Photocatalytic Conversion of Fructose to Lactic Acid by BiOBr/Zn@SnO2 Material
Abstract
:1. Introduction
2. Results and Discussion
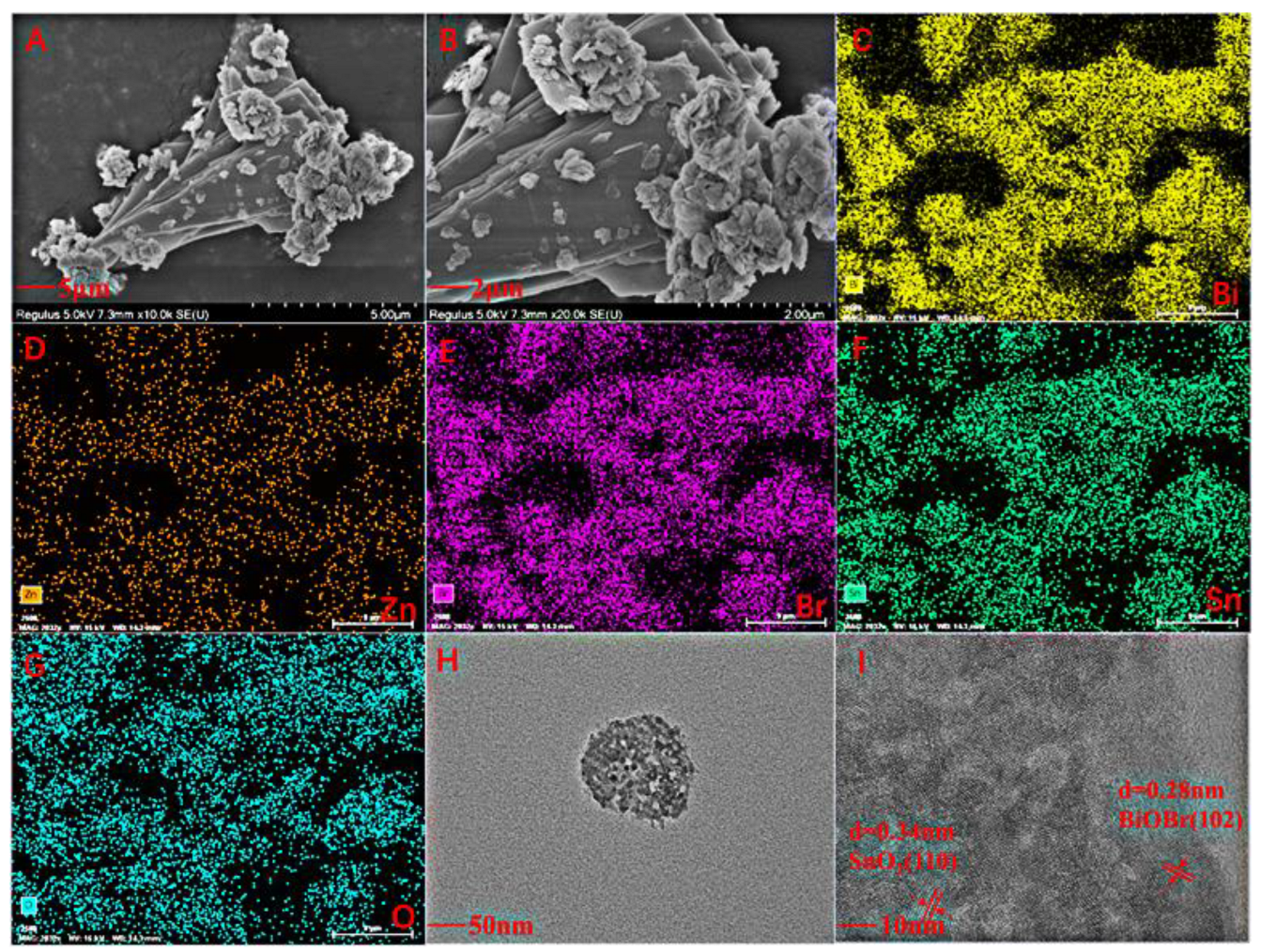
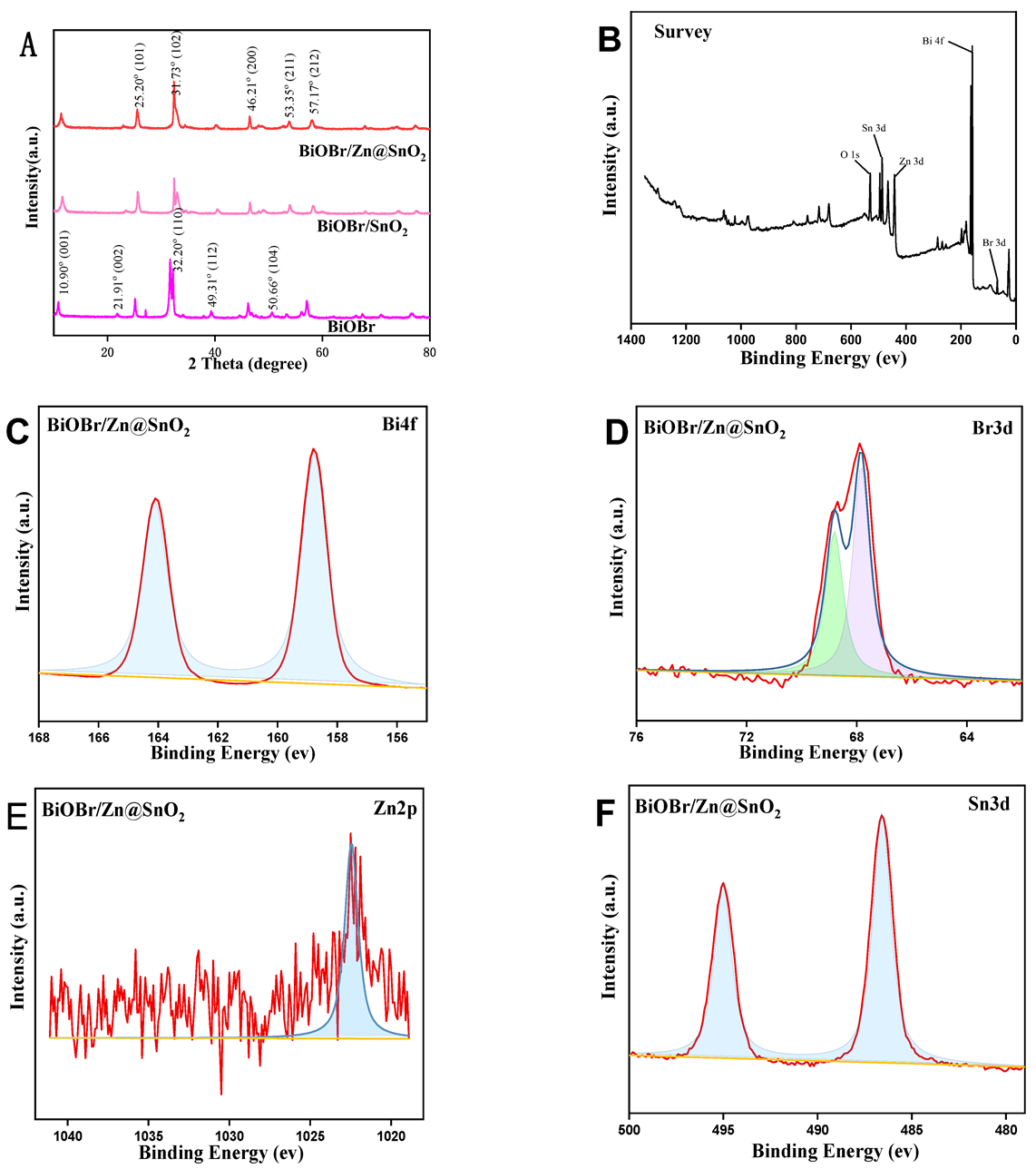
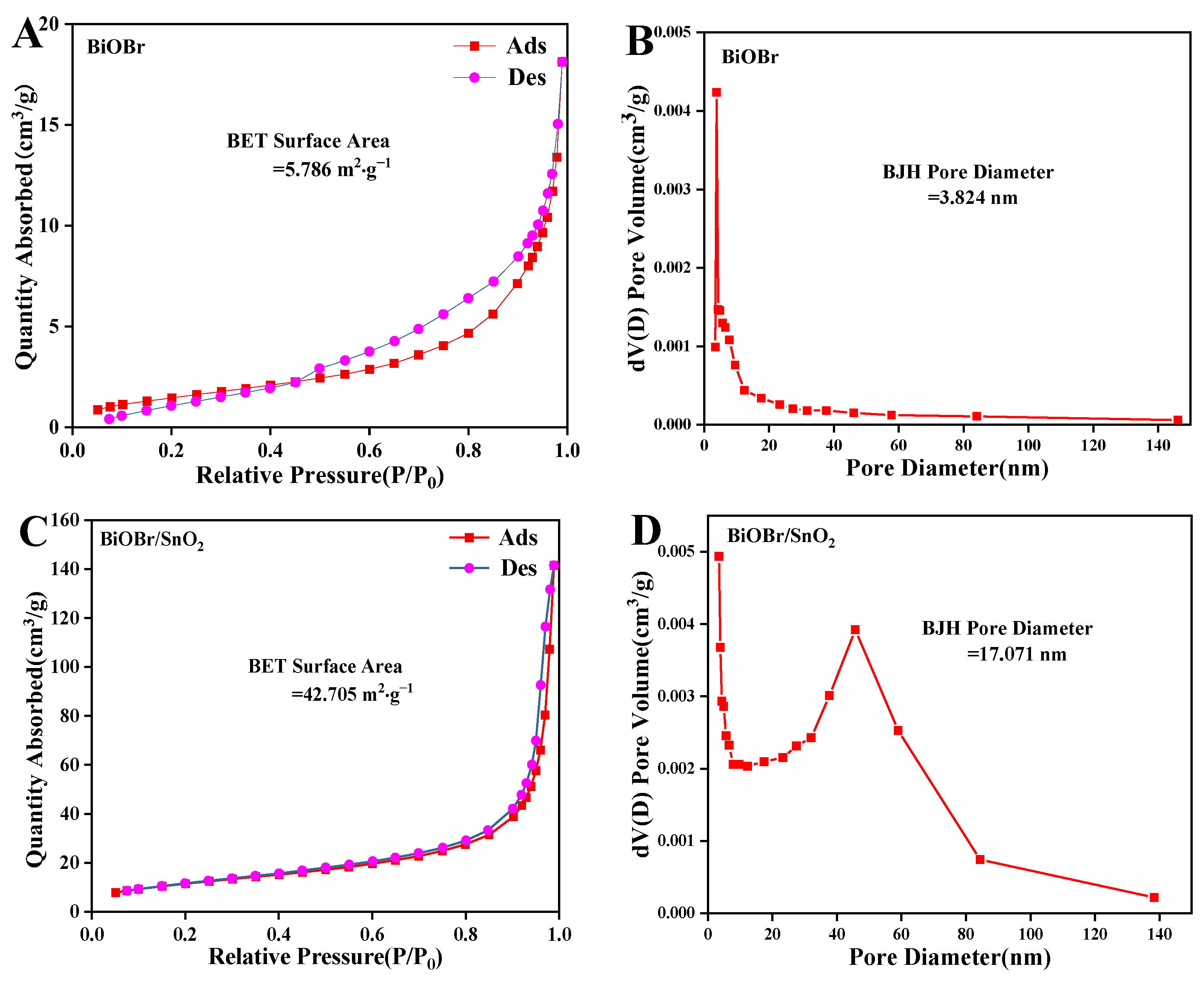
2.1. Characterization of BiOBr, BiOBr/SnO2, BiOBr/Zn@SnO2
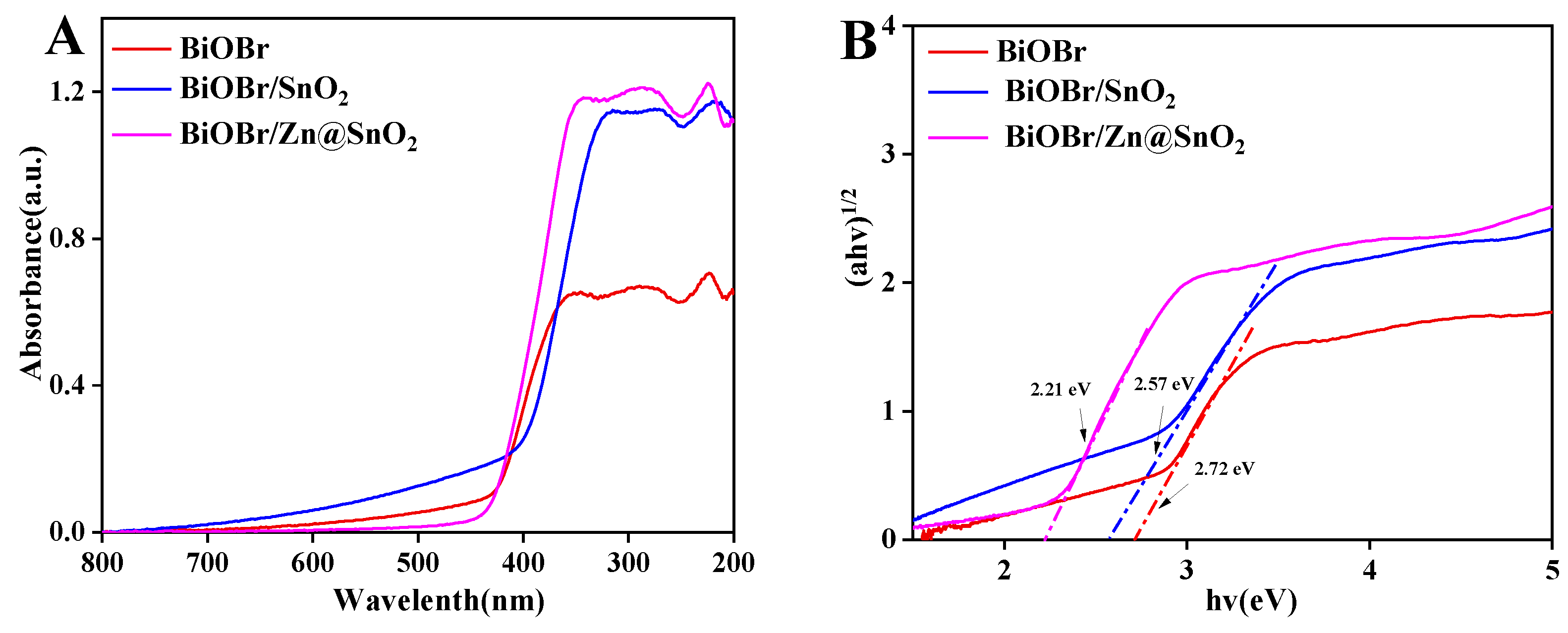
2.2. Optical Characterization of BiOBr, BiOBr/SnO2, BiOBr/Zn@SnO2
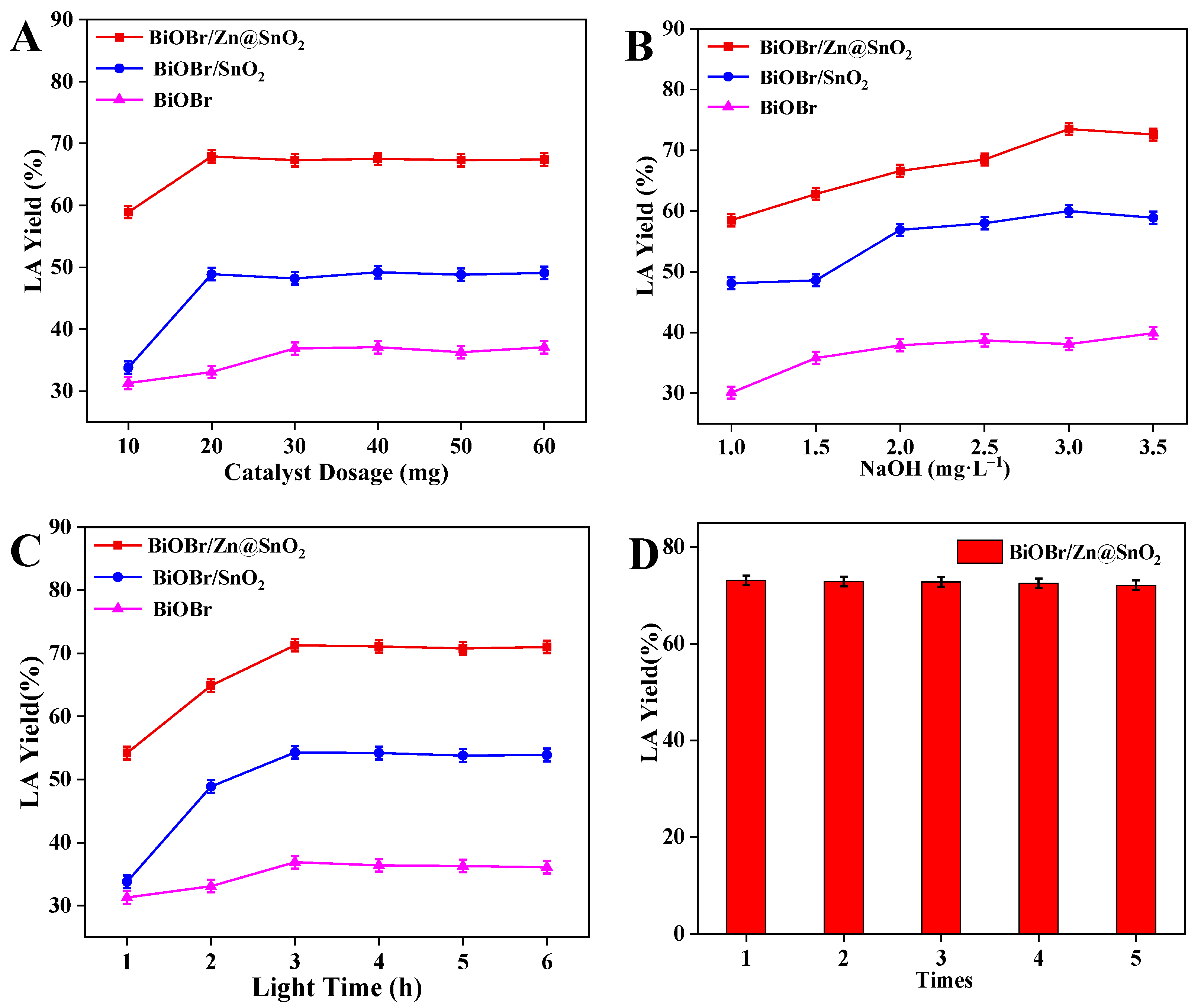
2.3. Investigating the Effect of Different Conditions on Fructose Photoproduction of Lactic Acid
2.4. Possible Pathways for Lactic Acid-Lactate Production by Fructose Photorefinery
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katryniok, B.; Paul, S.; Dumeignil, F. Highly efficient catalyst for the decarbonylation of lactic acid to acetaldehyde. Green Chem. 2010, 12, 1910–1913. [Google Scholar] [CrossRef]
- Purushothaman, R.K.P.; Van Haveren, J.; Van Es, D.; Melián-Cabrera, I.; Meeldijk, J.; Heeres, H. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal. B Environ. 2014, 147, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Eş, I.; Khaneghah, A.M.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production-a review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Deng, W.; Li, Y.; Zhang, Q.; Wang, Y. Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J. Energy Chem. 2019, 32, 138–151. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Wang, Y.; Zhang, S.; Gupta, K.M.; Hülsey, M.J.; Asakura, H.; Liu, L.; Han, Y.; Karp, E.M.; Beckham, G.T. Catalytic amino acid production from biomass-derived intermediates. Proc. Natl. Acad. Sci. USA 2018, 115, 5093–5098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, H.; Naghadeh, S.B.; Zhang, J.Z.; Fang, P. Visible light driven hydrogen evolution by photocatalytic reforming of lignin and lactic acid using one-dimensional NiS/CdS nanostructures. Appl. Catal. B Environ. 2018, 227, 229–239. [Google Scholar] [CrossRef]
- Kong, L.; Li, G.; Wang, H.; He, W.; Ling, F. Hydrothermal catalytic conversion of biomass for lactic acid production. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2008, 83, 383–388. [Google Scholar] [CrossRef]
- Huang, C.-W.; Nguyen, B.-S.; Wu, J.C.-S.; Nguyen, V.-H. A current perspective for photocatalysis towards the hydrogen production from biomass-derived organic substances and water. Int. J. Hydrogen Energy 2020, 45, 18144–18159. [Google Scholar] [CrossRef]
- Duan, Q.; Ji, J.; Hong, X.; Fu, Y.; Wang, C.; Zhou, K.; Liu, X.; Yang, H.; Wang, Z.-Y. Design of hole-transport-material free CH3NH3PbI3/CsSnI3 all-perovskite heterojunction efficient solar cells by device simulation. Sol. Energy 2020, 201, 555–560. [Google Scholar] [CrossRef]
- Chaves, A.; Azadani, J.; Alsalman, H.; Da Costa, D.; Frisenda, R.; Chaves, A.; Song, S.H.; Kim, Y.; He, D.; Zhou, J. Bandgap engineering of two-dimensional semiconductor materials. NPJ 2D Mater. Appl. 2020, 4, 29. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.-F.; Yong, X.; Kumar, P.; Palma, B.; Hu, Z.-Y.; Van Tendeloo, G.; Siahrostami, S.; Larter, S.; Zheng, D. Coproduction of hydrogen and lactic acid from glucose photocatalysis on band-engineered Zn1-xCdxS homojunction. Iscience 2021, 24, 102109. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhong, L.X.; Chen, W.; Huang, Y.; Chen, Z.; Xiao, D.; Zou, R.; Chen, L.; Hao, Q.; Liu, Z. Regulating the electron–hole separation to promote selective oxidation of biomass using ZnS@ Bi2S3 nanosheet catalyst. Appl. Catal. B Environ. 2021, 292, 120180. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Jin, D.; Jiao, G.; Yang, X.; Liu, K.; Zhou, J.; Sun, R. Copper oxide functionalized chitosan hybrid hydrogels for highly efficient photocatalytic-reforming of biomass-based monosaccharides to lactic acid. Appl. Catal. B Environ. 2021, 291, 120123. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Rasrendra, C.B.; Fachri, B.A.; Makertihartha, I.G.B.; Adisasmito, S.; Heeres, H.J. Catalytic conversion of dihydroxyacetone to lactic acid using metal salts in water. ChemSusChem 2011, 4, 768–777. [Google Scholar] [CrossRef]
- Wang, J.; Masui, Y.; Onaka, M. Conversion of triose sugars with alcohols to alkyl lactates catalyzed by Brønsted acid tin ion-exchanged montmorillonite. Appl. Catal. B Environ. 2011, 107, 135–139. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Xie, Y.; Wei, C.; Liu, C.; Song, F.; Sun, X.; Zhang, Y.; Cui, H. Synergistic catalysis of species in molten salt hydrate for conversion of cellulose to 5-hydroxymethylfurfural. Biomass Bioenergy 2022, 158, 106363. [Google Scholar] [CrossRef]
- Gómez Millán, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent advances in the catalytic production of platform chemicals from holocellulosic biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Dhepe, P.L. Fukuoka A: Cellulose conversion under heterogeneous catalysis. ChemSusChem Chem. Sustain. Energy Mater. 2008, 1, 969–975. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.; Li, M.; Zhang, S.; Bai, H.; Yang, L.; Zhang, S. One-pot hydrothermal synthesis of SnO2/BiOBr heterojunction photocatalysts for the efficient degradation of organic pollutants under visible light. ACS Appl. Mater. Interfaces 2018, 10, 28686–28694. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Tu, Y.; Chai, Y.; Yuan, R. Photoelectrochemical Assay Based on SnO2/BiOBr p–n Heterojunction for Ultrasensitive DNA Detection. Anal. Chem. 2021, 93, 12995–13000. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yuan, C.; Chen, R.; Wang, J.; Dong, F.; Li, J.; Sun, Y. Mechanisms of Interfacial Charge Transfer and Photocatalytic NO Oxidation on BiOBr/SnO2 p–n Heterojunctions. ACS Appl. Mater. Interfaces 2020, 12, 43741–43749. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, M.; Zhang, H.; Wang, B.; Peng, J.; Liu, G. One-Step Synthesis of Hierarchical Flower-like SnO2/BiOCOOH Microspheres with Enhanced Light Response for the Removal of Pollutants. Langmuir 2020, 36, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Tammanoon, N.; Wisitsoraat, A.; Tuantranont, A.; Liewhiran, C. Flame-made Zn-substituted SnO2 nanoparticulate compound for ultra-sensitive formic acid gas sensing. J. Alloy. Compd. 2021, 871, 159547. [Google Scholar] [CrossRef]
- Duresa, L.W.; Kuo, D.-H.; Bekena, F.T.; Kebede, W.L. Simple room temperature synthesis of oxygen vacancy-rich and In-doped BiOBr nanosheet and its highly enhanced photocatalytic activity under visible-light irradiation. J. Phys. Chem. Solids 2021, 156, 110132. [Google Scholar] [CrossRef]
- Lv, J.; Dai, K.; Zhang, J.; Liu, Q.; Liang, C.; Zhu, G. Facile constructing novel 2D porous g-C3N4/BiOBr hybrid with enhanced visible-light-driven photocatalytic activity. Sep. Purif. Technol. 2017, 178, 6–17. [Google Scholar] [CrossRef]
- Xiao, K.; Huang, H.; Tian, N.; Zhang, Y. Mixed-calcination synthesis of Bi2MoO6/g-C3N4 heterojunction with enhanced visible-light-responsive photoreactivity for RhB degradation and photocurrent generation. Mater. Res. Bull. 2016, 83, 172–178. [Google Scholar] [CrossRef]
- Kumaravelan, S.; Seshadri, S.; Suresh, R.; Ravichandran, K.; Sathishkumar, P.; Shanthaseelan, K.; Suganthi, N. Effect of Zn dopant on SnO2 nano-pyramids for photocatalytic degradation. Chem. Phys. Lett. 2021, 769, 138352. [Google Scholar] [CrossRef]
- Qiu, F.; Li, W.; Wang, F.; Li, H.; Liu, X.; Sun, J. In-situ synthesis of novel Z-scheme SnS2/BiOBr photocatalysts with superior photocatalytic efficiency under visible light. J. Colloid Interface Sci. 2017, 493, 1–9. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. A simple large-scale method for preparation of g-C3N4/SnO2 nanocomposite as visible-light-driven photocatalyst for degradation of an organic pollutant. Mater. Express 2015, 5, 309–318. [Google Scholar] [CrossRef]
- Suthakaran, S.; Dhanapandian, S.; Krishnakumar, N.; Ponpandian, N. Hydrothermal synthesis of surfactant assisted Zn doped SnO2 nanoparticles with enhanced photocatalytic performance and energy storage performance. J. Phys. Chem. Solids 2020, 141, 109407. [Google Scholar] [CrossRef]
- Ao, Y.; Wang, K.; Wang, P.; Wang, C.; Hou, J. Synthesis of novel 2D-2D pn heterojunction BiOBr/La2Ti2O7 composite photocatalyst with enhanced photocatalytic performance under both UV and visible light irradiation. Appl. Catal. B Environ. 2016, 194, 157–168. [Google Scholar] [CrossRef]
- Mao, W.; Bao, K.; Cao, F.; Chen, B.; Liu, G.; Wang, W.; Li, B. Synthesis of a CoTiO3/BiOBr heterojunction composite with enhanced photocatalytic performance. Ceram. Int. 2017, 43, 3363–3368. [Google Scholar] [CrossRef]
- Xiong, F.; Yin, L.-L.; Li, F.; Wu, Z.; Wang, Z.; Sun, G.; Xu, H.; Chai, P.; Gong, X.-Q.; Huang, W. Anatase TiO2 (001)-(1 × 4) Surface Is Intrinsically More Photocatalytically Active than the Rutile TiO2 (110)-(1 × 1) Surface. J. Phys. Chem. C 2019, 123, 24558–24565. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Zhang, L.; Liu, W.; Zhang, C.; Shi, Y.; Lin, H. Regulating superoxide radicals and light absorption ability for enhancing photocatalytic performance of MoS2@ Z by CeO2 rich in adsorbed oxygen. J. Clean. Prod. 2021, 322, 129059. [Google Scholar] [CrossRef]
- Ma, H.; Feng, J.; Jin, F.; Wei, M.; Liu, C.; Ma, Y. Where do photogenerated holes at the gC3N4/water interface go for water splitting: H2O or OH−? Nanoscale 2018, 10, 15624–15631. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; Shah, T. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]






| Materials | Specific Surface Area (m2·g−1) | Average Pore Sizes (nm) |
|---|---|---|
| BiOBr | 5.8 m2·g−1 | 3.8 nm |
| BiOBr/SnO2 | 42.7 m2·g−1 | 17.0 nm |
| BiOBr/Zn@SnO2 | 61.5 m2·g−1 | 17.1 nm |
| Material | Catalyst | Experimental Conditions | Product | Literature |
|---|---|---|---|---|
| Fructose | BiOBr/Zn@SnO2 | light 3 h | Lactic Acid 79.6% | This experiment |
| Glucose | Ni 400 ppm | 240 °C 120 s | Lactic Acid 8.37% | Technical Note Hydrothermal catalytic conversion of biomass for lactic acid production |
| Fructose | Sn-Beta zeolite | 140 °C | Lactic acid methyl ester 44% | Conversion of Sugars to Lactic Acid Derivatives Using Heterogeneous Zeotype Catalysts |
| Cellulose | Y-Beta zeolite | 220 °C 2 MPa N2 | Lactic Acid 49.2% | Efficient conversion of cellulose to lactic acid over yttrium modified siliceous Beta zeolites |
| Glucose | 0.15 M Ba(OH)2 | 60 °C 12 h | Lactic Acid 42.55% | Ca(OH)2 induced a controlled-release catalytic system for the efficient conversion of high-concentration glucose to lactic acid |
| Glucose | Zn0.6Cd0.4S | light 5 h | Lactic Acid 35% | Coproduction of hydrogen and lactic acid from glucose photocatalysis on band-engineered Zn 1−x Cd x S homojunction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.-J.; Zhang, Y.-H.; Wang, Z.; Yang, G.-H. Photocatalytic Conversion of Fructose to Lactic Acid by BiOBr/Zn@SnO2 Material. Catalysts 2022, 12, 719. https://doi.org/10.3390/catal12070719
Qin H-J, Zhang Y-H, Wang Z, Yang G-H. Photocatalytic Conversion of Fructose to Lactic Acid by BiOBr/Zn@SnO2 Material. Catalysts. 2022; 12(7):719. https://doi.org/10.3390/catal12070719
Chicago/Turabian StyleQin, Hong-Juan, Yu-Hang Zhang, Zhen Wang, and Gui-Hua Yang. 2022. "Photocatalytic Conversion of Fructose to Lactic Acid by BiOBr/Zn@SnO2 Material" Catalysts 12, no. 7: 719. https://doi.org/10.3390/catal12070719
APA StyleQin, H.-J., Zhang, Y.-H., Wang, Z., & Yang, G.-H. (2022). Photocatalytic Conversion of Fructose to Lactic Acid by BiOBr/Zn@SnO2 Material. Catalysts, 12(7), 719. https://doi.org/10.3390/catal12070719






