Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation
2.1.1. X-ray Diffraction and (XRD) Thermogravimetric Analysis (TGA)
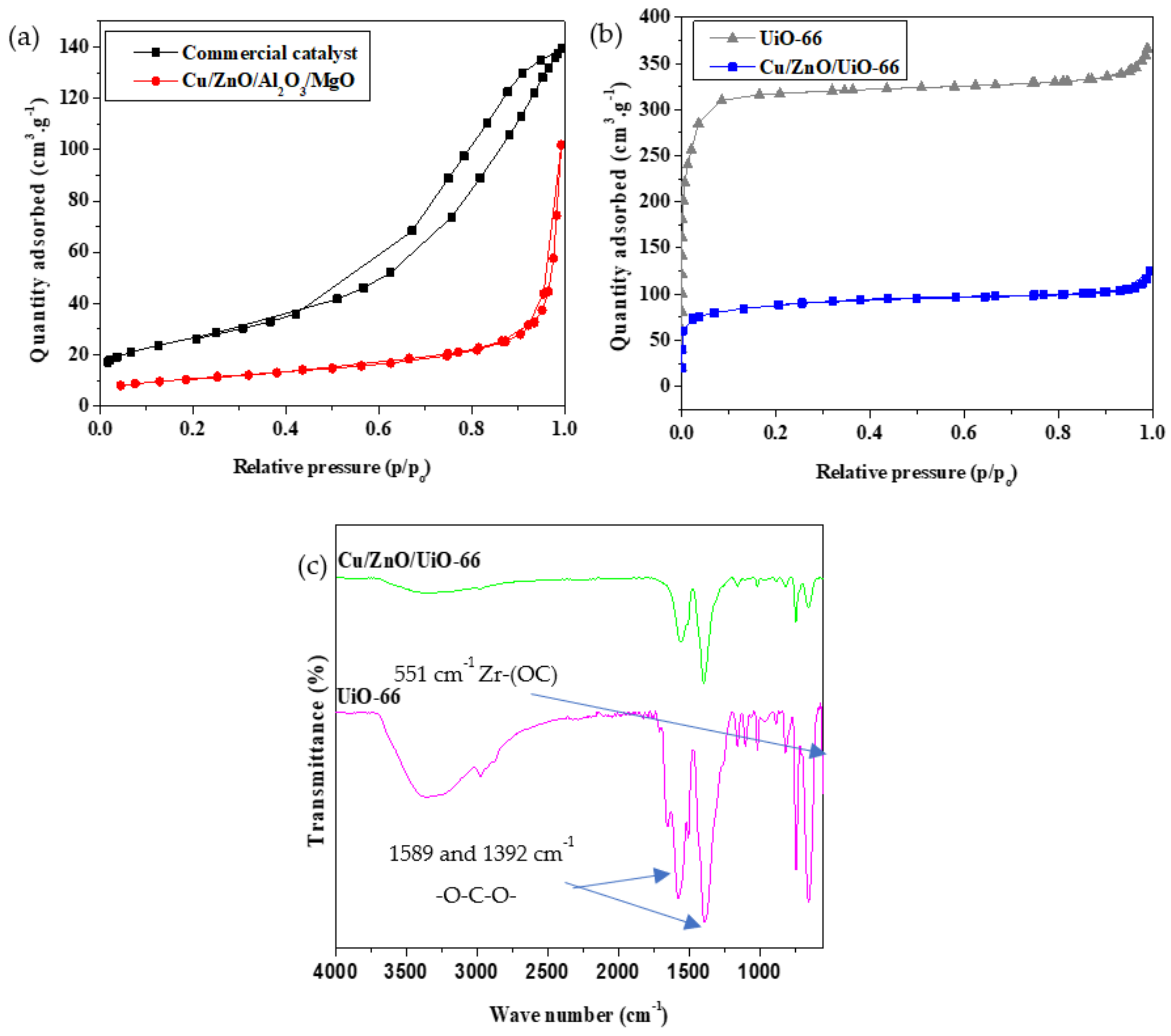
2.1.2. N2 Physisorption, Fourier Transform Infrared Spectroscopy (FTIR), and Metal Loading
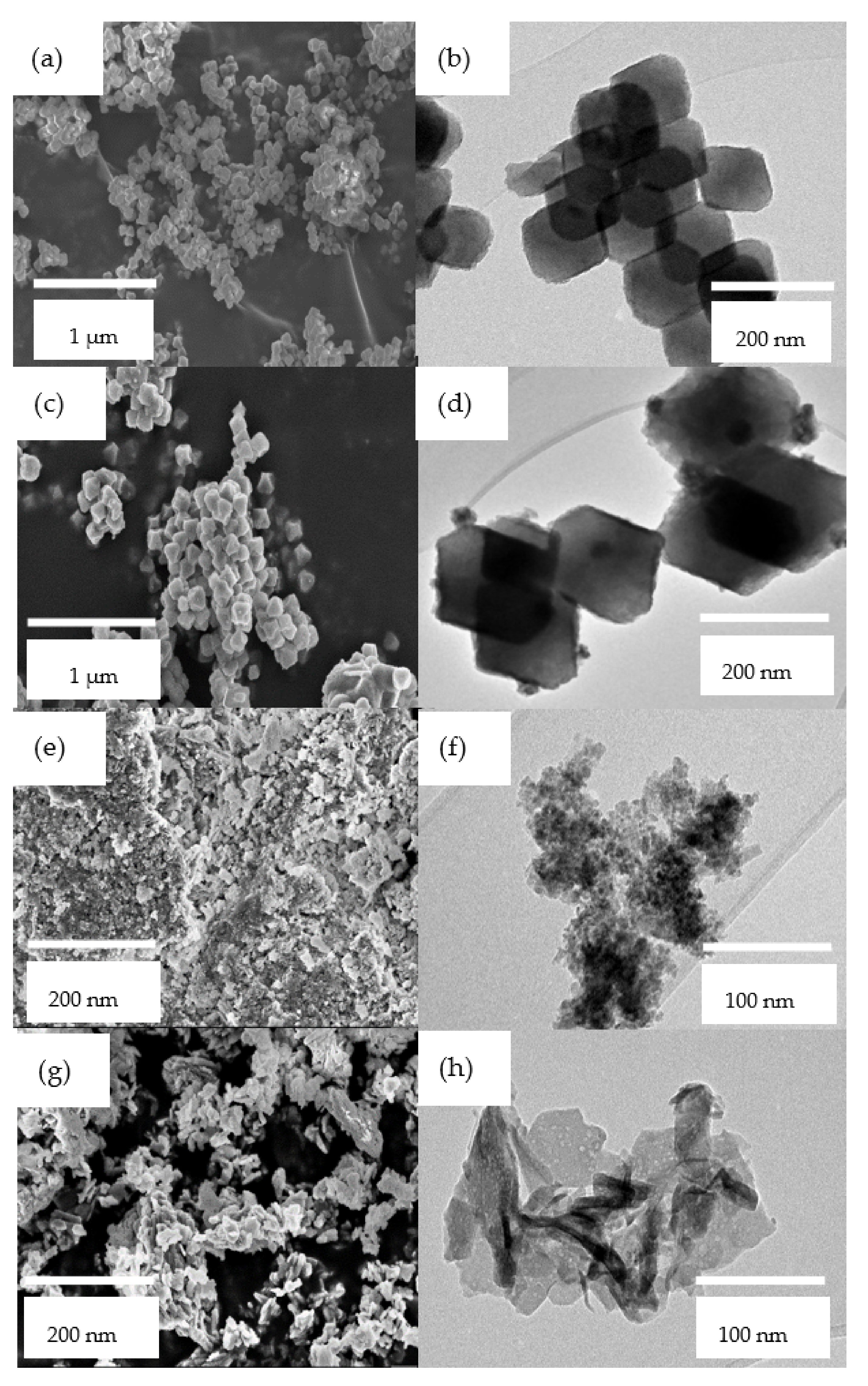
2.1.3. Electron Microscopies
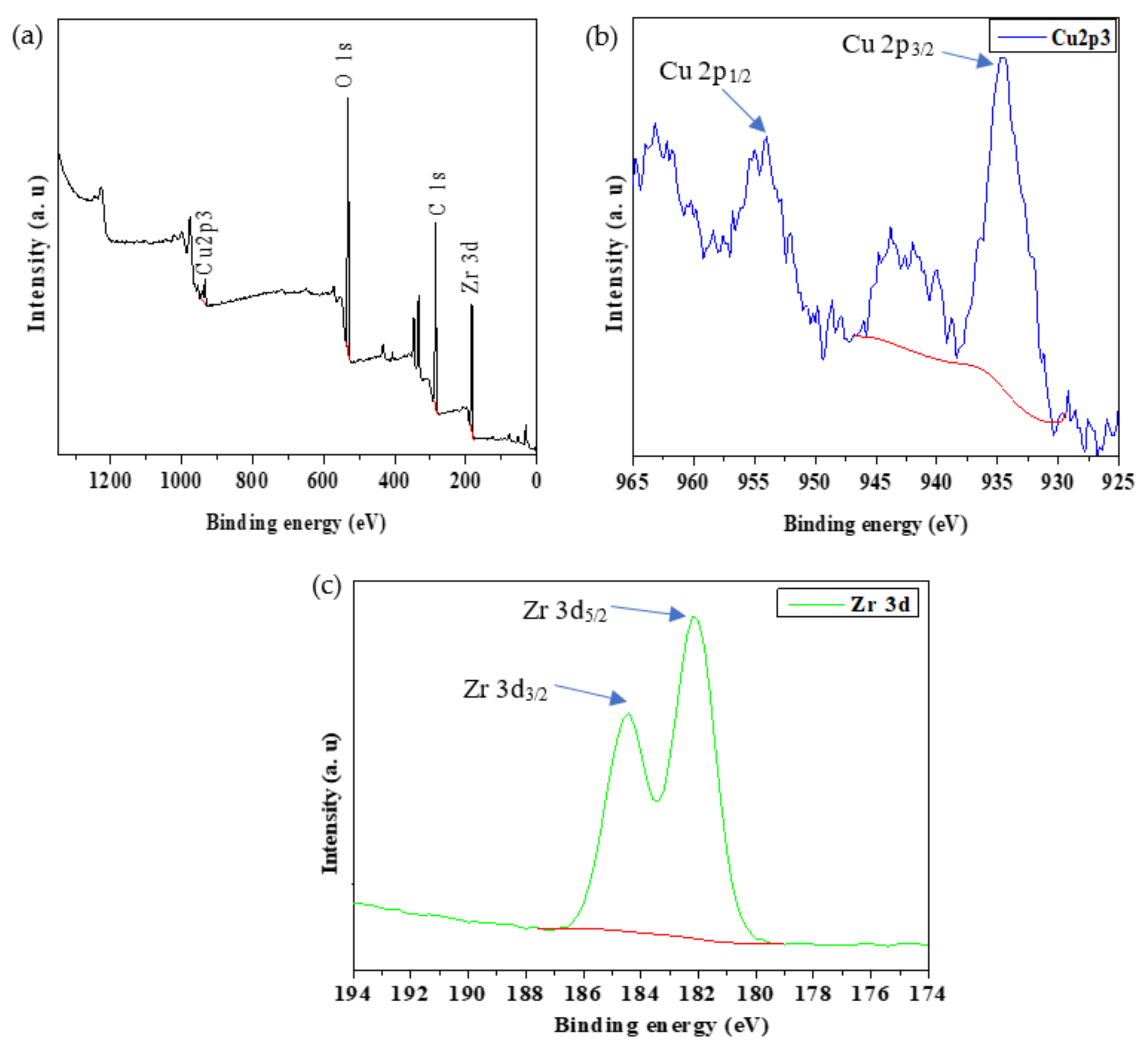
2.1.4. X-ray Photoelectron Spectroscopy (XPS)
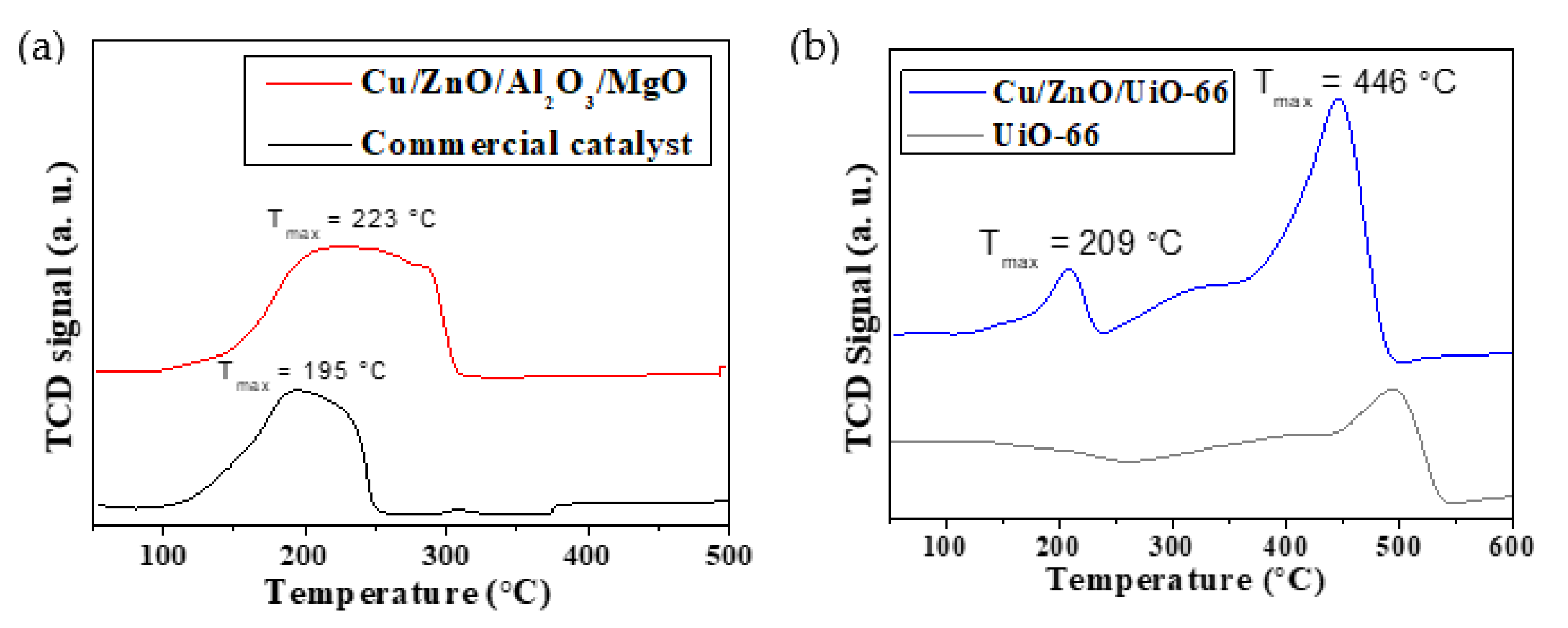
2.1.5. Hydrogen Temperature-Programmed Reduction (H2-TPR)
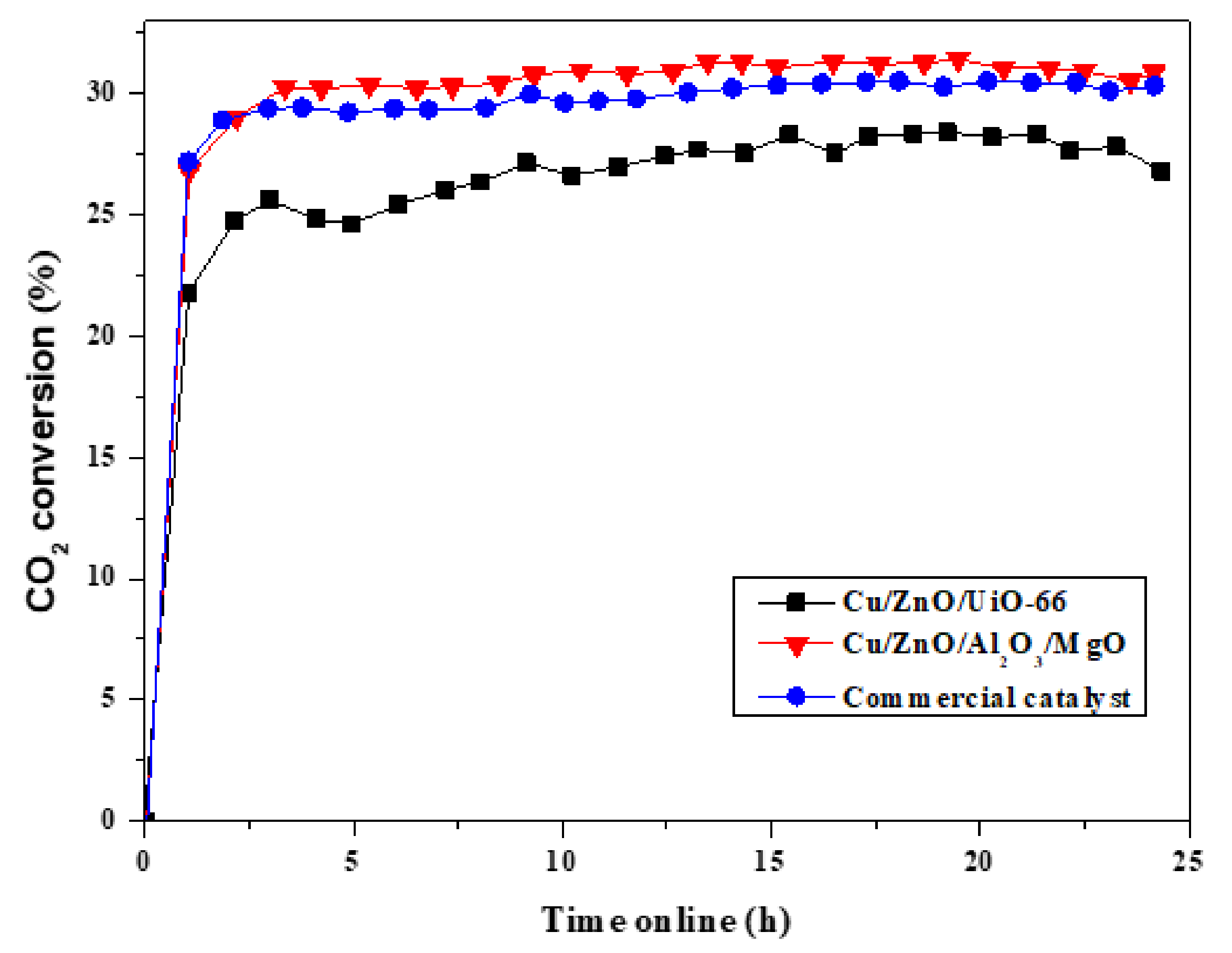
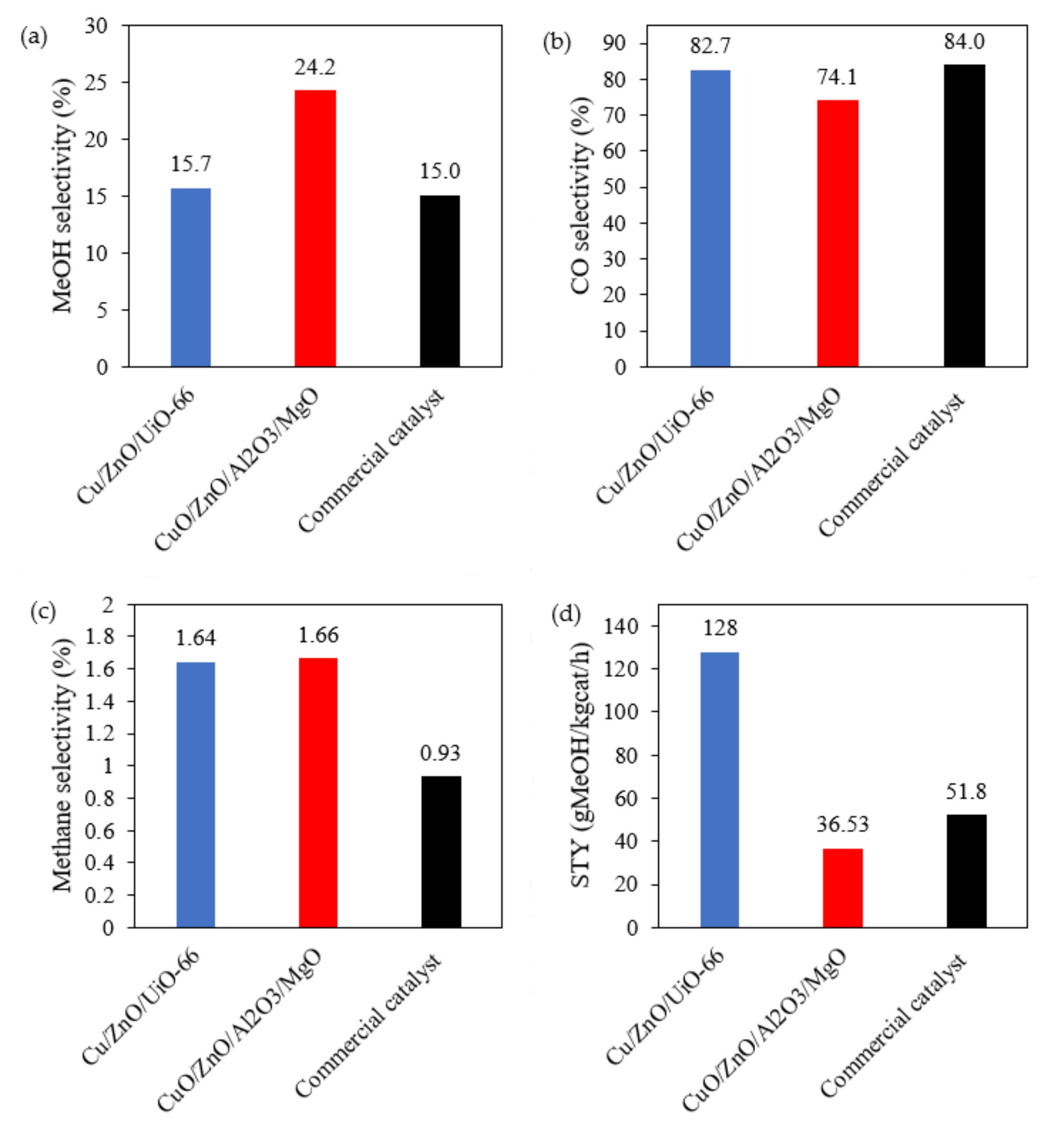
2.2. Catalyst Testing and Evaluation
Conversion, Selectivity and Productivity
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Catalysts’ Synthesis
3.2.1. Cu/ZnO/Al2O3/MgO Catalyst
3.2.2. UiO-66 (Zr) MOF Synthesis
3.2.3. UiO-66 (Zr) MOF-Supported Catalyst Synthesis
3.3. Characterisation
3.4. Catalyst Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tursunov, O.; Kustov, L.; Kustov, A. A Brief Review of Carbon Dioxide Hydrogenation to Methanol Over Copper and Iron Based Catalysts. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2017, 72, 30. [Google Scholar] [CrossRef] [Green Version]
- Fischer, H.; Wahlen, M.; Smith, J.; Mastroianni, D.; Deck, B. Ice core records of atmospheric CO2 around the last three glacial terminations. Science 1999, 283, 1712–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stawowy, M.; Ciesielski, R.; Maniecki, T.; Matus, K.; Łużny, R.; Trawczynski, J.; Silvestre-Albero, J.; Łamacz, A. CO2 Hydrogenation to Methanol over Ce and Zr Containing UiO-66 and Cu/UiO-66. Catalysts 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal-Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- Gesmanee, S.; Koo-Amornpattana, W. Catalytic hydrogenation of CO2 for methanol production in fixed-bed reactor using Cu-Zn supported on gamma-Al2O3. Energy Procedia 2017, 138, 739–744. [Google Scholar] [CrossRef]
- Bowker, M. Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Fichtl, M.B.; Schlereth, D.; Jacobsen, N.; Kasatkin, I.; Schumann, J.; Behrens, M.; Schlögl, R.; Hinrichsen, O. Kinetics of deactivation on Cu/ZnO/Al2O3 methanol synthesis catalysts. Appl. Catal. A Gen. 2015, 502, 262–270. [Google Scholar] [CrossRef]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Tan, Q.; Tian, C.; Pan, Y.; Sun, X.; Zhang, J.; Wu, D. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: Effect of reduction temperature. J. Catal. 2019, 379, 78–89. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Rak, Z.S. Reduction of greenhouse gas emissions by catalytic processes. Appl. Catal. B Environ. 2003, 41, 143–155. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Heterogeneous catalytic CO2 conversion to value-added hydrocarbons. Energy Environ. Sci. 2010, 3, 884–890. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.S. Beyond Oil and Gas: The Methanol Economy. John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki, P.R.; Howard, B.H.; Deng, X.; Matranga, C. Active Sites and Structure–Activity Relationships of Copper-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Agarwal, A.S.; Rode, E.; Sridhar, N.; Hill, D. Conversion of CO2 to Value-Added Chemicals: Opportunities and Challenges. In Handbook of Climate Change Mitigation and Adaptation; Chen, W.-Y., Suzuki, T., Lackner, M., Eds.; Springer: New York, NY, USA, 2014; pp. 1–40. [Google Scholar]
- Etim, U.J.; Song, Y.; Zhong, Z. Improving the Cu/ZnO-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol, and the Use of Methanol As a Renewable Energy Storage Media. Front. Earth Sci. 2020, 8. [Google Scholar] [CrossRef]
- Ahouari, H.; Soualah, A.; Le Valant, A.; Pinard, L.; Magnoux, P.; Pouilloux, Y. Methanol synthesis from CO2 hydrogenation over copper based catalysts. React. Kinet. Mech. Catal. 2013, 110, 131–145. [Google Scholar] [CrossRef]
- Qaderi, J. A brief review on the reaction mechanisms of CO2 hydrogenation into methanol. Int. J. Innov. Res. Sci. Stud. 2020, 3, 53–63. [Google Scholar] [CrossRef]
- Melián-Cabrera, I.; López Granados, M.; Fierro, J.L.G. Reverse Topotactic Transformation of a Cu–Zn–Al Catalyst during Wet Pd Impregnation: Relevance for the Performance in Methanol Synthesis from CO2/H2 Mixtures. J. Catal. 2002, 210, 273–284. [Google Scholar] [CrossRef]
- Wang, J.; Funk, S.; Burghaus, U. Indications for Metal-support Interactions: The Case of CO2Adsorption on Cu/ZnO(0001). Catal. Lett. 2005, 103, 219–223. [Google Scholar] [CrossRef]
- Waugh, K.C. Methanol Synthesis. Catal. Today 1992, 15, 51–75. [Google Scholar] [CrossRef]
- Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjær, C.F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 2016, 352, 969–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, M.S. The role of zinc oxide in Cu/ZnO catalysts for methanol synthesis and the water–gas shift reaction. Top. Catal. 1999, 8, 259. [Google Scholar] [CrossRef]
- Prieto, G.; Meeldijk, J.D.; de Jong, K.P.; de Jongh, P.E. Interplay between pore size and nanoparticle spatial distribution: Consequences for the stability of CuZn/SiO2 methanol synthesis catalysts. J. Catal. 2013, 303, 31–40. [Google Scholar] [CrossRef]
- An, X.; Li, J.; Zuo, Y.; Zhang, Q.; Wang, D.; Wang, J. A Cu/Zn/Al/Zr Fibrous Catalyst that is an Improved CO2 Hydrogenation to Methanol Catalyst. Catal. Lett. 2007, 118, 264–269. [Google Scholar] [CrossRef]
- Dalebout, R.; Visser, N.L.; Pompe, C.E.L.; de Jong, K.P.; de Jongh, P.E. Interplay between carbon dioxide enrichment and zinc oxide promotion of copper catalysts in methanol synthesis. J. Catal. 2020, 392, 150–158. [Google Scholar] [CrossRef]
- Lozano, L.A.; Faroldi, B.M.C.; Ulla, M.A.; Zamaro, J.M. Metal–Organic Framework-Based Sustainable Nanocatalysts for CO Oxidation. Nanomaterials 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Y.; Zhu, G.; Hungerford, J.T.; Bhattacharyya, S.; Lively, R.P.; Sholl, D.S.; Walton, K.S. Heat-Treatment of Defective UiO-66 from Modulated Synthesis: Adsorption and Stability Studies. J. Phys. Chem. C 2017, 121, 23471–23479. [Google Scholar] [CrossRef] [Green Version]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Kobayashi, H.; Taylor, J.M.; Mitsuka, Y.; Ogiwara, N.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kitagawa, H. Charge transfer dependence on CO2 hydrogenation activity to methanol in Cu nanoparticles covered with metal–organic framework systems. Chem. Sci. 2019, 10, 3289–3294. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-H.; Bae, J.W.; Prasad, P.S.S.; Oh, J.-H.; Jun, K.-W.; Song, S.-L.; Min, K.-S. Influence of Ga addition on the methanol synthesis activity of Cu/ZnO catalyst in the presence and absence of alumina. J. Ind. Eng. Chem. 2009, 15, 665–669. [Google Scholar] [CrossRef]
- Mota, N.; Guil-Lopez, R.; Pawelec, B.G.; Fierro, J.L.G.; Navarro, R.M. Highly active Cu/ZnO–Al catalyst for methanol synthesis: Eeffect of aging on its structure and activity. RSC Adv. 2018, 8, 20619–20629. [Google Scholar] [CrossRef] [Green Version]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, characterization and activity pattern of Cu–ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Fei, J.-H.; Yang, M.-X.; Hou, Z.-Y.; Zheng, X.-M. Effect of the Addition of Manganese and Zinc on the Properties of Copper-Based Catalyst for the Synthesis of Syngas to Dimethyl Ether. Energy Fuels 2004, 18, 1584–1587. [Google Scholar] [CrossRef]
- Wu, J.; Luo, S.; Toyir, J.; Saito, M.; Takeuchi, M.; Watanabe, T. Optimization of preparation conditions and improvement of stability of Cu/ZnO-based multicomponent catalysts for methanol synthesis from CO2 and H2. Catal. Today 1998, 45, 215–220. [Google Scholar] [CrossRef]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M.; Bessarabov, D. Modulated synthesis of zirconium-metal organic framework (Zr-MOF) for hydrogen storage applications. Int. J. Hydrogen Energy 2014, 39, 890–895. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Dyosiba, X.; Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Onyango, M.S. Feasibility of Varied Polyethylene Terephthalate Wastes as a Linker Source in Metal–Organic Framework UiO-66(Zr) Synthesis. Ind. Eng. Chem. Res. 2019, 58, 17010–17016. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, H.; Peng, Y.; Tan, Z.; Tang, B. Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surf. B Biointerfaces 2019, 178, 1–7. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Fujimoto, K. Development of highly stable catalyst for methanol synthesis from carbon dioxide. Appl. Catal. A Gen. 2014, 469, 306–311. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, S.; Xiong, W.; Liu, D.; Li, M.; He, B.; Fan, X.; Luo, D. Supported CuO catalysts on metal-organic framework (Cu-UiO-66) for efficient catalytic wet peroxide oxidation of 4-chlorophenol in wastewater. Microporous Mesoporous Mater. 2020, 291, 109703. [Google Scholar] [CrossRef]
- Behrens, M. Meso-and nano-structuring of industrial Cu/ZnO/(Al2O3) catalysts. J. Catal. 2009, 267, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Feng, C.; Liu, S.; Zhang, R. Synthesis of CuO catalyst derived from HKUST-1 temple for the low-temperature NH3-SCR process. Catal. Today 2018, 314, 122–128. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A Gen. 2012, 427–428, 24–34. [Google Scholar] [CrossRef]
- Alayoglu, S.; Beaumont, S.K.; Zheng, F.; Pushkarev, V.V.; Zheng, H.; Iablokov, V.; Liu, Z.; Guo, J.; Kruse, N.; Somorjai, G.A. CO2 hydrogenation studies on Co and CoPt bimetallic nanoparticles under reaction conditions using TEM, XPS and NEXAFS. Top. Catal. 2011, 54, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Chen, D.; Zafeiratos, S. A mini review of in situ near-ambient pressure XPS studies on non-noble, late transition metal catalysts. Catal. Sci. Technol. 2019, 9, 3851–3867. [Google Scholar] [CrossRef]
- Titus, D.; James Jebaseelan Samuel, E.; Roopan, S.M. Chapter 12—Nanoparticle characterization techniques. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–319. [Google Scholar]
- Yang, Y.; Xu, Y.; Ding, H.; Yang, D.; Cheng, E.; Hao, Y.; Wang, H.; Hong, Y.; Su, Y.; Wang, Y.; et al. Cu/ZnOx@UiO-66 synthesized from a double solvent method as an efficient catalyst for CO2 hydrogenation to methanol. Catal. Sci. Technol. 2021, 11, 4367–4375. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Joo, O.-S.; Jung, K.-D.; Moon, I.; Rozovskii, A.Y.; Lin, G.I.; Han, S.-H.; Uhm, S.-J. Carbon Dioxide Hydrogenation To Form Methanol via a Reverse-Water-Gas-Shift Reaction (the CAMERE Process). Ind. Eng. Chem. Res. 1999, 38, 1808–1812. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal–Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Portha, J.-F.; Parkhomenko, K.; Kobl, K.; Roger, A.-C.; Arab, S.; Commenge, J.-M.; Falk, L. Kinetics of Methanol Synthesis from Carbon Dioxide Hydrogenation over Copper–Zinc Oxide Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13133–13145. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Tsung, C.-K.; Yamada, Y.; Yang, P.; Somorjai, G.A. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009, 8, 126–131. [Google Scholar] [CrossRef] [PubMed]

| Sample | SSA (m2/g) | Pore Volume (cm3/g) | Cu wt% | Zn wt% |
|---|---|---|---|---|
| Commercial catalyst | 97 | 0.188 | 59.9 | 27.5 |
| Cu/ZnO/Al2O3/MgO | 38 | 0.103 | 61.7 | 27.7 |
| UiO-66 | 1238 | 0.471 | - | - |
| Cu/ZnO/UiO-66 | 561 | 0.200 | 12 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duma, Z.G.; Dyosiba, X.; Moma, J.; Langmi, H.W.; Louis, B.; Parkhomenko, K.; Musyoka, N.M. Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks. Catalysts 2022, 12, 401. https://doi.org/10.3390/catal12040401
Duma ZG, Dyosiba X, Moma J, Langmi HW, Louis B, Parkhomenko K, Musyoka NM. Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks. Catalysts. 2022; 12(4):401. https://doi.org/10.3390/catal12040401
Chicago/Turabian StyleDuma, Zama G., Xoliswa Dyosiba, John Moma, Henrietta W. Langmi, Benoit Louis, Ksenia Parkhomenko, and Nicholas M. Musyoka. 2022. "Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks" Catalysts 12, no. 4: 401. https://doi.org/10.3390/catal12040401
APA StyleDuma, Z. G., Dyosiba, X., Moma, J., Langmi, H. W., Louis, B., Parkhomenko, K., & Musyoka, N. M. (2022). Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks. Catalysts, 12(4), 401. https://doi.org/10.3390/catal12040401