Photodegradation under UV Light Irradiation of Various Types and Systems of Organic Pollutants in the Presence of a Performant BiPO4 Photocatalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Calculation Methods
2.4. Photocatalytic Experiments
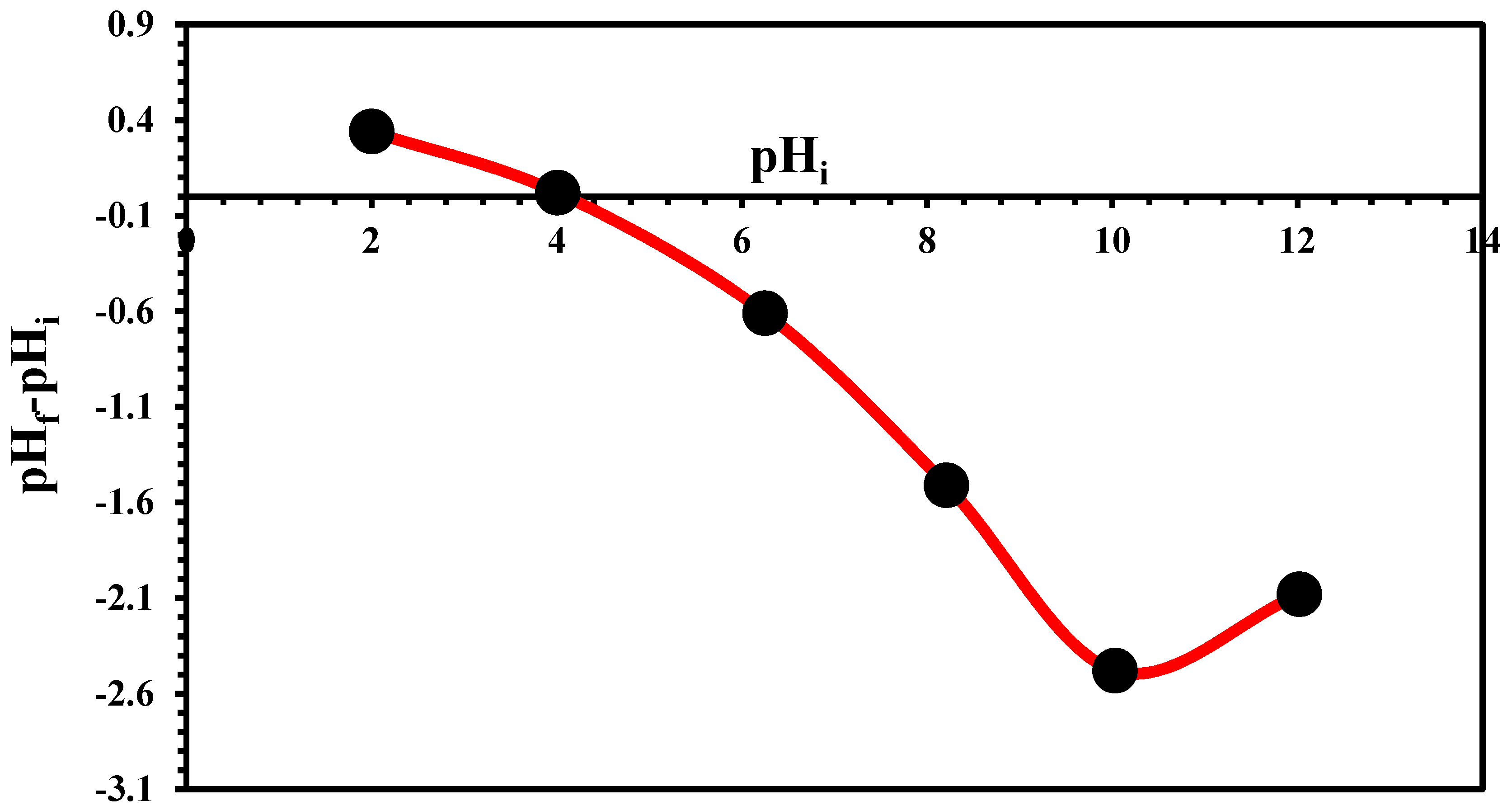
2.5. Point of Zero Charge Determination
2.6. Total Organic Carbon Analysis
3. Characterizations of the BiPO4 Photocatalyst
3.1. Structural Studies
3.2. Scanning Electron Microscopy
3.3. FT-IR Spectroscopy Analyses
3.4. UV-Vis Diffuse Reflectance Spectroscopy
3.5. Density Functional Theory Calculations Results
4. Evaluation of the Photocatalytic Activity of BiP-500
4.1. Photolysis and Adsorption Test of BiP-500
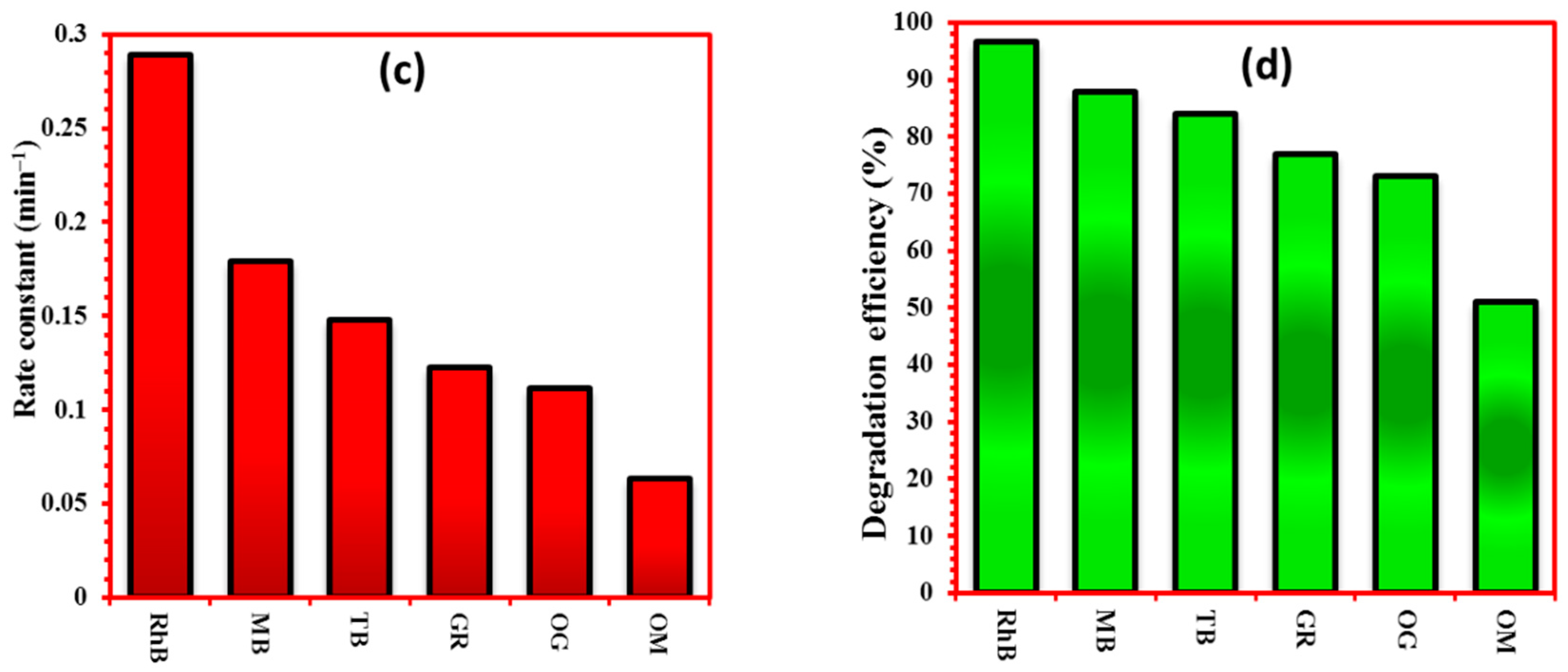
4.2. Photodegradation of Various Organic Dyes
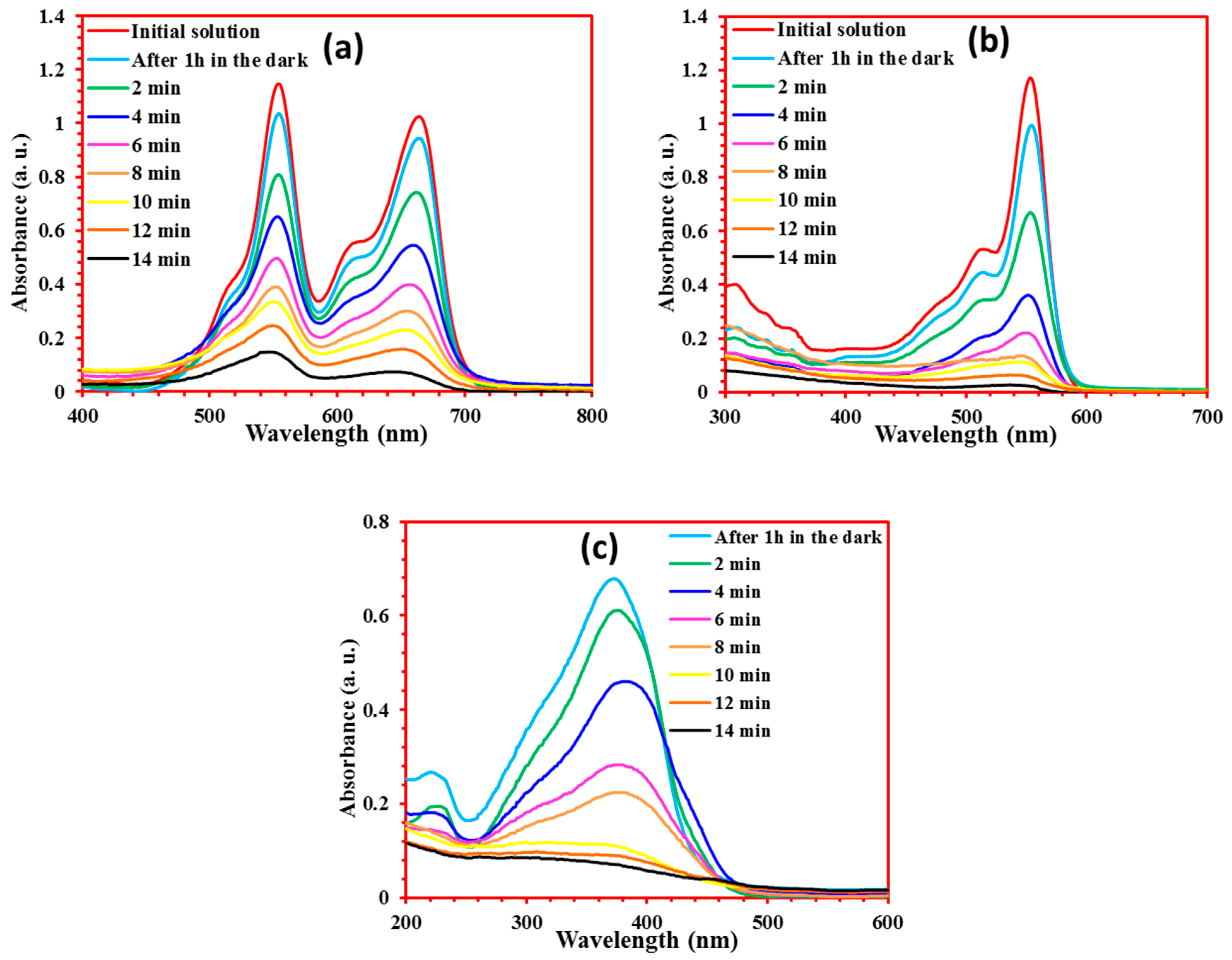
4.3. Photodegradation of Dyes Mixtures
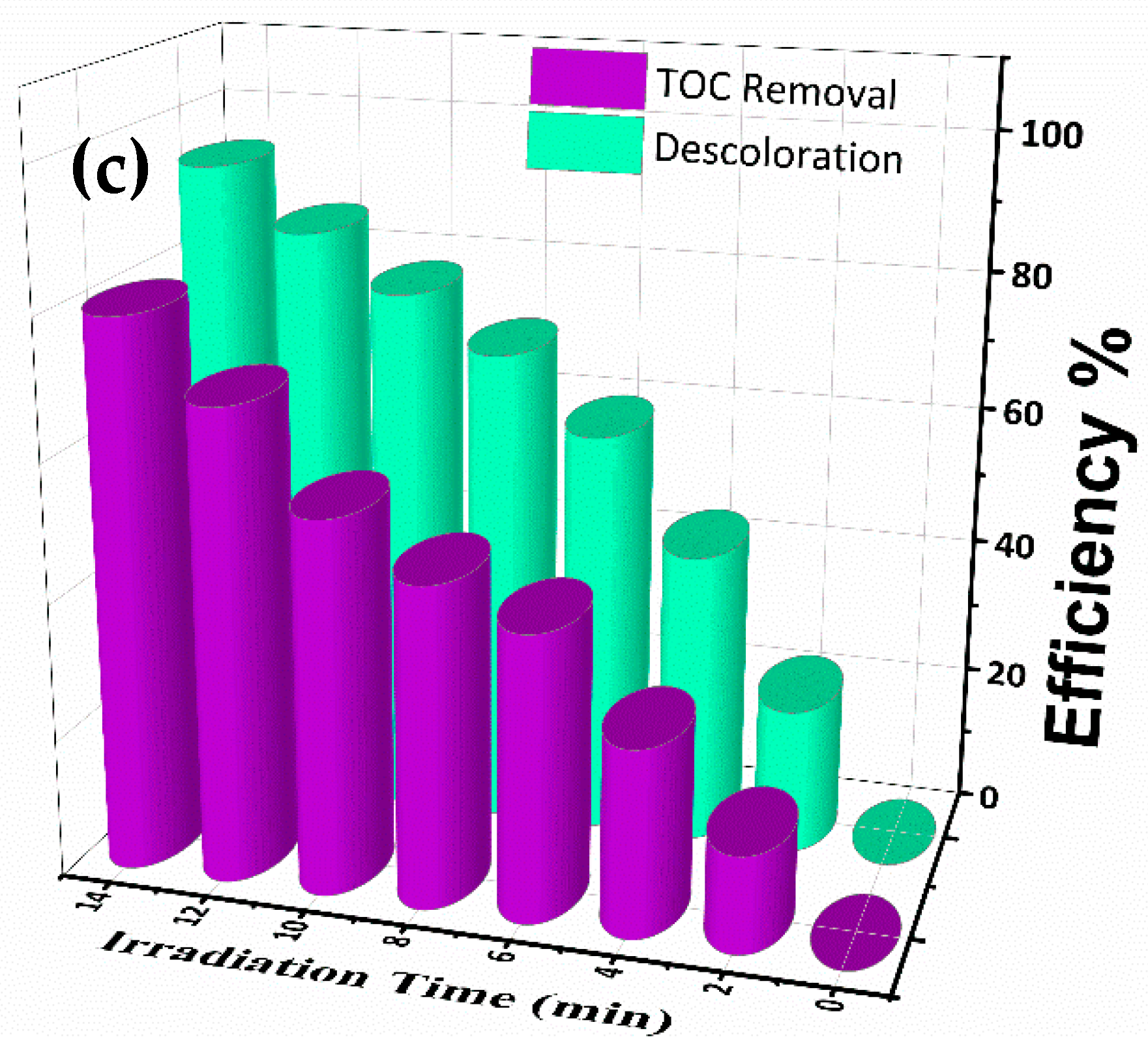
4.4. Mineralization of Pollutants RhB, OG and Mixture Dyes (RhB-MB)
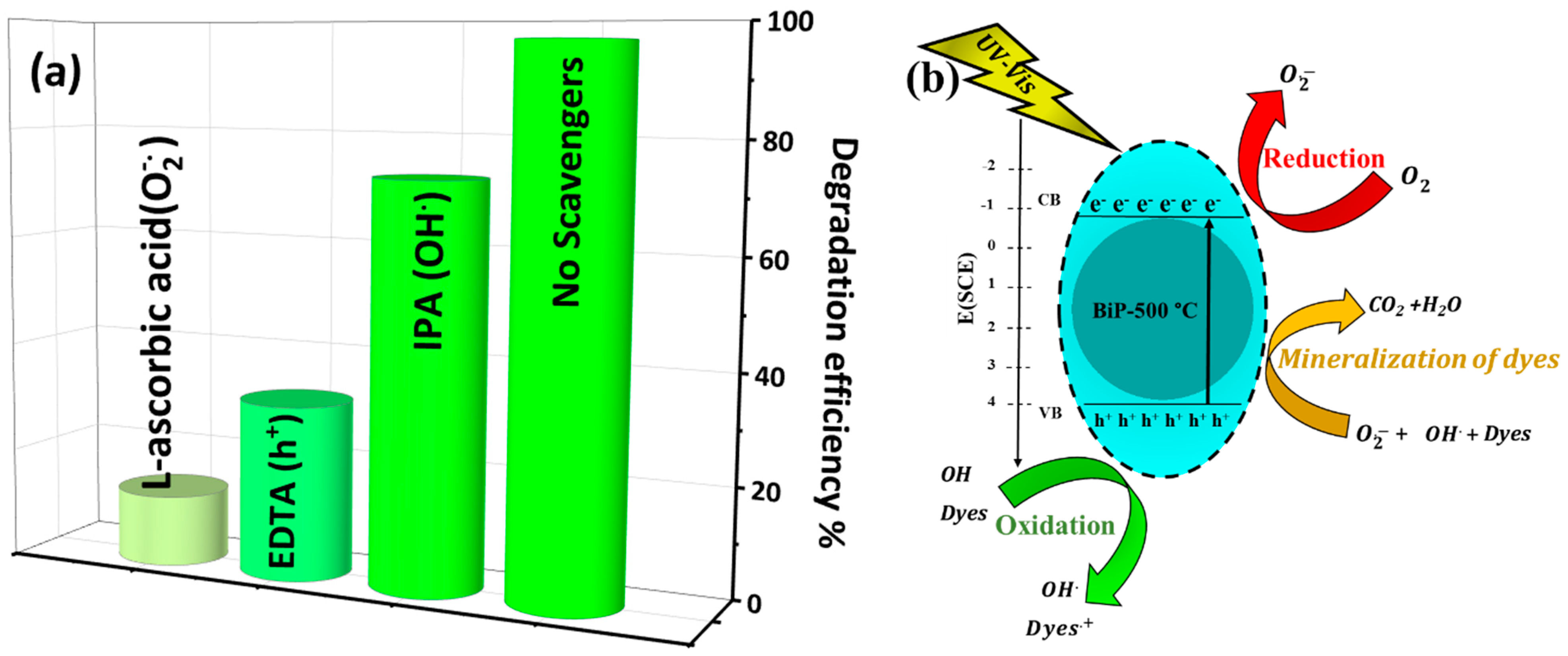
4.5. Role of Active Species
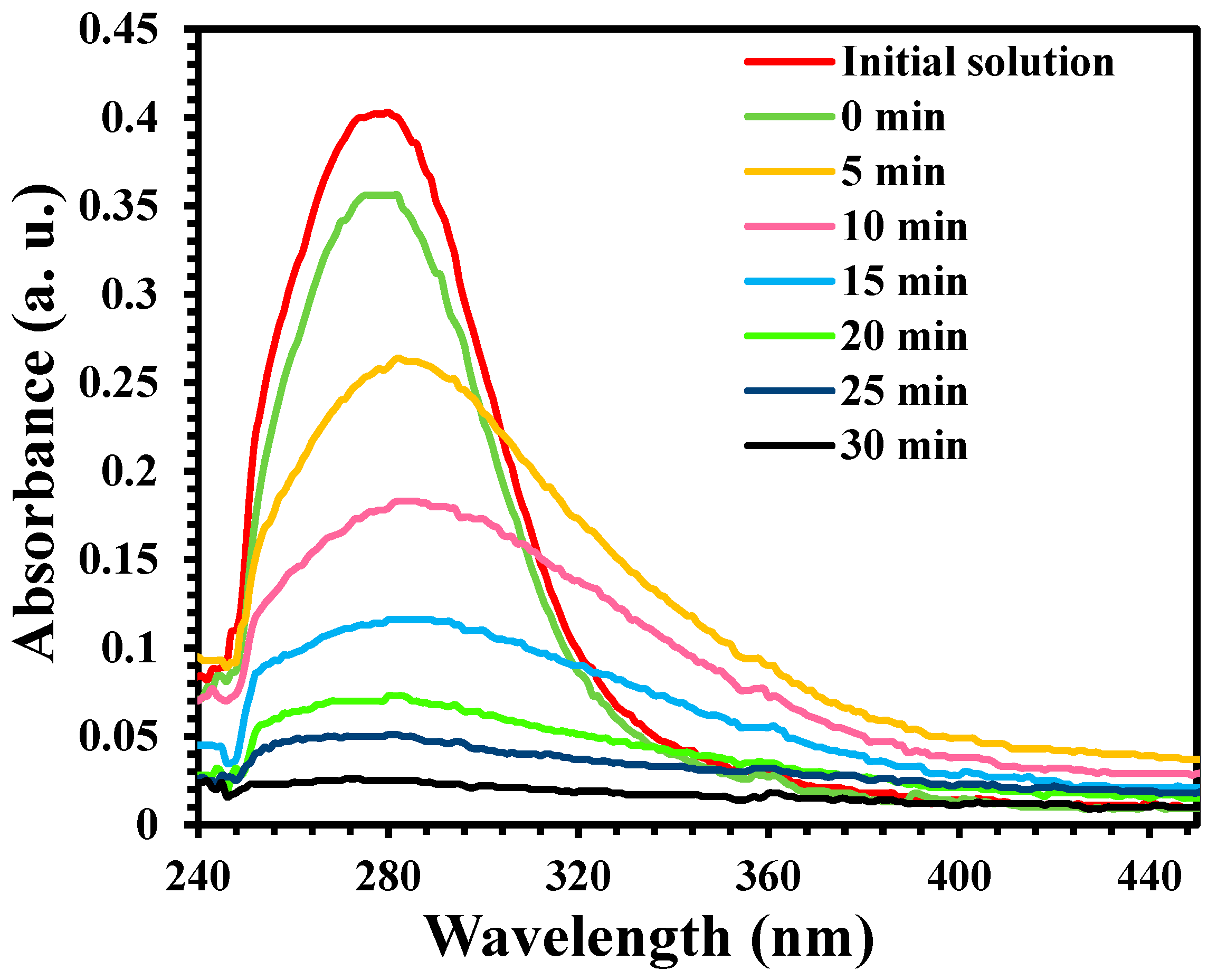
4.6. Photodegradation of Parathion-Methyl (PM)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, T.P.; Rodrigues, S.F.; Marques, G.N.; Costa, R.C.V.; Lopes, C.G.G.; Aranas, C.; Rojas, A.; Rangel, J.H.G.; Oliveira, M.M. Synthesis, Characterization, and Photocatalytic Investigation of CuFe2O4 for the Degradation of Dyes under Visible Light. Catalysts 2022, 12, 623. [Google Scholar] [CrossRef]
- Naciri, Y.; Hsini, A.; Ajmal, Z.; Bouddouch, A.; Bakiz, B.; Navío, J.; Albourine, A.; Valmalette, J.-C.; Ezahri, M.; Benlhachemi, A. Influence of Sr-doping on structural, optical and photocatalytic properties of synthesized Ca3(PO4)2. J. Colloid Interface Sci. 2020, 572, 269–280. [Google Scholar] [CrossRef]
- Chennah, A.; Naciri, Y.; Taoufyq, A.; Bakiz, B.; Bazzi, L.; Guinneton, F.; Villain, S.; Gavarri, J.R.; Benlhachemi, A. Electrodeposited zinc phosphate hydrate electrodes for electrocatalytic applications. J. Appl. Electrochem. 2018, 49, 163–177. [Google Scholar] [CrossRef]
- Amaterz, E.; Tara, A.; Bouddouch, A.; Taoufyq, A.; Bakiz, B.; Benlhachemi, A.; Jbara, O. Photo-electrochemical degradation of wastewaters containing organics catalysed by phosphate-based materials: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 843–872. [Google Scholar] [CrossRef]
- Ellouzi, I.; Bouddouch, A.; Bakiz, B.; Benlhachemi, A.; Oualid, H.A. Glucose-assisted ball milling preparation of silver-doped biphasic TiO2 for efficient photodegradation of Rhodamine B: Effect of silver-dopant loading. Chem. Phys. Lett. 2021, 770, 138456. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Gavarri, J.-R.; Valmalette, J.-C.; Benlhachemi, A. Customized synthesis of functional bismuth phosphate using different methods: Photocatalytic and photoluminescence properties enhancement. Nanotechnol. Environ. Eng. 2021, 6, 4. [Google Scholar] [CrossRef]
- Nethravathi, P.; Suresh, D. Silver-doped ZnO embedded reduced graphene oxide hybrid nanostructured composites for superior photocatalytic hydrogen generation, dye degradation, nitrite sensing and antioxidant activities. Inorg. Chem. Commun. 2021, 134, 109051. [Google Scholar] [CrossRef]
- Naseeb, F.; Ali, N.; Khalil, A.; Khan, A.; Asiri, A.M.; Kamal, T.; Bakhsh, E.M.; Ul-Islam, M. Photocatalytic degradation of organic dyes by U3MnO10 nanoparticles under UV and sunlight. Inorg. Chem. Commun. 2021, 134, 109075. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, R.; Zhang, Y.; Liu, X.; Xu, H. Preparation and photocatalytic properties of Bi2WO6/g-C3N4. Inorg. Chem. Commun. 2021, 132, 108761. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, A.W.; Zhu, Y. Visible-Light-Induced Degradation of Rhodamine B by Nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, Y. New Type of BiPO4 Oxy-Acid Salt Photocatalyst with High Photocatalytic Activity on Degradation of Dye. Environ. Sci. Technol. 2010, 44, 5570–5574. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, Y. A review of BiPO4, a highly efficient oxyacid-type photocatalyst, used for environmental applications. Catal. Sci. Technol. 2015, 5, 3071–3083. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Gavarri, J.; Ezahri, M.; Valmalette, J.; Benlhachemi, A. Role of thermal decomposition process in the photocatalytic or photoluminescence properties of BiPO4 polymorphs. Water Environ. Res. 2020, 92, 1874–1887. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Gavarri, J.-R.; Valmalette, J.-C.; Benlhachemi, A. Phase Transformation, Photocatalytic and Photoluminescent Properties of BiPO4 Catalysts Prepared by Solid-State Reaction: Degradation of Rhodamine B. Minerals 2021, 11, 1007. [Google Scholar] [CrossRef]
- Yang, S.; Xu, K.; Wang, H.; Yu, H.; Zhang, S.; Peng, F. Solution growth of peony-like copper hydroxyl-phosphate (Cu2(OH)PO4) flowers on Cu foil and their photocatalytic activity under visible light. Mater. Des. 2016, 100, 30–36. [Google Scholar] [CrossRef]
- Ge, M. Photodegradation of rhodamine B and methyl orange by Ag3PO4 catalyst under visible light irradiation. Chin. J. Catal. 2014, 35, 1410–1417. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Zhang, Y. Two Bi-based phosphate photocatalysts: Crystal structure, optical property and photocatalytic activity. Inorg. Chem. Commun. 2014, 44, 46–49. [Google Scholar] [CrossRef]
- Bagtache, R.; Abdmeziem, K.; Rekhila, G.; Trari, M. Synthesis and semiconducting properties of Na2MnPO4F. Application to degradation of Rhodamine B under UV-light. Mater. Sci. Semicond. Process. 2016, 51, 1–7. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Chen, C.; Lan, Y. Rapid photodegradation of methyl orange by oxalic acid assisted with cathode material of lithium ion batteries LiFePO4. J. Taiwan Inst. Chem. Eng. 2016, 62, 187–191. [Google Scholar] [CrossRef]
- Liu, J.; Geng, B.; Wang, S. Preparation and Usage of ZnS/Phosphate Heterostructured Hemispheres in Enhanced Photocatalytic Activities. Cryst. Growth Des. 2009, 9, 4384–4390. [Google Scholar] [CrossRef]
- Amaterz, E.; Tara, A.; Bouddouch, A.; Taoufyq, A.; Bakiz, B.; Lazar, F.; Gilliot, M.; Benlhachemi, A.; Bazzi, L.; Jbara, O. Hierarchical flower-like SrHPO4 electrodes for the photoelectrochemical degradation of Rhodamine B. J. Appl. Electrochem. 2020, 50, 569–581. [Google Scholar] [CrossRef]
- Amaterz, E.; Bouddouch, A.; Chennah, A.; Tara, A.; Taoufyq, A.; Bakiz, B.; Lazar, F.; Benlhachemi, A.; Bazzi, L.; Jbara, O. Heat treatment effect on the structure and morphology of strontium monoacid orthophosphate thin films. Mater. Today Proc. 2019, 22, 45–47. [Google Scholar] [CrossRef]
- Amaterz, E.; Bouddouch, A.; Tara, A.; Taoufyq, A.; Bakiz, B.; Benlhachemi, A.; Jbara, O. Correlation between photoluminescence and photoelectrochemical properties of SrHPO4/BaHPO4/FTO anode material. Opt. Mater. 2020, 109, 110268. [Google Scholar] [CrossRef]
- Akhsassi, B.; Bouddouch, A.; Naciri, Y.; Bakiz, B.; Taoufyq, A.; Favotto, C.; Villain, S.; Guinneton, F.; Benlhachemi, A. Enhanced photocatalytic activity of Zn3(PO4)2/ZnO composite semiconductor prepared by different methods. Chem. Phys. Lett. 2021, 783, 139046. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Xu, J.; Bai, X.; Zong, R.; Zhu, Y. Degradation and mineralization mechanism of phenol by BiPO4 photocatalysis assisted with H2O2. Appl. Catal. B Environ. 2013, 142–143, 561–567. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Zhang, Y.; Lv, X.; Wei, L.; Wang, X. Fabrication and luminescence of BiPO4:Tb3+/Ce3+ nanofibers by electrospinning. Superlattices Microstruct. 2016, 90, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Mooney-Slater, R.C.L. Polymorphic forms of bismuth phosphate. Z. Kristallographie-Crystall. Mater. 1962, 117, 371–385. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Shawabkeh, R.; Al-Harahsheh, A.; Tarawneh, K.; Batiha, M.M. Surface modification and characterization of Jordanian kaolinite: Application for lead removal from aqueous solutions. Appl. Surf. Sci. 2009, 255, 8098–8103. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Roisnel, FullProf. 98 and WinPLOTR: New windows 95/NT applications for diffraction commission for powder diffraction. Int. Union Crystallogr. Newsl. 1998, 20, 35–36. [Google Scholar]
- Romero, B.; Bruque, S.; Aranda, M.A.G.; Iglesias, J.E. Syntheses, Crystal Structures, and Characterization of Bismuth Phosphates. Inorg. Chem. 1994, 33, 1869–1874. [Google Scholar] [CrossRef]
- Bérar, J.-F.; Lelann, P. ESD’s and estimated probable error obtained in Rietveld refinements with local correlations. J. Appl. Crystallogr. 1991, 24, 1–5. [Google Scholar] [CrossRef]
- Vadivel, S.; Maruthamani, D.; Kumaravel, M.; Saravanakumar, B.; Paul, B.; Dhar, S.S.; Muthuraj, V. Supercapacitors studies on BiPO4 nanoparticles synthesized via a simple microwave approach. J. Taibah Univ. Sci. 2017, 11, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Li, G.; Zheng, J.; Li, L.; Wang, H.; Yang, L. Preparation and polymorph-sensitive luminescence properties of BiPO4:Eu, Part I: Room-temperature reaction followed by a heat treatment. CrystEngComm 2011, 13, 6251–6257. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L.; Yang, L.; Zheng, J.; Li, G. Exploring the unique electrical properties of metastable BiPO4 through switchable phase transitions. CrystEngComm 2013, 15, 609–615. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, K.; Zhao, H.; Zhu, J. UV-light driven photocatalytic performance of hydrothermally-synthesized hexagonal CePO4 nanorods. Solid State Sci. 2017, 72, 28–32. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Tian, Q.; Wu, W. Efficient Visible Light Formaldehyde Oxidation with 2D p-n Heterostructure of BiOBr/BiPO4 Nanosheets at Room Temperature. ACS Sustain. Chem. Eng. 2017, 5, 5008–5017. [Google Scholar] [CrossRef]
- Hizhnyi, Y.A.; Nedilko, S.; Chornii, V.; Slobodyanik, M.; Zatovsky, I.; Terebilenko, K. Electronic structures and origin of intrinsic luminescence in Bi-containing oxide crystals BiPO4, K3Bi5(PO4)6, K2Bi(PO4)(MoO4), K2Bi(PO4)(WO4) and K5Bi(MoO4)4. J. Alloys Compd. 2014, 614, 420–435. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Khraisheh, M.; Allen, S.; Ahmad, M. The removal of dyes from textile wastewater: A study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J. Environ. Manag. 2003, 69, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; Driscoll, C.T.; Bhanot, A. The zero point of charge of silica—Alumina oxide suspensions. J. Colloid Interface Sci. 1984, 97, 55–61. [Google Scholar] [CrossRef]
- Milonjić, S.K.; Kopečni, M.M.; Ilić, Z.E. The point of zero charge and adsorption properties of natural magnetite. J. Radioanal. Nucl. Chem. 1983, 78, 15–24. [Google Scholar] [CrossRef]
- Pan, C.; Xu, J.; Chen, Y.; Zhu, Y. Influence of OH-related defects on the performances of BiPO4 photocatalyst for the degradation of rhodamine B. Appl. Catal. B Environ. 2012, 115–116, 314–319. [Google Scholar] [CrossRef]
- Becerro, A.I.; Criado, J.; Gontard, L.C.; Obregón, S.; Fernández, A.; Colón, G.; Ocaña, M. Bifunctional, Monodisperse BiPO4-Based Nanostars: Photocatalytic Activity and Luminescent Applications. Cryst. Growth Des. 2014, 14, 3319–3326. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Ding, Y.; Zhang, Y.; Lu, Z.; Sun, H.; Chen, R. Microwave synthesis of BiPO4 nanostructures and their morphology-dependent photocatalytic performances. J. Colloid Interface Sci. 2011, 363, 497–503. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Lv, Y.; Ling, Q.; Liu, D.; Zhu, Y. Enhancement of photocatalytic activity for BiPO4via phase junction. J. Mater. Chem. A 2014, 2, 13041–13048. [Google Scholar] [CrossRef]
- Yan-Yan, Z.; Yan-Fang, L.; Yan-Hui, L.; Hua, W.; Qiang, L.; Yong-Fa, Z. Reflux Preparation and Photocatalytic Performance of Bismuth Phosphate Nanorods. Acta Phys.-Chim. Sin. 2013, 29, 576–584. [Google Scholar] [CrossRef]
- Zangiabadi, M.; Mehrabi, F.; Nasiripur, P.; Baghersad, M.H. Visible-light-driven photocatalytic degradation of methyl parathion as chemical warfare agent simulant by NiO/Bi2MoO6 heterojunction photocatalyst. J. Mol. Struct. 2022, 1256, 132472. [Google Scholar] [CrossRef]

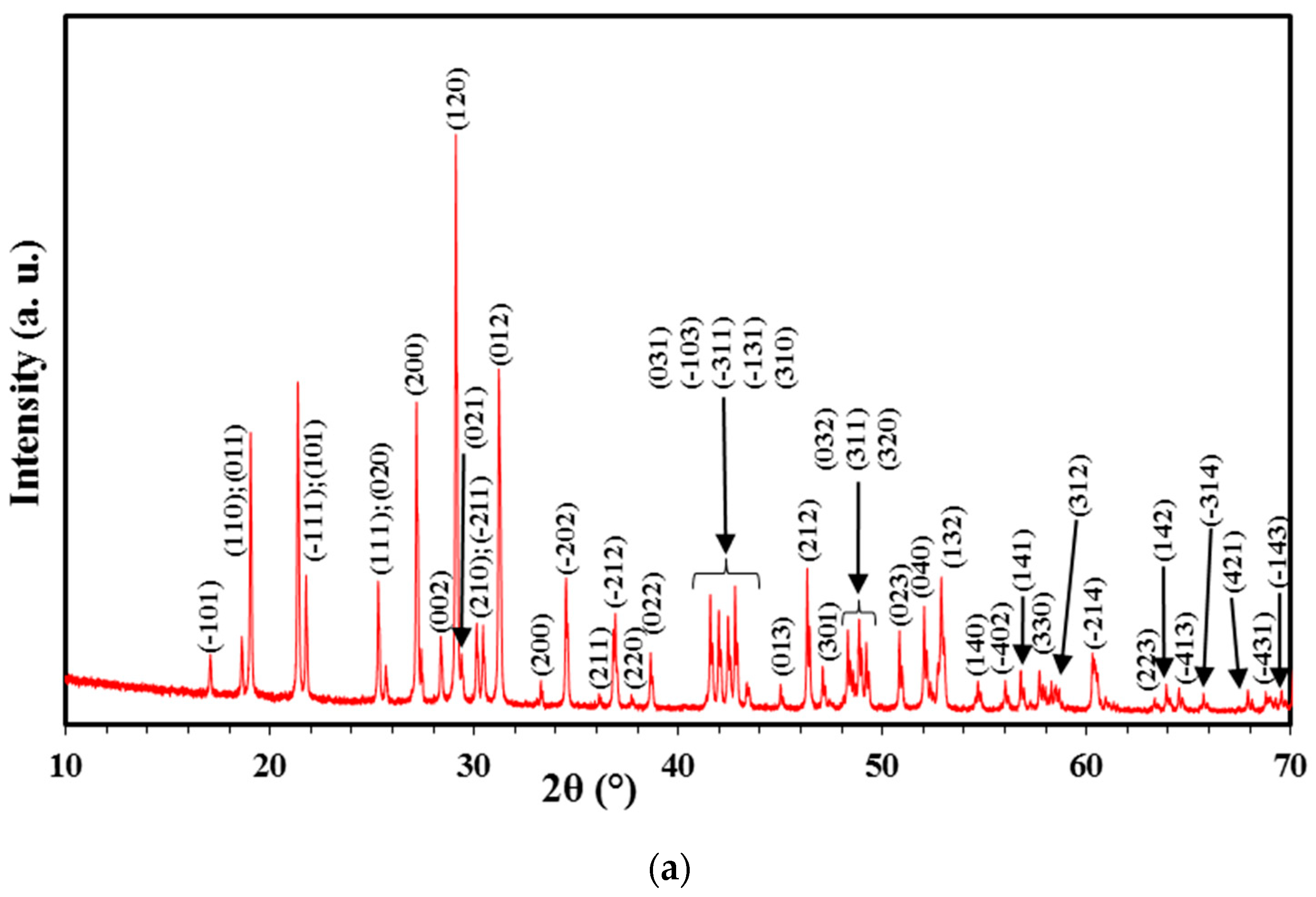
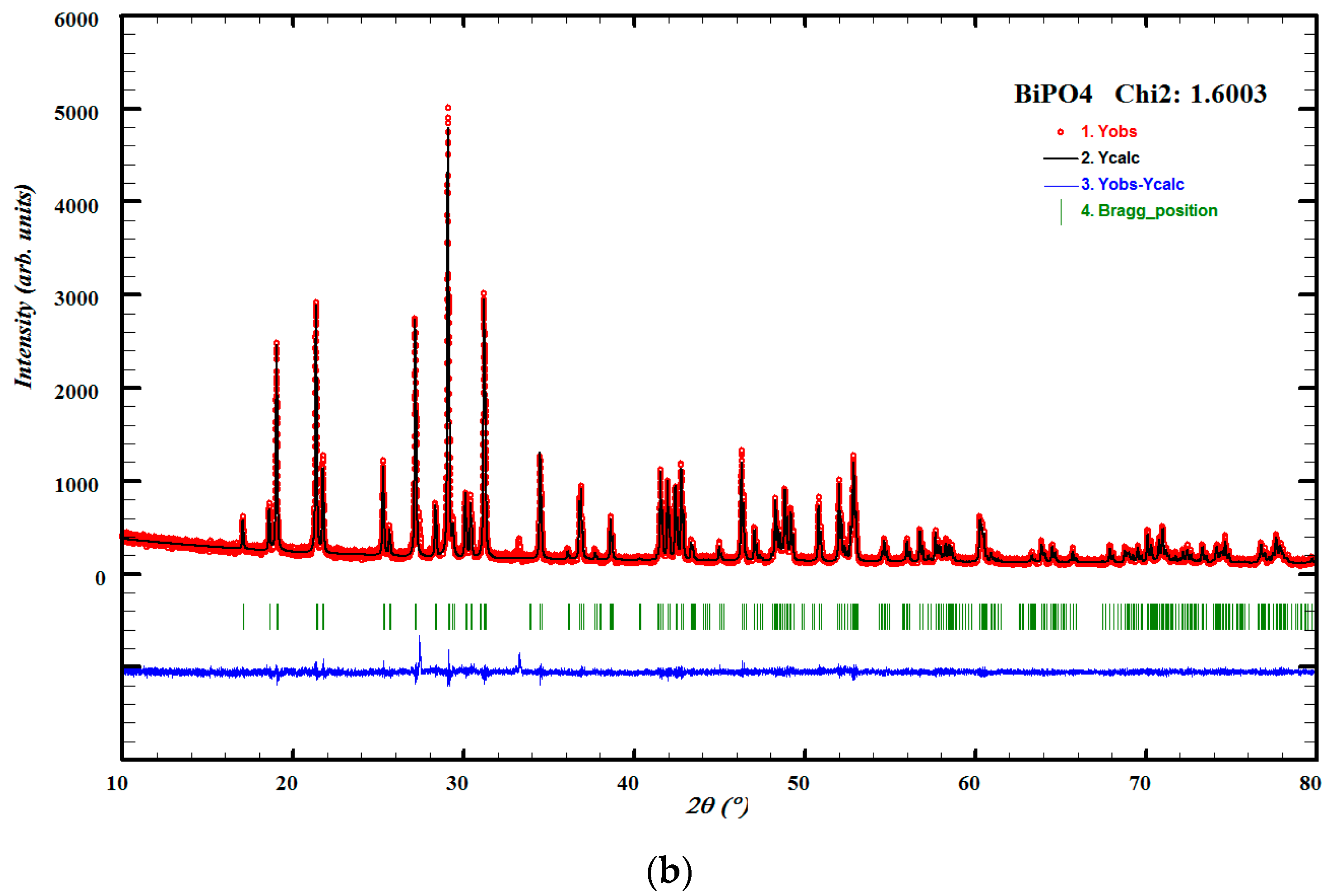
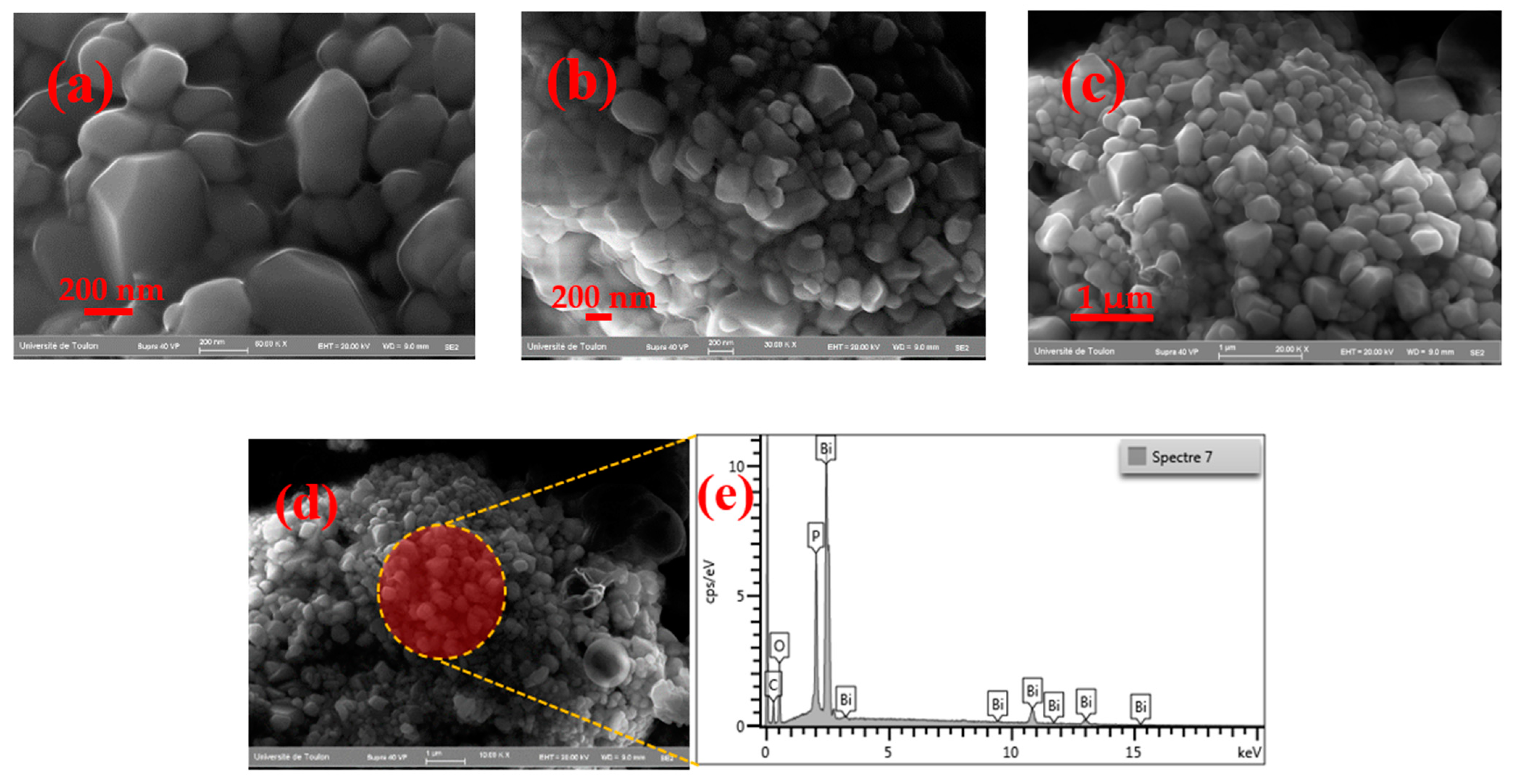

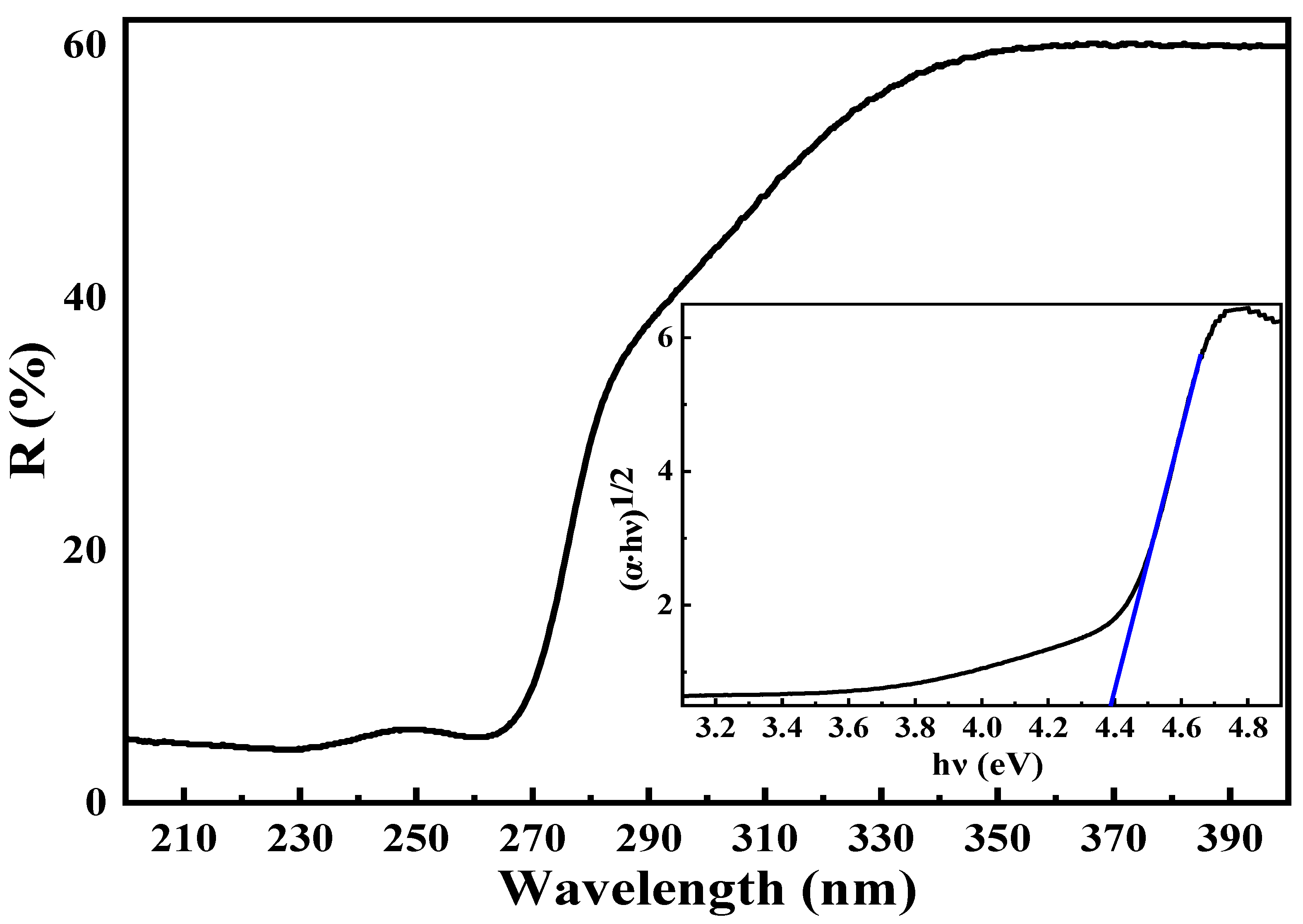
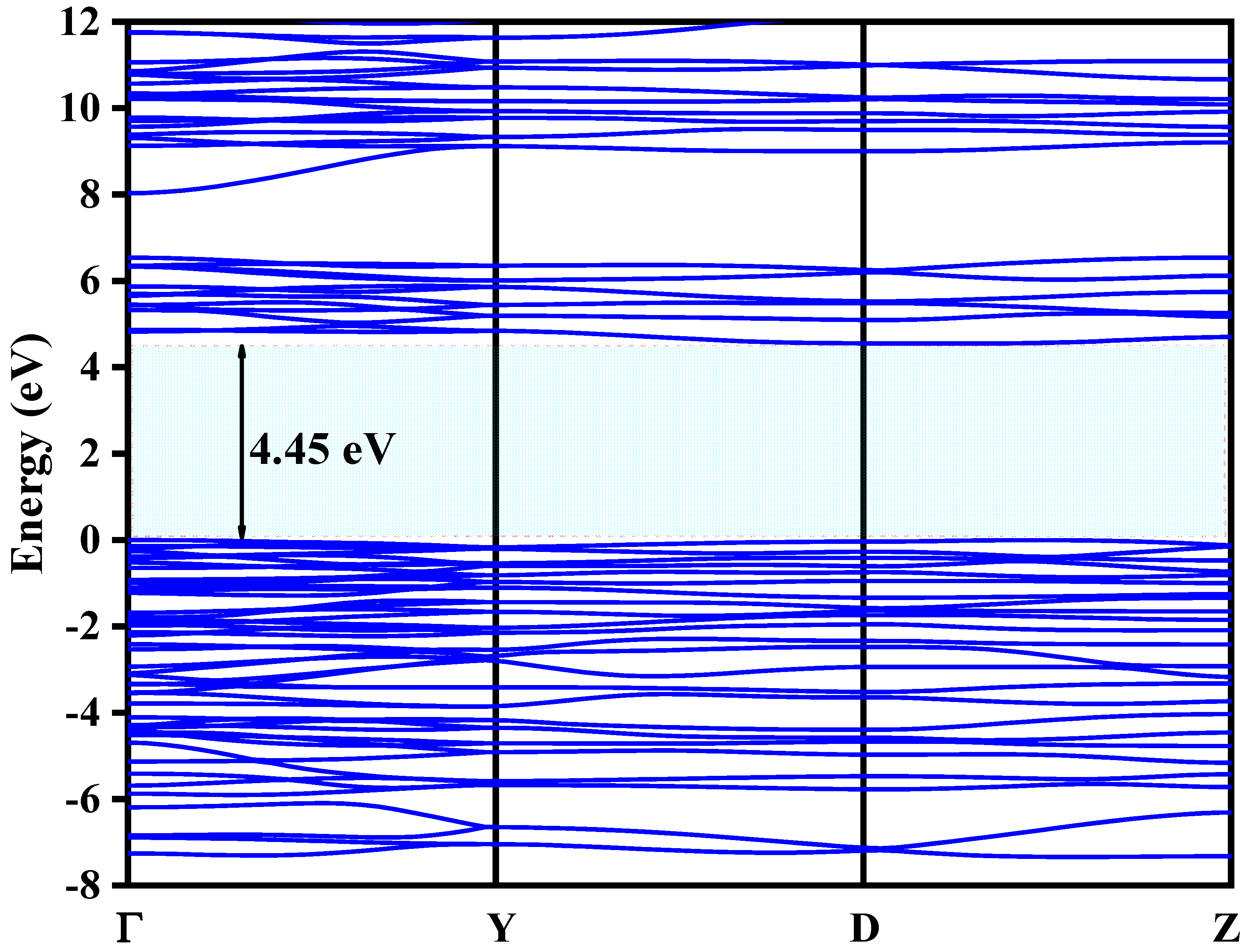
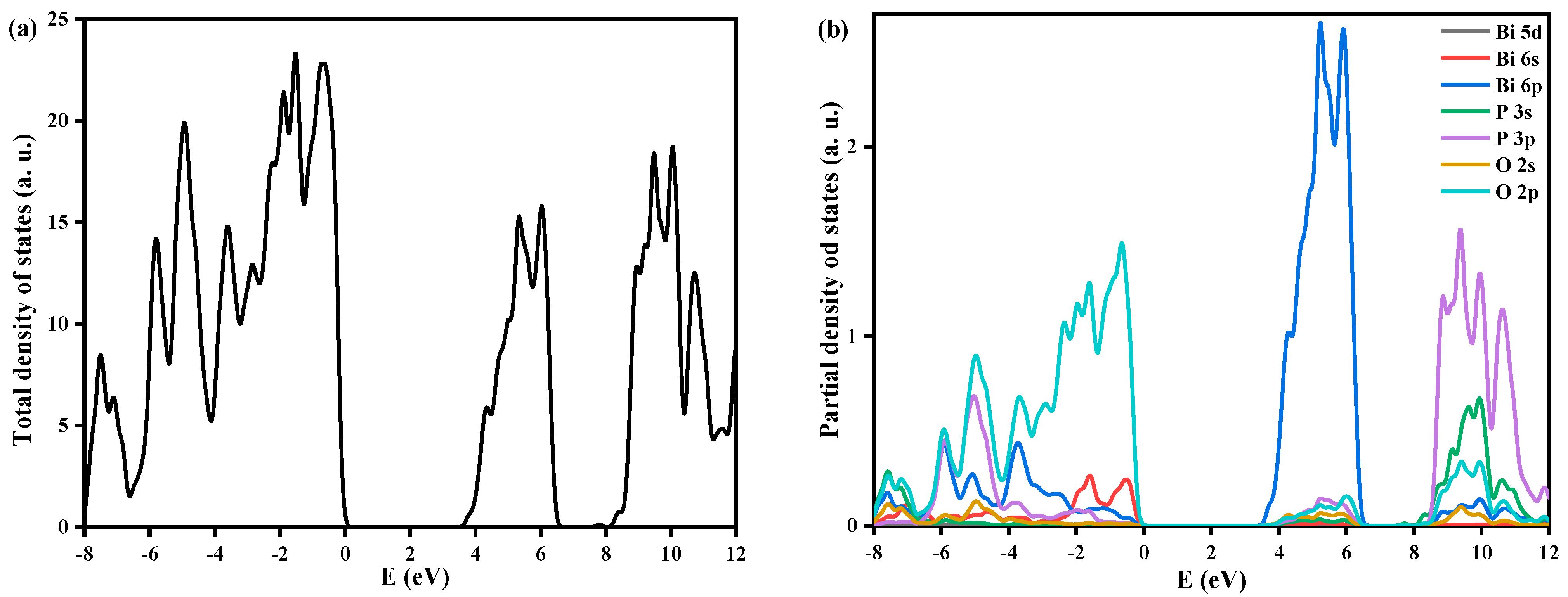


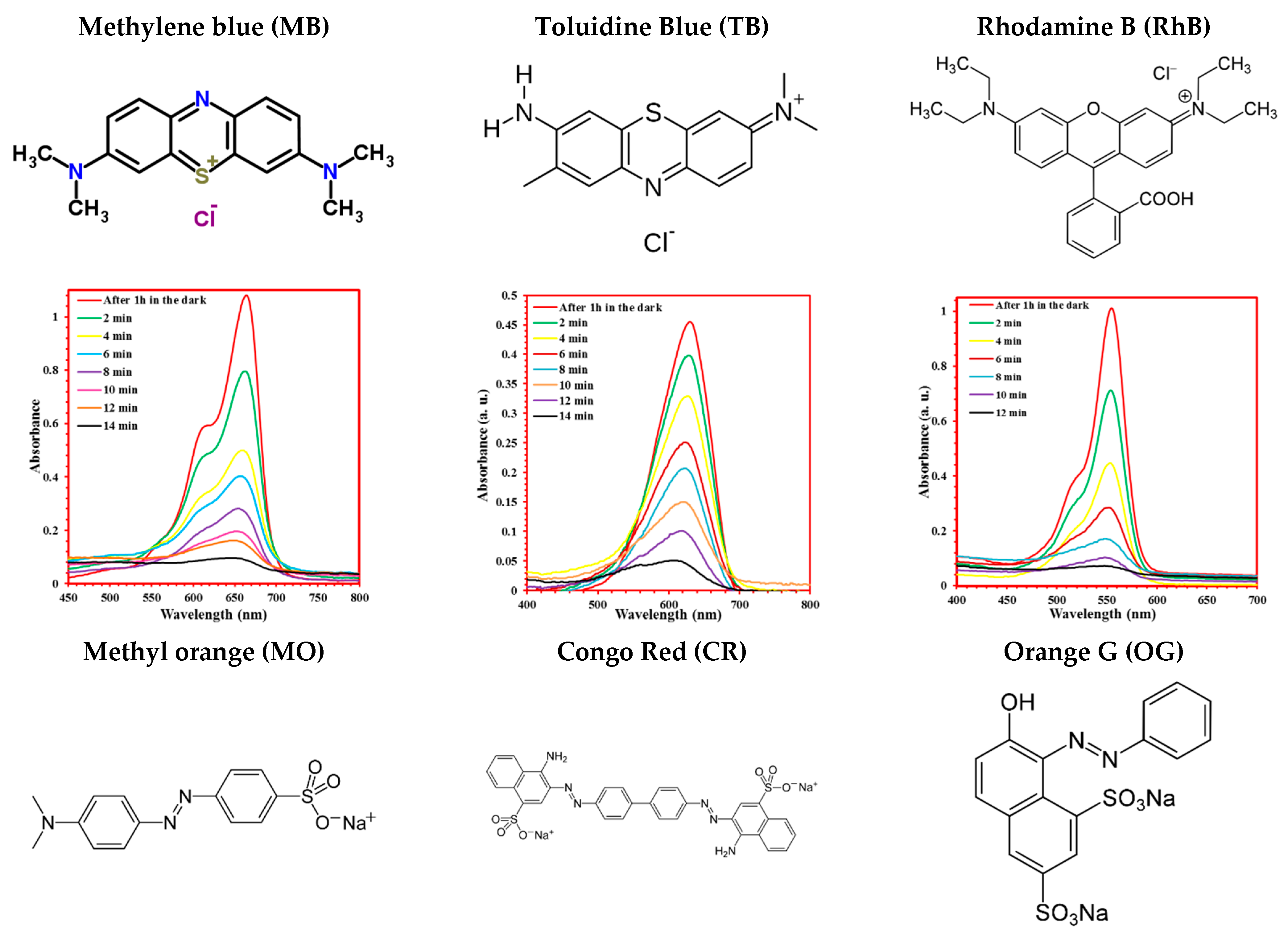

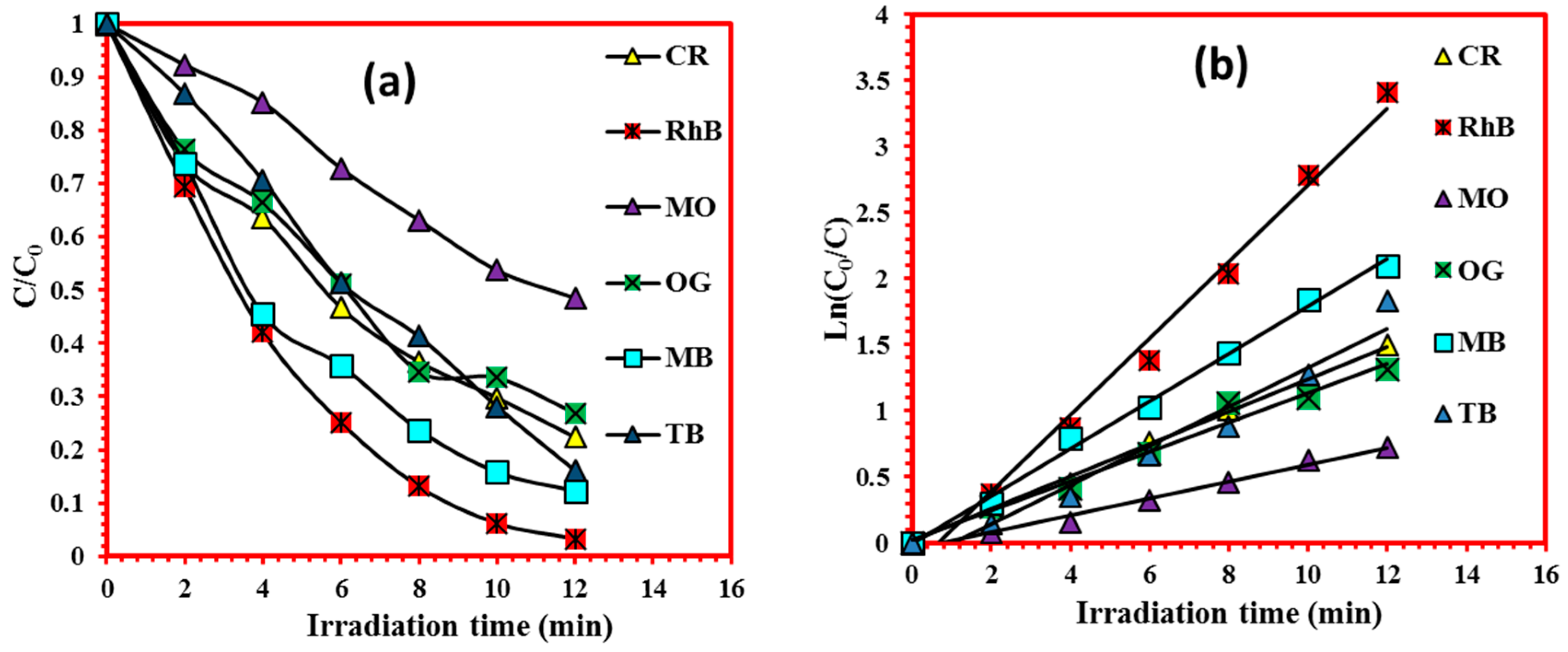

| Cell Parameters (10−10 m) Volume (10−30 m) Standard Deviations in Parentheses: ( ) | Lattice System, Space Group | Reference JCPDS 80-0209 | ||
|---|---|---|---|---|
| a = 6.7553(1) | Monoclinic P21/n | a = 6.7626(1) | ||
| b = 6.9419(1) | b = 6.9516(1) | |||
| c = 6.4772(1) | c = 6.4822(8) | |||
| β = 103.690(1) | β = 103.736(1) | |||
| V = 295.115(8) | V = 296.018(8) | |||
| RB = 100. {∑|Ikobs − Ikicalc|/∑|Ikobs|} | =3.5% | |||
| RF = 100. {∑|Fkobs − Fkicalc|/∑|Fkobs│} | =2.9% | |||
| Rp = 100. {∑|yiobs − yicalc|/∑|yiobs|} | =5.5% | |||
| Rwp = 100. {|∑ wi|yiobs − yicalc|2/∑ wi|yiobs|2]1/2} | =7.7% | |||
| Rexp = 100. {[(N − P + C)/∑ wi|yiobs|2]1/2} | =6.2% | |||
| Where N, P and C are the number of observations, parameters and constraints, respectively. | ||||
| Atom (Wyckoff) | x | y | z | Biso (Å2) (*) |
| Bi | 0.2855(3) | 0.1453(3) | 0.0864(3) | 0.56(5) |
| P | 0.296(2) | 0.161(2) | 0.615(2) | 0.84(27) |
| O1 | 0.263(3) | −0.002(2) | 0.438(3) | 0.33(67) |
| O2 | 0.377(3) | 0.344(4) | 0.515(3) | 1.24(65) |
| O3 | 0.458(3) | 0.105(3) | 0.815(3) | 1.13(68) |
| O4 | 0.115(3) | 0.198(3) | 0.709(3) | 1.37(72) |
| Areal Parameters of the BiP-500 Catalyst | |
|---|---|
| Mass of photocatalyst (in mg) | 100 |
| Crystallite size D in nm | 250 |
| Exposed surface Sexp (m2) | 0.35 |
| SSA (m2/g) | 3.52 |
| -Specific surface areas of crystallites in the form of a sphere: Exposed surface: Sexp = (6/D)(m/µ); specific surface area: SSA = (6/Dµ); m = total mass of photocatalyst; µ = theoretical density of the material from crystallographic data. | |
| Catalyst | Pollutant Examined | Synthesis Method | Operating Conditions (C0; Light Source) | Degradation Efficiency; Time | Ref. |
|---|---|---|---|---|---|
| BiPO4 | RhB | Hydrothermal | 5 ppm, UV 254 nm | kapp = 0.1225 min−1, 30 min | [46] |
| BiPO4 | RhB | Solvothermal | 5 ppm, UV 254 nm | kapp = 0.53 h−1, 180 min | [47] |
| BiPO4 | MO | Microwave | 10 ppm, 500 W Xe lamp | kapp =0.035 min−1 | [48] |
| BiPO4 | MB | Coprecipitation | 15 ppm, UV 254 nm | kapp = 0.1089 min−1 | [49] |
| BiPO4 | MB | Flux | 5 ppm, UV 254 nm | kapp = 0.193 min−1 | [50] |
| BiPO4 | RhB | Coprecipitation | 5 ppm, UV 254 nm | 98%, 160 min | [14] |
| BiP-500 | RhB | Solid-state | 5 ppm, UV 254 nm | 96.7%, 12 min | This study |
| BiP-500 | MB | -- | -- | 87.8%, 12 min | This study |
| BiP-500 | TB | -- | -- | 84%, 12 min | This study |
| BiP-500 | CR | -- | -- | 77%, 12 min | This study |
| BiP-500 | OG | -- | -- | 73%, 12 min | This study |
| BiP-500 | MO | -- | -- | 51%, 12 min | This study |
| BiP-500 | PM | Solid-state | 10 ppm, UV 254 nm | 93%, 30 min | This study |
| Brute Formula | C8H10NO5PS |
|---|---|
| Chemical structure | 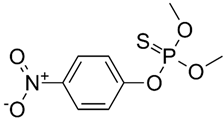 |
| Chemical name | O,O-Dimethyl O-(p-nitrophenyl) phosphorothioate |
| Molecular mass | 263.8 g/mol |
| Physical state | Crystallized solid |
| Water solubility | 55 mg/L |
| Melting point | 35–36 °C |
| λmax | 278 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouddouch, A.; Akhsassi, B.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Villain, S.; Guinneton, F.; El Aamrani, A.; Gavarri, J.-R.; Benlhachemi, A. Photodegradation under UV Light Irradiation of Various Types and Systems of Organic Pollutants in the Presence of a Performant BiPO4 Photocatalyst. Catalysts 2022, 12, 691. https://doi.org/10.3390/catal12070691
Bouddouch A, Akhsassi B, Amaterz E, Bakiz B, Taoufyq A, Villain S, Guinneton F, El Aamrani A, Gavarri J-R, Benlhachemi A. Photodegradation under UV Light Irradiation of Various Types and Systems of Organic Pollutants in the Presence of a Performant BiPO4 Photocatalyst. Catalysts. 2022; 12(7):691. https://doi.org/10.3390/catal12070691
Chicago/Turabian StyleBouddouch, Abdessalam, Brahim Akhsassi, Elhassan Amaterz, Bahcine Bakiz, Aziz Taoufyq, Sylvie Villain, Frédéric Guinneton, Abdelaziz El Aamrani, Jean-Raymond Gavarri, and Abdeljalil Benlhachemi. 2022. "Photodegradation under UV Light Irradiation of Various Types and Systems of Organic Pollutants in the Presence of a Performant BiPO4 Photocatalyst" Catalysts 12, no. 7: 691. https://doi.org/10.3390/catal12070691
APA StyleBouddouch, A., Akhsassi, B., Amaterz, E., Bakiz, B., Taoufyq, A., Villain, S., Guinneton, F., El Aamrani, A., Gavarri, J.-R., & Benlhachemi, A. (2022). Photodegradation under UV Light Irradiation of Various Types and Systems of Organic Pollutants in the Presence of a Performant BiPO4 Photocatalyst. Catalysts, 12(7), 691. https://doi.org/10.3390/catal12070691









