2.1. Preparation and SiO2 Encapsulation of SnZnCu Microspheres
The SnZnCu microspheres were prepared by a molten LiCl–KCl–CsCl eutectic-based metal emulsion method [
24]. Since the melting points of bulk Cu are about 1080 °C, in the previous work, an annealing temperature of 1000 °C was chosen to melt micro-sized Cu powder [
24]. In this work, Cu powder was replaced by Cu nanowires. As shown in
Figure S1 (see
Supplementary Materials), Cu nanowires with an average diameter of about 120 nm have an initial melting temperature of about 500 °C, and at 700 °C all Cu nanowires, whether their diameters are large or small, are melted. Therefore, an annealing temperature of 750 °C was used to melt Cu nanowires.
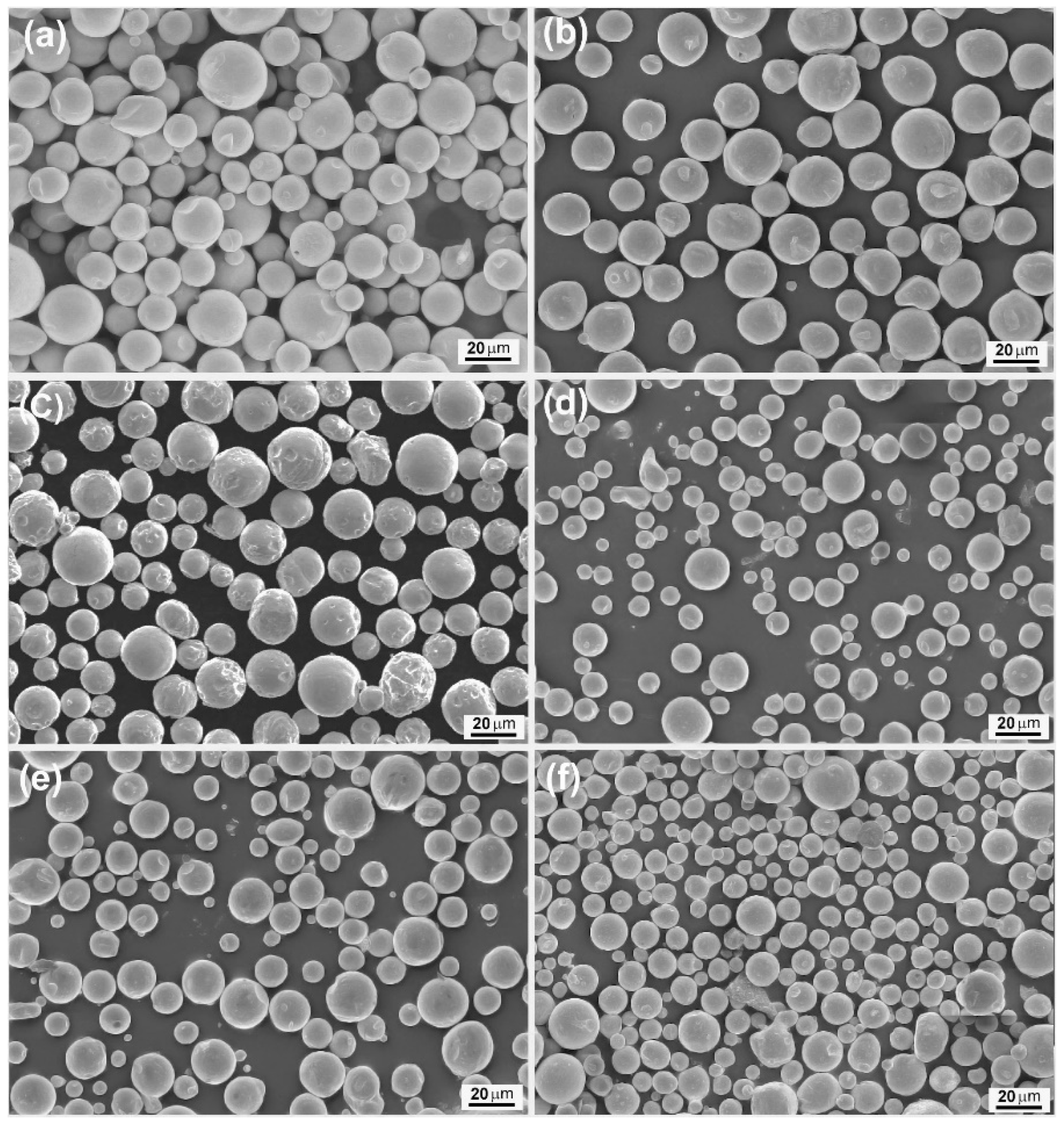
Figure 1 presents the SEM images of some SnZnCu samples (namely SnZn
0.04Cu
0.015, SnZn
0.04Cu
0.02, and SnZn
0.04Cu
0.03) prepared by the molten salt-based metal emulsion method. For the sake of comparison, the SEM images of Sn, SnCu
0.015, and SnZn
0.04 are also shown. It is obvious from these SEM images that all the products of different chemical compositions are dominantly composed of spherical microparticles, and the diameters of these microspheres are typically in the range of several micrometers to more than twenty micrometers under the employed experimental conditions. Since the metal microspheres originate from rapid solidification of the metal droplets in the molten salts, this result suggests that the Sn-based liquid metal can be ultrasonically emulsified into the molten LiCl–KCl–CsCl eutectic as micro-sized droplets, and such molten salt-based metal emulsion method is an effective way for preparing well-shaped SnZnCu microspheres.
Encapsulation of SnZnCu microspheres in SiO
2 is achieved by a sol–gel method [
25].
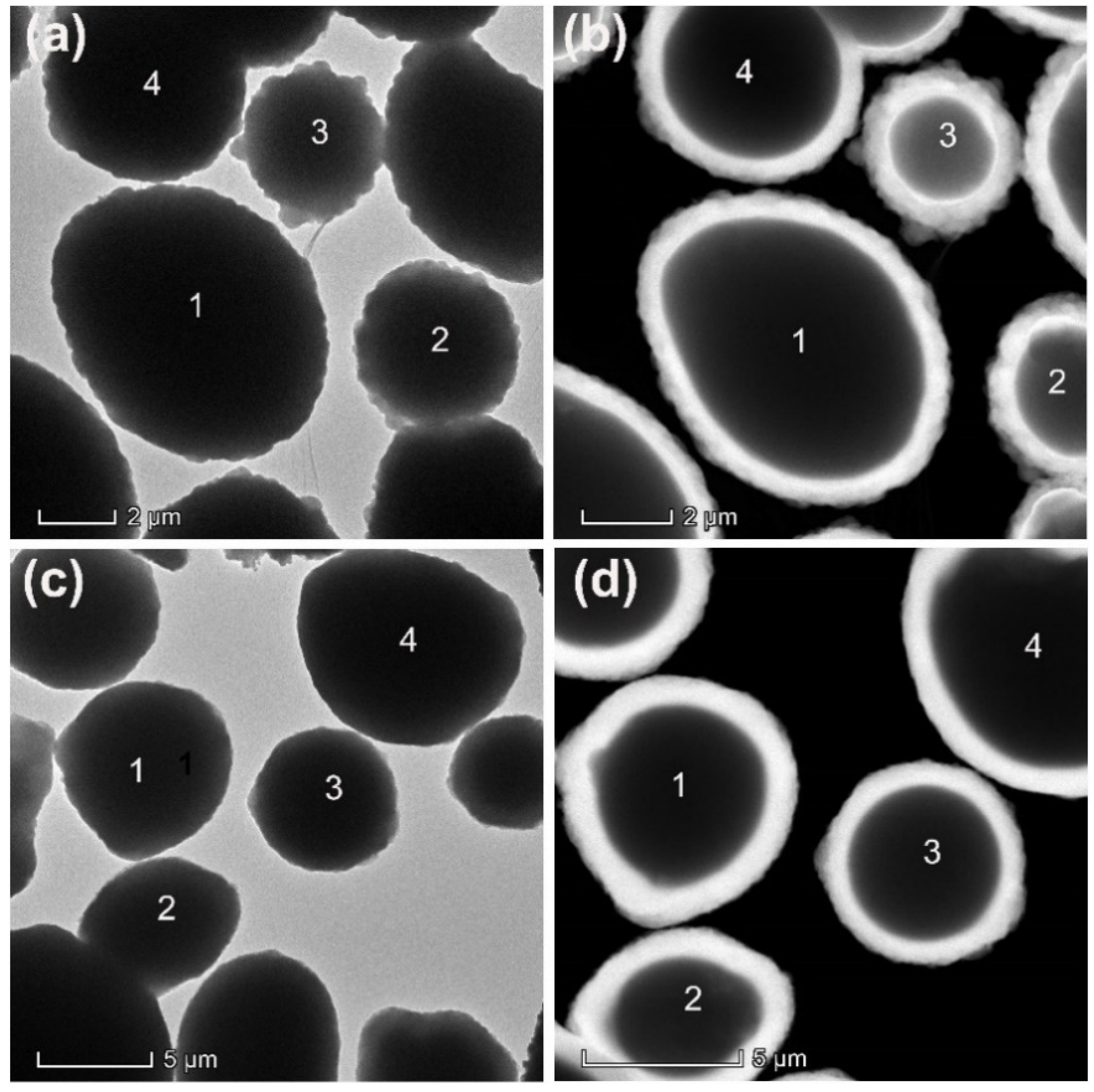
Figure 2 presents the SEM, TEM, and scanning transmission electron microscopy (STEM) images of SnZn
0.04Cu
0.015 after being encapsulated in SiO
2. It is clear from the TEM and STEM images that these metal microspheres are coated with a uniform layer of SiO
2 whose thickness is about 260 nm under the employed experimental conditions. In addition, the thickness of the SiO
2 layer can be easily raised by simply repeating the encapsulation procedure. As shown in
Figure 3, the thickness of the SiO
2 layer can be raised to more than 700 nm or 1 micrometer after repeating encapsulation three or five times. These results suggest that this sol–gel method is extremely effective for coating SnZnCu microspheres with a uniform SiO
2 layer of desired thickness. In order to ensure the practical application of SiO
2-encapsulated SnZnCu microspheres as PCMs for latent heat storage, the SiO
2 layer should have the ability to prevent the leakage of liquid metal cores during operation. The anti-leakage experiment is conducted at 400 °C, a temperature well above the melting point of SnZnCu microspheres. Since the metals might form a thin layer of oxide that may help to prevent their leakage if the microspheres are tested in air at 400 °C, the anti-leakage of the SiO
2 layer is evaluated in the atmosphere of N
2.
Figure 4 presents the SEM images of SiO
2-encapsulated SnZn
0.04Cu
0.015 microsphere (encapsulation times = 3) before and after calcination at 400 °C in N
2 for 6 h. No obvious change in the morphology of these microcapsules can be observed before and after calcination, indicating that the SiO
2 layer as the shell of SnZnCu microcapsules can prevent the leakage of liquid metals during operation.
2.2. Effect of Addition of Cu and Zn on Both Phase Composition of Sn Microspheres and the Undercooling of Sn Microcapsules
Thermodynamically, undercooling is an inherent feature of liquid to solid phase transition via homogeneous nucleation. Unlike heterogeneous nucleation which starts at nucleation sites on surfaces or pre-existing nuclei in the old phase, homogeneous nucleation needs the creation of new nuclei of very small size that are thermodynamically unstable and thus requires the undercooling of the medium. Doping of Sn with trace elements may introduce new clusters as nucleation sites or new phases whose surface can provide nucleation sites to induce heterogeneous nucleation and thus reduce the undercooling. Therefore, understanding the phase composition of doped Sn will help to identify which phase can provide more effective nucleation sites for heterogeneous nucleation.
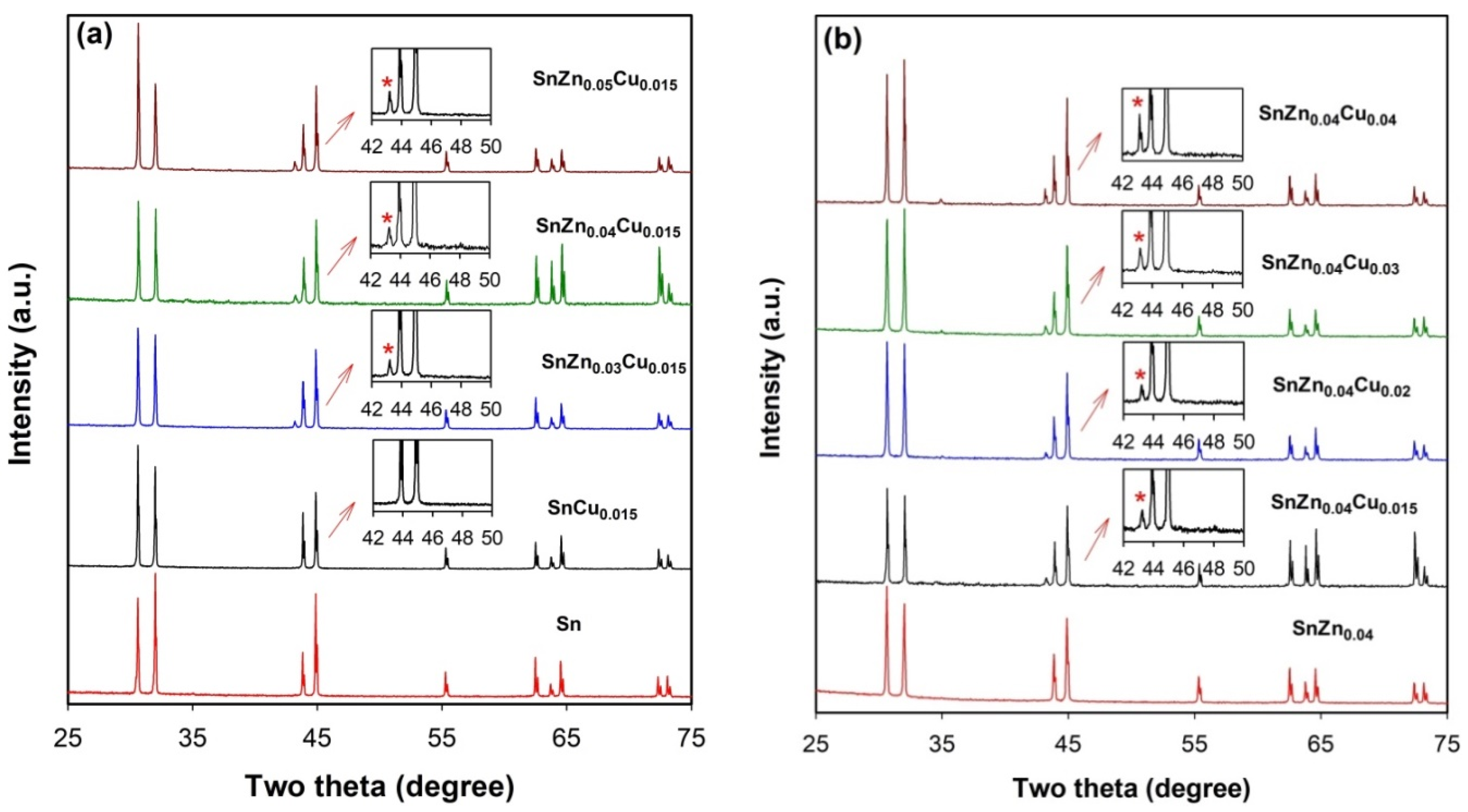
Figure 5 presents the XRD patterns of as-prepared SnZnCu microspheres with different amounts of Zn (
Figure 5a) or different amounts of Cu (
Figure 5b). For comparison, the XRD patterns of Sn, SnCu
0.015, and SnZn
0.04 are also shown. For all microspheres, the peaks observed at 2θ of 30.6°, 32.0°, 43.9°, 44.9°, 55.4°, 62.5°, 63.8°, 64.6°, 72.4°, and 73.1° can all be indexed to metal Sn with a tetragonal structure (space group I41/amd, JCPDS 04-0673) and correspond to the diffractions of (200), (101), (220), (211), (301), (112), (400), (321), (420), and (411) planes, respectively. The absence of the diffraction peaks of Sn oxides, Cu oxides, or Zn oxides indicates that the Ar gas protection can avoid the oxidation of metal droplets during ultrasonic emulsification. In the XRD patterns of SnCu
0.015 microspheres (
Figure 5a), no peaks which can be attributed to Cu or Sn–Cu intermetallic compounds can be observed. A similar phenomenon can be found in the XRD patterns of SnZn
0.04 microspheres where no diffraction peaks of Zn appear (
Figure 5b). According to the phase diagrams of Sn–Cu [
26] and Sn–Zn [
27], there is a eutectic reaction L = Cu
6Sn
5 + Sn at 1.66 at.% Cu and a temperature of 227 °C in the Sn–Cu system and a eutectic reaction L = Zn + Sn at about 14.9 at.% Zn and 199 °C in the Sn–Zn system. Hence, at a low solidification rate, Cu
6Sn
5 or Zn phase should be observed when the temperature of the system reaches room temperature. No Cu
6Sn
5 or Zn phase observed in SnCu
0.015 or SnZn
0.04 microspheres indicate that Cu or Zn is highly dispersed in the Sn matrix due to rapid cooling.
Owing to the much lower solubility of Cu in Sn compared to that of Zn in Sn at a temperature close to the melting point of Sn [
26,
27], however, new peaks appear in the XRD patterns of SnCu microspheres when the amount of Cu is raised to 3.0 at.% (
Figure S2). These peaks at 2θ of 30.1°, 42.3°, and 43.3° can be attributed to the diffractions of (22-1), (132), and (42-2) of the intermetallic compound Cu
6Sn
5 with a monoclinic structure (space group C2/c, JCPDS 45-1488). The formation of the Cu
6Sn
5 phase, rather than the Cu phase, is consistent with the result obtained from the Sn–Cu phase diagram for a system containing small amounts of Cu [
26]. However, such an intermetallic phase cannot be found when both Zn and Cu are added to Sn, e.g., 3.0–5.0 at.% of Zn is added into SnCu
0.015 (see
Figure 5a) or 1.5–4.0 at.% of Cu is added into SnZn
0.04 (see
Figure 5b). Instead, a new peak at 2θ of about 43.2° appears in the XRD patterns of these SnZnCu microspheres. Since this peak is absent in the XRD patterns of SnCu
0.015, SnCu
0.02, SnCu
0.03, and SnZn
0.04 microspheres, it is speculated that this peak should be related to the formation of another intermetallic phase Cu
5Zn
8 with a cubic structure and correspond to the diffraction of (330) plane (JCPDS 41-1435). This Cu
5Zn
8 phase has been reported to be formed via an interfacial reaction between the Sn–Zn solders and Cu substrate [
28] or by melting Cu and Zn powders in an electric arc discharge synthesis [
29]. In fact, the Cu
5Zn
8 phase is an equilibrium phase for a system containing 3.0–5.0 at.% of Zn and 1.5 at.% of Cu at room temperature based on the isothermal sections of the Sn–Zn–Cu system at 210 °C [
30]. The formation of the Cu
5Zn
8 phase in SnZnCu microspheres demonstrates that Cu atoms are preferentially segregated to Zn atoms, rather than Sn atoms. As will be shown below, the presence of the Cu
5Zn
8 phase is much more effective than the Cu
6Sn
5 phase for inducing heterogeneous nucleation that may significantly reduce the undercooling.
The undercooling in this work is defined as the difference between the onset temperatures of melting (T
onset,m) and solidification (T
onset,s) in DSC measurement [
20,
21,
23]. The value of the onset temperature of melting or solidification was determined by the intersection of the heat flow curve with the baseline during the melting or solidification process. As shown in
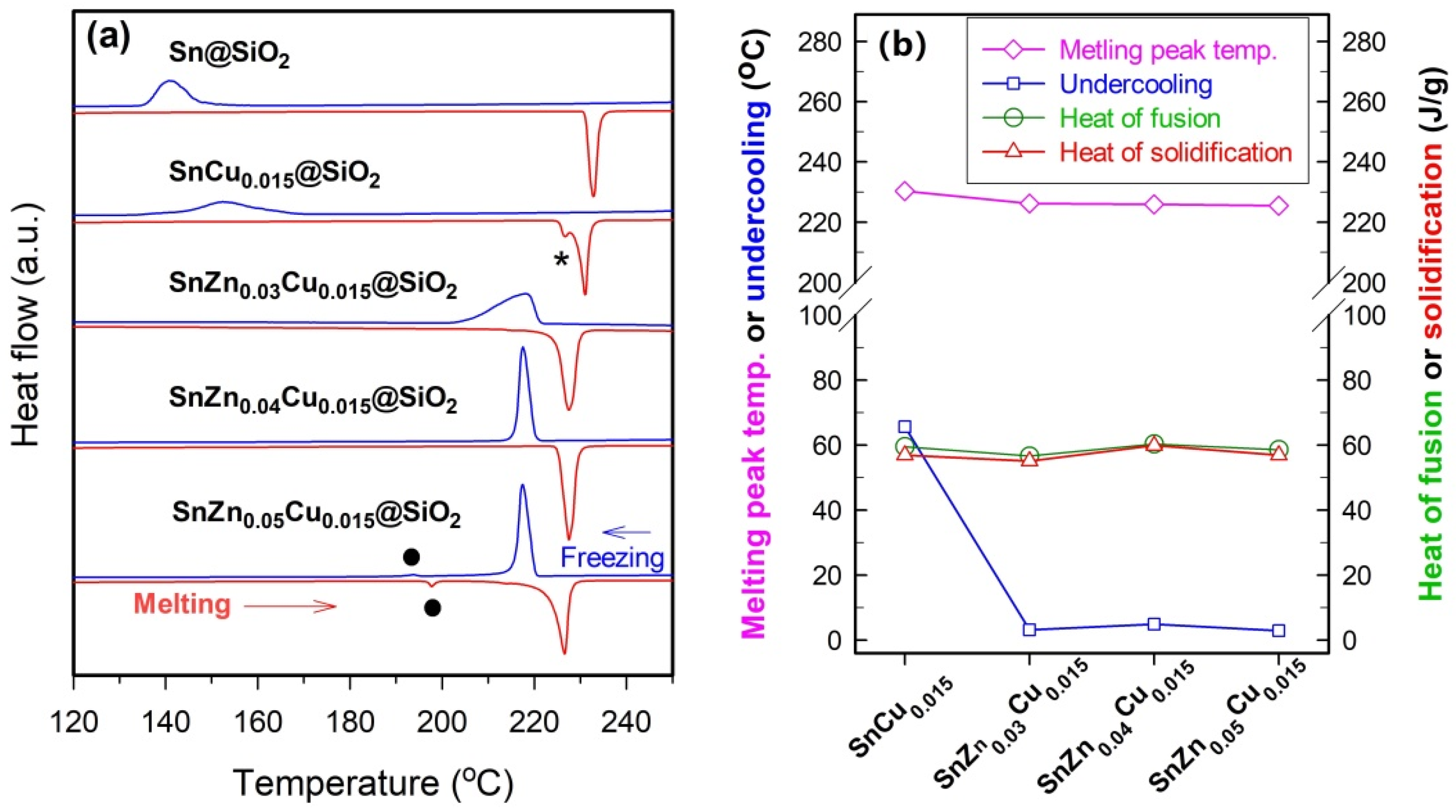
Figure 6a, the onset temperature of solidification of Sn in Sn@SiO
2 microspheres is about 147.0 °C and the undercooling obtained is around 83.7 °C. The very high undercooling observed for Sn@SiO
2 microspheres indicates that the homogenous nucleation dominates in the Sn solidification process despite the presence of SiO
2 shell. Although the addition of small amounts of Cu (1.5 at.%) may facilitate the solidification of Sn and thus SnCu
0.015@SiO
2 microspheres have a lower undercooling (about 57 °C) compared to Sn@SiO
2 microspheres (
Figure 6b), the resulting undercooling is still too high for practical application. In addition, a very broad freezing peak is observed in SnCu
0.015@SiO
2 microspheres. Since under the same experimental conditions, a wider melting/freezing peak implies a broader melting/freezing range despite that the value of the peak width depends on the heating/cooling rate and the mass of the sample measured, the broad freezing peak suggests a wide temperature range needed for totally releasing the latent heat. This phenomenon also exists in SnCu
0.02@SiO
2 or SnCu
0.03@SiO
2 microspheres with the presence of the Cu
6Sn
5 phase (
Figure S3 in
Supplementary Materials). These results hint that the Cu
6Sn
5 phase cannot provide very effective sites for rapid nucleation and solidification of liquid Sn.
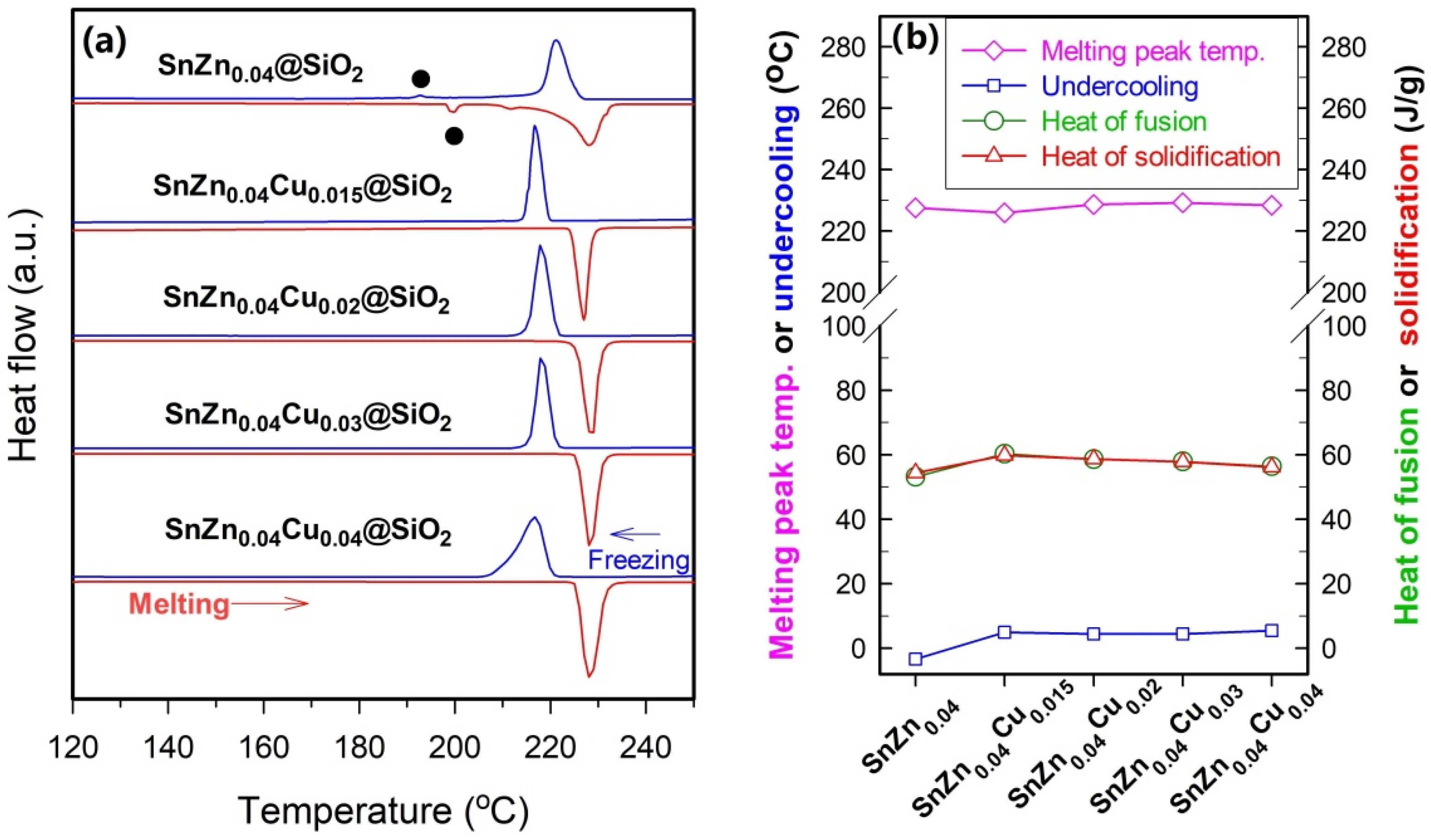
To further reduce the undercooling and narrow the freezing range, 3.0–5.0 at.% of Zn, which is much higher than 1.1 at.% used in Sn-based solders, is introduced into SnCu
0.015@SiO
2 microspheres (
Figure 6). The addition of 3.0–5.0 at.% Zn can significantly reduce undercooling. For example, the values of undercooling of SnZn
0.03Cu
0.015@SiO
2, SnZn
0.04Cu
0.015@SiO
2, and SnZn
0.05Cu
0.015@SiO
2 microspheres are about 3.2, 4.9, and 2.8 °C, respectively. Furthermore, the exothermic peak of SnZn
0.04Cu
0.015@SiO
2 or SnZn
0.05Cu
0.015@SiO
2 microspheres is much sharper than that of Sn@SiO
2 or SnCu
0.015@SiO
2 microspheres, suggesting that addition of 4.0 or 5.0 at.% Zn can greatly reduce the freezing range. As indicated by the difference in phase composition between SnCu
0.015@SiO
2 and SnZn
0.03–0.05Cu
0.015@SiO
2 microspheres (
Figure 5a), the significant promotion effect on the solidification of liquid Sn observed in these SnZnCu@SiO
2 microspheres should be related to the formation of the Cu
5Zn
8 phase. In addition, by comparing these observations with the result obtained from SnCu
0.03@SiO
2 microspheres in the presence of Cu
6Sn
5 phase, it is inferred that the Cu
5Zn
8 phase can provide much more effective sites for heterogeneous nucleation than the Cu
6Sn
5 phase. This result is similar to the observation in Sn–Cu–Ni solders that the Ni
3Sn
4 phase is more effective for undercooling suppression than the Cu
6Sn
5 phase [
22].
Besides reducing the undercooling and narrowing the freezing range, the addition of 4.0 at.% Zn also results in a sharp, symmetrical melting peak with its peak temperature being slightly lower than that of Sn@SiO
2 microspheres (
Figure 6a). The sharp melting peak hints at a very narrow melting range, which is a common feature of a pure substance or a eutectic mixture, hinting that Sn–Zn–Cu eutectic forms in SnZn
0.04Cu
0.015@SiO
2 microspheres probably because the chemical composition of SnZn
0.04Cu
0.015 microspheres is close to that of Sn–Zn–Cu eutectic. According to the method for determination of eutectic by DSC [
31], the speculation that the addition of 4 at.% Zn leads to a chemical composition close to the composition of Sn–Zn–Cu eutectic is supported by the result that when 3.0 or 5.0 at.% Zn is added, the melting peak is obviously broader than that of SnZn
0.04Cu
0.015@SiO
2 microspheres (
Figure 6a). In addition, when 5.0 at.% Zn is added, a pair of new but weak peaks appear in the melting and solidification processes (indicated by solid circles in
Figure 6a), indicating that 5.0 at.% Zn is obviously larger than the amount of Zn in the Sn–Zn–Cu eutectic. Based on the Sn–Zn phase diagram [
27], an endothermic peak temperature of around 197 °C hints at the formation of SnZn
0.15 eutectic in SnZn
0.05Cu
0.015@SiO
2 microspheres. It is also interesting to note that the chemical composition of SnZn
0.04Cu
0.015 microspheres is very similar to that of another Sn-based ternary eutectic, namely Sn–Ag–Cu eutectic with 3.5 wt.% Ag and 0.9 wt.% Cu (namely about 3.81 at.% Ag and 1.66 at.% Cu) [
32].
The above results demonstrate that addition of 4.0 at.% Zn is crucial for achieving a type of SnZnCu@SiO
2 microsphere (namely SnZn
0.04Cu
0.015@SiO
2 microsphere) with very low undercooling and narrow freezing/melting peak width via the formation of both Sn–Zn–Cu ternary eutectic and intermetallic phase Cu
5Zn
8. In order to further understand the effect of Cu amount on the phase change properties of SnZnCu@SiO
2 microspheres, the amount of Cu is raised from 1.5 to 4.0 at.% (
Figure 7). In the absence of Cu, the primary endothermic peak of SnZn
0.04@SiO
2 microspheres is broad and asymmetrical, and a pair of weak peaks appear in the melting and solidification processes (indicated by solid circles in
Figure 7a), hinting at the formation of a small amount of SnZn
0.15 eutectic. Based on the Sn–Zn phase diagram [
27], the observed broad and asymmetrical endothermic peak is consistent with the fact that its chemical composition greatly deviates from that of Sn–Zn eutectic and is the reason for a negative value (−3.41 °C) of undercooling obtained in SnZn
0.04@SiO
2 microspheres. It is interesting to find that raising the amount of Cu from 1.5 at.% to 2.0 or 3.0 at.% has no significant impact on both the undercooling and the freezing/melting peak width, and all three types of SnZnCu@SiO
2 microspheres, namely SnZn
0.04Cu
0.015@SiO
2, SnZn
0.04Cu
0.02@SiO
2, and SnZn
0.04Cu
0.03@SiO
2 microspheres, own a very low undercooling and sharp freezing/melting peaks. However, when the amount of Cu is raised to 4.0 at.%, both the melting peak and the freezing peak become broader compared to those of SnZn
0.04Cu
0.015@SiO
2 microspheres, suggesting that the chemical composition has obviously deviated from that of Sn–Zn–Cu ternary eutectic.
2.3. Thermal Cycling Stability of SnZnCu@SiO2 Microspheres and the Factors Governing Thermal Stability
The understanding of the thermal cycling stability of SnZnCu@SiO
2 microspheres is important for their long-term application. Since SnZn
0.04Cu
0.015@SiO
2, SnZn
0.04Cu
0.02@SiO
2, and SnZn
0.04Cu
0.03@SiO
2 microspheres show similar phase change properties despite having different amounts of Cu, the thermal cycling stability of all three samples is investigated by measuring their phase change properties after repeated heating-cooling cycles between 200 and 250 °C.
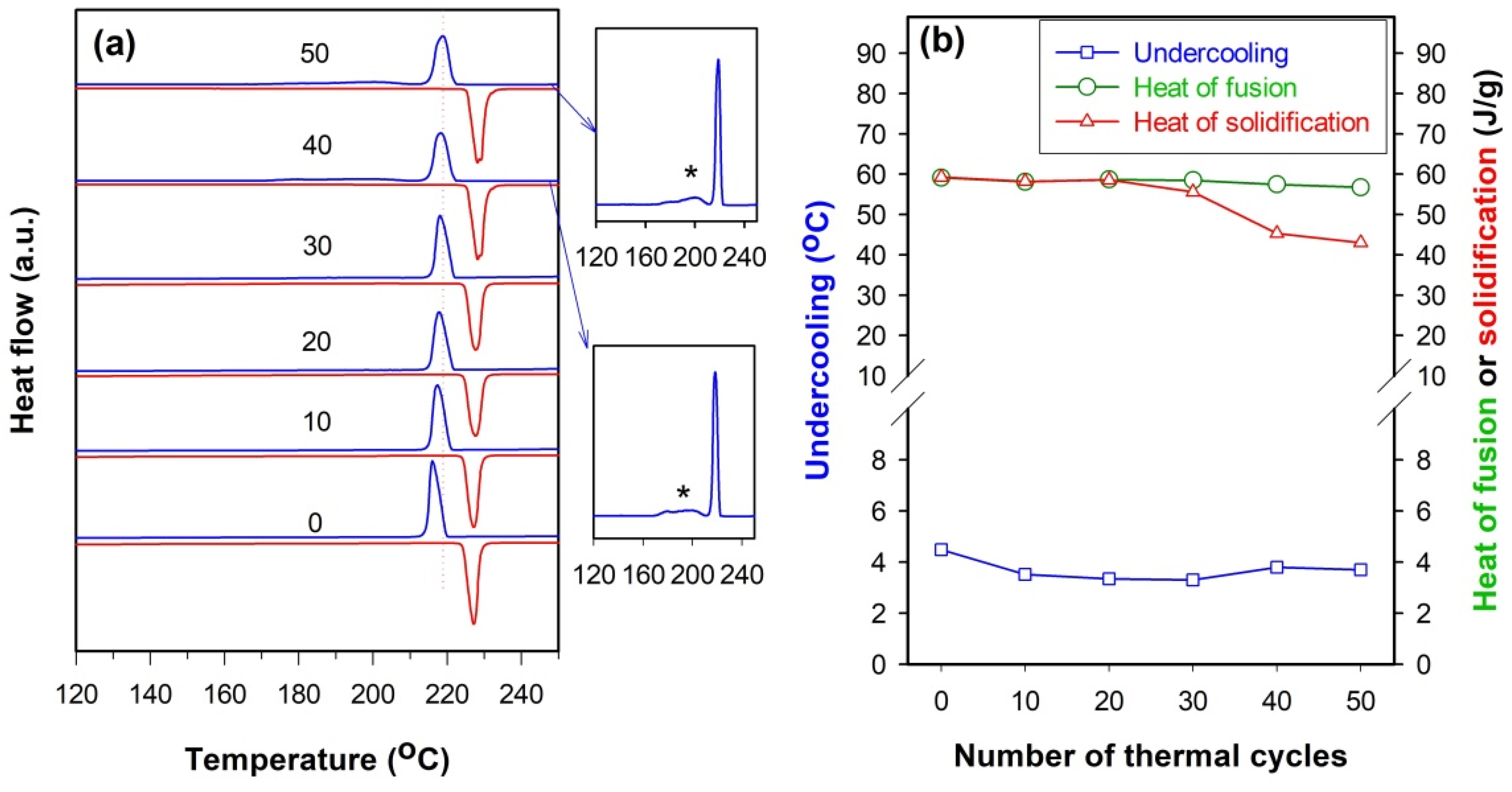
Figure 8a displays the DSC curves of the SnZn
0.04Cu
0.015@SiO
2 microspheres after being subjected to different numbers of thermal cycles. The plot of both the undercooling and the heat of fusion or solidification as a function of the number of thermal cycles is shown in
Figure 8b (note that the heat of solidification is calculated based on the first exothermic peak if there are two or more peaks appearing in the solidification process). It is clear from
Figure 8 that, although the freezing peak gradually shifts to the right and thus the undercooling decreases slightly as the number of thermal cycles increases to 30, there is no remarkable change in both heat of fusion and heat of solidification with the latter being almost equal to the former if the number of thermal cycles is not more than 30. When the number of thermal cycles reaches 40 or above, however, a new broad exothermic peak appears in the low-temperature region (indicated by a star in the insets of
Figure 8a) and thus results in an obvious decrease in heat of solidification that is calculated on the first exothermic peak (see
Figure 8b). These observations suggest that SnZn
0.04Cu
0.015@SiO
2 microspheres become thermally unstable after 40 thermal cycles.
In the thermal cycling test of the SnZn
0.04Cu
0.02@SiO
2 microspheres, a similar thermal instability can be observed (see
Figure 9). However, the broad exothermic peak is observed clearly only when the number of thermal cycles reaches more than 80 (see insets of
Figure 9a). This indicates that the SnZn
0.04Cu
0.02@SiO
2 microspheres are more thermally stable than the SnZn
0.04Cu
0.015@SiO
2 microspheres and a rise in the amount of Cu can improve the thermal cycling stability of SnZnCu@SiO
2 microspheres. The positive effect of the amount of Cu on the thermal cycling stability of SnZnCu@SiO
2 microspheres is further supported by the result obtained from the SnZn
0.04Cu
0.03@SiO
2 microspheres. As shown in
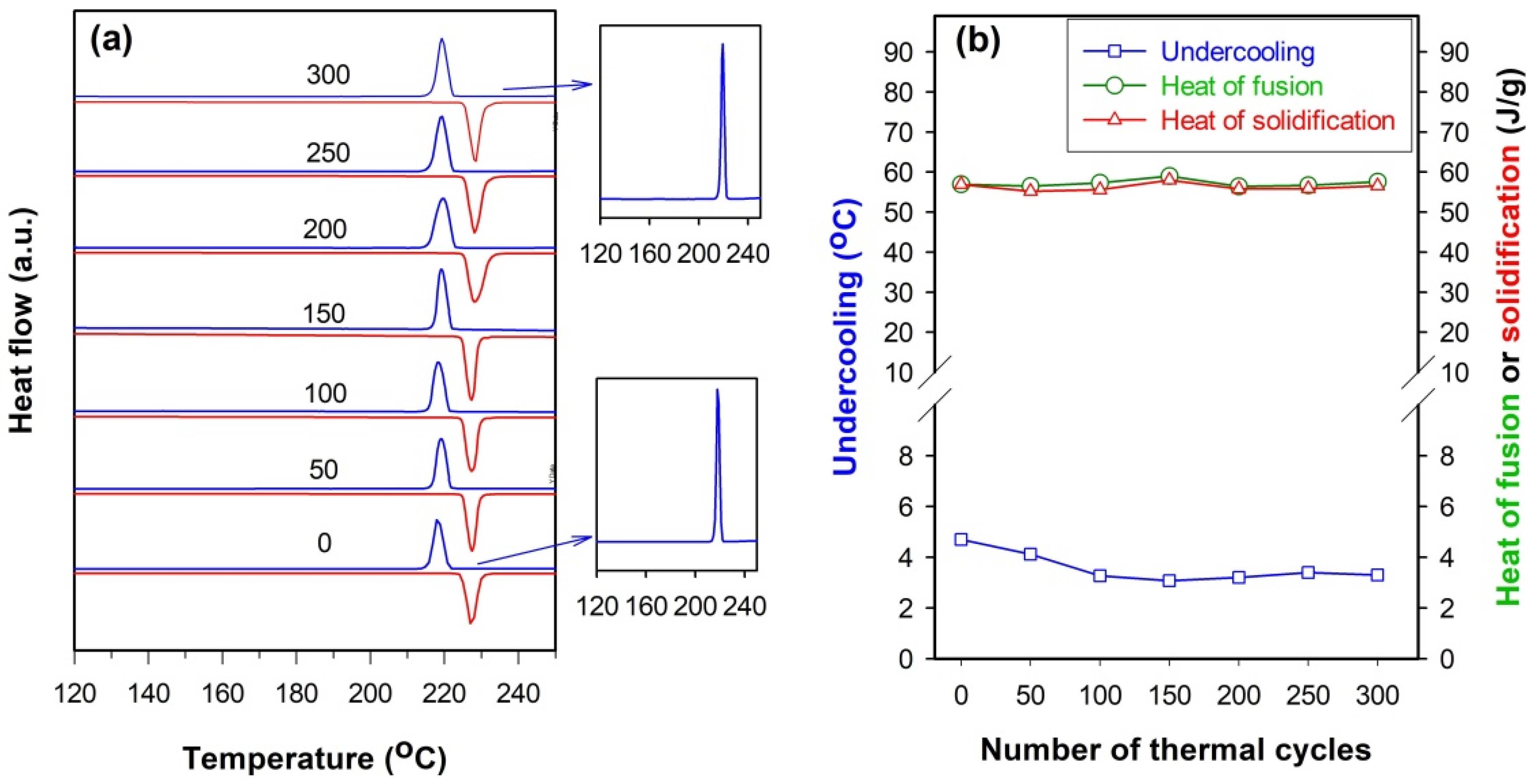
Figure 10, except that the undercooling decreases slightly as the number of thermal cycles increases to 150, there is almost no change in heat of fusion or solidification of SnZn
0.04Cu
0.03@SiO
2 microspheres even after 300 thermal cycles. Similar to the untreated sample, no broad exothermic peak appears in the solidification curve after 300 thermal cycles (see insets of
Figure 10a), hinting that the thermal cycling stability of the SnZnCu@SiO
2 microspheres can be remarkably improved as the amount of Cu increases to about 3.0 at.%.
The high thermal stability of the SnZn
0.04Cu
0.03@SiO
2 microspheres, as well as their very low undercooling and narrow freezing/melting peak width, suggests that they should be a promising PCM for latent heat storage.
Table 1 gives a comparison of the properties between SnZn
0.04Cu
0.03@SiO
2 microspheres and some PCMs with a melting point ranging from 220 to 240 °C, including encapsulated Sn and some nitrate salt-based PCMs. Besides having very poor thermal conductivity [
4], it is clear from
Table 1 that the latent heat of fusion on a volume basis of the nitrate-based PCMs with a melting point close to that of Sn is also less than that of SnZn
0.04Cu
0.03@SiO
2 microspheres, giving SnZn
0.04Cu
0.03@SiO
2 microspheres an edge over those nitrate salts as PCMs used in the case where a limited room is available for PCMs.
As has been mentioned above, a new exothermic peak normally appears in the solidification process when the SnZnCu@SiO
2 microspheres are not thermally stable. Compared with the first exothermic peak, this peak is much broader and appears in the lower temperature region. This feature demonstrates that the solidification of some liquid metal needs high undercooling and occurs slowly, which is similar to the solidification process in the SnCu
0.015@SiO
2 (see
Figure 6a) or SnCu
0.02@SiO
2 microspheres (see
Figure S3 in
Supplementary Materials). Since the Cu
5Zn
8 phase contributes significantly to both much lower undercooling and narrower freezing peak of SnZnCu@SiO
2 microspheres compared to those of SnCu@SiO
2 microspheres, it is speculated that the appearance of a broad exothermic peak indicates a possibility of the decomposition of this phase, which has been observed in the soldered Sn–Ag–Zn/Cu interface [
38]. Therefore, the Cu
5Zn
8 phase was investigated by XRD to understand the origin of thermal instability observed in the SnCuZn@SiO
2 microspheres after thermal cycling.
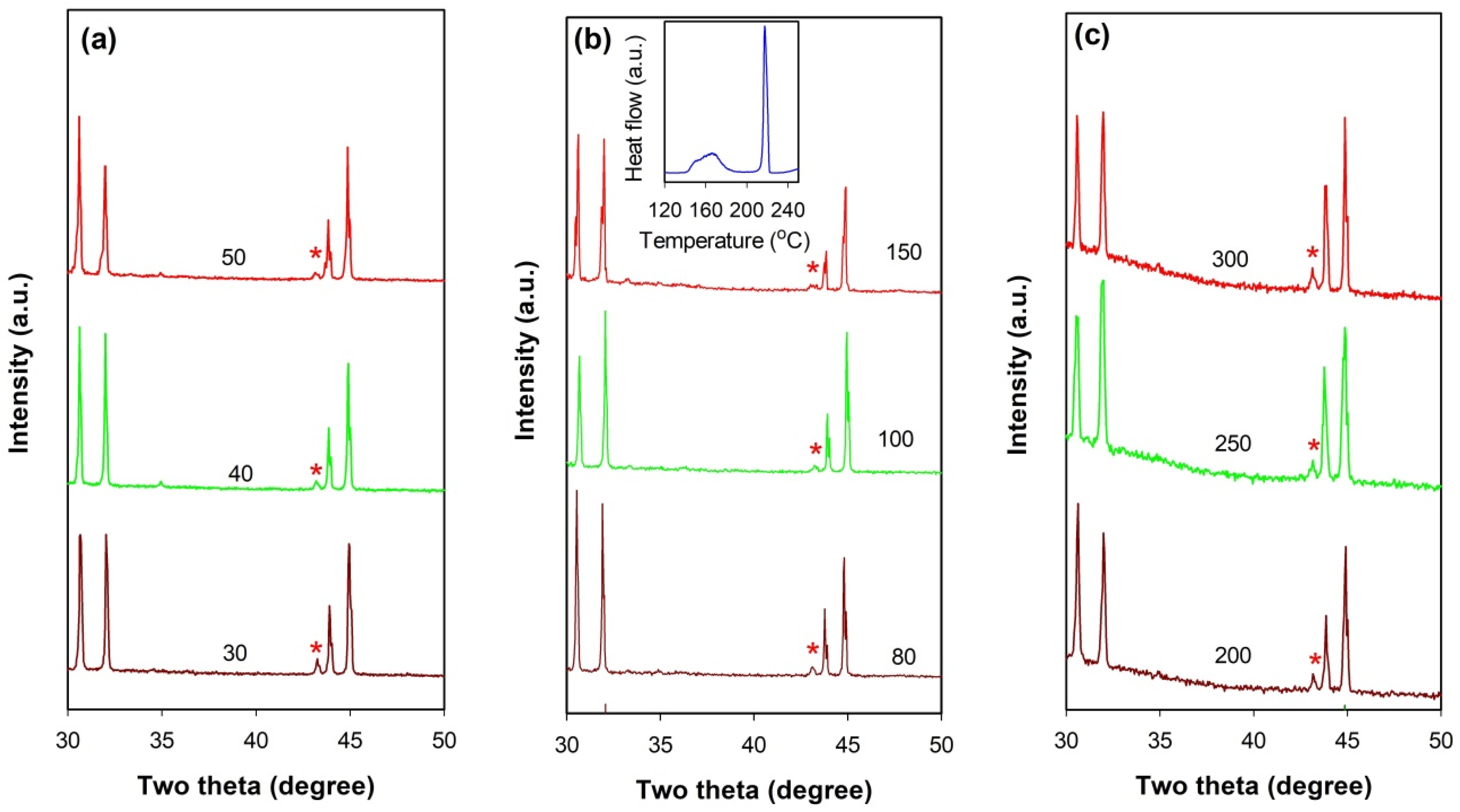
Figure 11 presents a comparison between the XRD patterns of thermally stable and unstable SnZnCu@SiO
2 microspheres after thermal cycling. For all three types of SnZnCu@SiO
2 microspheres, whether thermally stable or unstable, the Cu
5Zn
8 phase can be found in their XRD patterns (indicated by a red star). However, an obvious decrease in diffraction intensity of this phase is observed in the SnZn
0.04Cu
0.015@SiO
2 microspheres after 40 or 50 thermal cycles compared to those after 30 thermal cycles (
Figure 11a). The crystal size of the Cu
5Zn
8 phase estimated by Scherrer’s equation is reduced from about 50 nm (after 30 thermal cycles) to 30 nm (after 50 thermal cycles). A similar phenomenon can also be observed in the SnZn
0.04Cu
0.02@SiO
2 microspheres after 100 thermal cycles (
Figure 11b), especially the sample subjected to another 50 thermal cycles (namely 100 thermal cycles totally) which becomes more unstable as indicated by an obvious rise in the peak intensity of the second exothermic peak in the DSC curve (see inset of
Figure 11b). The crystal size of the Cu
5Zn
8 phase is reduced from about 45 nm (after 80 thermal cycles) to 20 nm (after 150 thermal cycles). However, this phenomenon cannot be observed in the SnZn
0.04Cu
0.03@SiO
2 microspheres after 300 thermal cycles (
Figure 11c). These observations imply the occurrence of the decomposition of Cu
5Zn
8, and the thermal cycling stability of SnZnCu@SiO
2 microspheres is strongly related to this phase. The fact that the decomposition of Cu
5Zn
8 does not occur in the SnZn
0.04Cu
0.03@SiO
2 microspheres after 300 thermal cycles demonstrates that an increase in the amount of Cu can improve its stability. This can be understood from the thermodynamic point of view. The decomposition of Cu
5Zn
8 during thermal cycling should take place at a temperature where Sn is liquefied. When the decomposition of Cu
5Zn
8 occurs, the Cu atoms formed will be highly dispersed into the liquid Sn phase as suggested by the above XRD result that no Cu phase is detected even at room temperature. Thermodynamically, therefore, an increase in the amount of Cu present in the liquid Sn phase is unfavorable for the decomposition of Cu
5Zn
8.
2.4. Application of SnZn0.04Cu0.03@SiO2 Microspheres in Fischer–Tropsch Synthesis for Stabilizing the Temperature of the Catalyst Bed
Fischer–Tropsch synthesis is an important process for converting coal or natural gas to liquid fuels. As a highly exothermic catalytic reaction (ΔH = −165 kJ/mol of CO) [
39], efficient heat removal from the packed bed is critical because an undesirable temperature rise may lead to catalyst deactivation, negative effect on product selectivity, and even thermal runaway. To facilitate the heat transfer across the catalyst bed, improving the thermal conductivity of the catalysts, e.g., depositing the catalyst on monolith [
40], growing the catalyst on metal foam [
41], and entrapping metal microfiber into the catalyst [
18], is a common strategy. In contrast, this work presents a tentative study on the possibility of using metal-based PCMs to improve the thermal stability of the catalyst bed by embedding SnZn
0.04Cu
0.03@SiO
2 microspheres into the Co/SiO
2 catalyst.
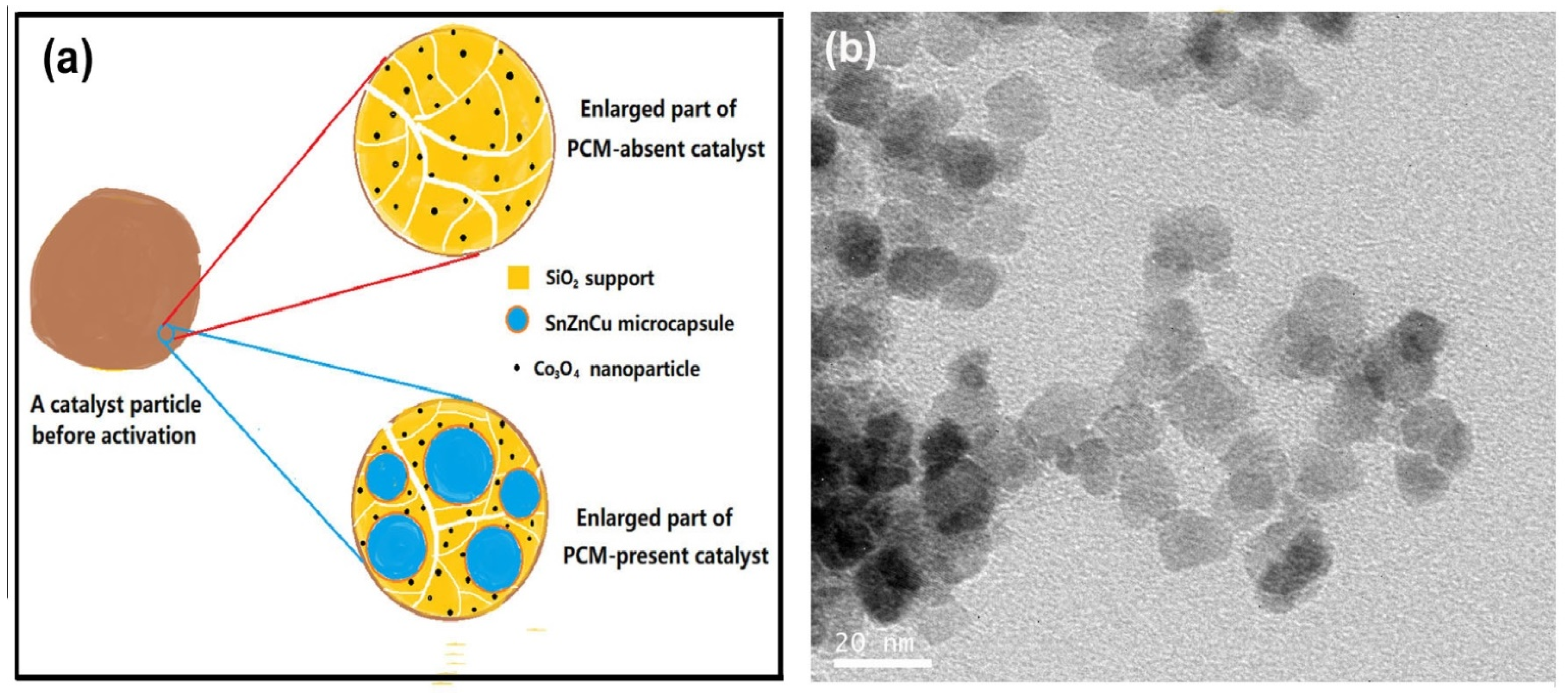
Figure 12a illustrates the structural difference between PCM-absent and PCM-present catalysts before activation. To make a reasonable comparison between PCM-absent and PCM-present catalysts, the pre-synthesized Co
3O
4 nanoparticles (see
Figure 12b), rather soluble cobalt salts, are used as the precursor to ensure that the size of active component Co in two catalysts is the same. In addition, the amount of Co loaded in the packed bed in two cases should be as close as possible.
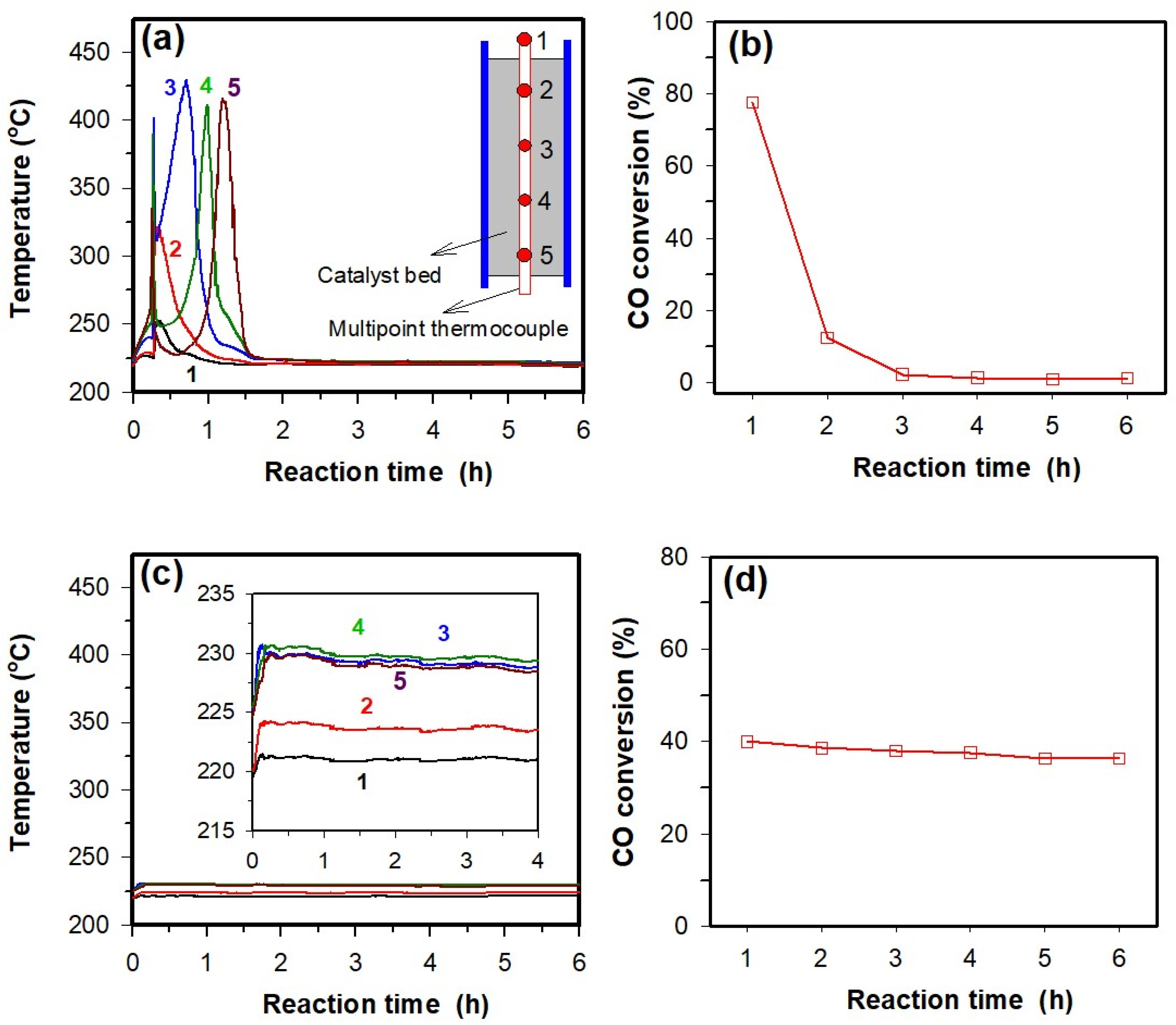
Figure 13 presents the temperature profiles at the centerline of the packed beds of two catalysts measured by a multi-point thermocouple (locations of thermocouple no. 1–5 is shown in the inset of
Figure 13a) and the CO conversions over two catalysts. For the PCM-absent Co/SiO
2 catalyst (
Figure 13a), the temperature of the catalyst bed increases rapidly at the beginning and hot spots with an amplitude of 100–200 °C can be observed. The hot spot is initially located at the top of the catalyst bed and then moves in a downstream direction, probably due to loss of catalyst activity with time. The maximum temperature difference in the axial direction is about 200 °C. After about 1.5 h, the catalyst bed reaches a steady temperature distribution with a negligible temperature difference in the axial direction, suggesting that the catalyst should be totally deactivated. This is confirmed by the data of catalytic activity measured (
Figure 13b), where the conversion of CO is reduced from about 80% to 1% when the catalyst bed reaches a steady temperature distribution. The rapid deactivation of the catalyst should be associated with the high temperature of the hot spot or thermal runaway which may accelerate re-oxidation [
18], carbon deposition [
42], or sintering of cobalt nanocrystals [
43]. As shown in the inset of
Figure 13c, however, the temperature profiles at the centerline of the packed bed of the PCM-present Co/SiO
2 catalyst are quite different from that of PCM-absent Co/SiO
2 catalyst. Hot spots can be suppressed and thus no thermal runaway is found, and the catalyst bed almost reaches a steady temperature distribution during the startup period. The maximum temperature difference in the axial direction is about 6.5 °C (see inset of
Figure 13c), much lower than the value of about 200 °C observed in the PCM-absent Co/SiO
2 catalyst bed. As a result, deactivation of the catalyst due to thermal runaway is not found and the CO conversion remains in a steady state with a value close to 40% (
Figure 13d). These observations demonstrate that the presence of Sn–Zn–Cu PCM in the catalyst can greatly improve the thermal stability of the catalyst bed in Fischer–Tropsch synthesis.
The positive effect on the thermal stability of the catalyst bed observed in the PCM-present Co/SiO
2 catalyst should be related to the fact that Sn–Zn–Cu PCM not only owns a much higher thermal conductivity than the amorphous SiO
2 but also has the ability to store thermal energy released by the reaction. First, because the thermal conductivities of both Cu and Zn (about 386 and 113 W⋅m
−1⋅K
−1 at 293 K, respectively [
19]) are higher than that of Sn (about 67 W⋅m
−1⋅K
−1 at 293 K), the Sn–Zn–Cu PCM should have a thermal conductivity higher than that of Sn. The much higher thermal conductivity of Sn–Zn–Cu PCM compared to the amorphous SiO
2 (about 1 W⋅m
−1⋅K
−1 at 300 K [
44]) allows the bed of the PCM-present catalyst a high heat removal rate (via heat transfer through the walls), which is critical for achieving a steady catalyst bed temperature distribution and avoiding thermal runaway in the case where high CO conversion is high (namely high heat generation rate). Second, since Sn–Zn–Cu PCM can absorb the heat of reaction via solid–liquid phase change when the temperature of catalyst bed reaches its melting point, the presence of PCM can help to suppress the formation of hot spots, especially the one caused by the unexpected, temporary variation in the operating conditions. In addition, since the Sn–Zn–Cu PCM can release the absorbed heat via liquid–solid phase transition, the use of a PCM-containing catalyst may also reduce the electric heating time of the reactor and thus save energy if the PID controlled reaction temperature is set slightly below the melting point of PCM.