Abstract
The Fenton reaction is a powerful method for removing refractory pollutants from water, yet it is restricted by shortcomings such as pH adjustments and generation of iron-containing sludge. In this study, a highly dispersed pyrite nanoplate supported on chitosan hydrochar was prepared through a simple one-pot hydrothermal method. The interactions between chitosan and Fe3+ suppressed the accumulation of FeS2 in the crystal growth period and led to the formation of pyrite nanoplates with many exposed (210) facets. Thus, it showed excellent Fenton-like activity and the removal efficiency of AR 73 reached 99.9% within 60 min. The catalyst could be used in a wide pH range of 3~10. Hydroxyl radicals are the main reactive oxygen species in this catalytic system. The self-reduction of generated Fe(III) species by sulfur via inner electron transfer promoted the Fe(II)/Fe(III) redox cycle, and the presence of graphene facilitated the adsorption of pollutants. This catalyst also showed good reuse performances as well as stability, which has promising prospects for practical use in wastewater treatment.
1. Introduction
In past decades, the treatment of refractory pollutants like pharmaceuticals, dyes, and phenols in water was viewed as a challenge for traditional wastewater treatment plants based on microbial metabolism [1]. Various advanced oxidized processes (AOPs) such as ozonation and photocatalysis involving strongly oxidizing species have been studied and evaluated [2,3]. In this context, Fenton reactions use ferrous sulfate to catalyze hydrogen peroxide to produce •OH at normal temperature and pressure. This process is widely used for industrial wastewater treatment due to its high effectiveness and simple operation [4]. However, the reactions require strictly acidic conditions (pH 3–4), and a large amount of iron-containing sludge is inevitably generated during the treatment process, leading to an increase both in costs and treatment difficulty [5].
Recent heterogeneous Fenton-like systems based on solid catalysts have shed light on how to improve traditional Fenton reactions [6]. The critical points lie in the rapid Fe(II)/Fe(III) redox cycle, particularly, the reduction of Fe(III) to Fe(II), which is usually viewed as the rate-determining step in the reactions (k = 0.001–0.01 M−1s−1) [7]. Therefore, adding electron-donating groups or reducing species can promote the reduction of Fe(III), thereby increasing the reaction rate and preventing ferric ions precipitation [8,9,10]. From this perspective, iron sulfide is more advantageous than iron oxides for activating H2O2 because of its reducing characteristics. Pyrite (FeS2) is the most abundant iron sulfide in nature. Several cases have been reported on the treatment of organic pollutants in wastewater by using natural pyrite and its modified materials as catalysts [11,12]. Zhang et al. [13] reported an enhanced Fenton reaction catalyzed by natural heterogeneous pyrite that exploited rapid Fe(III) reduction and its pH self adjustment. During the reaction, iron ions were continuously released into the bulk solution and reached a final concentration of 201 mg/L. However, it is difficult to further improve its catalytic activity by reducing particle size due to high hardness and phase impurities [14].
Hydrothermal synthesis is a promising method for obtaining nano-FeS2 with well-defined morphology and greater catalytic performance [15]. Liu et al. [16] demonstrated that hydrothermally synthesized pyrite showed greater activity for catalyzing H2O2 than its commercial counterpart. The small size and exposure of specific crystal faces promoted its high catalytic activity. Nevertheless, the direct use of these nanoparticles often introduces problems such as agglomeration and difficult recovery. Thus, a proper support such as graphene [17] is needed to ensure that nano-FeS2 maintains its activity and dispersibility.
Chitosan is an abundant and low-cost natural derivative that is widely used in food packaging, tissue engineering, drug delivery, and other fields because of its antimicrobial/antioxidant properties combined with its hydrophobicity [18,19]. Its ample amino and hydroxyl groups can serve as binding sites to anchor heavy metals and other pollutants; thus, various chitosan-based functional eco-materials have been designed for water and wastewater treatment [20,21,22]. Chitosan can be also used as a nitrogen-doped carbon precursor for the synthesis of supported materials. For example, Wang et al. [23] proposed a facile chitosan-induced hydrothermal and post-sintering method to synthesize hierarchical MoS2 nanoflowers anchored on N-doped carbon nanosheets. During the reaction, chitosan acts as the carbon source, soft template, and surfactant to control the formation of unique structures.
Inspired by these reports, in this study, a highly dispersed pyrite nanoplate supported on chitosan hydrochar was prepared as an efficient Fenton-like catalyst through a simple one-pot hydrothermal method. The interactions between chitosan and Fe3+ suppressed the accumulation of FeS2 in the crystal growth period and led to the formation of pyrite nanoplates with many exposed (210) facets. Its catalytic activity was promoted due to the self-reduction of Fe(III) by sulfur and the adsorption of the enhanced organic by graphene species. The catalyst also showed high pH adaptation as well as stability toward organic pollutant removal, which endows it with great practical potential use in wastewater treatment.
2. Results and Discussion
2.1. Pyrite Formation in the Catalyst
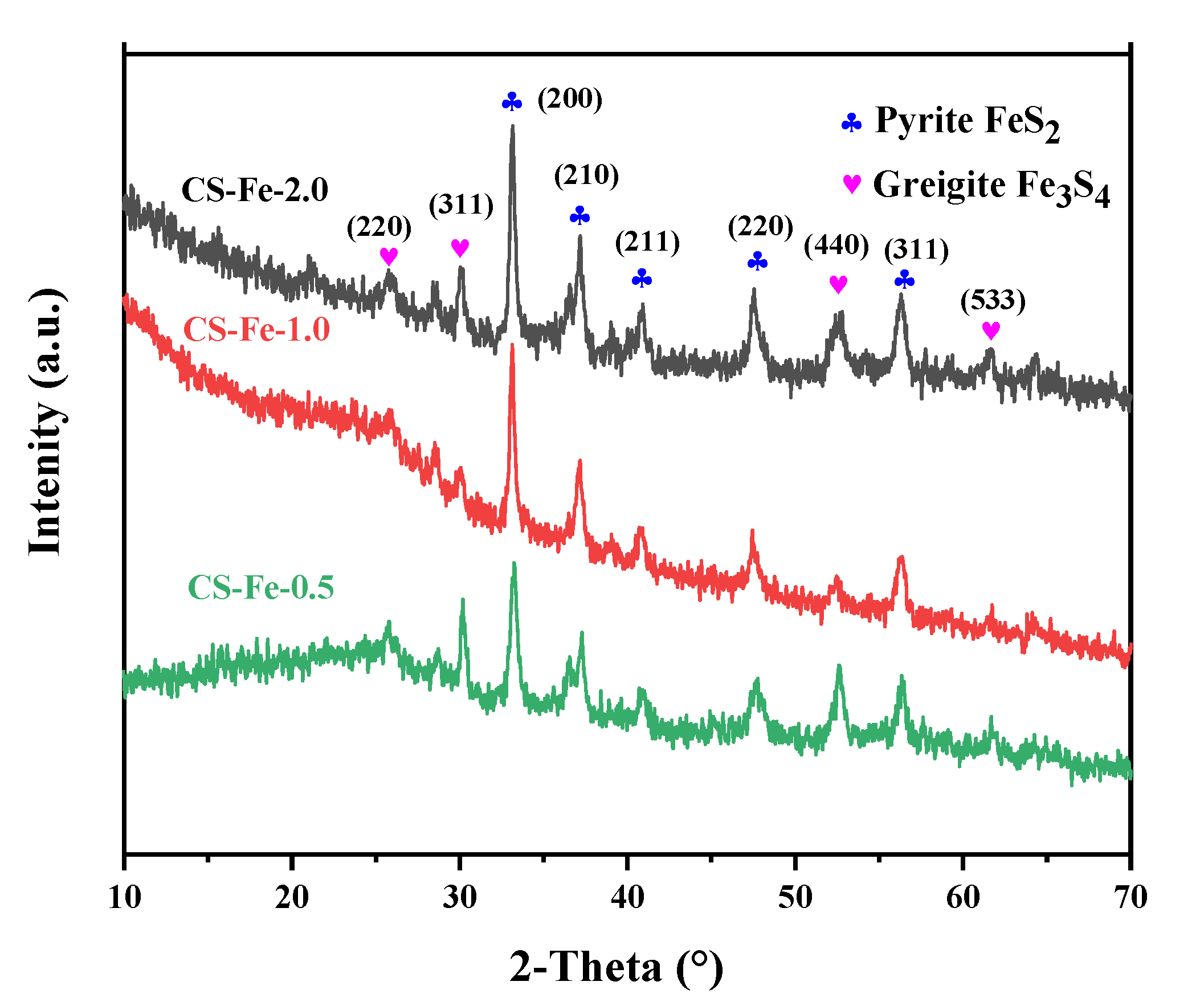
It can be seen from the XRD spectra in Figure 1 that the dominant iron species in CS-Fe-2 was pyrite (FeS2), and the peaks at 33.1°, 37.1°, 40.8°, 47.4°, and 56.3° corresponded to its characteristic (200), (210), (211), (220) and (311) crystal planes (JCD#42-1340). A small amount of Fe3S4 was also present in the catalyst, as indicated by the peaks at 25.5°, 29.9°, 52.3°, and 61.5°, which were well matched to the (220), (311), (440), and (533) crystal planes of greigite (JCD#16-0713). Greigite is often considered to be an intermediate during the formation of FeS2 phases, and it is a commonly associated mineral of pyrite in nature [24,25]. The existence of greigite indicates that the pyrite transformation process was interrupted or that the sulfur content is insufficient to form pyrite. In this study, the added S: Fe ratio exceeded the stoichiometric ratio, and upon increasing iron content, the peak intensity of CS-Fe-2 became significantly higher than that of CS-Fe-1 and CS-Fe-0.5. Therefore, it can be deduced that the formation of pyrite underwent the Fe3S4 intermediate pathway, which is described by Equations (1)–(4).
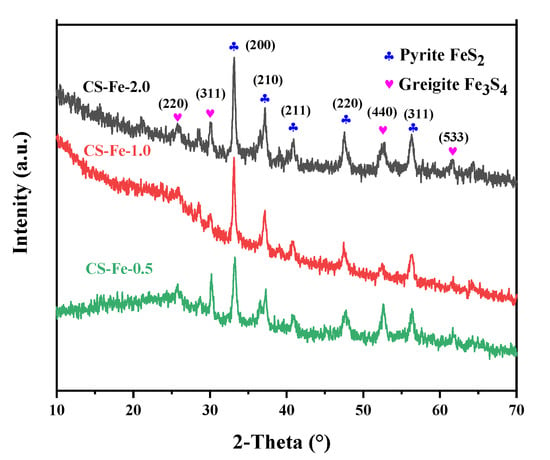
Figure 1.
XRD spectra of the prepared samples.
The reactions started with the hydrolysis of thiourea, which is commonly used in the hydrothermal synthesis of MoS2 or other metal sulfides [26,27]. Then, the resulting S2− reacted with iron and generated FeS. In a heated and sulfur-abundant environment, the oxidation-addition by sulfur or the Fe released from the solid occurred. This led to a phase transformation from FeS to Fe3S4, which was finally converted into the stable FeS2 phase [24].
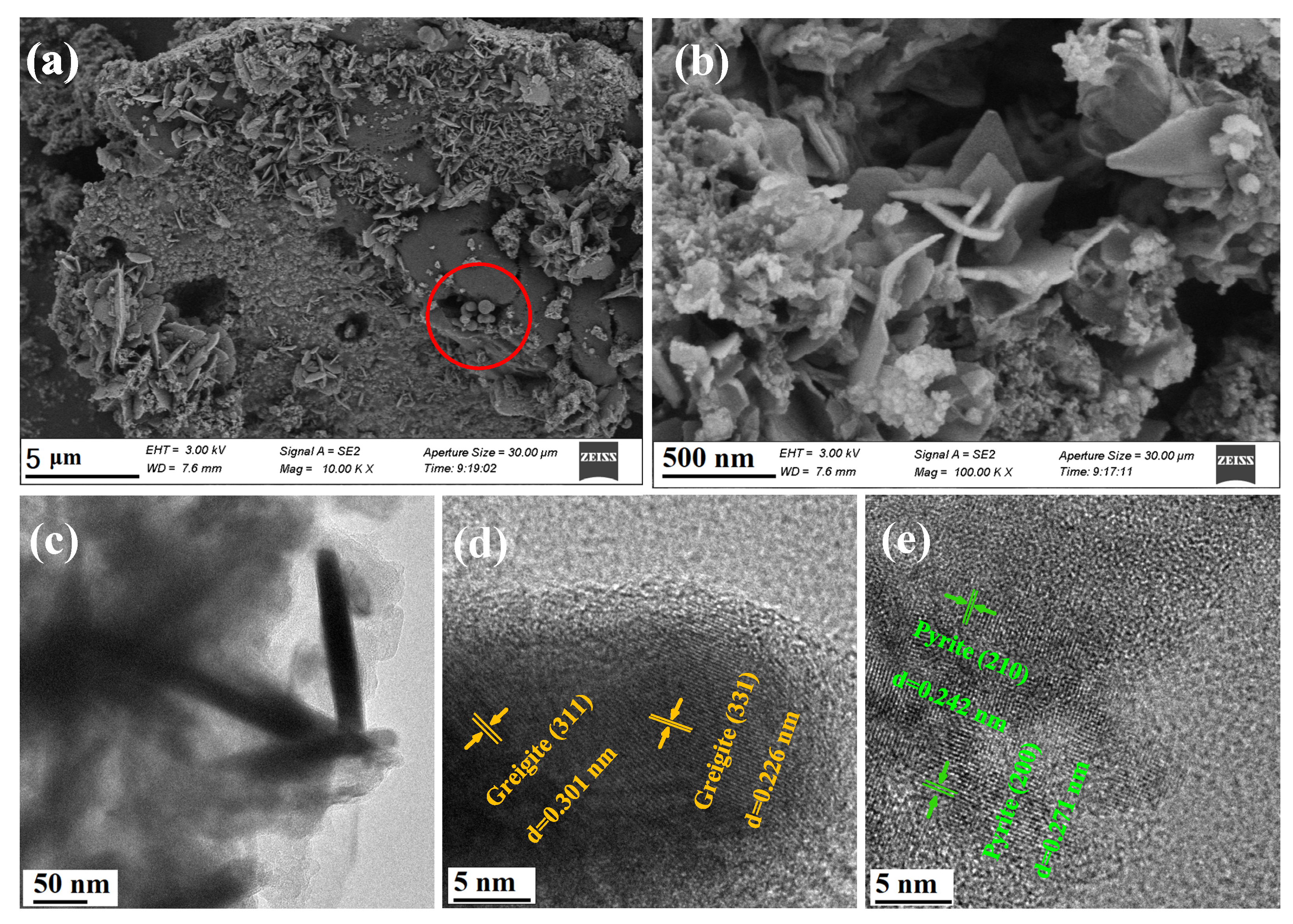
Pyrite has a strong crystallization tendency, and well-crystallized forms such as cubic (100), pentagonal dodecahedral (210), and octahedral (111) are often observed in natural sediments and laboratory synthesis [28]. However, in this study, the crystal growth of FeS2 was restricted due to the coordination and complexation of chitosan molecules with the iron precursors. In addition, compared with other studies [29], the hydrothermal reaction time was shorter, so planes with faster growth rates, such as the (210) facet, were predominant in the final crystals [30]. Consequently, as shown in Figure 2a,b, many quadrilateral and irregular pyrite nanoplates were embedded on the surface of the bulk material. The HRTEM images in Figure 2c,e show the presence of (210) and (200) crystal planes with a lattice spacing of 0.242 nm and 0.271 nm, respectively. He et al. [31] pointed out that the (210) plane of pyrite is a sulfur-rich crystal plane, and in terms of surface structure properties, its oxidation rate is higher than the common (100) and (111) planes. Therefore, the exposure of more (210) planes promoted the activation of H2O2. Greigite often aggregates into grape-like shapes due to strong magnetic interactions [32], so it is speculated that the aggregated spherical particles on the surface of the bulk material circled in Figure 2a were greigite. The HRTEM image in Figure 2d also displayed the (311) and (331) crystal planes of greigite with a lattice spacing of 0.301 nm and 0.226 nm, respectively.

Figure 2.
(a,b) SEM images of CS-Fe-2 at different magnification; (c) TEM images of CS-Fe-2; (d,e) HRTEM images of CS-Fe-2 at different positions.
2.2. Chemical Structure of the Catalyst
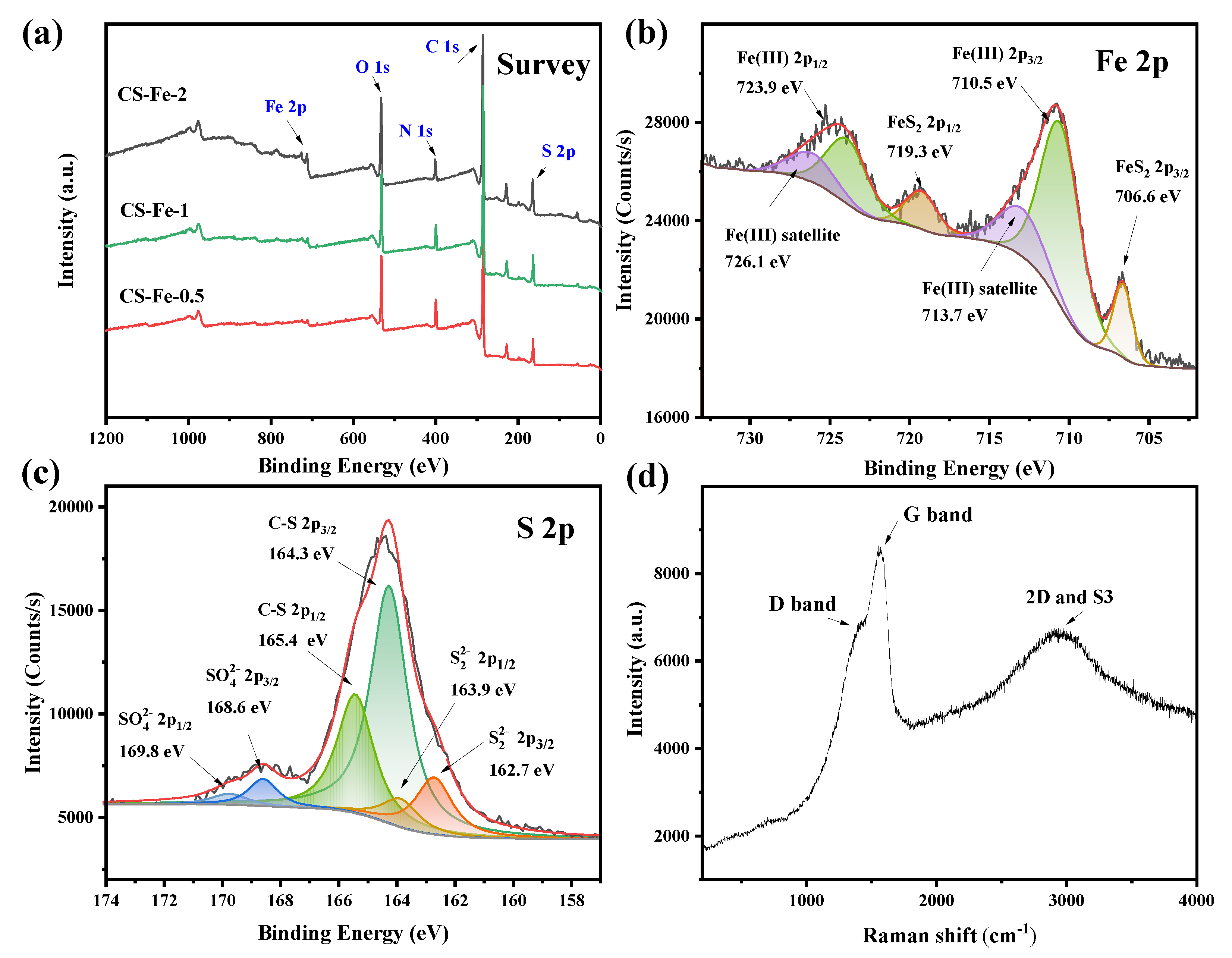
The surface chemical states of the prepared catalysts were analyzed by XPS, and the results are shown in Figure 3. The survey spectra showed that all catalysts contained C, N, O, S, and Fe elements. From the calculated contents in Table S1, the S:Fe ratio was greater than 2, and upon increasing the amount of Fe added to the precursor, both the S and O contents on the catalyst surface gradually increased. Peak fitting of the high-resolution spectra provided additional information about the valence states and chemical binding. As displayed in Figure 3b, surface Fe in CS-Fe-2 retains two characteristic peaks at 706.6 eV and 719.3 eV, corresponding to the 2p3/2 and 2p1/2 peaks of Fe(II) in FeS2, respectively. Another pair of peaks at 710.5 eV and 723.9 eV was ascribed to the 2p3/2 and 2p1/2 peaks of Fe(III), alongside their satellite peaks at 713.7 eV and 726.1 eV, respectively. FeS2 on the surface of the prepared catalyst was partially oxidized to form Fe(III)–O, giving rise to a higher surface oxygen content. Accordingly, the surface S spectrum includes peaks of S22− located at 162.7 eV and 163.9 eV, and the oxidized SO42− at 168.6 eV and 169.8 eV. A pair of strong peaks also appeared at 164.3 eV and 165.4 eV, which was attributed to the 2p3/2 and 2p1/2 peaks of C–S. These peaks indicated that sulfur in the precursor modified the surface of chitosan to generate thiol groups, which further combined with FeS2 to ensure the stability of the material.
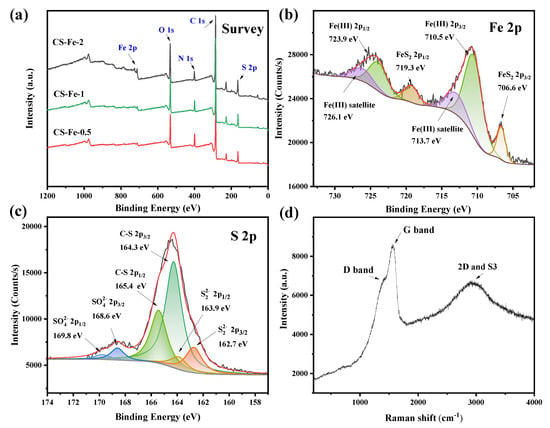
Figure 3.
(a) XPS survey spectra of the prepared samples; (b) Fe 2p spectra of CS-Fe-2; (c) S 2p spectra of CS-Fe-2; (d) Raman spectra of CS-Fe-2.
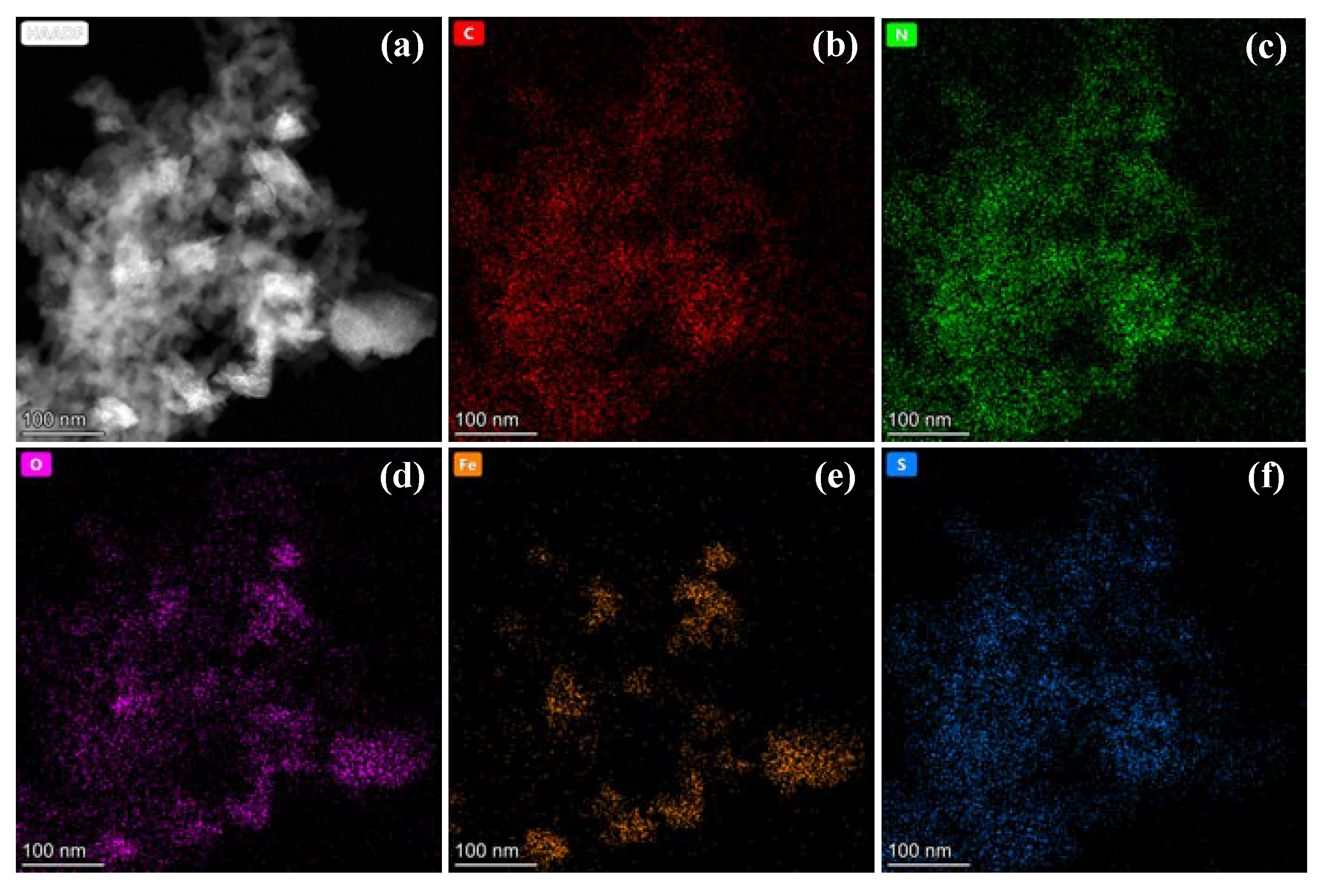
According to Table S1, the surface carbon contents of CS-Fe-2 reached 64.93 at%. The Raman spectra in Figure 3d showed a relatively strong G band at 1574 cm−1, a broad S3 band at 2923 cm−1, and a weak D band at about 1370 cm−1, implying the presence of a multilayered graphene structure with many defects [33]. The deconvoluted C 1s and N 1s spectra in Figure S1 revealed the existence of a few oxygen atoms but many nitrogen-containing functional groups bonded to the carbon skeleton. Consequently, as shown in the elemental mapping (Figure 4), the distribution of N was consistent with that of C, while O was more similar to Fe due to surface FeS2 oxidation.
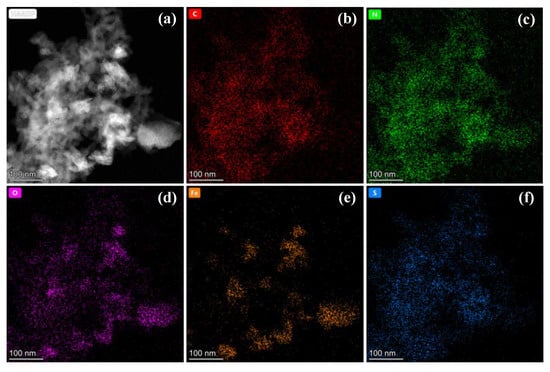
Figure 4.
(a) HADDF images and (b–f) elemental mapping of CS-Fe-2.
2.3. Catalytic Performances in Fenton-Like Reactions
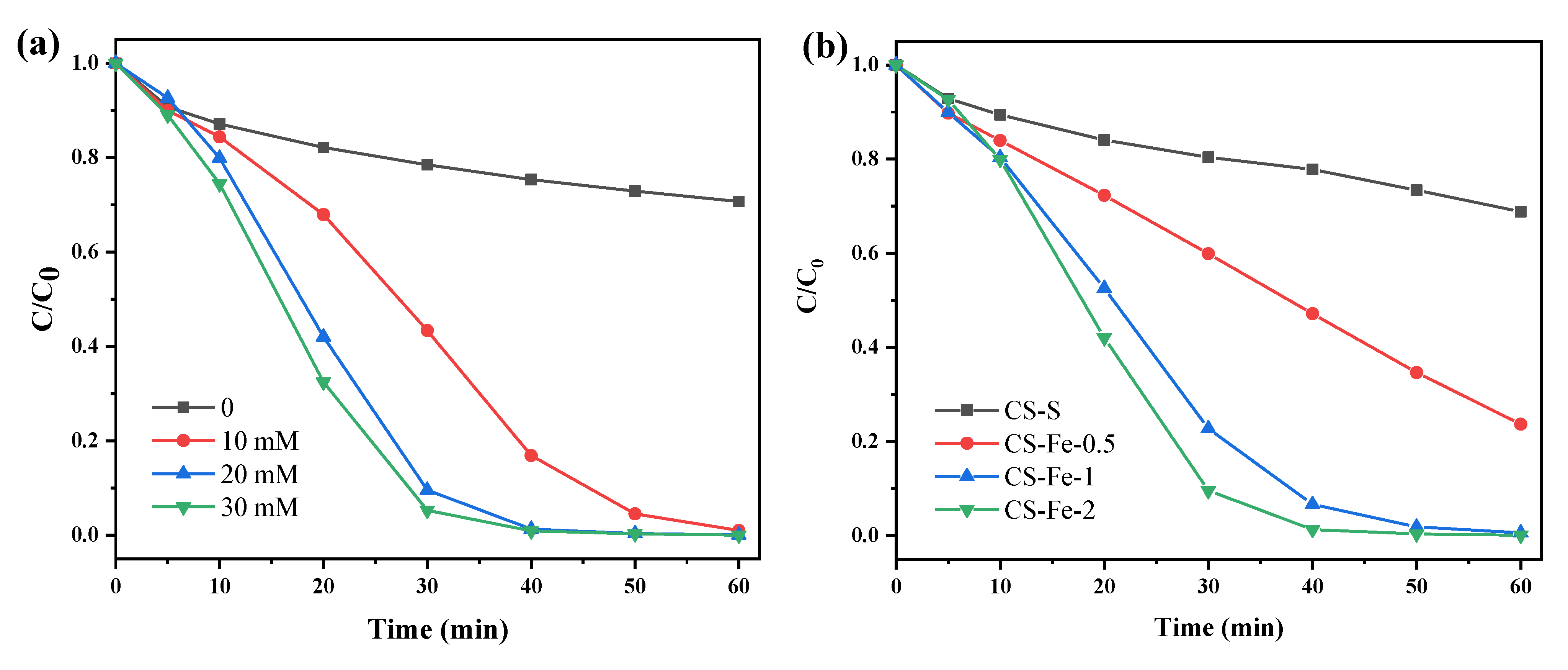
Acid red 73 (AR 73) is a widely used azo dye and was chosen as the model pollutant in this study [34]. As H2O2 was used as the precursor of reactive oxygen species (ROS), its optimum addition amount on the removal of AR 73 (50 mg/L) was investigated first. The results in Figure 5a show that without H2O2 addition, the adsorption removal efficiency of AR 73 by CS-Fe-2 after 60 min was only 29.31%. Because AR 73 has negatively charged and aromatic properties, this adsorption can be attributed to the combined effect of iron or graphene components on the surface. Upon increasing H2O2 dosage, the AR 73 removal rate gradually increased, indicating that the catalyst activated H2O2 to generate reactive oxygen species for the degradation of pollutants. However, when the H2O2 dosage was raised to 20 mmol/L, the removal efficiency of AR 73 reached 99.9% within 60 min, and a further increase to 30 mmol/L only contributed to a tiny growth in the removal rate. Therefore, 20 mmol/L was selected as the optimal H2O2 dosage in the following experiments.
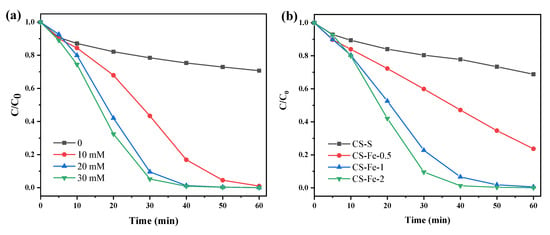
Figure 5.
(a) Degradation of AR 73 by CS-Fe-2 at different H2O2 dosages; (b) degradation of AR 73 by different catalysts at a H2O2 dosage of 20 mM (catalyst dosage: 1 g/L; AR 73: 50 mg/L; time: 60 min; pH: 6.8).
The catalytic performance of CS-Fe-2 was also compared with other prepared samples. Figure 5b shows that the removal efficiencies of AR 73 of 50 mg/L increased with increasing iron content in the catalyst. For CS-S, only 31.18% of total AR 73 was removed within 60 min, revealing the limited activation effect of S-modified chitosan hydrochar on H2O2. The reactions were fitted by the pseudo-first-order kinetics model (Figure S2), and the rate constants of CS-S, CS-Fe-0.5, CS-Fe-1, and CS-Fe-2 were 0.0056 ± 0.0004 (R2 = 0.9748), 0.0228 ± 0.0016 (R2 = 0.9709), 0.0870 ± 0.0079 (R2 = 0.9524), and 0.1250 ± 0.0101 (R2 = 0.9627) min−1, respectively. Moreover, the TOC removal efficiencies over the reactions are direct evidence to reflect the mineralization degree of AR 73, and they were measured to be 7.73%, 17.08%, 17.73%, and 23.95% for the four above-mentioned samples, respectively. From these results, it can be seen that the reaction rates, as well as TOC removal efficiencies, are positively correlated with the iron content in the catalysts, suggesting that the Fe species counts for the H2O2 activation, and consequently, CS-Fe-2 with a higher Fe content performed better than other samples.
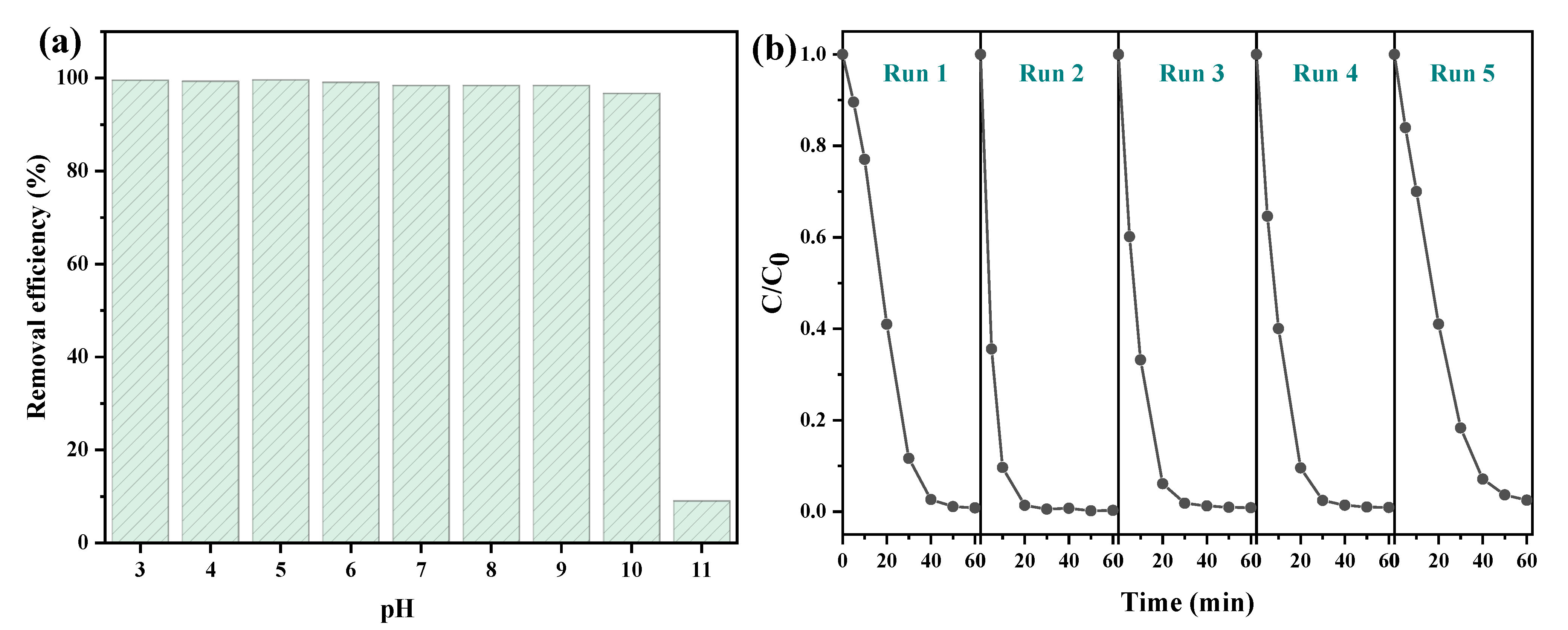
To explore the effect of pH on the catalytic performance, the removal of AR 73 by CS-Fe-2 was investigated over a wide pH range of 3–11. The results in Figure 6a show that the catalyst had excellent performance under both acidic and weakly alkaline conditions, and the removal efficiency of AR 73 maintained a level of 96.72% at pH 10. The reasons for this can be attributed to the generation of H+ through FeS2 oxidation, as reported previously [13], and consequently to the spontaneous adjustment of AR 73 solution pH. However, it seems that pH 11 exceeded this self-regulating ability. Under such strongly alkaline conditions, hydroxide precipitates may have formed on the surface of FeS2, and too much HO− captured the generated free radicals [35], resulting in a sharp decline in the removal efficiency of AR 73. Thus, it is suggested that the pyrite nanoplate supported on chitosan hydrochar be used at pH < 11.
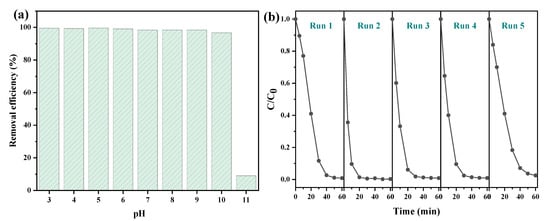
Figure 6.
The catalytic performance of CS-Fe-2 (a) under different pH values and (b) in recycle use at neutral pH values (AR 73: 50 mg/L; CS-Fe-2 dosage: 1 g/L; H2O2 dosage:20 mM; time: 60 min).
The stability of recycling is a vital factor for evaluating a catalyst’s performance. After the reactions, CS-Fe-2 was collected by centrifugation and drying, and then used for another cycle. In Figure 6b, there was only a 1.7% decay in the AR 73 removal efficiency after five cycles, indicating the excellent stability of the catalyst. The chemical structure of the used catalyst was studied by XPS, and the results are presented in Figures S3 and S4. The survey and Fe 2p spectra showed only slight changes, but the Fe contents (Table S1) increased from 2.49 at% to 2.97 at% after the reaction. The peaks of C–S 2p3/2 and SO42− 2p3/2 in the S 2p spectra shifted visibly towards the lower binding energies. In addition, the area ratio of the fitted SO42− 2p3/2 peaks to S22− increased from 0.38 to 0.89 after the reaction. These results demonstrate that the surface S atoms were partly oxidized to SO42− by H2O2 and other ROS, or due to inner electron transfer from reductive S to Fe3+, which could facilitate the Fenton-like reactions [36]. The released Fe could bind to the catalyst surface through complexation with thiol groups or electrostatic interactions due to SO42−. As a result, only 4.32 mg/L of total iron was leached to the bulk solution, which was much lower than that of other pyrite-based Fenton-like reactions [13,19,37].
2.4. Reaction Mechanism
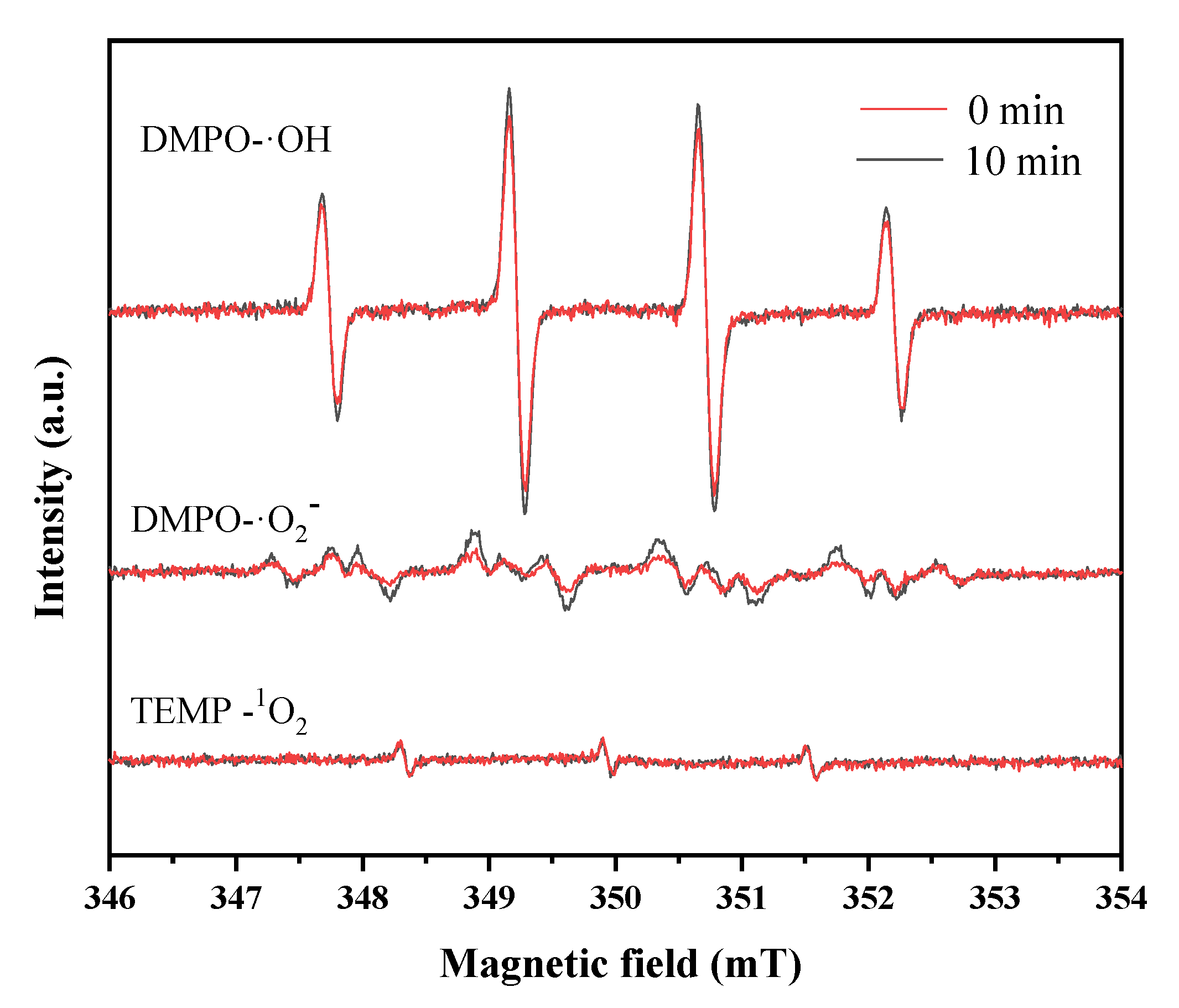
The electron spin resonance experiments using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and 2,2,6,6-tetramethyl-4-piperidinone (TEMP) as the trapping agents were conducted to identify the reactive oxygen species. Figure 7 displays typical DMPO-HO• signals with quartet peaks of 1:2:2:1 in water, while also showing the DMPO-•O2− multiplet in methanol, and the TEMP-1O2 triplet peaks in an aqueous solution. It can be seen that abundant HO• was produced in the catalyst system, while the amounts of O2−• and 1O2 were much lower. In traditional Fenton reactions, the regeneration of Fe2+ was believed to mainly depend on the reduction of Fe3+ by H2O2, but this process is accompanied by the generation of HO• [38]. The results in this study verified that CS-Fe-2 mainly catalyzed the generation of HO• from H2O2 for the degradation of pollutants, but H2O2 played a limited role in the reduction of Fe(III); therefore, the reductive S species may have been involved in the enhanced Fenton-like reactions.
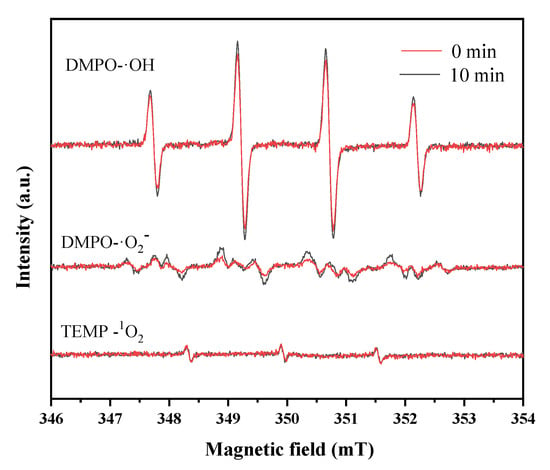
Figure 7.
ESR spectra of DMPO-•OH in aqueous solution; DMPO-•O2− in methanol solution, and TEMP-1O2 in aqueous solution.
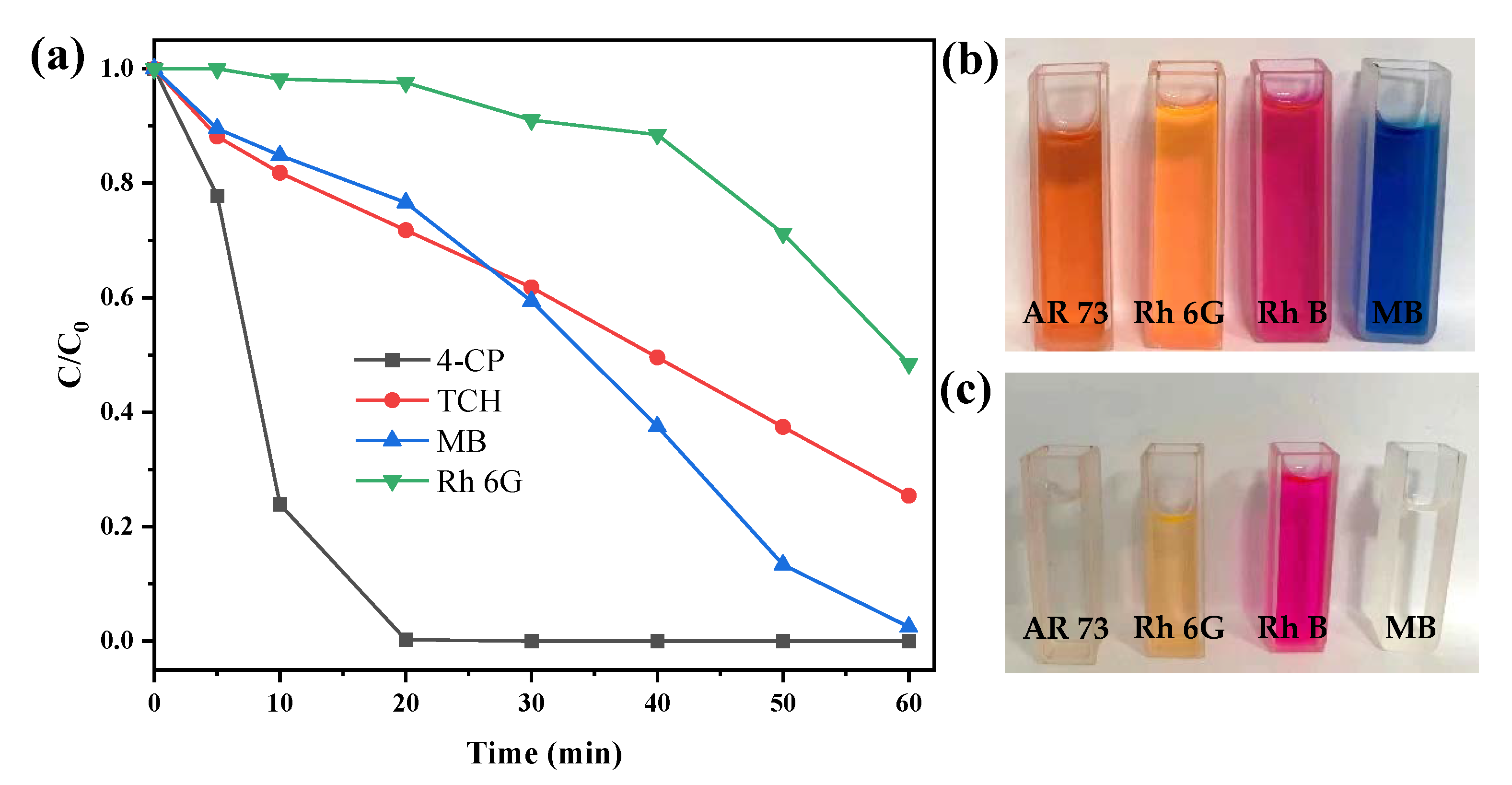
The experiments for the removal of other pollutants suggested additional possible mechanisms. It can be seen from Figure 8a that the removal behaviors of methylene blue (MB), rhodamine 6G (Rh 6G), p-chlorophenol (4-CP), and tetracycline hydrochloride (TCH) were significantly different. The removal efficiency of 4-CP was the best, reaching 99.74% in 20 min. At 60 min, MB, TCH, and Rh 6G were 97.5%, 74.60%, and 51.56%, respectively. Especially for Rh 6G, a hysteresis was observed during the first 30 min, and then the reaction accelerated. The reasons for this could be ascribed to the hydrophobic forces and short-range electrostatic interactions between the catalyst and the pollutant [39]. Figure S5 provides the chemical structure of the studied pollutants. AR 73 is an anionic dye that could interact with the positively charged pyrite through electrostatic adsorption. 4-CP is a somewhat hydrophobic small molecule that could be easily adsorbed by the graphene components as illustrated by the Raman spectra. Rh 6G and MB are cationic dyes, while TCH is an amphoteric molecule at pH 3.3–7.68 [40], thus, their electrostatic adsorption was inhibited, resulting in an initial reduction in the removal rate. Another cationic dye of rhodamine 6G (Rh B) was used to verify this speculation. As expected, it was not completely discolored within 60 min (Figure 8b,c, removal efficiency 65.5%). The reason for self-acceleration during the reaction could be the higher Fe contents on both the catalyst surface (Table S1) and in the bulk solution, therefore boosting the catalytic ability to generate more HO• and •O2− after 10 min, as observed in Figure 8.
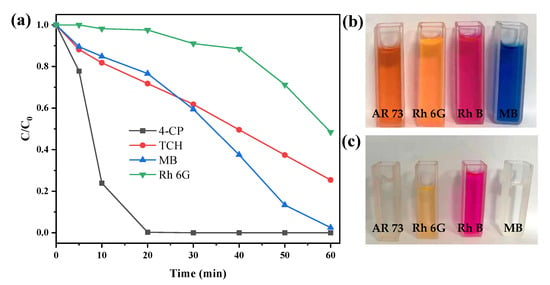
Figure 8.
(a) Degradation of AR 73 by CS-Fe-2; (b) the color of different dyes before degradation; (c) the color of different dyes after degradation (dyes: 50 mg/L; CS-Fe-2 dosage: 1 g/L; H2O2 dosage: 20 mM, time: 60 min; no pH adjustment).
Based on the above discussion, it can be concluded that the reaction mechanism may include: (i) the catalytic decomposition of H2O2 into HO• by the supported pyrite nanoplate (Equation (5)); (ii) the self-reduction of generated Fe(III) species by sulfur via inner electron transfer, as reported in many pyrite oxidation systems [36], or the Fe(III) reduction by H2O2 (Equations (6)–(7)); (iii) pollutant enrichment on the catalyst surface due to electrostatic interactions or hydrophobic forces with the graphene components, followed by degradation by HO• to various intermediates and even to the final mineralization.
During the reactions, many H+ ions were generated, which helped maintain the acidic environment near the catalyst, thus efficiently removing pollutants under both neutral and weakly alkaline conditions. Due to the oxidation of S species, some Fe was inevitably released from the crystal phase, which could then bind to the catalyst surface due to C-S or other groups. This stabilized the catalyst and enhanced its performance due to increased exposure to active Fe species.
3. Materials and Methods
3.1. Materials and Reagents
Acetic acid (AR), ferric chloride hexahydrate (FeCl3·6H2O, AR), and thiourea (CH₄N₂S, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Chitosan (CS, 200–400 mPa s) with a deacetylation degree of 91.2% was purchased from Zhejiang Golden-Shell Biochemical (Zhejiang, China). Acid Red 73 (AR 73) was purchased from Gracia Chemical Technology Co., Ltd., China. All reagents used in this study were analytical grade.
3.2. Catalyst Preparation
In a typical procedure, 0.45 g of chitosan was slowly dissolved in a 45 mL acetic acid solution (2 wt%). Then, 0.42 g of ferric chloride hexahydrate was added to the solution, followed by the addition of 0.5014 g of thiourea as the sulfur source. After being stirred magnetically for 30 min, the resulting homogeneous system was transferred into a 100 mL polytetrafluoroethylene hydrothermal reactor and kept at 200 °C for 12 h. After cooling and centrifugation, the sediment was washed with ethanol and water several times, and finally dried at 70 °C to obtain a supported pyrite Fenton catalyst, which was denoted as CS-Fe-2. Other samples were also prepared for comparison using the same procedure but different amounts of ferric salts (0, 0.1050, and 0.2100 g). These samples were named CS-S, CS-Fe-0.5, and CS-Fe-1, respectively.
3.3. Characterization Methods
The crystal phases of iron were first verified by powder X-ray diffraction (XRD) using an X’Pert PRO Multi-purpose X-ray Diffraction System (Bruker D8 Advance, Berlin, Germany) with Cu-Kα radiation (λ = 0.1541 nm). The catalyst morphology was characterized by scanning electron microscopy (SEM; FEI, Hillsboro, OR, USA) with an accelerating voltage of 3.0 kV, a working distance of 7.6 mm, and an aperture size of 30 μm. High-resolution transmission electron microscopy (HRTEM; FEI, USA) was carried out using a Tecnai G2 F30 equipped with a high angle annular field (HADDF) detector. The chemical structure was analyzed by X-ray photoelectron spectroscopy (XPS) on an ESCALAB 250Xi X-ray photoelectron spectrometer (Thermo Fisher, Waltham, MA, USA).
3.4. Performance Tests
The catalyst performance tests were carried out in a 250 mL conical flask containing 100 mL Acid Red 73 dye solution without pH adjustment (50 mg/L, pH ≈ 6.8). Unless otherwise specified, the dosages of catalyst and H2O2 were 1.0 g/L and 20 mM, respectively. The conical flask was sealed and shaken at 150 rpm and 25 °C. At specific intervals, the solution was extracted and filtered through a 0.45 μm filter. The residual AR 73 concentration was then immediately quantified by a UV-Vis spectrophotometer (Perkin Elmer Lambda 850, Akron, OH, USA) at 508 nm. The total organic carbon (TOC) was determined by a total organic carbon analyzer (Shimadzu TOC-L, Kyoto, Japan). The concentrations of leached ions during the reaction were determined by inductively coupled plasma mass spectroscopy (ICP-MS, Agilent 7700, Santa Clare, CA, USA). Electron spin resonance (ESR) experiments were recorded on an electron paramagnetic resonance spectrometer (Bruker EMX PLUS, Berlin, Germany) using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and 2,2,6,6-tetramethyl-4-piperidone (TEMP) as radical scavengers.
4. Conclusions
In this study, a highly dispersed pyrite nanoplate supported on chitosan hydrochar with many exposed (210) facets was prepared through a simple one-pot hydrothermal method. It showed excellent catalytic performances for Fenton-like reactions in a wide pH range. The rapid reduction of Fe(III) by sulfur species, pH self-adjusting ability by the generated H+, and the stabilization of the released Fe by surface thiol groups contributed to its high activity and stability. The presence of graphene facilitated the adsorption of pollutants. This study reports an improved pyrite-based Fenton system and provides new insights for practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12080858/s1, Table S1: Elements contents calculated from the EDS results; Figure S1: (a) C 1s and (b) N 1s XPS spectra of CS-Fe-2; Figure S2: Pseudo-first-order fitting of AR 73 degradation by different samples (AR 73: 50 mg/L; catalyst dosage: 1 g/L; H2O2: 20 mmol/L; pH: 6.8); Figure S3: (a) XPS survey and (b) Fe 2p spectra of the fresh and used CS-Fe-2; Figure S4: S 2p XPS spectra of the fresh and used CS-Fe-2; Figure S5: Chemical structures of (a) AR 73; (b) Rh B; (c) TCH; (d) Rh 6G; (e) 4-CP; (f) MB.
Author Contributions
Conceptualization, J.M.; methodology, A.S.; validation, J.M.; formal analysis, A.S., H.Z. and M.W.; investigation, A.S.; resources, J.M. and H.J.; data curation, H.Z. and M.W.; writing—original draft preparation, A.S.; writing—review and editing, J.M.; visualization, A.S.; supervision, J.M. and H.J.; project administration, K.Z.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No.52000155), the Zhejiang Provincial Natural Science Foundation of China (No. LQ20E080002), the Public Benefits Projects of Ningbo (No. 202002N3055), and the Major Technological Innovation Projects of Ningbo National High-tech Zone (New Material Technology City, No.20201CX050018).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China, Zhejiang Provincial Natural Science Foundation of China, Ningbo Science & Technology Bureau, and the Science and Technology Innovation Bureau of Ningbo National High-Tech Zone (New Material Technology City) as mentioned in the Funding Part. The authors also thank Shiyanjia Lab (www.shiyanjia.com) for their participation in catalyst characterization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, X.; Zhang, B.F.; Liu, H.J.; Chen, F.Y.; Li, A.Z.; Qu, J.H. Transformation characteristics of refractory pollutants in plugboard wastewater by an optimal electrocoagulation and electro-Fenton process. Chemosphere 2012, 87, 631–636. [Google Scholar] [CrossRef]
- Mustafa, M.; Kozyatnyk, I.; Gallampois, C.; Oesterle, P.; Tysklind, M. Regeneration of saturated activated carbon by electro-peroxone and ozonation: Fate of micropollutants and their transformation products. Sci. Total Environ. 2021, 776, 145723. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Omer, K.M.; Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Kwan, W.P.; Voelker, B.M. Decomposition of Hydrogen Peroxide and Organic Compounds in the Presence of Dissolved Iron and Ferrihydrite. Environ. Sci. Technol. 2002, 36, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramirez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions-A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Pan, Y.; Su, H.R.; Zhu, Y.T.; Molamahmood, F.V.; Long, M. CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals. Water Res. 2018, 145, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, M.H. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef]
- Bolobajev, J.; Trapido, M.; Goi, A. Improvement in iron activation ability of alachlor Fenton-like oxidation by ascorbic acid. Chem. Eng. J. 2015, 281, 566–574. [Google Scholar] [CrossRef]
- Dong, C.C.; Ji, J.H.; Shen, B.; Xing, M.Y.; Zhang, J.L. Enhancement of H2O2 Decomposition by the Co-catalytic Effect of WS2 on the Fenton Reaction for the Synchronous Reduction of Cr (VI) and Remediation of Phenol. Environ. Sci. Technol. 2018, 52, 11297–11308. [Google Scholar] [CrossRef]
- Farshchi, M.E.; Aghdasinia, H.; Khataee, A. Heterogeneous Fenton reaction for elimination of Acid Yellow 36 in both fluidized-bed and stirred-tank reactors: Computational fluid dynamics versus experiments. Water Res. 2019, 151, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, N.; Labiadh, L.; Oturan, M.A.; Oturan, N.; Gadri, A.; Ammar, S.; Brillas, E. Electrochemical mineralization of the antibiotic levofloxacin by electro-Fenton-pyrite process. Chemosphere 2015, 141, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, K.; Dai, C.; Zhou, X.; Si, H. An enhanced Fenton reaction catalyzed by natural heterogeneous pyrite for nitrobenzene degradation in an aqueous solution. Chem. Eng. J. 2014, 244, 438–445. [Google Scholar] [CrossRef]
- Khan, M.A.; Manasreh, M.O.; Kang, Y.-M. Synthesis, characterization and optoelectronic properties of iron pyrite nanohusks. Mater. Lett. 2014, 126, 181–184. [Google Scholar] [CrossRef]
- Kar, S.; Chaudhuri, S. Solvothermal synthesis of nanocrystalline FeS2 with different morphologies. Chem. Phys. Lett. 2004, 398, 22–26. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Ai, Z.; Zhang, L. Hydrothermal Synthesis of FeS2 as a High-Efficiency Fenton Reagent to Degrade Alachlor via Superoxide-Mediated Fe (II)/Fe (III) Cycle. ACS Appl. Mater. Inter. 2015, 7, 28534–28544. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-based smart hybrid materials: A physico-chemical perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes coated by chitosan for the controlled release of khellin. Polymers 2020, 12, 1766. [Google Scholar] [CrossRef]
- Liu, W.P.; Xu, L.L.; Li, X.F.; Shen, C.S.; Rashid, S.; Wen, Y.Z.; Liu, W.P.; Wu, X.H. High-dispersive FeS2 on graphene oxide for effective degradation of 4-chlorophenol. RSC Adv. 2015, 5, 2449–2456. [Google Scholar] [CrossRef]
- Shen, C.S.; Shen, Y.; Wen, Y.Z.; Wang, H.Y.; Liu, W.P. Fast and highly efficient removal of dyes under alkaline conditions using magnetic chitosan-Fe (III) hydrogel. Water Res. 2011, 45, 5200–5210. [Google Scholar] [CrossRef]
- Ma, J.Q.; Shen, Y.; Shen, C.S.; Wen, Y.Z.; Liu, W.P. Al-doping chitosan-Fe (III) hydrogel for the removal of fluoride from aqueous solutions. Chem. Eng. J. 2014, 248, 98–106. [Google Scholar] [CrossRef]
- Abu El-Soad, A.M.; Lazzara, G.; Abd El-Magied, M.O.; Cavallaro, G.; Al-Otaibi, J.S.; Sayyed, M.I.; Kovaleva, E.G. Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media. Int. J. Mol. Sci. 2022, 23, 2396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Tian, J.H.; Cheng, X.; Na, R.; Wang, D.D.; Shan, Z.Q. Chitosan-Induced Synthesis of Hierarchical Flower Ridge-like MoS2/N-Doped Carbon Composites with Enhanced Lithium Storage. ACS Appl. Mater. Inter. 2018, 10, 35953–35962. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.; Benning, L.G. Greigite: A true intermediate on the polysulfide pathway to pyrite. Geochem. Trans. 2007, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.T.; Nguyen, T.P.N.; Kim, C.D.; Park, C. Formation mechanisms of pyrite (FeS2) nano-crystals synthesized by colloidal route in sulfur abundant environment. Mater. Chem. Phys. 2014, 148, 1095–1098. [Google Scholar] [CrossRef]
- Duraisamy, S.; Ganguly, A.; Sharma, P.K.; Benson, J.; Davis, J.; Papakonstantinou, P. One-Step Hydrothermal Synthesis of Phase-Engineered MoS2/MoO3 Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2021, 4, 2642–2656. [Google Scholar] [CrossRef]
- Adhikari, S.; Mandal, S.; Kim, D.H. 1D/2D constructed Bi2S3/Bi2O2CO3 direct Z-Scheme heterojunction: A versatile photocatalytic material for boosted photodegradation, photoreduction and photoelectrochemical detection of water-based contaminants. J. Hazard. Mater. 2021, 418, 126263. [Google Scholar] [CrossRef]
- Alfonso, D.R. Computational Investigation of FeS2 Surfaces and Prediction of Effects of Sulfur Environment on Stabilities. J. Phys. Chem. C 2010, 114, 8971–8980. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.; Luo, S.X.; Yang, M.Z.; Zeng, P.; Qin, Z.H.; Yu, W.B.; Wan, Q. Facile Hydrothermal Synthesis of Nanocubic Pyrite Crystals Using Greigite Fe3S4 and Thiourea as Precursors. Minerals 2019, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Donnay, J.D.H.; Harker, D. A new law of crystal morphology extending the law of bravais. Am. Mineral. 1937, 22, 446–467. [Google Scholar]
- He, H.P.; Xian, H.Y.; Zhu, J.X.; Tan, W.; Liang, X.L.; Chen, M. Perspective of mineral reactivity from surfaces to crystal faces: A case study on the oxidation behavior differences among various crystal faces of pyrite. Acta Petrol. Sin. 2019, 35, 129–136. [Google Scholar]
- Jiang, W.T.; Horng, C.S.; Roberts, A.P.; Peacor, D.R. Contradictory magnetic polarities in sediments and variable timing of neoformation of authigenic greigite. Earth Planet. Sci. Lett. 2001, 193, 1–12. [Google Scholar] [CrossRef]
- Jiang, J.W.; Tang, H.; Wang, B.S.; Su, Z.B. Raman and infrared properties and layer dependence of the phonon dispersions in multilayered graphene. Phys. Rev. B 2008, 77, 235421. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Q.; Yang, Q.F.; Wen, Y.Z.; Liu, W.P. Fe-g-C3N4/graphitized mesoporous carbon composite as an effective Fenton-like catalyst in a wide pH range. Appl. Catal. B Environ. 2017, 201, 232–240. [Google Scholar] [CrossRef]
- Wang, N.; Tong, Z.; Zhang, G.; Peng, W. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef] [Green Version]
- Rimstidt, J.D.; Vaughan, D.J. Pyrite oxidation: A state-of-the-art assessment of the reaction mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Oral, O.; Kantar, C. Diclofenac removal by pyrite-Fenton process: Performance in batch and fixed-bed continuous flow systems. Sci. Total Environ. 2019, 664, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Guo, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Munoz, D.; Orellana, S.; Yanez, A.; Olea, A.F.; Oyarzun-Ampuero, F.; Moreno-Villoslada, I. Immobilization of rhodamine 6G in calcium alginate microcapsules based on aromatic-aromatic interactions with poly (sodium 4-styrenesulfonate). React. Funct. Polym. 2014, 81, 14–21. [Google Scholar] [CrossRef]
- de Lima, C.R.M.; Gomes, D.N.; de Morais, J.R.; Pereira, M.R.; Fonseca, J.L.C. Anionic and cationic drug sorption on interpolyelectrolyte complexes. Colloids Surf. B 2018, 170, 210–218. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).