Ionic Liquid/Deep Eutectic Solvent-Mediated Ni-Based Catalysts and Their Application in Water Splitting Electrocatalysis
Abstract
:1. Introduction
2. Using Ionic Liquids to Prepare Nickel-Based Electrocatalysts
| Catalyst | Morphology | Applied IL | Synthesis Method | Synthesis Condition | Catalytic Performance | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER | OER | Water Splitting | |||||||||||
| Electrolyte | ƞ (mV)@ Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | ƞ(mV)@ Current Density (mA cm−2) | TafelSlope(mV dec−1) | Electrolyte | Potential (V)@Current Density (mA cm−2) | ||||||
| NiO·Fe2O3 | Nanoparticles | [EMIM] NO3 | Hydrothermal method | 180 °C, 12 h | -- | -- | -- | 1 M KOH | -- | -- | -- | -- | [33] |
| NiFe hydroxides | Nanoparticles | [PMIM] BF4 | Coprecipitation | Room temperature, 24 h | -- | -- | -- | 1 M KOH | 300@10 | 54.4 | -- | -- | [34] |
| Ni2P | Big bulk grains with surface and interior thickly dotted with holes | 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) | Self-templated wet-chemical method | 120 °C, 12 h | -- | -- | -- | 1 M KOH | 246@10 | 46 | -- | -- | [35] |
| Ni12P5 | Hollow structure | -- | Reflow heating | 320 °C, 2 h | 0.5 M H2SO4 | 208@10 | 75 | -- | -- | -- | -- | -- | [36] |
| Ni2P | Rough surface morphology | -- | Ball milling method | 500 rpm, 6 h | 0.5 M H2SO4 | -- | 79 | -- | -- | -- | -- | -- | [37] |
| Ni2P | Netlike structure | -- | Hydrothermal method | 140 °C, 15 h | 1 M KOH | 85@10 | -- | 1 M KOH | 260@10 | 112 | -- | -- | [38] |
| Ni12P5 | Netlike structure | -- | Hydrothermal method | 200 °C, 15 h | 1 M KOH | 170@10 | -- | 1 M KOH | 240@10 | 106 | -- | -- | [38] |
| S-NiFeP | Nanoparticles | -- | Hydrothermal and annealing methods | 150 °C, 24 h; 300 ℃, 2 h | 1 M KOH | 56@10 | 38 | 1 M KOH | 201@10 | 41.2 | 1 M KOH | 1.5@10 | [39] |
| Ni2P | Nanoparticles | [BMIM] Tf2N | Reflow heating | 310 °C, 30 min | 0.5 M H2SO4 | 107@10 | 70 | -- | -- | -- | -- | -- | [40] |
| NiZn | Nanowires | [BMP] Tf2N | Electrodeposition and galvanic displacement reaction | −0.67 V (vs. Ag/Ag+) and 80 °C | -- | -- | -- | 1 M NaOH | -- | -- | -- | -- | [41] |
| NiFeP | Nanosheets | -- | Annealing method | 300 °C, 2 h | 1 M KOH | 130@20 | 78 | -- | -- | -- | -- | -- | [42] |
| NaYF4 | Spherical morphology | [BMIM][BF4] | Ionothermal method | 200 °C, 12 h | -- | -- | -- | -- | -- | -- | -- | -- | [43] |
| P,F-Ni1.5Co1.5N | Nanorods | [BMIM] PF6 | Hydrothermal method and annealing process | 150 °C, 10 h and 400 °C, 3 h in NH3 atmosphere | -- | -- | -- | 1 M KOH | 280@10 | 66.1 | -- | -- | [44] |
| CuxNi1-x | Flower-like morphology | [BMIM] PF6 | Hydrothermal method | 200 °C, 20 h | 1 M KOH | 88@10 | 91 | 1 M KOH | 198@10 | 76 | 1 M KOH | 1.58@10 | [45] |
| Ni2P wrapped by carbon fiber | Nanoparticles | [BMIM] PF6 | Inkjet printing technology | Using a commercial electrohydrodynamic inkjet printer | 1 M KOH | 117@10 | 92.0 | -- | -- | -- | -- | -- | [46] |
| NiP2 | Nanoparticles | [P4444]Cl | Microwave | 50 W, 1 min 50 s | 0.5 M H2SO4 | 102@10 | 46 | -- | -- | -- | -- | -- | [47] |
| Co2P | Shuttle shaped morphology | Trihexyl(tetradecyl)phosphonium tetrachlorocobaltate ([P66614]2[CoCl4]) | Annealing method | 400 °C, 2 h | 0.5 M H2SO4 | 150@10 | 47 | -- | -- | -- | -- | -- | [48] |
| Co2P | Spherical morphology | tetrabutylphosphonium tetrachlorocobaltate(II) ([P4444]2[CoCl4]) | Microwave | 6 min | 0.5 M H2SO4 | 135@10 | 58 | -- | -- | -- | -- | -- | [49] |
| Fe2P | Spherical morphology | trihexyl(tetradecyl)phosphonium tetrachloroferrate ([P(C6H13)3C14H29][FeCl4]) | Annealing method | 400 °C, 2 h | 0.5 M H2SO4 | 115@10 | 68 | -- | -- | -- | -- | -- | [50] |
| Ni2P4O12 | Nanoparticles | Octylamine/hypophosphorous | Annealing method | 400 °C, 2 h | 0.5 M H2SO4 | 116@10 | 97 | -- | -- | -- | -- | -- | [51] |
2.1. Ionic Liquids as Reaction Solvents and Templates to Prepare Nickel-Based Electrocatalysts
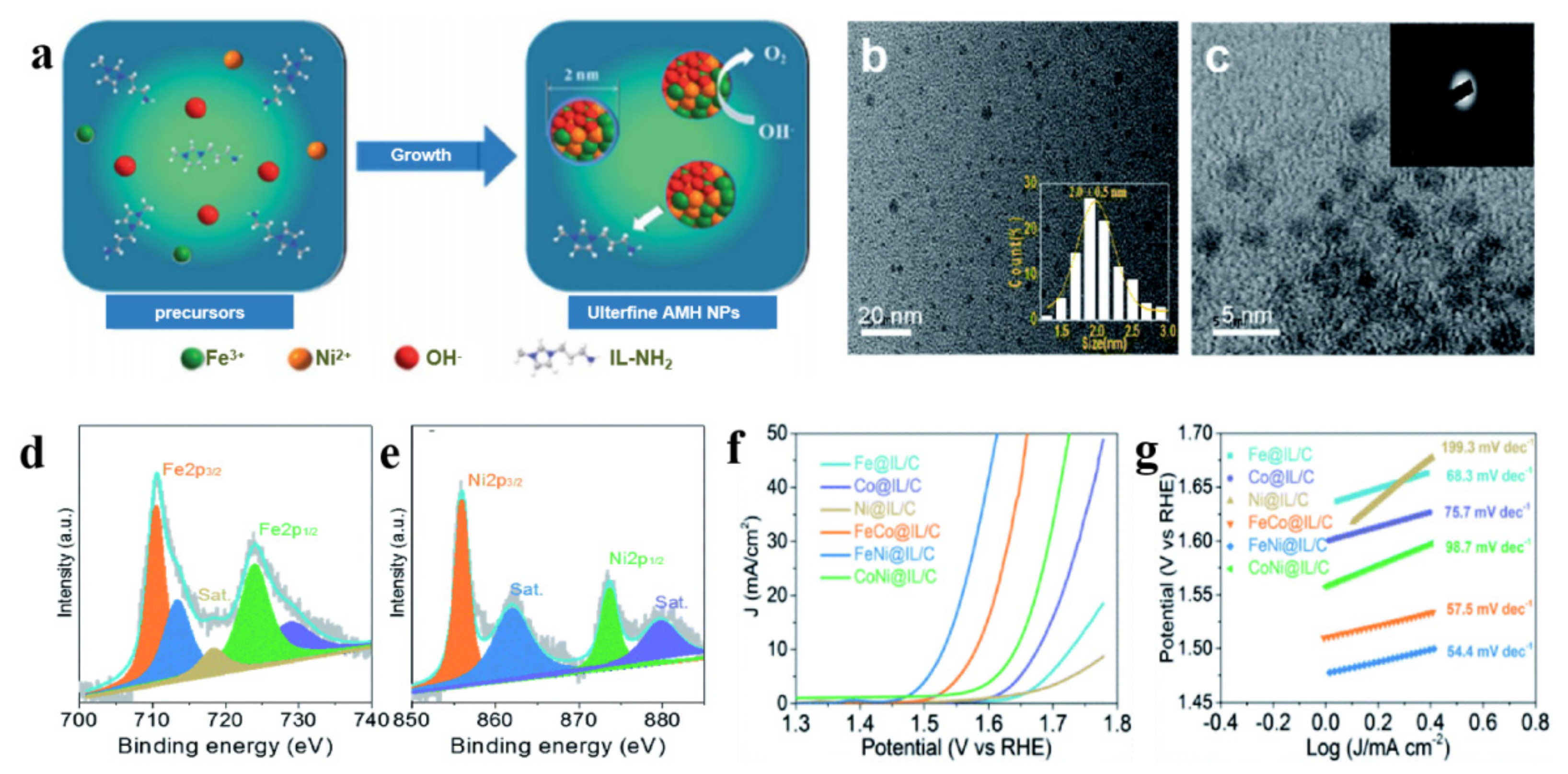
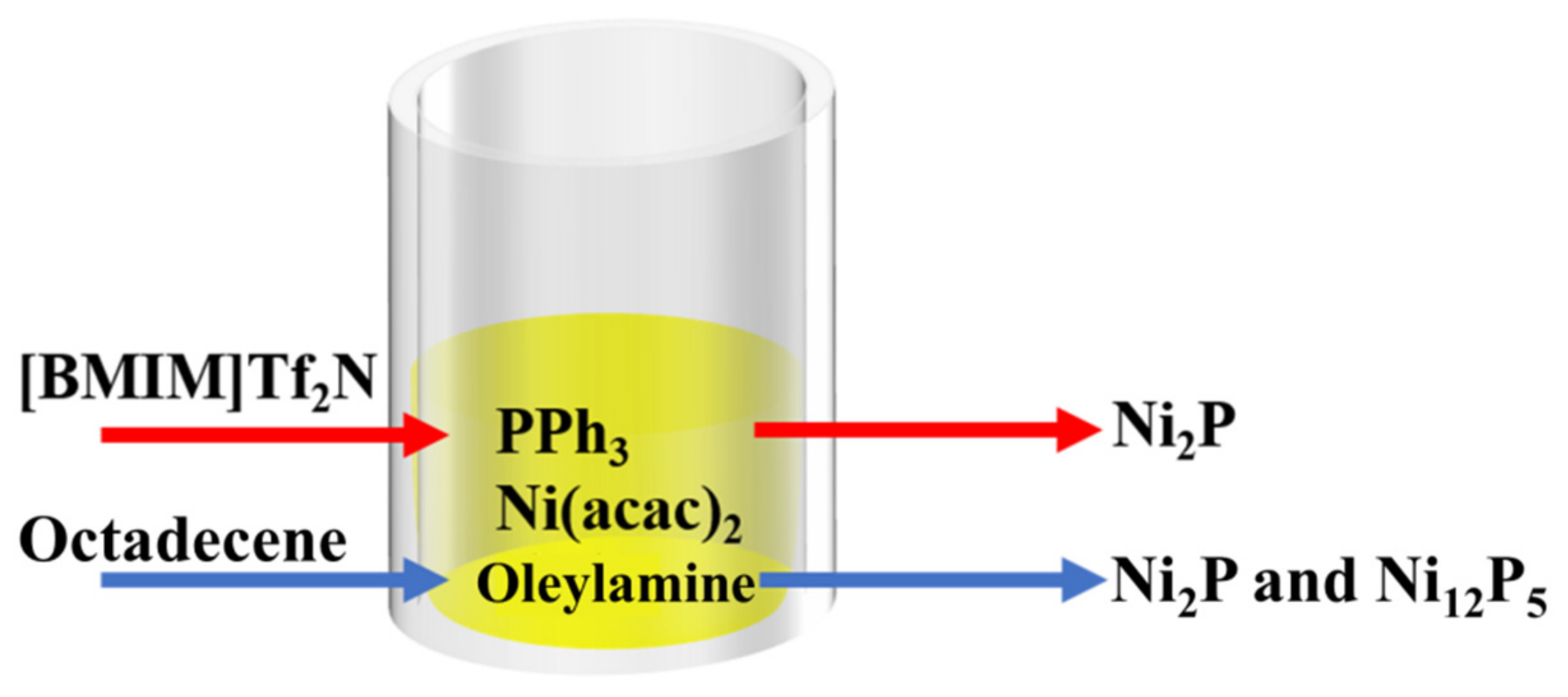
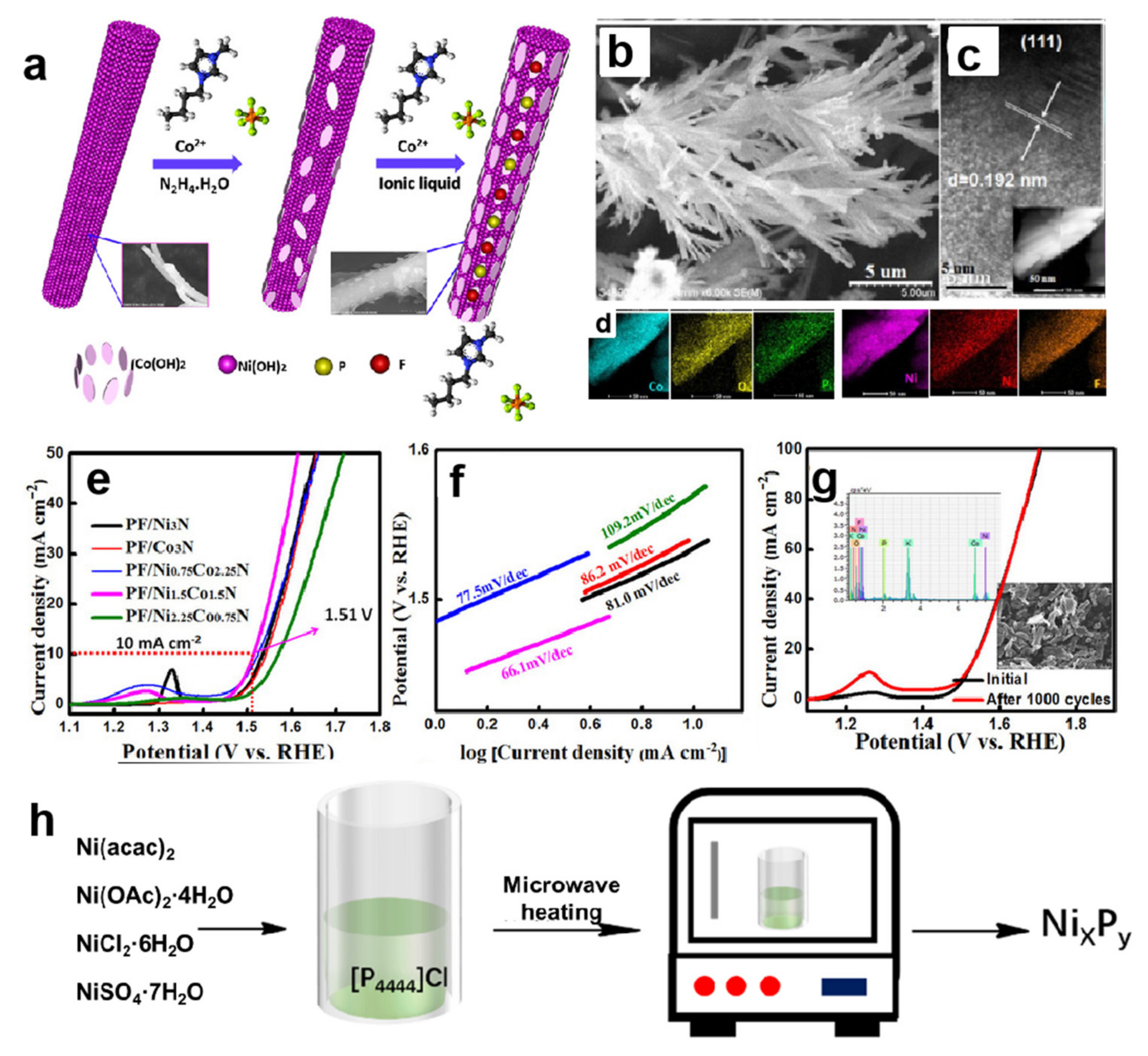
2.2. Ionic Liquids as Reaction Reagents to Prepare Nickel-Based Electrocatalysts
3. Using Deep Eutectic Solvents to Prepare Nickel-Based Electrocatalysts
| Catalyst | Morphology | Applied DES/IL | Synthesis Method | Synthesis Condition | Catalytic Performance | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER | OER | Water Splitting | |||||||||||
| Electrolyte | ƞ (mV)@ Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | ƞ(mV)@ Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | Potential (V)@ Current Density (mA cm−2) | ||||||
| NiFe | Nanoparticles | ChCl/EG | Galvanic replacement reaction | 80 °C, 5 h | -- | -- | 1 M KOH | 319@10 | 41.2 | -- | -- | [52] | |
| Ni | Nanoparticles | ChCl/EG | Galvanic replacement reaction | 80 °C, 5 h | 1 M KOH | 170@10 | 98.5 | -- | -- | -- | -- | -- | [53] |
| Ni3S2 | 3D hierarchically porous morphology | ChCl/EG | Galvanic replacement reaction | 80 °C, 5 h | 1 M KOH | 60.8@10 | 67.5 | -- | -- | -- | -- | -- | [54] |
| Ni3S2 | 3D hierarchically porous morphology | ChCl/EG | Galvanic replacement reaction | 80 °C, 5 h | 0.5 M H2SO4 | 63.5@10 | 91.6 | -- | -- | -- | -- | -- | [54] |
| Ni | Cubic morphology | ChCl/EG | Electrodeposition | −0.98 V (vs. Ag/Ag+), 900 s, 45 °C | 1 M KOH | 154@10 | 147 | -- | -- | -- | -- | -- | [55] |
| Metals | -- | Choline-based ILs | Selective extraction | -- | -- | -- | -- | -- | -- | -- | -- | -- | [56] |
| Ni/TiO2 | Spherical morphology | ChCl/EG | Electrodeposition | 5–15 mA cm−2, 40 °C | 1 M NaOH | -- | 122 | -- | -- | -- | -- | -- | [57] |
| Ni/Ni(OH)2 | Irregular morphology | ChCl/EG | Electrodeposition | −0.85 V (vs. Ag/Ag+), 70 °C | 1 M KOH | 110@10 | 83.9 | 1 M KOH | 290@10 | 120.9 | -- | -- | [58] |
| Cox-Ni(OH)2 | Ultrafine nanoparticles | ChCl/EG | Electrodeposition | −0.3 V to −1.1 V (vs. Ag/Ag+), 60 °C | 1 M KOH | 106@10 | 98.2 | 1 M KOH | 330@100 | 126.7 | -- | -- | [59] |
| NiSx | Cauliflflower-like morphology | ChCl/EG | Electrodeposition | −0.65 V (vs. Ag/Ag+), Room temperature | 1 M KOH | 54@10 | 54 | -- | -- | -- | -- | -- | [60] |
| Ni2P | Nanorods | [BMIM] Br | Ionothermal and calcining methods | 150 °C, 72 h; 700 °C, 2 h | -- | -- | -- | -- | -- | -- | -- | -- | [61] |
| NiPx | Nanoparticles | ChCl/EG | Electrodeposition | 0.54 V (vs. Ag/Ag+), Room temperature | 1 M KOH | 105@10 | 44.7 | -- | -- | -- | -- | -- | [62] |
| Ni-Cu | Nanosheets | ChCl/EG | Electrodeposition | −0.55 V to -0.90 V (vs. Ag/Ag+), 60 °C | 1 M KOH | 128@10 | 57.2 | -- | -- | -- | -- | -- | [63] |
| Ni-Co-Sn | Agglom erated nodular-grainsmorphology | ChCl/EG | Electrodeposition | 30 mA cm-2, Room temperature | 1 M KOH | -- | 121 | -- | -- | -- | -- | -- | [64] |
| Ni-Fe | Nanoparticles | ChCl/EG | Electrodeposition | −0.9 V (vs. Ag/Ag+) , 60 °C | 0.1 M KOH | 316@10 | 62 | -- | -- | -- | -- | -- | [65] |
| Ni-Mo-Cu | Irregular block-shaped particles | ChCl/urea | Electrodeposition | −1.2 V (vs. Ag/Ag+), 70 °C | 1 M KOH | 93@10 | 105 | -- | -- | -- | -- | -- | [66] |
| Ni-Mo | Microsphere | ChCl/EG | Electrodeposition | −0.95 V (vs. Ag/Ag+), 60 °C | 1 M KOH | 63@20 | 49 | 1 M KOH | 335@20 | 108 | 1 M KOH | 1.59@10 | [67] |
| S-NiFe2O4/Ni3Fe | Porous networks | ChCl/EG | Electrodeposition | −1.0 V (vs. Ag/Ag+), 60 °C | -- | -- | -- | 1 M KOH | 260@10 | 35 | 1 M KOH | 1.52@10 | [68] |
| NiCoxSy | Microsphere arrays | ChCl/EG | Electrodeposition | −0.80 V (vs. Ag/Ag+), 60 °C | 1 M KOH | 65@20 | 54 | 1 M KOH | 270@20 | 35 | 1 M KOH | 1.57@10 | [69] |
| (FeCoNiCuZn)(C2O4)· 2H2O | 2D morphology | PEG/oxalic acid | Ionothermal method | 160 °C, 8 h | -- | -- | -- | 1 M KOH | 334@10 | 67.93 | -- | -- | [70] |
| NiCo2O4@NiMoO4 | Nanorods | -- | Hydrothermal method | 120 °C, 10 h; 80 °C, 6 h | 1 M KOH | 170@10 | 184.25 | 1 M KOH | 300@10 | 94.06 | 1 M KOH | 1.65@10 | [71] |
| Ni0.33Co0.67S2 | Nanowires | -- | Hydrothermal and calcining methods | 90 °C, 8 h; 250 °C 2 h | 0.5 M H2SO4 | 73@10 | 44.1 | -- | -- | -- | -- | -- | [72] |
| NiCo2O4 | Nanooctahedron | ChCl/glycerol | Calcining method | 500 °C, 15 min, air atmosphere | -- | -- | -- | 1 M KOH | 320@10 | 67 | -- | -- | [73] |
| NiCo2S4 | Spherical sea urchin-like nanostructures | PEG 200/thiourea | Ionothermal method | 160.15 °C, 16 h | -- | -- | -- | 1 M KOH | 337@10 | 64 | -- | -- | [74] |
| Fe3S4 | Nanosheets | PEG 200/thiourea | Ionothermal method | 200 °C, 16 h | -- | -- | -- | -- | -- | -- | -- | -- | [75] |
| NiS/graphene | 2 D morphology | NiCl2· 6H2O/PEG 200 | Calcining method | 550 °C, 4 h, N2 atmosphere | 1 M KOH | 70@10 | 50.1 | 1 M KOH | 300@10 | 55.8 | 1 M KOH | 1.54@10 | [76] |
| NiS2/ graphene | Nanosphere | NiCl2· 6H2O/malonic acid | Calcining method | 550 °C, 4 h, N2 atmosphere | 1 M KOH | 57@10 | 47 | 1 M KOH | 294@10 | 54 | 1 M KOH | 1.52@10 | [77] |
| Ni2P/graphene | Nanoparticles | NiCl2· 6H2O/malonic acid | Calcining method | 400 °C, 4 h, N2 atmosphere | 1 M KOH | 103@10 | 56.5 | 1 M KOH | 275@20 | 56.2 | 1 M KOH | 1.51@10 | [78] |
| N-C/NiS2 | Sheet-like 2D nanostructures | NiCl2· 6H2O/urea | Calcining method | 550 °C, 4 h, N2 atmosphere | 1 M KOH | 78@10 | 63.4 | 1 M KOH | 264@10 | 51.3 | 1 M KOH | 1.53@10 | [79] |
| NiFe-LDH/N-C | flflower-like structure | NiCl2· 6H2O/ FeCl3· 6H2O/urea/water | Ionothermal method | 120 °C, 12 h | -- | -- | -- | 0.1 M KOH | 363@500 | 49.8 | -- | -- | [80] |
| NiFe-LDH | 2D morphology | FeCl3· 6H2O/urea | Dipping-redox method | 60 °C, 30 s | 1 M KOH | 160@10 | 42 | 1 M KOH | -- | -- | 1 M KOH | 1.61@10 | [81] |
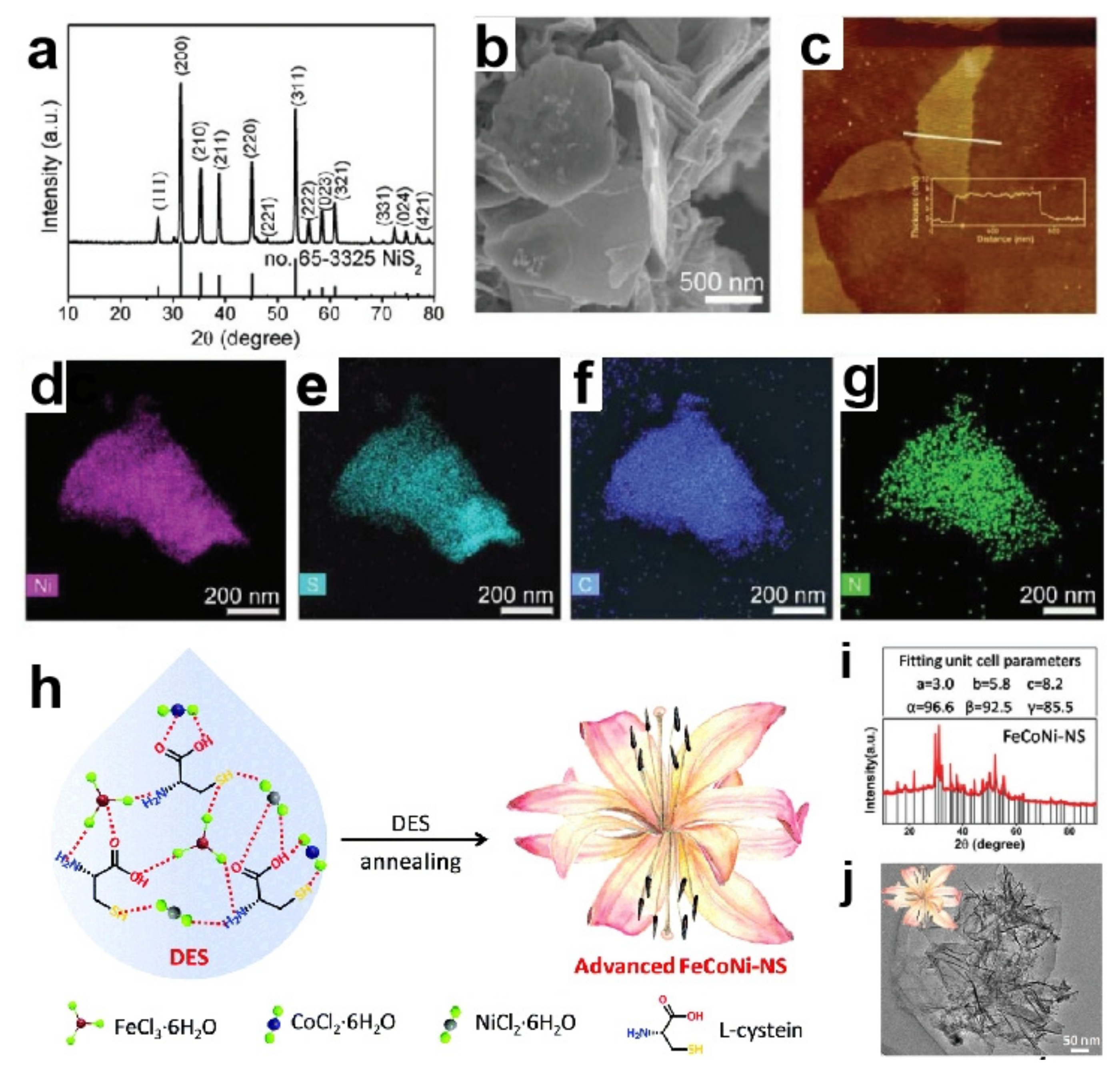
| N,S-FeCoNi | Oriented-grow flower | FeCl3· 6H2O/ CoCl2· 6H2O/ NiCl2· 6H2O/L-cysteine | Calcining method | 350 °C, 12 h, N2 atmosphere | -- | -- | -- | 1 M KOH | 251@10 | 58 | -- | -- | [82] |
| High-entropy metal phosphides | Nanoparticles | [P4444]Cl/ethylene glycol /five equimolar hydrated metal chlorides | Eutectic solvent method | in an inert atmosphere at 400 °C for 3 h | 1 M KOH | 136@10 | 85.5 | 1 M KOH | 320@10 | 60.8 | 1 M KOH | 1.78@100 | [83] |
3.1. Deep Eutectic Solvents as Solvents and Templates to Prepare Nickel-Based Electrocatalysts
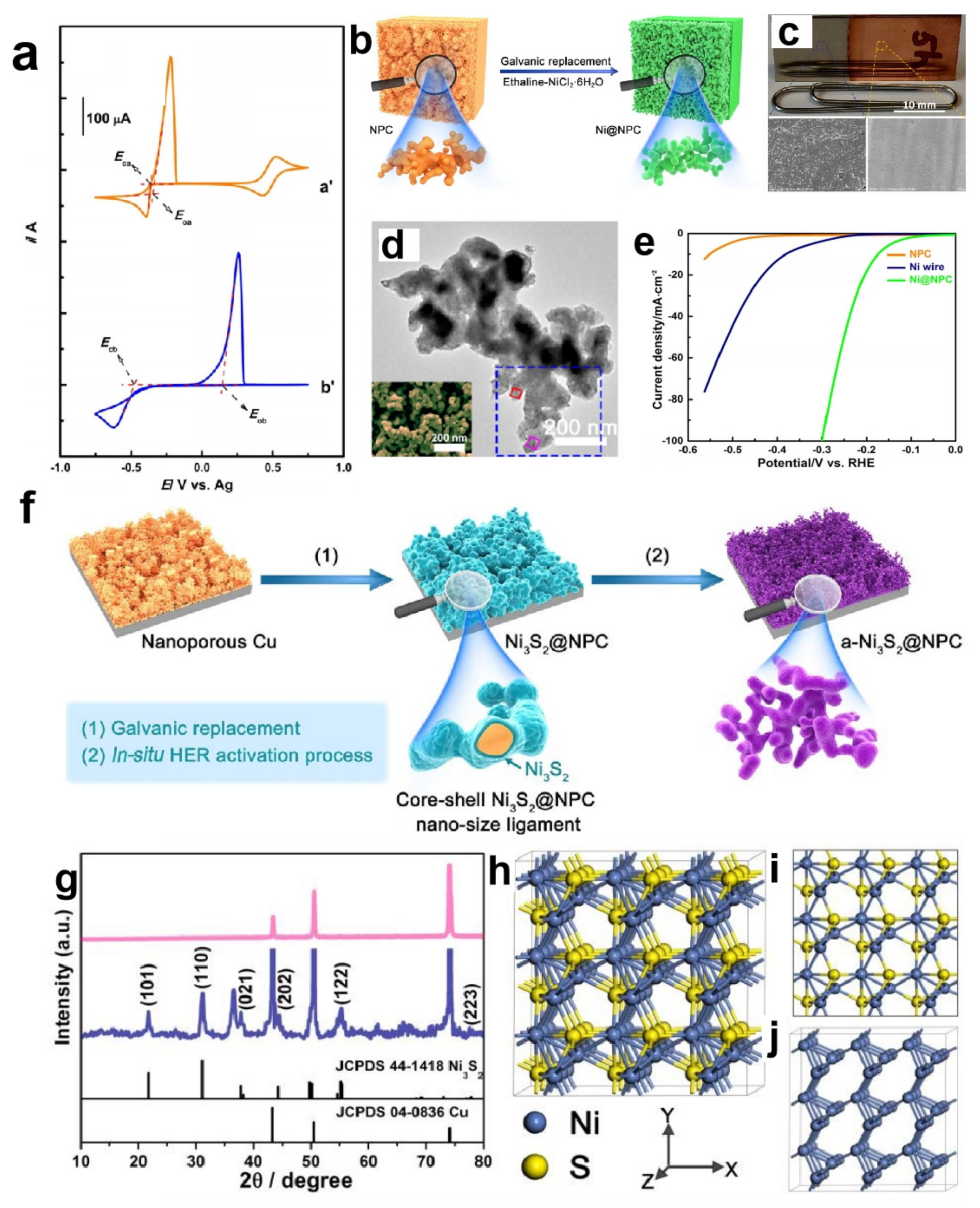
3.2. Deep Eutectic Solvents as Reaction Reagents to Prepare Nickel-Based Electrocatalysts
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.V.; Alhassan, S.M. Scalable solid-state synthesis of MoS2–NiS2/graphene nanohybrids as bifunctional electrocatalysts for enhanced overall water splitting. Mater. Adv. 2020, 1, 794. [Google Scholar] [CrossRef]
- Jeong, S.; Mai, H.D.; Nam, K.H.; Park, C.M.; Jeon, K.J. Self-healing graphene-templated platinum-nickel oxide heterostructures for overall water splitting. ACS Nano 2022, 16, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.J.; Zong, S.C.; Shen, S.H. Homojunction photocatalysts for water splitting. Nano Res. 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef] [PubMed]
- Ifkovits, Z.P.; Evans, J.M.; Meier, M.C.; Papadantonakis, K.M.; Lewis, N.S. Decoupled electrochemical water-splitting systems: A review and perspective. Energy Environ. Sci. 2021, 14, 4740–4759. [Google Scholar] [CrossRef]
- Yu, C.; Lu, J.J.; Luo, L.; Xua, F.; Shen, P.; Tsiakaras, P.; Yin, S. Bifunctional catalysts for overall water splitting: CoNi oxyhydroxide nanosheets electrodeposited on titanium sheets. Electrochim. Acta 2019, 301, 449–457. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Yoon, T.; Kim, K.S. Nickel-based electrocatalysts for energy-related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; Wang, Y.; Zou, Y.; Dong, C.L.; Huang, Y.C.; Xiao, Z.H.; Wei, Z.X.; Du, S.Q.; Chen, C.; et al. Activity origins and design principles of nickel-based catalysts for nucleophile electrooxidation. Chem 2020, 6, 2974–2993. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.X.; Yu, X.; Ma, Y.R. Graphdiyne reinforced multifunctional Cu/Ni bimetallic phosphides -graphdiyne hybrid nanostructure as high performance electrocatalyst for water splitting. J. Colloid Interface Sci. 2022, 628, 508–518. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shobukawa, H.; Tokuda, H.; Tabaata, S.; Watanabe, M. Preparation and transport properties of novel lithium ionic liquids. Electrochim. Acta 2004, 50, 305–309. [Google Scholar] [CrossRef]
- Dai, L.; Yu, S.; Shan, Y.; He, M. Novel room temperature inorganic ionic liquids. Eur. J. Inorg. Chem. 2004, 2004, 237–241. [Google Scholar] [CrossRef]
- Bhaisare, M.L.; Abdelhamid, H.N.; Wu, B.S.; Wu, H.F. Rapid and direct MALDI-MS identification of pathogenic bacteria from blood using ionic liquid-modified magnetic nanoparticles (Fe3O4@SiO2). J. Mater. Chem. B 2014, 2, 4671–4683. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.K.H.; Singla, M. Diverse applications of ionic liquid: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Gopal, J.; Wu, H.F. Synthesis and application of ionic liquid matrices (ILMs) for effective pathogenic bacteria analysis in matrix assisted laser desorption/ionization (MALDI-MS). Anal. Chim. Acta 2013, 767, 104–111. [Google Scholar] [CrossRef]
- Marfavi, Y.; AliAkbari, R.; Kowsari, E.; Sadeghi, B.; Ramakrishna, S. Application of ionic liquids in green energy-storage materials. In Ionic Liquid-Based Technologies for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 155–166. [Google Scholar] [CrossRef]
- Chen, T.; Liu, S.; Ying, H.; Li, Z.; Hao, J. Reactive ionic liquid enables the construction of 3D Rh particles with nanowire subunits for electrocatalytic nitrogen reduction. Chem. Asian J. 2020, 15, 1081–1087. [Google Scholar] [CrossRef]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Ratti, R.; Shirota, H. Ionic liquids: Synthesis and applications in catalysis. Adv. Chem. 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Holtmann, C.S.D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
- Millia, L.; Dall’Asta, V.; Ferrara, C.; Berbenni, V.; Quartarone, E.; Perna, F.M.; Capriati, V.; Mustarelli, P. Bio-inspired choline chloride-based deep eutectic solvents as electrolytes for lithium-ion batteries. Solid State Ion. 2018, 323, 44–48. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, H.; Zhao, Q.; Liang, Z. Use of microwave-assisted deep eutectic solvents to recycle lithium manganese oxide from Li-ion batteries. JOM 2021, 73, 2104–2110. [Google Scholar] [CrossRef]
- Roldán-Ruiz, M.J.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Highly efficient p-toluenesulfonic acid-based deep-eutectic solvents for cathode recycling of Li-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 5437–5445. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G.H. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Lovelock, K.R.J.; Armstrong, J.P.; Licence, P.; Jones, R.G. Vaporisation and thermal decomposition of dialkylimidazolium halide ion ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 1339–1353. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Shishov, A.; Chromá, R.; Vakh, C.; Kuchár, J.; Simon, A.; Andruch, V.; Bulatov, A. In situ decomposition of deep eutectic solvent as a novel approach in liquid-liquid microextraction. Anal. Chim. Acta 2019, 10, 49–55. [Google Scholar] [CrossRef]
- Thorat, G.M.; Jadhav, H.S.; Roy, A.; Chung, W.J.; Seo, J.G. Dual role of deep eutectic solvent as a solvent and template for the synthesis of octahedral cobalt vanadate for an oxygen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 16255–16266. [Google Scholar] [CrossRef]
- Xiong, Z.; Si, Y.; Li, M. Improving the catalytic performance of nickel-iron oxide to oxygen evolution reaction by refining its particles with the assistance of ionic liquid. Ionics 2017, 23, 789–794. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, S.; Yu, C.L.; Zhang, J.W.; Pan, X.L.; Li, G. Ionic liquid-assisted one-step preparation of ultrafine amorphous metallic hydroxide nanoparticles for the highly efficient oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 15767–15773. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Q.; Liu, Y.; Qin, Q.; Zhang, J.; Qi, K.; Chen, J.; Wang, Z.; Zheng, K.; Świerczek, K.; et al. Red phosphorus as self-template to hierarchical nanoporous nickel phosphides toward enhanced electrocatalytic activity for oxygen evolution reaction. Electrochim. Acta 2020, 332, 135500. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zhao, J.; Yang, K.; Liang, J.; Liu, D.; Hu, W.; Liu, D.; Liu, Y.; Liu, C. Monodispersed nickel phosphide nano crystals with different phases: Synthesis, characterization and electrocatalytic properties for hydrogen evolution. J. Mater. Chem. A 2015, 3, 1656–1665. [Google Scholar] [CrossRef]
- Kucernak, A.R.J.; Naranammalpuram Sundaram, V.N. Nickel phosphide: The effect of phosphorus content on hydrogen evolution activity and corrosion resistance in acidic medium. J. Mater. Chem. A 2014, 2, 17435–17445. [Google Scholar] [CrossRef] [Green Version]
- Menezes, P.; Indra, A.; Das, C.; Walter, C.; Göbel, C.; Gutkin, V.; Schmeiβer, D.; Driess, M. Uncovering the nature of active species of nickel phosphide catalysts in high-performance electrochemical overall water splitting. ACS Catal. 2017, 7, 103–109. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Su, H.; Hong, A.N.; Wang, Y.; Yang, H.; Ge, L.; Song, W.; Liu, J.; Ma, T.; et al. Electron redistributed S-doped nickel iron phosphides derived from one-step phosphatization of MOFs for significantly boosting electrochemical water splitting. Adv. Funct. Mater. 2022, 2, 200733. [Google Scholar] [CrossRef]
- Roberts, E.J.; Read, C.G.; Lewis, N.S.; Brutchey, R.L. Phase directing ability of an ionic liquid solvent for the synthesis of HER-active Ni2P nanocrystals. ACS Appl. Energy Mater. 2018, 1, 1823–1827. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, C.; Chen, P. Galvanic displacement on electrodeposited tangled Zn nanowire sacrificial template for preparing porous and hollow Ni electrodes in ionic liquid. J. Mol. Liq. 2020, 298, 112050. [Google Scholar] [CrossRef]
- Wang, J.; Ji, L.; Zuo, S.; Chen, Z. Hierarchically structured 3D integrated electrodes by galvanic replacement reaction for highly efficient water splitting. Adv. Energy Mater. 2017, 7, 1700107. [Google Scholar] [CrossRef]
- Chen, C.; Sun, L.; Li, Z.; Li, L.; Zhang, J.; Zhang, Y. Ionic liquid-based route to spherical NaYF4 nanoclusters with the assistance of microwave radiation and their multicolor upconversion luminescence. Langmuir 2018, 26, 8797–8803. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, Q.; Xu, G.; Ning, Y.; Huang, K.; He, F.; Wu, Z.; Zhang, J. Phosphorus and fluorine Co-doping induced enhancement of oxygen evolution reaction in bimetallic nitride nanorods arrays: Ionic liquid-driven and mechanism clarification. Chem. Eur. J. 2017, 23, 16862–16870. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Y.; Huang, K.; Li, K.; Wang, Q. Interfacial electronic structure and electrocatalytic performance modulation in Cu0.81Ni0.19 nanoflowers by heteroatom doping engineering using ionic liquid dopant. Appl. Surf. Sci. 2020, 500, 144052. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Z.; Zhang, Y.; Lv, Q.; Jing, F.; Chi, K.; Wang, S. Large-scale printing synthesis of transition metal phosphides encapsulated in N,P co-doped carbon as highly efficient hydrogen evolution cathodes. Nano Energy 2018, 51, 223–230. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, B.; Xi, Z.; Zhang, B.; Li, Z.; Zhang, H.; Li, Z.; Hao, J. Phosphonium-based ionic liquid: A new phosphorus source toward microwave-driven synthesis of nickel phosphide for efficient hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 1468–1477. [Google Scholar] [CrossRef]
- Tang, D.; Li, T.; Li, C.M. Metal and phosphonium-based ionic liquid: A new Co and P dual-source for synthesis of cobalt phosphide toward hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 1720–1726. [Google Scholar] [CrossRef]
- Li, T.; Tang, D.; Li, C.M. Microwave-assisted synthesis of cobalt phosphide using ionic liquid as Co and P dual-source for hydrogen evolution reaction. Electrochim. Acta 2019, 295, 1027–1033. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Zhao, Q.; Liu, D.; Li, C.M. The in situ preparation of iron phosphide using ionic liquids as iron and phosphorus sources for efficient hydrogen evolution reactions. RSC Adv. 2020, 10, 33026–33032. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, C.; Chen, T.; Zhao, X.; Li, Z.; Hao, J. A new phosphonium-based ionic liquid to synthesize nickel metaphosphate for hydrogen evolution reaction. Nanotechnology 2020, 31, 505402. [Google Scholar] [CrossRef]
- Xue, R.; Guo, M.; Wei, Z.; Zhang, Q. Deep eutectic solvent-induced synthesis of Ni–Fe catalyst with excellent mass activity and stability for water oxidation. Green Energy Environ. 2021, in press. [CrossRef]
- Yang, C.; Zhang, Q.B.; Abbott, A.P. Facile fabrication of nickel nanostructures on a copper-based template via a galvanic replacement reaction in a deep eutectic solvent. Electrochem. Commun. 2016, 70, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Gao, M.Y.; Zhang, Q.B.; Zeng, J.R.; Li, X.T.; Abbott, A.P. In-situ activation of self-supported 3D hierarchically porous Ni3S2 films grown on nanoporous copper as excellent pH-Universal electrocatalysts for hydrogen evolution reaction. Nano Energy 2017, 36, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Elsharkawya, S.; Hammad, S.; El-hallaga, I. Electrodeposition of Ni nanoparticles from deep eutectic solvent and aqueous solution promoting high stability electrocatalyst for hydrogen and oxygen evolution reactions. J. Solid State Electrochem. 2022, 26, 1501–1517. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Shikotra, P. Selective extraction of metals from mixed oxide matrixes using choline-based ionic liquids. Inorg. Chem. 2005, 44, 6497–6499. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.; Danilov, F.I. Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni/TiO2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 24604–24616. [Google Scholar] [CrossRef]
- Gao, M.; Sun, C.B.; Lei, H.; Zeng, J.; Zhang, Q.B. Nitrate-induced and in situ electrochemical activation synthesis of oxygen deficiencies-rich nickel/nickel (oxy)hydroxide hybrid films for enhanced electrocatalytic water splitting. Nanoscale 2018, 10, 17546–17551. [Google Scholar] [CrossRef]
- Sun, C.B.; Guo, M.W.; Siwal, S.S.; Zhang, Q.B. Efficient hydrogen production via urea electrolysis with cobalt doped nickel hydroxide-riched hybrid films: Cobalt doping effect and mechanism aspect. J. Catal. 2020, 381, 454–461. [Google Scholar] [CrossRef]
- Zeng, J.R.; Gao, M.Y.; Zhang, Q.B.; Yang, C.; Li, X.T.; Yang, W.Q.; Hua, Y.X.; Xu, C.Y.; Li, Y. Facile electrodeposition of cauliflower-like S-doped nickel microsphere films as highly active catalysts for electrochemical hydrogen evolution. J. Mater. Chem. A. 2017, 5, 15056–15064. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Feng, H.; Shen, J. Synthesis of nickel phosphide nano-particles in a eutectic mixture for hydrotreating reactions. J. Mater. Chem. 2011, 21, 8137–8145. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, J.; Lei, H.; Yang, W.; Zhang, Q. Direct electrodeposition of phosphorus-doped nickel superstructures from choline chloride–ethylene glycol deep eutectic solvent for enhanced hydrogen evolution catalysis. ACS Sustain. Chem. Eng. 2019, 7, 1529–1537. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Yu, Y.W.; Hua, Y.X.; Li, Y.; Dong, P. Electrochemical fabrication of porous Ni-Cu alloy nanosheets with high catalytic activity for hydrogen evolution. Electrochim. Acta 2016, 215, 609–616. [Google Scholar] [CrossRef]
- Vijayakumar, J.; Mohan, S.; Anand Kumar, S.; Suseendiran, S.R.; Pavithra, S. Electrodeposition of Ni–Co–Sn alloy from choline chloride-based deep eutectic solvent and characterization as cathode for hydrogen evolution in alkaline solution. Int. J. Hydrogen Energy 2013, 38, 10208–10214. [Google Scholar] [CrossRef]
- Vo, T.; Hidalgo, S.D.S.; Chiang, C. Controllable electrodeposition of binary metal films from deep eutectic solvent as an efficient and durable catalyst for the oxygen evolution reaction. Dalton Trans. 2019, 48, 14748–14757. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Geng, S.; Wang, S.; Rao, S.; Huang, Y.; Zou, X.; Zhang, Y.; Xu, Q.; Lu, X. Electrodeposition of NiMoCu coatings from roasted nickel matte in deep eutectic solvent for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 5704–5716. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Zeng, J.R.; Li, X.T.; Hua, Y.X.; Xu, C.Y.; Dong, P. Facile electrochemical preparation of self-supported porous Ni–Mo alloy microsphere films as efficient bifunctional electrocatalysts for water splitting. J. Mater. Chem. A 2017, 5, 5797–5805. [Google Scholar] [CrossRef]
- Gao, M.Y.; Zeng, J.R.; Zhang, Q.B.; Yang, C.; Li, X.T.; Hua, Y.X.; Xu, C.Y. Scalable one-step electrochemical deposition of nanoporous amorphous S-doped NiFe2O4/Ni3Fe composite films as highly efficient electrocatalysts for oxygen evolution with ultrahigh stability. J. Mater. Chem. A 2018, 6, 1551–1560. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Siwal, S.S.; Yang, W.; Fu, X.; Zhang, Q. Morphological and electronic modification of 3D porous nickel microsphere arrays by cobalt and sulfur dual synergistic modulation for overall water splitting electrolysis and supercapacitors. Appl. Surf. Sci. 2019, 491, 570–578. [Google Scholar] [CrossRef]
- Yang, H.Y.; Cheng, Z.F.; Wu, P.C.; Wei, Y.H.; Jiang, J.Y.; Xu, Q. Deep eutectic solvents regulation synthesis of multi-metal oxalate for electrocatalytic oxygen evolution reaction and supercapacitor applications. Electrochim. Acta 2022, 427, 140879. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, R.; Dai, J.; Xiang, J.; Wu, F. A hybrid NiCo2O4@NiMoO4 structure for overall water splitting and excellent hybrid energy storage. Dalton Trans. 2020, 49, 9668–9679. [Google Scholar] [CrossRef]
- Peng, Z.; Jia, D.; Al-Enizi, A.M.; Elzatahry, A.A.; Zheng, G. Electrocatalysts: From water oxidation to reduction: Homologous Ni–Co based nanowires as complementary water splitting electrocatalysts. Adv. Energy Mater. 2015, 5, 1402031. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, B.; Chen, T.; Ying, H.; Li, Z.; Hao, J. Deep eutectic solvent strategy enables an octahedral Ni–Co precursor for creating high-performance NiCo2O4 catalyst toward oxygen evolution reaction. Green Energy Environ. 2021, in press. [CrossRef]
- Jiang, J.; Yan, C.; Zhao, X.; Luo, H.; Xue, Z.; Mu, T. A PEGylated deep eutectic solvent for controllable solvothermal synthesis of porous NiCo2S4 for efficient oxygen evolution reaction. Green Chem. 2017, 19, 3023–3031. [Google Scholar] [CrossRef]
- Zhao, X.; Lan, X.; Yu, D.; Fu, H.; Liu, Z.; Mu, T. Deep eutectic-solvothermal synthesis of nanostructured Fe3S4 for electrochemical N2 fixation under ambient conditions. Chem. Commun. 2018, 54, 13010–13013. [Google Scholar] [CrossRef]
- Zhang, D.; Mou, H.; Lu, F.; Song, C.; Wang, D. Heterostructures as efficient bifunctional electrocatalysts for overall water splitting. Appl. Catal. B 2019, 254, 471–478. [Google Scholar] [CrossRef]
- Zhang, D.; Mou, H.; Chen, L.; Wang, D.; Song, C. Design and in-situ synthesis of unique catalyst via embedding graphene oxide shell membrane in NiS2 for efficient hydrogen evolution. Appl. Surf. Sci. 2020, 510, 145483. [Google Scholar] [CrossRef]
- Mou, H.; Wang, J.; Yu, D.; Zhang, D.; Lu, F.; Chen, L.; Wang, D.; Mu, T. A facile and controllable, deep eutectic solvent aided strategy for synthesis graphene encapsulated metal phosphides for enhanced electrocatalysis overall water splitting. J. Mater. Chem. A 2019, 7, 13455–13459. [Google Scholar] [CrossRef]
- Zhang, D.; Mou, H.; Chen, L.; Xing, G.; Wang, D.; Song, C. Surface/interface engineering N-doped carbon/NiS2 nanosheets for efficient electrocatalytic H2O splitting. Nanoscale 2020, 12, 3370–3376. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Sun, C.; Song, C.; Wang, D. One-step ionothermal accompanied thermolysis strategy for N-doped carbon quantum dots hybridized NiFe LDH ultrathin nanosheets for electrocatalytic water oxidation. Electrochim. Acta 2021, 391, 138932. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Zhang, H.; Li, Z.; Hao, J. Hydrated metal halide-based deep eutectic solvent-mediated NiFe layered double hydroxide: An excellent electrocatalyst for urea electrolysis and water splitting. Chem. Asian J. 2019, 14, 2995–3002. [Google Scholar] [CrossRef]
- Jiang, J.; Chang, L.; Zhao, W.; Tian, Q.; Xu, Q. An advanced FeCoNi nitro-sulfide hierarchical structure from deep eutectic solvents for enhanced oxygen evolution reaction. Chem. Commun. 2019, 55, 10174–10177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, Z.; Chen, W.; Wang, Y.; Mu, T. Eutectic synthesis of high-entropy metal phosphides for electrocatalytic water splitting. ChemSusChem 2020, 13, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.; Janiak, C. Metal nanoparticles in ionic liquids. Top. Curr. Chem. 2017, 375, 65. [Google Scholar] [CrossRef] [PubMed]
| Method | Limitations of Traditional Synthesis | Advantages of IL/DES Synthesis |
|---|---|---|
| Precipitation method | The products have poor dispersibility, being aggregate easily. |
|
| Sol-gel method |
|
|
| Solvothermal/hydrothermal method |
|
|
| Electrodeposition |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Bai, T.; Sun, Y.; Xin, B.; Zhang, S. Ionic Liquid/Deep Eutectic Solvent-Mediated Ni-Based Catalysts and Their Application in Water Splitting Electrocatalysis. Catalysts 2022, 12, 928. https://doi.org/10.3390/catal12080928
Zhang C, Bai T, Sun Y, Xin B, Zhang S. Ionic Liquid/Deep Eutectic Solvent-Mediated Ni-Based Catalysts and Their Application in Water Splitting Electrocatalysis. Catalysts. 2022; 12(8):928. https://doi.org/10.3390/catal12080928
Chicago/Turabian StyleZhang, Chenyun, Te Bai, Yefan Sun, Bingwei Xin, and Shengnan Zhang. 2022. "Ionic Liquid/Deep Eutectic Solvent-Mediated Ni-Based Catalysts and Their Application in Water Splitting Electrocatalysis" Catalysts 12, no. 8: 928. https://doi.org/10.3390/catal12080928
APA StyleZhang, C., Bai, T., Sun, Y., Xin, B., & Zhang, S. (2022). Ionic Liquid/Deep Eutectic Solvent-Mediated Ni-Based Catalysts and Their Application in Water Splitting Electrocatalysis. Catalysts, 12(8), 928. https://doi.org/10.3390/catal12080928







