Heterofunctional Methacrylate Beads Bearing Octadecyl and Vinyl Sulfone Groups: Tricks to Obtain an Interfacially Activated Lipase from Thermomyces lanuginosus and Covalently Attached to the Support
Abstract
1. Introduction
2. Results
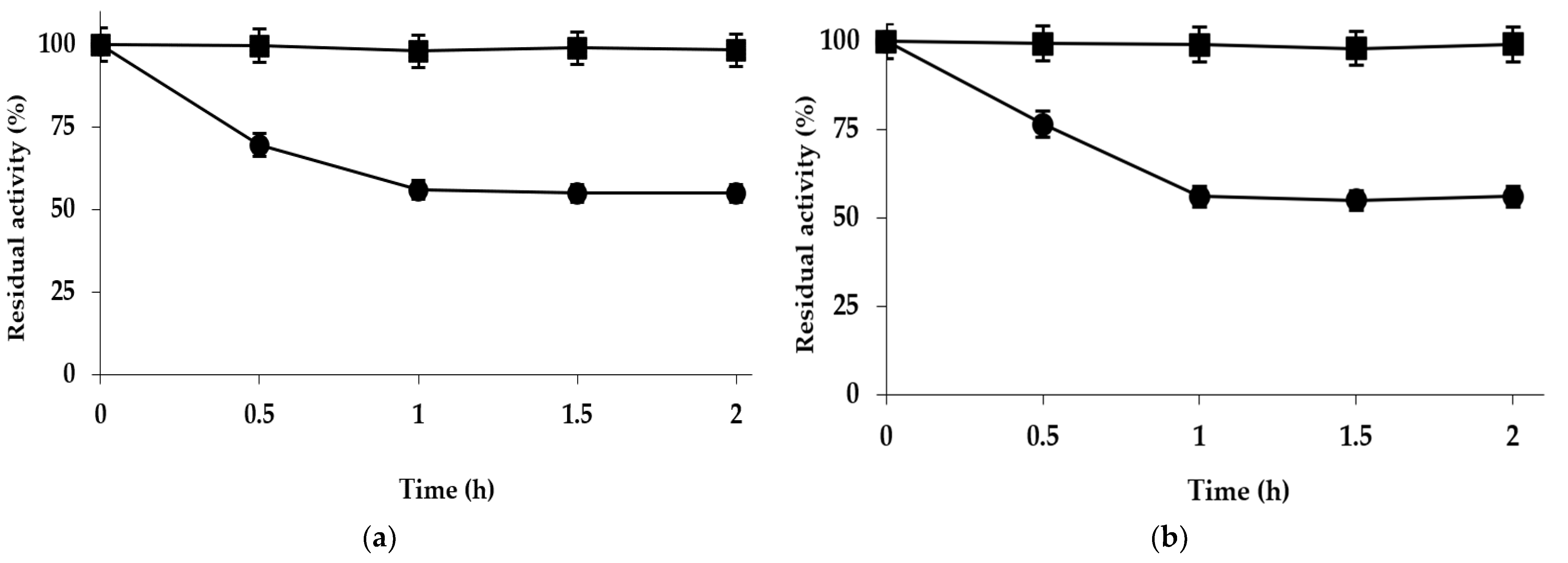
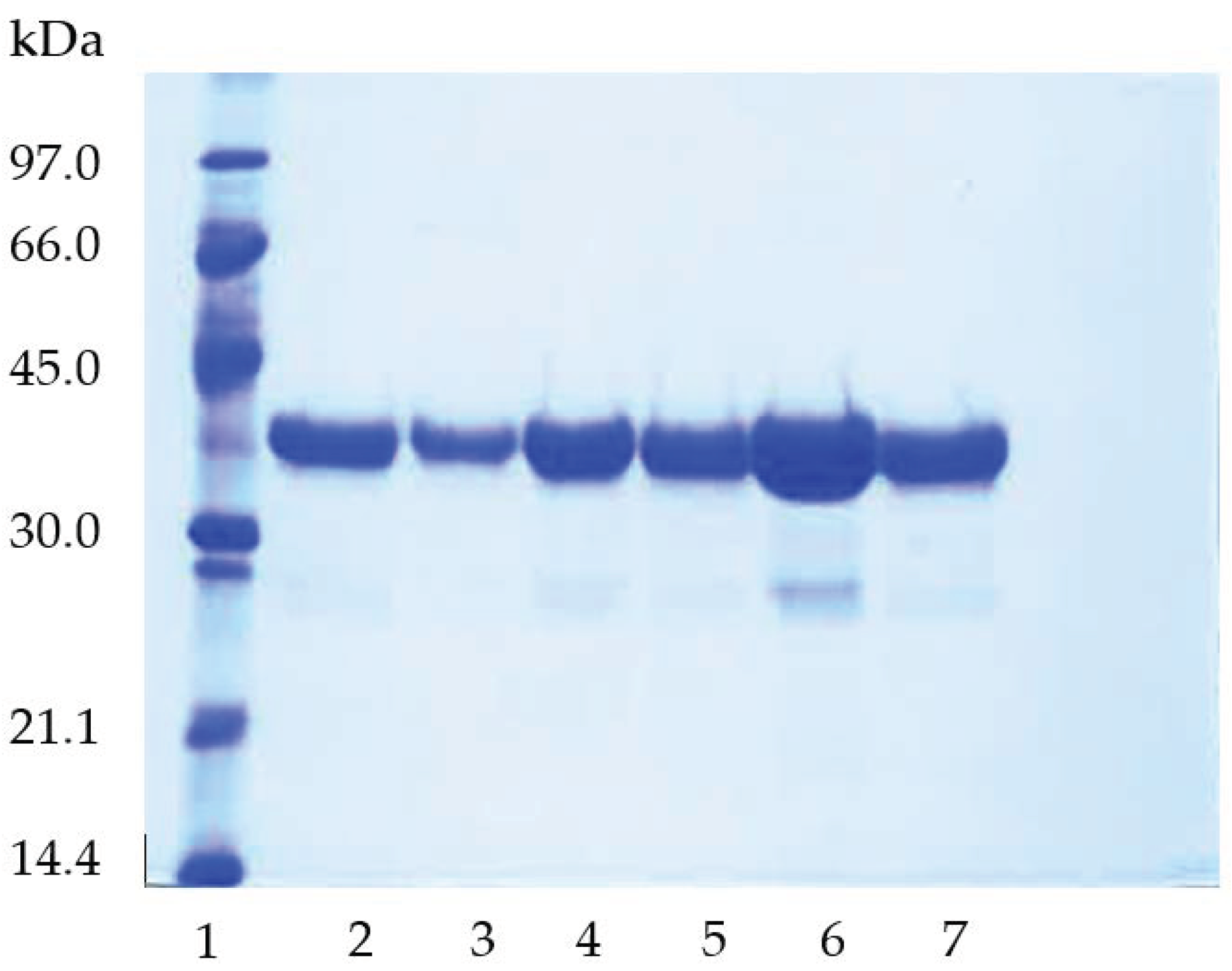
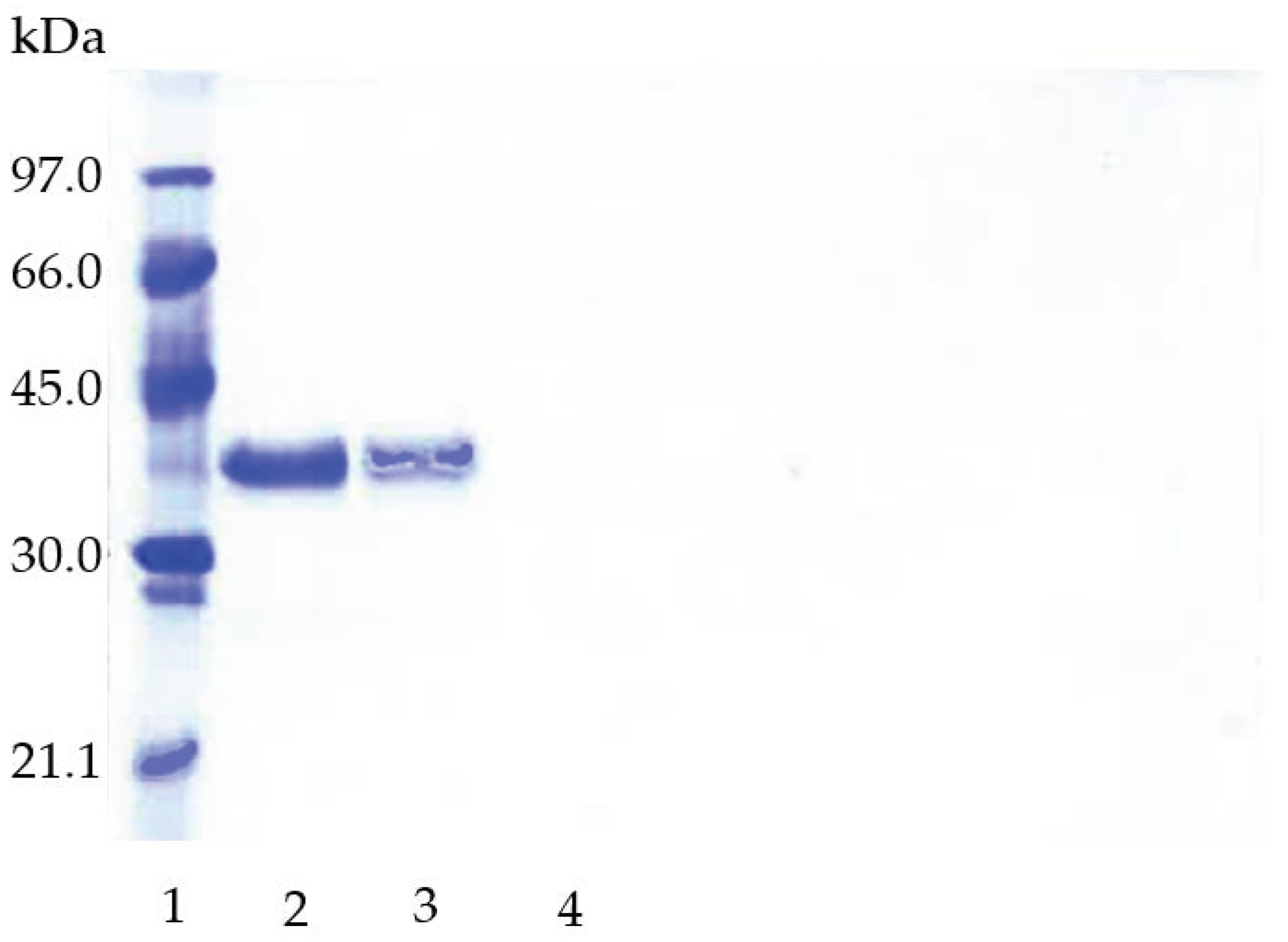
2.1. Immobilization of TLL on Purolite C18 and Purolite C18-VS
2.2. Preparation of Purolite C18-EDA-VS
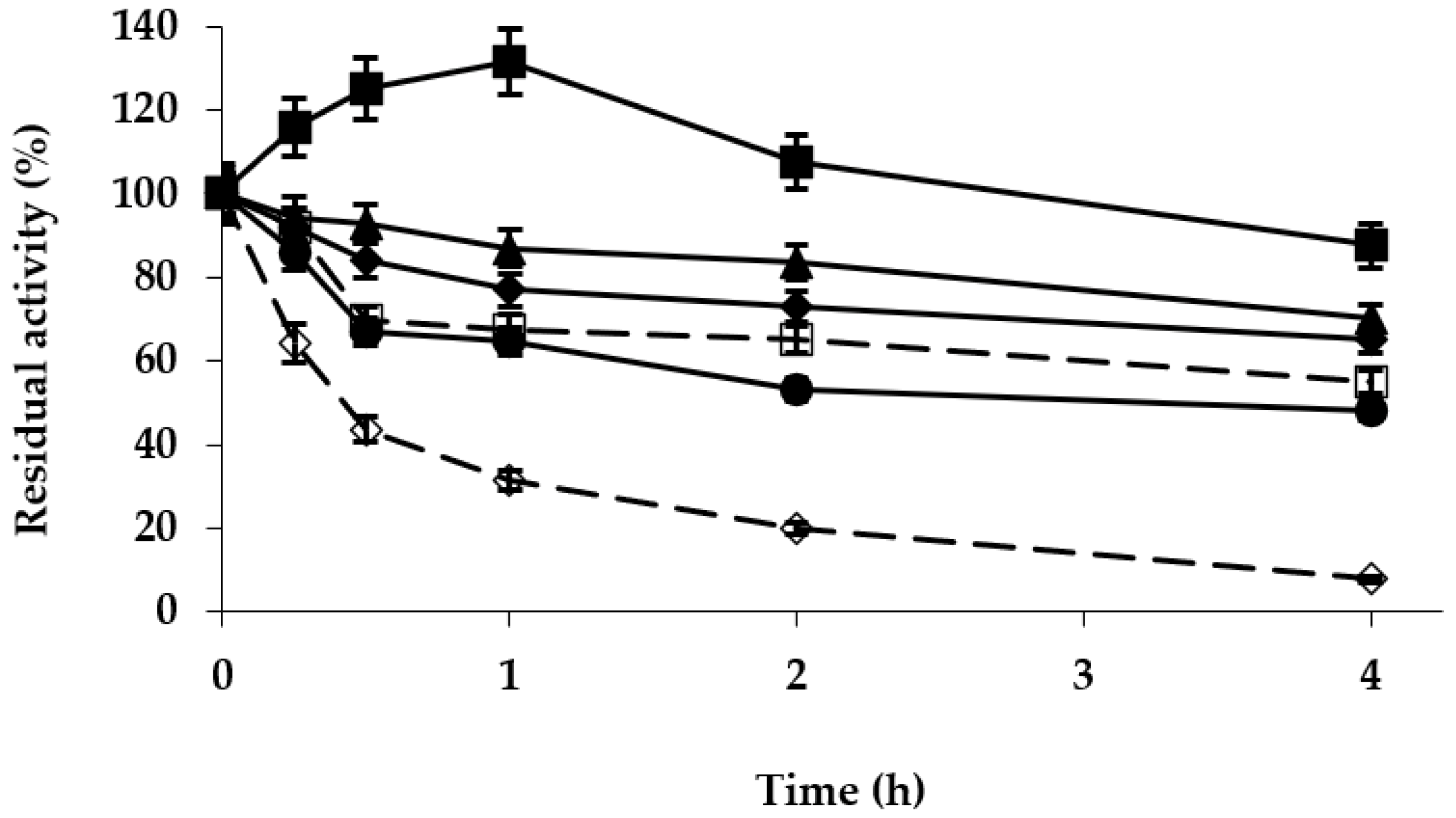
2.3. Optimization of the Purolite C18-EDA-VS-TLL Biocatalyst
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Wetting of Purolite C18 Beads
3.2.2. Preparation of Octadecyl-Vinyl Sulfone Purolite Beads
3.2.3. TLL Immobilization
Immobilization of Lipases on Wet Purolite C18 Beads
Immobilization of Lipases on Octadecyl-Vinyl Sulfone Purolite Beads
3.2.4. SDS-PAGE Analysis
3.2.5. Thermal Inactivation of the Different TLL Preparations
3.2.6. Enzyme Activity Assays
Hydrolysis of p-NPB
Hydrolysis of Triacetin
Hydrolysis of R- or S-Methyl Mandelate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vivek, K.; Sandhia, G.S.; Subramaniyan, S. Extremophilic lipases for industrial applications: A general review. Biotechnol. Adv. 2022, 60, 108002. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalek, S.A.; Yan, Y. Recent developments of lipase immobilization technology and application of immobilized lipase mixtures for biodiesel production. Biofuels Bioprod. Biorefin. 2022, 16, 1062–1094. [Google Scholar] [CrossRef]
- Nimkande, V.D.; Bafana, A. A review on the utility of microbial lipases in wastewater treatment. J. Water Process Eng. 2022, 46, 102591. [Google Scholar] [CrossRef]
- Patti, A.; Sanfilippo, C. Stereoselective promiscuous reactions catalyzed by lipases. Int. J. Mol. Sci. 2022, 23, 2675. [Google Scholar] [CrossRef] [PubMed]
- Remonatto, D.; Miotti, R.H., Jr.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Soni, S. Trends in lipase engineering for enhanced biocatalysis. Biotechnol. Appl. Biochem. 2021, 69, 265–272. [Google Scholar] [CrossRef]
- Ismail, A.R.; Kashtoh, H.; Baek, K.-H. Temperature-resistant and solvent-tolerant lipases as industrial biocatalysts: Biotechnological approaches and applications. Int. J. Biol. Macromol. 2021, 187, 127–142. [Google Scholar] [CrossRef]
- Mhetras, N.; Mapare, V.; Gokhale, D. Cold active lipases: Biocatalytic tools for greener technology. Appl. Biochem. Biotechnol. 2021, 193, 2245–2266. [Google Scholar] [CrossRef]
- Szymczak, T.; Cybulska, J.; Podleśny, M.; Frąc, M. Various perspectives on microbial lipase production using agri-food waste and renewable products. Agriculture 2021, 11, 540. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Priyanka, P.; Tan, Y.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2019, 41, 203–220. [Google Scholar] [CrossRef]
- Peña-García, C.; Martínez-Martínez, M.; Reyes-Duarte, D.; Ferrer, M. High throughput screening of esterases, lipases and phospholipases in mutant and metagenomic libraries: A review. Comb. Chem. High Throughput Screen. 2016, 19, 605–615. [Google Scholar] [CrossRef]
- Ferrer, M.; Bargiela, R.; Martínez-Martínez, M.; Mir, J.; Koch, R.; Golyshina, O.V.; Golyshin, P.N. Biodiversity for biocatalysis: A review of the α/β-hydrolase fold superfamily of esterases-lipases discovered in metagenomes. Biocatal. Biotransform. 2015, 33, 235–249. [Google Scholar] [CrossRef]
- Hamdan, S.H.; Maiangwa, J.; Ali, M.S.M.; Normi, Y.M.; Sabri, S.; Leow, T.C. Thermostable lipases and their dynamics of improved enzymatic properties. Appl. Microbiol. Biotechnol. 2021, 105, 7069–7094. [Google Scholar] [CrossRef]
- Kumar, R.; Goomber, S.; Kaur, J. Engineering lipases for temperature adaptation: Structure function correlation. Biochim. Biophys. Acta—Proteins Proteom. 2019, 1867, 140261. [Google Scholar] [CrossRef]
- Anobom, C.D.; Pinheiro, A.S.; De-Andrade, R.A.; Aguieiras, E.C.G.; Andrade, G.C.; Moura, M.V.; Almeida, R.V.; Freire, D.M. From structure to catalysis: Recent developments in the biotechnological applications of lipases. Biomed Res. Int. 2014, 2014, 684506. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Tang, Q.; Lan, D.; Wang, Y. Improving the catalytic activity and thermostability of MAS1 lipase by alanine substitution. Mol. Biotechnol. 2018, 60, 319–328. [Google Scholar] [CrossRef]
- Ravi, B.; Banerjee, U.; Mehrotra, S.; Mehrotra, R. Engineering lipases for enhanced catalysis. Curr. Chem. Biol. 2013, 7, 114–120. [Google Scholar] [CrossRef]
- Noro, J.; Cavaco-Paulo, A.; Silva, C. Chemical modification of lipases: A powerful tool for activity improvement. Biotechnol. J. 2022, 17, 2100523. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Enzyme immobilization and co-immobilization: Main framework, advances and some applications. Processes 2022, 10, 494. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Prata, A.S.; Forte, M.B.S. Enzyme immobilization: What have we learned in the past five years? Biofuels Bioprod. Biorefin. 2022, 16, 587–608. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Verger, R. ‘Interfacial activation’ of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.M.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature 1991, 351, 491–494. [Google Scholar] [CrossRef]
- van Tilbeurgh, H.; Egloff, M.-P.; Martinez, C.; Rugani, N.; Verger, R.; Cambillau, C. Interfacial activation of the lipase–procolipase complex by mixed micelles revealed by X-ray crystallography. Nature 1993, 362, 814–820. [Google Scholar] [CrossRef]
- Grochulski, P.; Li, Y.; Schrag, J.D.; Bouthillier, F.; Smith, P.; Harrison, D.; Rubin, B.; Cygler, M. Insights into interfacial activation from an open structure of Candida rugosa lipase. J. Biol. Chem. 1993, 268, 12843–12847. [Google Scholar] [CrossRef]
- Martinelle, M.; Holmquist, M.; Hult, K. On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim. Biophys. Acta—Lipids Lipid Metab. 1995, 1258, 272–276. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Savage, H.; Verma, C.S.; Turkenburg, J.P.; Lawson, D.M.; Svendsen, A.; Patkar, S. Structural origins of the interfacial activation in Thermomyces (Humicola) lanuginosa lipase. Biochemistry 2000, 39, 15071–15082. [Google Scholar] [CrossRef]
- Ericsson, D.J.; Kasrayan, A.; Johansson, P.; Bergfors, T.; Sandström, A.G.; Bäckvall, J.-E.; Mowbray, S.L. X-ray structure of Candida antarctica lipase A shows a novel lid structure and a likely mode of interfacial activation. J. Mol. Biol. 2008, 376, 109–119. [Google Scholar] [CrossRef]
- Palomo, J.M.; Peñas, M.M.; Fernández-Lorente, G.; Mateo, C.; Pisabarro, A.G.; Fernández-Lafuente, R.; Ramírez, L.; Guisán, J.M. Solid-phase handling of hydrophobins: Immobilized hydrophobins as a new tool to study lipases. Biomacromolecules 2003, 4, 204–210. [Google Scholar] [CrossRef]
- Wang, P.; He, J.; Sun, Y.; Reynolds, M.; Zhang, L.; Han, S.; Liang, S.; Sui, H.; Lin, Y. Display of fungal hydrophobin on the Pichia pastoris cell surface and its influence on Candida antarctica lipase B. Appl. Microbiol. Biotechnol. 2016, 100, 5883–5895. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General trend of lipase to self-assemble giving bimolecular aggregates greatly modifies the enzyme functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzym. Microb. Technol. 2017, 96, 30–35. [Google Scholar] [CrossRef]
- Hirata, D.B.; Albuquerque, T.L.; Rueda, N.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Fernandez-Lafuente, R. Evaluation of different immobilized lipases in transesterification reactions using tributyrin: Advantages of the heterofunctional octyl agarose beads. J. Mol. Catal. B Enzym. 2016, 133, 117–123. [Google Scholar] [CrossRef]
- Rueda, N.; Albuquerque, T.L.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; Dos Santos, J.C.S.; Barbosa, O.; Fernandez-Lafuente, R. Reversible immobilization of lipases on heterofunctional octyl-amino agarose beads prevents enzyme desorption. Molecules 2016, 21, 646. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.L.D.; Rueda, N.; Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Suescun, A.; Rueda, N.; Dos Santos, J.C.S.; Castillo, J.J.; Ortiz, C.; Torres, R.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of lipases on glyoxyl-octyl supports: Improved stability and reactivation strategies. Process Biochem. 2015, 50, 1211–1217. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Heterofunctional hydrophilic–hydrophobic porous silica as support for multipoint covalent immobilization of lipases: Application to lactulose palmitate synthesis. Langmuir 2014, 30, 3557–3566. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Improvement of efficiency in the enzymatic synthesis of lactulose palmitate. J. Agric. Food Chem. 2015, 63, 3716–3724. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalyzed by immobilized Geobacillus thermocatenulatus lipase. Catal. Today 2015, 255, 21–26. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Selectivity of R-α-monobenzoate glycerol synthesis catalyzed by Candida antarctica lipase B immobilized on heterofunctional supports. Process Biochem. 2015, 50, 1870–1877. [Google Scholar] [CrossRef]
- Boros, Z.; Weiser, D.; Márkus, M.; Abaháziová, E.; Magyar, Á.; Tomin, A.; Koczka, B.; Kovács, P.; Poppe, L. Hydrophobic adsorption and covalent immobilization of Candida antarctica lipase B on mixed-function-grafted silica gel supports for continuous-flow biotransformations. Process Biochem. 2013, 48, 1039–1047. [Google Scholar] [CrossRef]
- Vescovi, V.; Kopp, W.; Guisán, J.M.; Giordano, R.L.C.; Mendes, A.A.; Tardioli, P.W. Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino- glutaraldehyde spacer arms. Process Biochem. 2016, 51, 2055–2066. [Google Scholar] [CrossRef]
- Rios, N.S.; Mendez-Sanchez, C.; Arana-Peña, S.; Rueda, N.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Immobilization of lipase from Pseudomonas fluorescens on glyoxyl-octyl-agarose beads: Improved stability and reusability. Biochim. Biophys. Acta—Proteins Proteom. 2019, 1867, 741–747. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J.F.; Medina-Castillo, A.L.; Ramón-Márquez, T.; Arias-Martos, M.C.; Millán-Linares, M.C.; Pedroche, J.; Yust, M.D.M.; et al. Characterization of supports activated with divinyl sulfone as a tool to immobilize and stabilize enzymes via multipoint covalent attachment. Application to chymotrypsin. RSC Adv. 2015, 5, 20639–20649. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Rueda, N.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzym. Microb. Technol. 2015, 77, 1–7. [Google Scholar] [CrossRef]
- Souza, P.M.P.; Carballares, D.; Lopez-Carrobles, N.; Gonçalves, L.R.B.; Lopez-Gallego, F.; Rodrigues, S.; Fernandez-Lafuente, R. Enzyme-support interactions and inactivation conditions determine Thermomyces lanuginosus lipase inactivation pathways: Functional and florescence studies. Int. J. Biol. Macromol. 2021, 191, 79–91. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Torrestiana-Sánchez, B.; Dal Magro, L.; Virgen-Ortíz, J.J.; Suárez-Ruíz, F.J.; Rodrigues, R.C.; Fernandez-Lafuente, R. Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew. Energy 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernández-Lafuente, R.; Rodrigues, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sánchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fish waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Zaak, H.; Sassi, M.; Fernandez-Lafuente, R. A new heterofunctional amino-vinyl sulfone support to immobilize enzymes: Application to the stabilization of β-galactosidase from Aspergillus oryzae. Process Biochem. 2018, 64, 200–205. [Google Scholar] [CrossRef]
- Pinheiro, B.B.; Rios, N.S.; Rodríguez Aguado, E.; Fernandez-Lafuente, R.; Freire, T.M.; Fechine, P.B.A.; dos Santos, J.C.S.; Gonçalves, L.R.B. Chitosan activated with divinyl sulfone: A new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Peirce, S.; Tacias-Pascacio, V.G.; Cortes-Corberan, V.; Marzocchella, A.; Russo, M.E.; Fernandez-Lafuente, R. Reuse of anion exchangers as supports for enzyme immobilization: Reinforcement of the enzyme-support multiinteraction after enzyme inactivation. Process Biochem. 2016, 51, 1391–1396. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; Siar, E.-H.; Braham, S.A.; de Andrades, D.; Pedroche, J.; Millán, M.D.C.; Fernandez-Lafuente, R. Effect of amine length in the interference of the multipoint covalent immobilization of enzymes on glyoxyl agarose beads. J. Biotechnol. 2021, 329, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Ait Braham, S.; Hussain, F.; Morellon-Sterling, R.; Kamal, S.; Kornecki, J.F.; Barbosa, O.; Kati, D.E.; Fernandez-Lafuente, R. Cooperativity of covalent attachment and ion exchange on alcalase immobilization using glutaraldehyde chemistry: Enzyme stabilization and improved proteolytic activity. Biotechnol. Prog. 2019, 35, e2768. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.; Pedrero, S.; Fernandez-Lopez, L.; Lopez-Carrobles, N.; Gorines, B.; Otero, C.; Fernandez-Lafuente, R. Desorption of lipases immobilized on octyl-agarose beads and coated with ionic polymers after thermal inactivation. Stronger adsorption of polymers/unfolded protein composites. Molecules 2017, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.R.; Carballares, D.; Rocha-Martin, J.; Tardioli, P.W.; Fernandez-Lafuente, R. The immobilization protocol greatly alters the effects of metal phosphate modification on the activity/stability of immobilized lipases. Int. J. Biol. Macromol. 2022, 222, 2452–2466. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Carballares, D.; Tardioli, P.W.; Rocha-Martin, J.; Fernandez-Lafuente, R. Tuning immobilized commercial lipase preparations features by simple treatment with metallic phosphate salts. Molecules 2022, 27, 4486. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Velasco-Lozano, S.; Alcaraz-Fructuoso, M.T.; Sassi, M.; Lopez-Gallego, F.; Fernandez-Lafuente, R. Effect of high salt concentrations on the stability of immobilized lipases: Dramatic deleterious effects of phosphate anions. Process Biochem. 2017, 62, 128–134. [Google Scholar] [CrossRef]
- Lombardo, D.; Guy, O. Effect of alcohols on the hydrolysis catalyzed by human pancreatic carboxylic-ester hydrolase. Biochim. Biophys. Acta—Enzym. 1981, 657, 425–437. [Google Scholar] [CrossRef]
- Hernandez, K.; Garcia-Verdugo, E.; Porcar, R.; Fernandez-Lafuente, R. Hydrolysis of triacetin catalyzed by immobilized lipases: Effect of the immobilization protocol and experimental conditions on diacetin yield. Enzym. Microb. Technol. 2011, 48, 510–517. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization on octyl-agarose beads and some catalytic features of commercial preparations of lipase a from Candida antarctica (Novocor ADL): Comparison with immobilized lipase B from Candida antarctica. Biotechnol. Prog. 2019, 35, e2735. [Google Scholar] [CrossRef]

| Biocatalysts | Activity (U/g) | ||
|---|---|---|---|
| Triacetin | R-Mandelate | S-Mandelate | |
| Purolite C18-TLL | 548.7 ± 25.9 | 2.2 ± 0.2 | 0.32 ± 0.01 |
| Purolite C18-EDA-VS-TLL-Gly | 526.7 ± 20.5 | 2.0 ± 0.1 | 0.22 ± 0.01 |
| Purolite C18-EDA-VS-TLL-EDA | 479.6 ± 24.5 | 2.2 ± 0.1 | 0.11 ± 0.06 |
| Purolite C18-EDA-VS-TLL-Asp | 534.7 ± 30.0 | 2.0 ± 0.1 | 0.20 ± 0.01 |
| Purolite C18-EDA-VS-TLL-Cys | 327.5 ± 10.2 | 1.6 ± 0.1 | 0.09 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, J.R.; Carballares, D.; Rocha-Martin, J.; Alcántara, A.R.; Tardioli, P.W.; Fernandez-Lafuente, R. Heterofunctional Methacrylate Beads Bearing Octadecyl and Vinyl Sulfone Groups: Tricks to Obtain an Interfacially Activated Lipase from Thermomyces lanuginosus and Covalently Attached to the Support. Catalysts 2023, 13, 108. https://doi.org/10.3390/catal13010108
Guimarães JR, Carballares D, Rocha-Martin J, Alcántara AR, Tardioli PW, Fernandez-Lafuente R. Heterofunctional Methacrylate Beads Bearing Octadecyl and Vinyl Sulfone Groups: Tricks to Obtain an Interfacially Activated Lipase from Thermomyces lanuginosus and Covalently Attached to the Support. Catalysts. 2023; 13(1):108. https://doi.org/10.3390/catal13010108
Chicago/Turabian StyleGuimarães, José R., Diego Carballares, Javier Rocha-Martin, Andrés R. Alcántara, Paulo W. Tardioli, and Roberto Fernandez-Lafuente. 2023. "Heterofunctional Methacrylate Beads Bearing Octadecyl and Vinyl Sulfone Groups: Tricks to Obtain an Interfacially Activated Lipase from Thermomyces lanuginosus and Covalently Attached to the Support" Catalysts 13, no. 1: 108. https://doi.org/10.3390/catal13010108
APA StyleGuimarães, J. R., Carballares, D., Rocha-Martin, J., Alcántara, A. R., Tardioli, P. W., & Fernandez-Lafuente, R. (2023). Heterofunctional Methacrylate Beads Bearing Octadecyl and Vinyl Sulfone Groups: Tricks to Obtain an Interfacially Activated Lipase from Thermomyces lanuginosus and Covalently Attached to the Support. Catalysts, 13(1), 108. https://doi.org/10.3390/catal13010108











