1. Introduction
The increasing need for materials for various important applications, a growing world population, and the fact that our Earth’s resources are limited raise the question of future material supply. Several industry activities, policy initiatives, and research projects have recently been initiated in Europe with the aim to ensure an adequate supply of raw materials [
1]. Copper, the third metal by global production volume after iron and aluminum, is an essential material for emerging technologies such as electric cars, photovoltaic panels, and wind generators, which will increase copper demand [
2,
3]. The depletion of world reserves of high-grade minerals is causing an increase in the price of copper and, consequently, exploitation of lower-grade deposits. Unfortunately, increasing copper extraction and decreasing ore grades intensify energy use and generate a higher environmental impact, leading to the need to develop advanced mining and metallurgical technologies with lower environmental impacts [
4].
Chalcopyrite, the resource with the highest copper content, represents about 70–80% of known world copper reserves [
5,
6,
7]. Similar to other copper sulfides, this mineral is usually exploited in deposits with grades as low as 0.4–0.5% copper. The exploitation of these reserves is within the limits of economic viability using traditional pyrometallurgical treatment of sulfide concentrates. However, chalcopyrite is refractory in acid media, with low dissolution rates that hinder the hydrometallurgical route. This refractoriness is attributed to the formation of passive layers of elemental sulfur [
8,
9,
10,
11], copper-rich polysulfides [
12,
13], or iron salts [
14,
15] on the chalcopyrite surface particles. These coating layers have low electrical conductivity and can prevent the transfer of electrons and ions to the core of chalcopyrite, hindering copper extraction [
16].
The addition of several species to improve the extraction of copper from chalcopyrite in acidic sulfuric media has been investigated. For example, the addition of pyrite [
17,
18] NaCl [
19], manganese dioxide [
20,
21], nano-silica [
22], and carbon materials [
20,
23,
24] have demonstrated their capacity to increase the copper leaching. Nakazawa [
23] concluded that the improved kinetics of chalcopyrite leaching could be due to two factors: (1) decrease in the redox potential and (2) galvanic interaction between carbonaceous structures and chalcopyrite. Alvarez et al. [
25] studied the effects of different carbon materials on the leaching of copper and zinc from a complex sulfide mineral—rich in chalcopyrite, pyrite, and sphalerite. They concluded that the added carbon materials reduced the Eh of the leaching systems, but not all of them improved copper extraction. Despite the advances in recent years, there is a lack of knowledge about the mechanism involved in the leaching of sulfide minerals in the presence of carbon materials, as carbon materials with different properties can act as catalysts [
23,
24,
25]. Thus, the main objective of this research is to advance the knowledge of using carbon materials as potential catalysts in the oxidation of sulfide minerals in sulfuric/ferric solutions. For this research, two commercial carbon materials with different properties (CC and BC) were added to selected chalcopyrite ore (CPY) in three different ratios. An exhaustive characterization of the feedstocks and final solid residues was performed by XRD and SEM-EDS analysis. Additionally, CC and BC were treated with sulfuric/ferric solutions for 48 h at 90 °C in order to assess the interaction between the leaching agent and the carbon materials and to identify possible modifications to carbonaceous structures during the leaching process.
3. Discussion
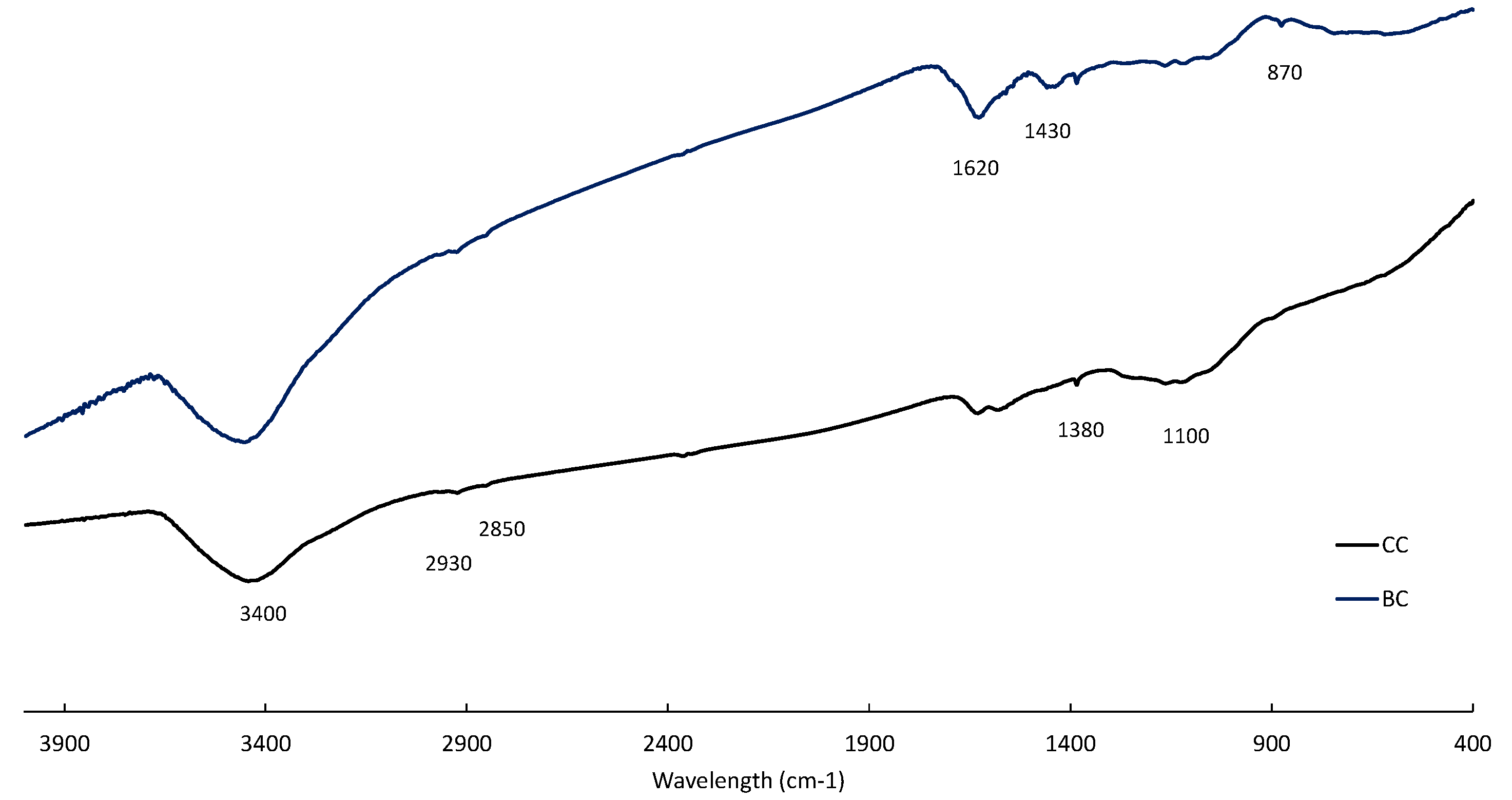
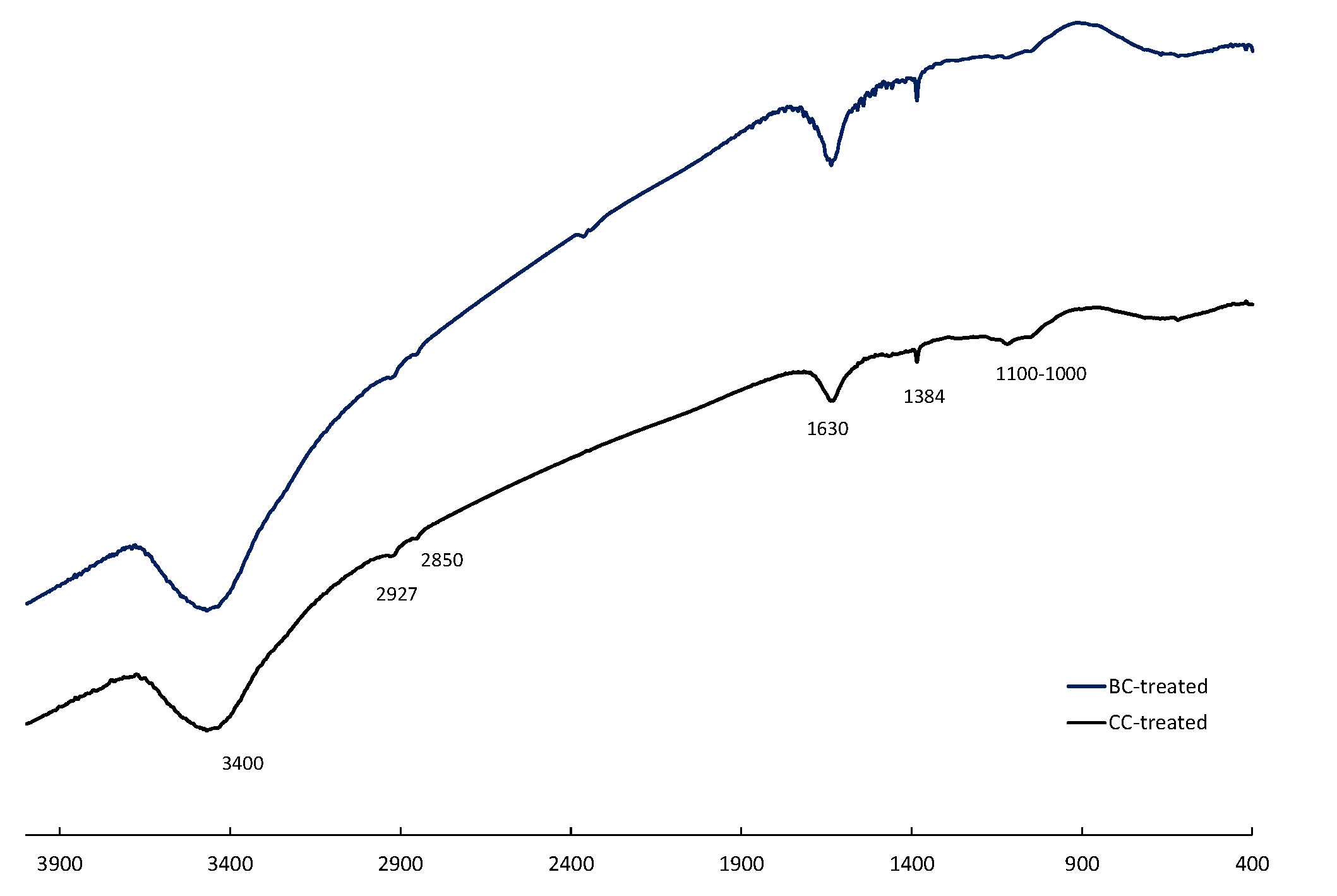
Previous work has shown that the presence of functional groups, especially hydroxyquinone and quinone groups, greatly influences the catalytic properties of carbon materials during oxidative leaching of sulfide minerals, allowing for reversible redox reactions [
27]. Hammes et al. [
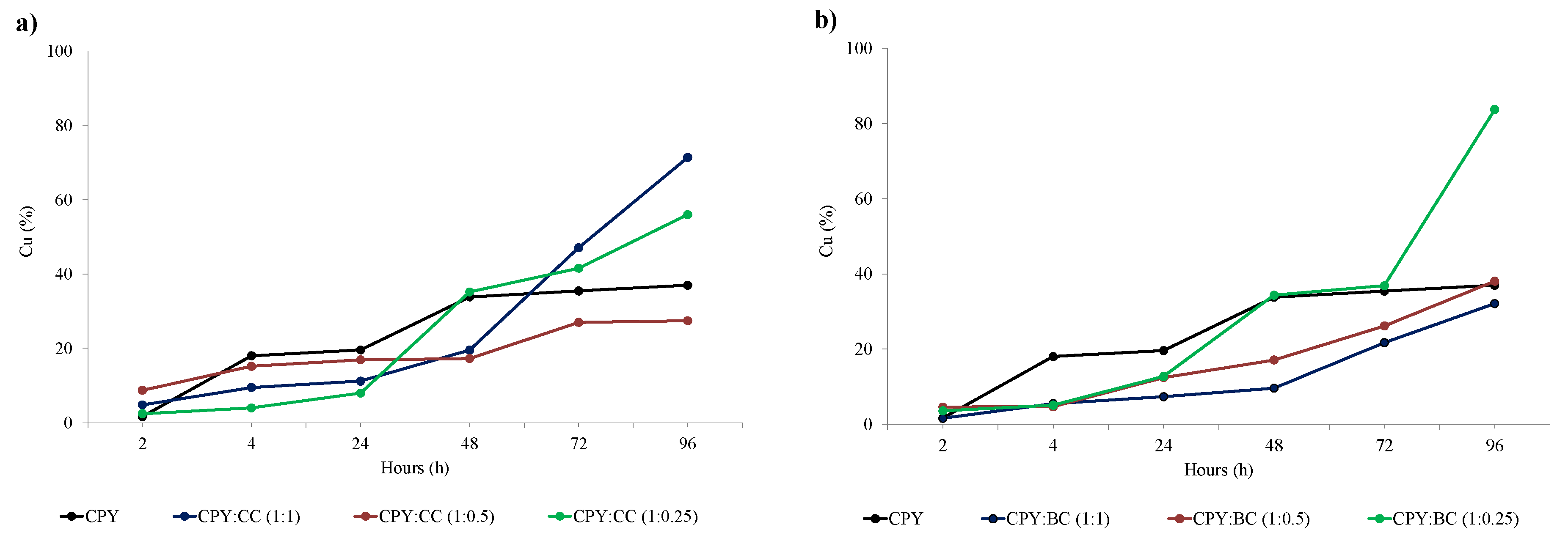
28] concluded that materials with highly condensed aromatic carbon rings have H/C ratios lower than 0.3, as is the case for CC. The O/C ratio of CC (0.11) was higher than that of BC (0.01), showing the presence of more oxygen functionalization in CC, related to such functional groups’ generation during activation processes of commercial activated carbons. Oxygen can appear on carbon surfaces in different functional groups, such as hydroxyl, carbonyl, carboxyl, or ester groups. The concentration of different functional groups and the oxygen availability can influence the catalytic properties of carbon materials. The addition of two carbon materials (CC and BC), despite their different properties, can increase copper extraction from chalcopyrite. The effectiveness of the extraction process depends on the properties of the carbon material and their proportion in the leaching system. In fact, it is possible to reach Cu extraction values of 91.1% (CPY:BC ratio 1:0.25) and of 99.1% (CPY:CC ratio 1:1). The percentages were higher than that obtained previously in the leaching of a complex sulfide mineral (52.6% chalcopyrite, 32.2% sphalerite, and 8.4% pyrite) [
25], indicating the influence of mineral composition on the addition of carbon materials and their proportion in the leaching system.
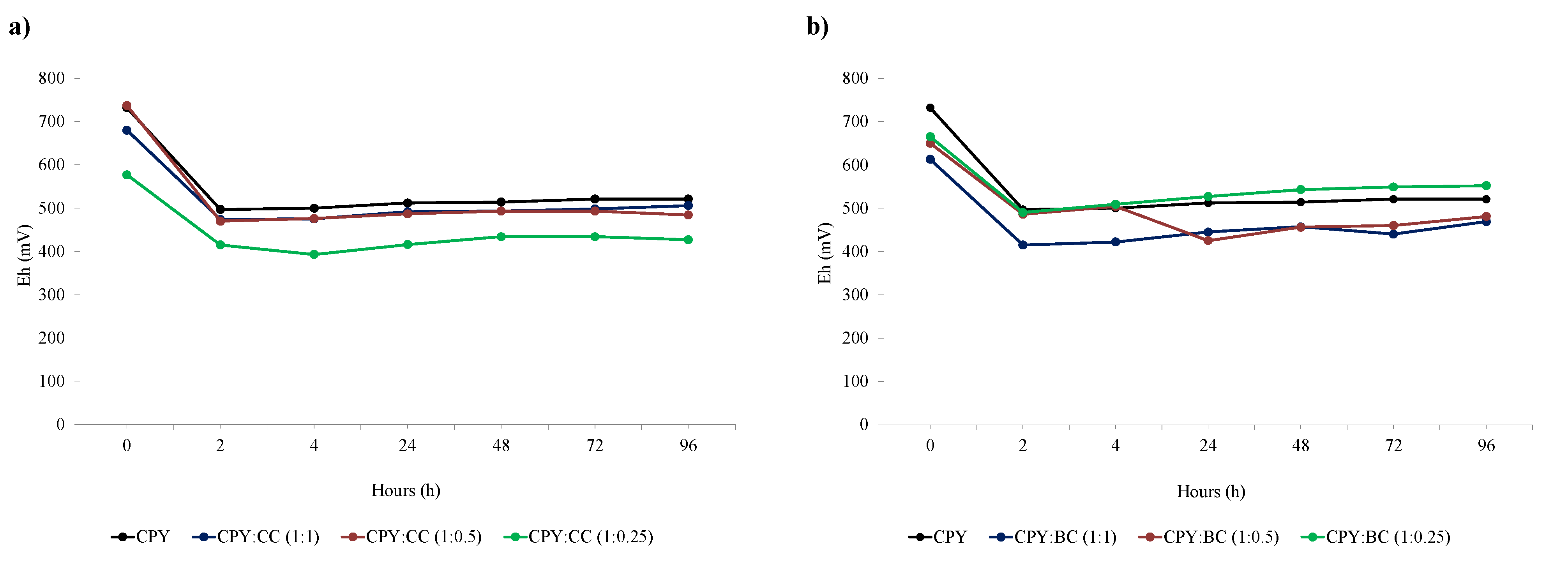
Figure 4 shows that there was an important increase in copper extraction for the CPY:CC ratio 1:1 (after 48 h of leaching) and for the CPY:BC 1:0.25 ratio (after 72 h of leaching). These results could indicate the formation of intermediate species during the first hours of extraction that are more amenable to oxidation. Previous works have concluded that copper extraction is mainly controlled by: (1) the redox potential of the leaching solution, (2) the concentration of ferric/ferrous ions, and (3) the formation of intermediate species that are oxidized in a lower potential region with higher ferrous concentrations that enhance copper extraction [
29]. Furthermore, it can be proposed that the surface of a carbon material can be modified by a sulfuric/Fe
3+ solution, increasing its content in oxygen functional groups and modifying its catalytic effect over time [
27]. For this reason, characterization of BC and CC was performed after treatment of carbon materials for 48 h with the leaching agent at 90 °C. Treatment with sulfuric/Fe
3+ solution modified the characteristics of CC and BC (
Table 2 and
Figure 5). It is important to note that the ash content decreased, particularly in BC, indicating the solubilization of the ash components.
For this reason, in the future use of carbon materials as catalysts in these leaching conditions, it will be of interest to prepare materials with a low ash content to avoid additional contamination of the leaching liquors. A significant increase in the O content of BC was observed, indicating the generation of oxygenated groups on the biochar. The sulfuric/Fe
3+ solution treatment also increased the S content and decreased the H/C ratio, probably due to the oxidation of more aromatic structures and the formation of –OH and −COOH groups. This result indicates that the BC sample can achieve catalytic properties during treatment with sulfuric/Fe
3+ acid due to the in-situ formation of active groups, in agreement with the increase in the recovery of copper from the CPY:CC 1:0.25 sample after 72 h of leaching (
Figure 4b).
In conclusion, adding either material (BC or CC) can increase copper extraction despite their differences in surface area, functional groups, and Eh values (
Table 2). This fact can be explained by the optimal ratios were different for each material, and biochar was oxidized during the leaching process with the formation of C=O and C-O groups such as quinones, carboxylic acids, ethers or esters, or phenolic groups. Nakazawa [
23] observed an increment from 28 to 90% in the extraction of copper after adding carbon black (surface area: 65 m
2/g, particle media diameter: 36 nm) to chalcopyrite during leaching with a sulfuric/ferric solution at 50 °C. Therefore, the surface area is not the only or main factor involved in the catalytic effect of carbon materials, as it is possible to use cheaper materials prepared without activation processes, such as the biochar used in the present research. Finally, it can be concluded that some copper is adsorbed onto the material’s surface and recovered by washing with an acid solution at the end of the leaching process (see
Table 3 and
Figure 4). Nakazawa [
23] used carbon black in sulfuric acid media at 50 °C and proposed that the enhanced leaching kinetics of chalcopyrite could be due to dissolution reactions at low redox potential joined to the galvanic interaction between carbon black structures and chalcopyrite. This author showed that the acceleration of chalcopyrite extraction did not occur without direct contact between particles of carbon black and chalcopyrite and that the addition of carbon black decreased the ferric/ferrous ratio in the early stages of extraction and lowered the redox potential below 600 mV. Other researchers [
14,
29] concluded that the redox potential is a key factor in the leaching of chalcopyrite and proposed a critical potential, suggesting that chalcopyrite dissolves through the formation of intermediate species. Cordoba et al. proposed that a high potential for extraction favors rapid precipitation of ferric materials such as jarosite and the corresponding passivation of chalcopyrite [
14]. Also, Hiroyoshi et al. noted that the redox potential must be low enough for Cu
2S formation and high enough for its subsequent oxidation [
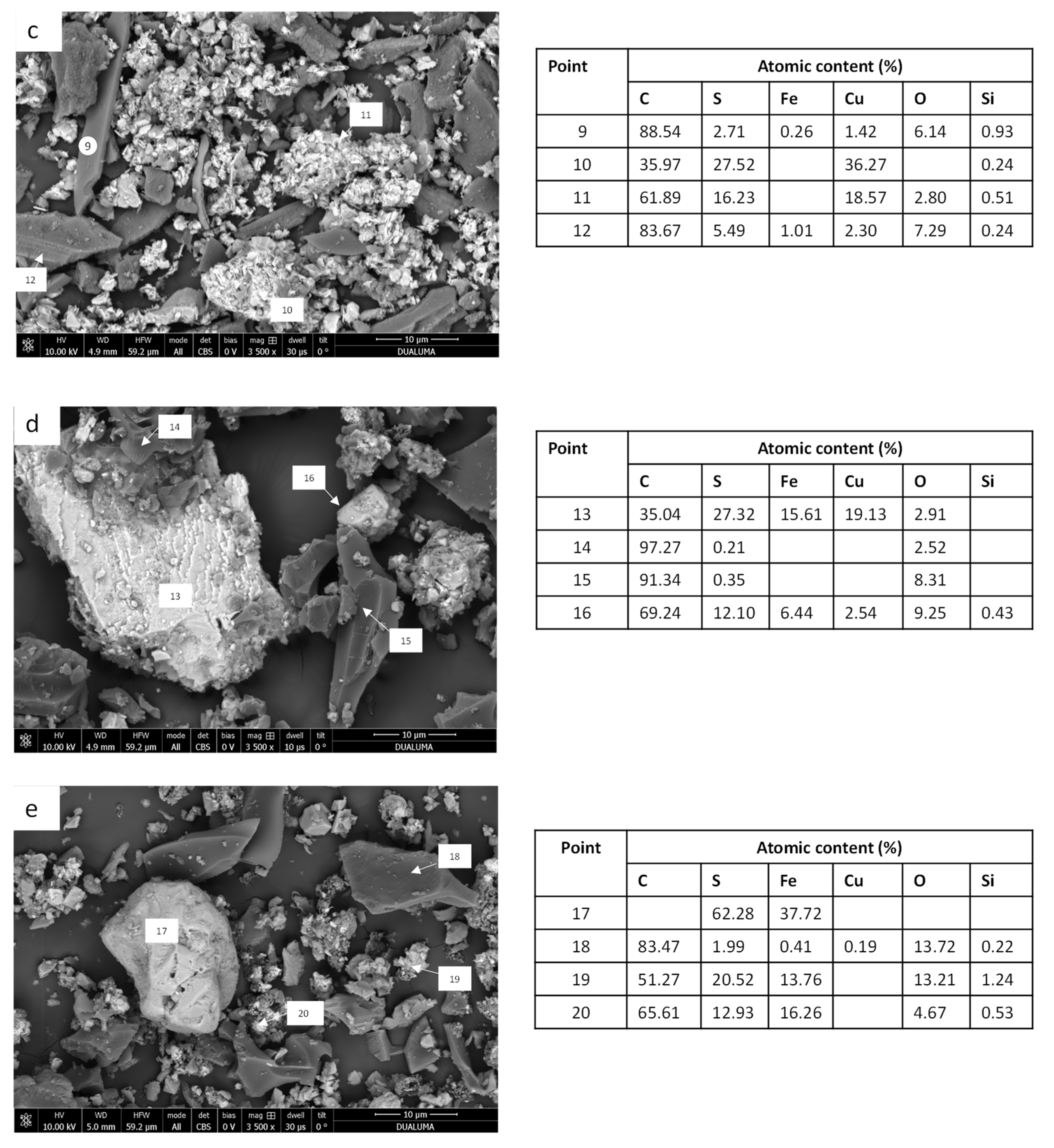
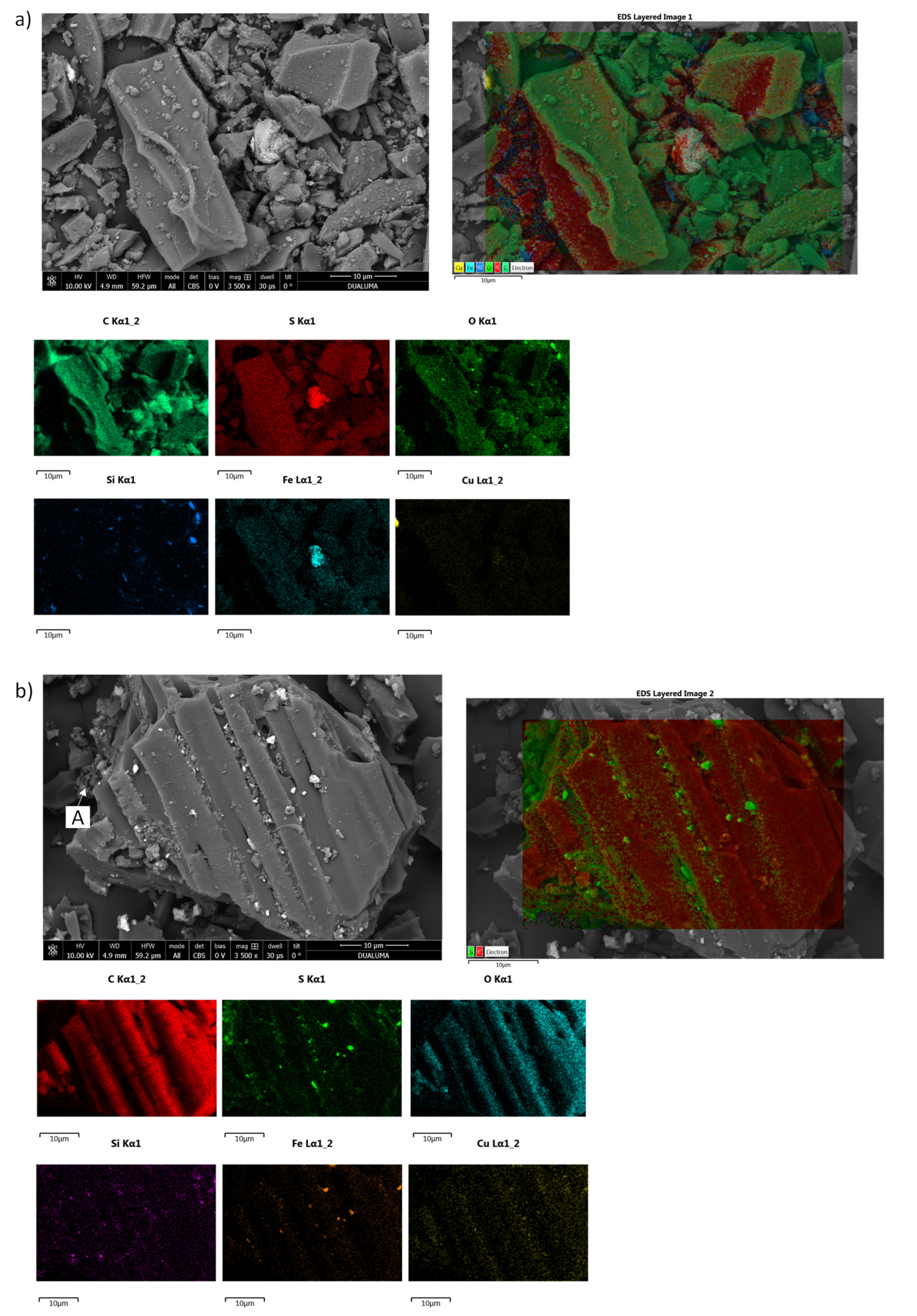
29]. This fact can explain the behavior of the treatment of the CPY:BC ratio 1:0.25. Briefly, Eh decreased during the first 4 h, increasing later from 509 to 552 mV. However, although the amount of copper extracted was higher for the CPY:CC ratio 1:1, Eh was similar during the leaching time. For each carbon material (CC or BC), the highest values of copper extraction were achieved when the ratio between the carbon material and the solution yielded the highest value of Eh. It can be concluded that factors such as galvanic interactions contribute to the catalytic effect of carbon. The final catalytic effect was a consequence of different physicochemical processes making an optimal ratio depending on the characteristics of carbon materials. In order to achieve a better understanding of the carbon material’s effect on copper extraction from chalcopyrite, the final residues were widely characterized.
The presence of CuS in the final residue of CPY:CC ratio 1:0.25 indicates that the addition of CC and the reduction of Eh modified the reaction mechanism, which was performed via intermediate CuS formation. Finally, the addition of CC at both ratios (1:1 and 1:0.25) seems to inhibit the formation of elemental sulfur. Lemos et al. [
30] found that activated carbons promote the oxidation of aqueous sulfide to form polysulfides, especially disulfide, and oxygenated sulfur species such as sulfite, sulfate, and thiosulfate but not elemental sulfur. Additionally, they concluded that this oxidation increases when the activated carbon contains oxygen groups on its surface, such as quinone/hydroquinone and carboxylic acids.
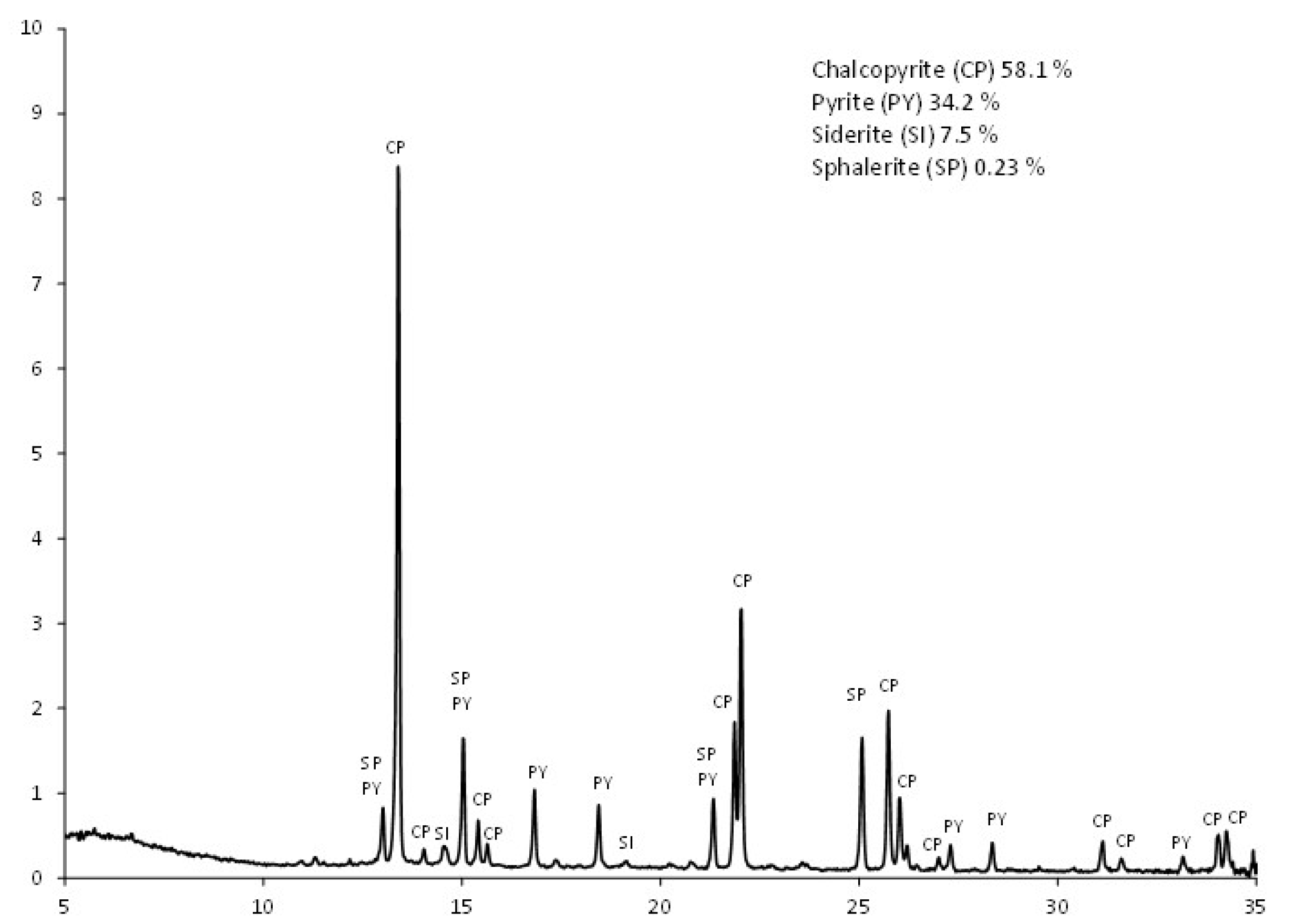
The presence of pyrite (89%) and small amounts of quartz (7.6%) and calcite (3.8%) as the unique mineralogical species in the final residue of extraction of CPY:CC ratio 1:1 (
Table 4) is of great importance for the future industrial implementation of processes based on the addition of activated carbon. This fact can limit the environmental impact of mine waste, as pyrite could be recovered from the waste by flotation processes. It is known that the oxidation of pyrite in mine ponds is responsible for the formation of acidic waters. Finally, it is interesting to note the presence of calcite after leaching of samples in acidic media. Its presence is higher in samples with CC and BC, probably due to their presence in its ashes. Previous works have shown that gypsum can precipitate on the surface of dissolving calcite in acidic media if the solutions have high sulfate concentrations and low pHs [
31,
32]. These coatings on the calcite’s surface can reduce contact between the solution and calcite, leading to decreased dissolution rates.
An interesting result is the influence of the carbon material on the elemental sulfur content. Similar results have been obtained previously using biomass-derived activated carbon as an additive in the leaching of sulfide minerals [
33]. The sulfur adsorption onto the carbon materials was higher for CC than for BC. This fact may play an important role in the leaching mechanism of chalcopyrite and other sulfide minerals, inhibiting the formation of a sulfur passivation layer on the surface of the mineral. Though BC does not adsorb sulfur, its addition to CPY in low ratios also increases the copper extraction, indicating that, galvanic interactions were probably the main factor influencing the catalytic effect of the carbon material. However, other characteristics of carbon materials, such as their capacity to adsorb sulfur and their promotion of sulfide oxidation, could have contributed to this positive effect on the total copper extraction. The presence of CC can inhibit the formation of elemental sulfur (
Table 4) due to its adsorption and probable combination with functional groups on the surface of CC. The surface area of BC was lower than that of CC (
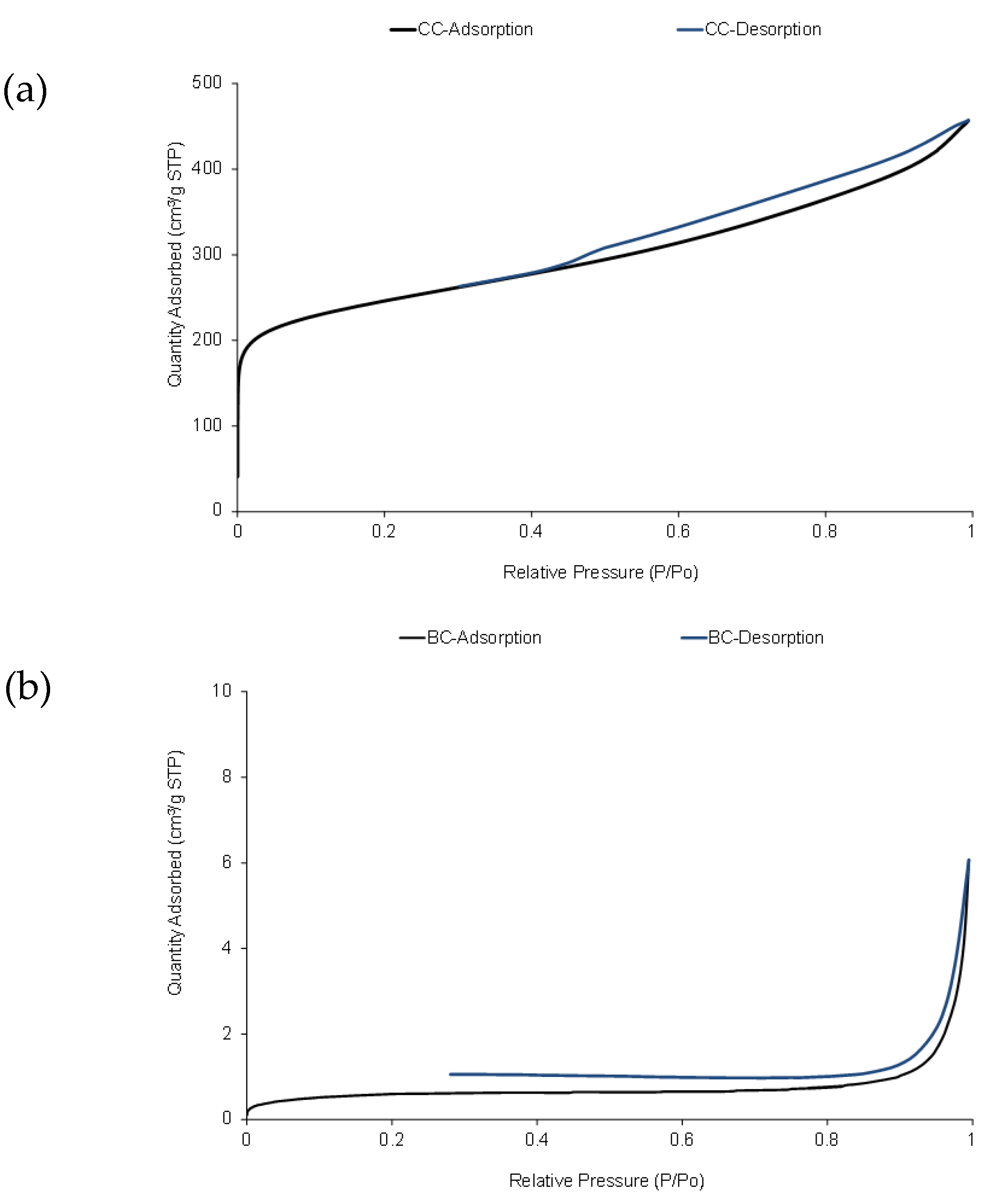
Figure 2); this fact could explain the differences observed in sulfur adsorption capacity between BC and CC. Jahromi and Ghahreman [
26] have shown that some carbon materials adsorb elemental sulfur onto their surfaces, facilitating the potential to remove sulfur from the leaching residue.
4. Materials and Methods
4.1. Samples Selection and Characterization
A chalcopyrite ore sample (CPY) was obtained from a copper sulfide deposit located in the Huelva region (Spain). CPY was air-dried and sieved below 50 µm using a Retsch RM 100 mortar mill. (Retsch-Verder Scientifc, Haan, Germany) The pH and redox potential (Eh) of CPY were determined with a CPY/distilled water ratio of 1:2.5. The pH was measured using a Crison micro pH 2000 ((Crison, Barcelona, Spain)), and the Eh (mV) was measured using a pH 60 DHS ((XS Instruments. Capry, Italy). Wavelength X-ray fluorescence (WDXRF) was performed SCAY-Malaga University in an ARL ADVANT XP + sequential model from THERMO (Thermo Fisher Scientific, Waltham, MA, EEUU)). Concentration data were obtained using the UNIQUANT integrated software ((Thermo Fisher Scientific, Waltham, EEUU). XRD was performed using a Bruker model D8 Advance A25 diffractometer (Bruker corporation, Billerica, MA, EEUU).
Two carbon materials, a commercial activated carbon (CC) supplied by Panreac (Barcelona, Spain) and commercial biochar (BC), were selected for this research [
25,
34]. The BC sample was air-dried, crushed, and sieved (<100 µm) using a ceramic mortar. The two samples, BC and CC, were characterized according to BET surface (SBET) (m
2 g
−1), pore volume (cm
3 g
−1), ash content (wt%), and elemental analysis (C, H, N, O, and S%). S
BET was analyzed by N
2 adsorption isotherms using an ASAP 2420 Porosimetry System by Micromeritics (Micromeritics, Dunstable, England). Elemental analysis was determined using a LECO CHNS 932 Analyzer (LECO corporation, St. Joseph, MI, EEUU) by dry combustion. The ash content was calculated by the combustion of samples in a Labsys Setaram TGA analyzer ((KEP Technologies, Mougins, France); 20 mg of each sample were heated at a rate of 15 °C min
−1 to 850 °C using 30 mL min
−1 of air. Oxygen was obtained by difference as 100% − (%C + %H + %N + %S + %Ash). Atomic ratios of H/C and O/C were calculated from the elemental analysis results. In addition, FTIR analysis was performed in a Vertex 70 spectrophotometer ((Vertex, Barcelona, Spain). For the acquisition of spectra, a standard spectral resolution of 4 cm
−1 was used in the spectral range of 4000–400 cm
−1, with 64 accumulations per sample.
4.2. Leaching Experiments
The leaching agent was a sulfuric acid solution with 5 g L
−1 of Fe (III). The purities of the sulfuric acid and Fe (III) sulfate hydrated supplied by Panreac (Barcelona, Spain) were 97% and 99.5%, respectively. In each experiment, approximately 5 g of CPY samples was mixed with 100 mL of leaching agent solution and added to a 500 mL glass flask. The CPY:carbon material ratios (weight:weight) were 1:1, 1:0.5, and 1:0.25, except for the control. The glass flask was introduced into a heating mantle with magnetic stirring (Nahita Serie 658, Auxiliab, Navarra, Spain; heating power of 250 W) and temperature control inside the glass flask. Experiments were carried out at 90 °C with a stirring speed of 250 rpm. At different times (2, 4, 24, 48, 72, and 96 h), 1 mL of the supernatant solution of each leaching experiment was withdrawn. To achieve that, the stirring was stopped at each time to let the sample stand and favor its decantation. One mL of the supernatant solution was removed, filtered, and transferred to a 25 mL graduated flask, and the rest of the flask was filled with distilled water. To maintain the same system conditions, 1 mL of the leaching agent solution was added after each extraction. Copper extraction from CPY was calculated as shown in Equation (1):
After 96 h, the stirring was stopped. When the samples reached room temperature, the pulp was filtered, and the solid residue was washed three times with 50 mL of H2SO4 solution at pH = 2 to recover the potential copper adsorbed onto the surface of the CC or BC. Total copper extraction was determined considering the copper content after leaching for 96 h and the copper content in the washed solutions. Copper was measured using an AAnalyst 400 PerkinElmer (AAS) spectrometer (Perkin Elmer, Waltham, MA, EEUU). The Eh of the leaching system was determined at different reaction times (0, 2, 4, 24, 48, 72, and 96 h) using a potentiometer of the model 60 DHS XS with an Ag/Ag+ electrode, and values were converted to the standard hydrogen electrode (SHE).
Leaching experiments and determination of Eh, pH, and copper extraction were performed in triplicate.
Furthermore, CC and BC were put in contact for 48 h with the leaching agent (without the CPY) at 90 °C in order to assess the interaction between the leaching agent and the carbon materials. This provided information about possible modifications to carbonaceous structures.
4.3. Characterization of Solid Residue
The final solid residue obtained after leaching of CPY with a sulfuric/ferric solution was analyzed by XRD (see
Section 2.1). In addition, SEM-EDS analysis was performed using a Helios Nanolab 650 dual beam microscope from FEI Companywith a Schottky field emission source for SEM (FESEM) and a Tomahawk focused ion beam (FIB) (FEI company, Hillsboro, OR, EEUU). The microscope was equipped with an energy-dispersive X-ray detector (EDS) and an electron-backscatter diffraction detector (EBSD) from Oxford Company (Oxford Lab products, Waples, San Diego, CA, EEUU). CC and BC after treatment with the leaching agent were characterized by FTIR, ash content, and elemental analysis.
5. Conclusions
The main conclusions of the present research are the following:
Commercial activated carbon or biochar in the appropriate ratio, despite their different properties, can increase the extraction of copper during sulfuric/ferric oxidation of chalcopyrite. Despite the general reduction of Eh in the presence of carbon, for each carbon material, the highest copper extraction is achieved in the mixture that gives the highest value of Eh.
Commercial activated carbon and biochar were oxidized during treatment with sulfuric/ferric solution, and biochar probably displayed catalytic properties in the leaching medium.
The final residue of chalcopyrite leaching in the presence of activated carbon with a ratio of 1:1 is mainly composed of pyrite and quartz with sulfur compounds on the surface of activated carbon particles. This result is of great importance in the future industrial implementation of this hydrometallurgical process for the treatment of chalcopyrite, as it can increase the extraction of copper and facilitate the recovery of pyrite and sulfur from the solid residue.
The carbon catalysts used are cost-effective and can be synthesized in a carbon-negative manner. Our research demonstrates how to recover copper from a refractory mineral, reducing the environmental impact of mine wastes while achieving a carbon offset.