Role of Post-Hydrothermal Treatment on the Microstructures and Photocatalytic Activity of TiO2-Based Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
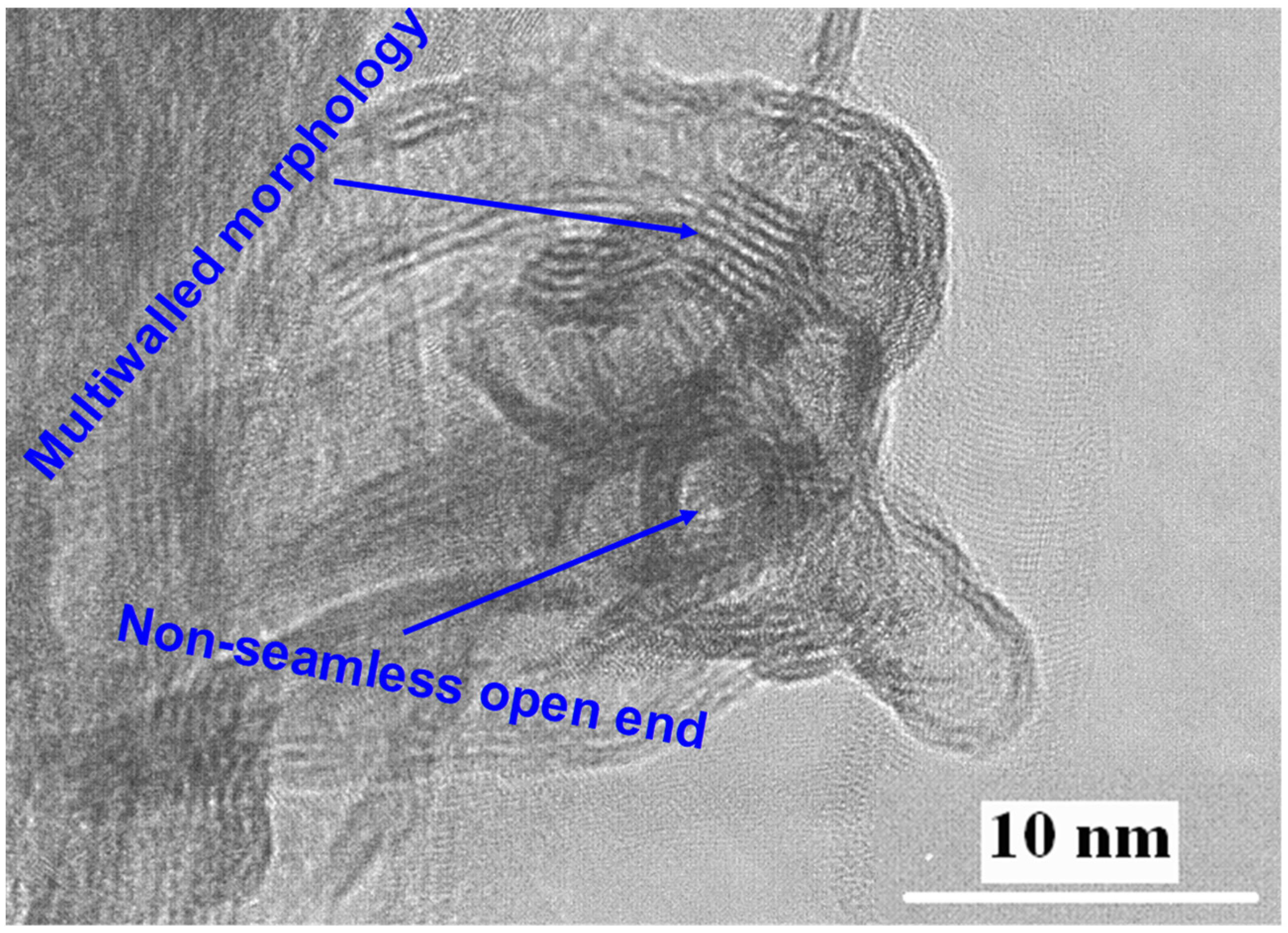
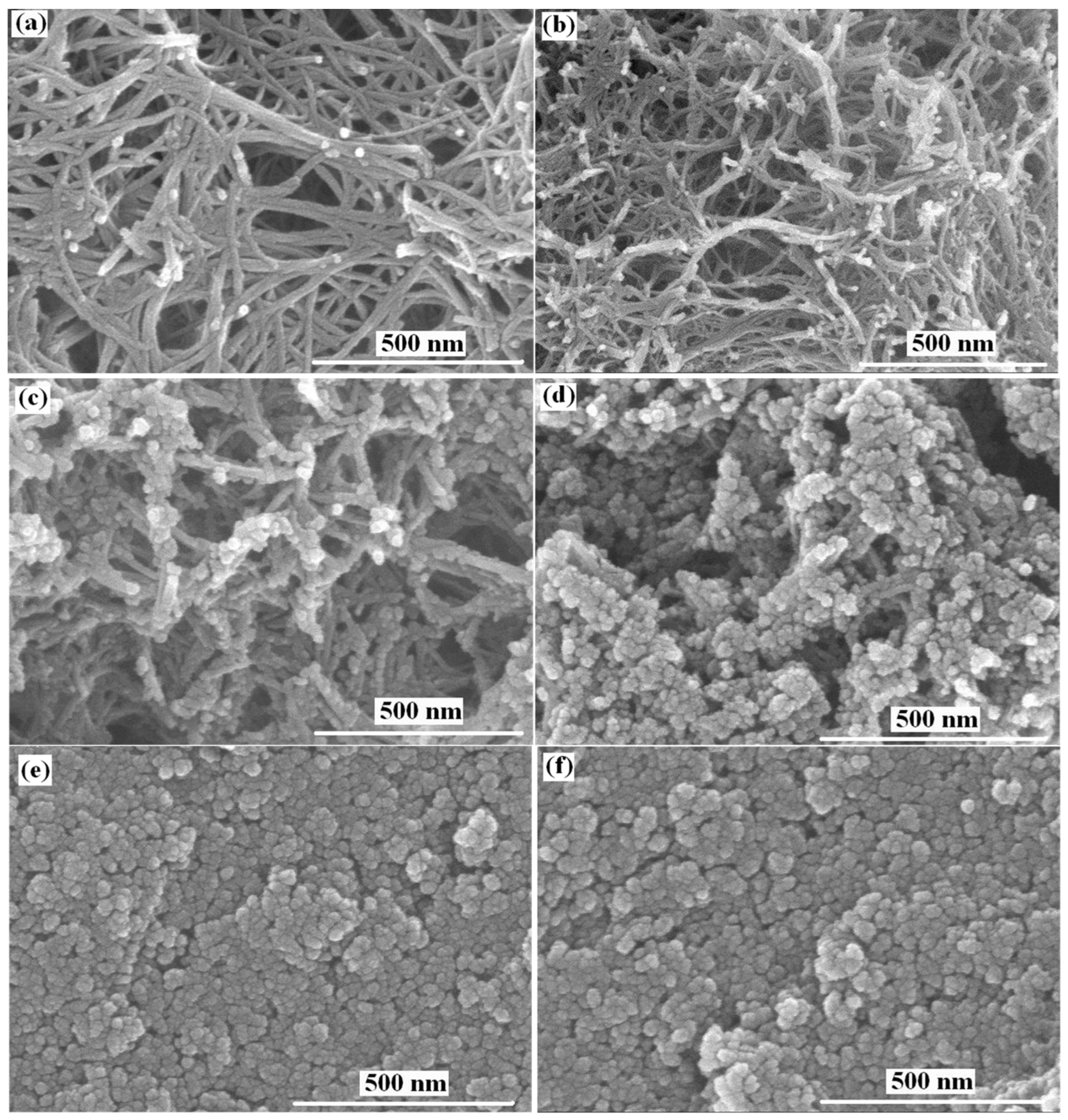
2.1. Impact of Post-Hydrothermal Treatment on Phase Structure and Shape and Morphology
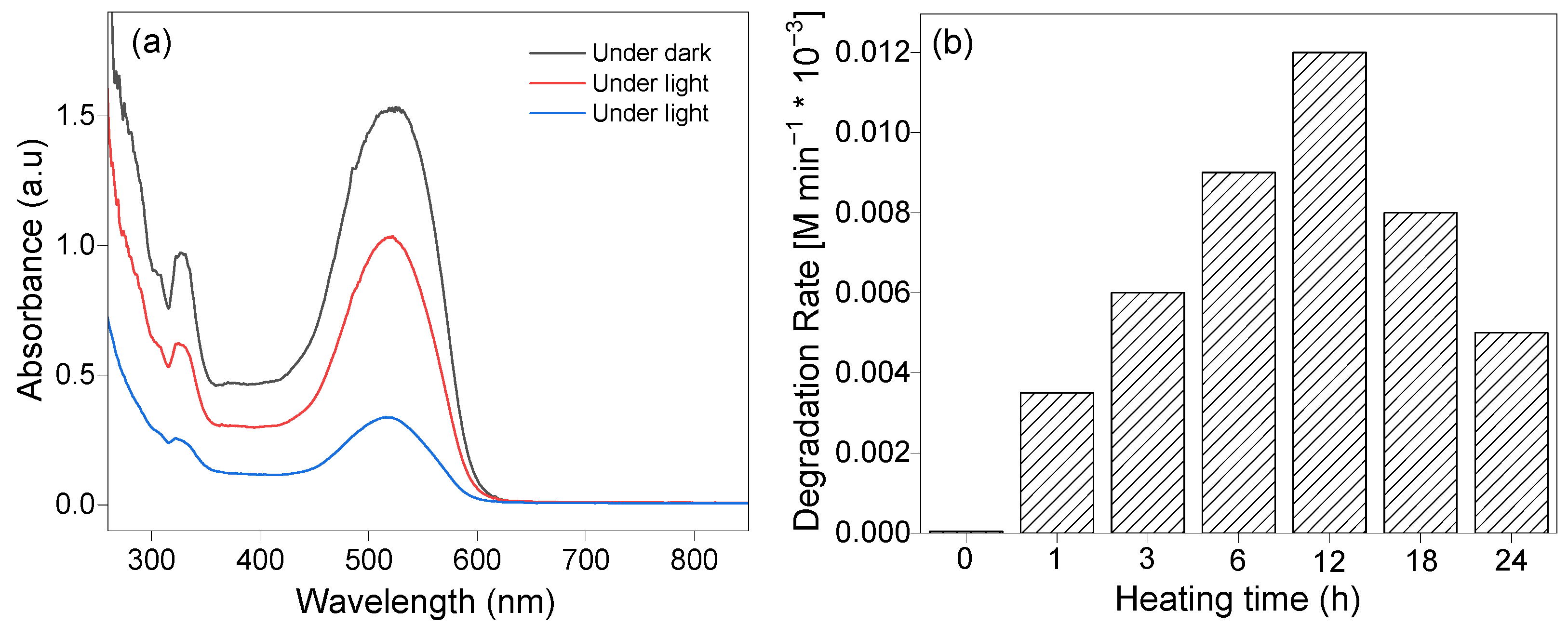
2.2. Impact of Post-Hydrothermal Processing on Photocatalytic Activity
2.3. Impact of pH on the Degradation Rate
2.4. Impact of pH on the Degradation Rate
2.5. Impact of Photocatalyst Loading on the Degradation Rate
2.6. Role of Conduction Band Electrons/O2−• and Holes/OH•
3. Materials and Methods
3.1. Synthesis
3.2. Structural Characterization
3.3. Evaluation of Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Everhart, B.M.; McAuley, B.; Mayyahi, A.A.; Tonyali, B.; Yucel, Y.; Amama, P.B. Photocatalytic NOx mitigation under relevant conditions using carbon nanotube-modified titania. Chem. Eng. J. 2022, 446, 136984. [Google Scholar] [CrossRef]
- Li, L.; Xiao, S.; Li, R.; Cao, Y.; Chen, Y.; Li, Z.; Li, G.; Li, H. Nanotube array-like WO3 photoanode with dual-layer oxygen-evolution cocatalysts for photoelectrocatalytic overall water splitting. ACS Appl. Energy Mater. 2018, 1, 6871–6880. [Google Scholar] [CrossRef]
- He, Q.; Qiao, S.; Zhou, Y.; Vajtai, R.; Li, D.; Ajayan, P.M.; Ci, L.; Song, L. Carbon nanotubes-based electrocatalysts: Structural regulation, support effect, and synchrotron-based characterization. Adv. Func. Mater. 2022, 32, 2106684. [Google Scholar] [CrossRef]
- Chang, K.C.; Zhou, Q.; Liu, K.; Li, L.; Zhang, R.; Liu, H.-J.; Kuo, T.-P. Eco-friendly, highly efficient ethanol-assisted supercritical preparation of an ultrathin ZnO nanotube. ACS Sustain. Chem. Eng. 2021, 9, 15478–15483. [Google Scholar] [CrossRef]
- Ivanoskii, A.L. Non-carbon nanotubes: Synthesis and simulation. Russ. Chem. Rev. 2022, 71, 175–194. [Google Scholar]
- Rempel, A.A.; Valeeva, A.A.; Vokhmintsev, A.S.; Weinstein, I.A. Titanium dioxide nanotubes: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2021, 90, 1397. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Humayun, M.; Wang, C.; Luo, W. Recent progress in the synthesis and applications of composite photocatalysts: A critical review. Small 2022, 6, 2101395. [Google Scholar] [CrossRef]
- Mohamedkhair, A.K.; Drmosh, Q.A.; Qamar, M.; Yamani, Z.H. Tuning structural properties of WO3 thin films for photoelectrocatalytic water oxidation. Catalysts 2021, 11, 381. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hezam, A.; Hossain, M.K.; Qamar, M.; Yamani, Z.H.; Byrappa, K. A novel Cs2O–Bi2O3–TiO2–ZnO heterostructure with direct Z-Scheme for efficient photocatalytic water splitting. Ceram. Int. 2019, 45, 23756–23764. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Airinei, A.; Olaru, N.; Rosca, I.; Koudoumas, E.; Suchea, M.P. Innovative Ag–TiO2 nanofibers with excellent photocatalytic and antibacterial actions. Catalysts 2021, 11, 1234. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of poly(titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Badmus, K.O.; Wewers, F.; Al-Abri, M.; Shahbaaz, M.; Petrik, L.F. Synthesis of oxygen deficient TiO2 for improved photocatalytic efficiency in solar radiation. Catalysts 2021, 11, 904. [Google Scholar] [CrossRef]
- Gyulavári, T.; Dusnoki, D.; Márta, V.; Yadav, M.; Abedi, M.; Sápi, A.; Kukovec, A.; Kónya, Z.; Pap, Z. Dependence of photocatalytic activity on the morphology of strontium titanates. Catalysts 2022, 12, 523. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, G. ; Armstrong, A.R.; Canales, J.; Bruce, P.G. Nanotubes with the TiO2-B structure. Chem. Commun. 2005, 19, 2454–2456. [Google Scholar] [CrossRef]
- Armstrong, A.R. ; Armstrong, G.; Canales, J.; Garcia, R.;Bruce, P.G. Lithium-ion intercalation into TiO2-B nanowires. Adv. Mater. 2005, 17, 862–865. [Google Scholar] [CrossRef]
- Wu, Z.; Hwang, I.; Cha, G.; Qin, S.; Tomanec, O.; Badura, Z.; Kment, S.; Zboril, R.; Schmuki, P. Optimized Pt single atom harvesting on TiO2 nanotubes—Towards a most efficient photocatalyst. Small 2022, 18, 2104892. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, X.; Lin, H.; Li, J.; Zhang, H.; Guo, J.; Jia, D.; Zhang, H. A novel carbon support: Few-layered graphdiyne-decorated carbon nanotubes capture metal clusters as effective metal-supported catalysts. Small 2021, 17, 2006442. [Google Scholar] [CrossRef]
- Wiel, L.; Li, H.; Rust, C.; Chen, J.; Flavel, B.S. Carbon nanotubes for photovoltaics: From lab to industry. Adv. Energy Mater. 2021, 11, 2002880. [Google Scholar]
- Kasuga, T. ; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Gautam, J.; Yang, J.-M.; Yang, B.l. Transition metal co-doped TiO2 nanotubes decorated with Pt nanoparticles on optical fibers as an efficient photocatalyst for the decomposition of hazardous gaseous pollutants. Colloid Surf. A 2022, 643, 128786. [Google Scholar] [CrossRef]
- Kim, E.; Do, K.H.; Wang, J.; Hong, Y.; Rangappa, A.P.; Reddy, D.A.; Kumar, D.P.; Kim, T.K. Construction of 1D TiO2 nanotubes integrated ultrathin 2D ZnIn2S4 nanosheets heterostructure for highly efficient and selective photocatalytic CO2 reduction. App. Surf. Sci. 2022, 587, 152895. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Chen, Z.; Li, J.; Li, X.; Huo, P.; Wang, Q. TiO2 modified g-C3N4 with enhanced photocatalytic CO2 reduction performance. Solid State Sci. 2020, 100, 106099. [Google Scholar] [CrossRef]
- Fabrication of PbS nanocrystal-sensitized ultrafine TiO2 nanotubes for efficient and unusual broadband-light-driven hydrogen production. Mater. Today Chem. 2020, 17, 100310. [CrossRef]
- Guo, J.Q.; Fan, Y.-P.; Dong, X.-S.; Ma, X.-M.; Yao, S.-L.; Xing, H.-J. Modified coal tailings with TiO2 nanotubes and their application for methylene blue removal. Colloid Surf. A 2021, 627, 127211. [Google Scholar] [CrossRef]
- Liu, W.; Borthwick, A.G.L.; Li, X.Z.; Ni, J.R. High photocatalytic and adsorptive performance of anatase-covered titanate nanotubes prepared by wet chemical reaction. Micropor. Mesopor. Mater. 2014, 186, 168–175. [Google Scholar] [CrossRef]
- Cheng, K.; Cai, Z.; Fu, J.; Sun, X.; Sun, W.; Chen, L.; Zhang, D.; Liu, W. Synergistic adsorption of Cu (II) and photocatalytic degradation of phenanthrene by a jaboticaba-like TiO2/titanate nanotube composite: An experimental and theoretical study. Chem. Eng. J. 2019, 358, 1155–1165. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, Z.; Wang, T.; O’Reilly, S.; Liu, W.; Zhao, D. A new type of cobalt-deposited titanate nanotubes for enhanced photocatalytic degradation of phenanthrene. Appl. Catal. B Environ. 2016, 187, 134–143. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Kim, D.H.; Jho, J.H.; Lee, K.S.; Lee, W.J.; Lee, H.G.; Kim, S.J. Effect of post treatments on the structure and thermal stability of titanate nanotubes. Nanotechnology 2006, 17, 5922–5929. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Lee, N.H.; Park, K.; Kim, D.H.; Lee, K.S.; Lee, W.J.; Kim, S.J. Preparation and photocatalytic activity of nanotubes obtained from titanium dioxide. Catal. Today 2008, 131, 3–14. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Gao, X.P.; Lan, Y.; Song, D.Y.; Xi, Y.X.; Zhao, J.C. Hydrogen titanate nanofibers covered with anatase nanocrystals: A delicate structure achieved by the wet chemistry reaction of the titanate nanofibers. J. Am. Chem. Soc. 2004, 126, 8380–8381. [Google Scholar] [CrossRef]
- Yang, C.; Olsen, T.; Lau, M.L.; Smith, K.A.; Hattar, K.; Sen, A.; Xiong, H. In situ ion irradiation of amorphous TiO2 nanotubes. J. Mater. Res. 2022, 37, 1144–1155. [Google Scholar] [CrossRef]
- Zhukovskii, Y.F.; Pugno, N.; Popov, A.I.; Balasubramanian, C.; Bellucci, S. Influence of F centres on structural and electronic properties of AlN single-walled nanotubes. J. Phys. Condens. Matter 2007, 19, 395021. [Google Scholar] [CrossRef] [Green Version]
- Zavala, M.Á.L.; Morales, S.A.L.; Ávila-Santos, M. Synthesis of stable TiO2 nanotubes: Effect of hydrothermal treatment, acid washing and annealing temperature. Heliyon 2017, 3, e00456. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sakulkhaemaruethai, S.; Yoshida, R.; Yoshikawa, S. Heat treatment effect on the structure of TiO2-derived nanotubes prepared by hydrothermal method. In Ceramic Nanomaterials and Nanotechnology III, Proceedings of the 106th Annual Meeting of The American Ceramic Society, Indianapolis, Indiana, USA 2004; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 159, pp. 185–192. [Google Scholar]
- J Suzuki, Y.; Yoshikawa, S. Synthesis and thermal analyses of TiO2-derived nanotubes prepared by the hydrothermal method. J. Mater. Res. 2004, 19, 982–985. [Google Scholar] [CrossRef]
- Qamar, M.; Muneer, M. A comparative photocatalytic activity of titanium dioxide and zinc oxide by investigating the degradation of vanillin. Desalination 2009, 249, 535–540. [Google Scholar] [CrossRef]
- Qamar, M.; Gondal, M.A.; Yamani, Z.H. Synthesis of highly active nanocrystalline WO3 and its application in laser-induced photocatalytic removal of a dye from water. Catal. Commun. 2009, 10, 1980–1984. [Google Scholar] [CrossRef]
- Bahnemann, D.; Muneer, M.; Haque, M. Titanium dioxide-mediated photocatalysed degradation of few selected organic pollutants in aqueous suspensions. Catal. Today 2007, 124, 133–148. [Google Scholar] [CrossRef]
- Muneer, M.; Qamar, M.; Saquib, M.; Bahnemann, D.W. Heterogeneous photocatalysed reaction of three selected pesticide derivatives, propham, propachlor and tebuthiuron in aqueous suspensions of titanium dioxide. Chemosphere 2005, 61, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Muneer, M.; Bahnemann, D.W. Semiconductor-mediated photocatalyzed degradation of a herbicide derivative, chlorotoluron, in aqueous suspensions. Environ. Sci. Technol. 2006, 40, 4765. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Muneer, M. TiO2-mediated photocatalytic degradation of a textile dye derivative, bromothymol blue, in aqueous suspensions. Dye. Pigment. 2007, 75, 443–448. [Google Scholar] [CrossRef]
- Singh, H.K.; Saquib, M.; Haque, M.M.; Muneer, M.; Bahnemann, D.W. Heterogeneous photocatalyzed degradation of uracil and 5-bromouracil in aqueous suspensions of titanium dioxide. J. Hazard. Mater. 2007, 142, 425–430. [Google Scholar] [CrossRef]
- Reda, S.M.; Khairy, M.; Mousa, M.A. Photocatalytic activity of nitrogen and copper doped TiO2 nanoparticles prepared by microwave-assisted sol-gel process. Arab. J. Chem. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Photocatalytic oxidation of paracetamol: Dominant reactants, intermediates, and reaction mechanisms. Environ. Sci. Technol. 2009, 43, 460–465. [Google Scholar] [CrossRef]
- Mohammadi, T.; Isfahani, A.Z. Photooxidation of salicylic acid with TiO2 and metal coated TiO2. Acta Chim. Slov. 2008, 55, 172–178. [Google Scholar]
- Carlotti, M.E.; Sapino, S.; Vione, D.; Minero, C.; Peira, E.; Trotta, M. Study on the photodegradation of salicylic acid in different vehicles in the absence and in the presence of TiO2. J. Dispers. Sci. Technol. 2007, 28, 805–818. [Google Scholar] [CrossRef]
- Lin, C.J.; Yang, W.T.; Chou, C.Y.; Liou, S.Y.H. Hollow mesoporous TiO2 microspheres for enhanced photocatalytic degradation of acetaminophen in water. Chemosphere 2016, 152, 490–495. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2-Pd and TiO2-Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Roşu, M.C.; Socaci, C.; Floare-Avram, V.; Borodi, G.; PogǍcean, F.; Coroş, M.; MǍgeruşan, L.; Pruneanu, S. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Sagadevan, S.; Oh, W.-C. Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite. Sci. Rep. 2022, 16, 5759. [Google Scholar] [CrossRef] [PubMed]



| Sample | BET Surface Area (m2g−1) | Anatase Crystallite Size (nm) |
|---|---|---|
| As-prepared | 254 | * |
| Acid-treated | 353 | * |
| 1 h | 262 | ~6 |
| 3 h | 182 | ~14 |
| 6 h | 119 | ~21 |
| 12 h | 107 | ~25 |
| 18 h | 94 | ~28 |
| 24 h | 92 | ~32 |
| Parameter | Value | Degradation Rates [M min−1 × 10−3] |
|---|---|---|
| Solution pH | 3.5 | 0.0120 |
| 5.6 | 0.0144 | |
| 7.9 | 0.0183 | |
| 10.4 | 0.0156 | |
| Substrate concentration (mM) | 0.15 | 0.0120 |
| 0.25 | 0.0167 | |
| 0.35 | 0.0268 | |
| 0.50 | 0.0323 | |
| 0.70 | 0.0231 | |
| Catalyst loading (g L−1) | 0.5 | 0.0080 |
| 1.0 | 0.0120 | |
| 2.0 | 0.0157 | |
| 3.0 | 0.0198 | |
| 4.0 | 0.0212 | |
| 5.0 | 0.0215 |
| Catalyst | Catalyst Loading | Pollutant | Pollutant Concentration | Rate/Degradation Rate | References |
|---|---|---|---|---|---|
| TiO2 (P25) | 1.0 g L−1 | Tebuthiuron | 0.5 mM | 0.012 × 10−3 M min−1 | [42,43] |
| TiO2 (UV100) | 1.0 g L−1 | Tebuthiuron | 0.5 mM | 0.004 × 10−3 M min−1 | [42,43] |
| TiO2 (UV100) | 1.0 g L−1 | Propachlor | 0.6 mM | 0.0016 × 10−3 M min−1 | [42,43] |
| TiO2 (P25) | 1.0 g L−1 | Chlortoluron | 0.25 mM | 0.013 × 10−3 M min−1 | [42,44] |
| TiO2 (UV100) | 1.0 g L−1 | Chlortoluron | 0.25 mM | 0.004 × 10−3 M min−1 | [42,44] |
| TiO2 (PC500) | 1.0 g L−1 | Chlortoluron | 0.25 mM | 0.002 × 10−3 M min−1 | [42,44] |
| TiO2 (UV100) | 1.0 g L−1 | Bromothymol Blue | 0.25 mM | 0.009 × 10−3 M min−1 | [42,45] |
| TiO2 (P25) | 1.0 g L−1 | 5-Bromouracil | 1.0 mM | 0.0028 × 10−3 M min−1 | [42,46] |
| TiO2 nanoparticles | 0.1 g L−1 | Methylene blue | 0.09 mM | 7.0 × 10−4 min−1 | [47] |
| TiO2 (P25) | 0.4 g L−1 | Paracetamol | 0.25 mM | 1.9 × 10−3 min−1 | [48] |
| TiO2 (P25) | 1.0 g L−1 | Salicylic acid | 0.1 mM | 1.9 × 10−6 M min−1 | [49] |
| TiO2 (anatase) | 0.5 g L−1 | Salicylic acid | 0.36 mM | 1.7 × 10−3 min−1 | [50] |
| Hollow mesoporous TiO2 microspheres | 0.1 g L−1 | Acetaminophen | 0.33 mM | 4.3 × 10−2 min−1 | [51] |
| TiO2–Pd | 1 g L−1 | Diclofenac | 0.17 mM | 5.0 × 10−2 min−1 | [52] |
| TiO2–Ag | 4.0 × 10−2 min−1 | ||||
| Graphene/TiO2-Ag composites | 4 mg | Amaranth | 0.02 mM | 5.8 × 10−2 min−1 | [53] |
| TiO2 nanoparticles | 1.0 g L−1 | Acid Blue-29 | 0.06 mM | 0.6 × 10−4 M min−1 | [54] |
| CdS | 1.0 g L−1 | Acid Blue-29 | 0.06 mM | 4.5 × 10−4 M min−1 | [54] |
| CdS-TiO2 | 1.0 g L−1 | Acid Blue-29 | 0.06 mM | 5.8 × 10−4 M min−1 | [54] |
| Ag–TiO2 nanofibers | 0.4 g L−1 | Methylene blue dye | 0.03 mM | 1.29 × 10−2 min−1 | [12] |
| TiO2-based nanotubes | 1.0 g L−1 | Amaranth | 0.15 mM | 0.012× 10−3 M min−1 | [This study] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qamar, M.; Zaidi, S.A.; Rafatullah, M.; Qutob, M.; Kim, S.-J.; Drmosh, Q.A. Role of Post-Hydrothermal Treatment on the Microstructures and Photocatalytic Activity of TiO2-Based Nanotubes. Catalysts 2022, 12, 702. https://doi.org/10.3390/catal12070702
Qamar M, Zaidi SA, Rafatullah M, Qutob M, Kim S-J, Drmosh QA. Role of Post-Hydrothermal Treatment on the Microstructures and Photocatalytic Activity of TiO2-Based Nanotubes. Catalysts. 2022; 12(7):702. https://doi.org/10.3390/catal12070702
Chicago/Turabian StyleQamar, Mohammad, Shabi Abbas Zaidi, Mohd Rafatullah, Mohammad Qutob, Sun-Jae Kim, and Qasem A. Drmosh. 2022. "Role of Post-Hydrothermal Treatment on the Microstructures and Photocatalytic Activity of TiO2-Based Nanotubes" Catalysts 12, no. 7: 702. https://doi.org/10.3390/catal12070702
APA StyleQamar, M., Zaidi, S. A., Rafatullah, M., Qutob, M., Kim, S.-J., & Drmosh, Q. A. (2022). Role of Post-Hydrothermal Treatment on the Microstructures and Photocatalytic Activity of TiO2-Based Nanotubes. Catalysts, 12(7), 702. https://doi.org/10.3390/catal12070702








