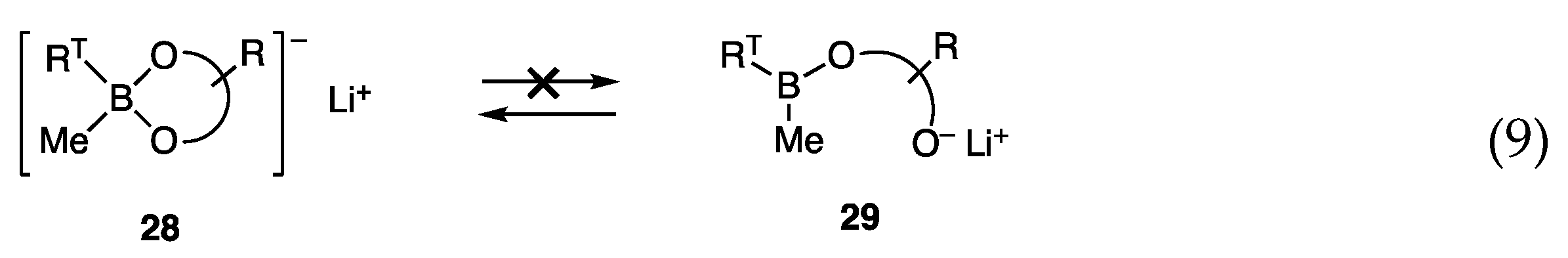
Although the allylic coupling reactions with trimethoxyborates [RT-B(OMe)3]− Li+ (7) proceeded in the presence of nickel catalysts, the required temperatures for the reaction were high (60–65 °C), and thus new borates with high reactivity at low temperatures were investigated. We postulated a low concentration of reactive species 7 in equilibrium with less reactive RTB(OMe)2 (27) and LiOMe (Scheme 4, Equation (8)), and envisaged that diol ligands in borates 28 would prevent the formation of the open form 29 by analogy with bidentate ligands that coordinate strongly with transition metals (Equation (9)).
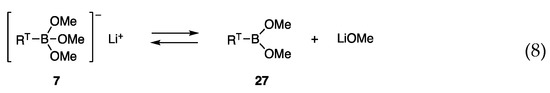
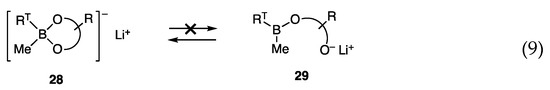
Scheme 4.
Borates in likely equilibria.
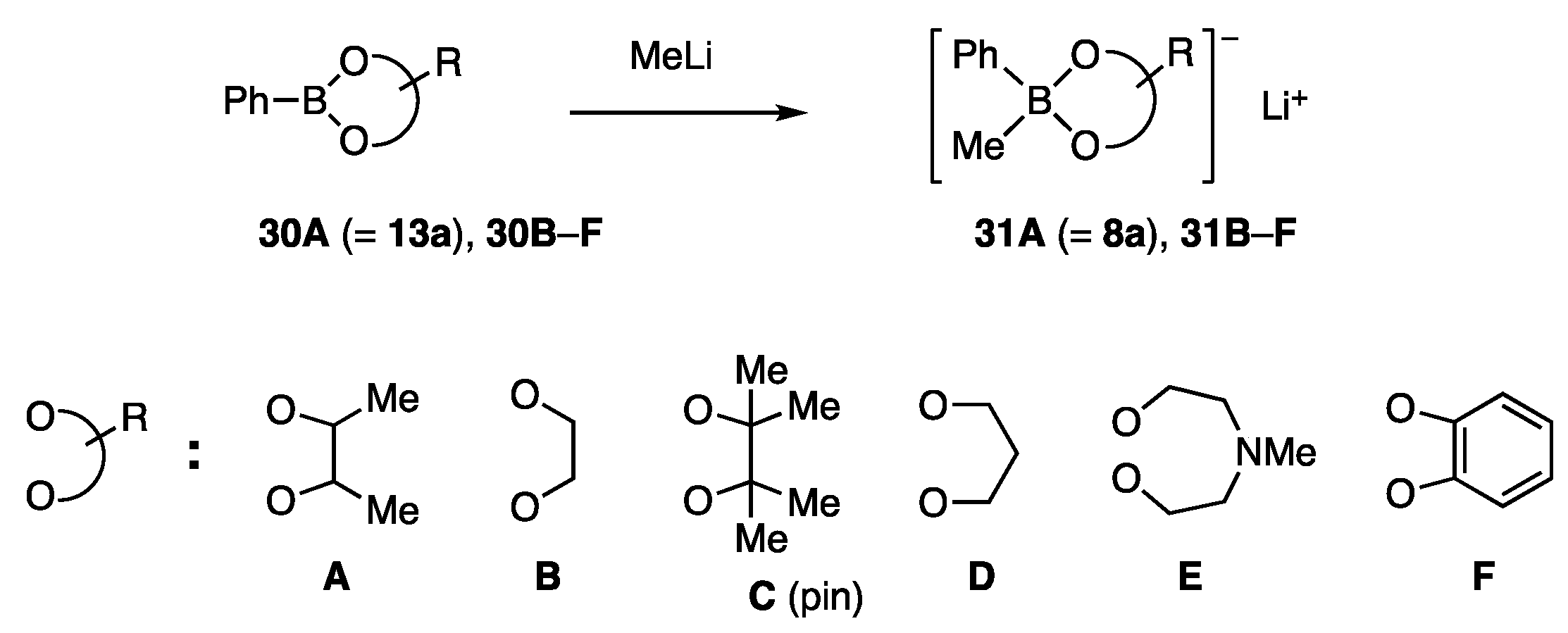
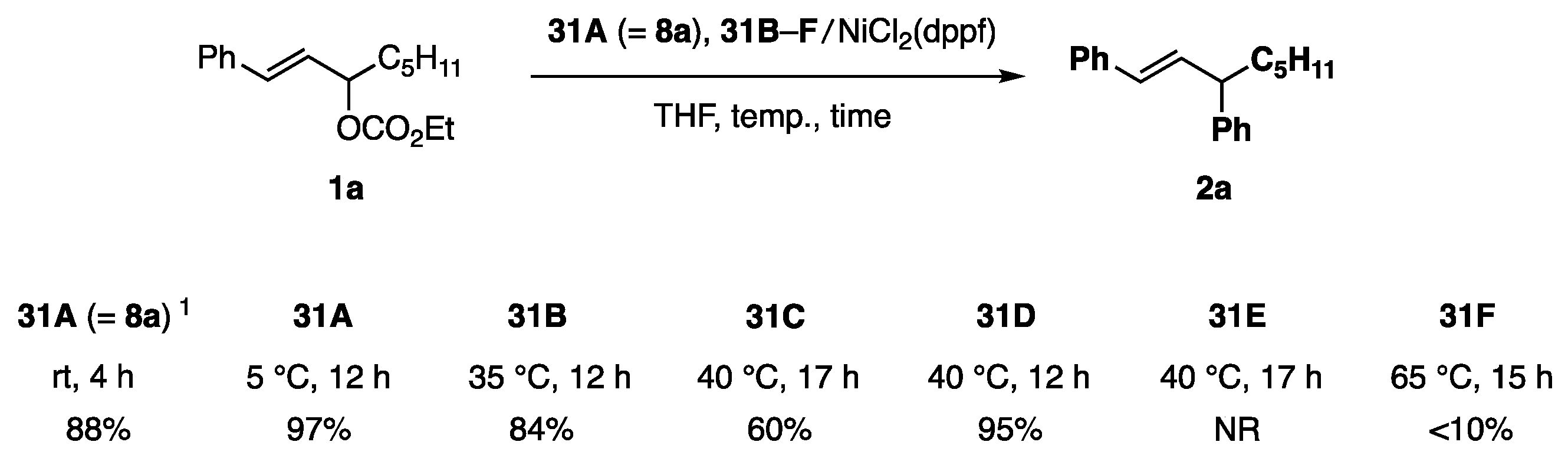
Phenylborates 31A (=8a) and 31B–F possessing various diol ligands A–F were chosen as test borates (Scheme 5). Among these ligands, C is known as a pin. The reactivity order of 31A (=8a) > 31B > 31D > 31C >> 31F > 31E was determined for the coupling reaction shown in Scheme 6. This order is consistent with the working hypothesis proposed in Scheme 4. Unanticipatedly, the methyl group on the diol ligand increased the reactivity (A vs. B), whereas the congested methyl groups were affected reversely because of the steric reason (A vs. C (pin)). The most active ligand A allowed the reaction to proceed even at 5 °C. NiCl2(PPh3)2 showed similar catalytic activity to NiCl2(dppf). In contrast, 31A/Pd catalysts were marginally productive.
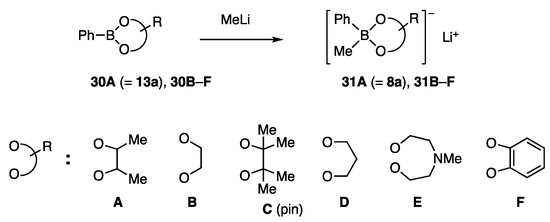
Scheme 5.
Phenylborates with diol ligands for allylic coupling.
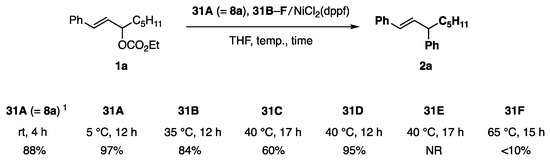
Scheme 6.
Allylic coupling reaction of 1a with phenylborates 31A–F. 1 2,3-Butanediol ligand consisted of dl- and meso-forms in a 4:1 ratio.
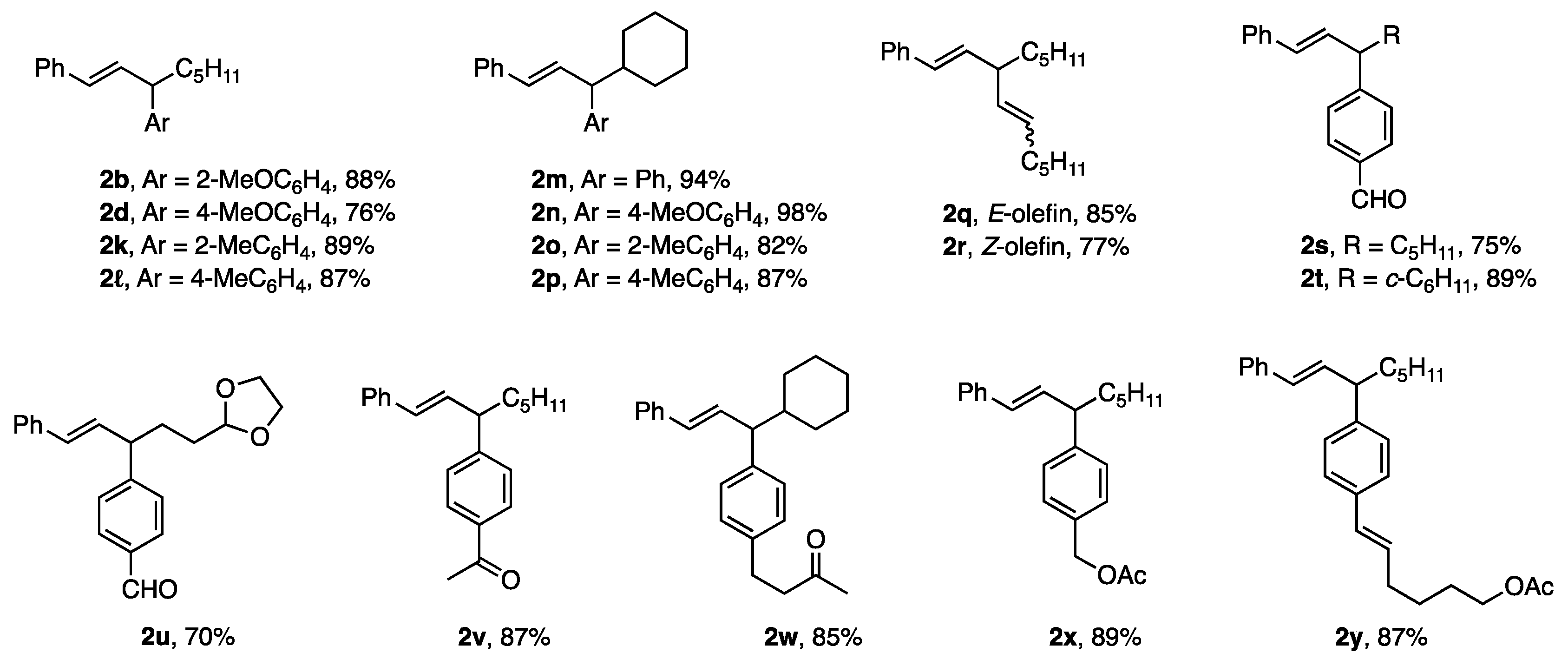
Various aryl borates with ligand A also showed high reactivity in allylic coupling to produce 2b–d with 2-, 3-, and 4-MeOC6H4 and 2e with 2-furyl in 85–99% yield (Figure 1). Products 2g–j demonstrated the compatibility of the borates with the ester groups present in the allylic substrates. The reaction of 1c with borates proceeded with inversion to afford 2i and 2j.
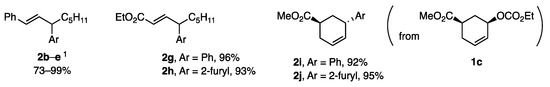
Figure 1.
Products 2b–j from borates with ligand A. 1 b, c, d: 2-, 3-, and 4-MeOC6H4; e and 2-fury.
4.3. Carbonyll Group-Friendly Zinc Borates
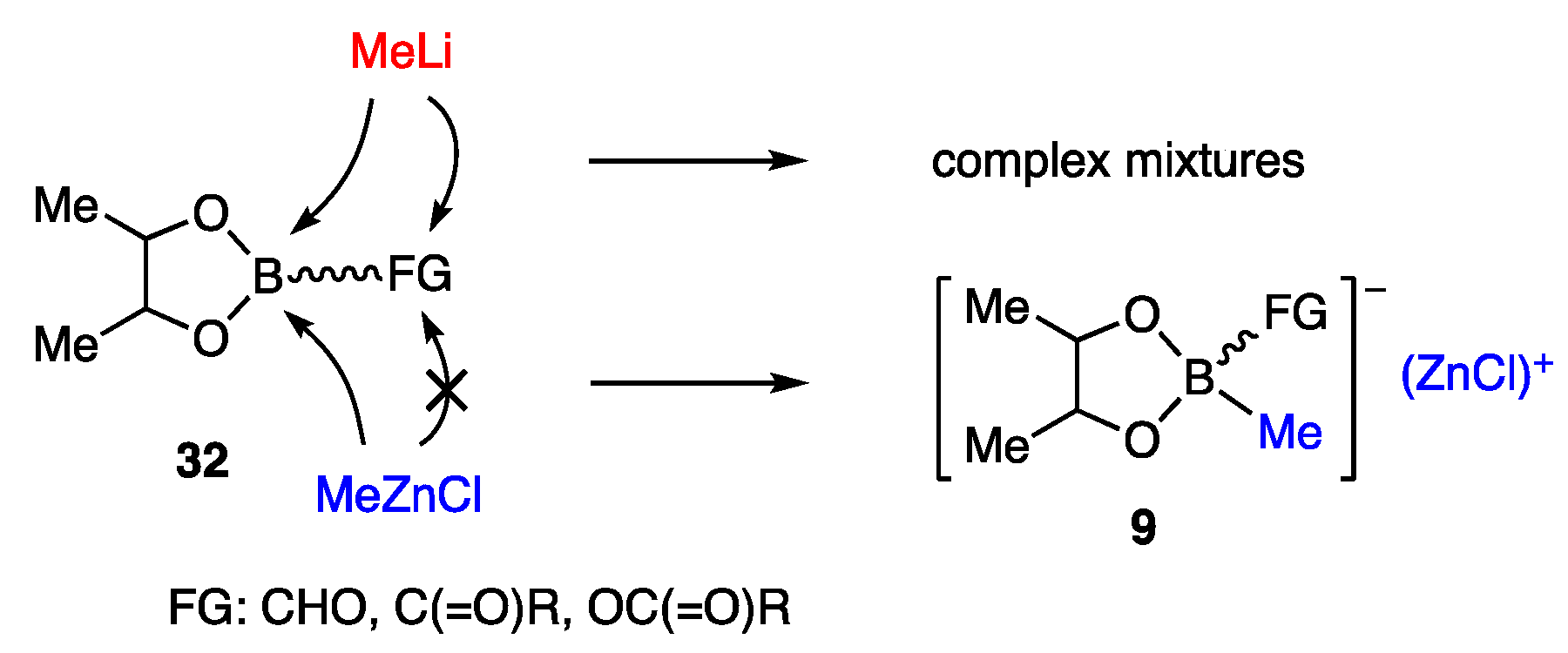
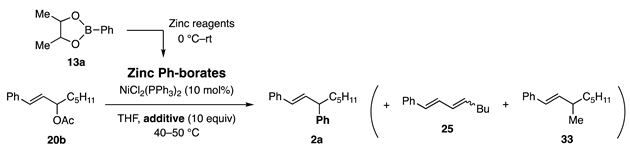
Since the carbonyl group in 32 was incompatible with MeLi, which is used for the transformation of boronate ester to borate, other organometallics that are reactive and friendly with such functional groups were explored (Scheme 7). Among the candidates examined, MeZnCl, upon addition to 32, provided zinc borate 9, which exhibited sufficient nucleophilicity for the allylic coupling [12].
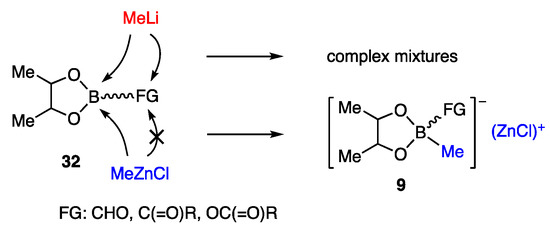
Scheme 7.
Reasons for developing zinc borates 9.
The reaction of acetate 20b with phenylborate 9a derived from 13a and MeZnCl at 40–50 °C produced 2a and byproducts (diene 25 and methyl coupling 33) (Table 2, entry 1). Similar results were obtained for the borates derived from MeZnBr (entry 2) and MeZnI. The use of postulated borates derived from MeZnF, n-BuZnCl, and Et2Zn was unsuccessful. Fortunately, the byproducts were eliminated by the addition of DMI or DMF (entries 3 and 4).

Table 2.
Allylic coupling reaction with zinc phenylborates.
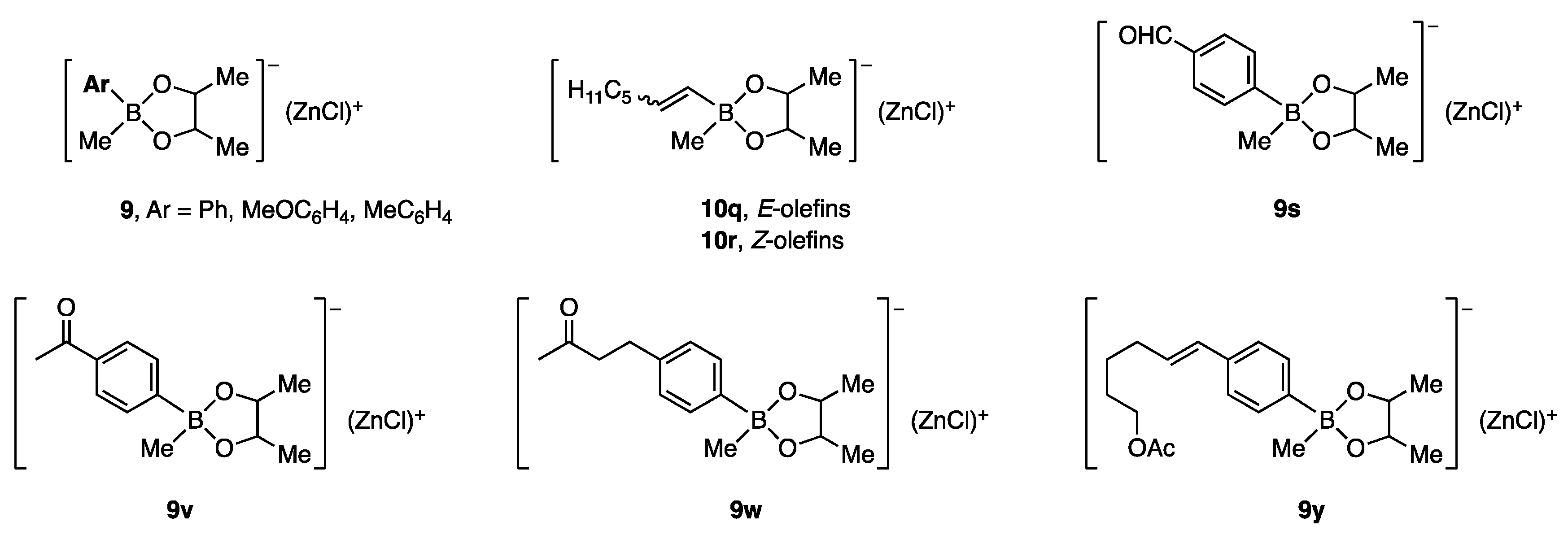
Under the reaction conditions used in entry 3 of Table 2, the zinc borates in Figure 2 underwent coupling reactions to give the products shown in Figure 3 in good to high yields. An anti-stereochemical course was established using cyclic substrates.
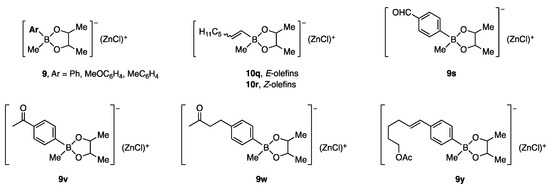
Figure 2.
Zinc borates prepared for allylic coupling reaction.
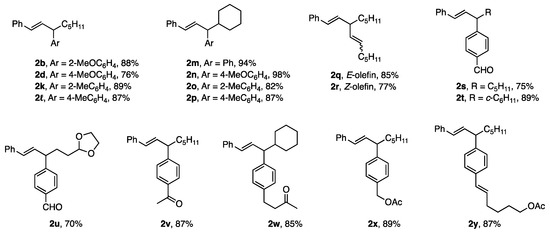
Figure 3.
Coupling products derived from zinc borates.
4.4. Allylic Coupling on Cyclopentenediol Monoacetate
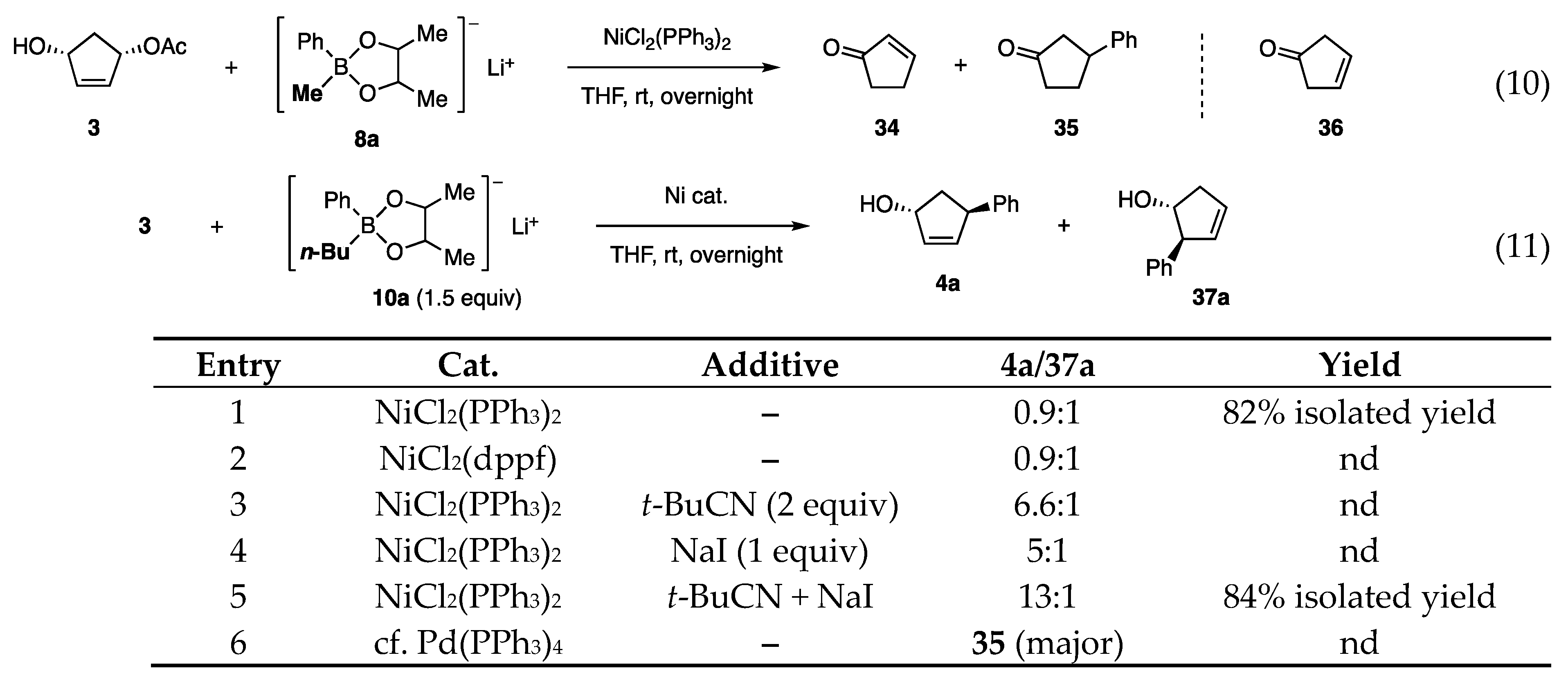
The Ni-catalyzed allylic coupling reaction with borates was then applied to cyclopentenediol monoacetate 3 (Scheme 1, Equation (2)) [13]. As substrate 3 is available in racemic as well as enantioenriched forms by several methods, the reaction was expected to provide new access to the cyclopentanoids such as prostaglandins. Unfortunately, an attempted reaction with phenylborate 8a (RT = Ph) gave a mixture of enone 34 and Ph ketone 35 (Scheme 8, Equation (10)). The likely steps to these byproducts involve predominant β-H elimination of the π-allyl Ni intermediate (derived from 3 and Ni) over the allylic coupling (step 1), isomerization of the resulting olefin 36 to enone 34 (step 2), and 1,4-addition probably catalyzed by the Ni catalyst (step 3). Reactions similar to steps 1 and 3 have been reported [16,17].
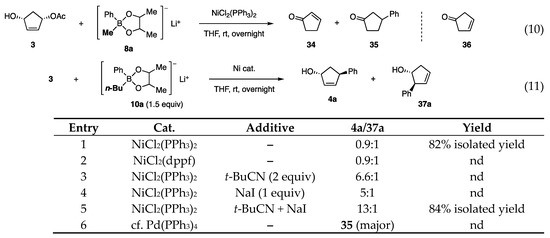
Scheme 8.
Reaction of cyclopentenyl acetate 3 with borates 8a and 10a 1. 1 Diol ligand consisted of dl- and meso-forms at a 4:1 ratio.
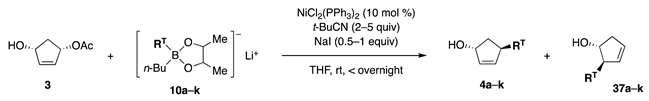
Based on the finding that the attachment of electron-donating Me groups to the diol ligand increased the nucleophilicity of borates (Scheme 6, 31A (=8a) vs. 31B), the Me ligand in 8a was replaced by the more electron-donating n-Bu. Indeed, the new phenylborate 10a transferred Ph to cyclopentenyl acetate 3 in the presence of NiCl2(PPh3)2 (Scheme 8, Equation (11), entry 1). However, a mixture of regioisomers 4a and 37a was produced at a 0.9:1 ratio. To solve this problem, catalysts, polar solvents, and inorganic salts were examined without any promising guidelines. Although NiCl2(dppf) did not change the product ratio (entry 2), t-BuCN and NaI favored the production of 4a (entries 3 and 4). Furthermore, the cooperative action between both additives resulted in a higher regioisomeric ratio of 13:1 (entry 5). In contrast, a Pd catalyst produced ketone 35 (entry 6).
The new reagent type consisting of 2,3-butanediol and n-Bu ligands was successfully applied to aryl borates 10b–e, which underwent a regioselective reaction in the presence of the two additives (t-BuCN and NaI) to produce 4b–e in good yields (Table 3, entries 2–5). Alkenyl borates 10f–k also afforded 4f–k (entries 6–11). Among the entries, the regioselectivity for 4h–j was especially high, whereas that for 4f and 4k was moderate. A hypothesis that accounts for this difference is the high nucleophilicity caused by the conjugation of the C–OTBS σ bond with the olefinic π-bond. In an additional entry, the MOM ether of 3 upon reaction with borate 10f afforded the MOM ethers of 4f and 37f at a ratio of 6:1 (equation not shown). This ratio was similar to that recorded for 3, indicating that the hydroxy group in 3 was not a regiocontroller.

Table 3.
Coupling reaction of cyclopentenyl acetate 3 with borates 10.
4.5. Determination of the Stereochemistry between RT and OH in the Major Products 4
Substituents OH and RT sterically affect the chemical shifts of Ha and Hb in 4 and Hc and Hd in cis isomer 38 (Figure 4) [18]. For example, Ha in 4 is cis to OH and trans to RT, whereas Hb is arranged in the opposite direction. This relationship (one cis, one trans) between Ha and Hb results in a small chemical shift difference (Δδ) of ca. 0.3 ppm. On the other hand, Hc in 38 is trans to OH and RT, whereas Hd is cis to these substituents, resulting in a large Δδ of 1 ppm. In our study, the stereochemistry of 4 was inverted to produce isomer 38 by the Mitsunobu inversion, and the vicinal protons (Ha/Hb, Hc/Hd) in the 1H NMR spectra were easily found based on the large coupling constants of ca. 14 Hz.

Figure 4.
Chemical shift difference (Δδ).
4.6. Synthetic Application of Allylic Coupling
4.6.1. Prostaglandin A2
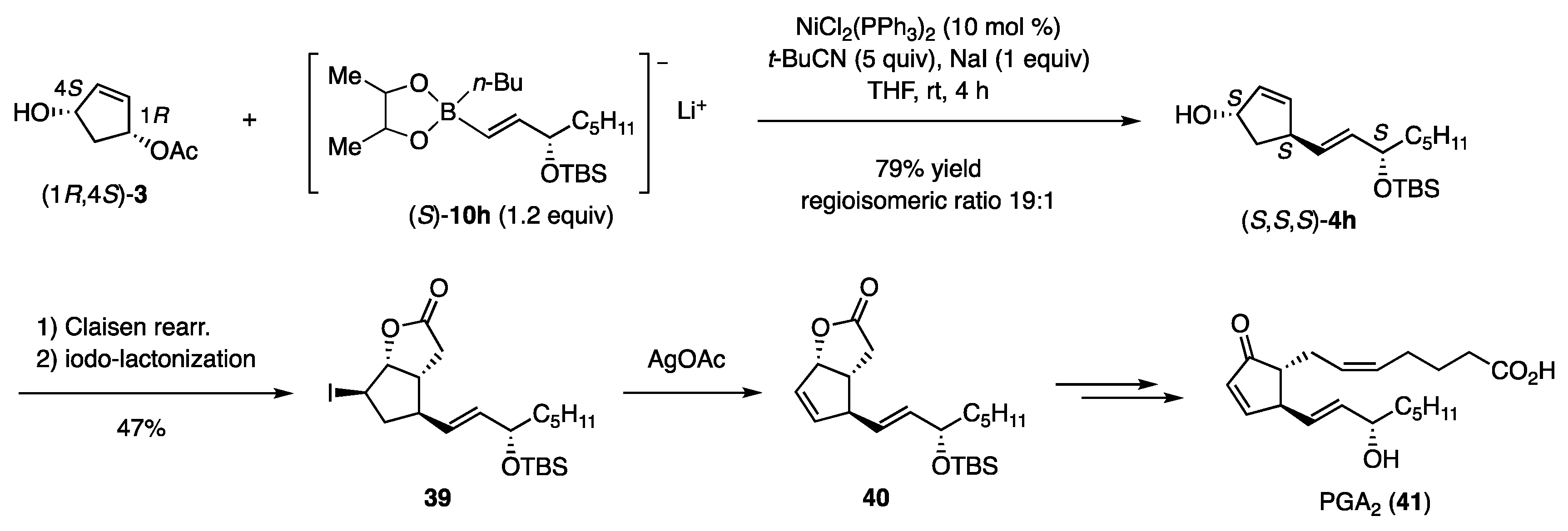
The coupling conditions disclosed in Table 3 were then applied to enantiomerically enriched (1R,4S)-3 and (S)-10h to afford (S,S,S)-4h in 79% yield with a regioisomeric ratio of 19:1 (Scheme 9) [13]. The product was transformed to iodolactone 39 through the Claisen rearrangement, followed by iodolactonization of the derived acid. Deiodination with AgOAc produced olefin 40, the intermediate in the synthesis of PGA2 (41) [19].
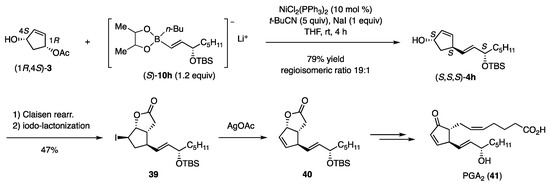
Scheme 9.
Coupling reactions and further conversion to PGA2.
4.6.2. Δ7-PGA1 Methyl Ester
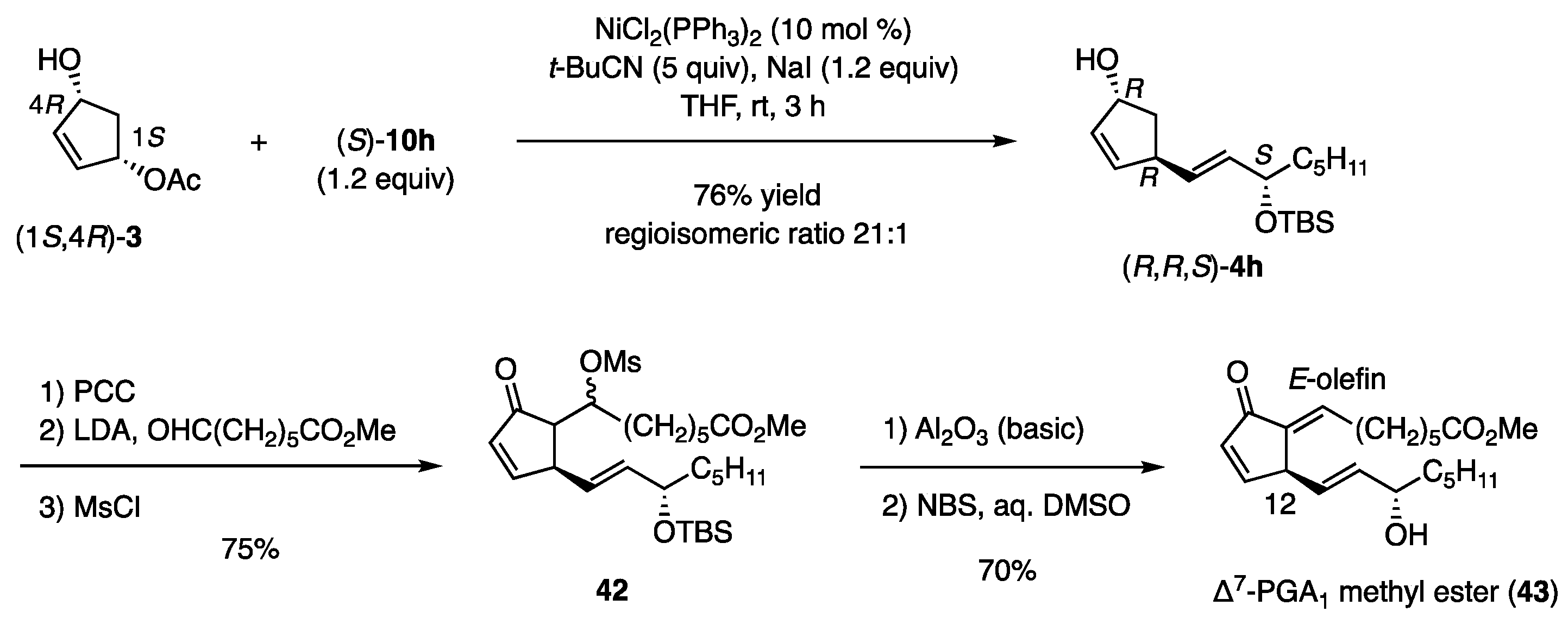
The coupling reaction also produced (R,R,S)-4h regioselectively from (1S,4R)-3 and (S)-10h (Scheme 10) [13]. This product was then transformed into mesylate 42 by oxidation to the ketone, followed by an aldol reaction under kinetic conditions and mesylation. The mesylate was eliminated using basic Al2O3, which afforded the E-olefin stereoselectively, irrespective of the aldol stereochemistry. Finally, desilylation to the target (43) [20] was achieved with NBS in aqueous DMSO, whereas Bu4NF caused deprotonation of the C12-hydrogen in the dienone core.
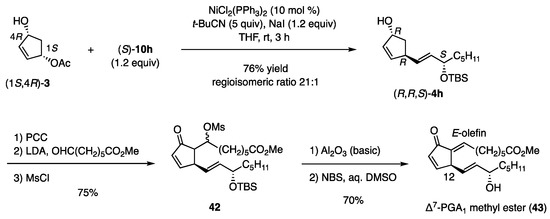
Scheme 10.
Synthesis of Δ7-PGA1 methyl ester.
4.6.3. Aristeromycin
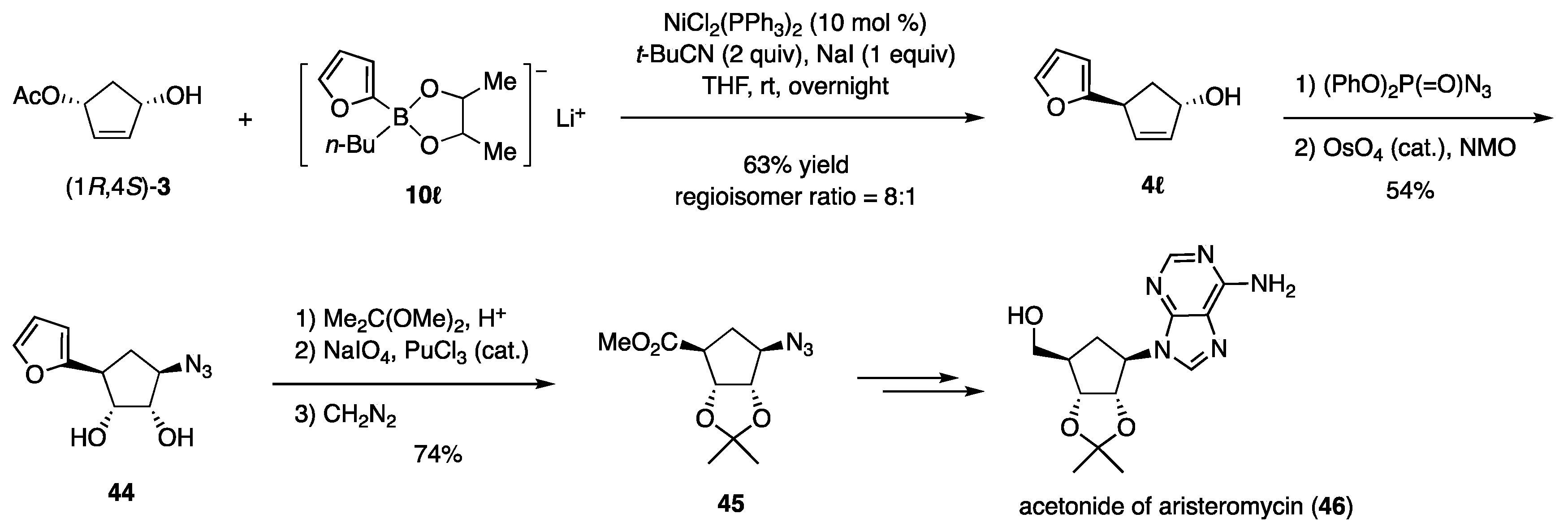
The furyl moiety as a precursor of CO2Me was installed to (1R,4S)-3 with furylborate 10ℓ (Scheme 11) [21]. The hydroxy group in the product 4ℓ was then replaced by N3 with inversion using (PhO)2P(=O)N3, and the furyl group was oxidatively cleaved to afford 45, which was transformed into the target 46 without difficulty.
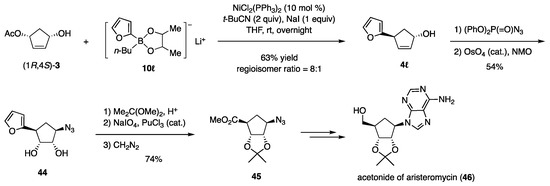
Scheme 11.
Synthesis of aristeromycin.
5. Alkenyl-Alkenyl Coupling Reaction
5.1. Optimal Alkanediol Ligand for Alkenyl Borates
The ready availability of allylic alcohol derivatives possessing a bromine atom at the cis position [22] was one of the reasons to investigate coupling reactions with alkenyl borates (Scheme 1, Equation (3)). Other reasons include the possibility of using the reaction for the synthesis of biologically active dienes and the stereoselective functionalization of the dienes. Although the low reactivity of the cis-bromides was a concern due to steric congestion with the OR (R = TBS, etc.), we expected compensation from the high reactivity of borates.
Borate 47A, with the most effective ligand A in the allylic coupling, was subjected to a test coupling with cis-bromide 5a. However, the reaction was capricious at rt and required heating at 40–45 °C to produce diene 6a (Table 4, entry 1), indicating that steric hindrance around the C–Br of 5a for oxidative addition and/or C–Ni–Br thereof for transmetalation was an obstacle to accessing Ni or the borate. Borate 47C with pin ligand C showed no reactivity (entry 2). Fortunately, ligand G exhibited the highest reactivity among the tested diols, affording 6a in 90% isolated yield (entry 4) [14]. An attempted PdCl2(PPh3)2-catalyzed coupling between 5a and 44G was unsuccessful.

Table 4.
Preliminary coupling reaction of (cis-bromo)olefin 5a with alkenyl borates possessing diol ligands.
The reactivity with ligand G was sufficient for the coupling of cis-bromides possessing bulkier TBDPS (t-BuPh2Si) than TBS (t-BuMe2Si) and a secondary alkyl group (c-C6H11), giving 6b and 6c in good yields. Furthermore, (2-Ph-vinyl)borate and a cis-borate afforded dienes 6d and 6e (Figure 5).
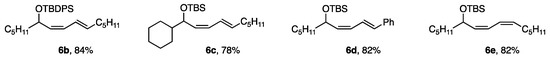
Figure 5.
Other dienes produced by the coupling reaction.
5.2. Synthetic Application of the Alkenyl-Alkenyl Coupling
5.2.1. Synthesis of 10,11-Dihydro-LTB4
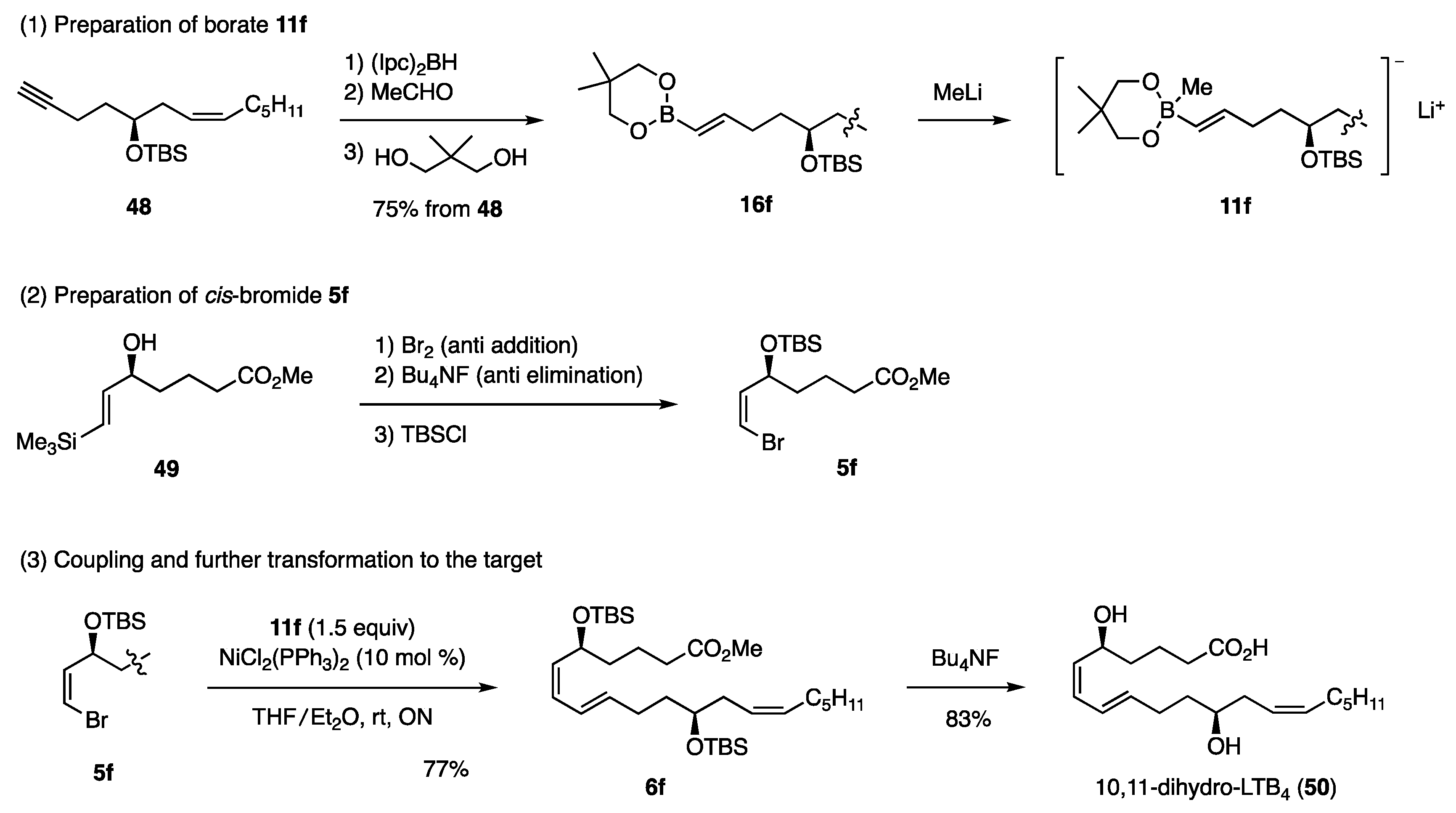
Based on the above findings for the synthesis of dienes, the necessary borate 11f and cis-bromide 5f were easily elucidated [23]. Among the existing methods for synthesizing borate 11f, the following method was selected based on the potential regio- and stereoselectivity (Scheme 12). The hydroboration of acetylene 48 with (Ipc)2BH (Ipc: diisopinocampheyl), followed by ligand exchange to EtO using MeCHO and transesterification, produced 16f, which upon reaction with MeLi afforded borate 11f. On the other hand, allylic alcohol 49 was prepared with high stereo- and enantioselectivity by the kinetic resolution using the Sharpless asymmetric epoxidation. Subsequently, 49 was converted to cis-bromide 5f [24]. The coupling reaction proceeded smoothly to afford 6f in 77% yield, and the subsequent desilylation of TBS with Bu4NF conveniently induced “formal hydrolysis” of the Me ester group to produce the target 50, which is an inflammatory mediator.
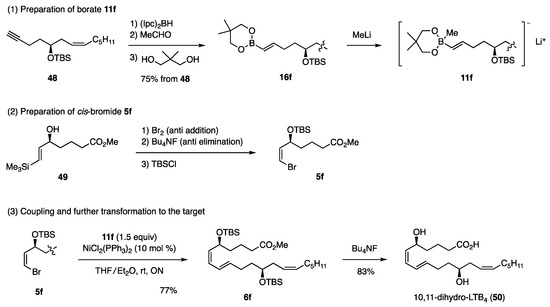
Scheme 12.
Synthesis of 10,11-dihydro-LTB4.
5.2.2. Synthesis of Korormicin
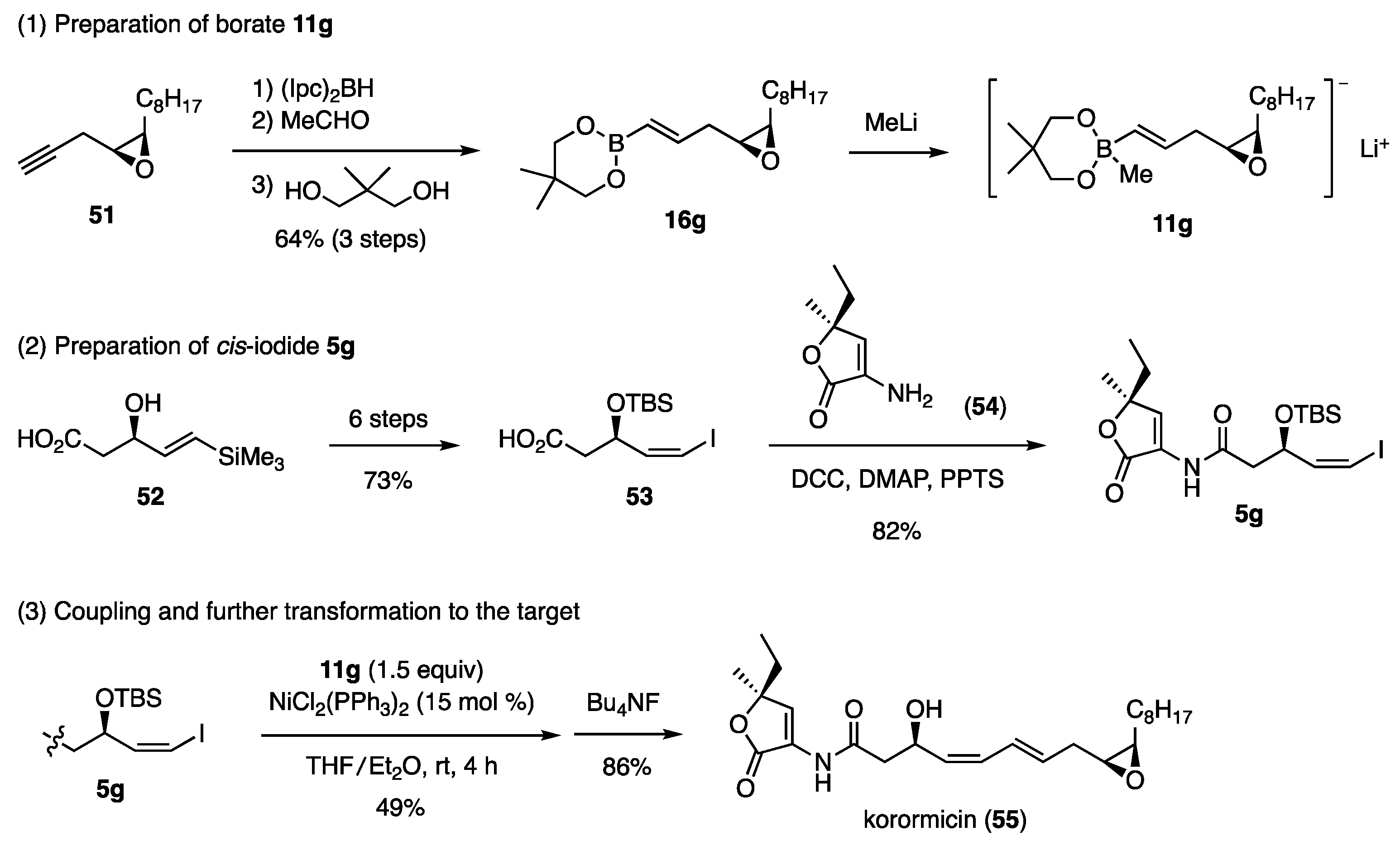
The title compound inhibits the growth of marine gram-negative bacteria. The planar structure of this compound was reported based on the NMR and mass spectral data. Consequently, four diastereomers were synthesized to determine the stereochemistry by comparing the [α]D values of the diastereomers with those published for natural korormicin. The synthesis of the diastereomer, which showed a close [α]D value to that of the natural one, is shown in Scheme 13 [25]. The necessary borate 11g and cis-iodide 5g, were synthesized through 51 and 52, respectively. Due to the structural specificity of 52 possessing the COOH group, the corresponding bromide could not be obtained. Instead, iodide 52 was transformed to 53 via iodolactonization. Acid 53 was then reacted with amine 54 under somewhat specific reaction conditions to afford amide 5g. The Ni-catalyzed coupling reaction of iodide 5g with borate 11g produced the TBS ether of korormicin, which upon desilylation afforded korormicin (55).
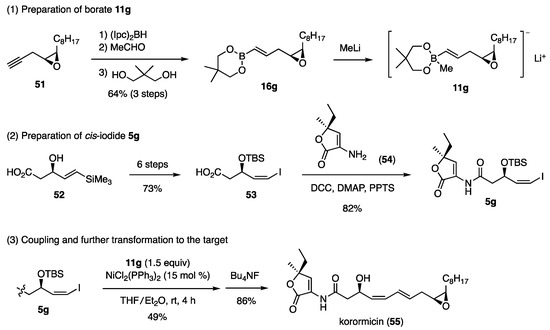
Scheme 13.
Synthesis of korormicin for determination of its stereochemistry.
5.3. Functionalization of the Alkenyl-Alkenyl Coupling Products
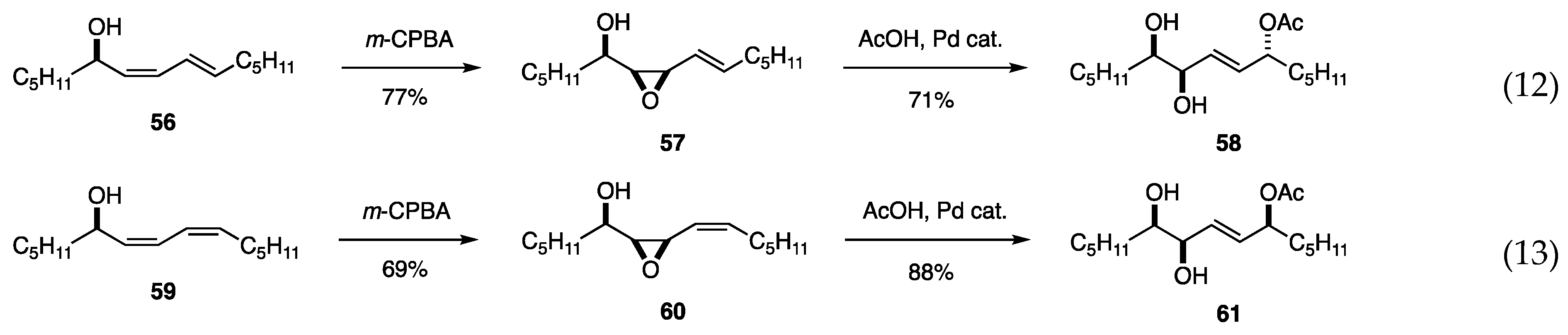
Since the epoxidation of cis allylic alcohols with m-CPBA proceeds stereoselectively, the regio- and stereoselectivity of the subsequent Pd-catalyzed reaction with AcOH were investigated for the synthesis of polyhydroxy compounds.
In practice, the epoxidation of alcohol 56 derived from 6a was stereoselective, and the subsequent Pd(PPh3)4-catalyzed reaction of the resulting epoxide 57 with AcOH produced dihydroxy acetate 58 with high regio- and stereoselectivity (Scheme 14, Equation (12)) [26]. Similarly, 59 was converted to 61 through 60 (Equation (13)). No cis olefins were produced. Several dienyl alcohols were also selectively converted (products not shown). A plausible mechanism involves the rapid isomerization of the anti-syn-π-allyl Pd intermediate formed from 60 to syn-syn-π-allyl Pd before the attack by AcOH.
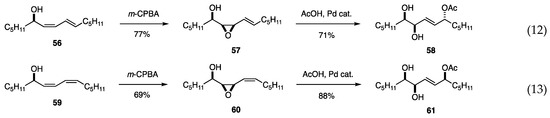
Scheme 14.
Functionalization of dienyl alcohols.
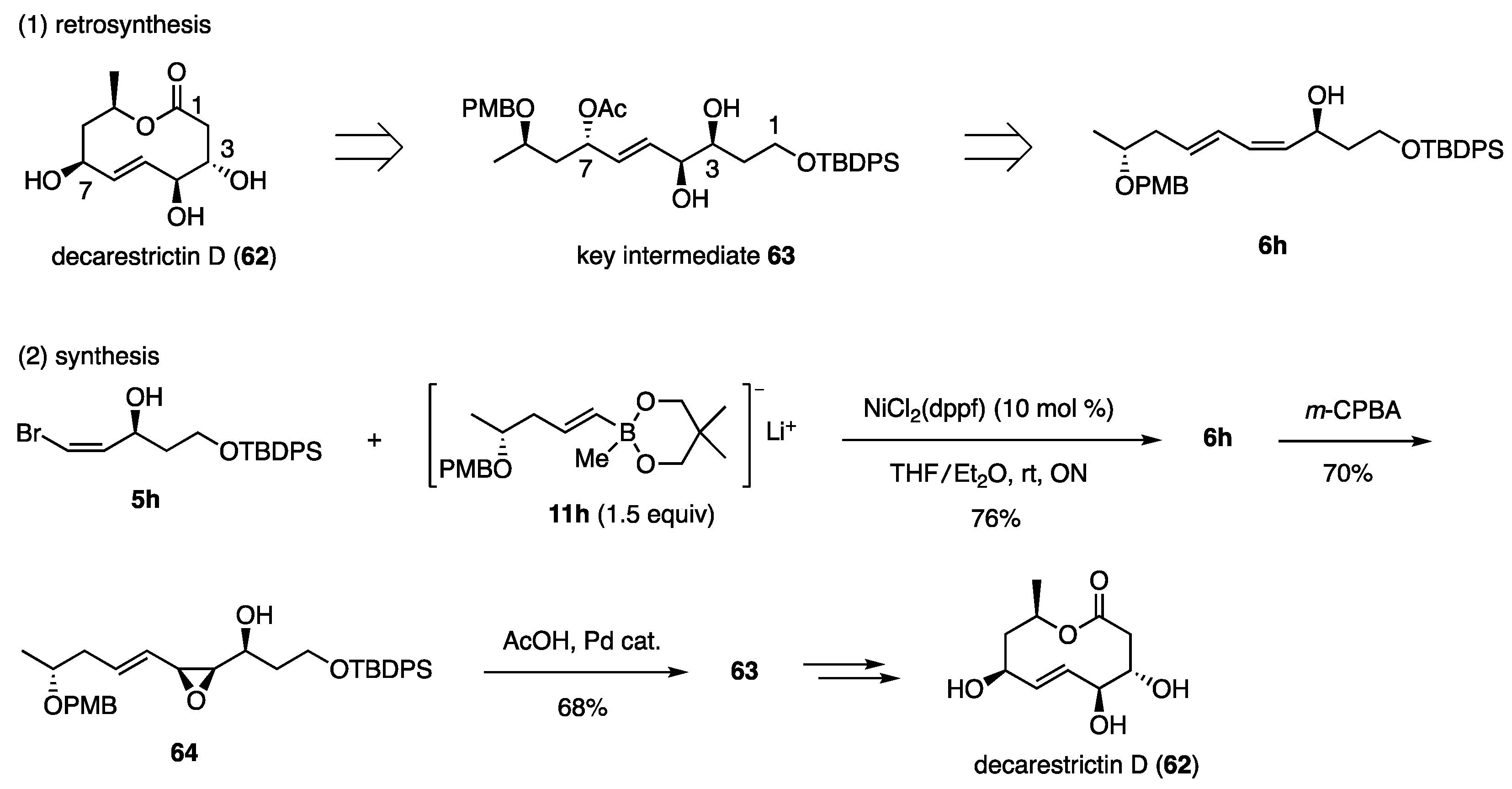
The same structural pattern was extracted from decarestrictin D (62), which exhibits inhibitory activity against HMG-CoA reductase. Based on the stereochemical relationship summarized in Scheme 14, dienyl alcohol 6h was designed as the precursor of the key intermediate 63 (Scheme 15) [26,27]. Coupling partners 5h and 11h were prepared using methods similar to those used for the synthesis of 10,11-dihydroxy-LTB4 (50) (Scheme 12). The use of cis-bromide 5h, which possesses a free hydroxy group, was a new case of the Ni-catalyzed coupling reactions. The reaction proceeded smoothly, requiring 1.5 equiv of 11h to produce 6h with a 76% yield. This result indicates that the borate was not affected by the free hydroxy group in 5h. The epoxidation of 6h with m-CPBA and subsequent Pd-catalyzed reaction with AcOH proceeded with high selectivity to afford the intermediate 63 with a 68% yield. Further transformation to decarestrictin D (62) was successful.
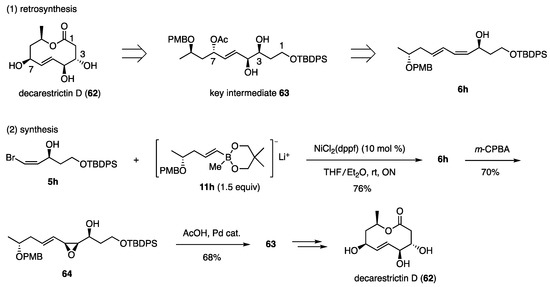
Scheme 15.
Synthesis of decarestrictin D 1. 1 PMB: p-MeOC6H4CH2, TBDPS: t-BuPh2Si.
5.4. C(sp2)–C(sp2) Coupling
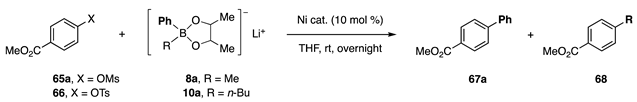
Borates with diol ligands have been applied to the coupling of other types of substrates with low reactivity. First, mesylates and tosylates were subjected to a coupling reaction with phenylborates 8a and 10a (Table 5) [15]. The former insufficiently produced a mixture of the desired product 67a and the Me derivative 68 (R = Me) in a ca. 1:1 ratio with 24–32% yields of 67a (entries 1 and 2). In contrast, borate 10a exhibited high reactivity, affording 67a with a 95% yield (entry 3). Tosylate 66 also underwent coupling to produce 67a with an 83% yield (entry 4).

Table 5.
Coupling reactions of mesylates.
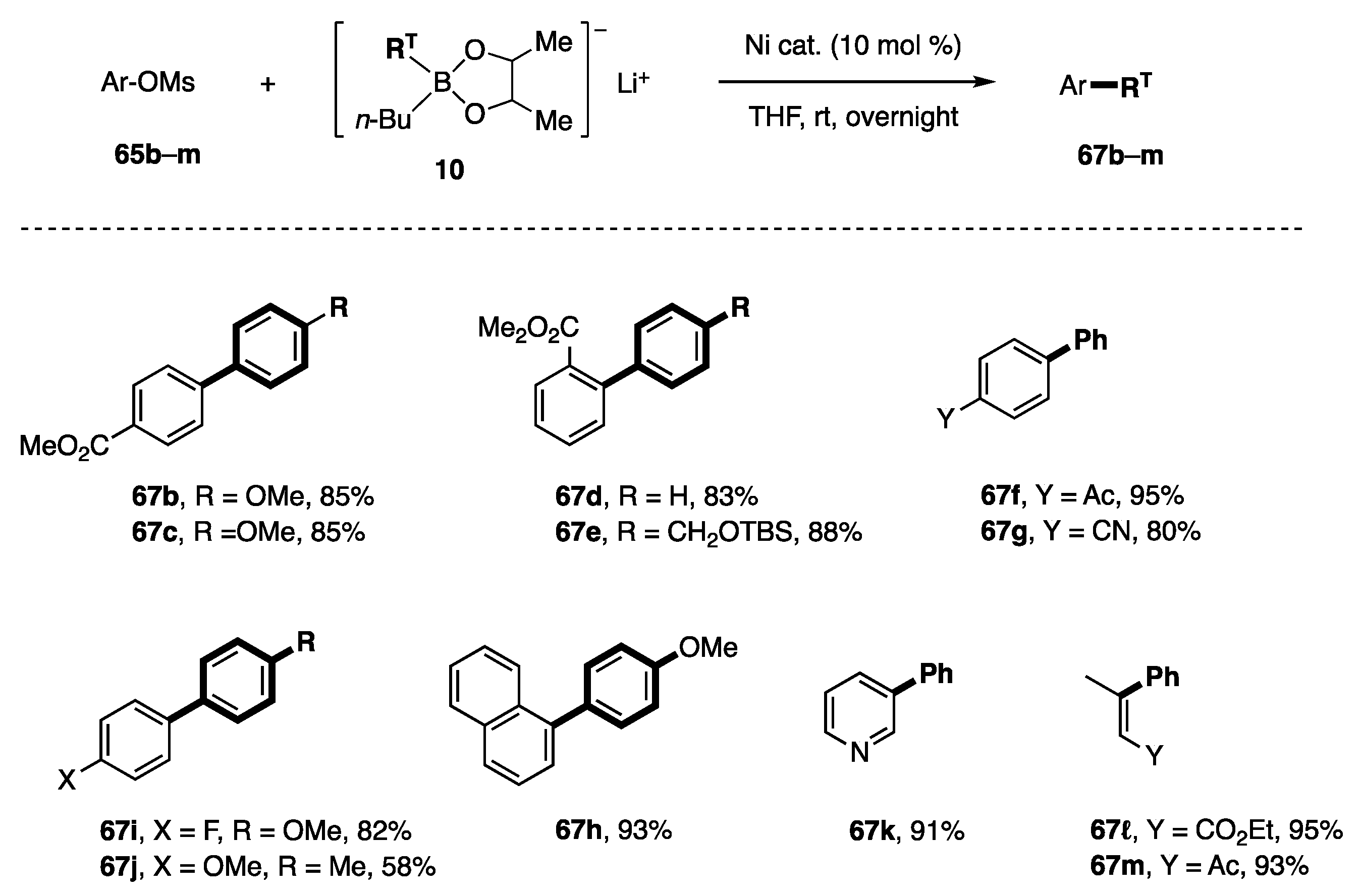
Several mesylates and borates of type 10 were subjected to the coupling conditions to afford the products shown in Scheme 16. Mesylates possessing electron-withdrawing groups, such as CO2Me, Ac, and CN, were good substrates. The CO2Me group at the ortho position was not an obstacle to the reaction (67d, 67e). The fluorine atom and the additional aromatic moiety were positive for the coupling. On the contrary, mesylate bearing the p-MeO group afforded 67j with a 58% yield. This result suggests that electron-donating groups play a somewhat negative role. In this regard, the high yields of 67k–m are not surprising.
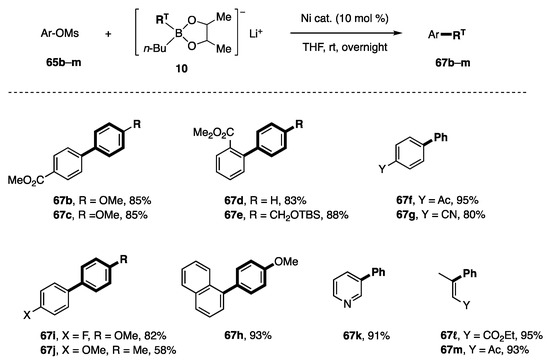
Scheme 16.
Coupling reaction of mesylates.
Although the coupling of mesylates with borates was successfully explored, we found little synthetic advantage because the same products could be synthesized using triflates. Therefore, investigation of the mesylates was discontinued.
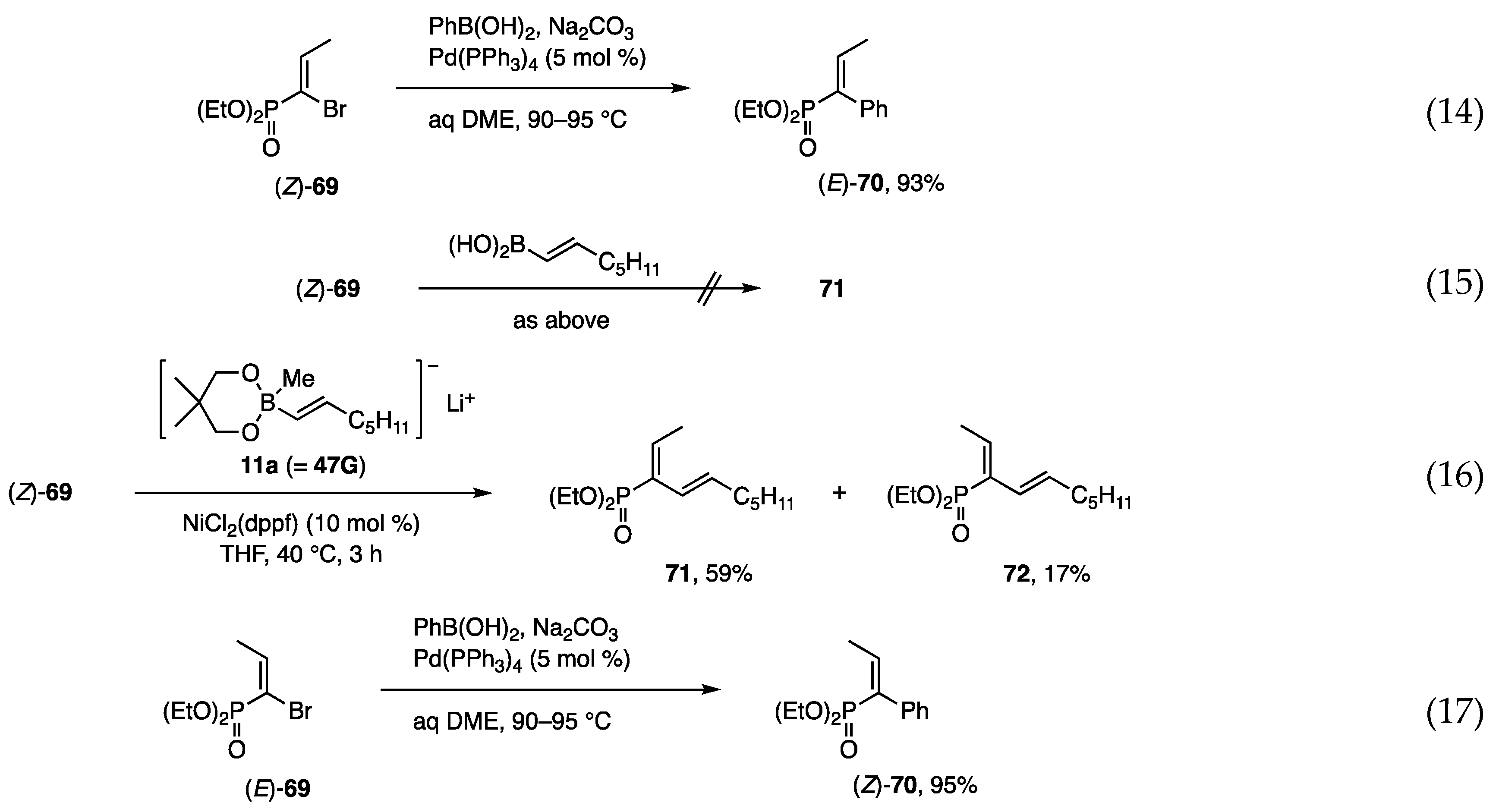
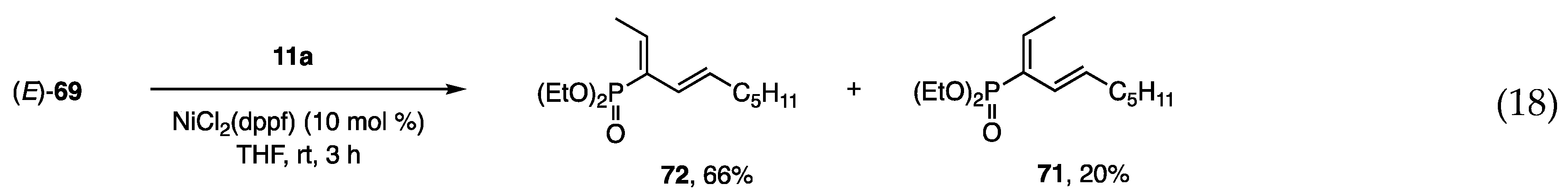
The construction of a carbon framework on small phosphonates was investigated starting with α-bromoalkenyl phosphonates [28,29], which were prepared by bromination of the corresponding olefins followed by dehydrobromination with Et3N. The Suzuki-Miyaura coupling of (Z)- and (E)-69 with PhB(OH)2 at 90–95 °C afforded (E)- and (Z)-70 in high yields (Scheme 17, Equations (14) and (17)), whereas a similar coupling with an alkenyl borane was unsuccessful (Equation (15)). In contrast, alkenyl borate 11a was reactive toward (Z)- and (E)-69 under the catalytic action of Ni to afford 71 as the major product from (Z)-69 and 72 from (E)-69, respectively (Equations (16) and (18)). Although the mixture of 71 and 72 was separated by chromatography, a more selective method is still necessary. Several entries that use different bromides and alkenyl borates yielded similar results.
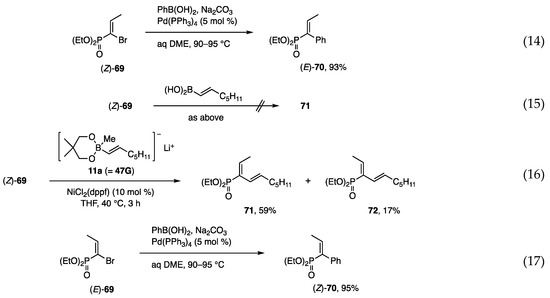
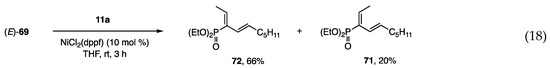
Scheme 17.
Coupling reactions of bromo-phosphonates with organometallics.
6. Beyond Borates
6.1. Limitation of Borates
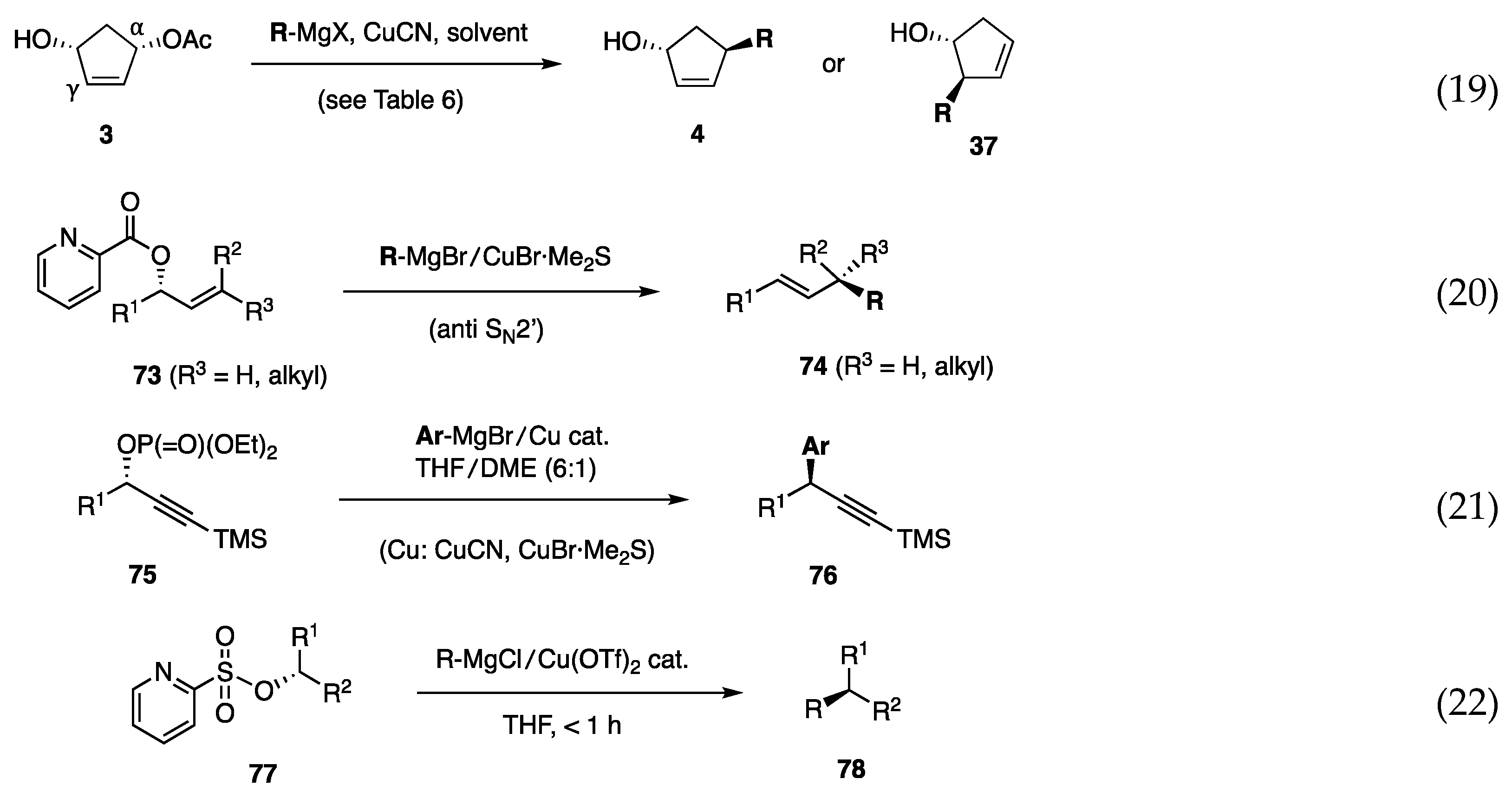
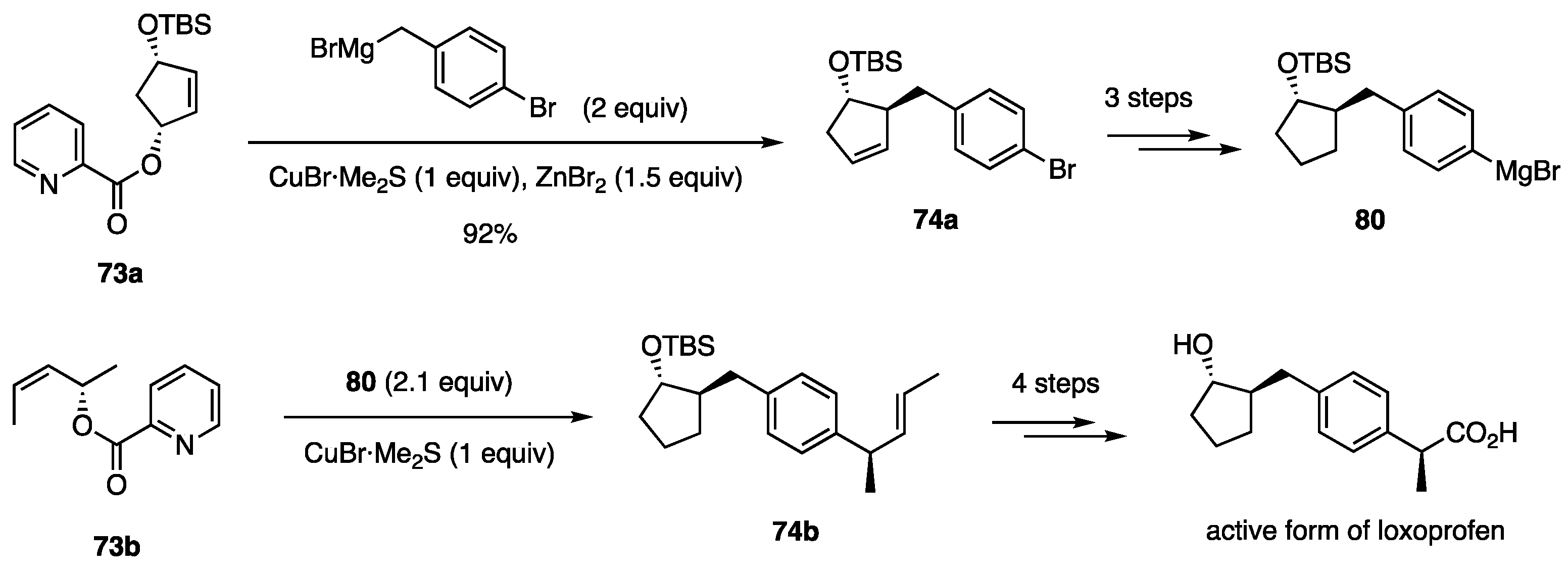
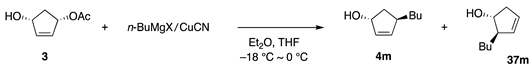
In the above study using borates, the development of alkyl borates for C–C bond formation was unsuccessful. Therefore, we focused on alkyl reagents derived from (alkyl)MgX and a copper salt and found that several combinations of (alkyl)MgX, CuCN, and solvent delivered an alkyl group to cyclopentenediol monoacetate 3 to afford either 4 or 37 with high regio- and stereoselectivity (Scheme 18, Equation (19)) [30]. Subsequently, aryl, alkenyl, and benzylic reagent systems were developed as described below. We also established allylic and propargylic substitutions on acyclic secondary carbons [Equations (20) and (21)]. Acyclic allylic substitution features the use of allylic picolinate 73 to yield the anti-SN2′ product 74 with regio- and stereoselectivity (Equation (20)) [31]. Propargylic substitution was realized using propargylic phosphate 75 and a Cu catalyst in THF/DME to produce 76 with high stereoselectivity (Equation (21)) [32]. In contrast to allylic and propargylic substrates, the substitution of secondary alkyl carbons was difficult because of the lack of reaction enhancers, such as allylic and propargylic unsaturation. Nevertheless, pyridinesulfonate 77 was invented for this purpose (Equation (22)) [33]. In the second part of this review, we briefly present the results of these reactions.
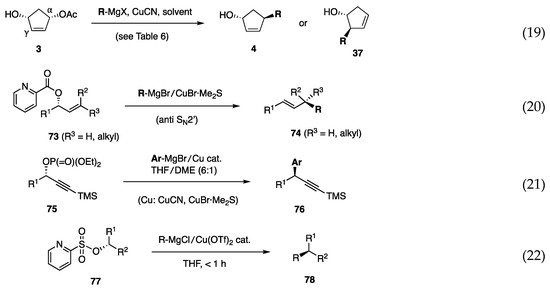
Scheme 18.
C–C bond forming reactions at secondary carbons.
6.2. Substitution of Cyclopentenediol Monoacetate 3
Although copper-based reagents generally undergo preferential substitution at the γ carbon (SN2′ reaction), 3 was not used as a substrate in the previous study of allylic substitution. In a preliminary study, reagents comprising n-BuMgX (X = Cl, Br)/CuCN in ratios of 1:1, 2:1, and 10:1 in Et2O and THF were systematically examined [30]. Reagents based on BuMgX (X = Cl, Br), mainly in Et2O, were highly γ selective, thereby producing 37m (Table 6). In contrast, n-BuMgCl/CuCN in 2:1 and 10:1 ratios in THF preferentially attacked the α carbon (entries 5 and 6). Surprisingly, the solvent (THF vs. Et2O) was decisive for the high regioselectivity (entries 5 and 6 vs. entries 2 and 3).

Table 6.
Allylic substitution with n-BuMgX/CuCN.
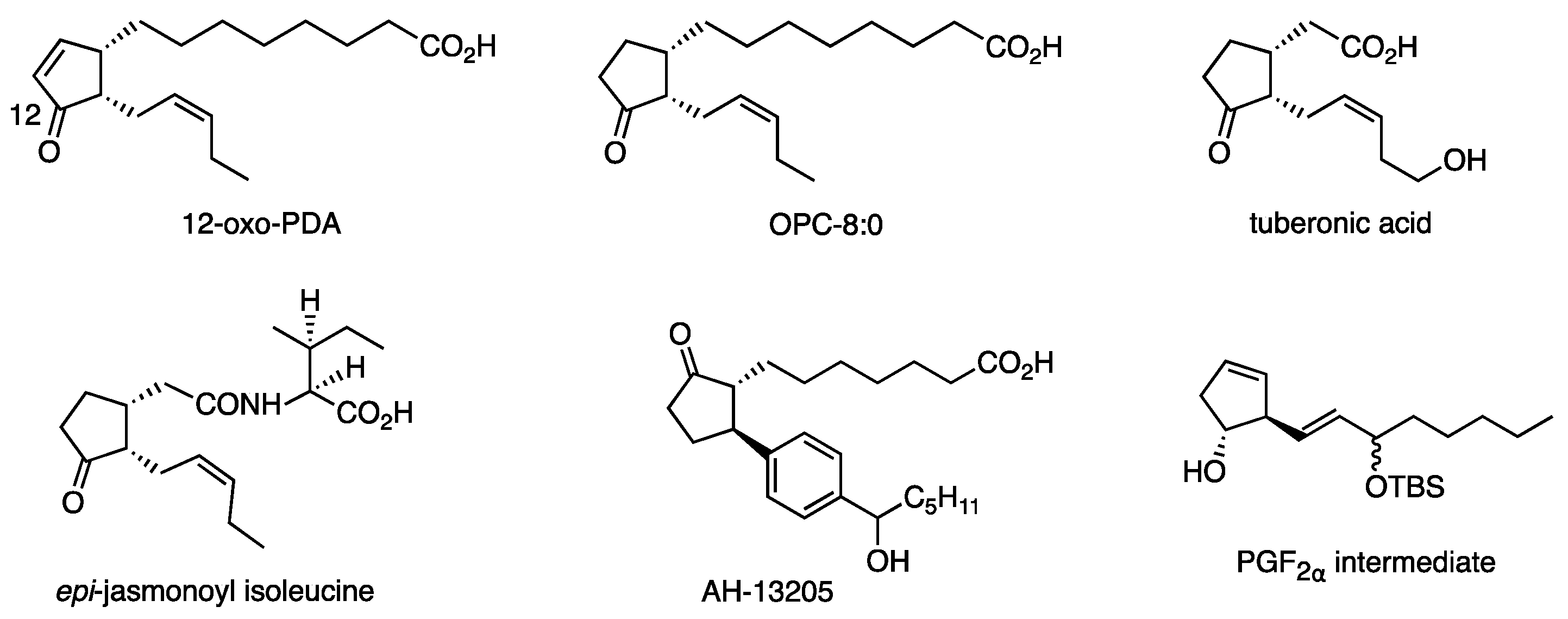
The combination of RMgX, CuX, and the solvent was further investigated to find aryl, alkenyl, and benzylic reagents. These protocols have been successfully used as key steps in the syntheses of plant hormones (such as 12-oxo-PDA and OPC-8:0 [34], tuberonic acid [35], isoleucine conjugate of epi-jasmonic acid [36]), AH-13205 (PG receptor agonist) [37], and PGF2α intermediates [38] (Figure 6). The ω side chain of Δ7-PGA1 methyl ester (43) (structure in Scheme 10) was installed to 3 with efficiency similar to that recorded with the borate [39].
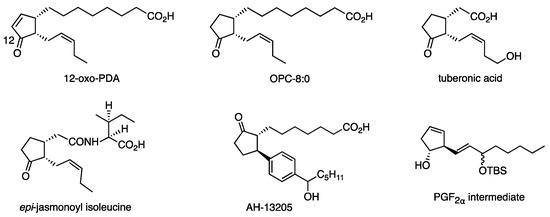
Figure 6.
Cyclopentanoids synthesized by using RMgX/CuCN.
6.3. Substitution of Secondary Allylic Picolinates
The reaction in Equation (20) in Scheme 18 features the picolinoxy leaving group, and requires the cis-olefin geometry (R2 ≠ H in 73) to obtain anti-SN2′ products with high selectivity [31]. This topic has been summarized in reviews [40,41]. In brief, substitution of 73a with PhMgBr (2 equiv) and CuBr·Me2S (0.5–1 equiv) afforded 74a with high regioselectivity (rs) and enantiospecificity (es) (=chirality transfer C.T.) (Equation (23)). Low temperatures were necessary for high es. Allylic picolinates are chemically stable and easily prepared by the DCC-assisted esterification of cis-allylic alcohols with picolinic acid (2-Py-CO2H). Although stable, this leaving group gains high reactivity by chelating to MgBr2, which is generated in situ from RMgBr and CuBr·Me2S. The substitution occurred not only with alkyl but also with aryl, alkenyl, and alkynyl reagents [42,43].


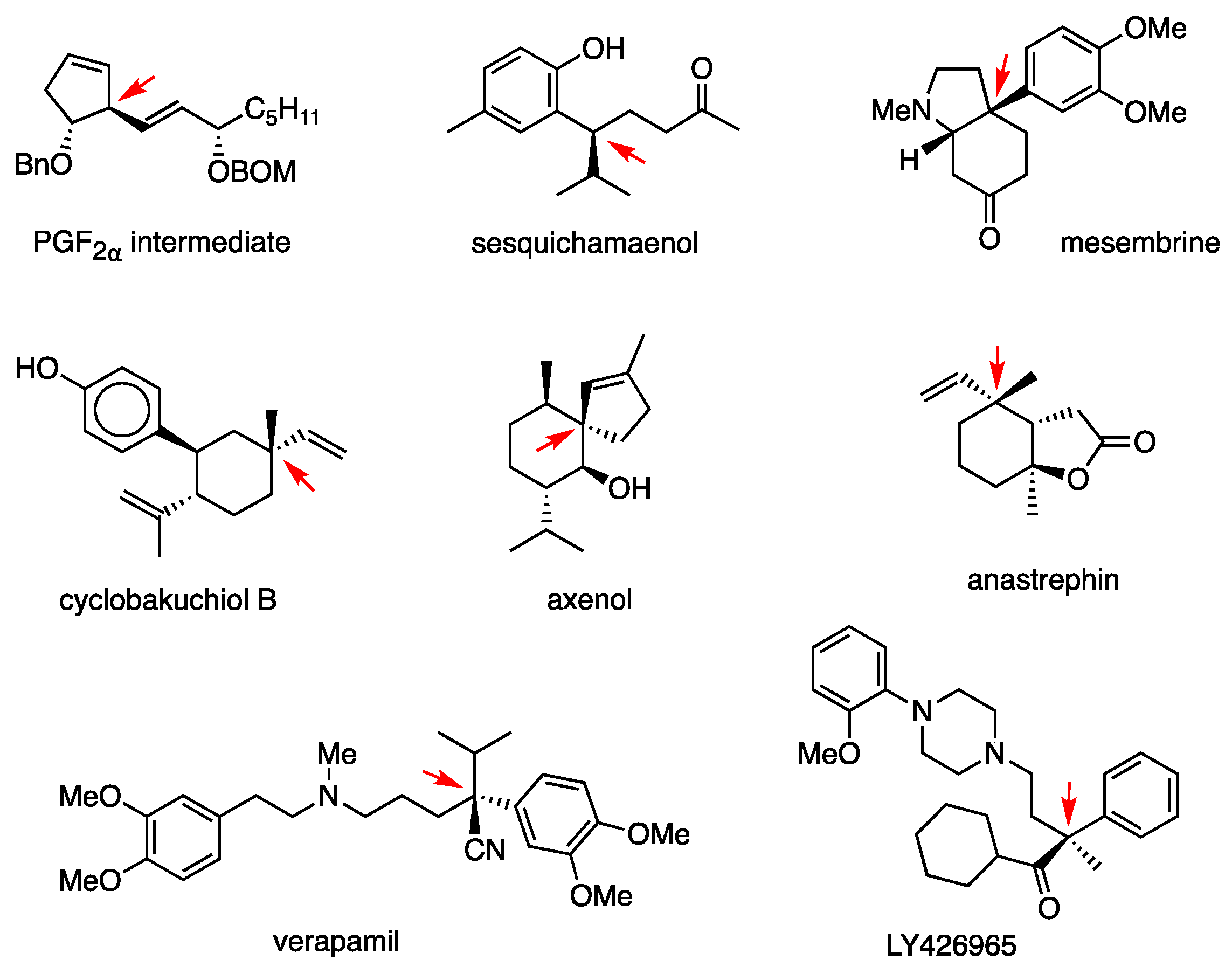
Using this substitution, the PGF2α intermediate was synthesized again (Figure 7) [43]. Other biologically active compounds synthesized are: sesquichamaenol [44], mesembrine [45], cyclobakuchiol B [46], axenol [47], anastrephin [48], verapamil [49], and LY426965 [50] (Figure 7). The active form of anti-inflammatory loxoprofen was synthesized by performing the allylic substitution twice (Scheme 19) [51].
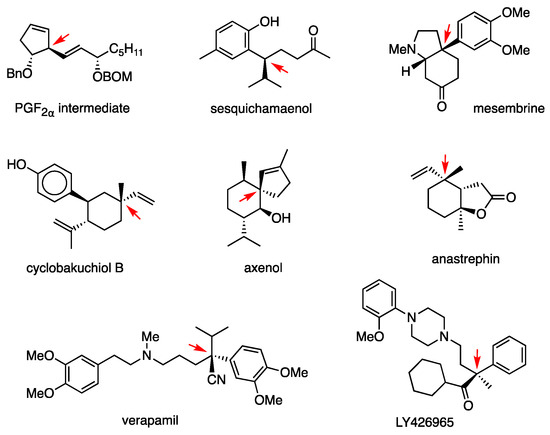
Figure 7.
Biologically active compounds synthesized by using RMgX/CuCN 1. 1 C–C bonds constructed by the anti-SN2′ reaction are indicated by a red arrow.
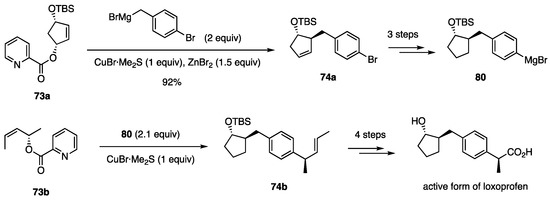
Scheme 19.
Synthesis of the active form of loxoprofen using allylic substitutions twice.
6.4. Substitution of Secondary Propargylic Phosphates
A reagent system derived from Grignard reagent/copper salt was next explored for the substitution of secondary propargylic alcohol derivatives (Scheme 18, Equation (21)). Although an attempted reaction of a propargylic picolinate and PhMgBr/CuCN in THF proceeded with high regioselectivity, 10–20% of the corresponding alcohol was coproduced. In contrast, phosphate 75a and PhMgBr (2.5 equiv)/CuCN (0.25 equiv) in THF/DME (6:1) gave products 76a (Ar = Ph) with high rs and es (Scheme 20) [32]. The use of CuCN as the catalyst and DME as the additional solvent was pivotal for producing 76b–f with high selectivity. The performance of CuBr·Me2S was as excellent as that of CuCN. The presence of TMS at the acetylene end was decisive for achieving the high regioselectivity as observed for the reaction of 75g. The phenyl group attached to the acetylene end of 75h also showed good regioselectivity.
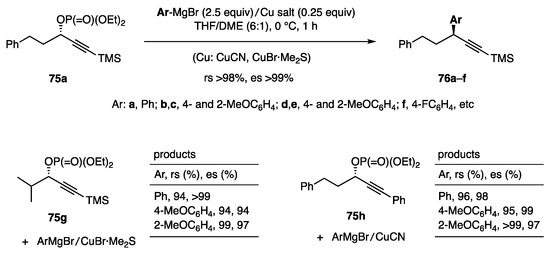
Scheme 20.
Substitution at secondary propargylic carbon.
In contrast, the reagents derived from PhMgBr (3 equiv) and Cu(acac)2 (1.5 equiv) gave allene 79 efficiently (Equation (24)).
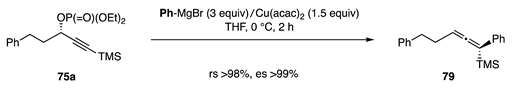

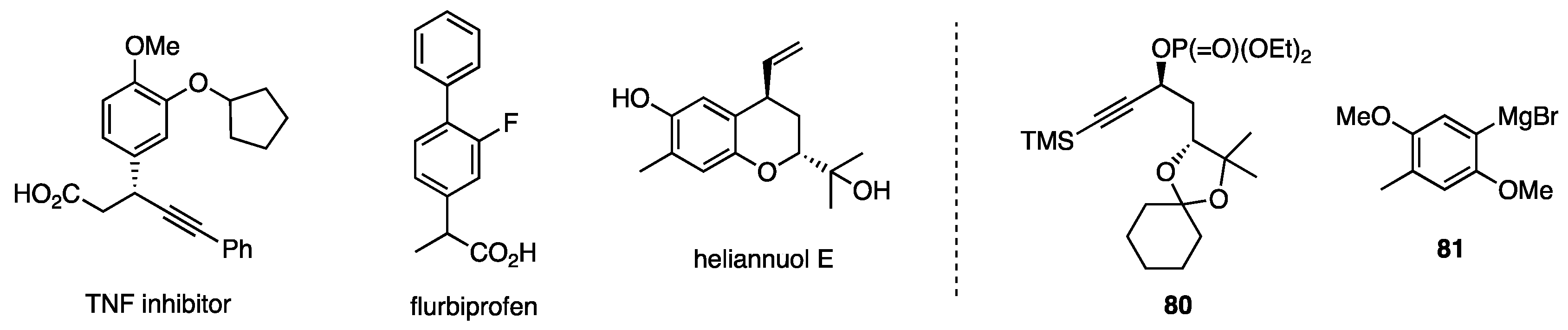
With the selective propargylic substitution in hand, the synthesis of TNF inhibitor and flurbiprofen (Figure 8) was easily designed [52], while the substitution of 80 with Grignard reagent 81 was used for the synthesis of heliannuol E [53].
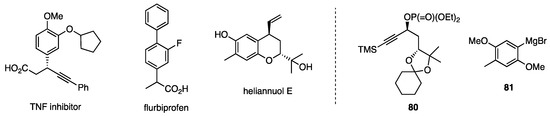
Figure 8.
Compounds synthesized by using propargylic substitution 1. 1 Phosphate 80 and Grignard reagent 81 were used for the synthesis of heliannuol E.
6.5. Substitution at Secondary Alkyl Carbons
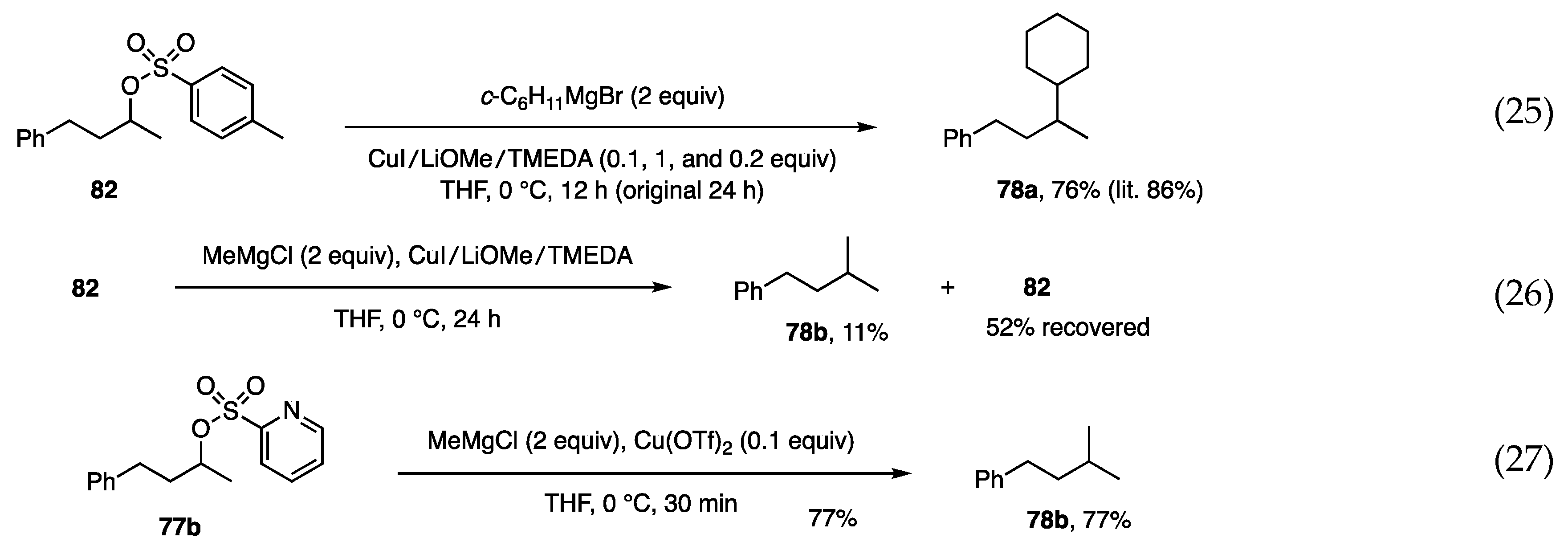
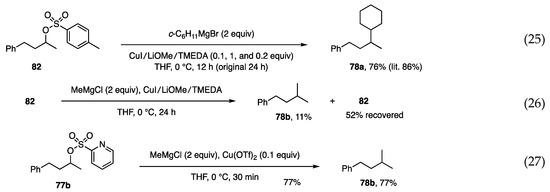
In contrast to the allylic and propargylic substitutions, the substitution on secondary alkyl carbons generally proceeds with difficulty because of the absence of a reaction promoter, such as alkenyl and alkynyl moieties. The substitution reported by Breit relies on an ester carbonyl group [54,55]. Consequently, it was not surprising to find only one publication by Liu, who employed tosylate, CuI, and additives [56]. Negishi applied this reaction to the synthesis of phthioceranic acid [57]. Having successfully reproduced the reaction of Liu (Scheme 21, Equation (25)), MeMgCl was used in the reaction to confirm the low reactivity of MeMgCl (Equation (26)). To explore a more reactive protocol, picolinate and phosphate leaving groups (2-pyridineCO2 and (EtO)2PO2) were examined, but unsuccessfully. As an extension of the concept using the pyridyl group, (2-pyridine)sulfonate 77b was next examined to find sufficient reactivity toward MeMgCl in the presence of Cu(OTf)2. The reaction was completed within 1 h to afford product 78b in 77% yield (Equation (27)) [33].
Scheme 21.
Substitution at secondary alkyl carbon by Liu (Equation (25)) and by us (Equations (26) and (27)).
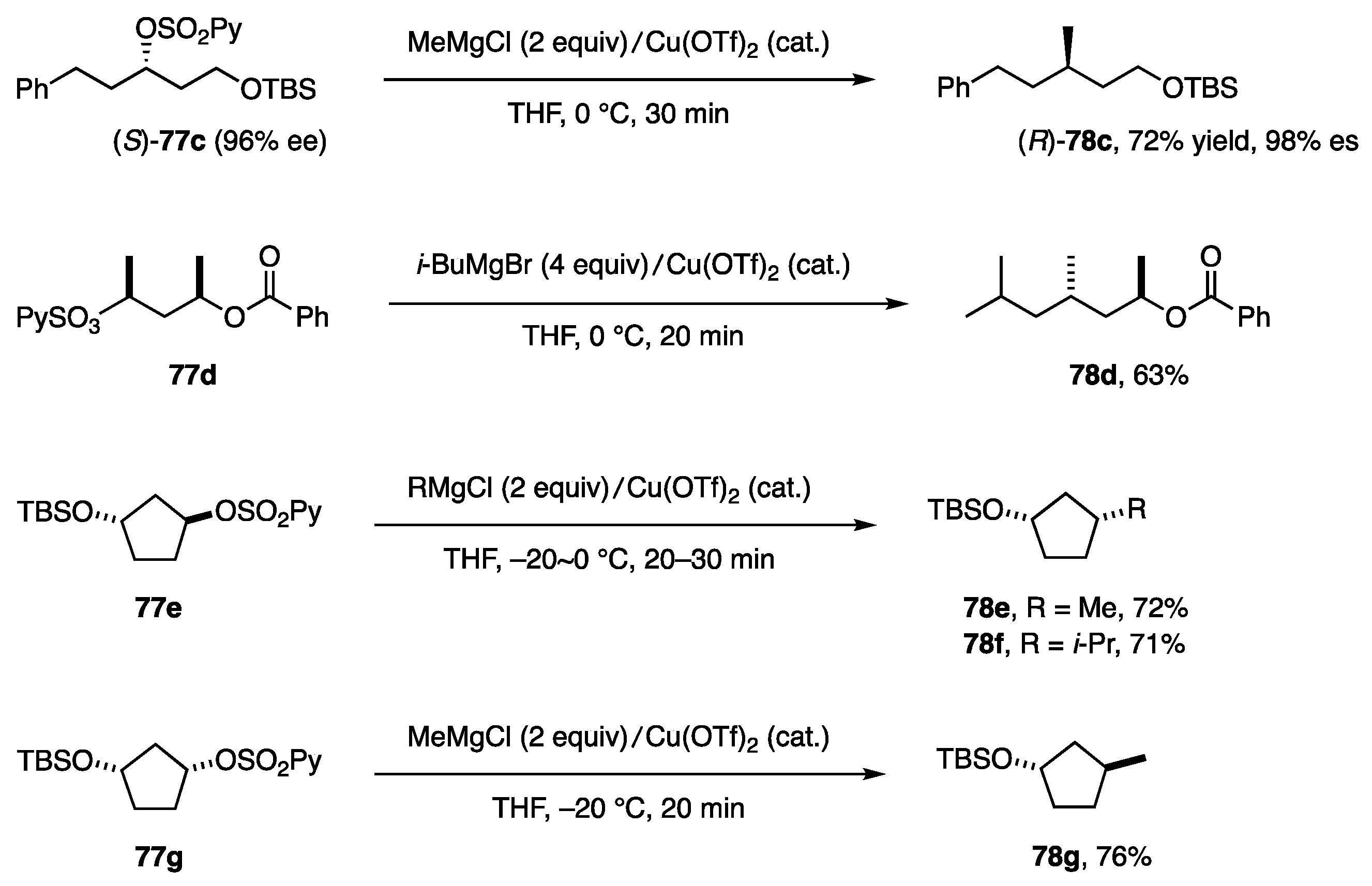
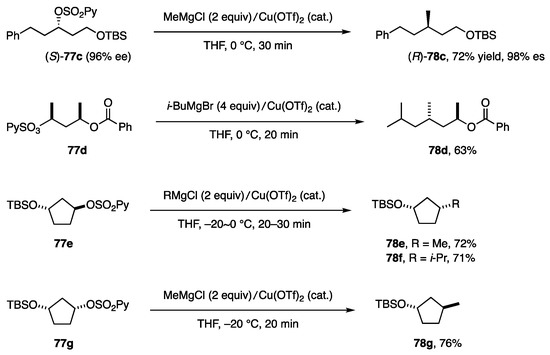
The substitution system shown in Equation (26) was applied to other (2-pyridine)sulfonates and RMgX to afford 78c–g in good yields with high stereoselectivity (Scheme 22).
Scheme 22.
Substitution on secondary alkyl carbons.
7. Summary
Allylic coupling reactions of secondary allylic carbonates and esters with aryl and alkenyl borates were found to be catalyzed by nickel complexes, such as NiCl2(PPh3) and NiCl2(dppf), and not by palladium catalysts. The alkanediol ligand on the borates was essential for producing high reactivity, which allowed the Ni-catalyzed coupling reaction between cyclopentenediol monoacetate 3 and borates. Furthermore, carbonyl group-friendly borates were synthesized from boronate esters and MeZnCl. Alkenyl borates were used for the construction of dienes from less reactive allylic alcohol derivatives with a bromine atom at the cis position. In contrast to the aryl and alkenyl borates, reactive alkyl borates were not developed. Instead, substitutions on secondary carbons with RMgX under copper-based and copper-catalyzed conditions were studied to obtain the following results: The regioselectivity in the allylic substitution of cyclopentenediol monoacetate 3 was highly controlled by the ratio of RMgX/CuCN, halogen X (Cl, Br) in RMgX, and the solvent (Et2O vs. THF). The substitution of acyclic allylic picolinates proceeded in anti-SN2′ mode. Propargylic phosphates underwent propargylic substitution with RMgBr and a Cu catalyst in THF-DME. In addition, the recent success in the substitution of secondary alkyl (2-pyridine)sulfonates with RMgX is presented. The reactions have been applied to the synthesis of biologically active compounds.
Funding
This review was funded by JSPS, KAKENHI Grant No. 20K05501. Fund for each research work described herein will be found in the publication.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Tsuji, J. Palladium Reagents and Catalysts: Innovations in Organic Synthesis; Wiley: Chichester, UK, 1995; pp. 1–574. ISBN 978-0-471-97202-0. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts: New Perspectives for the 21st Century; Wiley: Chichester, UK, 2004; pp. 1–670. ISBN 978-0-470-85032-9. [Google Scholar]
- Stork, G.; Tsuji, J. Lithium-Ammonia Reduction of α,β-Unsaturated Ketones. II. Formation and Alkylation of A β-Carbanion Intermediate. J. Am. Chem. Soc. 1961, 83, 2783–2784. [Google Scholar] [CrossRef]
- Stork, G.; Rosen, P.; Goldman, N.; Coombs, R.V.; Tsuji, J. Alkylation and Carbonation of Ketones by Trapping the Enolates from the Reduction of α,β-Unsaturated Ketones. J. Am. Chem. Soc. 1965, 87, 275–286. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suginome, H.; Suzuki, A. Novel and convenient method for the stereo-and regiospecific synthesis of conjugated alkadienes and alkenynes via the palladium-catalyzed cross-coupling reaction of 1-alkenylboranes with bromoalkenes and bromoalkynes. J. Am. Chem. Soc. 1985, 107, 972–980. [Google Scholar] [CrossRef]
- Pagett, A.B.; Lloyd-Jones, G.C. Suzuki–Miyaura Cross-Coupling. Org. React. 2019, 100, 547–620. [Google Scholar] [CrossRef]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2013, 43, 412–443. [Google Scholar] [CrossRef]
- Ghorai, D.; Cristòfol, À.; Kleij, A.W. Nickel-Catalyzed Allylic Substitution Reactions: An Evolving Alternative. Eur. J. Inorg. Chem. 2021, 2022, e202100820. [Google Scholar] [CrossRef]
- Kobayashi, Y. Modern Organonickel Chemistry; Tamaru., Y., Ed.; Wiley: Chichester, UK, 2005; Chapter 3; pp. 56–101. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ikeda, E. Nickel-catalysed substitution reactions of allylic carbonates with aryl- and alkenyl-borates. J. Chem. Soc. Chem. Commun. 1994, 1789–1790. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mizojiri, R.; Ikeda, E. Nickel-Catalyzed Coupling Reaction of 1,3-Disubstituted Secondary Allylic Carbonates and Lithium Aryl- and Alkenylborates. J. Org. Chem. 1996, 61, 5391–5399. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tokoro, Y.; Watatani, K. Zinc borates: Functionalized hard nucleophiles for coupling reaction with secondary allylic acetates. Eur. J. Org. Chem. 2000, 2000, 3825–3834. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Murugesh, M.G.; Nakano, M.; Takahisa, E.; Usmani, S.B.; Ainai, T. A New Method for Installation of Aryl and Alkenyl Groups onto a Cyclopentene Ring and Synthesis of Prostaglandins. J. Org. Chem. 2002, 67, 7110–7123. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakayama, Y.; Mizojiri, R. Nickel-catalyzed coupling reaction of sterically congested cis bromides and lithium alkenylborates. Tetrahedron 1998, 54, 1053–1062. [Google Scholar] [CrossRef]
- Kobayashi, Y.; William, A.D.; Mizojiri, R. Scope and limitation of the nickel-catalyzed coupling reaction between lithium borates and mesylate. J. Organometal. Chem. 2002, 653, 91–97. [Google Scholar] [CrossRef]
- Suzuki, M.; Oda, Y.; Noyori, R. Palladium(0) catalyzed reaction of 1,3-diene epoxides. A useful method for the site-specific oxygenation of 1,3-dienes. J. Am. Chem. Soc. 1979, 101, 1623–1625. [Google Scholar] [CrossRef]
- Grisso, B.A.; Johnson, J.R.; Mackenzie, P.B. Nickel-catalyzed, chlorotrialkylsilane-assisted conjugate addition of alkenyltributyltin reagents to α,β-unsaturated aldehydes. Evidence for a [1-[(trialkylsilyl)oxy]allyl]nickel(II) mechanism. J. Am. Chem. Soc. 1992, 114, 5160–5165. [Google Scholar] [CrossRef]
- Trost, B.M.; Verhoeven, T.R. Allylic alkylation. Palladium-catalyzed substitutions of allylic carboxylates. Stereo- and regiochemistry. J. Am. Chem. Soc. 1980, 102, 4730–4743. [Google Scholar] [CrossRef]
- Newton, R.F.; Reynolds, D.P.; Davies, J.; Kay, P.B.; Roberts, S.M.; Wallace, T.W. Enantiocomplementary total asymmetric syntheses of prostaglandin A2. J. Chem. Soc. Perkin Trans. 1983, 1, 683–685. [Google Scholar] [CrossRef]
- Noyori, R.; Suzuki, M. Organic Synthesis of Prostaglandins: Advancing Biology. Science 1993, 259, 44–45. [Google Scholar] [CrossRef]
- Tokoro, Y.; Kobayashi, Y. Realisation of highly stereoselective dihydroxylation of the cyclopentene in the synthesis of (−)-aristeromycin. Chem. Commun. 1999, 807–809. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Morita, M. Cutting-Edge of Organic Synthesis and Chemical Biology of Bioactive Molecules; Kobayashi, Y., Ed.; Springer Nature: Singapore, 2019; Chapter 9; pp. 193–231. ISBN 978-981-13-6243-9. Available online: https://link.springer.com/chapter/10.1007/978-981-13-6244-6_9 (accessed on 22 November 2022).
- Nakayama, Y.; Kumar, G.B.; Kobayashi, Y. Synthesis of 10,11-Dihydroleukotriene B4 Metabolites via a Nickel-Catalyzed Coupling Reaction of cis-Bromides and trans-Alkenyl Borates. J. Org. Chem. 2000, 65, 707–715. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shimazaki, T.; Taguchi, H.; Sato, F. Highly stereocontrolled total synthesis of leukotriene B4, 20-hydroxyleukotriene B4, leukotriene B3, and their analogs. J. Org. Chem. 1990, 55, 5324–5335. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yoshida, S.; Nakayama, Y. Total synthesis of korormicin. Eur. J. Org. Chem. 2001, 2001, 1873–1881. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yoshida, S.; Asano, M.; Takeuchi, A.; Acharya, H.P. Nickel-Catalyzed Coupling Producing (2Z)-2,4-Alkadien-1-ols, Conversion to (E)-3-Alkene-1,2,5-triol Derivatives, and Synthesis of Decarestrictine D. J. Org. Chem. 2007, 72, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Asano, M.; Yoshida, S.; Takeuchi, A. Stereoselective Synthesis of Decarestrictine D from a Previously Inaccessible (2Z,4E)-Alkadienyl Alcohol Precursor. Org. Lett. 2005, 7, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; William, A.D. Coupling Reactions of α-Bromoalkenyl Phosphonates with Aryl Boronic Acids and Alkenyl Borates. Org. Lett. 2002, 4, 4241–4244. [Google Scholar] [CrossRef]
- Kobayashi, Y.; William, A.D. Palladium- and Nickel-Catalyzed Coupling Reactions of α-Bromoalkenylphosphonates with Arylboronic Acids and Lithium Alkenylborates. Adv. Synth. Catal. 2004, 346, 1749–1757. [Google Scholar] [CrossRef]
- Ito, M.; Matsuumi, M.; Murugesh, M.G.; Kobayashi, Y. Scope and Limitation of Organocuprates, and Copper or Nickel Catalyst-Modified Grignard Reagents for Installation of an Alkyl Group onto cis-4-Cyclopentene-1,3-diol Monoacetate. J. Org. Chem. 2001, 66, 5881–5889. [Google Scholar] [CrossRef] [PubMed]
- Kiyotsuka, Y.; Acharya, H.P.; Katayama, Y.; Hyodo, T.; Kobayashi, Y. Picolinoxy Group, a New Leaving Group for anti SN2′ Selective Allylic Substitution with Aryl Anions Based on Grignard Reagents. Org. Lett. 2008, 10, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Takashima, Y.; Motoyama, Y.; Isogawa, Y.; Katagiri, K.; Tsuboi, A.; Ogawa, N. α- and γ-Regiocontrol and Enantiospecificity in the Copper-Catalyzed Substitution Reaction of Propargylic Phosphates with Grignard Reagents. Chem. Eur. J. 2020, 27, 3779–3785. [Google Scholar] [CrossRef]
- Shinohara, R.; Morita, M.; Ogawa, N.; Kobayashi, Y. Use of the 2-Pyridinesulfonyloxy Leaving Group for the Fast Copper-Catalyzed Coupling Reaction at Secondary Alkyl Carbons with Grignard Reagents. Org. Lett. 2019, 21, 3247–3251. [Google Scholar] [CrossRef]
- Ainai, T.; Matsuumi, M.; Kobayashi, Y. Efficient Total Synthesis of 12-oxo-PDA and OPC-8:0. J. Org. Chem. 2003, 68, 7825–7832. [Google Scholar] [CrossRef]
- Nonaka, H.; Ogawa, N.; Maeda, N.; Wang, Y.-G.; Kobayashi, Y. Stereoselective synthesis of epi-jasmonic acid, tuberonic acid, and 12-oxo-PDA. Org. Biomol. Chem. 2010, 8, 5212–5223. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Kobayashi, Y. Synthesis of the amino acid conjugates of epi-jasmonic acid. Amino Acids 2011, 42, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nakata, K.; Ainai, T. New Reagent System for Attaining High Regio-and Stereoselectivities in Allylic Displacement of 4-Cyclopentene-1,3-diol Monoacetate with Aryl- and Alkenylmagnesium Bromides. Org. Lett. 2004, 7, 183–186. [Google Scholar] [CrossRef]
- Nakata, K.; Kiyotsuka, Y.; Kitazume, T.; Kobayashi, Y. Realization of Anti-SN2′ Selective Allylation of 4-Cyclopentene-1,3-diol Monoester with Aryl- and Alkenyl-Zinc Reagents. Org. Lett. 2008, 10, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Kobayashi, Y. Aryl-and Alkenyllithium Preparations and Copper-Catalyzed Reaction between the Derived Magnesium Reagents and the Monoacetate of 4-Cyclopentene-1,3-diol. Org. Lett. 2005, 7, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y. Alkyl Pyridinesulfonates and Allylic Pyridinecarboxylates, New Boosters for the Substitution at Secondary Carbons. Heterocycles 2020, 100, 499. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shimoda, M. Cutting-Edge of Organic Synthesis and Chemical Biology of Bioactive Molecules; Kobayashi, Y., Ed.; Springer Nature: Singapore, 2019; Chapter 7; pp. 145–169. ISBN 978-981-13-6243-9. Available online: https://link.springer.com/chapter/10.1007/978-981-13-6244-6_7 (accessed on 22 November 2022).
- Kiyotsuka, Y.; Kobayashi, Y. Formation of Chiral C(sp3)−C(sp) Bond by Allylic Substitution of Secondary Allylic Picolinates and Alkynyl Copper Reagents. J. Org. Chem. 2009, 74, 7489–7495. [Google Scholar] [CrossRef]
- Wang, Q.; Kobayashi, Y. Allylic Substitution on Cyclopentene and -hexene Rings with Alkynylcopper Reagents. Org. Lett. 2011, 13, 6252–6255. [Google Scholar] [CrossRef]
- Kiyotsuka, Y.; Katayama, Y.; Acharya, H.P.; Hyodo, T.; Kobayashi, Y. New General Method for Regio- and Stereoselective Allylic Substitution with Aryl and Alkenyl Coppers Derived from Grignard Reagents. J. Org. Chem. 2009, 74, 1939–1951. [Google Scholar] [CrossRef]
- Ozaki, T.; Kobayashi, Y. Synthesis of (−)-mesembrine using the quaternary carbon-constructing allylic substitution. Org. Chem. Front. 2015, 2, 328–335. [Google Scholar] [CrossRef]
- Kawashima, H.; Kaneko, Y.; Sakai, M.; Kobayashi, Y. Synthesis of Cyclobakuchiols A, B, and C by Using Conformation-Controlled Stereoselective Reactions. Chem. Eur. J. 2013, 20, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ozaki, T. Concise Synthesis of (–)-Axenol by Using Stereocontrolled Allylic Substitution. Synlett 2015, 26, 1085–1088. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Wada, K.; Sakai, M.; Kawashima, H.; Ogawa, N. Efficient Synthesis of Anastrephin via the Allylic Substitution for Quaternary Carbon Construction. Synlett 2016, 27, 1428–1432. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saeki, R.; Nanba, Y.; Suganuma, Y.; Morita, M.; Nishimura, K. Synthesis of the Verapamil Intermediate through the Quaternary Carbon-Constructing Allylic Substitution. Synlett 2017, 28, 2655–2659. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamaguchi, K.; Morita, M. Regio-and stereoselective SN2′ reaction of an allylic picolinate in the synthesis of LY426965. Tetrahedron 2018, 74, 1826–1831. [Google Scholar] [CrossRef]
- Hyodo, T.; Kiyotsuka, Y.; Kobayashi, Y. Synthesis of the Active Form of Loxoprofen by Using Allylic Substitutions in Two Steps. Org. Lett. 2009, 11, 1103–1106. [Google Scholar] [CrossRef]
- Takashima, Y.; Isogawa, Y.; Tsuboi, A.; Ogawa, N.; Kobayashi, Y. Synthesis of a TNF inhibitor, flurbiprofen and an i-Pr analogue in enantioenriched forms by copper-catalyzed propargylic substitution with Grignard reagents. Org. Biomol. Chem. 2021, 19, 9906–9909. [Google Scholar] [CrossRef]
- Ogawa, N.; Uematsu, C.; Kobayashi, Y. Stereoselective Synthesis of (–)-Heliannuol E by α-Selective Propargyl Substitution. Synlett 2021, 32, 2071–2074. [Google Scholar] [CrossRef]
- Studte, C.; Breit, B. Zinc-Catalyzed Enantiospecific sp3–sp3 Cross-Coupling of α-Hydroxy Ester Triflates with Grignard Reagents. Angew. Chem. Int. Ed. 2008, 47, 5451–5455. [Google Scholar] [CrossRef]
- Brand, G.J.; Studte, C.; Breit, B. Iterative Synthesis of (Oligo) deoxypropionates via Zinc-Catalyzed Enantiospecific sp3−sp3 Cross-Coupling. Org. Lett. 2009, 11, 4668–4670. [Google Scholar] [CrossRef]
- Yang, C.-T.; Zhang, Z.-Q.; Liang, J.; Liu, J.-H.; Lu, X.-Y.; Chen, H.-H.; Liu, L. Copper-Catalyzed Cross-Coupling of Nonactivated Secondary Alkyl Halides and Tosylates with Secondary Alkyl Grignard Reagents. J. Am. Chem. Soc. 2012, 134, 11124–11127. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Oda, A.; Bobinski, T.; Li, H.; Matsueda, Y.; Negishi, E.-I. Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid. Angew. Chem. Int. Ed. 2015, 54, 9319–9322. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).