Covalent Modification of Iron Phthalocyanine into Skeleton of Graphitic Carbon Nitride and Its Visible-Light-Driven Photocatalytic Reduction of Nitroaromatic Compounds
Abstract
:1. Introduction
2. Experimental Section
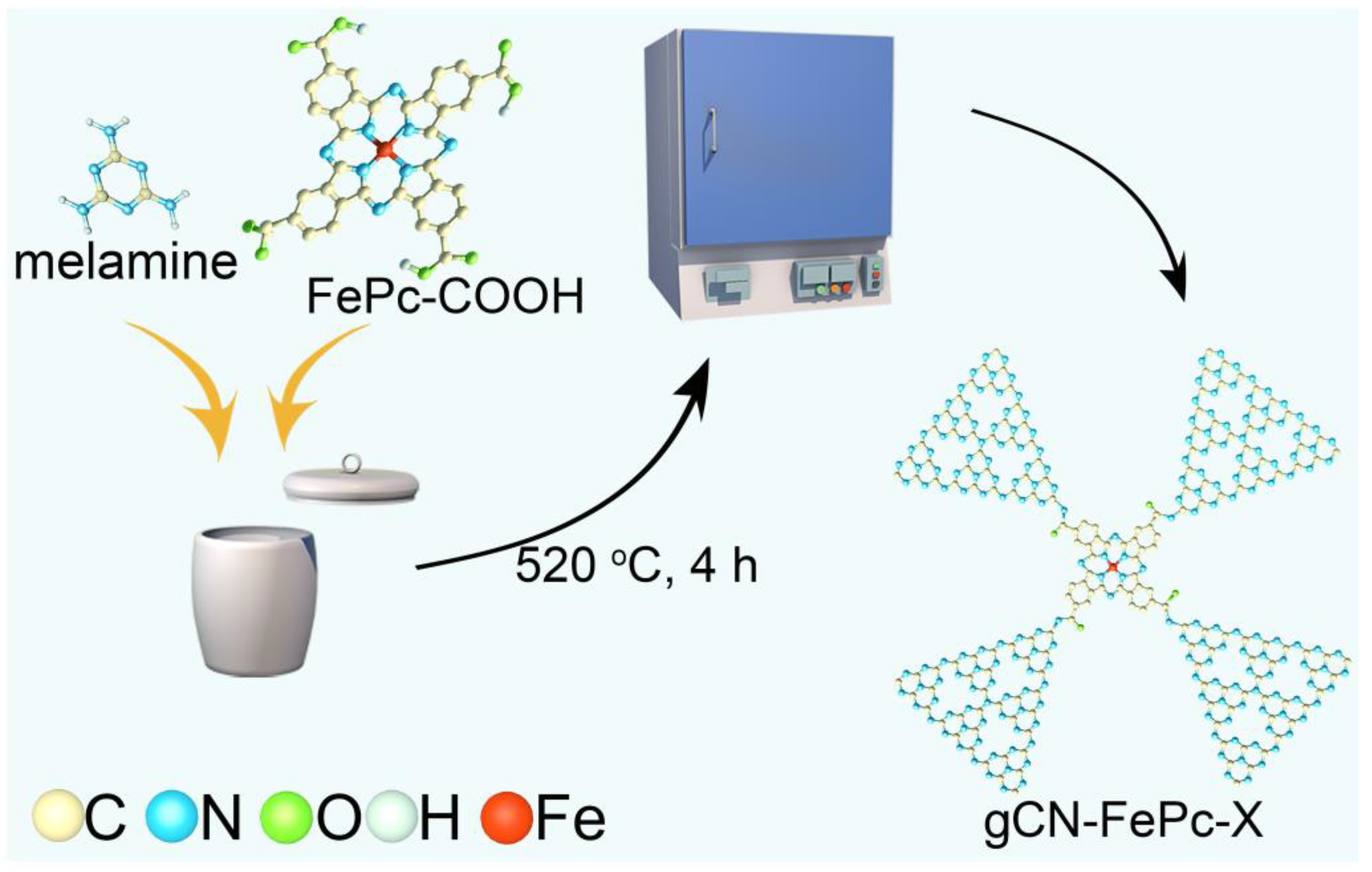
2.1. Synthesis of Photocatalysts
2.2. Characterizations
2.3. Photocatalytic Tests
2.4. Electrochemical Measurements
3. Results and Discussion
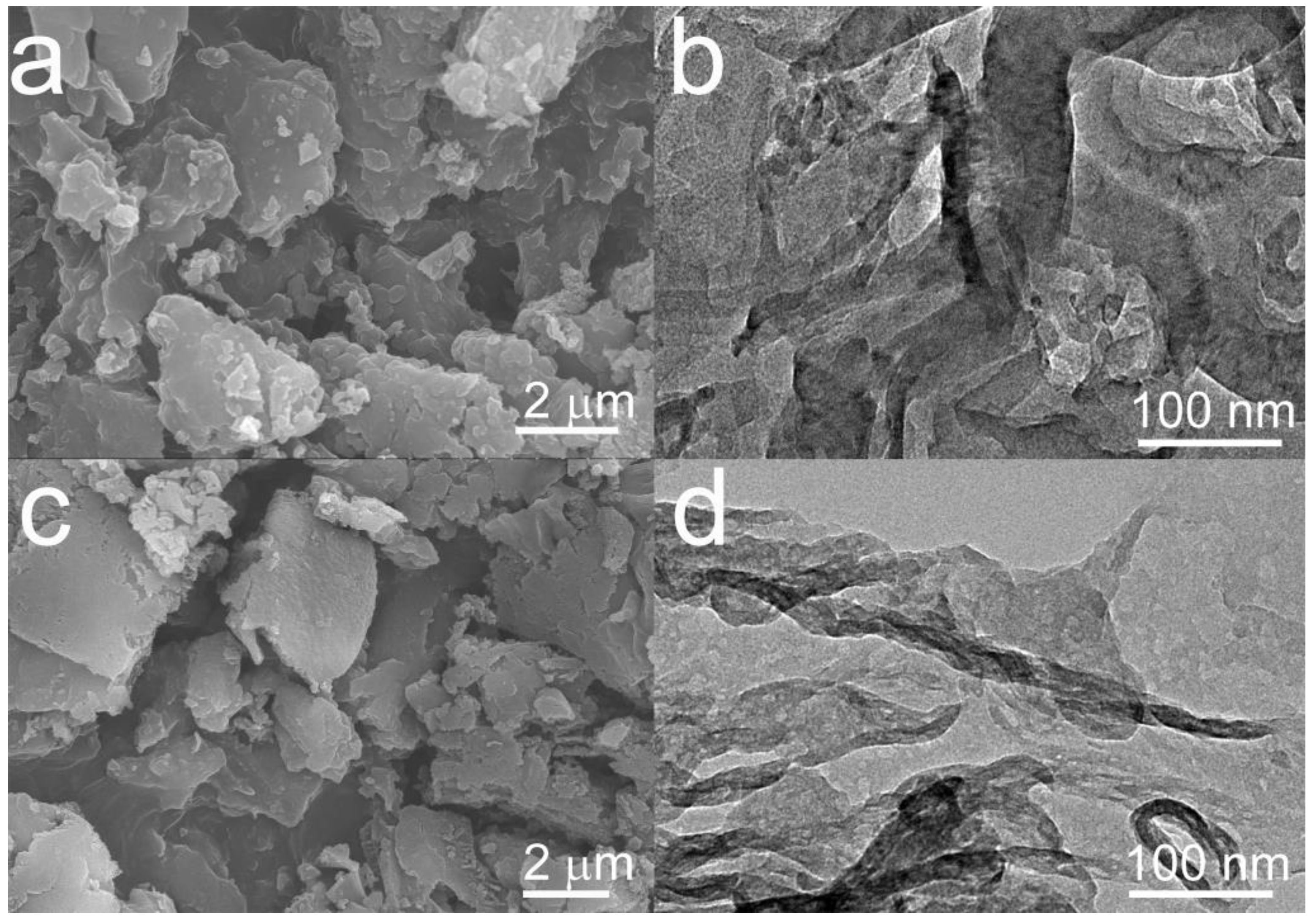
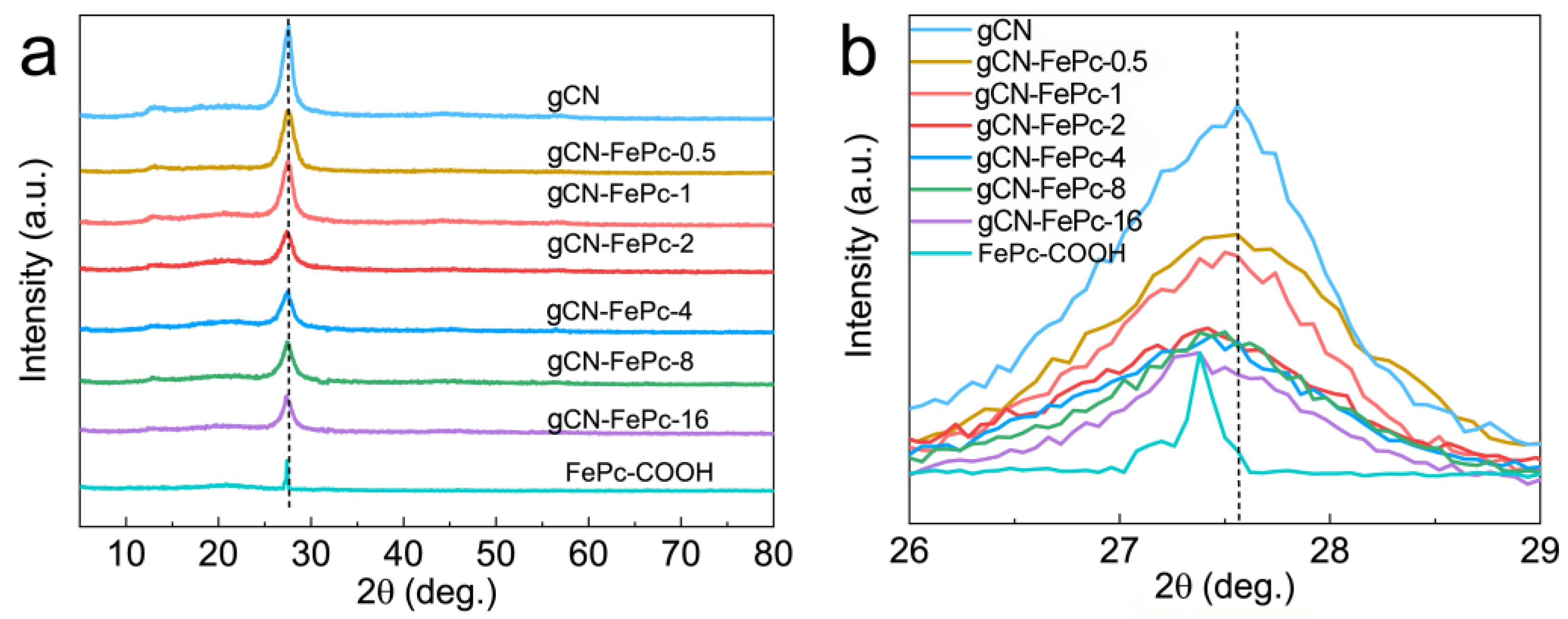
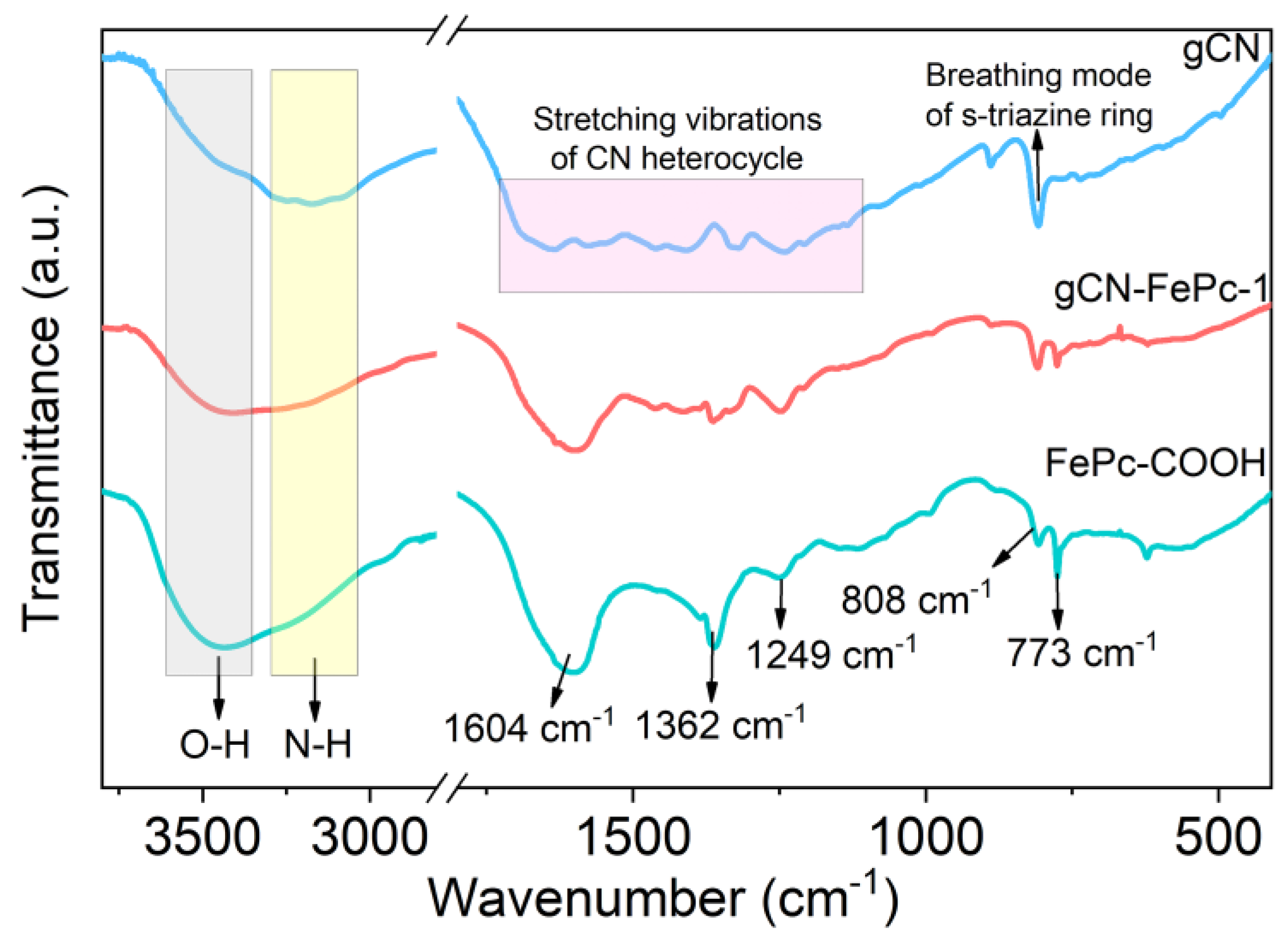
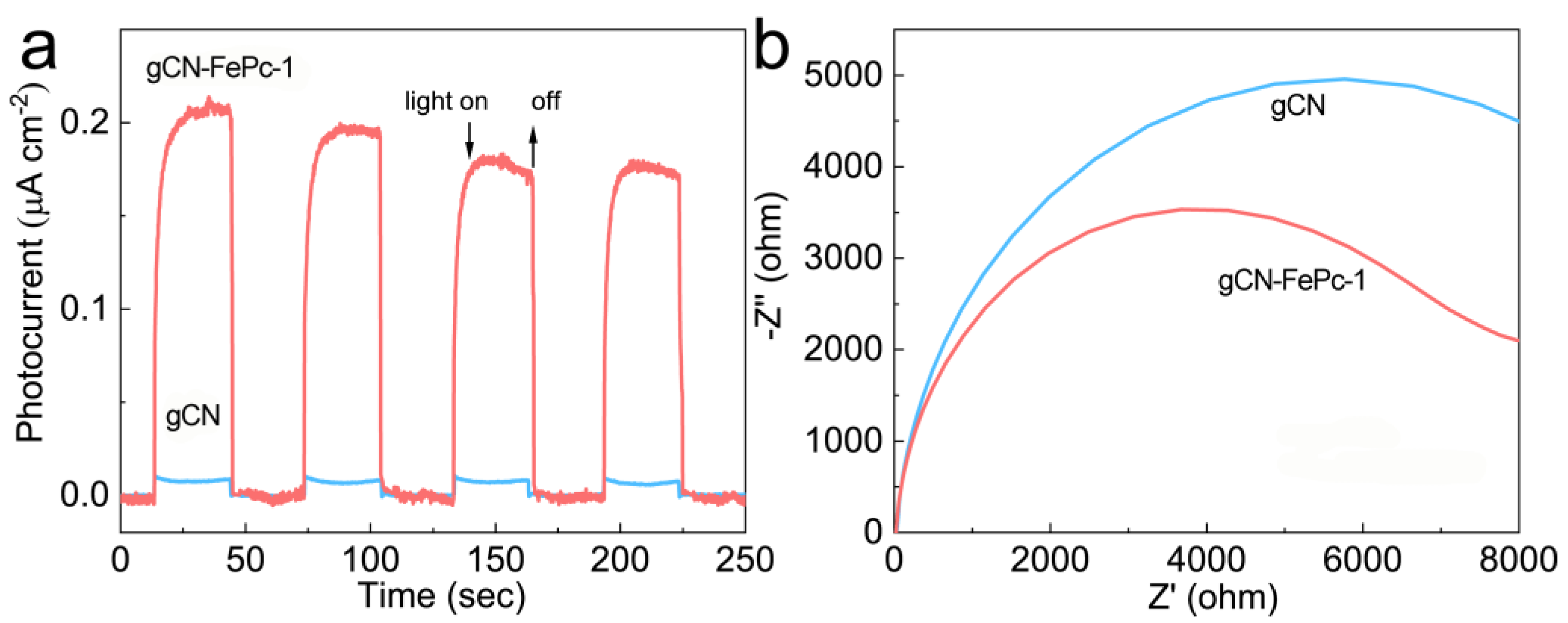
3.1. Morphology and Structure
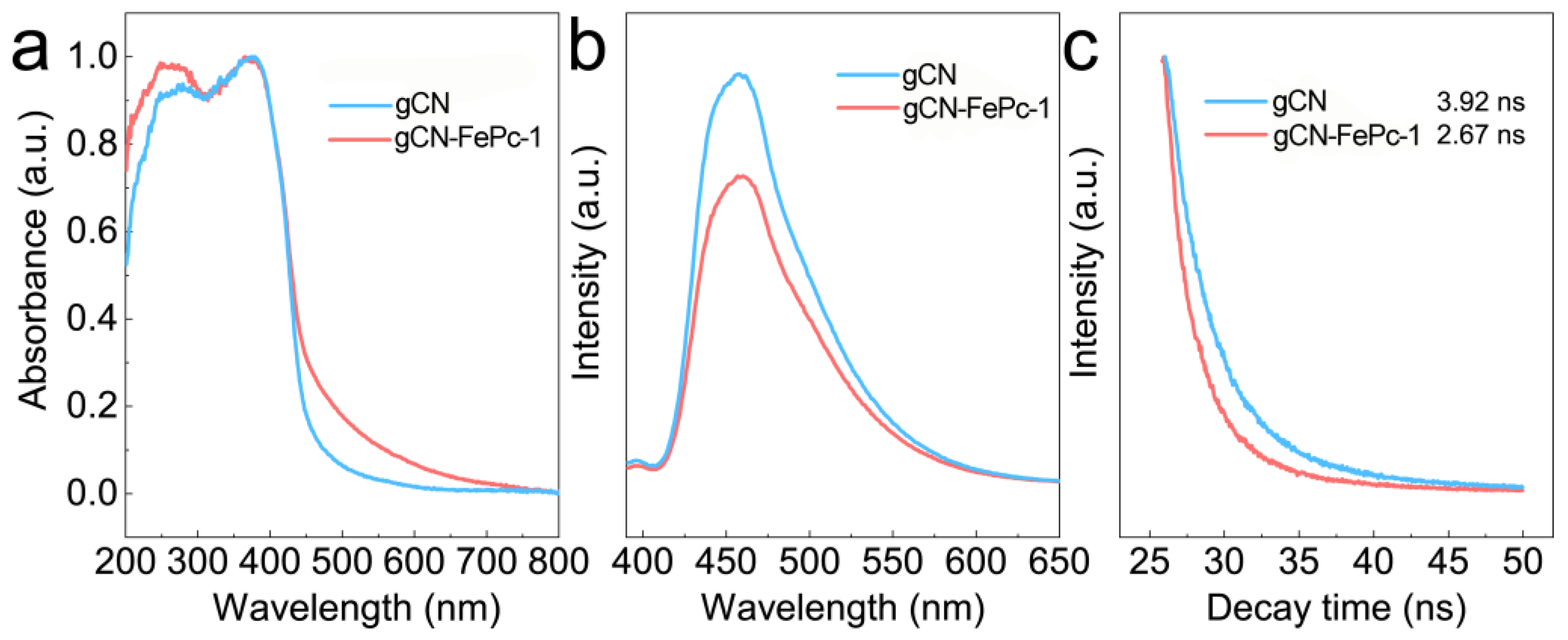
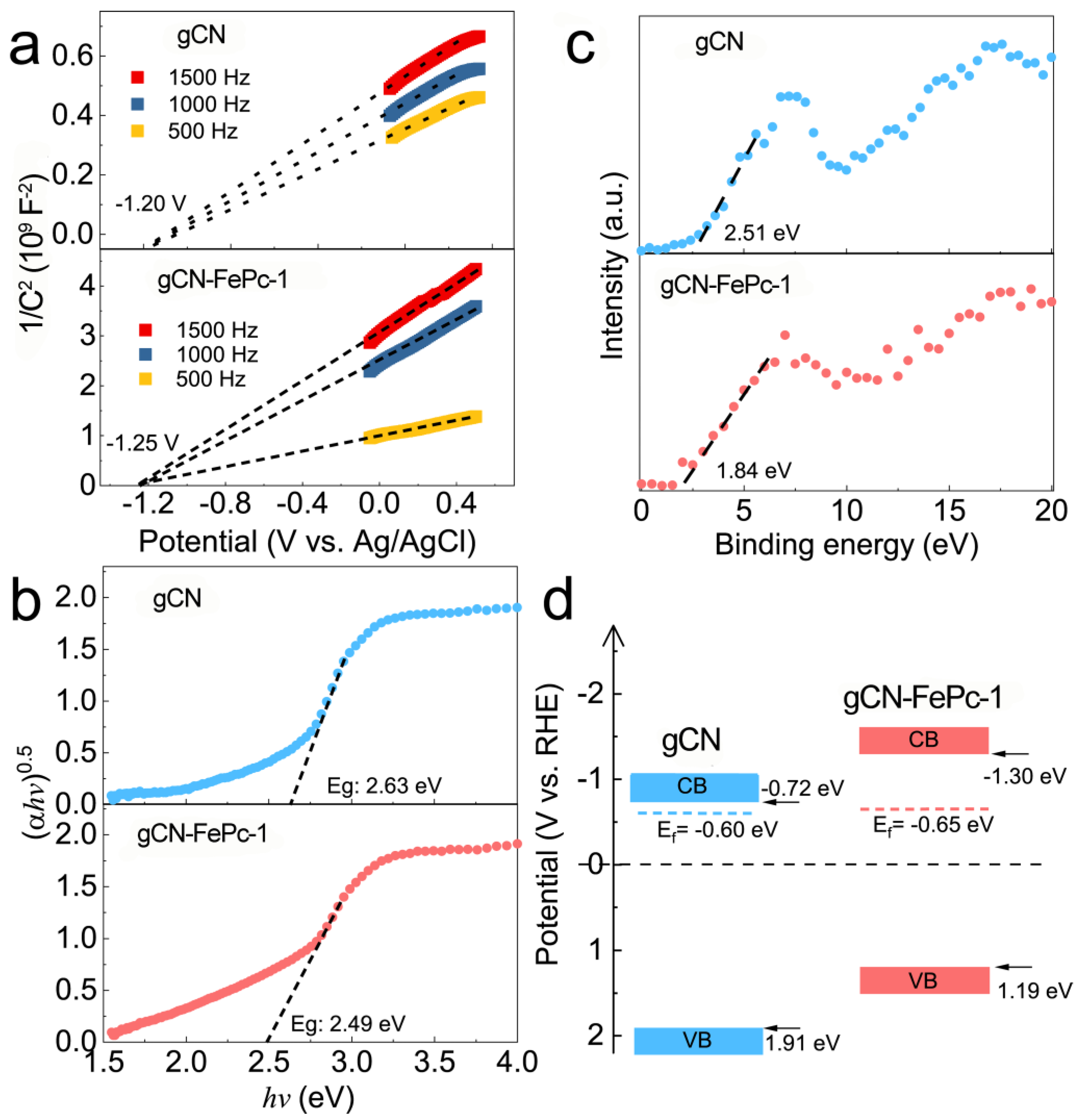
3.2. Optical Properties
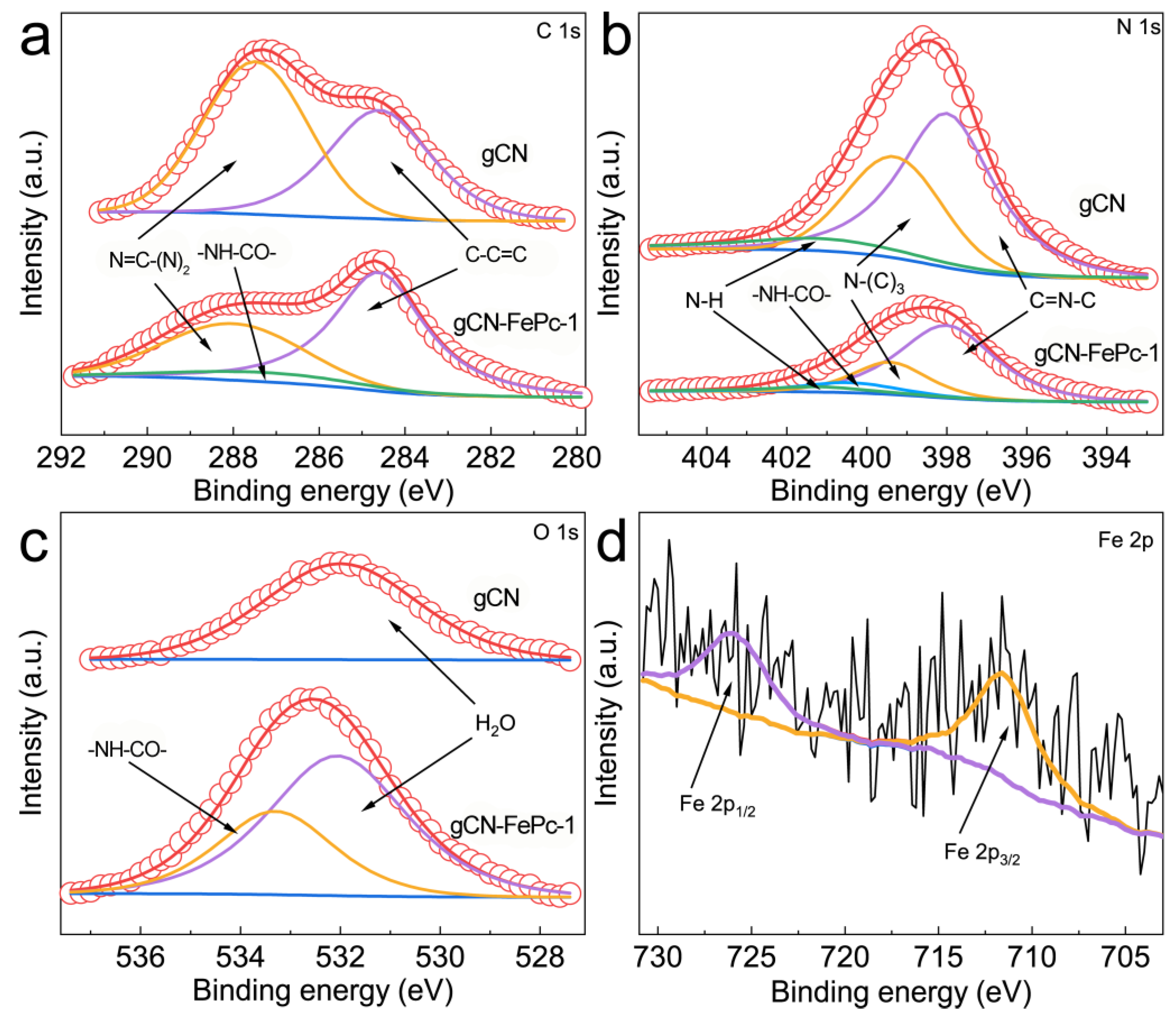
3.3. XPS Analysis
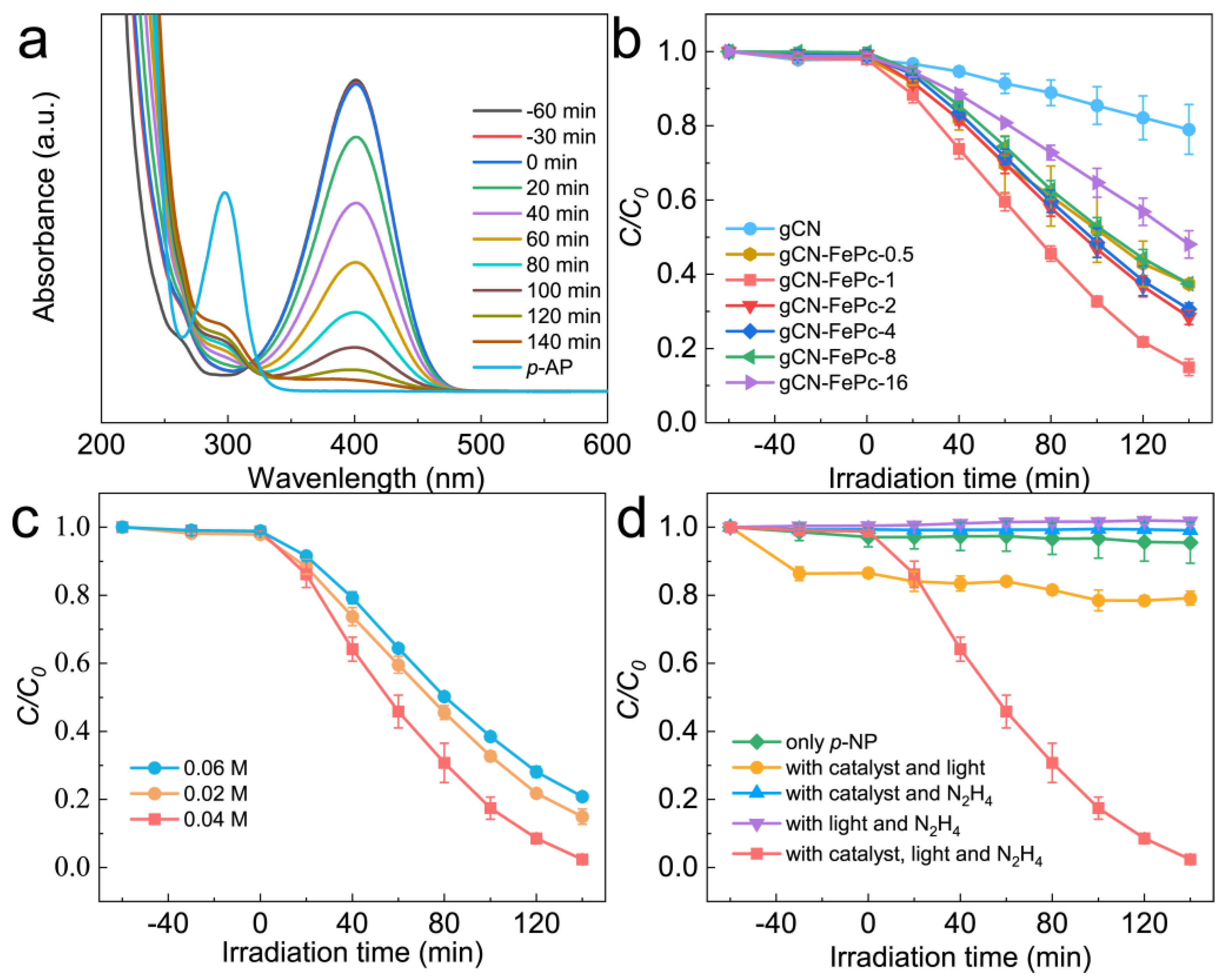
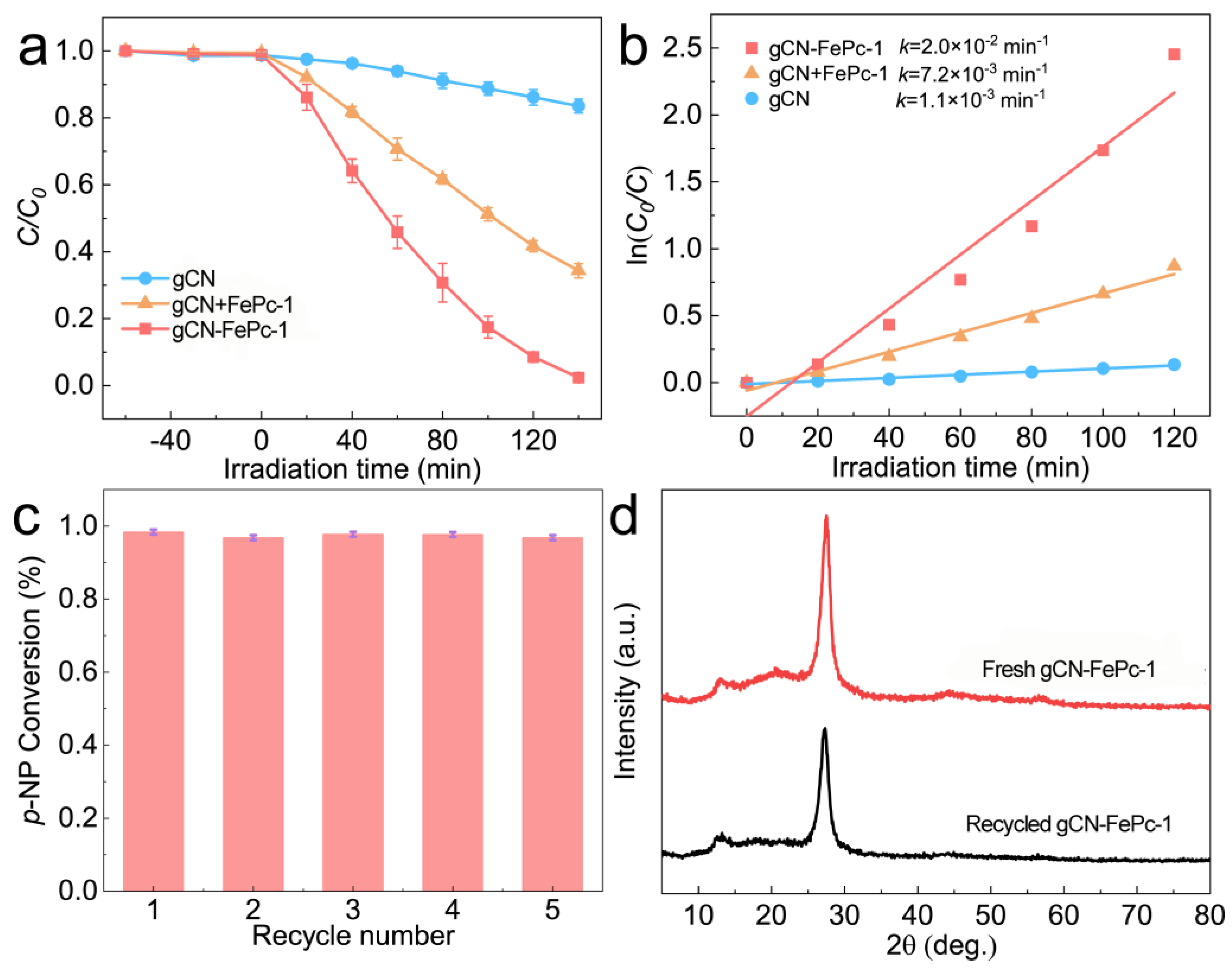
3.4. VLD Photocatalytic Performance for Reduction in NACs
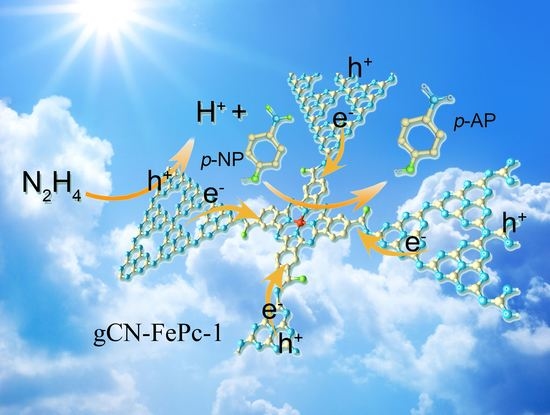
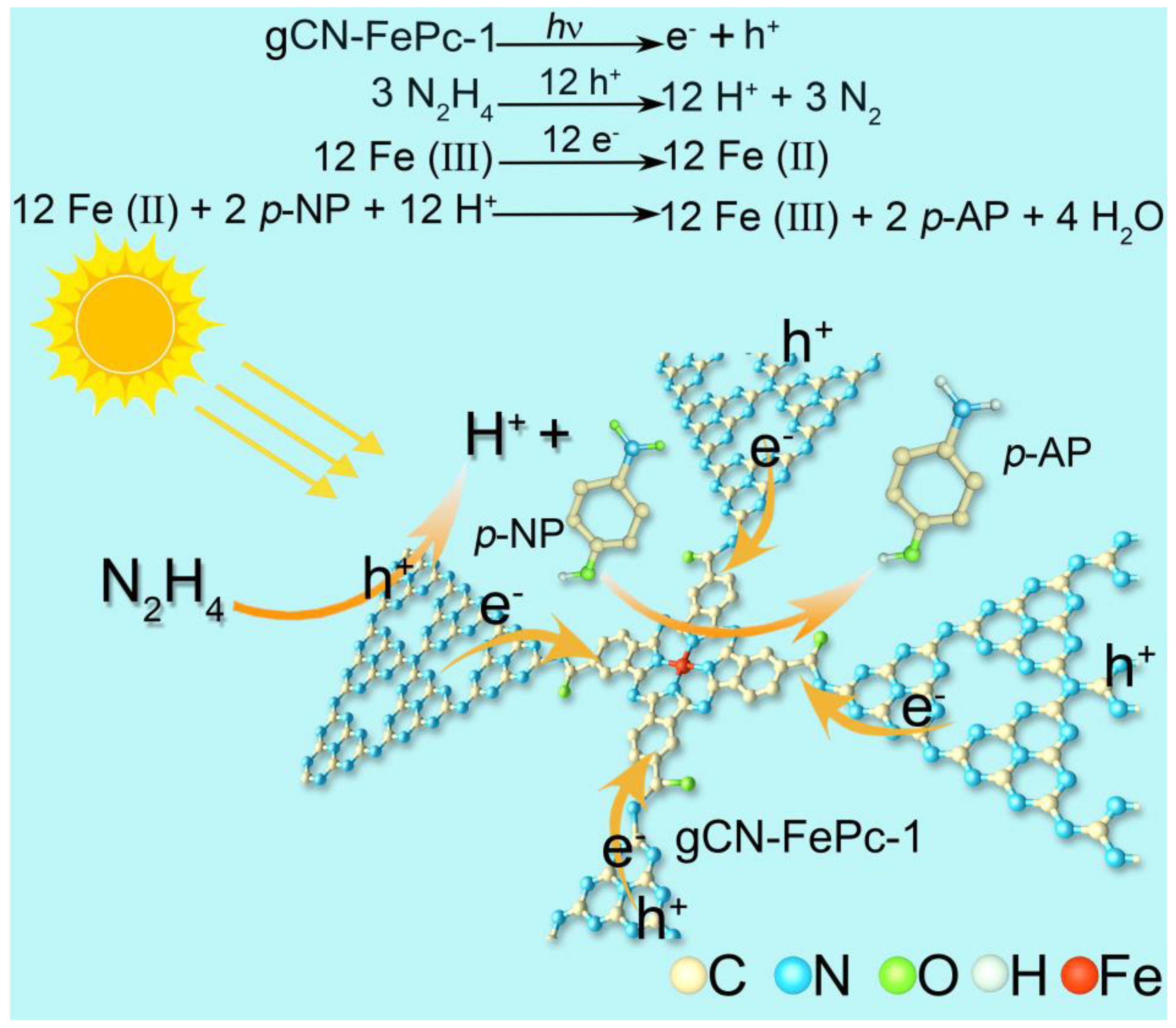
3.5. Plausible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Li, J.; Meng, R.; Jian, P.; Wang, L. Silver nanoparticles-decorated-Co3O4 porous sheets as efficient catalysts for the liquid-phase hydrogenation reduction of p-nitrophenol. J. Colloid Interf. Sci. 2019, 551, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Liu, J.; Guo, Z.; Li, P.; Bi, S.; Wang, Y.; Yang, Y.; Shen, W.; Wang, Y.; Xiao, Y.; et al. Simultaneous p-nitrophenol and nitrogen removal in PNP wastewater treatment: Comparison of two integrated membrane-aerated bioreactor systems. J. Hazard. Mater. 2019, 363, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, J.; Chen, R.; Cao, F.; Jin, B. Self-assembled BiOCl/Ti3C2T composites with efficient photo-induced charge separation activity for photocatalytic degradation of p-nitrophenol. Appl. Surf. Sci. 2020, 519, 146175. [Google Scholar] [CrossRef]
- Shi, D.; Zhu, G.; Zhang, X.; Zhang, X.; Li, X.; Fan, J. Ultra-small and recyclable zero-valent iron nanoclusters for rapid and highly efficient catalytic reduction of p-nitrophenol in water. Nanoscale 2019, 11, 1000–1010. [Google Scholar] [CrossRef]
- Bai, X.; Chen, D.; Li, Y.; Yang, X.; Zhang, M.; Wang, T.; Zhang, X.; Zhang, L.; Fu, Y.; Qi, X.; et al. Two-dimensional MOF-derived nanoporous Cu/Cu2O networks as catalytic membrane reactor for the continuous reduction of p-nitrophenol. J. Membr. Sci. 2019, 582, 30–36. [Google Scholar] [CrossRef]
- Huang, T.; Fu, Y.; Peng, Q.; Yu, C.; Zhu, J.; Yu, A.; Wang, X. Catalytic hydrogenation of p-nitrophenol using a metal-free catalyst of porous crimped graphitic carbon nitride. Appl. Surf. Sci. 2019, 480, 888–895. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, K.; Chen, Z.; Yang, J.; Geng, Z.; Zeng, J. Atomic-level insights into strain effect on p-nitrophenol reduction via Au@Pd core-shell nanocubes as an ideal platform. J. Catal. 2020, 381, 427–433. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt-Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4-nitrophenol reduction. Appl. Catal. B Environ. 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Cho, H.; Kwon, Y.; Lee, Y.; Park, Y.; Ji, H.; Lee, J. Morphological control of gold nanoparticles on exfoliated layers of layered double hydroxide: A reusable hybrid catalyst for the reduction of p-nitrophenol. Appl. Clay Sci. 2018, 156, 187–194. [Google Scholar] [CrossRef]
- Bae, S.; Gim, S.; Kim, H.; Hanna, K. Effect of NaBH4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol. Appl. Catal. B Environ. 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Xiao, G.; Li, P.; Zhao, Y.; Xu, S.; Su, H. Visible-light-driven chemoselective hydrogenation of nitroarenes to anilines in water through graphitic carbon nitride metal-free photocatalysis. Chem. Asian J. 2018, 13, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Wang, T.; Sun, L.; Du, Y.; Li, Y.; Li, H. Photocatalytic reduction of p-nitrophenol over plasmonic M (M = Ag, Au)/SnNb2O6 nanosheets. Appl. Surf. Sci. 2019, 466, 342–351. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, G.; Wang, Z.; Lu, Y.; Chen, J.; Matyjaszewski, K. Heteroatom-doped carbon dots (CDs) as a new class of metal-free photocatalysts for PET-RAFT polymerization under visible light and sunlight. Angew. Chem. Int. Ed. 2018, 130, 12213–12218. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, D.; Chen, Y.; Yan, Q.; Liu, C.Y.; Ling, K.; Liu, Y.; Lee, D.; Wu, X.; Senftle, T.P.; et al. Porphyrin-based donor–acceptor COFs as efficient and reusable photocatalysts for PET-RAFT polymerization under broad spectrum excitation. Chem. Sci. 2021, 12, 16092–16099. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloy Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Raaja Rajeshwari, M.; Kokilavani, S.; Sudheer Khan, S. Recent developments in architecturing the g-C3N4 based nanostructured photocatalysts: Synthesis, modifications and applications in water treatment. Chemosphere 2022, 291, 132735. [Google Scholar] [CrossRef]
- Liu, J.; Fu, W.; Liao, Y.; Fan, J.; Xiang, Q. Recent advances in crystalline carbon nitride for photocatalysis. J. Mater. Sci. Technol. 2021, 91, 224–240. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Wei, X.; Wang, L.; She, H.; Huang, J.; Wang, Q. Preparation of double-layered Co-Ci/NiFeOOH co-catalyst for highly meliorated PEC performance in water splitting. Adv. Powder Mater. 2022, 1, 100024. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Shanmugam, R.; Gangavarapu, R. Extending the π-electron conjugation in 2D planar graphitic carbon nitride: Efficient charge separation for overall water splitting. J. Mater. Chem. A 2019, 7, 3757–3771. [Google Scholar] [CrossRef]
- Sun, P.; Yu, H.; Liu, T.; Li, Y.; Wang, Z.; Xiao, Y.; Dong, X. Efficiently photothermal conversion in a MnOx-based monolithic photothermocatalyst for gaseous formaldehyde elimination. Chin. Chem. Lett. 2022, 33, 2564–2568. [Google Scholar] [CrossRef]
- Huang, R.; Wu, J.; Zhang, M.; Liu, B.; Zheng, Z.; Luo, D. Strategies to enhance photocatalytic activity of graphite carbon nitride-based photocatalysts. Mater. Des. 2021, 210, 110040. [Google Scholar] [CrossRef]
- Gao, C.; Wang, J.; Xu, H.; Xiong, Y. Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; El-Alami, W.; Song, Y.; Li, H.; Ajayan, P.; Xu, H. Emerging surface strategies on graphitic carbon nitride for solar driven water splitting. Chem. Eng. J. 2020, 382, 122812. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, L.; Chen, H.; Cai, L.; Tan, G.; Qiu, Y.; Xiang, Q.; Chen, G.; Lau, T.C.; Robert, M. Highly efficient photocatalytic reduction of CO2 to CO by in situ formation of a hybrid catalytic system based on molecular iron quaterpyridine covalently linked to carbon nitride. Angew. Chem. Int. Ed. Engl. 2022, 61, e202116832. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, W.; Xu, T.; Li, N.; Zhu, Z.; Wang, G.; Chen, W. Visible-light-assisted generation of high-valent iron-oxo species anchored axially on g-C3N4 for efficient degradation of organic pollutants. Chem. Eng. J. 2017, 328, 853–861. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and-mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772–6780. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A facile one-step synthesis of Fe-doped g-C3N4 nanosheets and their improved visible-light photocatalytic performance. Chemcatchem 2017, 9, 1708–1715. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Wang, R.; Wang, Z.; Lei, H.; Dong, X.; He, C. Highly dispersive and stable Fe3+ active sites on 2D graphitic carbon nitride nanosheets for efficient visible-light photocatalytic nitrogen fixation. J. Mater. Chem. A 2019, 7, 27547–27559. [Google Scholar] [CrossRef]
- Zheng, J.; Li, X.; Qin, Y.; Zhang, S.; Sun, M.; Duan, X.; Sun, H.; Li, P.; Wang, S. Zn phthalocyanine/carbon nitride heterojunction for visible light photoelectrocatalytic conversion of CO2 to methanol. J. Catal. 2019, 371, 214–223. [Google Scholar] [CrossRef]
- Bottari, G.; Trukhina, O.; Ince, M.; Torres, T. Towards artificial photosynthesis: Supramolecular, donor-acceptor, porphyrin- and phthalocyanine/carbon nanostructure ensembles. Coordin. Chem. Rev. 2012, 256, 2453–2477. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, W.; Li, N.; Xu, T.; Chen, W. Pyridyl-containing polymer blends stabilized iron phthalocyanine to degrade sulfonamides by enzyme-like process. Chem. Eng. J. 2017, 321, 58–66. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Huang, J.; Zhang, S.; Xu, X. Noble metal-free core-shell CdS/iron phthalocyanine Z-scheme photocatalyst for enhancing photocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2022, 115, 199–207. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, J.; Peng, W.; Radjenovic, P.; Zhang, H.; Tian, Z.; Li, J. Elucidating molecule-plasmon interactions in nanocavities with 2 nm spatial resolution and at the single-molecule level. Angew. Chem. Int. Ed. Engl. 2019, 58, 12133–12137. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, M.; Liu, C.; Xu, S.; Li, Z. Sulfur-doped graphitic carbon nitride decorated with zinc phthalocyanines towards highly stable and efficient photocatalysis. Appl. Catal. A Gen. 2016, 519, 107–115. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, J.; Yang, Z.; Wang, S.; Wang, H. Copper phthalocyanine-functionalized graphitic carbon nitride: A hybrid heterostructure toward photoelectrochemical and photocatalytic degradation applications. Chem. Asian J. 2016, 11, 1887–1891. [Google Scholar] [CrossRef]
- Yuan, A.; Lei, H.; Wang, Z.; Dong, X. Improved photocatalytic performance for selective oxidation of amines to imines on graphitic carbon nitride/bismuth tungstate heterojunctions. J. Colloid Interf. Sci. 2020, 560, 40–49. [Google Scholar] [CrossRef]
- Yao, C.; Yuan, A.; Wang, Z.; Lei, H.; Zhang, L.; Guo, L.; Dong, X. Amphiphilic two-dimensional graphitic carbon nitride nanosheets for visible-light-driven phase-boundary photocatalysis. J. Mater. Chem. A 2019, 7, 13071–13079. [Google Scholar] [CrossRef]
- Christendat, D.; David, M.; Morin, S.; Lever, A.; Kadish, K.; Shao, J. Synthesis and characterization of highly soluble hexadecachloro-and hexadecafluorophthalocyanine ruthenium (II) complexes. J. Porphyr. Phthalocya. 2005, 9, 626–636. [Google Scholar] [CrossRef]
- Qian, J.; Shen, C.; Yan, J.; Xi, F.; Dong, X.; Liu, J. Tailoring the electronic properties of graphene quantum dots by P doping and their enhanced performance in metal-free composite photocatalyst. J. Phys. Chem. C 2017, 122, 349–358. [Google Scholar] [CrossRef]
- Han, X.; Yuan, A.; Yao, C.; Xi, F.; Liu, J.; Dong, X. Synergistic effects of phosphorous/sulfur co-doping and morphological regulation for enhanced photocatalytic performance of graphitic carbon nitride nanosheets. J. Mater. Sci. 2018, 54, 1593–1605. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, W.; He, F.; Liu, K.; Cao, H.; Yan, H. One-step synthesis of nitrogen-defective graphitic carbon nitride for improving photocatalytic hydrogen evolution. J. Hazard Mater. 2021, 410, 124594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Yang, J.; Sun, Q.; Li, Q.; Yang, J. Iron phthalocyanine-graphene donor-acceptor hybrids for visible-light-assisted degradation of phenol in the presence of H2O2. Appl. Catal. B Environ. 2016, 192, 182–192. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, W.D. Photocatalytic hydrogen evolution over a nickel complex anchoring to thiophene embedded g-C3N4. J. Colloid. Interface Sci. 2021, 596, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Lei, H.; Xi, F.; Liu, J.; Qin, L.; Chen, Z.; Dong, X. Graphene quantum dots decorated graphitic carbon nitride nanorods for photocatalytic removal of antibiotics. J. Colloid Interface Sci. 2019, 548, 56–65. [Google Scholar] [CrossRef]
- Bao, J.; Jiang, X.; Huang, L.; Quan, W.; Zhang, C.; Wang, Y.; Wang, H.; Zeng, Y.; Zhang, W.; Ma, Y.; et al. Molybdenum disulfide loading on a Z-scheme graphitic carbon nitride and lanthanum nickelate heterojunction for enhanced photocatalysis: Interfacial charge transfer and mechanistic insights. J. Colloid Interface Sci. 2022, 611, 684–694. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, P.; Lai, C.; Zhang, C.; Zeng, G.; Huang, D.; Cheng, M.; Hu, L.; Xiong, W.; Wen, X.; et al. Rational design of graphic carbon nitride copolymers by molecular doping for visible-light-driven degradation of aqueous sulfamethazine and hydrogen evolution. Chem. Eng. J. 2019, 359, 186–196. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Z.; Shi, Y.; Cao, L.; Cheng, X.; Shi, W.; Chen, L.; Lin, X. A ragged porous hollow tubular carbon nitride towards boosting visible-light photocatalytic hydrogen production in water and seawater. Renew. Energ. 2022, 188, 1–10. [Google Scholar] [CrossRef]
- Bao, X.; Lv, X.; Wang, Z.; Wang, M.; Liu, M.; Dai, D.; Zheng, L.; Zheng, Z.; Cheng, H.; Wang, P.; et al. Nitrogen vacancy enhanced photocatalytic selective oxidation of benzyl alcohol in g-C3N4. Int. J. Hydrog. Energ. 2021, 46, 37782–37791. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, P.; Yu, Y.; Zhang, W. Integration of nickel complex as a cocatalyst onto in-plane benzene ring-incorporated graphitic carbon nitride nanosheets for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2020, 381, 122635. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. Primary and secondary oxygen-induced C1s binding energy shifts in x-ray photoelectron spectroscopy of polymers. Anal. Chem. 1992, 64, 1729–1736. [Google Scholar] [CrossRef]
- Ho, W.; Zhang, Z.; Lin, W.; Huang, S.; Zhang, X.; Wang, X.; Huang, Y. Copolymerization with 2,4,6-triaminopyrimidine for the rolling-up the layer structure, tunable electronic properties, and photocatalysis of g-C3N4. ACS Appl. Mater. Interfaces 2015, 7, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Sun, M.; Zhang, W. Polycyclic aromatic compounds-modified graphitic carbon nitride for efficient visible-light-driven hydrogen evolution. Carbon 2018, 134, 134–144. [Google Scholar] [CrossRef]
- Russat, J. Characterization of polyamic acid/polyimide films in the nanometric thickness range from spin-deposited polyamic acid. Surf. Interface Anal. 1988, 11, 414–420. [Google Scholar] [CrossRef]
- Duevel, R.; Corn, R. Amide and ester surface attachment reactions for alkanethiol monolayers at gold surfaces as studied by polarization modulation fourier transform infrared spectroscopy. Anal. Chem. 1992, 64, 337–342. [Google Scholar] [CrossRef]
- Uchida, E.; Uyama, Y.; Iwata, H.; Ikada, Y. XPS analysis of the poly (ethylene terephthalate) film grafted with acrylamide. J. Polym. Sci. Pol. Chem. 1990, 28, 2837–2844. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Najdovski, I.; Selvakannan, P.; Bhargava, S.; O’Mullane, A. Formation of nanostructured porous Cu-Au surfaces: The influence of cationic sites on (electro)-catalysis. Nanoscale 2012, 4, 6298–6306. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yuan, A.; Xiao, Y.; Yu, H.; Dong, X. Two-dimensional/two-dimensional Z-scheme photocatalyst of graphitic carbon nitride/bismuth vanadate for visible-light-driven photocatalytic synthesis of imines. Ceram. Int. 2020, 46, 16157–16165. [Google Scholar] [CrossRef]
- Han, X.; Yao, C.; Yuan, A.; Xi, F.; Dong, X.; Liu, J. Enhanced charge separation ability and visible light photocatalytic performance of graphitic carbon nitride by binary S., B co-doping. Mater. Res. Bull. 2018, 107, 477–483. [Google Scholar] [CrossRef]
- Sivula, K. Mott-Schottky analysis of photoelectrodes: Sanity checks are needed. ACS Energy Lett. 2021, 6, 2549–2551. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Xu, Y.; He, Q.; Yin, R.; Sun, P.; Dong, X. Phenanthroline bridging graphitic carbon nitride framework and Fe (II) ions to promote transfer of photogenerated electrons for selective photocatalytic reduction of nitrophenols. J. Colloid Interface Sci. 2022, 608, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
| Entry | Substrate | Reduced Product | Conversion (%) |
|---|---|---|---|
| 1 |  |  | 97 |
| 2 | 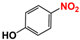 | 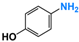 | 98 |
| 3 | 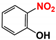 |  | 98 |
| 4 | 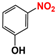 | 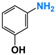 | 98 |
| 5 | 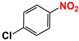 | 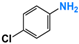 | 99 |
| 6 | 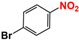 | 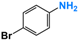 | 99 |
| 7 | 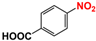 |  | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, J.; Liu, Y.; Zheng, W.; Zhou, B.; Dong, X. Covalent Modification of Iron Phthalocyanine into Skeleton of Graphitic Carbon Nitride and Its Visible-Light-Driven Photocatalytic Reduction of Nitroaromatic Compounds. Catalysts 2022, 12, 752. https://doi.org/10.3390/catal12070752
Qian J, Liu Y, Zheng W, Zhou B, Dong X. Covalent Modification of Iron Phthalocyanine into Skeleton of Graphitic Carbon Nitride and Its Visible-Light-Driven Photocatalytic Reduction of Nitroaromatic Compounds. Catalysts. 2022; 12(7):752. https://doi.org/10.3390/catal12070752
Chicago/Turabian StyleQian, Jiajia, Ying Liu, Weiran Zheng, Baocheng Zhou, and Xiaoping Dong. 2022. "Covalent Modification of Iron Phthalocyanine into Skeleton of Graphitic Carbon Nitride and Its Visible-Light-Driven Photocatalytic Reduction of Nitroaromatic Compounds" Catalysts 12, no. 7: 752. https://doi.org/10.3390/catal12070752
APA StyleQian, J., Liu, Y., Zheng, W., Zhou, B., & Dong, X. (2022). Covalent Modification of Iron Phthalocyanine into Skeleton of Graphitic Carbon Nitride and Its Visible-Light-Driven Photocatalytic Reduction of Nitroaromatic Compounds. Catalysts, 12(7), 752. https://doi.org/10.3390/catal12070752