Continuous Flow Glycolipid Synthesis Using a Packed Bed Reactor
Abstract
:1. Introduction
2. Results
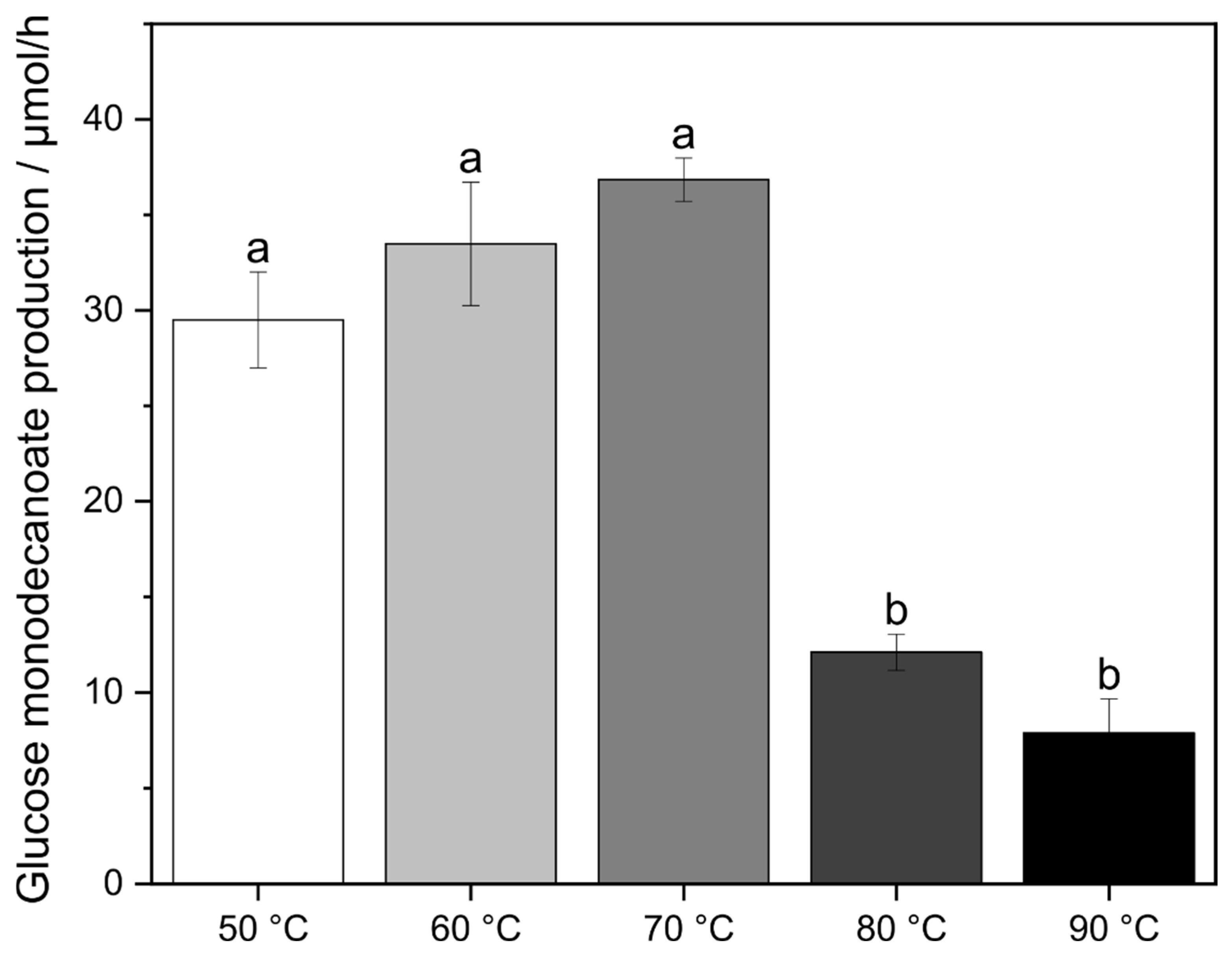
2.1. Influence of Temperature
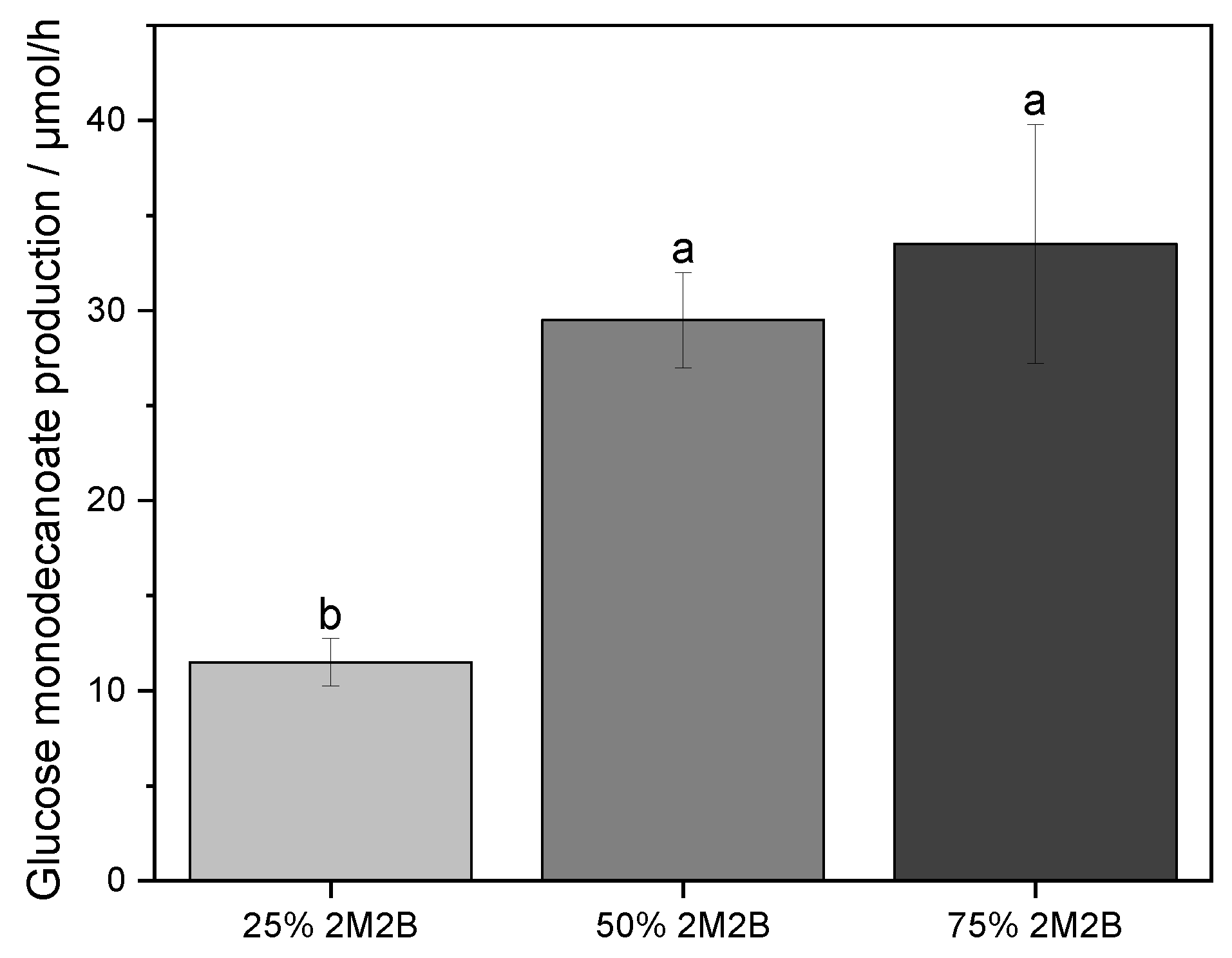
2.2. Ratio of the Aqueous Phase to Organic Phase
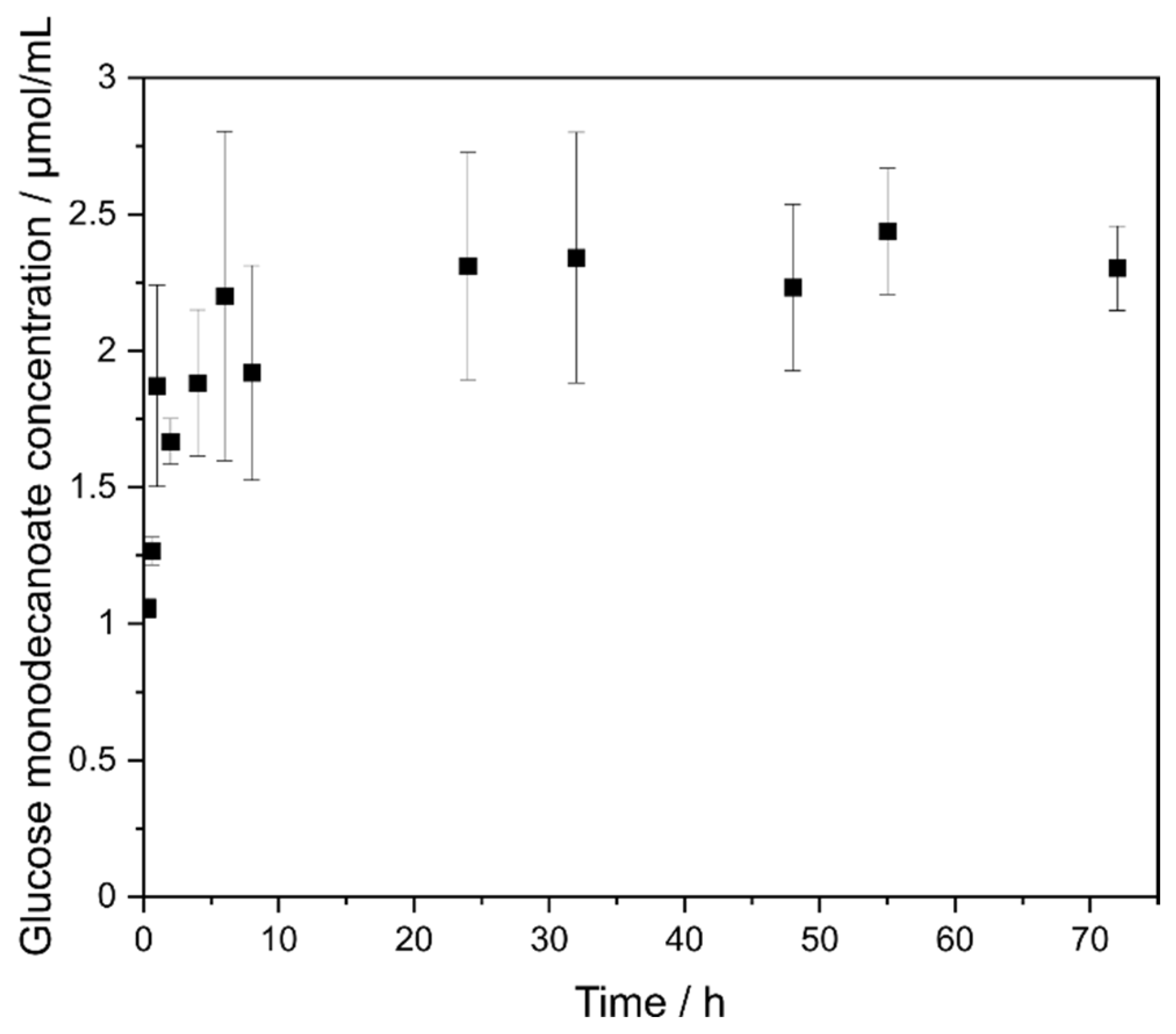
2.3. Sugar Concentration
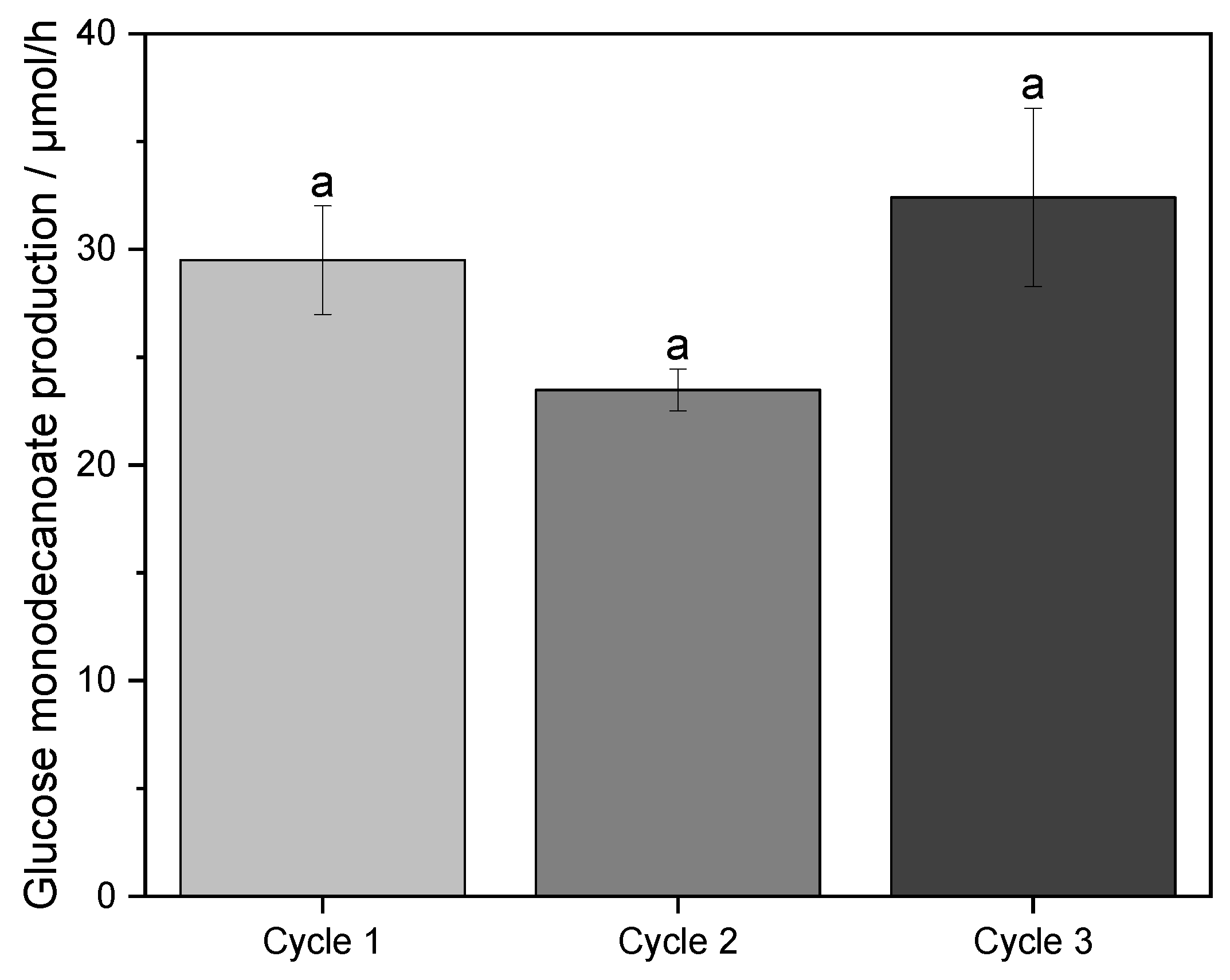
2.4. Sugar Recycling
3. Discussion
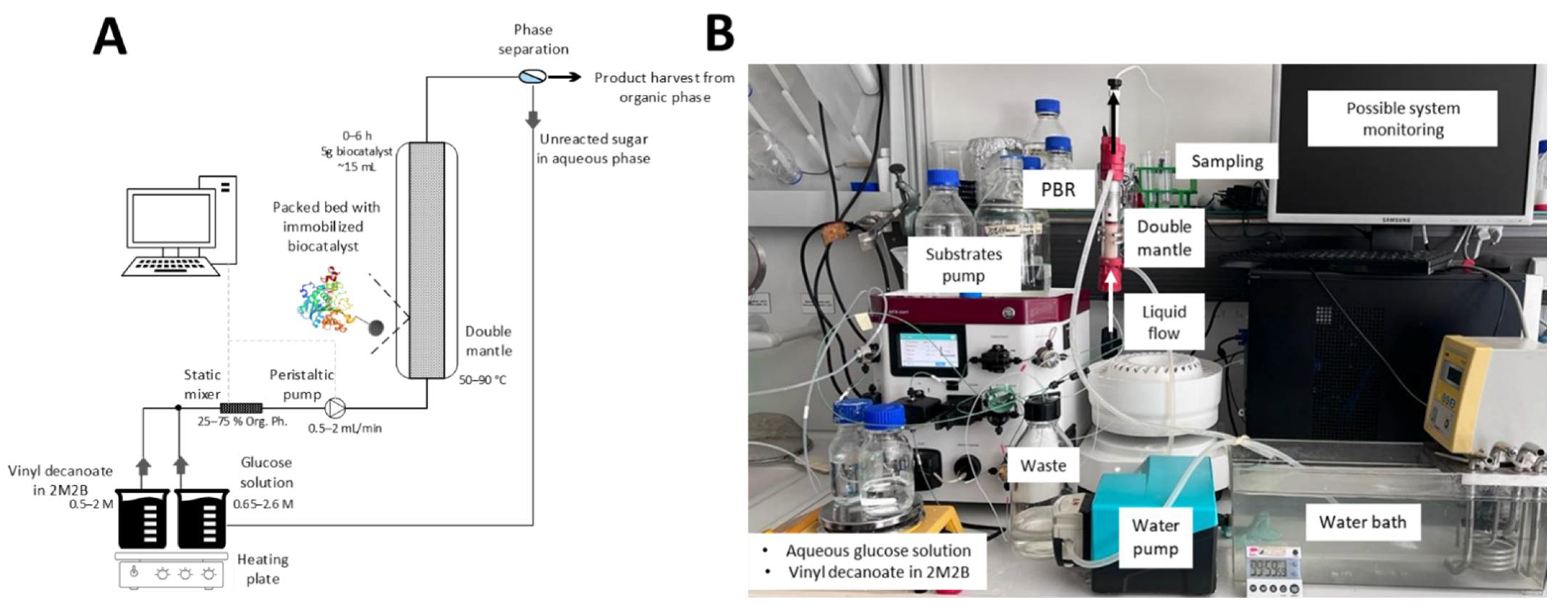
4. Materials and Methods
4.1. Enzymatic Synthesis of Glucose Monodecanoate in Continuous Packed Bed Mode Operation
4.2. Influence of Temperature
4.3. Influence of Glucose Concentration
4.4. Recycling of Glucose Solution
4.5. Quantification
4.6. Water-Activity Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef] [PubMed]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; da Silva R de, C.F.S.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Baker, I.J.A.; Matthews, B.; Suares, H.; Krodkiewska, I.; Furlong, D.N.; Grieser, F.; Drummond, C.I. Sugar fatty acid ester surfactants: Structure and ultimate aerobic biodegradability. J. Surfactants Deterg. 2000, 3, 1–11. [Google Scholar] [CrossRef]
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Carvalho, A.M.X.; Tótola, M.R.; Borges, A.C. Biodegradability of bacterial surfactants. Biodegradation 2011, 22, 585–592. [Google Scholar] [CrossRef]
- Johann, S.; Seiler, T.B.; Tiso, T.; Bluhm, K.; Blank, L.M.; Hollert, H. Mechanism-specific and whole-organism ecotoxicity of mono-rhamnolipids. Sci. Total Environ. 2016, 548–549, 155–163. [Google Scholar] [CrossRef]
- Dörjes, J. Experimentelle Untersuchungen zur Wirkung von Rohöl und Rohöl/Tensid-Gemischen im Ökosystem Wattenmeer. XVI. Zusammenfassung und Schlußfolgerungen. Senckenberg. Marit. Int. J. Mar. Sci. 1984, 16, 267–271. [Google Scholar]
- Poremba, K.; Gunkel, W.; Lang, S.; Wagner, F. Toxicity testing of synthetic and biogenic surfactants on marine microorganisms. Environ. Toxicol. Water Qual. 1991, 6, 157–163. [Google Scholar] [CrossRef]
- Hollenbach, R.; Völp, A.R.; Höfert, L.; Rudat, J.; Ochsenreither, K.; Willenbacher, N.; Syldatk, C. Interfacial and Foaming Properties of Tailor-Made Glycolipids—Influence of the Hydrophilic Head Group and Functional Groups in the Hydrophobic Tail. Molecules 2020, 25, 3797. [Google Scholar] [CrossRef]
- Hollenbach, R.; Oeppling, S.; Delavault, A.; Völp, A.R.; Willenbacher, N.; Rudat, J.; Ochsenreither, K.; Syldatk, C. Comparative study on interfacial and foaming properties of glycolipids in relation to the gas applied for foam generation. RSC Adv. 2021, 11, 34235–34244. [Google Scholar] [CrossRef]
- Garofalakis, G.; Murray, B.S.; Sarney, D.B. Surface activity and critical aggregation concentration of pure sugar esters with different sugar headgroups. J. Colloid Interface Sci. 2000, 229, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.S.; Vaidya, A.N.; Bal, A.S.; Kapur, R.; Juwarkar, A.; Khannan, P. Kinetics of biosurfactant production by Pseudomonas aeruginosa strain BS2 from industrial wastes. Biotechnol. Lett. 1996, 18, 263–268. [Google Scholar]
- Siebenhaller, S.; Grüninger, J.; Syldatk, C. Enzymatic Synthesis of Glycolipid Surfactants. In Lipid Modification by Enzymes and Engineered Microbes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–313. [Google Scholar]
- Grüninger, J.; Delavault, A.; Ochsenreither, K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochem. 2019, 87, 45–54. [Google Scholar] [CrossRef]
- Amodio, A.; Malyska, A.; Markouli, C.; Salinas, S.; Sanfelix, J.; VanHumbeeck, T. Mapping Study for the Development of Sustainable-by-Design Criteria. Eur. Comm. Dir. Res. Innov. 2021, 1, 19–35. Available online: https://op.europa.eu/en/publication-detail/-/publication/f679c200-a314-11eb-9585-01aa75ed71a1/languageen (accessed on 17 May 2022).
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Environmental Catalysis: Present and Future. ChemCatChem 2019, 11, 18–38. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Bio-based resources, bioprocesses and bioproducts in value creation architectures for bioeconomy markets and beyond—What really matters. EFB Bioecon. J. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Wohlgemuth, R.; Twardowski, T.; Aguilar, A. Bioeconomy moving forward step by step—A global journey. N. Biotechnol. 2021, 61, 22–28. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. The effect of water on enzyme action in organic media. J. Biol. Chem. 1988, 263, 8017–8021. [Google Scholar] [CrossRef]
- Arroyo, M.; Sánchez-Montero, J.M.; Sinisterra, J.V. Thermal stabilization of immobilized lipase B from Candida antarctica on different supports: Effect of water activity on enzymatic activity in organic media. Enzym. Microb. Technol. 1999, 24, 3–12. [Google Scholar] [CrossRef]
- Chamouleau, F.; Coulon, D.; Girardin, M.; Ghoul, M. Influence of water activity and water content on sugar esters lipase-catalyzed synthesis in organic media. J. Mol. Catal. A Chem. 2001, 11, 949–954. [Google Scholar] [CrossRef]
- Šabeder, S.; Habulin, M.; Knez, Ž. Lipase-catalyzed synthesis of fatty acid fructose esters. J. Food Eng. 2006, 77, 880–886. [Google Scholar] [CrossRef]
- Ganske, F.; Bornscheuer, U.T. Optimization of lipase-catalyzed glucose fatty acid ester synthesis in a two-phase system containing ionic liquids and t-BuOH. J. Mol. Catal. B Enzym. 2005, 36, 40–42. [Google Scholar] [CrossRef]
- Pöhnlein, M.; Ulrich, J.; Kirschhöfer, F.; Nusser, M.; Muhle-Goll, C.; Kannengiesser, B.; Brenner-Weiß, G.; Luy, B.; Liese, A.; Syldatk, C.; et al. Lipase-catalyzed synthesis of glucose-6-O-hexanoate in deep eutectic solvents. Eur. J. Lipid. Sci. Technol. 2015, 117, 161–166. [Google Scholar] [CrossRef]
- Hollenbach, R.; Bindereif, B.; van der Schaaf, U.S.; Ochsenreither, K.; Syldatk, C. Optimization of Glycolipid Synthesis in Hydrophilic Deep Eutectic Solvents. Front. Bioeng. Biotechnol. 2020, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Koumba Ibinga, S.K.; Fabre, J.F.; Bikanga, R.; Mouloungui, Z. Atypical Reaction Media and Organized Systems for the Synthesis of Low-Substitution Sugar Esters. Front. Chem. 2019, 7, 587. [Google Scholar] [CrossRef]
- Truppo, M.D. Biocatalysis in the Pharmaceutical Industry: The Need for Speed. ACS Med. Chem. Lett. 2017, 8, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Mangiagalli, M.; Ami, D.; de Divitiis, M.; Brocca, S.; Catelani, T.; Natalello, A.; Lotti, M. Short-chain alcohols inactivate an immobilized industrial lipase through two different mechanisms. Biotechnol. J. 2022, 2100712. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Carvalho, H.; Natalello, A.; Ferrario, V.; Pennati, M.L.; Barbiroli, A.; Lotti, M.; Pleiss, J.; Brocca, S. Diverse effects of aqueous polar co-solvents on Candida antarctica lipase B. Int. J. Biol. Macromol. 2020, 150, 930–940. [Google Scholar] [CrossRef]
- José, C.; Briand, L.E. Deactivation of Novozym® 435 during the esterification of ibuprofen with ethanol: Evidences of the detrimental effect of the alcohol. React. Kinet. Mech. Catal. 2010, 99, 17–22. [Google Scholar] [CrossRef]
- José, C.; Bonetto, R.D.; Gambaro, L.A.; Guauque Torres, M.D.P.; Foresti, M.L.; Ferreira, M.L.; Briand, L.E. Investigation of the causes of deactivation-degradation of the commercial biocatalyst Novozym® 435 in ethanol and ethanol-aqueous media. J. Mol. Catal. B Enzym. 2017, 71, 95–107. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Alcántara, A.R.; Domínguez de María, P.; Littlechild, J.A.; Schürmann, M.; Sheldon, R.A.; Wohlgemuth, R. Biocatalysis as Key to Sustainable Industrial Chemistry. ChemSusChem 2022, 15, 202102709. [Google Scholar]
- Burek, B.O.; Dawood, A.W.H.; Hollmann, F.; Liese, A.; Holtmann, D. Process Intensification as Game Changer in Enzyme Catalysis. Front. Catal. 2022, 2, 1–18. [Google Scholar] [CrossRef]
- Lindeque, R.M.; Woodley, J.M. Reactor selection for effective continuous biocatalytic production of pharmaceuticals. Catalysts 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Strniša, F.; Urbič, T.; Žnidaršič-Plazl, P.; Plazl, I. Process Intensification and Miniaturization of Chemical and Biochemical Processes. Comput. Aided Chem. Eng. 2019, 46, 1801–1806. [Google Scholar]
- Žnidaršič-Plazl, P. The Promises and the Challenges of Biotransformations in Microflow. Biotechnol. J. 2019, 14, 1800580. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef]
- Jones, E.; McClean, K.; Housden, S.; Gasparini, G.; Archer, I. Biocatalytic oxidase: Batch to continuous. Chem. Eng. Res. Des. 2012, 90, 726–731. [Google Scholar] [CrossRef]
- Delavault, A. Lipase-Catalysed Synthesis of Glycolipids-Utilization of Innovative Media, Reactors and Substrates. Repository KITopen. 2021. Available online: https://publikationen.bibliothek.kit.edu/1000136597 (accessed on 17 May 2022).
- Khaled, N.; Montet, D.; Pina, M.; Graille, J. Fructose oleate synthesis in a fixed catalyst bed reactor. Biotechnol. Lett. 1991, 13, 167–172. [Google Scholar] [CrossRef]
- Arcens, D.; Grau, E.; Grelier, S.; Cramail, H.; Peruch, F. Impact of Fatty Acid Structure on CALB-Catalyzed Esterification of Glucose. Eur. J. Lipid. Sci. Technol. 2020, 122, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Galonde, N.; Brostaux, Y.; Richard, G.; Nott, K.; Jerôme, C.; Fauconnier, M.L. Use of response surface methodology for the optimization of the lipase-catalyzed synthesis of mannosyl myristate in pure ionic liquid. Process Biochem. 2013, 48, 1914–1920. [Google Scholar] [CrossRef]
- Katsoura, M.H.; Polydera, A.C.; Katapodis, P.; Kolisis, F.N.; Stamatis, H. Effect of different reaction parameters on the lipase-catalyzed selective acylation of polyhydroxylated natural compounds in ionic liquids. Process Biochem. 2007, 42, 1326–1334. [Google Scholar] [CrossRef]
- Csajági, C.; Szatzker, G.; Rita Toke, E.; Ürge, L.; Darvas, F.; Poppe, L. Enantiomer selective acylation of racemic alcohols by lipases in continuous-flow bioreactors. Tetrahedron Asymmetry 2008, 19, 237–246. [Google Scholar] [CrossRef]
- Novak, U.; Lavric, D.; Žnidaršič-Plazl, P. Continuous lipase B-catalyzed isoamyl acetate synthesis in a two-liquid phase system using Corning® AFRTM module coupled with a membrane separator enabling biocatalyst recycle. J. Flow. Chem. 2016, 6, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- Arcos, J.A.; Hill, C.G.; Otero, C. Kinetics of the lipase-catalyzed synthesis of glucose esters in acetone. Biotechnol. Bioeng. 2001, 73, 104–110. [Google Scholar] [CrossRef]
- Cauglia, F.; Canepa, P. The enzymatic synthesis of glucosylmyristate as a reaction model for general considerations on “sugar esters” production. Bioresour. Technol. 2008, 99, 4065–4072. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T.; Chisti, Y. Lipase mediated synthesis of sugar fatty acid esters. Process Biochem. 2011, 46, 2079–2090. [Google Scholar] [CrossRef]
- Bornscheuer, U.; Herar, A.; Kreye, L.; Wendel, V.; Capewell, A.; Meyer, H.H.; Scheper, T.; Kolisis, F.N. Factors affecting the lipase catalyzed transesterification reactions of 3-hydroxy esters in organic solvents. Tetrahedron Asymmetry 1993, 4, 1007–1016. [Google Scholar] [CrossRef]
- Cheng, Y.; Tsai, S. Effects of water activity and alcohol concentration on the kinetic resolution of lipase-catalyzed acyl transfer in organic solvents. Enzym. Microb. Technol. 2003, 32, 362–368. [Google Scholar] [CrossRef]
- Delavault, A.; Opochenska, O.; Laneque, L.; Soergel, H.; Muhle-Goll, C.; Ochsenreither, K.; Syldatk, C. Lipase-catalyzed production of sorbitol laurate in a “2-in-1” deep eutectic system: Factors affecting the synthesis and scalability. Molecules 2021, 26, 2759. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Koo, Y.M.; Ha, S.H. Influence of ionic liquids under controlled water activity and low halide content on lipase activity. Korean J. Chem. Eng. 2008, 25, 1456–1462. [Google Scholar] [CrossRef]
- Zuorro, A. Water activity prediction in sugar and polyol systems using theoretical molecular descriptors. Int. J. Mol. Sci. 2021, 22, 11044. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollenbach, R.; Muller, D.; Delavault, A.; Syldatk, C. Continuous Flow Glycolipid Synthesis Using a Packed Bed Reactor. Catalysts 2022, 12, 551. https://doi.org/10.3390/catal12050551
Hollenbach R, Muller D, Delavault A, Syldatk C. Continuous Flow Glycolipid Synthesis Using a Packed Bed Reactor. Catalysts. 2022; 12(5):551. https://doi.org/10.3390/catal12050551
Chicago/Turabian StyleHollenbach, Rebecca, Delphine Muller, André Delavault, and Christoph Syldatk. 2022. "Continuous Flow Glycolipid Synthesis Using a Packed Bed Reactor" Catalysts 12, no. 5: 551. https://doi.org/10.3390/catal12050551
APA StyleHollenbach, R., Muller, D., Delavault, A., & Syldatk, C. (2022). Continuous Flow Glycolipid Synthesis Using a Packed Bed Reactor. Catalysts, 12(5), 551. https://doi.org/10.3390/catal12050551





