Recent Advances in Metal-Based Molecular Photosensitizers for Artificial Photosynthesis
Abstract
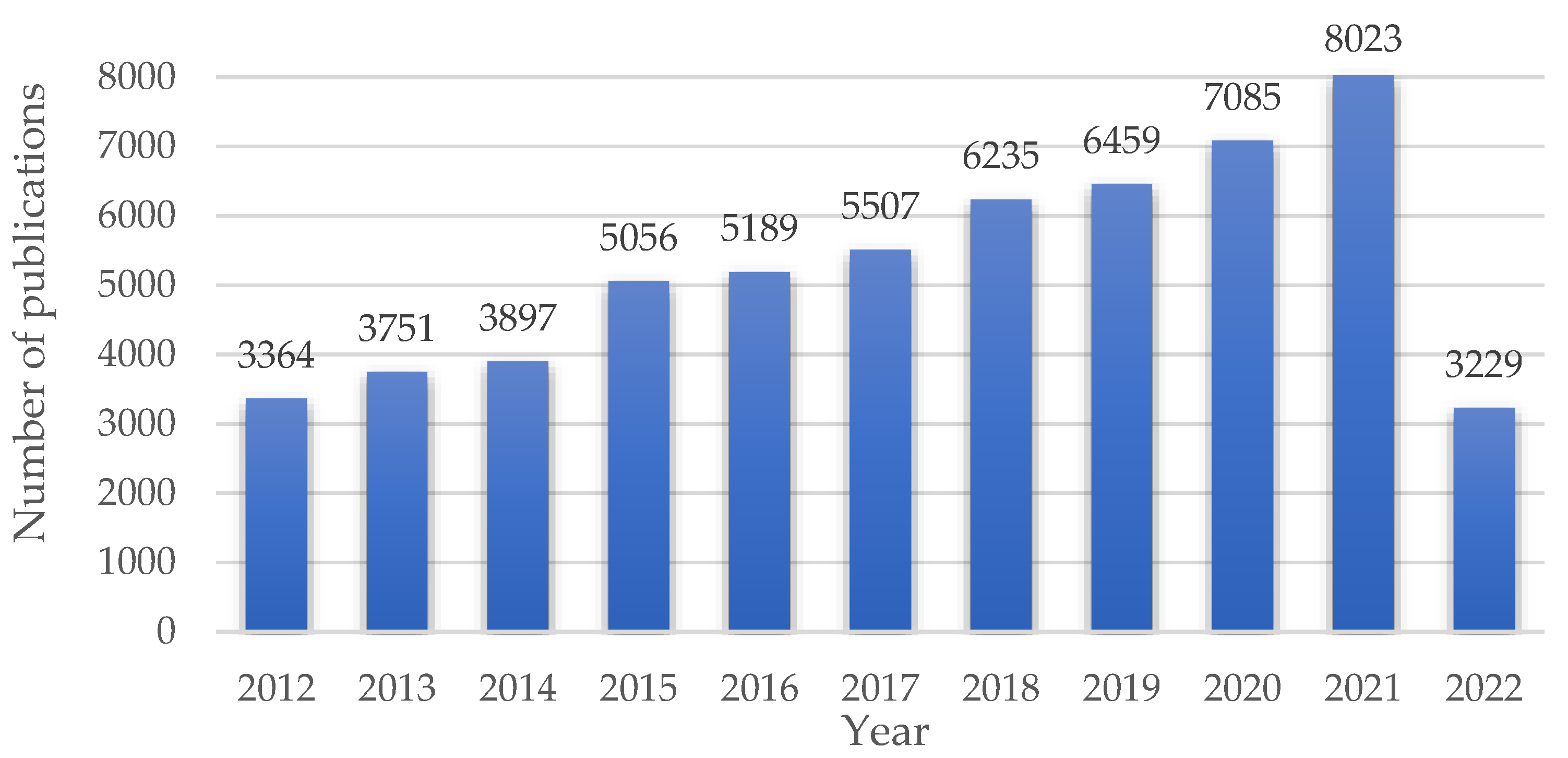
:1. Introduction
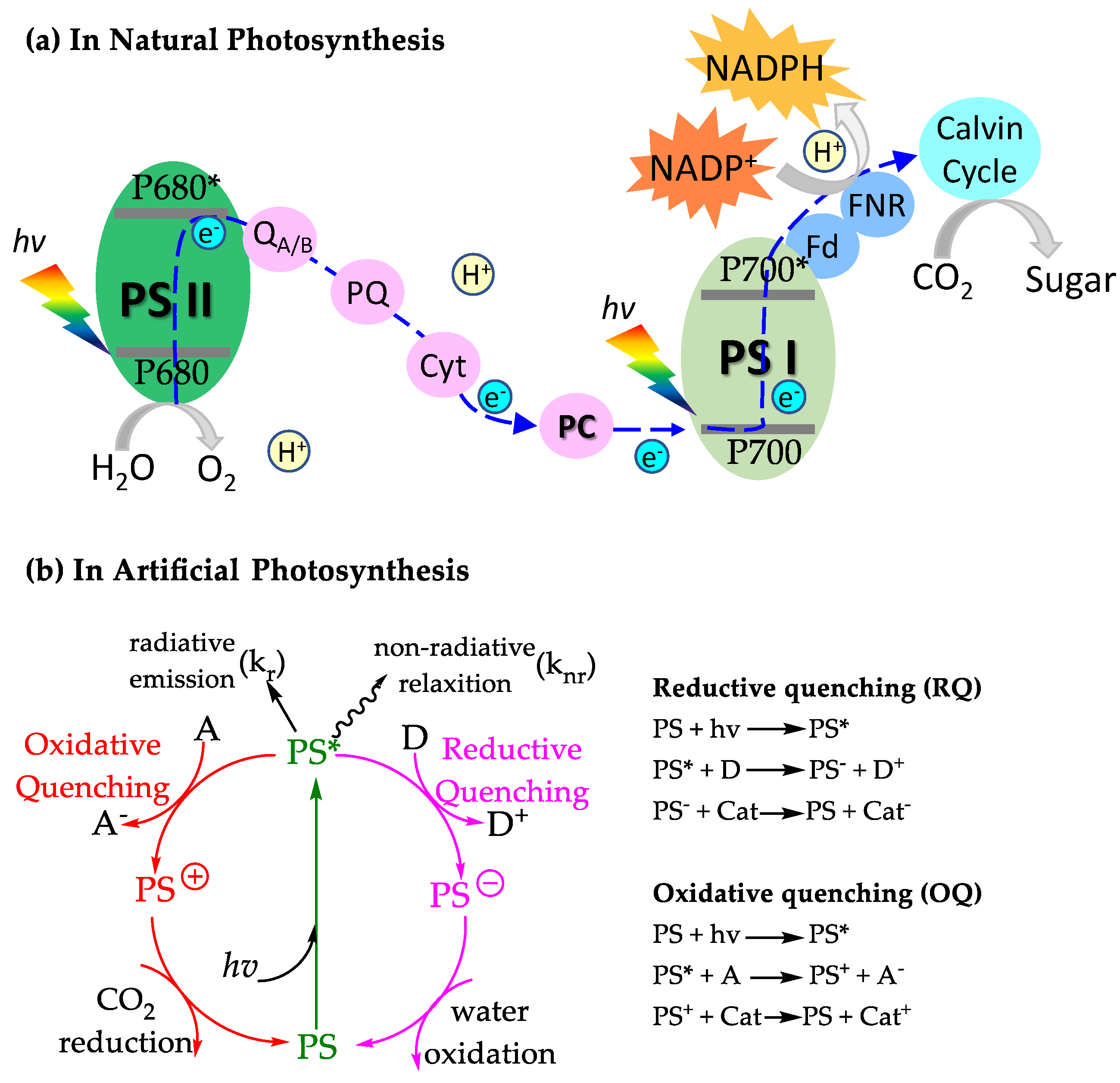
1.1. Photosynthesis in Nature
1.2. The Role of Photosensitizers in Artificial Photosynthesis
1.3. A General Scheme for Photosensitizer-Involved Photocatalysis
2. Parameters for Evaluating Photosensitizers
2.1. UV-Vis Absorption
2.2. Ground-State Redox Potential
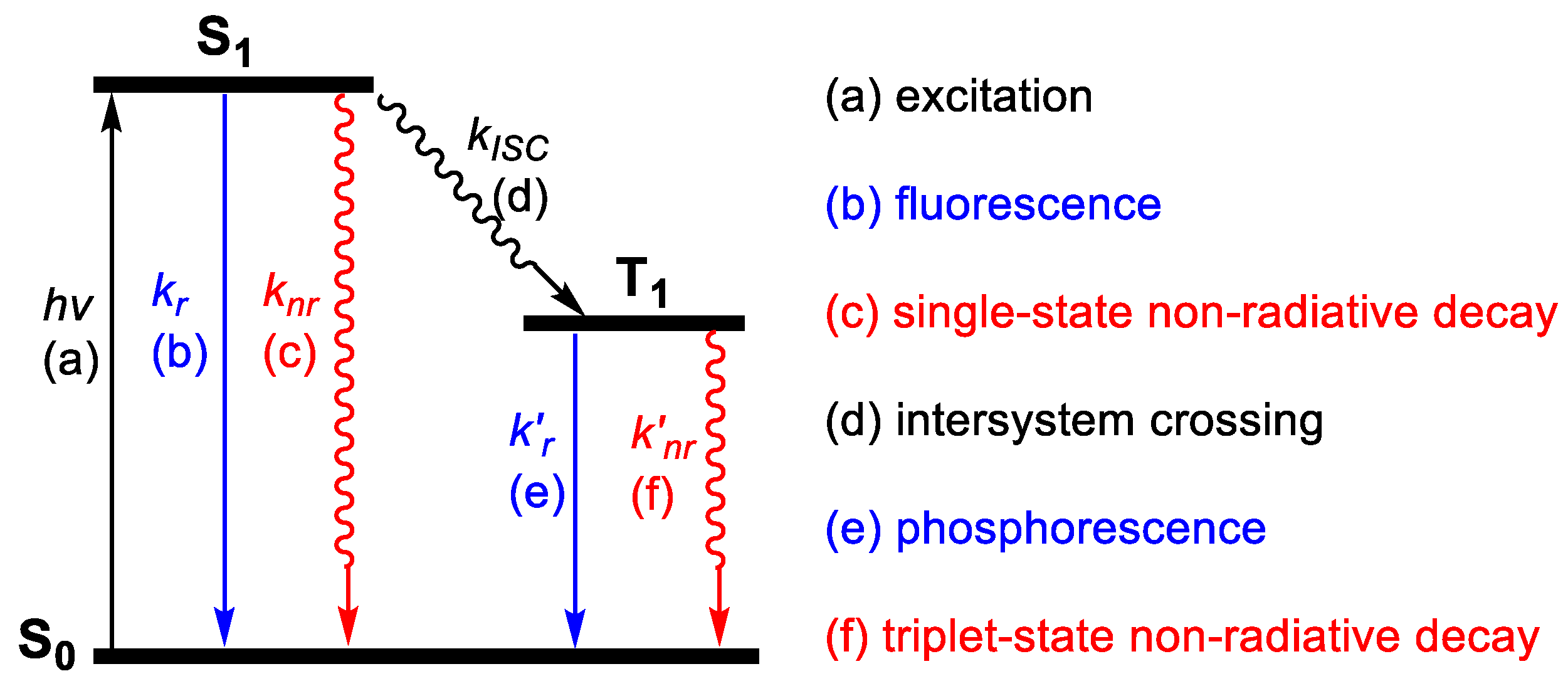
2.3. Steady-State Emission
2.4. Excited-State Redox Potential
2.5. Lifetime
2.6. Marcus Electron-Transfer Theory
2.7. Stern-Volmer Quenching
2.8. Turnover Frequency (TOF)
3. Noble-Metal-Based Photosensitizers
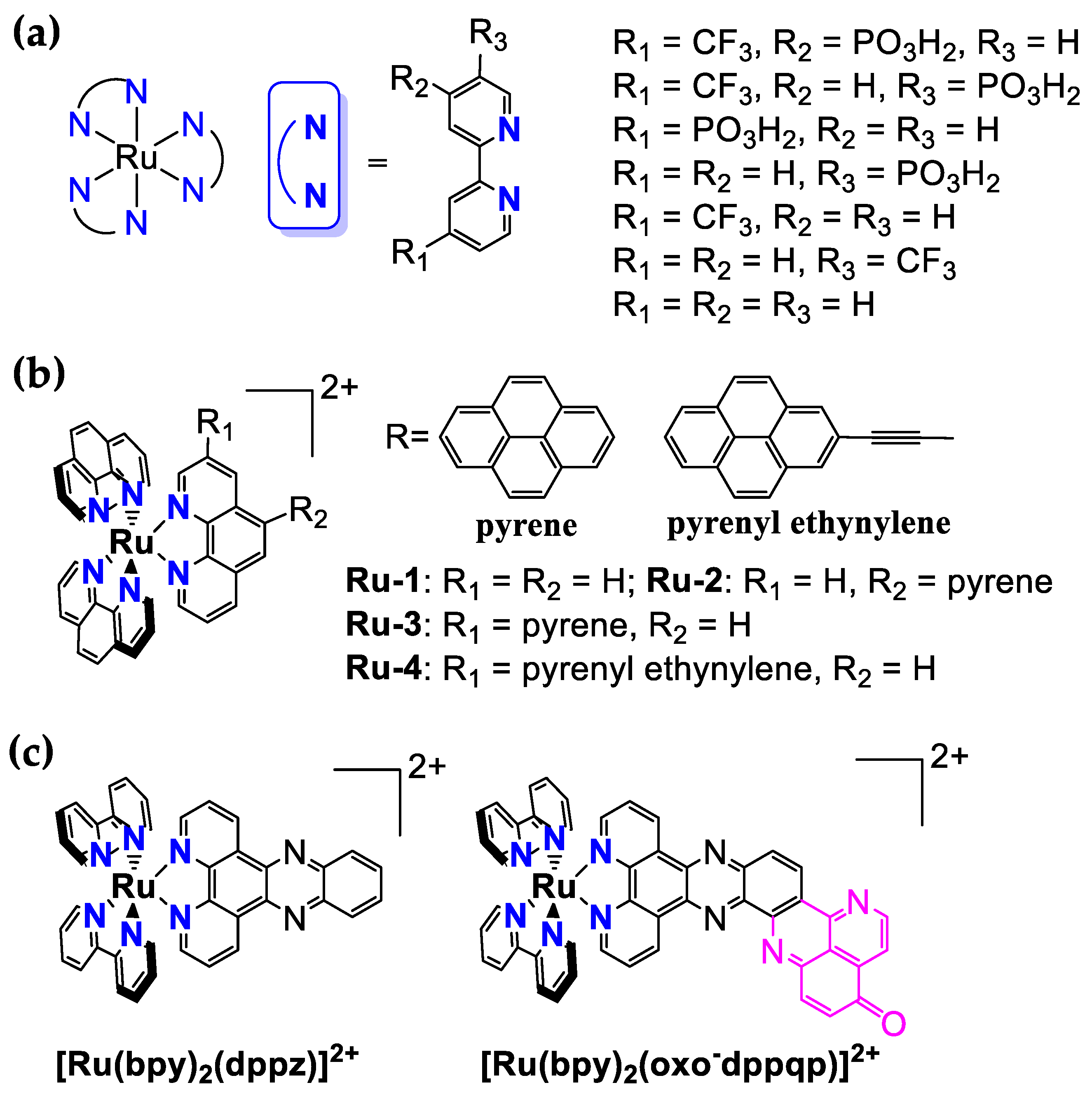
3.1. Ru-Based Photosensitizers
3.2. Ir-Based Photosensitizers
3.3. Re-Based Photosensitizers
4. Noble-Metal-Free Photosensitizers
4.1. Mn-Based Photosensitizers
4.2. Fe-Based Photosensitizers
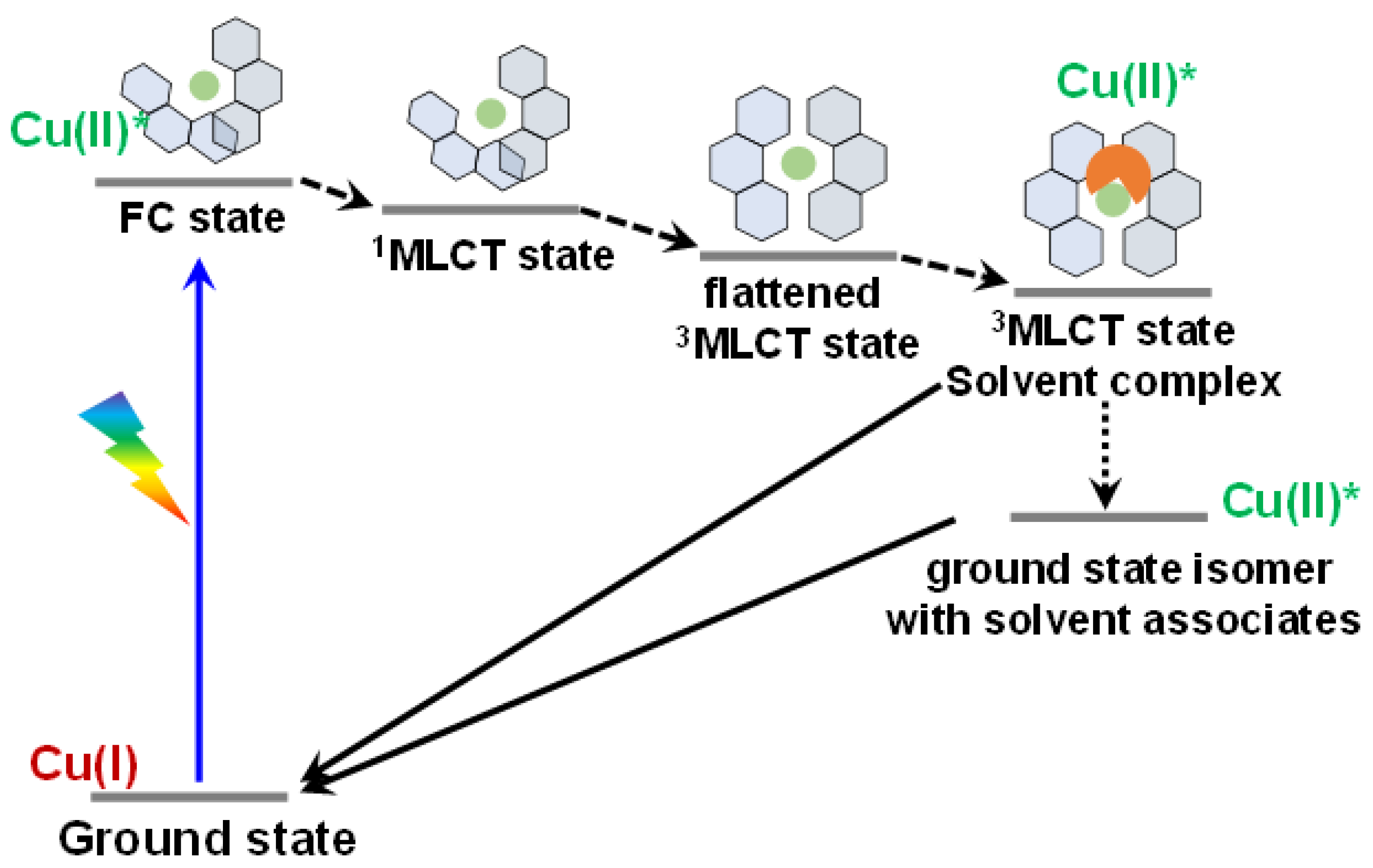
4.3. Cu-Based Photosensitizers
4.4. Other Photosensitizers
5. Conclusions and Outlook
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kharecha, P.A.; Hansen, J.E. Implications of “peak oil” for atmospheric CO2 and climate. Glob. Biogeochem. Cycles 2008, 22, GB3012. [Google Scholar] [CrossRef] [Green Version]
- Stern, D.I.; Kander, A. The role of energy in the industrial revolution and modern economic growth. Energy J. 2012, 33, 3. [Google Scholar] [CrossRef] [Green Version]
- Mayumi, K. Temporary emancipation from land: From the industrial revolution to the present time. Ecol. Econ. 1991, 4, 35–56. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sust. Energ. Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable energy and geopolitics: A review. Renew. Sust. Energ. Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Ren, D.; Loo, N.W.X.; Gong, L.; Yeo, B.S. Continuous production of ethylene from carbon dioxide and water using intermittent sunlight. ACS Sustain. Chem. Eng. 2017, 5, 9191–9199. [Google Scholar] [CrossRef]
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial photosynthesis for sustainable fuel and chemical production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. [Google Scholar] [CrossRef]
- Ji, X.; Su, Z.; Wang, P.; Ma, G.; Zhang, S. Integration of artificial photosynthesis system for enhanced electronic energy-transfer efficacy: A case study for solar-energy driven bioconversion of carbon dioxide to methanol. Small 2016, 12, 4753–4762. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, Y.; Nie, R.; Chen, F.; Zhu, Z.; Wang, J.; Jing, H. Artificial photosynthesis of methanol from carbon dioxide and water via a Nile red-embedded TiO2 photocathode. J. Mater. Chem. A 2017, 5, 5495–5501. [Google Scholar] [CrossRef]
- Stoll, T.; Castillo, C.E.; Kayanuma, M.; Sandroni, M.; Daniel, C.; Odobel, F.; Fortage, J.; Collomb, M.N. Photo-induced redox catalysis for proton reduction to hydrogen with homogeneous molecular systems using rhodium-based catalysts. Coord. Chem. Rev. 2015, 304, 20–37. [Google Scholar] [CrossRef]
- Puntoriero, F.; La Ganga, G.; Cancelliere, A.M.; Campagna, S. Recent progresses in molecular-based artificial photosynthesis. Curr. Opin. Green Sustain. Chem. 2022, 36, 100636. [Google Scholar] [CrossRef]
- Berardi, S.; Drouet, S.; Francàs, L.; Gimbert-Suriñach, C.; Guttentag, M.; Richmond, C.; Stoll, T.; Llobet, A. Molecular artificial photosynthesis. Chem. Soc. Rev. 2014, 43, 7501–7519. [Google Scholar] [CrossRef]
- Wang, C.; O’Hagan, M.P.; Willner, B.; Willner, I. Bioinspired artificial photosynthetic systems. Chem. Eur. J. 2022, 28, e202103595. [Google Scholar] [CrossRef]
- Barber, J.; Tran, P.D. From natural to artificial photosynthesis. J. R. Soc. Interface 2013, 10, 20120984. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Gunlazuardi, J.; Ivandini, T.A.; Umar, A. Photocatalytic conversion of CO2 using earth-abundant catalysts: A review on mechanism and catalytic performance. Renew. Sust. Energ. Rev. 2019, 113, 109246. [Google Scholar] [CrossRef]
- Puntoriero, F.; Sartorel, A.; Orlandi, M.; La Ganga, G.; Serroni, S.; Bonchio, M.; Scandola, F.; Campagna, S. Photoinduced water oxidation using dendrimeric Ru (II) complexes as photosensitizers. Coord. Chem. Rev. 2011, 255, 2594–2601. [Google Scholar] [CrossRef]
- Dogutan, D.K.; Nocera, D.G. Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis. Acc. Chem. Res. 2019, 52, 3143–3148. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Yu, Z.T.; Chen, D.Q.; Zou, Z.G. Metal-complex chromophores for solar hydrogen generation. Chem. Soc. Rev. 2017, 46, 603–631. [Google Scholar] [CrossRef]
- Shon, J.H.; Teets, T.S. Molecular photosensitizers in energy research and catalysis: Design principles and recent developments. ACS Energy Lett. 2019, 4, 558–566. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2013. [Google Scholar]
- Islam, A.; Sugihara, H.; Arakawa, H. Molecular design of ruthenium (II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J. Photochem. Photobiol. A 2003, 158, 131–138. [Google Scholar] [CrossRef]
- Wang, L.; Shaffer, D.W.; Manbeck, G.F.; Polyansky, D.E.; Concepcion, J.J. High-redox-potential chromophores for visible-light-driven water oxidation at low pH. ACS Catal. 2019, 10, 580–585. [Google Scholar] [CrossRef]
- Vlcek, A.; Dodsworth, E.S.; Pietro, W.J.; Lever, A. Excited state redox potentials of ruthenium diimine complexes; correlations with ground state redox potentials and ligand parameters. Inorg. Chem. 1995, 34, 1906–1913. [Google Scholar] [CrossRef]
- Eberhart, M.S.; Phelan, B.T.; Niklas, J.; Sprague-Klein, E.A.; Kaphan, D.M.; Gosztola, D.J.; Chen, L.X.; Tiede, D.M.; Poluektov, O.G.; Mulfort, K.L. Surface immobilized copper (i) diimine photosensitizers as molecular probes for elucidating the effects of confinement at interfaces for solar energy conversion. ChemComm 2020, 56, 12130–12133. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Wu, X.; Sun, L. Copper-based homogeneous and heterogeneous catalysts for electrochemical water oxidation. Nanoscale 2020, 12, 4187–4218. [Google Scholar] [CrossRef]
- Tran, T.T.; Pino, T.; Ha-Thi, M.H. Watching intermolecular light-induced charge accumulation on naphthalene diimide by tris (bipyridyl) ruthenium (II) photosensitizer. J. Phys. Chem. C 2019, 123, 28651–28658. [Google Scholar] [CrossRef]
- Andreiadis, E.S.; Chavarot-Kerlidou, M.; Fontecave, M.; Artero, V. Artificial photosynthesis: From molecular catalysts for light-driven water splitting to photoelectrochemical cells. Photochem. Photobiol. 2011, 87, 946–964. [Google Scholar] [CrossRef]
- Chen, H.C.; Hetterscheid, D.G.; Williams, R.M.; van der Vlugt, J.I.; Reek, J.N.; Brouwer, A.M. Platinum (ii)–porphyrin as a sensitizer for visible-light driven water oxidation in neutral phosphate buffer. Energy Environ. 2015, 8, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zeng, Y.; Chen, J.; Yu, T.; Zhang, X.; Yang, G.; Li, Y. Intramolecular triplet–triplet energy transfer enhanced triplet–triplet annihilation upconversion with a short-lived triplet state platinum (II) terpyridyl acetylide photosensitizer. RSC Adv. 2015, 5, 70640–70648. [Google Scholar] [CrossRef]
- Liu, Y.T.; Li, Y.R.; Wang, X.; Bai, F.Q. Theoretical investigation of NˆCˆN-coordinated Pt (II) and Pd (II) complexes for long-lived two-photon photodynamic therapy. Dyes Pigm. 2017, 142, 55–61. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Li, C.; Xu, S.; Hou, X.; Wu, P. Simultaneously broadened visible light absorption and boosted intersystem crossing in platinum-doped graphite carbon nitride for enhanced photosensitization. ACS Appl. Mater. 2019, 11, 20770–20777. [Google Scholar] [CrossRef]
- Irikura, M.; Tamaki, Y.; Ishitani, O. Development of a panchromatic photosensitizer and its application to photocatalytic CO2 reduction. Chem. Sci. 2021, 12, 13888–13896. [Google Scholar] [CrossRef]
- Lainé, P.P.; Campagna, S.; Loiseau, F. Conformationally gated photoinduced processes within photosensitizer–acceptor dyads based on ruthenium (II) and osmium (II) polypyridyl complexes with an appended pyridinium group. Coord. Chem. Rev. 2008, 252, 2552–2571. [Google Scholar] [CrossRef]
- Summers, P.A.; Calladine, J.A.; Ghiotto, F.; Dawson, J.; Sun, X.Z.; Hamilton, M.L.; Towrie, M.; Davies, E.S.; McMaster, J.; George, M.W. Synthesis and photophysical study of a [NiFe] hydrogenase biomimetic compound covalently linked to a Re-diimine photosensitizer. Inorg. Chem. 2016, 55, 527–536. [Google Scholar] [CrossRef]
- Juris, A.; Barigelletti, F.; Balzani, V.; Belser, P.; Von Zelewsky, A. New photosensitizers of the ruthenium-polypyridine family for the water splitting reaction. Isr. J. Chem. 1982, 22, 87–90. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Zhang, B.; Zhou, X.; Yu, F.; Sun, L. Visible light-driven water oxidation promoted by host–guest interaction between photosensitizer and catalyst with a high quantum efficiency. J. Am. Chem. Soc. 2015, 137, 4332–4335. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Wang, Y.; Bai, L.; Yu, F.; Sun, L. Visible-light-driven water oxidation on a photoanode by supramolecular assembly of photosensitizer and catalyst. ChemPlusChem 2016, 81, 1056–1059. [Google Scholar] [CrossRef]
- Limburg, B.; Bouwman, E.; Bonnet, S. Rate and stability of photocatalytic water oxidation using [Ru(bpy)3]2+ as photosensitizer. ACS Catal. 2016, 6, 5273–5284. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, M.V.; Sherman, B.D.; Coppo, R.L.; Wang, D.; Marquard, S.L.; Wee, K.R.; Murakami Iha, N.Y.; Meyer, T.J. Evaluation of chromophore and assembly design in light-driven water splitting with a molecular water oxidation catalyst. ACS Energy Lett. 2016, 1, 231–236. [Google Scholar] [CrossRef]
- Pickens, R.N.; Neyhouse, B.J.; Reed, D.T.; Ashton, S.T.; White, J.K. Visible light-activated co release and 1O2 photosensitizer formation with Ru (II), Mn (I) complexes. Inorg. Chem. 2018, 57, 11616–11625. [Google Scholar] [CrossRef]
- Ashford, D.L.; Glasson, C.R.; Norris, M.R.; Concepcion, J.J.; Keinan, S.; Brennaman, M.K.; Templeton, J.L.; Meyer, T.J. Controlling ground and excited state properties through ligand changes in ruthenium polypyridyl complexes. Inorg. Chem. 2014, 53, 5637–5646. [Google Scholar] [CrossRef]
- Wang, P.; Dong, R.; Guo, S.; Zhao, J.; Zhang, Z.M.; Lu, T.B. Improving photosensitization for photochemical CO2-to-CO conversion. Natl. Sci. Rev. 2020, 7, 1459–1467. [Google Scholar] [CrossRef]
- Skaisgirski, M.; Guo, X.; Wenger, O.S. Electron accumulation on naphthalene diimide photosensitized by [Ru (2,2′-Bipyridine)3]2+. Inorg. Chem. 2017, 56, 2432–2439. [Google Scholar] [CrossRef]
- Kuss-Petermann, M.; Orazietti, M.; Neuburger, M.; Hamm, P.; Wenger, O.S. Intramolecular light-driven accumulation of reduction equivalents by proton-coupled electron transfer. J. Am. Chem. Soc. 2017, 139, 5225–5232. [Google Scholar] [CrossRef]
- Aslan, J.M.; Boston, D.J.; MacDonnell, F.M. Photodriven multi-electron storage in disubstituted RuII dppz analogues. Chem.–Eur. J. 2015, 21, 17314–17323. [Google Scholar] [CrossRef]
- Lefebvre, J.F.; Schindler, J.; Traber, P.; Zhang, Y.; Kupfer, S.; Gräfe, S.; Baussanne, I.; Demeunynck, M.; Mouesca, J.M.; Gambarelli, S. An artificial photosynthetic system for photoaccumulation of two electrons on a fused dipyridophenazine (dppz)–pyridoquinolinone ligand. Chem. Sci. 2018, 9, 4152–4159. [Google Scholar] [CrossRef] [Green Version]
- Mills, I.N.; Porras, J.A.; Bernhard, S. Judicious design of cationic, cyclometalated Ir (III) complexes for photochemical energy conversion and optoelectronics. Acc. Chem. Res. 2018, 51, 352–364. [Google Scholar] [CrossRef]
- Luo, Y.; Maloul, S.; Schönweiz, S.; Wächtler, M.; Streb, C.; Dietzek, B. Yield—not only lifetime—of the photoinduced charge-separated state in iridium complex–polyoxometalate dyads impact their hydrogen evolution reactivity. Chem. Eur. J. 2020, 26, 8045–8052. [Google Scholar] [CrossRef]
- Luo, Y.; Maloul, S.; Endres, P.; Schönweiz, S.; Ritchie, C.; Wächtler, M.; Winter, A.; Schubert, U.S.; Streb, C.; Dietzek, B. Organic linkage controls the photophysical properties of covalent photosensitizer–polyoxometalate hydrogen evolution dyads. Sustain. Energy Fuels 2020, 4, 4688–4693. [Google Scholar] [CrossRef]
- Shon, J.H.; Teets, T.S. Potent bis-cyclometalated iridium photoreductants with β-diketiminate ancillary ligands. Inorg. Chem. 2017, 56, 15295–15303. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.H.; Kim, D.; Rathnayake, M.D.; Sittel, S.; Weaver, J.; Teets, T.S. Photoredox catalysis on unactivated substrates with strongly reducing iridium photosensitizers. Chem. Sci. 2021, 12, 4069–4078. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.H.; Kim, D.; Gray, T.G.; Teets, T.S. β-Diketiminate-supported iridium photosensitizers with increased excited-state reducing power. Inorg. Chem. Front. 2021, 8, 3253–3265. [Google Scholar] [CrossRef]
- Rohacova, J.; Ishitani, O. Rhenium (I) trinuclear rings as highly efficient redox photosensitizers for photocatalytic CO2 reduction. Chem. Sci. 2016, 7, 6728–6739. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, T.; Ishitani, O. Modulation of the photophysical, photochemical, and electrochemical properties of Re (I) diimine complexes by interligand interactions. Acc. Chem. Res. 2017, 50, 2673–2683. [Google Scholar] [CrossRef] [Green Version]
- Oppelt, K.; Mosberger, M.; Ruf, J.; Fernández-Terán, R.; Probst, B.; Alberto, R.; Hamm, P. Shedding light on the molecular surface assembly at the nanoscale level: Dynamics of a Re (I) carbonyl photosensitizer with a co-adsorbed cobalt tetrapyridyl water reduction catalyst on ZrO2. J. Phys. Chem. C 2020, 124, 12502–12511. [Google Scholar] [CrossRef]
- Fernández-Terán, R.; Sévery, L. Living long and prosperous: Productive intraligand charge-transfer states from a rhenium (I) terpyridine photosensitizer with enhanced light absorption. Inorg. Chem. 2020, 60, 1334–1343. [Google Scholar] [CrossRef]
- Wenger, O.S. A bright future for photosensitizers. Nat. Chem. 2020, 12, 323–324. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takizawa, S.Y.; Hirahara, M. Photofunctional molecular assembly for artificial photosynthesis: Beyond a simple dye sensitization strategy. Coord. Chem. Rev. 2022, 467, 214624. [Google Scholar] [CrossRef]
- Wenger, O.S. Photoactive complexes with earth-abundant metals. J. Am. Chem. Soc. 2018, 140, 13522–13533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant. Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.J. Manganese-based materials inspired by photosynthesis for water-splitting. Materials 2011, 4, 1693–1704. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Chen, R.; Fan, F.; Fu, P.; Yin, H.; Wang, X.; Wang, Z.; Du, P.; Li, C. Mimicking the key functions of photosystem II in artificial photosynthesis for photoelectrocatalytic water splitting. J. Am. Chem. Soc. 2018, 140, 3250–3256. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Yao, R.; Li, Y.; Zhang, C. Artificial Mn4Ca clusters with exchangeable solvent molecules mimicking the oxygen-evolving center in photosynthesis. Angew. Chem. Int. Ed. 2019, 131, 3979–3982. [Google Scholar] [CrossRef]
- Herr, P.; Kerzig, C.; Larsen, C.B.; Häussinger, D.; Wenger, O.S. Manganese (I) complexes with metal-to-ligand charge transfer luminescence and photoreactivity. Nat. Chem. 2021, 13, 956–962. [Google Scholar] [CrossRef]
- Wegeberg, C.; Wenger, O.S. Luminescent chromium (0) and manganese (i) complexes. Dalton Trans. 2022, 51, 1297–1302. [Google Scholar] [CrossRef]
- Zhang, W.; Alonso-Mori, R.; Bergmann, U.; Bressler, C.; Chollet, M.; Galler, A.; Gawelda, W.; Hadt, R.G.; Hartsock, R.W.; Kroll, T. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 2014, 509, 345–348. [Google Scholar] [CrossRef]
- Auböck, G.; Chergui, M. Sub-50-fs photoinduced spin crossover in [Fe(bpy)3]2+. Nat. Chem. 2015, 7, 629–633. [Google Scholar] [CrossRef]
- Jay, R.M.; Eckert, S.; Vaz da Cruz, V.; Fondell, M.; Mitzner, R.; Föhlisch, A. Covalency-driven preservation of local charge densities in a metal-to-ligand charge-transfer excited iron photosensitizer. Angew. Chem. Int. Ed. 2019, 58, 10742–10746. [Google Scholar] [CrossRef] [Green Version]
- Huber-Gedert, M.; Nowakowski, M.; Kertmen, A.; Burkhardt, L.; Lindner, N.; Schoch, R.; Herbst-Irmer, R.; Neuba, A.; Schmitz, L.; Choi, T.K. Fundamental characterization, photophysics and photocatalysis of a base metal iron (II)-cobalt (III) dyad. Chem. Eur. J. 2021, 27, 9905–9918. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, T.G.U.; Ghobadi, A.; Demirtas, M.; Buyuktemiz, M.; Ozvural, K.N.; Yildiz, E.A.; Erdem, E.; Yaglioglu, H.G.; Durgun, E.; Dede, Y. Building an iron chromophore incorporating prussian blue analogue for photoelectrochemical water oxidation. Chem. Eur. J. 2021, 27, 8966–8976. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Bai, Y.; Zhang, P.; Han, Q.; Mitzi, D.B.; Therien, M. Electronic structure and photophysics of a supermolecular iron complex having a long MLCT-state lifetime and panchromatic absorption. Proc. Natl. Acad. Sci. USA 2020, 117, 20430–20437. [Google Scholar] [CrossRef] [PubMed]
- Zobel, J.P.; Bokareva, O.S.; Zimmer, P.; Wölper, C.; Bauer, M.; González, L. Intersystem crossing and triplet dynamics in an iron(II) N-heterocyclic carbene photosensitizer. Inorg. Chem. 2020, 59, 14666–14678. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Müller, P.; Burkhardt, L.; Schepper, R.; Neuba, A.; Steube, J.; Dietrich, F.; Flörke, U.; Mangold, S.; Gerhards, M. N-heterocyclic carbene complexes of Iron as photosensitizers for light-Induced water reduction. Eur. J. Inorg. Chem. 2017, 2017, 1504–1509. [Google Scholar] [CrossRef]
- Duchanois, T.; Etienne, T.; Cebrián, C.; Liu, L.; Monari, A.; Beley, M.; Assfeld, X.; Haacke, S.; Gros, P.C. An iron-based photosensitizer with extended excited-state lifetime: Photophysical and photovoltaic properties. Eur. J. Inorg. Chem. 2015, 2015, 2469–2477. [Google Scholar] [CrossRef]
- Zimmer, P.; Burkhardt, L.; Friedrich, A.; Steube, J.; Neuba, A.; Schepper, R.; Müller, P.; Flörke, U.; Huber, M.; Lochbrunner, S. The connection between NHC ligand count and photophysical properties in Fe(II) photosensitizers: An experimental study. Inorg. Chem. 2018, 57, 360–373. [Google Scholar] [CrossRef]
- Kunnus, K.; Vacher, M.; Harlang, T.C.; Kjær, K.S.; Haldrup, K.; Biasin, E.; van Driel, T.B.; Pápai, M.; Chabera, P.; Liu, Y. Vibrational wavepacket dynamics in Fe carbene photosensitizer determined with femtosecond X-ray emission and scattering. Nat. Commun. 2020, 11, 634. [Google Scholar] [CrossRef] [Green Version]
- Wehry, E.; Sundararajan, S. Intersystem crossing and internal conversion from the lowest charge-transfer singlet excited state of the (2, 9-dimethyl-1, 10-phenanthroline) copper (I) cation. ChemComm 1972, 20, 1135–1136. [Google Scholar]
- Ahn, B.T.; McMillin, D.R. Studies of photoinduced electron transfer from bis (2,9-dimethyl-1, 10-phenanthroline) copper (I). Inorg. Chem. 1978, 17, 2253–2258. [Google Scholar] [CrossRef]
- Phifer, C.C.; McMillin, D.R. The basis of aryl substituent effects on charge-transfer absorption intensities. Inorg. Chem. 1986, 25, 1329–1333. [Google Scholar] [CrossRef]
- Eggleston, M.K.; Fanwick, P.E.; Pallenberg, A.J.; McMillin, D.R. A twist on the copper center in the crystal structure of [Cu(dnpp)2] PF6 and the charge-transfer excited state?(dnpp= 2,9-Dineopentyl-1,10-phenanthroline). Inorg. Chem. 1997, 36, 4007–4010. [Google Scholar] [CrossRef]
- Gandhi, B.A.; Green, O.; Burstyn, J.N. Facile oxidation-based synthesis of sterically encumbered four-coordinate bis (2, 9-di-tert-butyl-1,10-phenanthroline) copper (I) and related three-coordinate copper (I) complexes. Inorg. Chem. 2007, 46, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, M.; Kayanuma, M.; Planchat, A.; Szuwarski, N.; Blart, E.; Pellegrin, Y.; Daniel, C.; Boujtita, M.; Odobel, F. First application of the HETPHEN concept to new heteroleptic bis (diimine) copper (I) complexes as sensitizers in dye sensitized solar cells. Dalton Trans. 2013, 42, 10818–10827. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The emergence of copper (I)-based dye sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef] [Green Version]
- Kohler, L.; Hayes, D.; Hong, J.; Carter, T.J.; Shelby, M.L.; Fransted, K.A.; Chen, L.X.; Mulfort, K.L. Synthesis, structure, ultrafast kinetics, and light-induced dynamics of CuHETPHEN chromophores. Dalton Trans. 2016, 45, 9871–9883. [Google Scholar] [CrossRef]
- Kohler, L.; Hadt, R.G.; Hayes, D.; Chen, L.X.; Mulfort, K.L. Synthesis, structure, and excited state kinetics of heteroleptic Cu (I) complexes with a new sterically demanding phenanthroline ligand. Dalton Trans. 2017, 46, 13088–13100. [Google Scholar] [CrossRef] [Green Version]
- Zerk, T.J.; Bernhardt, P.V. Redox-coupled structural changes in copper chemistry: Implications for atom transfer catalysis. Coord. Chem. Rev. 2018, 375, 173–190. [Google Scholar] [CrossRef]
- Levi, G.; Biasin, E.; Dohn, A.O.; Jónsson, H. On the interplay of solvent and conformational effects in simulated excited-state dynamics of a copper phenanthroline photosensitizer. Phys. Chem. Chem. Phys. 2020, 22, 748–757. [Google Scholar] [CrossRef]
- Zhang, Y.; Zedler, L.; Karnahl, M.; Dietzek, B. Excited-state dynamics of heteroleptic copper (I) photosensitizers and their electrochemically reduced forms containing a dipyridophenazine moiety–a spectroelectrochemical transient absorption study. Phys. Chem. Chem. Phys. 2019, 21, 10716–10725. [Google Scholar] [CrossRef]
- Velasco, L.; Llanos, L.; Levín, P.; Vega, A.; Yu, J.; Zhang, X.; Lemus, L.; Aravena, D.; Moonshiram, D. Structure and excited-state dynamics of dimeric copper (I) photosensitizers investigated by time-resolved X-ray and optical transient absorption spectroscopy. Phys. Chem. Chem. Phys. 2021, 23, 3656–3667. [Google Scholar] [CrossRef] [PubMed]
- Mara, M.W.; Fransted, K.A.; Chen, L.X. Interplays of excited state structures and dynamics in copper (I) diimine complexes: Implications and perspectives. Coord. Chem. Rev. 2015, 282, 2–18. [Google Scholar] [CrossRef]
- Stroscio, G.D.; Ribson, R.D.; Hadt, R.G. Quantifying entatic states in photophysical processes: Applications to copper photosensitizers. Inorg. Chem. 2019, 58, 16800–16817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosko, M.C.; Wells, K.A.; Hauke, C.E.; Castellano, F.N. Next generation cuprous phenanthroline MLCT photosensitizer featuring cyclohexyl substituents. Inorg. Chem. 2021, 60, 8394–8403. [Google Scholar] [CrossRef]
- Garakyaraghi, S.; McCusker, C.E.; Khan, S.; Koutnik, P.; Bui, A.T.; Castellano, F.N. Enhancing the visible-light absorption and excited-state properties of Cu(I) MLCT excited states. Inorg. Chem. 2018, 57, 2296–2307. [Google Scholar] [CrossRef]
- Takeda, H.; Kamiyama, H.; Okamoto, K.; Irimajiri, M.; Mizutani, T.; Koike, K.; Sekine, A.; Ishitani, O. Highly efficient and robust photocatalytic systems for CO2 reduction consisting of a Cu (I) photosensitizer and Mn(I) catalysts. J. Am. Chem. Soc. 2018, 140, 17241–17254. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Whang, D.R.; Park, S.Y. Designing highly efficient CuI photosensitizers for photocatalytic H2 evolution from water. ChemSusChem 2017, 10, 1883–1886. [Google Scholar] [CrossRef]
- Heberle, M.; Tschierlei, S.; Rockstroh, N.; Ringenberg, M.; Frey, W.; Junge, H.; Beller, M.; Lochbrunner, S.; Karnahl, M. Heteroleptic Copper Photosensitizers: Why an extended π-system does not automatically lead to enhanced hydrogen production. Chem. Eur. J. 2017, 23, 312–319. [Google Scholar] [CrossRef]
- Giereth, R.; Frey, W.; Junge, H.; Tschierlei, S.; Karnahl, M. Copper photosensitizers containing P^ N ligands and their influence on photoactivity and stability. Chem. Eur. J. 2017, 23, 17432–17437. [Google Scholar] [CrossRef]
- Takeda, H.; Ohashi, K.; Sekine, A.; Ishitani, O. Photocatalytic CO2 reduction using Cu(I) photosensitizers with a Fe(II) catalyst. J. Am. Chem. Soc. 2016, 138, 4354–4357. [Google Scholar] [CrossRef]
- Zhang, Y.; Heberle, M.; Wächtler, M.; Karnahl, M.; Dietzek, B. Determination of side products in the photocatalytic generation of hydrogen with copper photosensitizers by resonance Raman spectroelectrochemistry. RSC Adv. 2016, 6, 105801–105805. [Google Scholar] [CrossRef] [Green Version]
- Takeda, H.; Monma, Y.; Sugiyama, H.; Uekusa, H.; Ishitani, O. Development of visible-light driven Cu (I) complex photosensitizers for photocatalytic CO2 reduction. Front. Chem. 2019, 7, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giereth, R.; Reim, I.; Frey, W.; Junge, H.; Tschierlei, S.; Karnahl, M. Remarkably long-lived excited states of copper photosensitizers containing an extended π-system based on an anthracene moiety. Sustain. Energy Fuels 2019, 3, 692–700. [Google Scholar] [CrossRef]
- Müller, C.; Schulz, M.; Obst, M.; Zedler, L.; Gräfe, S.; Kupfer, S.; Dietzek, B. Role of MLCT States in the Franck–Condon Region of Neutral, Heteroleptic Cu (I)–4 H-imidazolate Complexes: A Spectroscopic and Theoretical Study. J. Phys. Chem. A 2020, 124, 6607–6616. [Google Scholar] [CrossRef]
- Pann, J.; Roithmeyer, H.; Viertl, W.; Pehn, R.; Bendig, M.; Dutzler, J.; Kriesche, B.; Brüggeller, P. Phosphines in artificial photosynthesis: Considering different aspects such as chromophores, water reduction catalysts (WRCs), water oxidation catalysts (WOCs), and dyads. Sustain. Energy Fuels 2019, 3, 2926–2953. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, K.; Wang, L.; Wang, L.; Fan, Z. Porphyrin-based heterogeneous photocatalysts for solar energy conversion. Chin. Chem. Lett. 2021, 33, 33–60. [Google Scholar] [CrossRef]
- Al Mogren, M.M.; Ahmed, N.M.; Hasanein, A.A. Molecular modeling and photovoltaic applications of porphyrin-based dyes: A review. J. Saudi Chem. Soc. 2020, 24, 303–320. [Google Scholar] [CrossRef]
- Ramasamy, S.; Bhagavathiachari, M.; Suthanthiraraj, S.A.; Pichai, M. Mini review on the molecular engineering of photosensitizer: Current status and prospects of metal-free/porphyrin frameworks at the interface of dye-sensitized solar cells. Dyes Pigm. 2022, 203, 110380. [Google Scholar] [CrossRef]
- Zarrabi, N.; Poddutoori, P.K. Aluminum (III) porphyrin: A unique building block for artificial photosynthetic systems. Coord. Chem. Rev. 2021, 429, 213561. [Google Scholar] [CrossRef]
- Giannoudis, E.; Benazzi, E.; Karlsson, J.; Copley, G.; Panagiotakis, S.; Landrou, G.; Angaridis, P.; Nikolaou, V.; Matthaiaki, C.; Charalambidis, G. Photosensitizers for H2 evolution based on charged or neutral Zn and Sn porphyrins. Inorg. Chem. 2020, 59, 1611–1621. [Google Scholar] [CrossRef]
- Luo, G.F.; Biniuri, Y.; Chen, W.H.; Wang, J.; Neumann, E.; Marjault, H.B.; Nechushtai, R.; Winkler, M.; Happe, T.; Willner, I. Modelling photosynthesis with ZnII-protoporphyrin all-DNA G-quadruplex/aptamer scaffolds. Angew. Chem. Int. Ed. 2020, 132, 9248–9255. [Google Scholar] [CrossRef]
- Kunnus, K.; Li, L.; Titus, C.J.; Lee, S.J.; Reinhard, M.E.; Koroidov, S.; Kjær, K.S.; Hong, K.; Ledbetter, K.; Doriese, W.B. Chemical control of competing electron transfer pathways in iron tetracyano-polypyridyl photosensitizers. Chem. Sci. 2020, 11, 4360–4373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
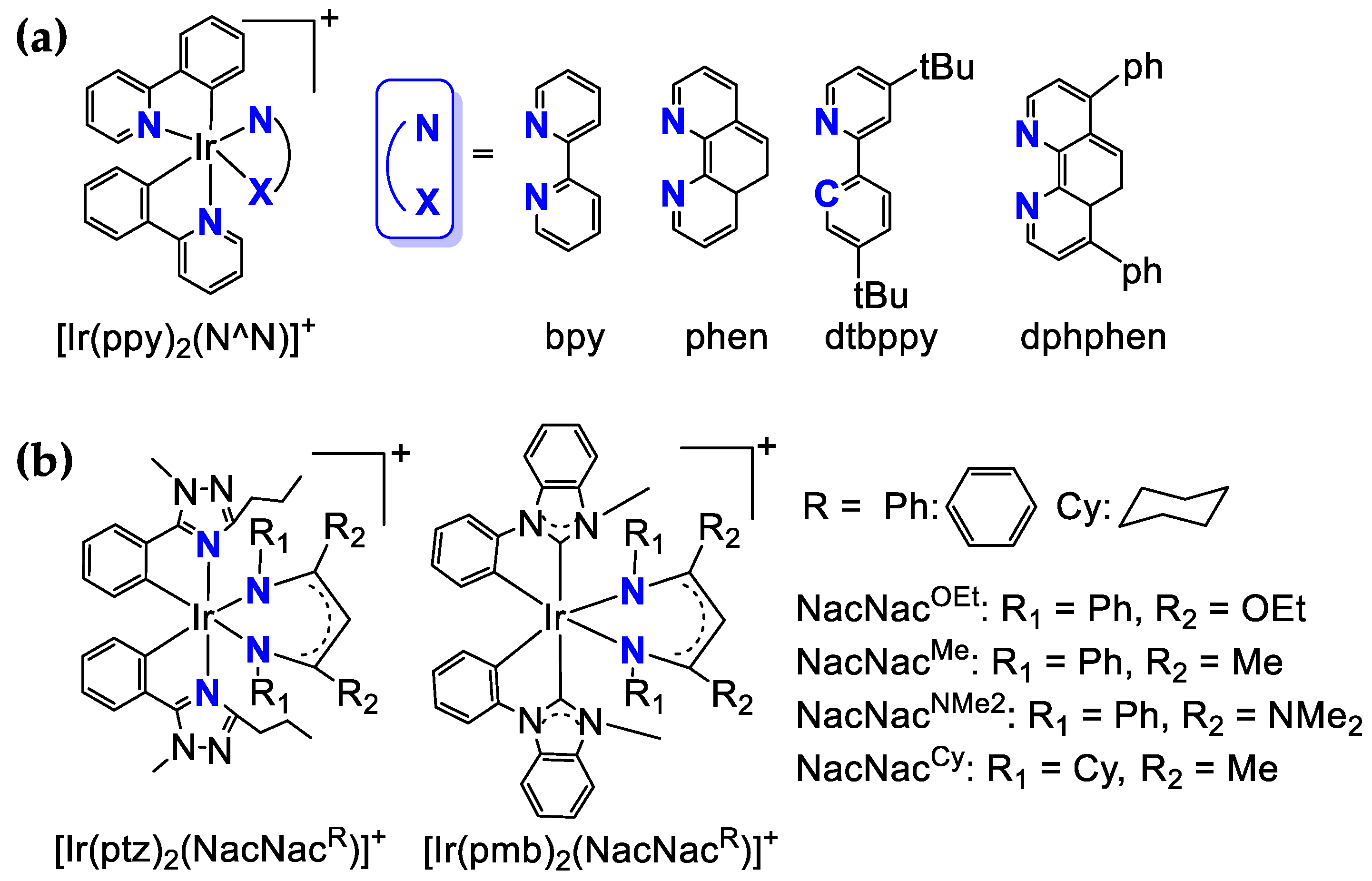
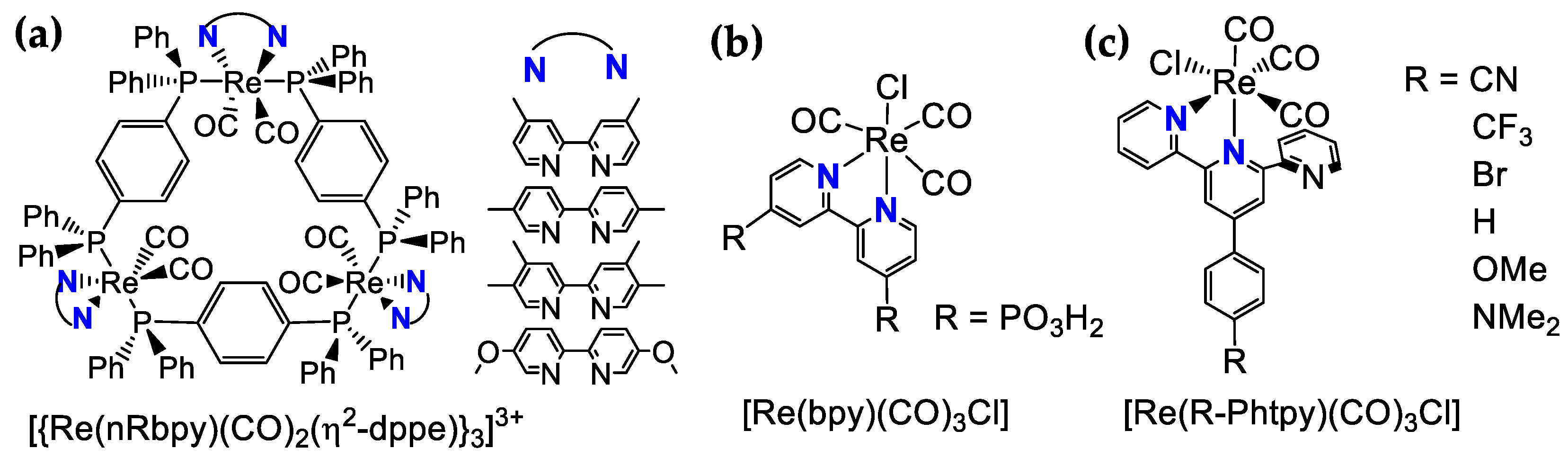

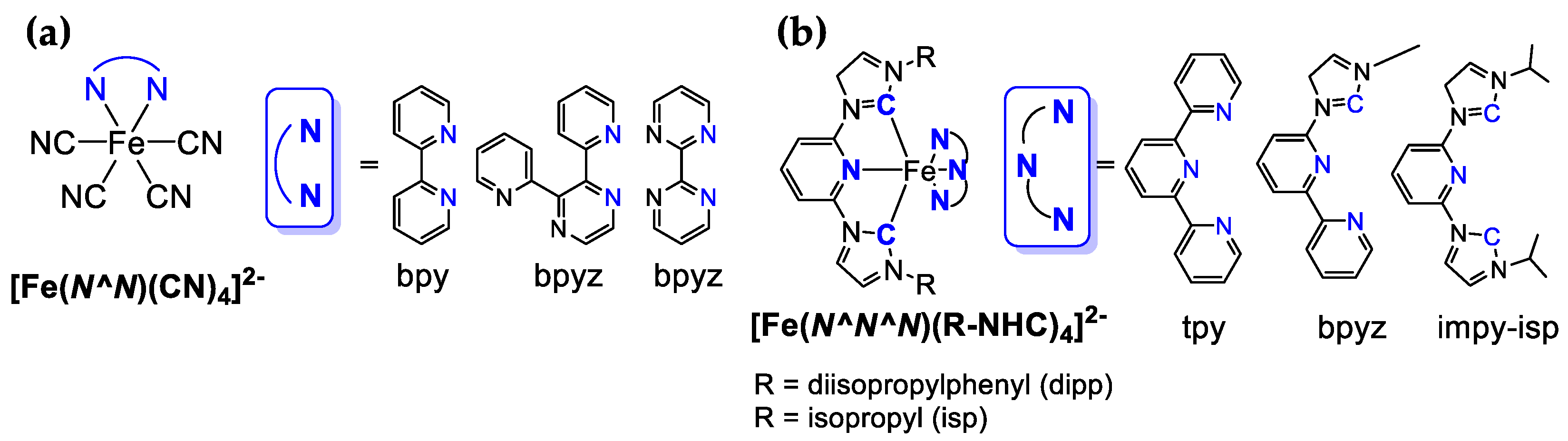
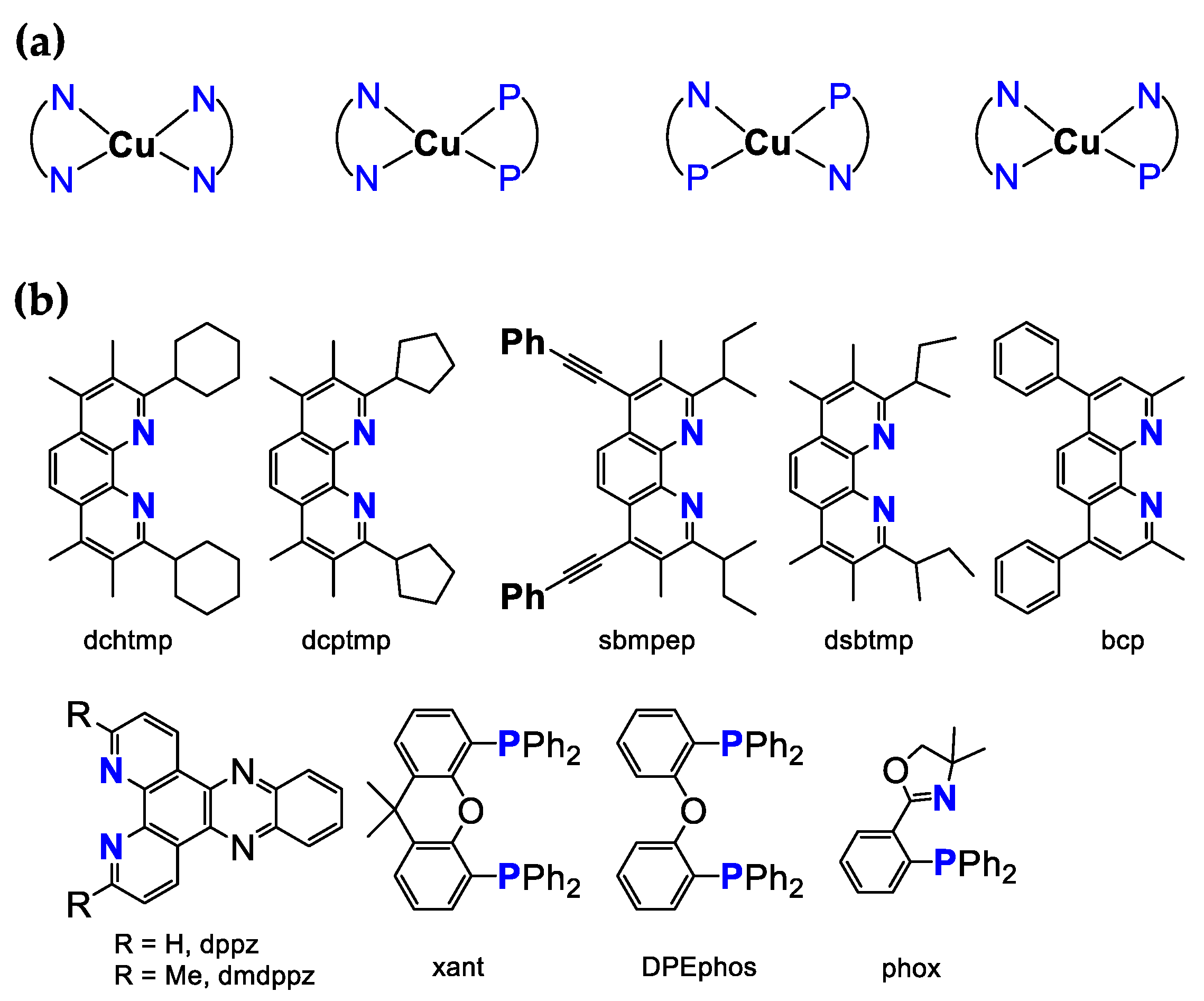
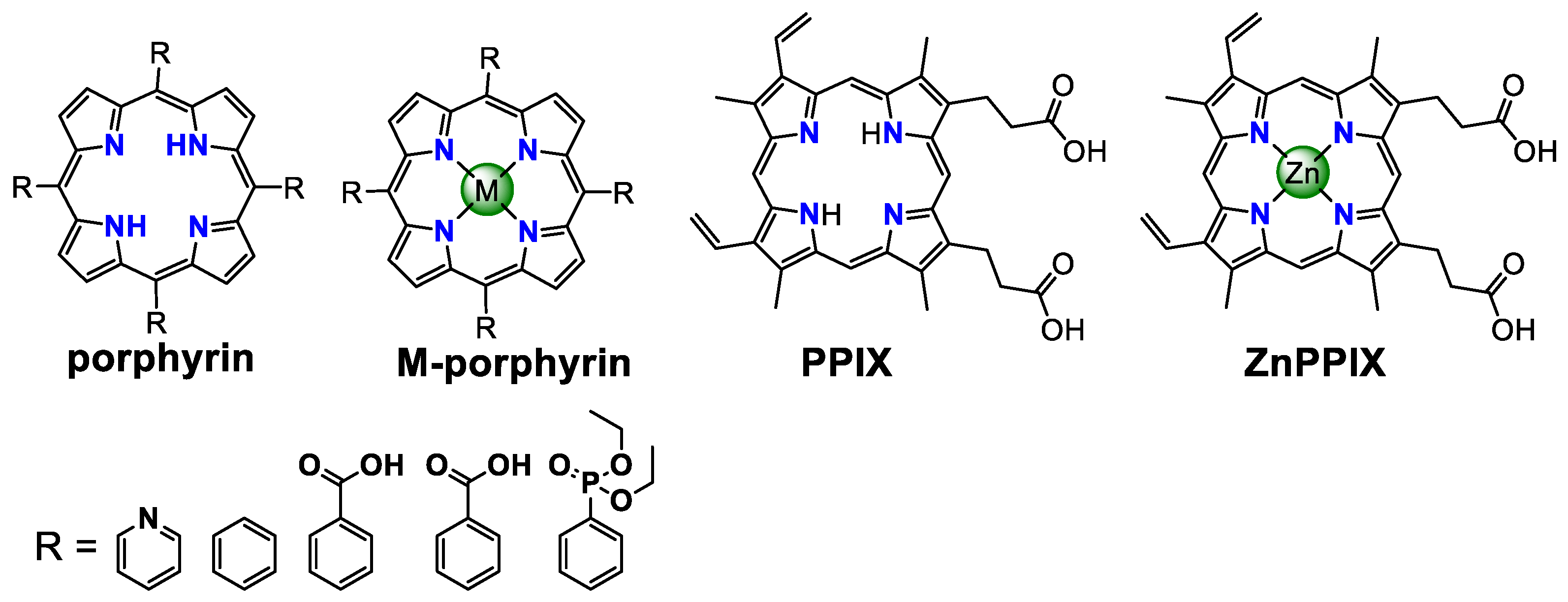
| Photosensitizer | Solvent * | E1/2 (V) | λabs (nm) | λem (nm) | Φ | TON h | TOF h | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| [Ru(bpy)3]2+ | 0.10 M HClO4 | 1.26 a | 454 | 611 | 580 ns | 0.042 | / | / | [24] |
| [Ru(4-CF3-4′-PO3H2-bpy)3]2+ | 0.10 M HClO4 | 1.60 a | 461 | 624 | 667 ns | 0.0334 | 75 | 0.24/s | [24] |
| [Ru(4-CF3-5′-PO3H2-bpy)3]2+ | 0.10 M HClO4 | 1.60 a | 468 | 640 | 222 ns | 0.0123 | 90 | 0.19/s | [24] |
| [Ru(4-PO3H2-bpy)3]2+ | 0.10 M HClO4 | 1.34 a | 458 | 628 | 534 ns | 0.0379 | 70 | 0.21/s | [24] |
| [Ru(5-PO3H2-bpy)3]2+ | 0.10 M HClO4 | 1.34 a | 467 | 640 | 147 ns | 0.0128 | 55 | 0.21/s | [24] |
| [Ru(4-CF3-bpy)3]2+ | 0.10 M HClO4 | 1.51 a | 455 | 617 | 731 ns | 0.0141 | 85 | 0.21/s | [24] |
| [Ru(5-CF3-bpy)3]2+ | 0.10 M HClO4 | 1.53 a | 464 | 630 | 258 ns | 0.0238 | 140 | 0.24/s | [24] |
| [Ru(phen)3]2+ | CH3CN | 1.29 b | 447 | 595 | 0.4 µs | / | 66 | 5.5/10 h | [45] |
| [Ru(phen)2(5-pyrenylphen)3]2+ | CH3CN | 1.36 b | 447 | 595 | 32 µs | / | 452 | 37.6/10 h | [45] |
| [Ru(phen)2(3-pyrenylphen)3]2+ | CH3CN | 1.36 b | 391 | 632 | 68.2 µs | / | 1120 | 93.3/10 h | [45] |
| [Ru(phen)2(3-pyrenyl ethynylenephen)3]2+ | CH3CN | 1.40 b | 415 | 668 | 118.7 µs | / | 120 | 10/10 h | [45] |
| [Ru(bpy)2(dppz)]2+ | CH3CN | 0.84 c,e | 450 | / | / | / | / | / | [49] |
| [Ru(bpy)2(oxo-dppqp)]2+ | CH3CN | 0.86 c,e | 417 | / | / | / | / | / | [49] |
| [Ir(ppy)2(bpy)]+ | CH3CN | 1.25 d | / | 585 | / | / | 275 | / | [50] |
| [Ir(ppy)2(phen)]+ | CH3CN | 1.24 d | / | 579 | / | / | 195 | / | [50] |
| [Ir(ppy)2(dphphen)]+ | CH3CN | 1.23 d | / | 587 | / | / | / | / | [50] |
| [Ir(ppy)2(NacNacCy)]+ | CH3CN | −0.39 c | / | / | / | / | / | / | [54] |
| [Ir(ppy)2(NacNac NMe2)]+ | CH3CN | −0.26 c | 511 f | 634 | 0.75 µs | 0.16 | / | / | [53] |
| [Ir(ppy)2(NacNacMe)]+ | CH3CN | −0.07 c | 460 f | 595 | 0.2 µs | 0.053 | / | / | [53] |
| [Ir(ppy)2(NacNacOEt)]+ | CH3CN | 0.03 c | 456 f | 571 | 2 µs | 0.23 | / | / | [53] |
| [Ir(ptz)2(NacNacNMe2)]+ | CH3CN | −0.22 c | 380 | 576 | 0.13 µs | 0.025 | / | / | [55] |
| [Ir(ptz)2(NacNacCy)]+ | CH3CN | −0.41 c | 375 | 642 | 0.068 µs | 0.0032 | / | / | [55] |
| [Ir(pmb)2(NacNacNMe2)]+ | CH3CN | −0.26 c | 430 | 591 | 0.85 µs | 0.039 | / | / | [55] |
| [Ir(pmb)2(NacNaccy)]+ | CH3CN | −0.43 c | 439 | 675 | 0.12 µs | 0.0027 | / | / | [55] |
| [{Re(4-dmbpy)(CO)2(η2-dppe)}s3]3+ | DMF | −1.73 g | 398 | 598 | 1.57 µs | 0.12 | 19.68 | / | [56] |
| [{Re(5-dmbpy)(CO)2(η2-dppe)}3]3+ | DMF | −1.82 g | 390 | 561 | 2.32 µs | 0.36 | 14.2 | / | [56] |
| [{Re(4,5-dmbpy)(CO)2(η2-dppe)}3]3+ | DMF | −1.91 g | 384 | 545 | 3.58 µs | 0.60 | 6.31 | / | [56] |
| [{Re(4-OMebpy)(CO)2(η2-dppe)}3]3+ | DMF | −1.89 g | 380 | 543 | 7.77 µs | 0.66 | 2.13 | / | [56] |
| Re(CN-Phtpy)(CO)3Cl | DMF | −1.61 c | 389 | 667 | 0.582 ns | / | / | / | [59] |
| Re(CF3-Phtpy)(CO)3Cl | DMF | −1.66 c | 387 | 666 | 0.79 ns | / | / | / | [59] |
| Re(Br-Phtpy)(CO)3Cl | DMF | −1.70 c | 382 | 663 | 1.35 ns | / | / | / | [59] |
| Re(H-Phtpy)(CO)3Cl | DMF | −1.73 c | 380 | 652 | 1.53 ns | / | 580 | ~2.5/s | [59] |
| Re(OMe-Phtpy)(CO)3Cl | DMF | −1.77 c | 378 | 647 | 2.26 ns | / | / | / | [59] |
| Re(NMe2-Phtpy)(CO)3Cl | DMF | −1.81 c | 425 | 532 | 380 ns | / | 2130 | ~150/s | [59] |
| Element | Abundance | Element | Abundance |
|---|---|---|---|
| Ru | Mn | 0.091 | |
| Re | Fe | 4.7 | |
| Os | Ni | ||
| Ir | Cu | 0.005 | |
| Pt | Zn | 0.007 |
| Photosensitizer | Solvent | E1/2 (V) | λabs (nm) | λem (nm) | Φ | Reference | |
|---|---|---|---|---|---|---|---|
| [Mn(Lbi)3]+ | CH3CN | 1.05 a | 385 | 485 | 0.74 ns | 0.05% | [68] |
| [Mn(Ltri)2]+ | CH3CN | 1.00 a | 395 | 525 | 1.73 ns | 0.03% | [68] |
| [Fe(bpy)(CN)4]2− | CH3OH | / | 514 b | / | 0.22 ps | / | [113] |
| [Fe(bpyz)(CN)4]2− | CH3OH | / | 590 b | / | 0.61 ps | / | [113] |
| [Fe(bpym)(CN)4]2− | CH3OH | / | 608 b | / | 16.9 ps | / | [113] |
| [Fe(tpy)(dipp-NHC)]2− | CH3CN | 0.56 c | 379, 503, 538 | / | / | / | [78] |
| [Fe(isp-NHC)2]2− | CH3CN | 0.43 c | 392, 458 | / | / | / | [78] |
| [Fe(bpyz)(dipp-NHC)]2− | CH3CN | 0.46 c | 376, 409, 466, 506, 538 | / | / | / | [78] |
| [Cu(dsbtmp)2]+ | CH3CN | 0.428 c | 445 | 649 | 1.5 µs | 2.9% | [95] |
| [Cu(dchtmp)2]+ | CH3CN | 0.43 c | / | 650 | 1.5 µs | 2.6% | [95] |
| [Cu(sbmpep)2]+ | CH2Cl2 | 0.69 c | 491 | 687 | 1.4 µs | 2.7% | [96] |
| [Cu(xant)(dppz)]+ | CH3CN | 0.815, 1.026 c | 376, 394 | / | 4380 ns | / | [104] |
| [Cu(xant)(dmdppz)]+ | CH3CN | 0.926, 1.228 c | 383, 400 | / | 1250 ns, 3785 ns | / | [104] |
| [Cu(xant)(dmphen)]+ | CH3CN | 0.82 c | 378 | / | 64 ns | / | [104] |
| [Cu(DPEphos)(bcp)]+ | CH3CN | / | 384 | 590 | 0.99 µs | 2.7% | [103] |
| [ZnTMePyP4+]Cl4 | 1:1 CH3CN/H2O | 1.28 d | 438 | 627/667 | 85 µs | / | [111] |
| Sn(OH)2TPyP | 1:1 CH3CN/H2O | 1.38 d | 417 | 597/650 | 78 µs | / | [111] |
| Sn(Cl2)TPP-[COOMe]4 | 1:1 CH3CN/H2O | 1.29 d | 425 | 604/660 | 70 µs | / | [111] |
| Sn(Cl2)TPP-[PO(OEt)2]4 | 1:1 CH3CN/H2O | 1.32 d | 424 | 605/659 | / | / | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L. Recent Advances in Metal-Based Molecular Photosensitizers for Artificial Photosynthesis. Catalysts 2022, 12, 919. https://doi.org/10.3390/catal12080919
Wang L. Recent Advances in Metal-Based Molecular Photosensitizers for Artificial Photosynthesis. Catalysts. 2022; 12(8):919. https://doi.org/10.3390/catal12080919
Chicago/Turabian StyleWang, Lei. 2022. "Recent Advances in Metal-Based Molecular Photosensitizers for Artificial Photosynthesis" Catalysts 12, no. 8: 919. https://doi.org/10.3390/catal12080919
APA StyleWang, L. (2022). Recent Advances in Metal-Based Molecular Photosensitizers for Artificial Photosynthesis. Catalysts, 12(8), 919. https://doi.org/10.3390/catal12080919






