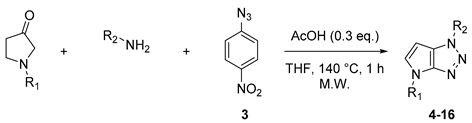
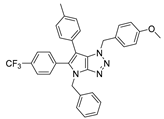
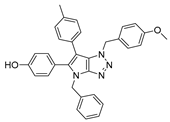
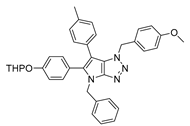
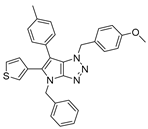
4.2. General Procedure (A) for 4–16
In a microwave vial already filled with anhydrous Toluene (0.25 M) and molecular sieves (3 Å), were successively added pyrrolidinone (1.0 eq.), amine (3.0 eq.), 1-Azido-4-nitrobenzene (5.0 eq.), and acetic acid (0.3 eq.). The vial was finally capped and stirred 1 h at 140 °C under microwave irradiation. The resulting mixture was reduced in a vacuum and filtered on charcoal. The crude product was purified by flash silica gel column chromatography using DCM, then PE/EtOAc mixtures to obtain the desired compound.
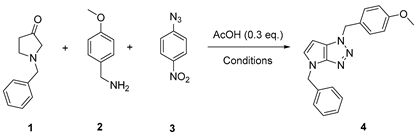
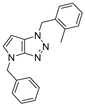
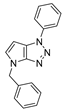
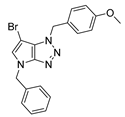
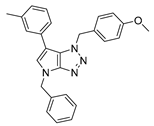
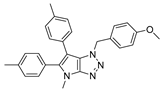
4.2.1. 4-Benzyl-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (4)
The reaction was carried out as described in general procedure A using 4-methoxybenzylamine (206 mg, 1.5 mmol, 3.0 eq.), 1-benzyl-3-pyrrolidinone (88.0 mg, 0.5 mmol, 1.0 eq.), 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9.0 mg, 0.15 mmol, 0.3 eq.), and 50 mg of molecular sieves (3 Å) in anhydrous Toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 4 as a white solid (119.0 mg, 75%). Rf = 0.27 (PE/EtOAc: 70/30). Mp 89–91 °C. 1H NMR (400 MHz, CDCl3): δ 7.32–7.26 (m, 7H), 6.88 (d, J = 8.7 Hz, 2H), 6.78 (d, J = 3.1 Hz, 1H), 5.55 (d, J = 3.1 Hz, 1H), 5.52 (s, 2H), 5.23 (s, 2H), 3.80 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 159.8 (Cq), 150.9 (Cq), 137.0 (Cq), 130.3 (CHAr), 130.1 (2 × CHAr), 128.9 (2 × CHAr), 128.1 (CHAr), 127.9 (Cq), 127.9 (2 × CHAr), 127.0 (Cq), 114.3 (2 × CHAr), 88.6 (CHAr), 55.4 (CH3), 53.3 (CH2), 50.6 (CH2). IR (ATR diamond, cm−1) ν: 3101, 3038, 2928, 2836, 1611, 1431, 1302, 1184, 1084, 751, 637. HRMS (EI+) m/z calcd for C18H19N4O [M+H]+: 319.1553, found: 319.1555.
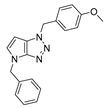
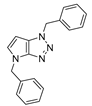
4.2.2. 1,4-Dibenzyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (5)
The reaction was carried out as described in general procedure A using benzylamine (161 mg, 1.5 mmol, 3.0 eq.), 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.), 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 5 as a yellow solid (110 mg, 76%). Rf = 0.25 (PE/EtOAc: 80/20). Mp 107–109 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.38–7.22 (m, 10H), 6.79 (d, J = 3.2 Hz, 1H), 5.59 (s, 2H), 5.57 (d, J = 3.2 Hz, 1H), 5.24 (s, 2H). 13C NMR (101 MHz, Chloroform-d): δ 150.9 (Cq), 137.0 (Cq), 134.9 (Cq), 130.4 (CHAr), 129.0 (2 × CHAr), 128.9 (2 × CHAr), 128.5 (CHAr), 128.5 (2 × CHAr), 128.1 (CHAr), 128.0 (Cq), 127.8 (2 × CHAr), 88.5 (CHAr), 53.7 (CH2), 50.6 (CH2). IR (ATR diamond, cm−1) ν: 3085, 3032, 2971, 1520, 1313, 1169, 1075, 938, 727, 694. HRMS (EI+) m/z calcd for C18H17N4 [M+H]+: 289.1448, found: 289.1450.
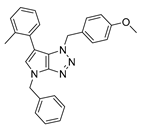
4.2.3. 4-Benzyl-1-(4-methylbenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (6)
The reaction was carried out as described in general procedure A using 4-methylbenzylamine (182 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 6 as a white solid (103 mg, 72%). Rf = 0.21 (PE/EtOAc: 80/20). Mp 70–72 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.32–7.20 (m, 7H), 7.15 (m, 2H), 6.78 (d, J = 3.2 Hz, 1H), 5.57 (d, J = 3.2 Hz, 1H), 5.53 (s, 2H), 5.22 (s, 2H), 2.32 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 150.8 (Cq), 138.3 (Cq), 137.0 (Cq), 131.9 (Cq), 130.3 (CHAr), 129.6 (2 × CHAr), 128.9 (2 × CHAr), 128.5 (2 × CHAr), 128.0 (CHAr), 127.9 (Cq), 127.8 (2 × CHAr), 88.5 (CHAr), 53.5 (CH2), 50.6 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 3098, 3030, 2934, 2838, 1612, 1494, 1351, 1111, 1082, 972, 818, 773. HRMS (EI+) m/z calcd for C19H19N4 [M+H]+: 303.1604, found: 303.1608.
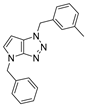
4.2.4. 4-Benzyl-1-(3-methylbenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (7)
The reaction was carried out as described in general procedure A using 3-methylbenzylamine (182 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 7 as a white solid (105 mg, 73%). Rf = 0.21 (PE/EtOAc: 80/20). Mp 58–60 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.32–7.21 (m, 6H), 7.13 (m, 3H), 6.79 (d, J = 3.2 Hz, 1H), 5.59 (d, J = 3.2 Hz, 1H), 5.55 (s, 2H), 5.24 (s, 2H), 2.33 (s, 3H, H). 13C NMR (101 MHz, Chloroform-d): δ 150.9 (Cq), 138.7 (Cq), 137.0 (Cq), 134.8 (Cq), 130.4 (CHAr), 129.3 (CHAr), 129.2 (CHAr), 128.9 (2 × CHAr), 128.8 (CHAr), 128.1 (CHAr), 128.0 (Cq), 127.8 (2 × CHAr), 125.6 (CHAr), 88.5 (CHAr), 53.7 (CH2), 50.6 (CH2), 21.5 (CH3). IR (ATR diamond, cm−1) ν: 3028, 2927, 1518, 1366, 1201, 1099, 938, 770, 691. HRMS (EI+) m/z calcd for C19H19N4 [M+H]+: 303.1604, found: 303.1609.
4.2.5. 4-Benzyl-1-(2-methylbenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (8)
The reaction was carried out as described in general procedure A using 4-methylbenzylamine (182 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 8 as a white solid (107 mg, 76%). Rf = 0.21 (PE/EtOAc: 80/20). Mp 73–75 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.36–7.19 (m, 9H), 6.75 (d, J = 3.2 Hz, 1H), 5.60 (s, 2H), 5.35 (d, J = 3.2 Hz, 1H), 5.23 (s, 2H), 2.38 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 150.7 (Cq), 137.5 (Cq), 137.0 (Cq), 132.7 (Cq), 130.9 (CHAr), 130.3 (CHAr), 129.9 (CHAr), 129.0 (CHAr), 128.9 (2 × CHAr), 128.0 (CHAr), 128.0 (Cq), 127.8 (2 × CHAr), 126.4 (CHAr), 88.5 (CHAr), 52.0 (CH2), 50.6 (CH2), 19.2 (CH3). IR (ATR diamond, cm−1) ν: 3029, 2922, 1519, 1453, 1343, 1175, 1074, 924, 734, 696. HRMS (EI+) m/z calcd for C19H19N4 [M+H]+: 303.1604, found: 303.1605.
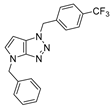
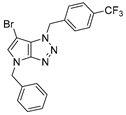
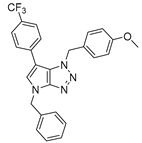
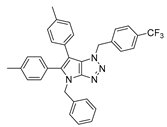
4.2.6. 4-Benzyl-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (9)
The reaction was carried out as described in general procedure A using 4-(trifluoromethyl)benzylamine (263 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 9 as a beige solid (91 mg, 51%). Rf = 0.13 (PE/EtOAc: 80/20). Mp 105–107 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.61 (d, J = 8.0 Hz, 2H), 7.41 (d, J = 8.0 Hz, 2H), 7.37–7.24 (m, 5H), 6.85 (d, J = 3.2 Hz, 1H), 5.68–5.63 (m, 3H), 5.26 (s, 2H). 13C NMR (101 MHz, Chloroform-d): δ 150.9 (Cq), 139.1 (Cq), 136.8 (Cq), 131.32–130.24 (m, Cq and CHAr), 129.0 (2 × CHAr), 128.5 (2 × CHAr), 128.2 (CHAr), 127.9 (Cq), 127.9 (2 × CHAr), 126.00 (q, J = 3.7 Hz, 2 × CHAr), 124.03 (d, J = 272.2 Hz, Cq), 88.1 (CHAr), 53.0 (CH2), 50.7 (CH2). 19F NMR (376 MHz, Chloroform-d): δ −62.7. IR (ATR diamond, cm−1) ν: 2169, 1990, 1521, 1327, 1157, 1066, 1018, 819, 744, 715. HRMS (EI+) m/z calcd for C19H16F3N4 [M+H]+: 357.1322, found: 357.1323.
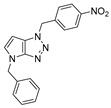
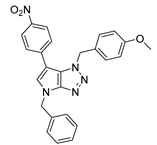
4.2.7. 4-Benzyl-1-(4-nitrobenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (10)
The reaction was carried out as described in general procedure A using 4-nitrobenzylamine hydrochloride (283 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 10 as a white solid (13 mg, 8%). Rf = 0.13 (PE/EtOAc: 80/20). Mp 123–125 °C. 1H NMR (400 MHz, Chloroform-d): δ 8.21 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 8.5 Hz, 2H), 7.35–7.28 (m, 5H), 6.88 (d, J = 3.2 Hz, 1H), 5.74–5.67 (m, 3H), 5.27 (s, 2H). 13C NMR (101 MHz, Chloroform-d): δ 150.9 (Cq), 148.1 (Cq), 142.3 (Cq), 136.7 (Cq), 131.1 (CHAr), 130.1 (Cq), 129.0 (2 × CHAr), 128.8 (2 × CHAr), 128.2 (CHAr), 127.9 (2 × CHAr), 124.3 (2 × CHAr), 87.9 (CHAr), 52.7 (CH2), 50.8 (CH2). IR (ATR diamond, cm−1) ν: 3062, 2937, 2850, 1518, 1341, 1241, 1105, 932, 873, 783, 696, 619. HRMS (EI+) m/z calcd for C19H16N5O2 [M+H]+: 334.1299, found: 334.1297.
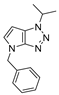
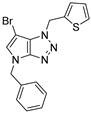
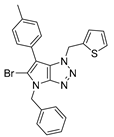
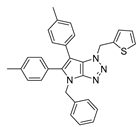
4.2.8. 4-Benzyl-1-(thiophen-2-ylmethyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (11)
The reaction was carried out as described in general procedure A using 2-thiophènemethylbenzylamine (170 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 11 as a brown solid (105 mg, 71%). Rf = 0.22 (PE/EtOAc: 80/20). Mp 65–67 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.31 (m, 6H), 7.16 (m, 1H), 7.02 (m, 1H), 6.85 (d, J = 3.2 Hz, 1H), 5.80 (s, 2H), 5.71 (d, J = 3.2 Hz, 1H), 5.27 (s, 2H). 13C NMR (101 MHz, Chloroform-d): δ 150.8 (Cq), 136.9 (Cq), 136.7 (Cq), 130.5 (CHAr), 128.9 (2 × CHAr), 128.2 (CHAr), 128.1 (CHAr), 127.8 (2 × CHAr), 127.7 (Cq), 127.2 (CHAr), 126.7 (CHAr), 88.5 (CHAr), 50.6 (CH2), 48.0 (CH2). IR (ATR diamond, cm−1) ν: 3101, 2918, 1600, 1520, 1360, 1273, 1172, 1029, 853, 751, 694. HRMS (EI+) m/z calcd for C16H15N4S [M+H]+: 295.1012, found: 295.1013.
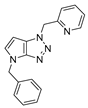
4.2.9. 4-Benzyl-1-(pyridin-2-ylmethyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (12)
The reaction was carried out as described in general procedure A using pyridin-2-ylmethanamine (162 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-Azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 12 as a yellow solid (101 mg, 70%). Rf = 0.22 (PE/EtOAc: 50/50). Mp 79–81 °C. 1H NMR (400 MHz, Chloroform-d): δ 8.59 (dd, J = 5.0, 1.8 Hz, 1H), 7.61 (td, J = 7.7, 1.8 Hz, 1H), 7.34–7.25 (m, 5H), 7.21 (dd, J = 7.7, 5.0 Hz, 1H), 7.09 (d, J = 7.7 Hz, 1H), 6.85 (d, J = 3.2 Hz, 1H), 5.80 (d, J = 3.2 Hz, 1H), 5.74 (s, 2H), 5.25 (s, 2H). 13C NMR (101 MHz, Chloroform-d): δ 155.2 (Cq), 150.8 (Cq), 149.5 (CHAr), 137.2 (CHAr), 136.8 (Cq), 130.6 (CHAr), 128.8 (2 × CHAr), 128.3 (Cq), 128.0 (CHAr), 127.8 (2 × CHAr), 123.1 (CHAr), 122.2 (CHAr), 88.5 (CHAr), 55.1 (CH2), 50.6 (CH2). IR (ATR diamond, cm−1) ν: 3091, 3032, 2956, 2933, 2359, 1612, 1514, 1300, 1171, 1027, 832, 759. HRMS (EI+) m/z calcd for C17H16N5 [M+H]+: 290.1400, found: 290.1405.
4.2.10. 4-Benzyl-1-propyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (14)
The reaction was carried out as described in general procedure A using propylamine (89 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 14 as an orange oil (96 mg, 80%). Rf = 0.15 (PE/EtOAc: 80/20). 1H NMR (400 MHz, Chloroform-d): δ 7.39–7.21 (m, 5H), 6.88 (d, J = 3.2 Hz, 1H), 5.96 (d, J = 3.2 Hz, 1H), 5.26 (s, 2H), 4.39 (t, J = 7.4 Hz, 2H), 1.99 (h, J = 7.4 Hz, 2H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, Chloroform-d): δ 150.8 (Cq), 137.1 (Cq), 130.3 (CHAr), 128.9 (2 × CHAr), 128.0 (Cq and CHAr), 127.8 (2 × CHAr), 88.1 (CHAr), 51.4 (CH2), 50.6 (CH2), 23.0 (CH2), 11.5 (CH3). IR (ATR diamond, cm−1) ν: 3031, 2956, 2933, 2875, 1733, 1519, 1355, 1268, 1144, 1026, 900, 731, 696. HRMS (EI+) m/z calcd for C14H17N4 [M+H]+: 241.1448, found: 241.1448.
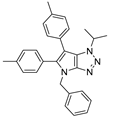
4.2.11. 4-Benzyl-1-isopropyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (15)
The reaction was carried out as described in general procedure A using isopropylamine (89 mg, 1.5 mmol, 3.0 eq.) as amine, 1-benzyl-3-pyrrolidinone (88 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 15 as a brown oil (78 mg, 65%). Rf = 0.26 (PE/EtOAc: 80/20). 1H NMR (400 MHz, Chloroform-d): δ 7.40–7.25 (m, 5H), 6.90 (d, J = 3.2 Hz, 1H), 6.00 (d, J = 3.2 Hz, 1H), 5.27 (s, 2H), 4.95 (hept, J = 6.8 Hz, 1H), 1.64 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, Chloroform-d): δ 150.8 (Cq), 137.0 (Cq), 129.9 (CHAr), 128.8 (2 × CHAr), 128.0 (CHAr), 127.8 (2 × CHAr), 126.2 (Cq), 88.7 (CHAr), 52.5 (CH), 50.5 (CH2), 22.3 (2 × CH3). IR (ATR diamond, cm−1) ν: 3030, 2977, 2931, 1518, 1488, 1388, 1242, 1077, 1028, 727, 697. HRMS (EI+) m/z calcd for C14H17N4 [M+H]+: 241.1448, found: 241.1452.
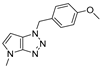
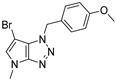
4.2.12. 1-(4-Methoxybenzyl)-4-methyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (16)
The reaction was carried out as described in general procedure A using 4-methoxybenzylamine (206 mg, 1.5 mmol, 3.0 eq.) as amine, 1-methyl-3-pyrrolidinone (50 mg, 0.5 mmol, 1.0 eq.) as pyrrolidinone, 1-Azido-4-nitrobenzene (410.2 mg, 2.5 mmol, 5.0 eq.), acetic acid (9 mg, 0.15 mmol, 0.3 eq.), and 50 mg molecular sieves (3 Å) in anhydrous toluene (0.25 M). The crude mixture was purified by flash chromatography on silica gel using first DCM and then (PE/EtOAc: 80/20) to afford 16 as a yellow oil (58 mg, 48%). Rf = 0.17 (PE/EtOAc: 70/30). 1H NMR (400 MHz, Chloroform-d): δ 7.26 (d, J = 8.6 Hz, 2H), 6.88 (d, J = 8.6 Hz, 2H), 6.76 (d, J = 3.1 Hz, 1H), 5.54 (d, J = 3.1 Hz, 1H), 5.52 (s, 2H), 3.79 (s, 3H), 3.77 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.7 (Cq), 151.0 (Cq), 131.3 (CHAr), 129.8 (2 × CHAr), 127.6 (Cq), 127.0 (Cq), 114.2 (2 × CHAr), 87.7 (CHAr), 55.3 (CH3), 53.1 (CH2), 33.0 (CH3). IR (ATR diamond, cm−1) ν: 2162, 2002, 1612, 1512, 1303, 1222, 1174, 1028, 819, 740, 702. HRMS (EI+) m/z calcd for C13H15N4O [M+H]+: 243.1240, found: 243.1245.
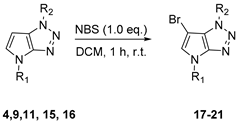
4.3. General Procedure (B) for the Bromination of C-6 Position of 1,4-dihydropyrrolo[2,3-d][1,2,3]triazole Derivatives 17–21
To a solution of corresponding 1,4-dihydropyrrolo[2,3-d][1,2,3]triazole derivative (1.0 eq.) in DCM (0.05 M) was added N-bromosuccinimide (1.0 eq.) and the mixture was stirred 1 h at room temperature. The resulting mixture was quenched using water and phases were separated. The aqueous phase was extracted with DCM and combined organic phases were washed with brine and dried over MgSO4. After being concentrated under vacuum conditions, the residue was purified by flash chromatography on silica gel affording the desired 6-bromo-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole derivative.
4.3.1. 4-Benzyl-6-bromo-1-(4-Methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (17)
The reaction was carried out as described in general procedure B using 4-benzyl-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 4 (67 mg, 0.21 mmol, 1.0 eq.), and NBS (39 mg, 0.21 mmol, 1.0 eq.) in DCM (0.05 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 17 as a beige solid (78 mg, 93%). Rf = 0.55 (PE/EtOAc: 70/30). Mp 88–90 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.38–7.27 (m, 7H), 6.85 (d, J = 8.7 Hz, 2H), 6.82 (s, 1H), 5.57 (s, 2H), 5.20 (s, 2H), 3.78 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.8 (Cq), 150.3 (Cq), 136.2 (Cq), 129.7 (2 × CHAr), 129.4 (CHAr), 129.1 (2 × CHAr), 128.4 (CHAr), 128.1 (2 × CHAr), 127.8 (Cq), 126.1 (Cq), 114.3 (2 × CHAr), 74.7 (Cq), 55.4 (O-CH3), 52.5 (CH2), 51.0 (CH2). IR (ATR diamond, cm−1) ν: 3098, 2934, 2858, 1612, 1463, 1280, 1027, 928, 759, 636. HRMS (EI+) m/z calcd for C19H18BrN4O [M+H]+: 397.0659, found: 397.0655.
4.3.2. 4-Benzyl-6-bromo-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (18)
The reaction was carried out as described in general procedure B using 4-benzyl-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 9 (241 mg, 0.68 mmol, 1.0 eq.), and NBS (130 mg, 0.68 mmol, 1.0 eq.) in DCM (0.05 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 18 as a yellow solid (257 mg, 87%). Rf = 0.70 (PE/EtOAc: 70/30). Mp 93–95 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.60 (d, J = 8.0 Hz, 2H), 7.48 (d, J = 8.0 Hz, 2H), 7.41–7.28 (m, 5H), 6.86 (s, 1H), 5.69 (s, 2H), 5.22 (s, 2H). 13C NMR (101 MHz, Chloroform-d): δ 150.2 (Cq), 139.5 (Cq), 136.0 (Cq), 130.8 (d, J = 32.5 Hz, Cq), 129.7 (CHAr), 129. (2 × CHAr), 128.5 (CHAr), 128.4 (2 × CHAr), 128.1 (2 × CHAr), 126.2 (Cq), 126.0 (q, J = 3.8 Hz, 2 × CHAr), 124.0 (d, J = 272.2 Hz, Cq), 74.5 (Cq), 52.3 (CH2), 51.0 (CH2). 19F NMR (376 MHz, Chloroform-d): δ −62.7. IR (ATR diamond, cm−1) ν: 1323, 1155, 1111, 1066, 789, 776, 726, 698. HRMS (EI+) m/z calcd for C19H15BrF3N4 [M+H]+: 435.0427, found: 435.0423.
4.3.3. 4-Benzyl-6-bromo-1-(thiophen-2-ylmethyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (19)
The reaction was carried out as described in general procedure B using 4-benzyl-1-(thiophen-2-ylmethyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 11 (62 mg, 0.21 mmol, 1.0 eq.), and NBS (39 mg, 0.21 mmol, 1.0 eq.) in DCM (0.05 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 19 as a yellow pale solid (68 mg, 90%). Rf = 0.27 (PE/EtOAc: 80/20). Mp: 96–96 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.38–7.22 (m, 6H), 7.20–7.13 (m, 1H), 6.98–6.93 (m, 1H), 6.84 (s, 1H), 5.81 (s, 2H), 5.20 (s, 2H). 13C NMR (101 MHz, Chloroform-d): δ 150.1 (Cq), 137.6 (Cq), 136.2 (Cq), 129.6 (CHAr), 129.1 (2 × CHAr), 128.5 (CHAr), 128.1 (2 × CHAr), 127.9 (CHAr), 127.2 (CHAr), 126.7 (CHAr), 126.0 (Cq), 74.8 (Cq), 51.0 (CH2), 47.5 (CH2). IR (ATR diamond, cm−1) ν: 3107, 2164, 2015, 1516, 1334, 1180, 1070, 929, 748, 669. HRMS (EI+) m/z calcd for C16H14BrN4S [M+H]+: 373.0117, found: 373.0114.
4.3.4. 4-Benzyl-6-bromo-1-isopropyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (20)
The reaction was carried out as described in general procedure B using 4-benzyl-1-isopropyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 15 (50 mg, 0.21 mmol, 1.0 eq.), and NBS (39 mg, 0.21 mmol, 1.0 eq.) in DCM (0.05 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 20 as an orange oil (48 mg, 70%). Rf = 0.38 (PE/EtOAc: 80/20). 1H NMR (400 MHz, Chloroform-d): δ 7.35–7.25 (m, 5H), 6.87 (s, 1H), 5.20 (s, 2H), 5.04 (hept, J = 6.8 Hz, 1H), 1.67 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, Chloroform-d): δ 150.3 (Cq), 136.2 (Cq), 129.0 (CHAr), 128.9 (2 × CHAr), 128.0 (CHAr), 127.9 (2 × CHAr), 124.9 (Cq), 74.6 (Cq), 52.9 (CH), 50.7 (CH2), 22.8 (2 × CH3). IR (ATR diamond, cm−1) ν: 2978, 1512, 1454, 1344, 1178, 1145, 1126, 1062, 923, 729. HRMS (EI+) m/z calcd for C14H16BrN4 [M+H]+: 319.0553, found: 319.0554.
4.3.5. 6-bromo-1-(4-methoxybenzyl)-4-methyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (21)
The reaction was carried out as described in general procedure B using 1-(4-methoxybenzyl)-4-methyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 16 (51 mg, 0.21 mmol, 1.0 eq.), and NBS (39 mg, 0.21 mmol, 1.0 eq.) in DCM (0.05 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 21 as a white solid (61 mg, 90%). Rf = 0.30 (PE/EtOAc: 70/30). Mp 123–125 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.33 (d, J = 8.6 Hz, 2H), 6.85 (d, J = 8.6 Hz, 2H), 6.82 (s, 1H), 5.57 (s, 2H), 3.77 (s, 3H), 3.76 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.7 (Cq), 150.5 (Cq), 130.5 (CHAr), 129.6 (2 × CHAr), 127.9 (Cq), 126.0 (Cq), 114.3 (2 × CHAr), 73.8 (Cq), 55.4 (CH3), 52.5 (CH2), 33.4 (CH3). IR (ATR diamond, cm−1) ν: 2980, 2160, 1610, 1514, 1300, 1251, 1078, 1028, 948, 819, 750. HRMS (EI+) m/z calcd for C13H14BrN4O [M+H]+: 321.0346, found: 321.0340.
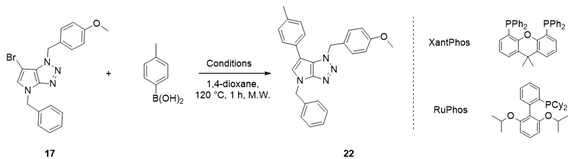
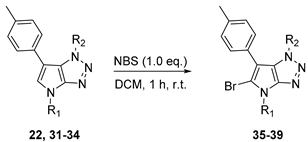
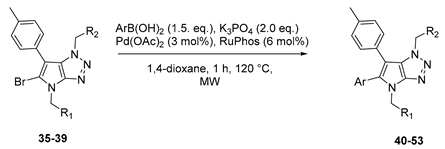
4.4. General Procedure (C): Suzuki–Miyaura Cross-Coupling in C-6 Position of 6-bromo-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole Derivative 22–33
A solution of corresponding 6-bromo-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole derivative (1.0 eq.), potassium phosphate tribasic (2.0 eq.), and corresponding aryl boronic acid (1.5 eq.) in dry 1,4-dioxane (0.15 M) was degassed by argon bubbling for 15 min. Pd (OAc)2 (0.03 eq.) and RuPhos (0.06 eq.) were added and the mixture was heated at 120 °C for 1 h under microwave irradiation. The reaction mixture was filtered through a pad of celite, and the filtrate was reduced to dryness under vacuum. The residue was taken up in DCM, washed with water and dried over MgSO4. After being concentrated under vacuum conditions, the residue was purified by flash chromatography on silica gel affording the desired 6-Arylated 1,4-dihydropyrrolo[2,3-d][1,2,3]triazole derivative.
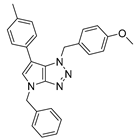
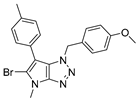
4.4.1. 4-Benzyl-1-(4-methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (22)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 17 (70 mg, 0.18 mmol, 1.0 eq.), p-tolyl boronic acid (37 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd (OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 22 as a white solid (66 mg, 90%). Rf = 0.25 (PE/EtOAc: 70/30). Mp 91–93 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.37 (m, 4H), 7.34–7.29 (m, 1H), 7.21 (d, J = 8.1 Hz, 2H), 7.15 (d, J = 8.1 Hz, 2H), 7.06 (d, J = 8.7 Hz, 2H), 6.97 (s, 1H), 6.75 (d, J = 8.7 Hz, 2H), 5.67 (s, 2H), 5.31 (s, 2H), 3.76 (s, 3H), 2.38 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 151.4 (Cq), 136.8 (Cq), 136.2 (Cq), 130.4 (Cq), 129.5 (2 × CHAr), 129.0 (2 × CHAr), 128.8 (2 × CHAr), 128.2 (Cq), 128.2 (CHAr), 128.0 (2 × CHAr), 127.4 (2 × CHAr), 127.4 (CHAr), 125.8 (Cq), 114.1 (2 × CHAr), 107.2 (Cq), 55.4 (CH3), 53.1 (CH2), 50.7 (CH2), 21.2 (CH3). IR (ATR diamond, cm−1) ν: 2930, 2835, 1611, 1535, 1245, 1174, 1071, 945, 733, 597. HRMS (EI+) m/z calcd for C26H25N4O [M+H]+: 409.2023, found: 409.2021.
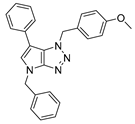
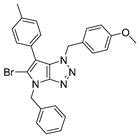
4.4.2. 4-Benzyl-1-(4-methoxybenzyl)-6-phenyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (23)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 17 (70 mg, 0.18 mmol, 1.0 eq.), phenyl boronic acid (33 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd(OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 23 as a white solid (65 mg, 92%). Rf = 0.47 (PE/EtOAc: 70/30). Mp 127–129 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.36–7.20 (m, 10H), 7.01 (d, J = 8.3 Hz, 2H), 6.96 (s, 1H), 6.74 (d, J = 8.3 Hz, 2H), 5.65 (s, 2H), 5.29 (s, 2H), 3.73 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 151.5 (Cq), 136.7 (Cq), 133.4 (Cq), 129.0 (2 × CHAr), 128.9 (2 × CHAr), 128.8 (2 × CHAr), 128.2 (CHAr), 128.2 (Cq), 128.1 (2 × CHAr), 127.5 (3 × CHAr), 126.6 (CHAr), 125.7 (Cq), 114.2 (2 × CHAr), 107.3 (Cq), 55.4 (CH3), 53.1 (CH2), 50.7 (CH2). IR (ATR diamond, cm−1) ν: 2162, 2009, 1512, 1348, 1300, 1251, 1026, 908, 792, 669, 582. HRMS (EI+) m/z calcd for C25H23N4O [M+H]+: 395.1866, found: 395.1865.
4.4.3. 4-Benzyl-1-(4-methoxybenzyl)-6-(4-methoxyphenyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (24)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 17 (70 mg, 0.18 mmol, 1.0 eq.), p-methoxyphenyl boronic acid (41 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd (OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 24 as a beige solid (37 mg, 48%). Rf = 0.37 (PE/EtOAc: 70/30). Mp 87–89 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.38–7.27 (m, 5H), 7.20 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.7 Hz, 2H), 6.89 (s, 1H), 6.86 (d, J = 8.7 Hz, 2H), 6.75 (d, J = 8.7 Hz, 2H), 5.62 (s, 2H), 5.28 (s, 2H), 3.82 (s, 3H), 3.74 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 158.6 (Cq), 151.4 (Cq), 136.9 (Cq), 129.0 (2 × CHAr), 128.8 (2 × CHAr), 128.8 (2 × CHAr), 128.2 (Cq), 128.1 (CHAr), 128.0 (2 × CHAr), 127.1 (CHAr), 125.9 (Cq), 125.8 (Cq), 114.3 (2 × CHAr), 114.1 (2 × CHAr), 106.8 (Cq), 55.5 (CH3), 55.3 (CH3), 53.0 (CH2), 50.6 (CH2). IR (ATR diamond, cm−1) ν: 2924, 2167, 2029, 1514, 1300, 1176, 1028, 948, 835, 785, 736, 675, 592. HRMS (EI+) m/z calcd for C26H25N4O2 [M+H]+: 425.1972, found: 425.1967.
4.4.4. 4-Benzyl-1-(4-methoxybenzyl)-6-(m-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (25)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-Methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 22 (70 mg, 0.18 mmol, 1.0 eq.), m-tolyl boronic acid (37 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd (OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 25 as a yellow oil (54 mg, 73%). Rf = 0.25 (PE/EtOAc: 70/30). 1H NMR (400 MHz, Chloroform-d): δ 7.37–7.27 (m, 5H), 7.24–7.18 (m, 1H), 7.13–7.08 (m, 2H), 7.07–7.01 (m, 3H), 6.97 (s, 1H), 6.79–6.74 (d, J = 8.7 Hz, 2H), 5.66 (s, 2H), 5.30 (s, 2H), 3.74 (s, 3H), 2.31 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 151.5 (Cq), 138.4 (Cq), 136.8 (Cq), 133.2 (Cq), 129.0 (2 × CHAr), 128.7 (CHAr), 128.7 (2 × CHAr), 128.2 (Cq), 128.2 (CHAr), 128.1 (CHAr), 128.0 (2 × CHAr), 127.5 (CHAr), 127.3 (CHAr), 125.7 (Cq), 124.5 (CHAr), 114.2 (2 × CHAr), 107.4 (Cq), 55.4 (CH3), 53.1 (CH2), 50.7 (CH2), 21.5 (CH3). IR (ATR diamond, cm−1) ν: 3034, 2920, 1608,1531, 1506, 1350, 1246, 1029, 914, 779. HRMS (EI+) m/z calcd for C26H25N4O [M+H]+: 409.2023, found: 409.2020.
4.4.5. 4-Benzyl-1-(4-methoxybenzyl)-6-(o-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (26)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-Methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 17 (70 mg, 0.18 mmol, 1.0 eq.), o-tolyl boronic acid (37 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd (OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 26 as a yellow oil (24 mg, 33%). Rf = 0.25 (PE/EtOAc: 70/30). 1H NMR (400 MHz, Chloroform-d): δ 7.35–7.28 (m, 5H), 7.25–7.20 (m, 2H), 7.18–7.12 (m, 1H), 7.10–7.06 (m, 1H), 6.77 (d, J = 8.7 Hz, 2H), 6.75 (s, 1H), 6.61 (d, J = 8.7 Hz, 2H), 5.36 (s, 2H), 5.28 (s, 2H), 3.72 (s, 3H), 2.10 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 149.0 (Cq), 137.1 (Cq), 137.0 (Cq), 132.6 (Cq), 131.1 (CHAr), 130.2 (CHAr), 129.4 (2 × CHAr), 129.0 (2 × CHAr), 128.6 (CHAr), 128.1 (CHAr), 127.9 (2 × CHAr), 127.8 (Cq), 127.6 (CHAr), 126.9 (Cq), 125.7 (CHAr), 113.9 (2 × CHAr), 105.0 (Cq), 55.4 (CH3), 52.9 (CH2), 50.7 (CH2), 20.6 (CH3). IR (ATR diamond, cm−1) ν: 2924, 1610, 1454, 1348, 1246, 1157, 1029, 796, 768. HRMS (EI+) m/z calcd for C26H25N4O [M+H]+: 409.2023, found: 409.2019.
4.4.6. 4-Benzyl-1-(4-methoxybenzyl)-6-(4-(trifluoromethyl)phenyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (28)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 17 (70 mg, 0.18 mmol, 1.0 eq.), 4-(trifluoromethyl)phenyl boronic acid (51 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd (OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 28 as a yellow solid (47 mg, 57%). Rf = 0.42 (PE/EtOAc: 70/30). Mp 111–113 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.53 (d, J = 8.1 Hz, 2H), 7.40–7.29 (m, 7H), 7.04 (s, 1H), 7.00 (d, J = 8.7 Hz, 2H), 6.76 (d, J = 8.7 Hz, 2H), 5.67 (s, 2H), 5.31 (s, 2H), 3.73 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.5 (Cq), 151.7 (Cq), 137.1 (Cq), 136.4 (Cq), 129.1 (2 × CHAr), 128.5 (2 × CHAr), 128.4 (CHAr), 128.2 (Cq), 128.1 (2 × CHAr), 128.0 (CHAr), 127.8 (Cq), 127.3 (2 × CHAr), 125.8 (q, J = 3.8 Hz, 2 × CHAr), 125.4 (Cq), 121.7 (d, J = 272.0 Hz, Cq), 114.3 (2 × CHAr), 106.1 (Cq), 55.4 (CH3), 53.4 (CH2), 50.9 (CH2). 19F NMR (376 MHz, Chloroform-d): δ −62.31. IR (ATR diamond, cm−1) ν: 2931, 2179, 1980, 1612, 1514, 1325, 1251, 1101, 1016, 925, 839, 694. HRMS (EI+) m/z calcd for C26H22F3N4O [M+H]+: 463.1740, found: 463.1741.
4.4.7. 4-Benzyl-1-(4-methoxybenzyl)-6-(thiophen-3-yl)1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (29)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 17 (50 mg, 0.126 mmol, 1.0 eq.), 3-thienyl boronic acid (25 mg, 0.189 mmol, 1.5 eq.), potassium phosphate tribasic (54 mg, 0.253 mmol, 2.0 eq.), Pd (OAc)2 (0.9 mg, 0.004 mmol, 0.03 eq.), and RuPhos (3.5 mg, 0.008 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 29 as a yellow oil (30 mg, 60%). Rf = 0.33 (PE/EtOAc: 70/30). 1H NMR (400 MHz, Chloroform-d): δ 7.40–7.30 (m, 6H), 7.04 (m, 4H), 6.99 (s, 1H), 6.80 (d, J = 8.2 Hz, 2H), 5.68 (s, 2H), 5.30 (s, 2H), 3.76 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 151.2 (Cq), 136.7 (Cq), 133.6 (Cq), 129.0 (2 × CHAr), 128.6 (2 × CHAr), 128.2 (CHAr), 128.1 (Cq), 128.0 (2 × CHAr), 127.7 (CHAr), 127.5 (CHAr), 126.2 (Cq), 125.7 (Cq), 119.9 (CHAr), 114.2 (2 × CHAr), 102.0 (Cq), 55.3 (CH3), 52.9 (CH2), 50.7 (CH2). IR (ATR diamond, cm−1) ν: 2926, 2837, 1961, 1610, 1512, 1340, 1246, 1149, 1029, 848, 794, 648. HRMS (EI+) m/z calcd for C23H20N4OS [M+H]+: 401.1431, found: 401.1433.
4.4.8. 4-(4-Benzyl-1-(4-methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazol-6-yl)phenol (30)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-Methoxybenzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 17 (70 mg, 0.18 mmol, 1.0 eq.), p-hydroxyphenyl boronic acid (37 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd (OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 30 as an orange solid (21 mg, 29%). Rf = 0.20 (PE/EtOAc: 70/30). Mp 175–177 °C. 1H NMR (400 MHz, Acetone-d6) δ 8.31 (bs, 1H), 7.42 (d, J = 7.0 Hz, 2H), 7.38–7.25 (m, 6H), 7.02 (d, J = 8.6 Hz, 2H), 6.85 (d, J = 8.5 Hz, 2H), 6.77 (d, J = 8.6 Hz, 2H), 5.68 (s, 2H), 5.32 (s, 2H), 3.71 (s, 3H). 13C NMR (101 MHz, Acetone-d6): δ 160.3 (Cq), 157.0 (Cq), 152.1 (Cq), 138.7 (Cq), 129.7 (Cq), 129.5 (4 × CHAr), 129.4 (2 × CHAr), 128.7 (2 × CHAr), 128.6 (CHAr), 128.1 (CHAr), 126.3 (Cq), 125.7 (Cq), 116.5 (2 × CHAr), 114.7 (2 × CHAr), 107.6 (Cq), 55.5 (CH3), 53.4 (CH2), 51.0 (CH2). IR (ATR diamond, cm−1) ν: 2952, 2924, 1612, 1512, 1249, 1175, 1029, 839, 779, 657. HRMS (EI+) m/z calcd for C25H23N4O2 [M+H]+: 411.1816, found: 411.1814.
4.4.9. 4-Benzyl-1-(thiophen-2-ylmethyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (31)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(thiophen-2-ylmethyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 19 (203 mg, 0.54 mmol, 1.0 eq.), p-tolyl boronic acid (111 mg, 0.82 mmol, 1.5 eq.), potassium phosphate tribasic (236 mg, 1.09 mmol, 2.0 eq.), Pd (OAc)2 (3.7 mg, 0.02 mmol, 0.03 eq.), and RuPhos (16 mg, 0.03 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 31 as a yellow oil (191 mg, 91%). Rf = 0.18 (PE/EtOAc: 80/20). 1H NMR (400 MHz, Chloroform-d): δ 7.35–7.25 (m, 7H), 7.20–7.15 (m, 3H), 6.96 (s, 1H), 6.86–6.82 (m, 1H), 6.81–6.78 (m, 1H), 5.86 (s, 2H), 5.29 (s, 2H), 2.37 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 151.4 (Cq), 138.3 (Cq), 136.8 (Cq), 136.4 (Cq), 130.4 (Cq), 129.7 (2 × CHAr), 129.0 (2 × CHAr), 128.2 (CHAr), 128.0 (2 × CHAr), 127.5 (CHAr), 127.4 (2 × CHAr), 127.0 (CHAr), 126.9 (CHAr), 126.2 (CHAr), 125.5 (Cq), 107.2 (Cq), 50.7 (CH2), 48.5 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2927, 2156, 1535, 1514, 1435, 1344, 1232, 1180, 929, 852, 792, 748, 619, 578. HRMS (EI+) m/z calcd for C23H21N4S [M+H]+: 385.1481, found: 385.1483.
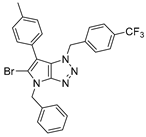
4.4.10. 4-Benzyl-6-(p-tolyl)-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (32)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 18 (100 mg, 0.23 mmol, 1.0 eq.), p-tolyl boronic acid (48 mg, 0.34 mmol, 1.5 eq.), potassium phosphate tribasic (98 mg, 0.46 mmol, 2.0 eq.), Pd (OAc)2 (1.6 mg, 0.007 mmol, 0.03 eq.), and RuPhos (6.5 mg, 0.014 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 32 as a white solid (85 mg, 83%). Rf = 0.36 (PE/EtOAc: 80/20). Mp 143–145 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.48 (d, J = 8.0 Hz, 2H), 7.38–7.28 (m, 5H), 7.18 (d, J = 8.0 Hz, 2H), 7.15–7.07 (m, 4H), 6.96 (s, 1H), 5.74 (s, 2H), 5.29 (s, 2H), 2.34 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 151.4 (Cq), 140.0 (Cq), 136.6 (Cq), 136.5 (Cq), 130.36 (d, J = 32.6 Hz, Cq), 130.1 (Cq), 129.6 (2 × CHAr), 129.0 (2 × CHAr), 128.3 (CHAr), 128.1 (2 × CHAr), 127.8 (2 × CHAr), 127.7 (CHAr), 127.3 (2 × CHAr), 125.9 (Cq), 125.8 (q, J = 3.8 Hz, 2 × CHAr), 124.0 (d, J = 272.2 Hz, Cq), 107.1 (Cq), 53.0 (CH2), 50.8 (CH2), 21.2 (CH3). 19F NMR (376 MHz, Chloroform-d): δ −62.7. IR (ATR diamond, cm−1) ν: 2916, 2848, 2160, 1512, 1325, 1159, 1112, 1066, 1016, 933, 823, 702, 628. HRMS (EI+) m/z calcd for C26H21F3N4 [M+H]+: 447.1791, found: 447.1793.
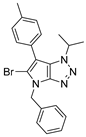
4.4.11. 4-Benzyl-1-isopropyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (33)
The reaction was carried out as described in general procedure C using 4-benzyl-6-bromo-1-isopropyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 20 (130 mg, 0.41 mmol, 1.0 eq.), p-tolyl boronic acid (84 mg, 0.61 mmol, 1.5 eq.), potassium phosphate tribasic (173 mg, 0.82 mmol, 2.0 eq.), Pd (OAc)2 (2.7 mg, 0.012 mmol, 0.03 eq.), and RuPhos (11.4 mg, 0.0024 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 33 as a yellow oil (110 mg, 82%). Rf = 0.23 (PE/EtOAc: 80/20). 1H NMR (400 MHz, Chloroform-d): δ 7.38–7.17 (m, 9H), 6.91 (s, 1H), 5.26 (s, 2H), 4.87 (hept, J = 6.8 Hz, 1H), 2.37 (s, 3H), 1.60 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, Chloroform-d): δ 151.1 (Cq), 136.9 (Cq), 136.3 (Cq), 130.8 (Cq), 129.5 (2 × CHAr), 128.8 (2 × CHAr), 128.0 (CHAr), 128.0 (2 × CHAr), 127.7 (2 × CHAr), 127.2 (CHAr), 125.3 (Cq), 106.9 (Cq), 52.7 (CH), 50.6 (CH2), 22.8 (2 × CH3), 21.2 (CH3). IR (ATR diamond, cm−1) ν: 2981, 2920, 1573, 1535, 1512, 1452, 1348, 1174, 1157, 10101, 1028, 921, 821, 786. HRMS (EI+) m/z calcd for C21H23N4 [M+H]+: 331.1917, found: 331.1915.
4.4.12. 1-(4-Methoxybenzyl)-4-methyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (34)
The reaction was carried out as described in general procedure C using 6-bromo-1-(4-Methoxybenzyl)-4-methyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 21 (58 mg, 0.18 mmol, 1.0 eq.), p-tolyl boronic acid (37 mg, 0.27 mmol, 1.5 eq.), potassium phosphate tribasic (76 mg, 0.36 mmol, 2.0 eq.), Pd (OAc)2 (1.21 mg, 0.0054 mmol, 0.03 eq.), and RuPhos (5 mg, 0.011 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (1000/0) to 70/30) to afford 34 as a white solid (39 mg, 65%). Rf = 0.11 (PE/EtOAc: 70/30). Mp 115–117 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.19 (d, J = 8.1 Hz, 2H), 7.14 (d, J = 8.1 Hz, 2H), 7.01 (d, J = 8.7 Hz, 2H), 6.92 (s, 1H), 6.75 (d, J = 8.7 Hz, 2H), 5.64 (s, 2H), 3.83 (s, 3H), 3.74 (s, 3H), 2.37 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 151.7 (Cq), 136.2 (Cq), 130.5 (Cq), 129.6 (2 × CHAr), 128.7 (2 × CHAr), 128.5 (CHAr), 128.3 (Cq), 127.4 (2 × CHAr), 125.6 (Cq), 114.2 (2 × CHAr), 106.6 (Cq), 55.4 (O-CH3), 53.1 (CH2), 33.2 (N-CH3), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2160, 2015, 1612, 1514, 1249, 1190, 1049, 1018, 815, 754. HRMS (EI+) m/z calcd for C20H21N4O [M+H]+: 333.1710, found: 333.1705.
4.5. General Procedure (D) for the Bromination of C-5 Position of C-6 Arylated 1,4-dihydropyrrolo[2,3-d][1,2,3]triazole Derivatives 35–39
To a solution of corresponding 1,4-dihydropyrrolo[2,3-d][1,2,3]triazole derivative (1.0 eq.) in DCM (0.015 M) was added N-bromosuccinimide (1.0 eq.), and the mixture was stirred 1 h at room temperature. The resulting mixture was quenched using water and phases were separated. The aqueous phase was extracted with DCM and combined organic phases were washed with brine and dried over MgSO4. After being concentrated under vacuum conditions, the residue was purified by flash chromatography on silica gel affording the desired bis 5-bromo-6-Aryl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole derivative.
4.5.1. 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (35)
The reaction was carried out as described in general procedure D using 4-benzyl-1-(4-methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 22 (100 mg, 0.245 mmol, 1.0 eq.) and NBS (47 mg, 0.245 mmol, 1.0 eq.) in DCM (0.015 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 35 as a white solid (100 mg, 86%). Rf = 0.28 (PE/EtOAc: 70/30). Mp 127–129 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.43–7.36 (m, 2H), 7.36–7.24 (m, 3H), 7.20 (s, 4H), 6.86 (d, J = 8.5 Hz, 2H), 6.68 (d, J = 8.5 Hz, 2H), 5.45 (s, 2H), 5.40 (s, 2H), 3.73 (s, 3H), 2.41 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 149.2 (Cq), 137.4 (Cq), 136.6 (Cq), 129.8 (2 × CHAr), 129.3 (2 × CHAr), 129.2 (2 × CHAr), 129.0 (Cq), 128.9 (2 × CHAr), 128.1 (2 × CHAr), 128.0 (CHAr), 127.7 (Cq), 125.2 (Cq), 114.1 (2 × CHAr), 113.5 (Cq), 106.8 (Cq), 55.4 (CH3), 52.8 (CH2), 49.4 (CH2), 21.4 (CH3). IR (ATR diamond, cm−1) ν: 2996, 1610, 1537, 1513, 1337, 1243, 1179, 833, 799. HRMS (EI+) m/z calcd for C26H24BrN4O [M+H]+: 487.1128, found: 487.1126.
4.5.2. 4-Benzyl-5-bromo-6-(p-tolyl)-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3] triazole (36)
The reaction was carried out as described in general procedure D using 4-benzyl-6-(p-tolyl)-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 32 (85 mg, 0.19 mmol, 1.0 eq.), and NBS (36 mg, 0.19 mmol, 1.0 eq.) in DCM (0.015 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 36 as a white solid (80 mg, 80%). Rf = 0.45 (PE/EtOAc: 80/20). Mp 157–159 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.45–7.38 (m, 4H), 7.37–7.28 (m, 3H), 7.17 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 7.02 (d, J = 8.0 Hz, 2H), 5.57 (s, 2H), 5.42 (s, 2H), 2.40 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 149.1 (Cq), 139.4 (Cq), 137.6 (Cq), 136.4 (Cq), 130.43 (d, J = 32.5 Hz, Cq), 129.7 (2 × CHAr), 129.4 (2 × CHAr), 128.9 (2 × CHAr), 128.8 (Cq), 128.1 (CHAr), 128.1 (2 × CHAr), 128.0, (2 × CHAr), 125.7 (q, J = 3.7 Hz, 2 × CHAr), 125.4 (Cq), 124.0 (d, J = 271.6 Hz, Cq), 113.9 (Cq), 106.7 (Cq), 52.7 (CH2), 49.5 (CH2), 21.4 (CH3). 19F NMR (376 MHz, Chloroform-d): δ −62.7. IR (ATR diamond, cm−1) ν: 2925, 2175, 1927, 1514, 1465, 1421, 1323, 1190, 933, 825, 792, 721. HRMS (EI+) m/z calcd for C26H20BrF3N4 [M+H]+: 525.0896, found: 525.0894.
4.5.3. 4-Benzyl-5-bromo-1-(thiophen-2-ylmethyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (37)
The reaction was carried out as described in general procedure D using 4-Benzyl-1-(thiophen-2-ylmethyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 31 (160 mg, 0.41 mmol, 1.0 eq.), and NBS (78 mg, 0.41 mmol, 1.0 eq.) in DCM (0.015 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 37 as a white solid (170 mg, 90%). Rf = 0.22 (PE/EtOAc: 90/10). Mp 128–130 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.42–7.36 (m, 2H), 7.34–7.23 (m, 7H), 7.16–7.13 (m, 1H), 6.83–6.74 (m, 1H), 6.63–6.58 (m, 1H), 5.69 (s, 2H), 5.41 (s, 2H), 2.42 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 149.1 (Cq), 137.6 (Cq), 137.5 (Cq), 136.5 (Cq), 129.7 (2 × CHAr), 129.5 (2 × CHAr), 129.0 (Cq), 128.9 (2 × CHAr), 128.1 (3 × CHAr), 127.2 (CHAr), 126.9 (CHAr), 126.4 (CHAr), 125.0 (Cq), 113.6 (Cq), 106.8 (Cq), 49.5 (CH2), 47.9 (CH2), 21.4 (CH3). IR (ATR diamond, cm−1) ν: 2162, 1980, 1514, 1460, 1334, 1182, 1056, 1029, 906, 831, 740, 665, 611, 590. HRMS (EI+) m/z calcd for C23H20BrN4S [M+H]+: 463.0587, found: 463.0590.
4.5.4. 4-Benzyl-5-bromo-1-isopropyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (38)
The reaction was carried out as described in general procedure D using 4-Benzyl-1-isopropyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 33 (90 mg, 0.27 mmol, 1.0 eq.), and NBS (52 mg, 0.27 mmol, 1.0 eq.) in DCM (0.015 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 38 as a white solid (100 mg, 90%). Rf = 0.51 (PE/EtOAc: 80/20). Mp 127–129 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.48–7.42 (m, 2H), 7.38–7.26 (m, 7H), 5.45 (s, 2H), 4.74 (p, J = 6.7 Hz, 1H), 2.44 (s, 3H), 1.50 (d, J = 6.7 Hz, 6H). 13C NMR (101 MHz, Chloroform-d): δ 148.9 (Cq), 137.5 (Cq), 136.7 (Cq), 130.0 (2 × CHAr), 129.6 (Cq), 129.4 (2 × CHAr), 128.8 (2 × CHAr), 128.1 (2 × CHAr), 128.0 (CHAr), 124.7 (Cq), 113.4 (Cq), 106.6 (Cq), 52.7 (CH3), 49.4 (CH2), 22.6 (2 × CH3), 21.4 (CH3). IR (ATR diamond, cm−1) ν: 3058, 2988, 1509, 1454, 1341, 1156, 1109, 1066, 788, 711. HRMS (EI+) m/z calcd for C21H22BrN4 [M+H]+: 409.1022, found: 409.1024.
4.5.5. 5-bromo-1-(4-Methoxybenzyl)-4-methyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (39)
The reaction was carried out as described in general procedure D using 1-(4-Methoxybenzyl)-4-methyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 34 (60 mg, 0.18 mmol, 1.0 eq.) and NBS (34 mg, 0.18 mmol, 1.0 eq.) in DCM (0.015 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 39 as a white solid (70 mg, 95%). Rf = 0.33 (PE/EtOAc: 70/30). Mp 153–155 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.24–7.14 (m, 4H), 6.84 (d, J = 8.7 Hz, 2H), 6.68 (d, J = 8.7 Hz, 2H), 5.45 (s, 2H), 3.83 (s, 3H), 3.73 (s, 3H), 2.42 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 149.5 (Cq), 137.3 (Cq), 129.7 (2 × CHAr), 129.3 (2 × CHAr), 129.1 (Cq), 129.0 (2 × CHAr), 127.8 (Cq), 124.9 (Cq), 114.2 (Cq), 114.1 (2 × CHAr), 106.3 (Cq), 55.4 (O-CH3), 52.8 (CH2), 32.4 (N-CH3), 21.4 (CH3). IR (ATR diamond, cm−1) ν: 2160, 2015, 1612, 1514, 1249, 1190, 1049, 1018, 815, 764, 754. HRMS (EI+) m/z calcd for C20H20BrN4O [M+H]+: 411.0815, found: 411.0813.
4.6. General Procedure (E): Suzuki–Miyaura Cross-Coupling in C-5 Position of 5-bromo-6-aryl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole Derivative 40–53
A solution of corresponding 5-bromo-6-aryl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole derivative (1.0 eq.), potassium phosphate tribasic (2.0 eq.), and corresponding aryl boronic acid (1.5 eq.) in dry 1,4-dioxane (0.15 M) was degassed by argon bubbling for 15 min. Pd (OAc)2 (0.03 eq.) and RuPhos (0.06 eq.) were added and the mixture was heated at 120 °C for 1 h under microwave irradiation. The reaction mixture was filtered through a pad of celite, and the filtrate was reduced to dryness under vacuum. The residue was taken up in DCM, washed with water and dried over MgSO4. After being concentrated under vacuum conditions, the residue was purified by flash chromatography on silica gel affording the desired 5,6-arylated 1,4-dihydropyrrolo[2,3-d][1,2,3] triazole derivative.
4.6.1. 4-Benzyl-1-(4-methoxybenzyl)-5,6-di-p-tolyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (40)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (80 mg, 0.16 mmol, 1.0 eq.), p-tolyl boronic acid (34 mg, 0.25 mmol, 1.5 eq.), potassium phosphate tribasic (70 mg, 0.33 mmol, 2.0 eq.), Pd (OAc)2 (1.1 mg, 0.005 mmol, 0.03 eq.), and RuPhos (4.6 mg, 0.010 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 40 as a white solid (61 mg, 75%). Rf = 0.23 (PE/EtOAc: 70/30). Mp 125–127 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.25–7.17 (m, 3H), 7.13–7.03 (m, 6H), 7.00 (d, J = 7.8 Hz, 2H), 6.95–6.90 (m, 4H), 6.70 (d, J = 8.2 Hz, 2H), 5.52 (s, 2H), 5.19 (s, 2H), 3.72 (s, 3H), 2.33 (s, 3H), 2.30 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.3 (Cq), 140.4 (Cq), 138.4 (Cq), 137.7 (Cq), 135.9 (Cq), 131.1 (2 × CHAr), 130.3 (Cq), 129.8 (2 × CHAr), 129.2 (2 × CHAr), 129.1 (2 × CHAr), 129.0 (2 × CHAr), 128.6 (2 × CHAr), 128.3 (Cq), 128.0 (Cq), 127.6 (2 × CHAr), 127.5 (CHAr), 126.7 (Cq), 114.0 (2 × CHAr), 104.6 (Cq), 55.3 (CH3), 52.6 (CH2), 48.3 (CH2), 21.5 (CH3), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 3028, 2927, 1509, 1513, 1329, 1244, 1179, 1049, 948, 924, 754. HRMS (EI+) m/z calcd for C33H31N4O [M+H]+: 499.2492, found: 499.247.
4.6.2. 4-Benzyl-1-(4-methoxybenzyl)-5-(m-tolyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (41)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), m-tolyl boronic acid (17 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd (OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 41 as a white solid (40 mg, 97%). Rf = 0.35 (PE/EtOAc: 70/30). Mp 147–149 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.28–7.12 (m, 7H), 7.02 (d, J = 7.9 Hz, 2H), 7.00–6.92 (m, 6H), 6.74 (d, J = 8.6 Hz, 2H), 5.55 (s, 2H), 5.21 (s, 2H), 3.76 (s, 3H), 2.33 (s, 3H), 2.27 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.3 (Cq), 151.3 (Cq), 140.5 (Cq), 138.0 (Cq), 137.8 (Cq), 135.9 (Cq), 131.9 (CHAr), 130.9 (Cq), 130.3 (Cq), 129.8 (2 × CHAr), 129.3 (CHAr), 129.2 (2 × CHAr), 129.0 (2 × CHAr), 128.6 (2 × CHAr), 128.5 (CHAr), 128.3 (CHAr), 128.3 (Cq), 127.7 (2 × CHAr), 127.6 (CHAr), 126.7 (Cq), 114.0 (2 × CHAr), 104.7 (Cq), 55.4 (CH3), 52.7 (CH2), 48.5 (CH2), 21.5 (CH3), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2008, 1513, 1328, 1189, 1072, 983, 926, 783, 737, 589. HRMS (EI+) m/z calcd for C33H31N4O [M+H]+: 499.2492, found: 499.2495.
4.6.3. 4-Benzyl-1-(4-methoxybenzyl)-5-(o-tolyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (42)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), o-tolyl boronic acid (17 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd (OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 42 as a white solid (36 mg, 88%). Rf = 0.45 (PE/EtOAc: 80/20). Mp 102–104 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.31–7.24 (m, 1H), 7.23–7.08 (m, 6H), 7.03–6.92 (m, 6H), 6.88 (d, J = 7.8 Hz, 2H), 6.72 (d, J = 8.3 Hz, 2H), 5.56 (q, ABX, 2H), 5.05 (s, 2H), 3.73 (s, 3H), 2.27 (s, 3H), 1.70 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.3 (Cq), 151.0 (Cq), 139.1 (Cq), 138.9 (Cq), 137.3 (Cq), 135.8 (Cq), 132.1 (CHAr), 130.5 (Cq), 130.4 (Cq), 130.3 (CHAr), 129.3 (CHAr), 129.2 (2 × CHAr), 129.1 (2 × CHAr), 129.0 (2 × CHAr), 128.5 (2 × CHAr), 128.2 (Cq), 128.1 (2 × CHAr), 127.6 (CHAr), 126.4 (Cq), 125.7 (CHAr), 114.0 (2 × CHAr), 105.1 (Cq), 55.3 (CH3), 52.7 (CH2), 48.2 (CH2), 21.2 (CH3), 19.7 (CH3). IR (ATR diamond, cm−1) ν: 2162, 2046, 1992, 1517, 1436, 1253, 1186, 1031, 815, 698, 553. HRMS (EI+) m/z calcd for C33H31N4O [M+H]+: 499.2492, found: 499.2491.
4.6.4. 4-(Benzyl-1-(4-Methoxybenzyl)-5-phenyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (43)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), phenyl boronic acid (15 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd (OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 43 as a white solid (30 mg, 76%). Rf = 0.30 (PE/EtOAc: 70/30). Mp 166–168 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.36–7.28 (m, 3H), 7.27–7.17 (m, 5H), 7.13–7.07 (m, 2H), 7.03 (d, J = 7.8 Hz, 2H), 7.00–6.92 (m, 4H), 6.74 (d, J = 8.4 Hz, 2H), 5.56 (s, 2H), 5.23 (s, 2H), 3.76 (s, 3H), 2.33 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.3 (Cq), 151.3 (Cq), 140.2 (Cq), 137.6 (Cq), 136.0 (Cq), 131.3 (2 × CHAr), 131.0 (Cq), 130.2 (Cq), 129.8 (2 × CHAr), 129.2 (2 × CHAr), 129.0 (2 × CHAr), 128.6 (2 × CHAr), 128.5 (CHAr), 128.5 (2 × CHAr), 128.2 (Cq), 127.6 (2 × CHAr), 127.5 (CHAr), 126.7 (Cq), 114.0 (2 × CHAr), 104.9 (Cq), 55.4 (CH3), 52.7 (CH2), 48.4 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2960, 2160, 1610, 1514, 1354, 1249, 1193, 1028, 918, 771, 736. HRMS (EI+) m/z calcd for C32H29N4O [M+H]+: 485.2336, found: 485.2367.
4.6.5. 4-(Benzyl-1-(4-methoxybenzyl)-5-(4-methoxyphenyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (44)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), 4-methoxyphenyl boronic acid (19 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd (OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 44 as a white solid (35 mg, 83%). Rf = 0.17 (PE/EtOAc: 80/20). Mp 159–161 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.27–7.20 (m, 3H), 7.14–7.05 (m, 4H), 7.01 (d, J = 7.8 Hz, 2H), 6.98–6.91 (m, 4H), 6.82 (d, J = 8.3 Hz, 2H), 6.72 (d, J = 8.2 Hz, 2H), 5.52 (s, 2H), 5.19 (s, 2H), 3.80 (s, 3H), 3.73 (s, 3H), 2.31 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.7 (Cq), 159.3 (Cq), 151.1 (Cq), 140.2 (Cq), 137.8 (Cq), 135.8 (Cq), 132.5 (2 × CHAr), 130.4 (Cq), 129.8 (2 × CHAr), 129.2 (2 × CHAr), 129.0 (2 × CHAr), 128.6 (2 × CHAr), 128.3 (Cq), 127.6 (2 × CHAr), 127.5 (CHAr), 126.7 (Cq), 123.2 (Cq), 114.0 (2 × CHAr), 113.9 (2 × CHAr), 104.6 (Cq), 55.3 (2 × CH3), 52.6 (CH2), 48.3 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2918, 2162, 2015, 1514, 1431, 1178, 1026, 815, 777, 731, 698. HRMS (EI+) m/z calcd for C33H31N4O [M+H]+: 515.2441, found: 515.2443.
4.6.6. 4-(Benzyl-1-(4-methoxybenzyl)-6-(p-tolyl)- 5-(4-(trifluoromethyl)phenyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (45)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), 4-(trifluoromethyl)phenyl boronic acid (24 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd (OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 45 as a white solid (40 mg, 88%). Rf = 0.16 (PE/EtOAc: 80/20). Mp 135–137 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.53 (d, J = 8.0 Hz, 2H), 7.29–7.20 (m, 5H), 7.08–7.01 (m, 4H), 6.95–6.87 (m, 4H), 6.70 (d, J = 8.2 Hz, 2H), 5.52 (s, 2H), 5.21 (s, 2H), 3.73 (s, 3H), 2.33 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.4 (Cq), 151.7 (Cq), 138.4 (Cq), 137.3 (Cq), 136.6 (Cq), 134.8 (Cq), 131.5 (2 × CHAr), 130.4 (d, J = 32.6 Hz, Cq), 129.9 (2 × CHAr), 129.6 (Cq), 129.3 (2 × CHAr), 129.2 (2 × CHAr), 128.8 (2 × CHAr), 128.0 (Cq), 127.8 (CHAr), 127.4 (2 × CHAr), 126.6 (Cq), 125.4 (q, J = 3.5 Hz, Cq), 123.88 (d, J = 234.4 Hz, 2 × CHAr), 114.0 (2 × CHAr), 105.8 (Cq), 55.4 (CH3), 52.7 (CH2), 48.7 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2929, 2158, 1977, 1612, 1512, 1317, 1163, 1058, 817, 669. HRMS (EI+) m/z calcd for C33H28F3N4O [M+H]+: 553.2210, found: 553.2211.
4.6.7. 4-(Benzyl-1-(4-methoxybenzyl)-5-(4-nitrophenyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (46)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), 4-nitrophenyl boronic acid (21 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd (OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.8 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 46 as a white solid (21 mg, 48%). Rf = 0.19 (PE/EtOAc: 80/20). Mp 77–79 °C. 1H NMR (400 MHz, Chloroform-d): δ 8.13 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 7.25–7.24 (m, 3H), 7.10–7.04 (m, 4H), 6.91 (m, 4H), 6.73 (d, J = 8.2 Hz, 2H), 5.54 (s, 2H), 5.28 (s, 2H), 3.76 (s, 3H), 2.36 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.5 (Cq), 152.2 (Cq), 147.4 (Cq), 137.8 (Cq), 137.5 (Cq), 137.1 (Cq), 137.0 (Cq), 131.9 (2 × CHAr), 130.0 (2 × CHAr), 129.5 (2 × CHAr), 129.2 (2 × CHAr), 129.2 (Cq), 128.9 (2 × CHAr), 128.0 (CHAr), 127.8 (2 × Cq), 127.2 (2 × CHAr), 126.7 (Cq), 123.7 (2 × CHAr), 114.1 (2 × CHAr), 106.7 (Cq), 55.4 (CH3), 52.8 (CH2), 48.9 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2922, 2850, 2160, 1598, 1344, 1246, 1176, 1029, 802. HRMS (EI+) m/z calcd for C33H28N5O3 [M+H]+: 530.2187, found: 530.2186.
4.6.8. 4-(Benzyl-1-(4-methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazol-5-yl)phenol (47)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), 4-hydroxyphenyl boronic acid (17 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd(OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 47 as a white solid (20 mg, 49%). Rf = 0.08 (PE/EtOAc: 80/20). Mp 195–197 °C. 1H NMR (400 MHz, Acetone-d6): δ 8.69 (bs, 1H), 7.26–7.17 (m, 3H), 7.13–7.02 (m, 8H), 6.90 (d, J = 8.3 Hz, 2H), 6.83 (d, J = 8.2 Hz, 2H), 6.73 (d, J = 8.3 Hz, 2H), 5.55 (s, 2H), 5.22 (s, 2H), 3.71 (s, 3H), 2.29 (s, 3H). 13C NMR (101 MHz, Acetone-d6): δ 160.3 (Cq), 158.7 (Cq), 151.7 (Cq), 141.0 (Cq), 139.0 (Cq), 136.4 (Cq), 133.4 (2 × CHAr), 131.5 (Cq), 130.6 (2 × CHAr), 129.8 (2 × CHAr), 129.7 (2 × CHAr), 129.5 (Cq), 129. (2 × CHAr), 128.2 (CHAr), 128.1 (2 × CHAr), 127.1 (Cq), 122.8 (Cq), 116.2 (2 × CHAr), 114.6 (2 × CHAr), 105.2 (Cq), 55.5 (CH3), 53.0 (CH2), 48.6 (CH2), 21.1 (CH3). IR (ATR diamond, cm−1) ν: 2177, 2031, 1971, 1612, 1454, 1064, 1026, 927, 794, 759, 723, 696. HRMS (EI+) m/z calculated for C32H29N4O2 [M+H]+: 501.2285, found: 501.2285.
4.6.9. 4-Benzyl-1-(4-methoxybenzyl)-5-(4-((tetrahydro-2H-pyran-2-yl)oxy)phenyl)6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazol-5-yl) (48)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), 4-(2-tetrahydropyranyloxy)phenylboronic acid (27 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd(OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 48 as a white solid (46 mg, 96%). Rf = 0.10 (PE/EtOAc: 80/20). Mp 167–169 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.28–7.17 (m, 3H), 7.17–7.04 (m, 4H), 7.04–6.89 (m, 8H), 6.71 (d, J = 8.3 Hz, 2H), 5.52 (s, 2H), 5.39 (m, 1H), 5.19 (s, 2H), 3.92 (m, 1H), 3.73 (s, 3H), 3.62 (m, 1H), 2.31 (s, 3H), 1.99 (m, 1H), 1.86 (m, 2H), 1.71–1.57 (m, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.3 (Cq), 157.3 (Cq), 151.0 (Cq), 140.2 (Cq), 137.7 (Cq), 135.8 (Cq), 132.4 (2 × CHAr), 130.3 (Cq), 129.8 (2 × CHAr), 129.1 (2 × CHAr), 129.0 (2 × CHAr), 128.6 (2 × CHAr), 128.3 (Cq), 127.6 (2 × CHAr), 127.5 (CHAr), 126.7 (Cq), 124.0 (Cq), 116.3 (2 × CHAr), 114.0 (2 × CHAr), 104.6 (Cq), 96.6 (CH), 62.5 (CH2), 55.3 (CH3), 52.6 (CH2), 48.3 (CH2), 30.5 (CH2), 25.2 (CH2), 21.3 (CH3), 19.0 (CH2). IR (ATR diamond, cm−1) ν: 2941, 2166, 1608, 1514, 1467, 1244, 1172, 1020, 918, 817. HRMS (EI+) m/z calcd for C37H37N4O3 [M+H]+: 585.2860, found: 585.2862.
4.6.10. 4-Benzyl-1-(4-methoxybenzyl)-5-(thiophen-3-yl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3] triazole (49)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(4-Methoxybenzyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 35 (40 mg, 0.08 mmol, 1.0 eq.), 3-thienyl boronic acid (16 mg, 0.12 mmol, 1.5 eq.), potassium phosphate tribasic (35 mg, 0.16 mmol, 2.0 eq.), Pd(OAc)2 (0.67 mg, 0.003 mmol, 0.03 eq.), and RuPhos (2.80 mg, 0.006 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 49 as a white solid (22 mg, 55%). Rf = 0.27 (PE/EtOAc: 80/20). Mp 121–123 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.28–7.20 (m, 4H), 7.13 (d, J = 7.2 Hz, 2H), 7.05 (m, 3H), 6.97–6.90 (m, 4H), 6.81 (d, J = 5.0 Hz, 1H), 6.71 (d, J = 8.3 Hz, 2H), 5.52 (s, 2H), 5.25 (s, 2H), 3.73 (s, 3H), 2.33 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.3 (Cq), 151.2 (Cq), 137.8 (Cq), 136.2 (Cq), 135.0 (Cq), 131.2 (Cq), 130.2 (Cq), 129.7 (2 × CHAr), 129.5 (CHAr), 129.1 (2 × CHAr), 129.1 (2 × CHAr), 128.7 (2 × CHAr), 128.2 (Cq), 127.6 (CHAr), 127.4 (2 × CHAr), 126.5 (CHAr), 126.5 (Cq), 125.8 (CHAr), 114.0 (2 × CHAr), 105.2 (Cq), 55.3 (CH3), 52.7 (CH2), 48.4 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1): δ 159.3 (Cq), 151.2 (Cq), 137.8 (Cq), 136.2 (Cq), 135.0 (Cq), 131.2 (Cq), 130.2 (Cq), 129.7 (2 × CHAr), 129.5 (CHAr), 129.1 (2 × CHAr), 129.1 (2 × CHAr), 128.7 (2 × CHAr), 128.2 (Cq), 127.6 (CHAr), 127.4 (2 × CHAr), 126.5 (CHAr), 126.5 (Cq), 125.8 (CHAr), 114.0 (2 × CHAr), 105.2 (Cq), 55.3 (CH3), 52.7 (CH2), 48.4 (CH2), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2929, 2158, 1982, 1612, 1514, 1327, 1249, 1182, 1029, 788, 752, 696, 640. HRMS (EI+) m/z calcd for C30H27N4OS [M+H]+: 491.1900, found: 491.1803.
4.6.11. 4-Benzyl-1-(thiophen-2-ylmethyl)-5,6-di-p-tolyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (50)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-(thiophen-2-ylmethyl)-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 37 (100 mg, 0.22 mmol, 1.0 eq.), p-tolyl boronic acid (46 mg, 0.33 mmol, 1.5 eq.), potassium phosphate tribasic (94 mg, 0.44 mmol, 2.0 eq.), Pd(OAc)2 (1.5 mg, 0.007 mmol, 0.03 eq.), and RuPhos (6.2 mg, 0.013 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 50 as a white solid (100 mg, 96%). Rf = 0.33 (PE/EtOAc: 80/20). Mp 158–160 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.25–6.99 (m, 14H), 6.83–6.78 (m, 1H), 6.66–6.63 (m, 1H), 5.76 (s, 2H), 5.19 (s, 2H), 2.35 (s, 3H), 2.32 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 151.1 (Cq), 140.6 (Cq), 138.5 (Cq), 138.3 (Cq), 137.7 (Cq), 136.0 (Cq), 131.1 (2 × CHAr), 130.4 (Cq), 129.7 (2 × CHAr), 129.3 (2 × CHAr), 129.2 (2 × CHAr), 128.6 (2 × CHAr), 127.9 (Cq), 127.6 (2 × CHAr), 127.6 (CHAr), 127.1 (CHAr), 126.9 (CHAr), 126.5 (Cq), 126.2 (CHAr), 104.7 (Cq), 48.4 (CH2), 47.8 (CH2), 21.5 (CH3), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2978, 1512, 1496, 1454, 1344, 1178, 1145, 1126, 1062, 923, 729, 680. HRMS (EI+) m/z calcd for C30H27N4S [M+H]+: 475.1951, found: 475.1955.
4.6.12. 4-Benzyl-5,6-di-p-tolyl-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (51)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-6-(p-tolyl)-1-(4-(trifluoromethyl)benzyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 36 (60 mg, 0.114 mmol, 1.0 eq.), p-tolyl boronic acid (24 mg, 0.171 mmol, 1.5 eq.), potassium phosphate tribasic (49 mg, 0.228 mmol, 2.0 eq.), Pd(OAc)2 (0.8 mg, 0.003 mmol, 0.03 eq.), and RuPhos (3.2 mg, 0.007 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 51 as a white solid (41 mg, 70%). Rf = 0.36 (PE/EtOAc: 70/30). Mp 177–179 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.43 (d, J = 8.0 Hz, 2H), 7.25–7.18 (m, 3H), 7.09 (m, 8H, H-9), 6.97 (d, J = 7.8 Hz, 2H), 6.86 (d, J = 7.8 Hz, 2H), 5.63 (s, 2H), 5.21 (s, 2H), 2.34 (s, 3H), 2.29 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 151.2 (Cq), 140.8 (Cq), 140.0 (Cq), 138.6 (Cq), 137.6 (Cq), 136.1 (Cq), 131.1 (2 × CHAr), 130.1 (Cq), 129.9 (d, J = 29.6 Hz, Cq), 129.7 (2 × CHAr), 129.3 (2 × CHAr), 129.1 (2 × CHAr), 128.7 (2 × CHAr), 128.0 (2 × CHAr), 127.8 (Cq), 127.6 (3 × CHAr), 126.9 (Cq), 125.6 (q, J = 3.7 Hz, 2 × CHAr), 124.1 (d, J = 272.1 Hz, Cq), 104.6 (Cq), 52.6 (CH2), 48.4 (CH2), 21.5 (CH3), 21.2 (CH3). 19F NMR (376 MHz, Chloroform-d): δ −62.7. IR (ATR diamond, cm−1) ν: 2196, 2025, 1514, 1323, 1122, 1066, 931, 794, 678, 586. HRMS (EI+) m/z calcd for C33H27F3N4 [M+H]+: 537.2260, found: 537.2262.
4.6.13. 4-Benzyl-1-isopropyl-5,6-di-p-tolyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (52)
The reaction was carried out as described in general procedure E using 4-Benzyl-5-bromo-1-isopropyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 38 (70 mg, 0.171 mmol, 1.0 eq.), p-tolyl boronic acid (35 mg, 0.257 mmol, 1.5 eq.), potassium phosphate tribasic (73 mg, 0.342 mmol, 2.0 eq.), Pd(OAc)2 (1.2 mg, 0.005 mmol, 0.03 eq.), and RuPhos (4.8 mg, 0.01 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (80/20) to afford 52 as a white solid (56 mg, 78%). Rf = 0.51 (PE/EtOAc: 80/20). Mp 137–139 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.26–7.01 (m, 13H), 5.20 (s, 2H), 4.78 (p, J = 6.7 Hz, 1H), 2.34 (s, 3H), 2.30 (s, 3H), 1.52 (d, J = 6.7 Hz, 6H). 13C NMR (101 MHz, Chloroform-d): δ 150.9 (Cq), 140.3 (Cq), 138.3 (Cq), 137.8 (Cq), 136.0 (Cq), 131.2 (2 × CHAr), 130.8 (Cq), 130.0 (2 × CHAr), 129.2 (2 × CHAr), 129.1 (2 × CHAr), 128.6 (2 × CHAr), 128.1 (Cq), 127.7 (2 × CHAr), 127.5 (CHAr), 126.3 (Cq), 104.6 (Cq), 52.4 (CH), 48.4 (CH2), 22.7 (2 × CH3), 21.5 (CH3), 21.3 (CH3). IR (ATR diamond, cm−1): δ 150.9 (Cq), 140.3 (Cq), 138.3 (Cq), 137.8 (Cq), 136.0 (Cq), 131.2 (2 × CHAr), 130.8 (Cq), 130.0 (2 × CHAr), 129.2 (2 × CHAr), 129.1 (2 × CHAr), 128.6 (2 × CHAr), 128.1 (Cq), 127.7 (2 × CHAr), 127.5 (CHAr), 126.3 (Cq), 104.6 (Cq), 52.4 (CH), 48.4 (CH2), 22.7 (2 × CH3), 21.5 (CH3), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2980, 2160, 1514, 1350, 1199, 1165, 1103, 952, 845, 777, 669. HRMS (EI+) m/z calcd for C28H29N4 [M+H]+: 421.2387, found: 421.2390.
4.6.14. 1-(4-Methoxybenzyl)-4-methyl-5,6-di-p-tolyl-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole (53)
The reaction was carried out as described in general procedure E using 5-bomo-1-(4-Methoxybenzyl)-4-methyl-6-(p-tolyl)-1,4-dihydropyrrolo[2,3-d][1,2,3]triazole 39 (50 mg, 0.12 mmol, 1.0 eq.), p-tolyl boronic acid (25 mg, 0.18 mmol, 1.5 eq.), potassium phosphate tribasic (52 mg, 0.24 mmol, 2.0 eq.), Pd(OAc)2 (0.8 mg, 0.004 mmol, 0.03 eq.), and RuPhos (3.3 mg, 0.007 mmol, 0.06 eq.) in dry 1,4-dioxane (0.15 M). The crude mixture was purified by flash chromatography on silica gel using (PE/EtOAc) from (100/0) to (70/30) to afford 53 as a white solid (48 mg, 95%). Rf = 0.33 (PE/EtOAc: 70/30). Mp 179–181 °C. 1H NMR (400 MHz, Chloroform-d): δ 7.18–7.10 (m, 4H), 7.01 (d, J = 7.9 Hz, 2H), 6.96–6.88 (m, 4H), 6.71 (d, J = 8.7 Hz, 2H), 5.52 (s, 2H), 3.73 (s, 3H), 3.67 (s, 3H), 2.36 (s, 3H), 2.32 (s, 3H). 13C NMR (101 MHz, Chloroform-d): δ 159.3 (Cq), 140.4 (Cq), 138.3 (Cq), 135.9 (Cq), 130.9 (2 × CHAr), 130.5 (Cq), 129.9 (2 × CHAr), 129.3 (2 × CHAr), 129.1 (4 × CHAr), 128.3 (Cq), 128.0 (Cq), 126.4 (Cq), 114.0 (2 × CHAr), 55.4 (CH3), 52.6 (CH2), 31.6 (CH3), 21.5 (CH3), 21.3 (CH3). IR (ATR diamond, cm−1) ν: 2922, 1610, 1512, 1390, 1247, 1176, 1033, 1020, 775, 682. HRMS (EI+) m/z calcd for C27H27N4O [M+H]+: 423.2179, found: 423.2178.