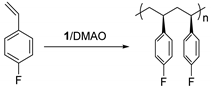
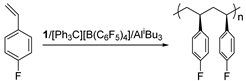Abstract
Isospecific polymerization of polar styrenes is a challenge of polymer science. Particularly challenging are monomers bearing electron-withdrawing substituents or bulky substituents. Here, we report the coordination polymerization of halide- and amino-functionalized styrenes including para-fluorostyrene (pFS), para-chlorostyrene (pClS), para-bromostyrene (pBrS), and para-(N,N-diethylamino)styrene (DMAS) using 2,2′-sulfur-bridged bis(phenolate) titanium precursor (1). The combination of 1 and [Ph3C][B(C6F5)4] and AliBu3 provides crystalline poly(pFS)s with perfect isotacticity (mmmm > 95%) and high molecular weights (≤16.0 × 104 g mol−1). Upon activation with a large excess of DMAO, 1 reaches polymerization activity of 5.58 × 105 g molTi−1 h−1 producing isotactic poly(pFS)s featuring higher molecular weights (≤39.6 × 104 g mol−1). The distinguished performance of the 1/DMAO system has been extended to the polymerization of pClS and pBrS, both usually involve halogen abstraction during the polymerization, to produce isotactic and high molecular weight (Mn = 32.2 × 104 vs. 13.7 × 104 g mol−1) polymers in good activities (2.18 × 105 vs. 1.31 × 105 g molTi−1 h−1). Surprisingly, 1/DMAO is nearly inactive for DMAS polymerization, on contrary, the system 1/[Ph3C][B(C6F5)4]/AliBu3 displays isoselectivity (mmmm > 95%) albeit in a moderate activity.
1. Introduction
The stereoselective polymerization of styrenes has made significant progress in recent years due to the development of novel transition metal catalysts [1,2,3,4,5] since Ishihara et al. first discovered that half-sandwich titanium complexes could catalyse the syndiospecific polymerization of styrene in 1985 [6,7]. Much effort has been expended pursuing the synthesis of stereospecific catalysts for the (co)polymerization of styrene derivatives bearing polar groups [8,9,10,11,12], such as halogen [13,14,15,16,17], alkoxyl [18,19,20,21,22,23], aminoalkyl [24,25,26], etc., which will endow the resultant polymers with distinguished functionalities such as improved surface properties, self-healing, and shape-memory abilities [27,28,29,30,31]. Group 4 metal-based catalysts have achieved some limited success in the polymerization of polar styrenes because they are easily poisoned by the polar groups [32,33,34]. The half-titanocene Cp*Ti(TEA)/MMAO is reported to show high catalytic activity and perfect syndioselectivity for the polymerization of amino-functionalized styrenes [35]. Ti(CH2Ph)4/MAO catalyses para-methoxystyrene polymerization with low activity, to give an atactic polymer [36]. With respect to the polymerization of halostyrene, titanium catalysts usually produce atactic polymers or a mixture of atactic and syndiotactic polymers, albeit with low catalytic activity [37,38,39,40]. Recently, the group-3 half-sandwich scandium complexes, especially those rare-earth metal-based complexes attached to the constrained geometry pyridyl-methylene-fluorenyl ligands, have shown excellent catalytic activity towards a broad range of polar styrenes to give syndiotactic polymers [5,8].
In contrast, the isotactic polymerization of polar styrene monomers develops relatively slowly [17,41], mainly due to the lack of highly efficient catalysts, which struggle to provide high activity while maintaining the sterically crowded coordination sphere required by the isotactic selectivity, although isotactic-enriched polystyrene has been synthesized long before with Ziegler–Natta catalysts or anionic catalysts [42,43]. However, the potential applications of functionalized isotactic polystyrenes as optically active, helical, etc., functional materials have led researchers to expend a lot of effort in this research field. In 2015, the first homogeneous single-site β-diketiminato rare-earth metal catalysts were developed by our group, showing high isoselectivity for ortho-methoxystyrene without masking reagents via the unique “self-assisted” mechanism; however, they are nearly inactive towards other polar styrene derivatives [44,45]. Recently, we developed a series of racemic isopropylidene-bridged bis(benz[e]indenyl) rare-earth metal alkyl complexes, which served as effective catalysts for the isoselective polymerizations of styrene, para/meta-methoxystyrenes, para-methylthiostyrene, and para-vinylphenyldimethylsilanol, etc. [46]. Unfortunately, they were virtually inert to the halide- and amino-functionalized styrenes. Probably because the coordination sphere of these catalysts is more open to allow more monomer coordination, in particular in the inert M-σ-X (X = functional group) mode. The bulkier ansa-bridged bis(indenyl) allyl yttrium and neodymium complexes developed by Carpentier’s group are also inactive [47,48], where the vacant coordination site is too crowded to facilitate the coordination of bulky amino-functionalized styrenes. In addition, the low Lewis acidity of neutral catalysts inhibits the coordination of the electron-deficient double bond of halostyrene to the metal ion. Thus, we turned to the 2,2′-sulfur-bridged bis(phenolato) titanium dichloro complex with a higher Lewis acidity (1) (Figure 1) [49,50,51,52], an isospecific catalyst for styrene polymerization reported by Okuda and co-workers, and examined its catalytic performance for the polymerization of polar styrenes, and in particular, of halostyrenes, since the resultant halogen styrene polymers feature improved corrosion resistance, heat resistance, flame retardancy, etc. [53,54,55,56,57]. Moreover, the halogen groups can be easily converted into other functionalities to impart different properties to the polymers [58]. In addition, this catalyst performs well in catalysing the (co)polymerization of conjugated dienes [59,60], which provides a possible route for the direct synthesis of halide-functionalized butyl rubber with faster vulcanization, high energy absorption, and low elastic modulus [61,62], and halide-functionalized butadiene-styrene rubber possessing better compatibility with polar fillers which could be used to fabricate tires with low-rolling resistance and good wet-skid resistance [63,64,65,66]. Herein, we report the polymerization behaviour of para-fluorostyrene (pFS), para-chlorostyrene (pClS), para-bromostyrene (pBrS), and para-(N,N-diethylamino)styrene (DMAS) by using complex 1 activated by organoborate, alkyl aluminium, MAO and dried MAO (DMAO).
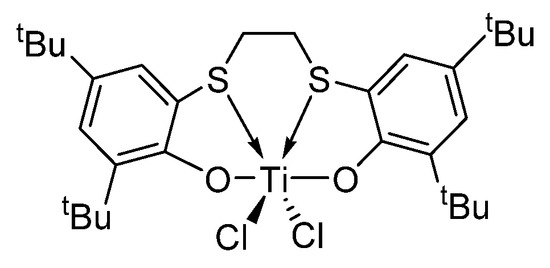
Figure 1.
Structure of [OSSO]-type titanium complex 1.
2. Results and Discussion
At first, a combination of 1 with [Ph3C][B(C6F5)4] and AliBu3 was chosen as the catalyst for polymerization of para-fluorostyrene (pFS) because organoborate-based activators are reported to be more efficient reagents than MAO. The polymerization was performed at 25 °C in a toluene solution to reach 54% conversion in 30 min. On increasing the reaction temperature from 25 to 80 °C, the highest catalytic activity of 1.12 × 105 g molTi−1 h−1 was observed at 40 °C (Table 1, entries 1–4), probably because the Ti(IV) active species is readily reduced to the inert Ti(III) by aluminium alkyls at high reaction temperatures. Subsequently, a kinetics investigation was carried out at 40 °C under a pFS-to-1 ratio of 1000:1. Increasing the reaction time from 15 to 120 min resulted in an obvious increase in monomer conversion from 30% to 79% (Table 1, entries 5–7). The molecular weight distributions of the resultant polymers consequently broadened from 1.53 to 1.89, but the molecular weights (Mn = 15.1–16.0 × 104 g mol−1) remained nearly constant (Figure 2), indicating the chain transfer reaction accompanying the polymerization process.

Table 1.
Isoselective polymerization of pFS with complex 1, AliBu3 and [Ph3C][B(C6F5)4].
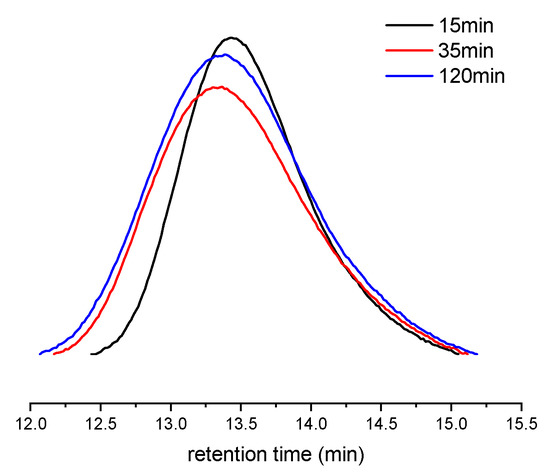
Figure 2.
GPC curves of poly(pFS)s obtained at different polymerization time (Table 1, entries 5–7).
The catalytic system 1/MAO has been reported to exhibit high catalytic activity for isospecific styrene polymerization [49]. Therefore, complex 1 activated by 2000 equivalents of MAO was utilized to catalyse pFS polymerization at 40 °C; however, only 25% of the monomer was consumed in 2 h (Table 2, entry 1). In contrast, the combination of 1 and 2000 equivalents of DMAO showed a much higher catalytic activity (5.51 × 105 g molTi−1 h−1 vs. 1.51 × 105 g molTi−1 h−1) with 90% monomer conversion under identical conditions (Table 2, entry 2). This was attributed mainly to the absence of free AlMe3, which is able to interact with the active species leading to an inactive dimethyl-bridged species [67,68,69]. Notably, a large excess of DMAO against complex 1 is necessary in order to obtain a highly active species for pFS polymerization. Whenever all complex 1 molecules were converted into the cationic active species, further increasing DMAO loading amount did not improve the catalytic activity (Table 2, entries 2, 6, and 7). Increasing reaction temperature accelerated the polymerization process to a certain degree, but too-high a temperature led to declined polymerization activity from 5.51 × 105 g molTi−1 h−1 at 40 °C to 4.54 × 105 g molTi−1 h−1 at 60 °C, along with a dramatic decrease in molecular weight from 37.4 × 105 g molTi−1 to 13.4 × 105 g molTi−1 h−1 (Table 2, entries 2, 8 and 9).

Table 2.
Isoselective polymerization of pFS with complex 1 and DMAO.
All of the resulting poly(pFS)s are nearly insoluble in toluene, tetrahedrofuran (THF), chloroform, etc., at room temperature, but readily soluble in chlorobenzene, acetylene tetrachloride, etc., at high temperature. NMR spectroscopy analysis unambiguously indicated that the poly(pFS)s produced by both the catalytic systems 1/AliBu3/[Ph3C][B(C6F5)4] and 1/DMAO have an isotactic microstructure, evidenced by the quintet centred at δ 2.14 ppm and the multiplet centred at δ 1.52 ppm assigned to the methine and asymmetric methylene protons, respectively (Figure 3a). The perfect isotacticity is further confirmed by the sharp singlets at δ 43.78 ppm for methylene carbon and δ 41.03 ppm for methine carbon. All the peaks of the fluorine-substituted phenyl carbons split into doublets as a result of coupling with 19F nuclei (Figure 3b). The ipso-carbon C3 shows a doublet at δ 141.62 ppm with a coupling constant of 4JC–F = 3 Hz. The coupling constants of ortho-carbon C4 (δ 128.94 ppm, 3JC–F = 8 Hz), meta-carbon C5 (δ 115.12 ppm, 2JC–F = 21 Hz) and para-carbon C6 (δ 161.55 ppm, 1JC–F = 244 Hz) are significantly enlarged due to gradually closing to the fluorine atom. The obtained isotactic polymer shows a high glass-transition temperature of around 104 °C and a melting temperature in the range of 242.9–247.8 °C (Figure S20–S28), which are much lower than those observed in syndiotactic poly(pFS) [14].
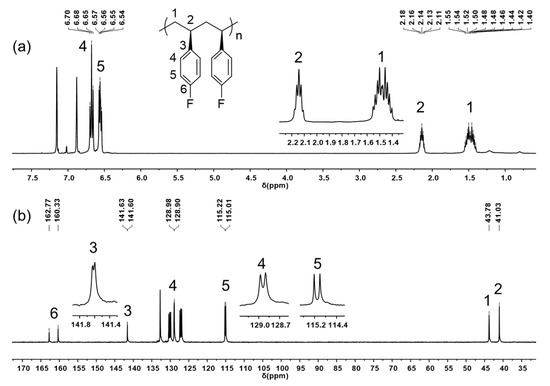
Figure 3.
1H NMR spectrum (a) and 13C NMR spectrum (b) of isotactic poly(pFS) (C6Cl2D4, 110 °C) (Table 1, entry 2).
Stimulated by the above results, the polymerizations of other chloro-styrene derivatives were studied using the system 1/DMAO. In a previous report, chloro- and bromo-substituted styrenes needed to be polymerized at low temperatures (≤0 °C) because the scandium cationic active species readily cleaved the C-X (X = Cl or Br) bond of halostyrene to generate the inert metal-halide species at high temperatures [15]. Surprisingly to us, para-chlorostyrene (pClS) was also converted into a perfect isotactic polymer at 40 °C with 63% conversion in 2 h (Table 3, entry 1). This may be due to the Lewis acidity of titanium cationic active species being lower than that of scandium, which would not cause C-X bond cleavage at higher temperatures. Even para-bromostyrene (pBrS) polymerization under similar conditions reached 57% monomer conversion in a prolonged reaction time (4 h) (Table 3, entry 2). Their polymerization activities relate mainly to the electronics of the monomers following the trend pFS > pClS > pBrS, which is consistent with the natural bond orbital (NBO) charge of β-CH2 of these monomers; pBrS has the lowest electron density (−0.339) as compared to pClS (−0.346) and pFS (−0.353) (Figure S38). On the other hand, Cl and Br atoms with larger atomic radii may also adversely affect the polymerization. Unexpectedly, 1/DMAO was virtually inactive for the polymerization of para-(N,N-diethylamino)styrene (DMAS). Switching to the catalytic system of 1/AliBu3/[Ph3C][B(C6F5)4], surprisingly, effective polymerization was achieved by converting 82% DMAS in 30 min in an isospecific manner, although the bulky dimethylamino group significantly decreased the activity (Table 3, entry 4). Thermal analyses revealed all the resultant isotactic poly(pClS), poly(pBrS), and poly(DMAS) possess higher glass transition temperatures of 124.7, 135.1, and 130.7 °C, respectively, but the melting points were not observed in the DSC curves despite their perfect isotacticity (mmmm > 95%) (Figure S39–S43).

Table 3.
Isoselective polymerization of styrene derivatives with complex 1.
3. Conclusions
We have demonstrated that the isospecific polymerizations of halostyrene and amino-functionalized styrenes with high activity have been achieved using titanium bisphenolate catalyst 1. Compared with scandium, yttrium, and lutetium rare-earth metal-based catalysts, titanium catalysts have suitable Lewis acidity, which not only compensates for the low coordination ability of the double bond on halostyrenes, but also does not cause C-X bond cleavage at high temperatures. The polymerization activity is strongly influenced by the cocatalysts and the reaction temperature. Upon activation with AliBu3 and [Ph3C][B(C6F5)4], complex 1 furnishes perfect isospecific poly(pFS) with high molecular weight and narrow molecular weight distribution for the first time; however, the polymerization activity is relatively low. When DMAO is used as the cocatalyst, the pFS polymerization process is greatly accelerated under a suitable reaction temperature, resulting in an isotactic product with a higher molecular weight and a narrow molecular weight distribution. A reaction temperature over 40 °C is not detrimental to isoselectivity but decreases the polymerization activity and the molecular weight, probably due to the reduction of Ti (IV) to Ti (III) by alkyl aluminium and the chain transfer reaction. In addition, the combination of 1/DMAO also shows high catalytic activity and perfect isoselectivity for the polymerization of pClS and pBrS, but the isospecific polymerization of DMAS is only accomplished by the system 1/[Ph3C][B(C6F5)4]/AliBu3. This work paves a new avenue to access isotactic polyhalostyrenes, which can be easily transferred to other functionalized isotactic polystyrenes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12040439/s1, Experimental procedures; Figure S1: 1H NMR spectrum of poly(pFS) (400MHz, C6Cl2D4, 110 oC) (Table 1, entry 1); Figure S2: 13C NMR spectrum of poly(pFS) (100MHz, C6Cl2D4, 110 oC) (Table 1, entry 1); Figure S3: 1H NMR spectrum of poly(pFS) (400MHz, C6Cl2D4, 110 oC) (Table 1, entry 3); Figure S4: 13C NMR spectrum of poly(pFS) (100MHz, C6Cl2D4, 110 oC) (Table 1, entry 3); Figure S5: 1H NMR spectrum of poly(pFS) (400MHz, C6Cl2D4, 110 oC) (Table 1, entry 4); Figure S6: 13C NMR spectrum of poly(pFS) (100MHz, C6Cl2D4, 110 oC) (Table 1, entry 4); Figure S7: The DSC curve of poly(pFS) (Table 1, entry 1); Figure S8: The DSC curve of poly(pFS) (Table 1, entry 2); Figure S9: The DSC curve of poly(pFS) (Table 1, entry 3); Figure S10: The DSC curve of poly(pFS) (Table 1, entry 4); Figure S11: The DSC curve of poly(pFS) (Table 1, entry 5); Figure S12: The DSC curve of poly(pFS) (Table 1, entry 6); Figure S13: The DSC curve of poly(pFS) (Table 1, entry 7); Figure S14: The GPC curve of poly(pFS) (Table 1, entry 1); Figure S15: The GPC curve of poly(pFS) (Table 1, entry 2); Figure S16: The GPC curve of poly(pFS) (Table 1, entry 3); Figure S17: The GPC curve of poly(pFS) (Table 1, entry 4); Figure S18: 1H NMR spectrum of poly(pFS) (400MHz, C6Cl2D4, 110 oC) (Table 2, entry 2); Figure S19: 13C NMR spectrum of poly(pFS) (100MHz, C6Cl2D4, 110 oC) (Table 2, entry 2); Figure S20: The DSC curve of poly(pFS) (Table 2, entry 1); Figure S21: The DSC curve of poly(pFS) (Table 2, entry 2); Figure S22: The DSC curve of poly(pFS) (Table 2, entry 3); Figure S23: The DSC curve of poly(pFS) (Table 2, entry 4); Figure S24: The DSC curve of poly(pFS) (Table 2, entry 5); Figure S25: The DSC curve of poly(pFS) (Table 2, entry 6); Figure S26: The DSC curve of poly(pFS) (Table 2, entry 7); Figure S27: The DSC curve of poly(pFS) (Table 2, entry 8); Figure S28: The DSC curve of poly(pFS) (Table 2, entry 9); Figure S29: The GPC curve of poly(pFS) (Table 2, entry 1); Figure S30: The GPC curve of poly(pFS) (Table 2, entry 2); Figure S31: The GPC curve of poly(pFS) (Table 2, entry 3); Figure S32: The GPC curve of poly(pFS) (Table 2, entry 4); Figure S33: The GPC curve of poly(pFS) (Table 2, entry 5); Figure S34: The GPC curve of poly(pFS) (Table 2, entry 6); Figure S35: The GPC curve of poly(pFS) (Table 2, entry 7); Figure S36: The GPC curve of poly(pFS) (Table 2, entry 8); Figure S37: The GPC curve of poly(pFS) (Table 2, entry 9); Figure S38: NBO charge of the monomers. (a) St; (b) pFS; (c) pClS; (d) pBrS; Figure S39: 1H NMR spectrum of poly(pClS) (400MHz, C6Cl2D4, 110 oC) (Table 3, entry 1); Figure S40: 13C NMR spectrum of poly(pClS) (100MHz, C6Cl2D4, 110 oC) (Table 3, entry 1); Figure S41: 1H NMR spectrum of poly(pBrS) (400MHz, C6Cl2D4, 110 oC) (Table 3, entry 2); Figure S42: 13C NMR spectrum of poly(pBrS) (100MHz, C6Cl2D4, 110 oC) (Table 3, entry 2); Figure S43: 13C NMR spectrum of poly(DMAS) (100MHz, CDCl3, 25 oC) (Table 3, entry 4); Figure S44: The DSC curve of poly(pClS) (Table 3, entry 1); Figure S45: The DSC curve of poly(pBrS) (Table 3, entry 2); Figure S46: The DSC curve of poly(DMAS) (Table 3, entry 4); Figure S47: The GPC curve of poly(pClS) (Table 3, entry 1); Figure S48: The GPC curve of poly(pBrS) (Table 3, entry 2); Figure S49: The GPC curve of poly(DMAS) (Table 3, entry 4).
Author Contributions
Conceptualization, Q.W. and S.L.; methodology, Q.W., Z.Z. and Y.J.; formal analysis, Q.W. and S.L.; investigation, Q.W. and Z.Z.; resources, D.C.; data curation, Q.W.; writing—original draft preparation, Q.W. and S.L.; writing—review and editing, S.L. and D.C.; project administration, S.L. and D.C.; funding acquisition, S.L., Y.Z. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Department of Science and Technology of Shanxi Province, grant number 2021JLM-40 and the National Natural Science Foundation of China, grant number 52073275.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirillov, E.; Lehmann, C.W.; Razavi, A.; Carpentier, J.F. Highly Syndiospecific Polymerization of Styrene Catalyzed by Allyl Lanthanide Complexes. J. Am. Chem. Soc. 2004, 126, 12240–12241. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Baldamus, J.; Hou, Z. Scandium Half-Metallocene-Catalyzed Syndiospecific Styrene Polymerization and Styrene−Ethylene Copolymerization: Unprecedented Incorporation of Syndiotactic Styrene−Styrene Sequences in Styrene−Ethylene Copolymers. J. Am. Chem. Soc. 2004, 126, 13910–13911. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Z.; Cui, D.; Liu, X. Precisely Controlled Polymerization of Styrene and Conjugated Dienes by Group 3 Single-Site Catalysts. Chemcatchem 2018, 10, 42–61. [Google Scholar] [CrossRef]
- Jaroschik, F.; Shima, T.; Li, X.; Mori, K.; Ricard, L.; Le Goff, X.-F.; Nief, F.; Hou, Z. Synthesis, Characterization, and Reactivity of Mono(phospholyl)lanthanoid(III) Bis(dimethylaminobenzyl) Complexes. Organometallics 2007, 26, 5654–5660. [Google Scholar] [CrossRef]
- Wu, Z.; Fu, X. Stereospecific Coordination Polymerization of Vinyl Monomers Mediated by Rare earth Metal Complexes. Sci. Sin. Chim. 2020, 50, 1654–1671. [Google Scholar] [CrossRef]
- Ishihara, N.; Seimiya, T.; Kuramoto, M.; Uoi, M. Crystalline Syndiotactic Polystyrene. Macromolecules 1986, 19, 2464–2465. [Google Scholar] [CrossRef]
- Ishihara, N.; Kuramoto, M.; Uoi, M. Stereospecific Polymerization of Styrene Giving the Syndiotactic Polymer. Macromolecules 1988, 21, 3356–3360. [Google Scholar] [CrossRef]
- Cui, D. Studies on Homo- and Co-polymerizations of Polar and Non-polar Monomers Using Rare-earth Metal Catalysts. Acta. Polym. Sin. 2020, 51, 12–29. [Google Scholar]
- Liu, D.; Wang, M.; Chai, Y.; Wan, X.; Cui, D. Self-Activated Coordination Polymerization of Alkoxystyrenes by a Yttrium Precursor: Stereocontrol and Mechanism. ACS Catal. 2019, 9, 2618–2625. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Wang, Z.; Cui, D. Development of Group 3 Catalysts for Alternating Copolymerization of Ethylene and Styrene Derivatives. ACS Catal. 2018, 8, 6086–6093. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Y.; Zhang, K.; Cai, Z.; Li, S.; Cui, D. DMAO-activated Rare-earth Metal Catalysts for Styrene and Its Derivative Polymerization. Chin. J. Polym. Sci. 2021, 39, 1185–1190. [Google Scholar] [CrossRef]
- Liu, D.; Wang, R.; Wang, M.; Wu, C.; Wang, Z.; Yao, C.; Liu, B.; Wan, X.; Cui, D. Syndioselective Coordination Polymerization of Unmasked Polar Methoxystyrenes Using a Pyridenylmethylene Fluorenyl Yttrium Precursor. Chem. Commun. 2015, 51, 4685–4688. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Jiao, N.; Jiang, L.; Li, Y.; Hou, Z. Scandium-Catalyzed Syndiospecific Polymerization of Halide-Substituted Styrenes and Their Copolymerization with Styrene. Macromolecules 2017, 50, 8398–8405. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Liu, J.; Liu, D.; Cui, D. Rapid Syndiospecific (Co)Polymerization of Fluorostyrene with High Monomer Conversion. Chem. Eur. J. 2017, 23, 18151–18155. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Liu, D.; Liu, B.; Cui, D. Highly Syndioselective Coordination (Co)Polymerization of para-Chlorostyrene. Macromolecules 2020, 53, 8333–8339. [Google Scholar] [CrossRef]
- Wang, T.; Liu, D.; Cui, D. Highly Syndioselective Coordination (Co)Polymerization of ortho-Fluorostyrene. Macromolecules 2019, 52, 9555–9560. [Google Scholar] [CrossRef]
- Carlo, F.D.; Capacchione, C.; Schiavo, V.; Proto, A. Reactivity of Styrene and Substituted Styrenes in the Presence of A Homogeneous Isospecific Titanium Catalyst. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1486–1491. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Zhai, J.; Wang, F.; Pan, L.; Shi, X. Imidazoline-2-imine Functionalized Fluorenyl Rare-Earth Metal Complexes: Synthesis and Their Application in the Polymerization of ortho-Methoxystyrene. Organometallics 2021, 40, 3323–3330. [Google Scholar] [CrossRef]
- Song, C.; Chen, J.; Fu, Z.; Yan, L.; Gao, F.; Cao, Q.; Li, H.; Yan, X.; Chen, S.; Zhang, S.; et al. Syndiospecific Polymerization of o-Methoxystyrene and Its Silyloxy or Fluorine-Substituted Derivatives by HNC-Ligated Scandium Catalysts: Synthesis of Ultrahigh-Molecular-Weight Functionalized Polymers. Macromolecules 2021, 54, 10838–10849. [Google Scholar] [CrossRef]
- Wang, R.; Liu, D.; Li, X.; Zhang, J.; Cui, D.; Wan, X. Synthesis and Stereospecific Polymerization of a Novel Bulky Styrene Derivative. Macromolecules 2016, 49, 2502–2510. [Google Scholar] [CrossRef]
- Cui, L.; Chen, M.; Chen, C.; Liu, D.; Jian, Z. Systematic Studies on (Co)Polymerization of Polar Styrene Monomers with Palladium Catalysts. Macromolecules 2019, 52, 7197–7206. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Wu, C.; Chen, J.; Zhang, J.; Cui, D.; Wan, X. Effect of the tactic structure on the chiroptical properties of helical vinylbiphenyl polymers. Polym. Chem. 2019, 10, 3887–3894. [Google Scholar] [CrossRef]
- Shi, X.; Nishiura, M.; Hou, Z. Simultaneous Chain-Growth and Step-Growth Polymerization of Methoxystyrenes by Rare-Earth Catalysts. Angew. Chem. Int. Ed. 2016, 55, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Li, Y. Syndiospecific Coordination (Co)polymerization of Carbazole-substituted Styrene Derivatives Using the Scandium Catalyst System. Polym. Chem. 2021, 12, 6291–6299. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, F.; Shi, Z.; Li, Y.; Hou, Z. Syndiotactic Poly(aminostyrene)-Supported Palladium Catalyst for Ketone Methylation with Methanol. Chemcatchem 2017, 9, 3827–3832. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, F.; Li, Y.; Hou, Z. Synthesis of Amino-containing Syndiotactic Polystyrene as Efficient Polymer Support for Palladium Nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 5–9. [Google Scholar] [CrossRef]
- Yang, K.; Niu, H.; Shi, Z.; Tan, R.; Li, T.; Shen, K.; Li, Y. Convenient Synthesis of Versatile Syndiotactic Polystyrene Materials Containing Pendant Alkenyl Groups with a Scandium Catalyst System. Polym. Chem. 2018, 9, 3709–3713. [Google Scholar] [CrossRef]
- Guo, F.; Wang, B.; Ma, H.; Li, T.; Li, Y. Scandium-catalyzed Synthesis of Si–H-containing Syndiotactic Polystyrene and Its Functionalized Polymer Carrying Pendant Perylene Bisimide Units. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 735–739. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Cui, D. Statistically Syndioselective Coordination (Co)polymerization of 4-Methylthiostyrene. Macromolecules 2016, 49, 781–787. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, Y.; Li, S.; Cui, D. Highly Syndioselective Coordination (Co)polymerization of Isopropenylstyrene. Polym. Chem. 2018, 9, 4476–4482. [Google Scholar] [CrossRef]
- Li, S.; Yan, F.; Zhang, Z.; Dou, Y.; Zhang, W.; Cui, D. Syndioselective Polymerization of Vinylnaphthalene. Macromol. Rapid. Commun. 2019, 40, 1900061. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.; Tomotsu, N. Syndiotactic Polystyrene Catalysts and Polymerization. Prog. Polym. Sci. 2002, 27, 1925–1982. [Google Scholar] [CrossRef]
- Schellenberg, J. Recent Transition Metal Catalysts for Syndiotactic Polystyrene. Prog. Polym. Sci. 2009, 34, 688–718. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Kirillov, E.; Carpentier, J.F. Group 3 and 4 Single-site Catalysts for Stereospecific Polymerization of Styrene. Coordin. Chem. Rev. 2008, 252, 2115–2136. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Han, Y.; Do, Y. Syndiotactic Polymerization of Amino-functionalized Styrenes Using (Pentamethylcyclopentadienyl)titanatrane/MMAO Catalyst System. Bull. Korean Chem. Soc. 2004, 25, 1648–1652. [Google Scholar]
- Grassi, A.; Longo, P.; Proto, A.; Zambelli, A. Reactivity of Some Substituted Styrenes in the Presence of a Syndiotactic Specific Polymerization Catalyst. Macromolecules 1989, 22, 104–108. [Google Scholar] [CrossRef]
- Soga, K.; Nakatani, H.; Monoi, T. Copolymerization of Styrene and Substituted Styrenes with Tetramenthoxytitanium-methylaluminoxane Catalyst. Macromolecules 1990, 23, 953–957. [Google Scholar] [CrossRef]
- Napoli, M.; Grisi, F.; Longo, P. Half-Titanocene-Based Catalysts in the Syndiospecific Polymerization of Styrenes: Possible Oxidation States of the Titanium Species and Geometries of the Active Sites. Macromolecules 2009, 42, 2516–2522. [Google Scholar] [CrossRef]
- Galdi, N.; Albunia, A.R.; Oliva, L.; Guerra, G. Polymorphism of Syndiotactic Poly(p-fluoro-styrene). Polymer 2009, 50, 1901–1907. [Google Scholar] [CrossRef]
- Longo, P.; Proto, A.; Zambelli, A. Syndiotactic Specific Polymerization of Styrene: Driving Energy of the Steric Control and Reaction Mechanism. Macromol. Chem. Phys. 1995, 196, 3015–3029. [Google Scholar] [CrossRef]
- Zhou, Q.; Liang, H.; Wei, W.; Meng, C.; Long, Y.; Zhu, F. Synthesis of Amphiphilic Diblock Copolymers of Isotactic Polystyrene-block-isotactic Poly(p-hydroxystyrene) Using a Titanium Complex with an [OSSO]-type Bis(phenolate) Ligand and Sequential Monomer Addition. RSC Adv. 2017, 7, 19885–19893. [Google Scholar] [CrossRef] [Green Version]
- Natta, G.; Pino, P.; Corradini, P.; Danusso, F.; Mantica, E.; Mazzanti, G.; Moraglio, G. Crystalline High Polymers of α-Olefins. J. Am. Chem. Soc. 1955, 77, 1708–1710. [Google Scholar] [CrossRef]
- Maréchal, J.M.; Carlotti, S.; Shcheglova, L.; Deffieux, A. Stereospecific Anionic Polymerization of Styrene Initiated by R2Mg/ROMt ‘ate’ Complexes. Polymer 2004, 45, 4641–4646. [Google Scholar] [CrossRef]
- Liu, D.; Yao, C.; Wang, R.; Wang, M.; Wang, Z.; Wu, C.; Lin, F.; Li, S.; Wan, X.; Cui, D. Highly Isoselective Coordination Polymerization of ortho-Methoxystyrene with β-Diketiminato Rare-Earth-Metal Precursors. Angew. Chem. Int. Ed. 2015, 54, 5205–5209. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Wang, L.; Liu, D.; Wang, Z.; Run, M.; Cui, D. Polar-Group Activated Isospecific Coordination Polymerization of ortho-Methoxystyrene: Effects of Central Metals and Ligands. Chem. Eur. J. 2019, 25, 2043–2050. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Li, S.; Cui, D. Isospecific (Co)polymerization of Unmasked Polar Styrenes by Neutral Rare-Earth Metal Catalysts. Angew. Chem. Int. Ed. 2022, 61, e202112966. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Kirillov, E.; Roisnel, T.; Razavi, A.; Vuillemin, B.; Carpentier, J.F. Highly Isospecific Styrene Polymerization Catalyzed by Single-Component Bridged Bis(indenyl) Allyl Yttrium and Neodymium Complexes. Angew. Chem. Int. Ed. 2007, 46, 7240–7243. [Google Scholar] [CrossRef]
- Annunziata, L.; Rodrigues, A.S.; Kirillov, E.; Sarazin, Y.; Okuda, J.; Perrin, L.; Maron, L.; Carpentier, J.F. Isoselective Styrene Polymerization Catalyzed by ansa-Bis(indenyl) Allyl Rare Earth Complexes. Stereochemical and Mechanistic Aspects. Macromolecules 2011, 44, 3312–3322. [Google Scholar] [CrossRef]
- Capacchione, C.; Proto, A.; Ebeling, H.; Mulhaupt, R.; Moller, K.; Spaniol, T.P.; Okuda, J. Ancillary Ligand Effect on Single-Site Styrene Polymerization: Isospecificity of Group 4 Metal Bis(phenolate) Catalysts. J. Am. Chem. Soc. 2003, 125, 4964–4965. [Google Scholar] [CrossRef]
- Beckerle, K.; Manivannan, R.; Spaniol, T.P.; Okuda, J. Living Isospecific Styrene Polymerization by Chiral Benzyl Titanium Complexes That Contain a Tetradentate [OSSO]-Type Bis(phenolato) Ligand. Organometallics 2006, 25, 3019–3026. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Saito, Y.; Watanabe, T.; Ishii, A. Highly Active and Isospecific Styrene Polymerization Catalyzed by Zirconium Complexes Bearing Aryl-substituted [OSSO]-Type Bis(phenolate) Ligands. Polymers 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradiso, V.; Capaccio, V.; Lamparelli, D.H.; Capacchione, C. [OSSO]-bisphenolate Metal Complexes: A Powerful and Versatile Tool in Polymerization Catalysis. Coordin. Chem. Rev. 2021, 429, 213644. [Google Scholar] [CrossRef]
- McNeill, I.; Coskun, M. Structure and Stability of Halogenated Polymers: Part 4—Chain Brominated Polystyrene. Polym. Degrad. Stab. 1989, 25, 1–9. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Sumida, Y.; Muggee, J.; Vogl, O. Head-to-head Polymers: 19. Chlorination of Cis-1,4-polybutadiene. Polymer 1982, 23, 1805–1814. [Google Scholar] [CrossRef]
- Wiacek, M.; Jurczyk, S.; Kurcok, M.; Janeczek, H.; SchabBalcerzak, E. Synthesis of Polystyrene Modified with Fluorine Atoms: Monomer Reactivity Ratios and Thermal Behavior. Polym. Eng. Sci. 2014, 54, 1170–1181. [Google Scholar] [CrossRef]
- Sessions, L.; Cohen, B.; Grubbs, R. Alkyne-Functional Polymers through Sonogashira Coupling to Poly(4-bromostyrene). Macromolecules 2007, 40, 1926–1933. [Google Scholar] [CrossRef]
- Jaymand, M. Recent Progress in the Chemical Modification of Syndiotactic Polystyrene. Polym. Chem. 2014, 5, 2663–2690. [Google Scholar] [CrossRef]
- Shin, J.; Chang, Y.; Nguyen, T.; Noh, S.; Bae, C. Hydrophilic Functionalization of Syndiotactic Polystyrene via A Combination of Electrophilic Bromination and Suzuki–Miyaura Reaction. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4335–4343. [Google Scholar] [CrossRef]
- Buonerba, A.; Fienga, M.; Milione, S.; Cuomo, C.; Grassi, A.; Proto, A.; Capacchione, C. Binary Copolymerization of p-Methylstyrene with Butadiene and Isoprene Catalyzed by Titanium Compounds Showing Different Stereoselectivity. Macromolecules 2013, 46, 8449–8457. [Google Scholar] [CrossRef]
- Milione, S.; Cuomo, C.; Capacchione, C.; Zannoni, C.; Grassi, A.; Proto, A. Stereoselective Polymerization of Conjugated Dienes and Styrene−Butadiene Copolymerization Promoted by Octahedral Titanium Catalyst. Macromolecules 2007, 40, 5638–5643. [Google Scholar] [CrossRef]
- Dall Asta, A.; Ragin, L. Nonlinear Behavior of Dynamic Systems with High Damping Rubber Devices. Eng. Struct. 2008, 30, 3610–3618. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Huang, Z. Static and Dynamic Mechanical Properties of Chlorobutyl Rubber Composites with Variable Sulfur/Accelerator Ratio. Mater. Des. 2011, 284–286, 1938–1941. [Google Scholar] [CrossRef]
- Cohen, R.E.; Ramos, A.R. Homogeneous and Heterogeneous Blends of Polybutadiene, Polyisoprene, and Corresponding Diblock Copolymers. Macromolecules 1979, 12, 131–134. [Google Scholar] [CrossRef]
- Halasa, A.F. Preparation and Characterization of Solution SIBR via Anionic Polymerization. Rubber Chem. Technol. 1997, 70, 295–308. [Google Scholar] [CrossRef]
- Halasa, A.F.; Gross, B.B.; Hsu, W.L. Multiple Glass Transition Terpolymers of Isoprene, Butadiene, and Styrene. Rubber Chem. Technol. 2010, 83, 380–390. [Google Scholar] [CrossRef]
- Zhu, S.H.; Chan, C.M.; Wong, S.C.; Mai, Y.W. Mechanical Properties of PVC/SBR Blends Compatibilized by Acrylonitrile-Butadiene Rubber and Covulcanization. Polym. Eng. Sci. 1999, 39, 1998–2006. [Google Scholar] [CrossRef]
- Bochmann, M. The Chemistry of Catalyst Activation: The Case of Group 4 Polymerization Catalysts. Organometallics 2010, 29, 4711–4740. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P.; Voskoboynikov, A.Z.; Lancaster, S.J.; Bochmann, M. Formation and Structures of Hafnocene Complexes in MAO- and AlBui3/CPh3[B(C6F5)4]-Activated Systems. Organometallics 2008, 27, 6333–6342. [Google Scholar] [CrossRef]
- Bochmann, M.; Lancaster, S.J. Monomer–Dimer Equilibria in Homo- and Heterodinuclear Cationic Alkylzirconium Complexes and Their Role in Polymerization Catalysis. Angew. Chem. Int. Ed. 1994, 33, 1634–1637. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).